Abstract
Background
Galectin‐3 (GAL3), a protein encoded by the LGALS3 gene, plays diverse roles in cancer initiation, progression, and drug resistance. Accordingly, high GAL3 expression in tumor cells is associated with poor prognosis in non‐small cell lung cancer (NSCLC). However, the prognostic impact of GAL3 expression on patients with resected NSCLC receiving platinum‐based adjuvant chemotherapy (AC) remains unclear.
This study aimed to determine the prognostic significance of GAL3 expression in NSCLC patients receiving platinum‐based AC.
Methods
The study included 111 patients with completely resected stages II and IIIA NSCLC who were receiving platinum‐based AC. GAL3 expression in cancer cells was evaluated immunohistochemically according to H‐score (“histo score), with a score of ≥170 considered as high expression. The correlation of GAL3 expression with clinicopathological characteristics and survival was subsequently evaluated.
Results
In survival analysis, GAL3 expression was significantly associated with recurrence‐free survival (RFS) and overall survival (OS). In multivariate analysis, GAL3 expression was an independent predictive factor of RFS rather than OS.
Conclusions
GAL3 expression is a reliable biomarker to predict the prognosis of completely resected NSCLC patients receiving platinum‐based AC.
Keywords: adjuvant chemotherapy, biomarker, galectin‐3, immunohistochemistry, non‐small cell lung cancer
High galectin‐3 expression is associated with poor prognosis in patients with NSCLC. Thus, galectin‐3 expression is a reliable marker in predicting clinical outcomes in patients with completely resected NSCLC treated with platinum‐based adjuvant chemotherapy. This study provides a further understanding of the tumorigenesis and biology of NSCLC, which might be useful in detecting novel prognostic markers or therapeutic targets in completely resected stages II and IIIA NSCLC receiving platinum‐based AC for improving survival outcomes.
INTRODUCTION
Primary lung cancer is the leading cause of cancer‐related mortality worldwide. 1 Although surgical resection is the optimal treatment modality for early‐stage non‐small cell lung cancer (NSCLC), the five‐year survival rates for resectable NSCLC remain unsatisfactory, ranging from 19% for stage IIIA to 63% for stage IA. 2 Adjuvant platinum‐based chemotherapy (AC) improves the survival of patients with completely resected stages II and IIIA NSCLC. However, its effect is still limited, yielding additional improvement of only 4%–15% in five‐year overall survival (OS). 3 , 4 Thus, a further understanding of the tumorigenesis and biology of lung cancer might be useful to detect novel prognostic markers or therapeutic targets in NSCLC for improving survival outcomes.
Galectins are carbohydrate‐binding proteins characterized by their binding affinity for β‐galactoside and by conserved sequences in the carbohydrate‐binding site. 5 They can be classified as prototype, chimera, and tandem. 6 , 7 Human galectin‐3 (GAL3), which is encoded by LGALS3, is the only chimera galectin expressed ubiquitously in healthy adult tissues and various tumor types. 7 , 8 , 9 GAL3 is localized mainly in the cytoplasm and nucleus; however, it is equally expressed on the cell surface and secreted via a nonclassical pathway. 7 , 8 , 9 GAL3 promotes tumor aggressiveness, including increased proliferation, antiapoptotic traits, angiogenesis, tumor cell motility, and metastatic activity. 8 , 9 , 10 , 11 GAL3 is responsible for various biological processes in several cancer types.
In NSCLC, high GAL3 expression in tumor cells is associated with tumor progression and poor prognosis. 12 , 13 , 14 In addition, GAL3 induces chemoresistance by regulating apoptotic activity through phosphorylation and translocation of GAL3, regulation of mitochondrial membrane potential, and regulation of cell survival and caspase pathways. 15 Further, we previously reported that serum anti‐GAL3 autoantibody levels effectively predicted the efficacy of platinum‐based chemotherapy for advanced NSCLC. 16 However, no study has investigated the efficacy of GAL3 as a predictor of AC efficacy in patients with resected NSCLC. Therefore, this study aimed to analyze the association of GAL3 expression with the clinicopathological characteristics and prognosis of patients with surgically resected stages II and IIIA NSCLC and administered adjuvant chemotherapy. To achieve this, we immunohistochemically evaluated GAL3 expression in tumor tissues and conducted a survival analysis.
METHODS
Ethics statements
The study was approved by the Ethics Committee of the Kitasato University School of Medicine (B17‐332) and conducted according to the tenets of the Declaration of Helsinki and its later amendments. All patients were approached based on the approved ethical guidelines, agreed to participate, and could refuse entry and discontinue participation at any time. All participants provided written consent.
