Abstract
Background
We report a subgroup analysis of afatinib with respect to its efficacy, safety, and the long‐term survival of patients in a Named Patient Use program at a single institution.
Methods
We analyzed 60 patients with stage IV non‐small cell lung cancer (NSCLC) who had been treated with ≥1 line of platinum‐based chemotherapy and had activating epidermal growth factor receptor (EGFR) mutations or disease control for ≥6 months with prior EGFR inhibitors. Afatinib was started on a daily dose of 50 mg, which was decreased according to the adverse events and tolerability.
Results
A total of 13 patients achieved partial remission, whereas 33, 12, and two showed stable disease, had progression, and were not evaluable, respectively, resulting in an objective response rate and disease control rate of 21.7% and 76.7%, respectively. The median progression‐free survival (PFS) was 5.4 (95% confidence interval [CI]: 4.0–7.7) months and median overall survival (OS) was 10.1 (8.5–13.6) months. Toxicities leading to drug discontinuation were experienced by four patients (6.7%). Grade 3 diarrhea occurred in 10 patients (16.7%), and afatinib dose reductions were required in 35 patients. The PFS and OS were significantly longer for patients whose dose was reduced to 40 or 30 mg than for those without dose reduction (7.0 vs 3.1 months and 13.5 vs 8.1 months, respectively, p < 0.05).
Conclusions
The efficacy of afatinib was similar to that identified in the global data without unexpected adverse events. Survival analyses support the currently approved dose of afatinib as first‐line treatment for NSCLC.
Keywords: afatinib; carcinoma, non‐small‐cell lung; EGFR
In non‐small cell carcinoma, a 40 mg or lower daily dose of afatinib prolonged progression‐free survival and overall survival more than the 50‐mg daily dose.

INTRODUCTION
More than 80% of lung cancer is non‐small cell lung cancer (NSCLC), which is mostly diagnosed at stage IV and for which medical treatment is the mainstay. 1 Platinum‐based doublet chemotherapy has been the main treatment option until approximately 20 years. 2 However, many target gene mutations have since been discovered, and various targeted agents are now the current standard of care as first‐line treatment for tumors harboring driver mutations. Recently, immune checkpoint inhibitors (ICIs) have replaced, or been combined with, platinum doublet chemotherapy as first‐line treatment for tumors without driver mutations. 3
In patients with NSCLC with activating epidermal growth factor receptor (EGFR) mutations, EGFR tyrosine kinase inhibitors (TKIs), such as gefitinib or erlotinib, have been shown to improve the objective response rate (ORR) and progression‐free survival (PFS) in patients compared with conventional chemotherapy. 4 , 5 , 6 In spite of favorable outcomes with EGFR TKIs, NSCLC eventually progresses via acquired resistance mechanisms after approximately one year. Although many resistance mechanisms have been discovered, the EGFR T790M mutation in exon 20 has been reported to be responsible for approximately 50% of cases. 7 Afatinib is an irreversible blocker of the ErbB family of proteins and shows antitumor activity on the T790M mutation in vitro. 8
In the first phase 3 Lux‐Lung 1 trial comparing the efficacy of afatinib with that of a placebo in patients with advanced NSCLC who had previously received at least 12 weeks of EGFR inhibitor treatment, afatinib improved PFS and quality of life (QOL), although there was no difference in overall survival (OS). 9 Therefore, patients with NSCLC with disease progression after the failure of EGFR TKIs have been enrolled into the compassionate use (Named Patient Use, NPU) program since 2011. The NPU program was established by Boehringer Ingelheim and global and Korean data have recently been reported. 10 , 11 Here, we report subgroup data of patients enrolled in the NPU program from the authors' institution focusing on the efficacy and long‐term survival according to the dose of afatinib.
METHODS
Study design and eligibility
The NPU program (1200.148) was an open‐label, multicenter, single‐arm study that recruited 3966 patients from 41 countries, 10 including 377 patients at eight institutions in Korea. 11 The aim of this program was to provide early access to afatinib for patients unable to use other treatments, or who were considered ineligible for other afatinib clinical trials. As there was no predefined primary efficacy objective, the sample size was not calculated.
Patients ≥18 years of age were eligible for the NPU program if they had pathologically confirmed NSCLC with progressive disease following at least one line of cisplatin‐based chemotherapy. The enrolled patients were expected to have received at least six months of treatment with erlotinib or gefitinib or have activating EGFR mutations. We used the PNA Clamp EGFR Mutation Detection Kit (Panagene Inc.) to detect EGFR gene mutations by real‐time PCR, based on the methods of a previous study. 12 Mutational analysis of the EGFR gene was not mandated in this study.
The study was approved by the institutional review board of our institution (2011–104) and the Korean Ministry of Food and Drug Safety, and was conducted according to International Conference on Harmonization Good Clinical Practice guidelines. All patients provided written informed consent before participating in the study.
Treatment
Afatinib was commenced at a daily dose of 50 mg which was administered until the occurrence of progressive disease (PD), withdrawal of consent, or withdrawal owing to severe adverse events (AEs). The drug dose was decreased to 40 mg and 30 mg if patients experienced >grade 3 AEs, assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI‐CTCAE) version 3.0. No additional dose reduction was allowed below 30 mg and treatment was discontinued if the AEs were not controlled by dose reduction and adequate supportive therapies.