Patient selection and specimens
We retrospectively evaluated 111 patients with completely resected stages II and IIIA NSCLC who were administered AC between January 2003 and December 2014 at the Kitasato University Hospital. Patients who received preoperative chemotherapy and/or radiotherapy were excluded. The histological diagnosis was based on the 2015 World Health Organization Classification of Lung and Pleural Tumors criteria. 17 The pathological stage was determined according to the 8th edition of the TNM classification. 18 We retrospectively reviewed the clinicopathological characteristics, including age at surgical resection, sex, tumor differentiation, histological type, pathological TNM (p‐TNM) and stage, smoking habits, nodal status, intratumoral vascular invasion, intratumoral lymphatic invasion, pleural invasion, adjuvant chemotherapy administration, recurrence‐free survival (RFS), and OS. RFS was calculated as the interval between surgery and recurrence or death from the disease. OS was defined as the duration from the date of surgery to the date of death or the end of the follow‐up. Patients who died of other causes or were lost to follow‐up were censored.
Evaluation of immunohistochemical stain
We sliced 10% formalin‐fixed and paraffin‐embedded tissues and cell lines into 3 μm thick sections and stained them with hematoxylin and eosin. To evaluate GAL3 expression, these sections were additionally immunostained using the Bond‐MAX Automated Immunohistochemistry system and Bond Polymer Refine Detection Kit (DC 9800; Leica Biosystems) based on our previous study with minor modifications. 19 First, the sections were deparaffinized and pretreated with Bond Epitope Retrieval Solution 2 (Leica Biosystems) at 100°C for 20 min. After washing, peroxidase blocking was performed for 10 min. The tissues were subsequently washed again and incubated with prediluted anti‐GAL3 monoclonal antibody (clone 9C4, Leica Biosystems) for 30 min. The sections were then incubated with Bond Polymer (Leica Biosystems) for 10 min, developed with DAB chromogen for 10 min, and finally counterstained with hematoxylin for 5 min. Negative controls were reacted with the Bond primary antibody diluent replacing the first antibody.
GAL3 cytoplasmic expression on tumor cells was evaluated and scored using the following scale: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The percentage of tumor cells expressing GAL3 was calculated and multiplied by the staining score and added in five different areas to obtain a semi‐quantitative histological score (H‐score [“histo” score]; maximum value: 300, corresponding to 100% of tumor cells positive for GAL3 and an overall staining score of 3). 20 In a preliminary study, we assessed the discriminate value of each cutoff point in semi‐quantitative H‐score regarding clinicopathological parameters and prognosis of the patients.
We previously studied the immunohistochemical expression of nestin and ATP‐binding cassette subfamily G member 2 (ABCG2) as cancer stem cell (CSC) markers and the expression of E‐cadherin and vimentin as epithelial‐to‐mesenchymal transition (EMT) markers in 86 patients with completely resected stage II and IIIA NSCLC treated with AC. 21 These 86 patients are included in the 111 patients of the present study. Thus, we investigated the correlation between the GAL3 expression and stem cell markers or EMT markers in the 86 patients by the kappa coefficient analysis. 22 The details of the immunostaining procedure and their staining evaluation have been previously described. 23
Two blinded investigators (S.I. and S.Y.) independently evaluated all specimens. Discordance was reviewed and discussed until a consensus was obtained.
Statistical analysis
The data cutoff was January 2019. The relationships between GAL3 expression and clinicopathological parameters were assessed using the Chi‐squared test or Fisher's exact test, as appropriate. Cumulative survival was estimated using the Kaplan–Meier method and compared between the high (≥170 H‐score) and low (<170 H‐score) GAL3 expression groups using the log‐rank test. The five‐year cumulative survival probability was estimated using the life table method with the interval length set at one month.
A multivariate analysis of RFS and OS was conducted using the Cox‐proportional hazards model. GAL3 expression, histological type, and p‐TNM stage were entered into the multivariate model. The association of GAL3 with EMT and stem cell markers was evaluated using kappa coefficient. Statistical analyses were performed using SPSS version 25.0 software (SPSS) without kappa coefficient.
Kappa coefficients were estimated with EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 3.0.2) based on a modified version of R commander (version 3.5–2). 22
RESULTS
Clinicopathological patient characteristics
The cohort comprised 69 men and 42 women with a median age of 62 years (range, 39–75 years). The clinicopathological characteristics of the patients are summarized in Table 1. There were 72 (64.9%) smokers, and 81 (73.0%), 24 (21.6%), three (2.7%), two (1.8%), and one (0.9%) patients had adenocarcinoma (AD), squamous cell carcinoma, pleomorphic carcinoma, large cell neuroendocrine carcinomas, and adenosquamous carcinoma, respectively. The median follow‐up time was 61 months (5–185 months), and no treatment‐related death occurred. At the end of follow‐up, 60 patients were alive, 40 had died of lung cancer, five died of other causes, and six were lost to follow‐up. Of the six patients lost to follow‐up, all were lost due to discontinuing hospital attendance and could not be contacted.
TABLE 1.
Patient characteristics (n = 111)
| Characteristics | n (%) |
|---|---|
| Age, years | |
| Median age (range) | 62 (39–75) |
| <60 | 42 (37.8) |
| ≥60 | 69 (62.2) |
| Sex | |
| Male | 69 (62.2) |
| Female | 42 (37.8) |
| Smoking status | |
| Never smoker | 39 (35.1) |
| Smoker | 72 (64.9) |
| Histological type | |
| AD | 81 (73.0) |
| Non‐AD | 30 (27.0) |
| p‐TNM stage | |
| Stage II | 35 (31.5) |
| Stage IIIA | 76 (68.5) |
| Chemotherapeutic regimen | |
| CBDCA based | 28 (25.2) |
| CDDP based | 83 (74.8) |
| Number of treatment cycle | |
| 1–2 | 15 (13.5) |
| 3–4 | 96 (86.5) |
| Survival status | |
| Alive | 60 (54.1) |
| Lung cancer‐related death | 40 (36.0) |
| Other causes of death | 5 (4.5) |
| Unknown | 6 (5.4) |
Abbreviations: AD, adenocarcinoma; CBDCA, carboplatin; CDDP, cisplatin; p‐TNM, pathological TNM eighth edition.
GAL3 expression in NSCLCs
In normal lung tissues, GAL3 cytoplasmic staining was observed in bronchial epithelial cells and in fibroblasts, lymphocytes, and macrophages at the tumor stroma. In NSCLCs, GAL3 was predominantly observed in varying degrees in the cytoplasm of tumor cells. In total, 61 patients showed high GAL3 expression. The representative images for GAL3 with H‐score of 0, 100, and 300 are shown in Figure 1(a)–(c), respectively. The relationships between GAL3 expression and clinicopathological characteristics are summarized in Table 2. High‐GAL3 expression was significantly correlated with adenocarcinoma (p < 0.001). In contrast, there was no significant correlation with age, sex, smoking habits, tumor size, nodal status, vascular invasion, lymphatic invasion, pleural invasion, AC regimen, or number of treatment cycle. Regarding histological subtypes, high GAL3 expression was detected in six of 11 (54.5%) patients with acinar subtype, one lepidic (100%), four of six micropapillary (66.7%), 37 of 51 (72.5%) papillary, five of 10 solid (50%), and none of the two invasive mucinous (0%) ADs.
FIGURE 1.
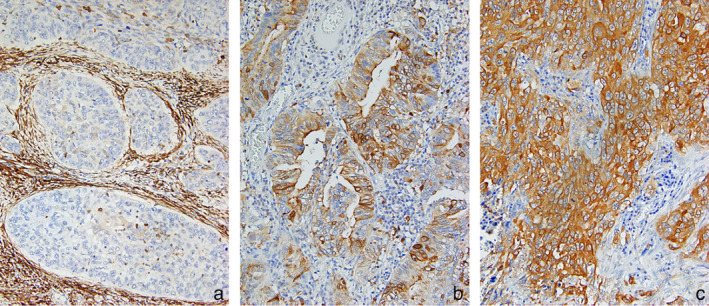
GAL3 expression in non‐small cell lung cancers (NSCLCs). Cytoplasmic expression of GAL3 was observed in tumor cells at various intensities, depending on the case. The H‐score of GAL3 expression in tumor cells of (a), (b), and (c) were 0, 100, and 300, respectively. Original magnification ×400
TABLE 2.
Association between GAL3 expression and clinicopathological parameters
| Clinicopathological parameters | GAL3 expression | Total | p‐value | |
|---|---|---|---|---|
| High (n = 61) | Low (n = 50) | |||
| Age, years | 0.226 | |||
| <60 | 20 (47.6) | 22 (52.4) | 42 | |
| ≥60 | 41 (59.4) | 28 (40.6) | 69 | |
| Sex | 0.45 | |||
| Male | 36 (52.2) | 33 (47.8) | 69 | |
| Female | 25 (59.5) | 17 (40.5) | 42 | |
| Smoking status | 0.531 | |||
| Never smoker | 23 (59.0) | 16 (41.0) | 39 | |
| Smoker | 38 (52.8) | 34 (47.2) | 72 | |
| Histological type | <0.001 | |||
| AD | 53 (65.4) | 28 (34.6) | 81 | |
| Non‐AD | 8 (26.7) | 22 (73.3) | 30 | |
| p‐TNM stage | 0.082 | |||
| Stage II | 15 (42.9) | 20 (57.1) | 35 | |
| Stage IIIA | 46 (60.5) | 30 (39.5) | 76 | |
| Tumor size, cm | 0.077 | |||
| ≤3 | 27 (65.8) | 14 (34.2) | 41 | |
| >3 | 34 (48.6) | 36 (51.4) | 70 | |
| Nodal status | 0.087 | |||
| N0 | 9 (39.1) | 14 (60.9) | 23 | |
| N1/N2/N3 | 52 (59.1) | 36 (40.9) | 88 | |
| Vascular invasion | 0.823 | |||
| No | 12 (57.1) | 9 (42.9) | 21 | |
| Yes | 49 (54.4) | 41 (45.6) | 90 | |
| Lymphatic invasion | 0.085 | |||
| No | 8 (38.1) | 13 (61.9) | 21 | |
| Yes | 53 (58.9) | 37 (41.1) | 90 | |
| Pleural invasion | 0.147 | |||
| No | 21 (46.7) | 24 (53.3) | 45 | |
| Yes | 40 (60.6) | 26 (39.4) | 66 | |
| Chemotherapeutic regimen | 0.294 | |||
| CBDCA based | 13 (46.4) | 15 (53.6) | 28 | |
| CDDP based | 48 (57.8) | 35 (42.2) | 83 | |
| Number of treatment cycles | 0.124 | |||
| 1–2 | 11 (73.3) | 4 (26.7) | 15 | |
| 3–4 | 50 (52.1) | 46 (47.9) | 96 | |
Note: Data are presented as number (percentage).
Abbreviations: CBDCA, carboplatin; CDDP, cisplatin; p‐TNM, pathological TNM eighth edition.
Similarly, no correlation was observed between the kappa coefficient and GAL3 expression and the nestin (κ = 0.033), ABCG2 (κ = 0.054), E‐cadherin (κ = 0.188), and vimentin (κ = 0.037) in patients with completely resected stage II and IIIA NSCLC who were administered platinum‐based AC.
Kaplan–Meier estimate of survival in patients with high and low GAL3 expression
All patients were included in the survival analysis. The five‐year cumulative survival probability was 71.1% in the overall cohort. A cumulative survival probability of 50% was not reached. Compared with low GAL3 expression, high GAL3 expression was correlated with significantly poorer RFS (p = 0.001; Figure 2(a)) and OS (p = 0.015; Figure 2(b)). The five‐year survival probability was significantly different between the low and the high GAL3 expression groups (80.0% vs. 63.9%, p = 0.015).
FIGURE 2.
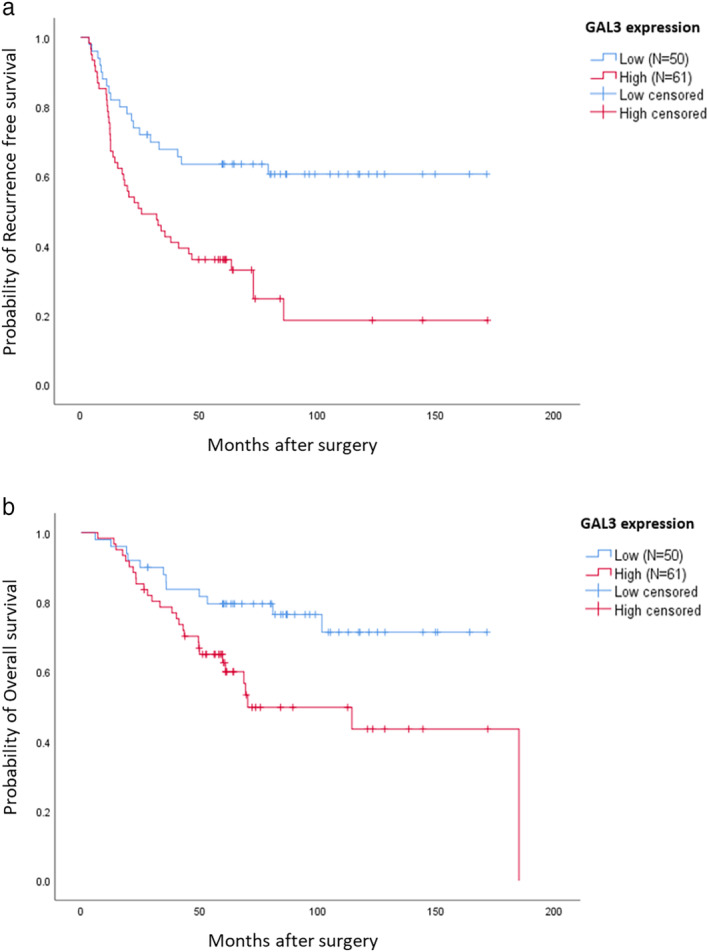
Cumulative survival of patients with non‐small cell lung cancer (NSCLC) according to GAL3 expression, as estimated using the Kaplan–Meier method. Panels (a) and (b) show the recurrence‐free survival (RFS) and overall survival (OS), respectively. High GAL3 expression was significantly associated with a shorter recurrence‐free survival (p = 0.001) and overall survival (p = 0.015) in patients with resected NSCLC who received platinum‐based adjuvant chemotherapy
Regarding predictors of RFS, univariate analysis indicated that histological type (HR, 0.286; 95% confidence interval (CI): 0.135–0.604; p < 0.001), p‐TNM stage (HR, 2.422; 95% CI: 1.287–4.557; p = 0.006), and GAL3 expression (HR, 2.542; 95% CI: 1.468–4.399; p < 0.001) were significant predictors (Table 3). Additionally, multivariate analysis indicated that histological type (hazard ratio [HR], 0.392; 95% CI: 0.179–0.859; p = 0.019), p‐TNM stage (HR, 2.004; 95% CI: 1.055–3.804; p = 0.034), and GAL3 expression (HR, 1.923; 95% CI: 1.083–3.413; p = 0.026) were significant predictors (Table 3).
TABLE 3.
Univariate and multivariate analyses of the effect of GAL3 expression on recurrence‐free survival (RFS)
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p‐value | HR | 95% CI | p‐value | |
| GAL3 expression | ||||||
| High vs. low | 2.542 | 1.468–4.399 | <0.001 | 1.923 | 1.083–3.413 | 0.026 |
| Age, years | ||||||
| ≥60 vs. <60 | 1.062 | 0.634–1.780 | 0.818 | Not included | ||
| Sex | ||||||
| Male vs. female | 1.724 | 1.045–2.846 | 0.33 | Not included | ||
| Smoking status | ||||||
| Smoker vs. never smoker | 0.682 | 0.410–1.133 | 0.139 | Not included | ||
| Histological type | ||||||
| AD vs. non‐AD | 0.286 | 0.135–0.604 | < 0.001 | 0.392 | 0.179–0.859 | 0.019 |
| p‐TNM stage | ||||||
| Stage III vs. stage II | 2.422 | 1.287–4.557 | 0.006 | 2.004 | 1.055–3.804 | 0.034 |
| Chemotherapeutic regimen | ||||||
| CDDP vs. CBDCA based | 0.84 | 0.480–1.468 | 0.54 | Not included | ||
| Number of treatment cycles | ||||||
| 3–4 vs. 1–2 | 0.934 | 0.444–1.965 | 0.858 | Not included | ||
Note: All analyses were performed using Cox proportional hazard regression.
Abbreviations: CBDCA, carboplatin; CDDP, cisplatin; 95% CI, 95% confidence interval; HR, hazard ratio.
Histological type (HR, 0.332; 95% CI: 0.130–0.851; p = 0.022), p‐TNM stage (HR, 3.612; 95% CI: 1.411–9.242; p = 0.007), and GAL3 expression (HR, 2.296; 95% CI: 1.156–4.562; p = 0.018) were significant predictors of poor OS in the univariate analysis (Table 4). However, unlike RFS, p‐TNM (HR, 2.948; 95% CI: 1.136–7.652; p = 0.026) was the only predictor of poor OS in the multivariate analysis, and GAL3 was not an independent predictor of poor OS (HR, 1.708; 95% CI: 0.831–3.513; p = 0.146; Table 4).
TABLE 4.
Univariate and multivariate analyses of the effect of GAL3 expression on overall survival (OS)
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p‐value | HR | 95% CI | p‐value | |
| GAL3 expression | ||||||
| High vs. low | 2.296 | 1.156–4.562 | 0.018 | 1.708 | 0.831–3.513 | 0.146 |
| Age, years | ||||||
| ≥60 vs. <60 | 1.562 | 0.790–3.088 | 0.2 | Not included | ||
| Sex | ||||||
| Male vs. female | 1.379 | 0.732–2.599 | 0.32 | Not included | ||
| Smoking status | ||||||
| Smoker vs. never smoker | 0.784 | 0.411–1.495 | 0.46 | Not included | ||
| Histological type | ||||||
| AD vs. non‐AD | 0.332 | 0.130–0.851 | 0.022 | 0.485 | 0.180–1.311 | 0.154 |
| p‐TNM stage | ||||||
| Stage III vs. stage II | 3.612 | 1.411–9.242 | 0.007 | 2.948 | 1.136–7.652 | 0.026 |
| Chemotherapeutic regimen | ||||||
| CDDP vs. CBDCA based | 0.653 | 0.335–1.274 | 0.212 | Not included | ||
| Number of treatment cycles | ||||||
| 3–4 vs. 1–2 | 0.605 | 0.267–1.372 | 0.229 | Not included | ||
Note: All analyses were performed using Cox proportional hazard regression.
Abbreviations: AD, adenocarcinoma; CBDCA, carboplatin; CDDP, cisplatin; 95% CI, 95% confidence interval; HR, hazard ratio; non‐AD, nonadenocarcinoma.
DISCUSSION
Members of the galectin family are reported to mediate cell adhesion, regulate cell growth, and trigger or inhibit apoptosis. 23 GAL3 expression has a prognostic impact in various carcinomas. High GAL3 expression is associated with poor prognosis in tongue, thyroid, ovarian, colorectal, hepatocellular, and prostate cancers. Conversely, low GAL3 expression is associated with poor prognosis in neuroblastoma and breast cancers. Meanwhile, the prognostic impact of GAL3 expression according to tumor localization is interesting. 24 , 25 Moderate expression of GAL3 has been found to be localized in both the nucleus and cytoplasm in normal prostate glands, whereas it is not usually expressed or decreased in prostate cancer cells. GAL3 has consistently been found to be excluded from the nucleus and only present in the cytoplasm in cancer cells. Van den Brule et al. 24 demonstrated that cytoplasmic expression of GAL3 in prostate cancer cells is an independent predictor of disease progression. They speculated that although GAL3 might be involved in antitumor activities when present in the nucleus, it favors tumor progression when expressed in the cytoplasm. Similar findings have been reported for colorectal cancer 26 and endometrial cancer. 27
However, the prognostic impact of GAL3 expression and localization in NSCLC are controversial. 14 , 28 , 29 , 30 Kosacka et al. reported that GAL3 expression was observed only in the cytoplasm of tumor cells and that it had no correlation with clinicopathological findings in their study. 30 A similar result was reported by Puglisi et al. 29 They described that the main pattern of GAL3 expression was cytoplasmic, and GAL3 was only present in a small percentage of the nucleus. Similarly, they described that all cases with nuclear GAL3 also showed cytoplasmic immune reactivity. In contrast, Mathieu et al. 28 reported that compared to cytoplasmic expression, nuclear expression of GAL3 was associated with a shorter RFS in their study. However, the majority of studies reported that GAL3 expression was observed in both the cytoplasm and nucleus of tumor cells, and its expression was evaluated as the sum without distinguishing their localizations. 31 , 32 , 33 The abovementioned studies included patients with distant metastases or those treated with nonradical resection. The heterogeneity of the patient population or treatment might have influenced the controversial outcomes.
Regarding NSCLC patients who underwent curative resection, Szöke et al. 14 studied the lectin‐binding capacities of the tumor cells with labeled GAL3 and suggested that among patients with stage II NSCLC receiving AC, those with high GAL3 expression have shorter OS than those with low GAL3 expression. Platinum‐based AC in patients with resected NSCLC aims to eradicate micrometastasis and improve survival. In this study, we observed that GAL3 was mainly expressed in the cytoplasm of tumor cells, is associated with poorer prognosis, and is an independent prognostic factor for RFS in stage II and IIIA NSCLC patients with completely resected tumors receiving AC. Although GAL3 expression was not an independent prognostic factor for OS, it showed a tendency to prolong the prognosis. These findings were consistent with those reported by Szöke et al., who used lectin immunochemistry.
Regarding chemoresistance, CSCs or cancer‐initiating cells are supposedly the causative factors of disease relapse. Although traditional chemotherapy can kill most cancer cells, it fails to target CSCs. 34 GAL3 expression is associated with EMT or CSC status. 35 In oral tongue squamous cell carcinoma, GAL3 overexpression significantly increased cell proliferation, migration, and invasion capabilities and induced EMT phenotypes. In contrast, knockdown of galectin‐3 using siRNA inhibited their cell proliferation, migration, and invasion capabilities and reduced EMT phenotypes. In our study, there was no association between GAL3 expression and EMT or stemness markers. Accordingly, it is reasonable to say that the association between GAL3 expression and EMT is controversial. Therefore, further studies are warranted to determine whether there is a significant association between them.
Several studies have indicated a strong correlation between EMT status and the expression of immune checkpoint molecules, especially PD‐L1, in multiple solid cancers, including NSCLC, breast cancer, extrahepatic cholangiocarcinoma, head and neck squamous cell carcinomas, and esophageal squamous cancer. 36 Kim et al. examined the expression of EMT markers and PD‐L1 levels in pulmonary adenocarcinoma patients and reported that SNAIL and vimentin were positively correlated with PD‐L1 expression. 37 These findings may indicate that patients with mesenchymal phenotypes are more likely to benefit from PD‐1/PD‐L1 immunotherapy. Another study established that PD‐L1 expression is significantly elevated in patients with mesenchymal phenotype than in those with the epithelial phenotype of lung adenocarcinoma in both TCGA and PROSPECT databases. In addition, E‐cadherin expression was determined to be strongly negatively correlated with PD‐L1 expression. 38 These data indicate that EMT status may be a cobiomarker with PD‐L1 level for the prognosis of cancer patients and a potential predictive biomarker to guide the selection of patients who are likely to benefit from checkpoint blockade through PD‐1 inhibitors. Our study indicates that GAL3 may act as an independent prognostic factor for the RFS of platinum‐based AC; this implies that NSCLC patients with high GAL3 expression and mesenchymal phenotype may show stronger resistance to platinum‐based chemotherapy. Thus, a clinical trial is required to validate the efficacy of AC with PD‐L1 inhibitors in patients with high GAL3 expression and EMT phenotype.
This study has several limitations. First, because it was retrospective and performed at a single institution with only a small number of squamous NSCLC patients, the results cannot be considered definitive. Second, the role of GAL3 in the invasive capability of cancer cells was not clarified. Hence, the prognostic significance of GAL3 expression requires confirmation in larger patient populations.
In conclusion, GAL3 expression is a reliable biomarker for the prediction of clinical outcomes in patients with completely resected NSCLC treated with platinum‐based AC. Identifying the subgroup of patients most likely to respond to a platinum‐based AC is the first step towards precision treatment of this patient population.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported in part by a Japan Society for the Promotion of Science grant‐in‐aid for scientific research (C), JSPS KAKENHI Grant Numbers 17K08279 and 15K21359, the Research Project Grant Numbers 2020‐1029 and 2019‐1030 from the school of Allied Health Sciences, Kitasato University.
Kusuhara S, Igawa S, Ichinoe M, et al. Prognostic significance of galectin‐3 expression in patients with resected NSCLC treated with platinum‐based adjuvant chemotherapy. Thorac Cancer. 2021;12:1570–1578. 10.1111/1759-7714.13945
REFERENCES
- 1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. [DOI] [PubMed] [Google Scholar]
- 2. Van Rens MT, de la Riviere AB, Elbers HR, van Den Bosch JM. Prognostic assessment of 2,361 patients who underwent pulmonary resection for non‐small cell lung cancer, stage I, II, and IIIA. Chest. 2000;117:374–9. [DOI] [PubMed] [Google Scholar]
- 3. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J, et al. Cisplatin‐based adjuvant chemotherapy in patients with completely resected non‐small‐cell lung cancer. N Engl J Med. 2004;350:351–60. [DOI] [PubMed] [Google Scholar]
- 4. Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. Vinorelbine plus cisplatin vs. observation in resected non‐small‐cell lung cancer. N Engl J Med. 2005;352:2589–97. [DOI] [PubMed] [Google Scholar]
- 5. Mazurek N, Sun YJ, Price JE, Ramdas L, Schober W, Nangia‐Makker P, et al. Phosphorylation of galectin‐3 contributes to malignant transformation of human epithelial cells via modulation of unique sets of genes. Cancer Res. 2005;65:10767–75. [DOI] [PubMed] [Google Scholar]
- 6. Hughes RC. The galectin family of mammalian carbohydrate‐binding molecules. Biochem Soc Trans. 1997;25:1194–8. [DOI] [PubMed] [Google Scholar]
- 7. Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. [DOI] [PubMed] [Google Scholar]
- 8. Cardoso AC, Andrade LN, Bustos SO, Chammas R. Galectin‐3 determines tumor cell adaptive strategies in stressed tumor microenvironments. Front Oncol. 2016;6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahmed H, AlSadek DM. Galectin‐3 as a potential target to prevent cancer metastasis. Clin Med Insights Oncol. 2015;9:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akahani S, Nangia‐Makker P, Inohara H, Kim HR, Raz A. Galectin‐3: a novel anti‐apoptotic molecule with a functional BH1 (NWGR) domain of Bcl‐2 family. Cancer Res. 1997;57:5272–6. [PubMed] [Google Scholar]
- 11. Nakahara S, Raz A. Regulation of cancer‐related gene expression by galectin‐3 and the molecular mechanism of its nuclear import pathway. Cancer Metastasis Rev. 2007;26:605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuo HY, Hsu HT, Chen YC, Chang YW, Liu FT, Wu CW. Galectin‐3 modulates the EGFR signalling‐mediated regulation of Sox2 expression via c‐Myc in lung cancer. Glycobiology. 2016;26:155–65. [DOI] [PubMed] [Google Scholar]
- 13. Chung LY, Tang SJ, Wu YC, Sun GC, Liu HY, Sun KH. Galectin‐3 augments tumor initiating property and tumorigenicity of lung cancer through interaction with beta‐catenin. Oncotarget. 2015;6:4936–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Szöke T, Kayser K, Trojan I, Kayser G, Furak J, Tiszlavicz L, et al. The role of microvascularization and growth/adhesion‐regulatory lectins in the prognosis of non‐small cell lung cancer in stage II. Eur J Cardiothorac Surg. 2007;31:783–7. [DOI] [PubMed] [Google Scholar]
- 15. Fukumori T, Kanayama HO, Raz A. The role of galectin‐3 in cancer drug resistance. Drug Resist Updat. 2007;10:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yanagita K, Nagashio R, Ryuge S, Katono K, Jiang SX, Tsuchiya B, et al. Serum anti‐Gal‐3 autoantibody is a predictive marker of the efficacy of platinum‐based chemotherapy against pulmonary adenocarcinoma. Asian Pac J Cancer Prev. 2015;16:7959–65. [DOI] [PubMed] [Google Scholar]
- 17. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–60. [DOI] [PubMed] [Google Scholar]
- 18. Asamura H, Chansky K, Crowley J, Goldstraw P, Rusch VW, Vansteenkiste JF, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10:1675–84. [DOI] [PubMed] [Google Scholar]
- 19. Nomura M, Matsumoto K, Shimizu Y, Ikeda M, Amano N, Nishi M, et al. TROY expression is associated with pathological stage and poor prognosis in patients treated with radical cystectomy. Cancer Biomark. 2019;24:91–6. [DOI] [PubMed] [Google Scholar]
- 20. Igawa S, Ryuge S, Ichinoe M, Nakashima H, Otani S, Nakahara Y, et al. Impact of EGFR‐tyrosine kinase inhibitors on postoperative recurrent non‐small‐cell lung cancer harboring EGFR mutations. Oncol Res Treat. 2017;40:7–13. [DOI] [PubMed] [Google Scholar]
- 21. Ryuge S, Sato Y, Nagashio R, Hiyoshi Y, Katono K, Igawa S, et al. Prognostic significance of nestin expression in patients with resected non‐small cell lung cancer treated with platinum‐based adjuvant chemotherapy; relationship between nestin expression and epithelial to mesenchymal transition related markers. PLoS One. 2017;12:e0173886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 23. Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med (Berl). 1998;76:402–12. [DOI] [PubMed] [Google Scholar]
- 24. van den Brule FA, Waltregny D, Liu FT, Castronovo V. Alteration of the cytoplasmic/nuclear expression pattern of galectin‐3 correlates with prostate carcinoma progression. Int J Cancer. 2000;89:361–7. [DOI] [PubMed] [Google Scholar]
- 25. Sanjuan X, Fernandez PL, Castells A, Castronovo V, van den Brule F, Liu FT, et al. Differential expression of galectin 3 and galectin 1 in colorectal cancer progression. Gastroenterology. 1997;113:1906–15. [DOI] [PubMed] [Google Scholar]
- 26. Lotz MM, Andrews CW, Korzelius CA, Lee EC, Steele GD, Clarke A, et al. Decreased expression of Mac‐2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc Natl Acad Sci U S A. 1993;90:3466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van den Brule FA, Buicu C, Berchuck A, Bast RC, Deprez M, Liu FT, et al. Expression of the 67‐kD laminin receptor, galectin‐1, and galectin‐3 in advanced human uterine adenocarcinoma. Hum Pathol. 1996;27:1185–91. [DOI] [PubMed] [Google Scholar]
- 28. Mathieu A, Saal I, Vuckovic A, Ransy V, Vereerstraten P, Kaltner H, et al. Nuclear galectin‐3 expression is an independent predictive factor of recurrence for adenocarcinoma and squamous cell carcinoma of the lung. Mod Pathol. 2005;18:1264–71. [DOI] [PubMed] [Google Scholar]
- 29. Puglisi F, Minisini AM, Barbone F, Intersimone D, Aprile G, Puppin C, et al. Galectin‐3 expression in non‐small cell lung carcinoma. Cancer Lett. 2004;212:233–9. [DOI] [PubMed] [Google Scholar]
- 30. Kosacka M, Piesiak P, Kowal A, Golecki M, Jankowska R. Galectin‐3 and cyclin D1 expression in non‐small cell lung cancer. J Exp Clin Cancer Res. 2011;30:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buttery R, Monaghan H, Salter DM, Sethi T. Galectin‐3: differential expression between small‐cell and non‐small‐cell lung cancer. Histopathology. 2004;44:339–44. [DOI] [PubMed] [Google Scholar]
- 32. Zhou W, Chen X, Hu Q, Chen X, Chen Y, Huang L. Galectin‐3 activates TLR4/NF‐kappaB signaling to promote lung adenocarcinoma cell proliferation through activating lncRNA‐NEAT1 expression. BMC Cancer. 2018;18:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kataoka Y, Igarashi T, Ohshio Y, Fujita T, Hanaoka J. Predictive importance of galectin‐3 for recurrence of non‐small cell lung cancer. Gen Thorac Cardiovasc Surg. 2019;67:704–11. [DOI] [PubMed] [Google Scholar]
- 34. Ahmed N, Abubaker K, Findlay J, Quinn M. Cancerous ovarian stem cells: obscure targets for therapy but relevant to chemoresistance. J Cell Biochem. 2013;114:21–34. [DOI] [PubMed] [Google Scholar]
- 35. Kang HG, Kim DH, Kim SJ, Cho Y, Jung J, Jang W, et al. Galectin‐3 supports stemness in ovarian cancer stem cells by activation of the Notch1 intracellular domain. Oncotarget. 2016;7:68229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang Y, Zhan H. Communication between EMT and PD‐L1 signaling: new insights into tumor immune evasion. Cancer Lett. 2020;468:72–81. [DOI] [PubMed] [Google Scholar]
- 37. Kim S, Koh J, Kim MY, Kwon D, Go H, Kim YA, et al. PD‐L1 expression is associated with epithelial‐to‐mesenchymal transition in adenocarcinoma of the lung. Hum Pathol. 2016;58:7–14. [DOI] [PubMed] [Google Scholar]
- 38. Lou Y, Diao L, Cuentas ERP, Denning WL, Chen L, Fan YH, et al. Epithelial‐mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin Cancer Res. 2016;22:3630–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