Efficacy and safety assessment
Although there was no predefined primary efficacy objective, baseline tumor diameters were measured using computed tomography (CT) at initial screening. Follow‐up CT was performed every six weeks and the smallest diameters were used to assess the best response according to the Response Evaluation Criteria in Solid Tumor (RECIST) version 1.1. 13 The response was categorized into four groups: complete response, partial response (PR), stable disease (SD), and PD. OS and PFS were calculated from the date of afatinib treatment initiation. Patients visited the hospital every six or eight weeks, during which they were assessed for the following AEs: skin rash, diarrhea, fatigue, anorexia, mucositis, paronychia, and drug‐induced interstitial lung disease (DILD).
Statistical analysis
The following patient data were collected: sex, pathological diagnosis, EGFR mutation test results, prior treatment history, dose, AEs, tumor response, progression, and OS (from the day afatinib was started). We used R software (version 4.01, 2020‐06‐06), and median PFS and OS were estimated using the Kaplan–Meier method. 14 As the data set had sufficient follow‐up time to capture all PFS events and all but one OS events, the conventional reverse Kaplan–Meier method could not be used to calculate the median follow‐up time. We used time to end‐of‐study (from the day of afatinib initiation to the end of the survival analysis) to calculate the median follow‐up time. 15
RESULTS
Patient population
A total of 60 patients were registered in the NPU program from October 2011 to September 2014, and the duration of afatinib treatment ranged from 4 to 700 days. EGFR gene mutation tests of specimens collected at diagnosis were conducted in 37 patients (61.7%), and 18 (48.6%) exhibited activating mutations. Furthermore, 43 (71.7%) and 17 (28.3%) patients were treated with gefitinib and erlotinib, respectively. Afatinib was used as the third‐, fourth‐, fifth‐, and ≥ sixth‐line treatments in two (3.3%), 27 (45.0%), 19 (31.7%), and 12 (20.0%) patients, respectively.
Baseline characteristics of the patients are summarized in Table 1. Twenty‐five (41.7%) patients received a starting dose of 50 mg daily throughout their drug treatment. However, doses were reduced to 40 and 30 mg in 25 (41.7%) and 10 (16.7%) patients, respectively. Four (6.7%) patients discontinued the drug as a result of toxicity. After discontinuing afatinib, 28 patients (46.7%) received one to three lines of subsequent treatments, whereas 32 patients (53.3%) received supportive care only.
TABLE 1.
Demographics of patients treated with afatinib in the named patient use (NPU) program
| Total | Final dose 50 mg n = 25 (41.7%) | Final dose <50 mg n = 35 (58.3%) | p‐value | |
|---|---|---|---|---|
| Age (years) mean ± SD a | 64.3 ± 10.5 | 64.2 ± 10.5 | 64.3 ± 10.7 | NS b |
| Sex, female/male | 39/21 | 15/10 | 24/11 | NS |
| Histology, adeno/squamous | 59/1 | 24/1 | 35/0 | NS |
| Stage, III/ IV | 4/56 | 2/23 | 2/33 | NS |
| EGFR mutation | NS | |||
| Ex 19 del/L858R | 11/7 | 4/2 | 7/5 | |
| Negative | 19 | 7 | 12 | |
| Not tested | 23 | 12 | 11 | |
| Final dose (50/40/30) | 25/25/10 | 25/0/0 | 0/25/10 | |
| Prior TKI, gefitinib/erlotinib | 43/17 | 18/7 | 25/10 | NS |
| Line of afatinib treatment | NS | |||
| 3/4/5 | 2/27/19 | 0/8/9 | 2/19/10 | |
| 6/7/8/9/10 | 6/1/3/1/1 | 3/1//1/1 | 3/0/1/0/0 | |
| Subsequent treatment | NS | |||
| Supportive care only | 32 | 15 | 17 | |
| One more regimen | 17 | 7 | 10 | |
| Two or three regimens | 11 | 3 | 8 | |
| Response rate (%) | 21.7 | 16.0 | 25.7 | NS |
| Disease control rate (%) | 76.7 | 64.0 | 85.7 | |
| PR/SD/PD/NE (number) c | 13/33/12/2 | 4/12/7/2 | 9/21/5/0 |
SD, standard deviation.
NS, not significant.
PR, partial remission; SD, stable disease; PD, progressive disease; NE, not evaluable.
Efficacy
Treatment response was evaluated in all the patients (n = 60) administered afatinib, but the duration of treatment in two patients was not sufficient to evaluate the treatment efficacy. Among all the patients, including those who were nonevaluable, 13 (21.7%), 33 (55.0%), and 12 (20.0%) showed PR, SD, and PD, respectively. Thus, afatinib demonstrated a 21.7% and 76.7% ORR and disease control rate (DCR), respectively.
All patients experienced disease progression within 25.4 months of follow‐up, and the median PFS was 5.4 (95% confidence interval [CI]: 4.0 to 7.7) months. The PFS was significantly longer in patients with dose reduction to 40 or 30 mg (median 7.0, 95% CI: 5.1 to 9.3 months, n = 35) than in those without dose reduction (median 3.1, 95% CI: 1.9 to 6.3, n = 25, log rank p = 0.002, Figure 1(a) and (b)).
FIGURE 1.
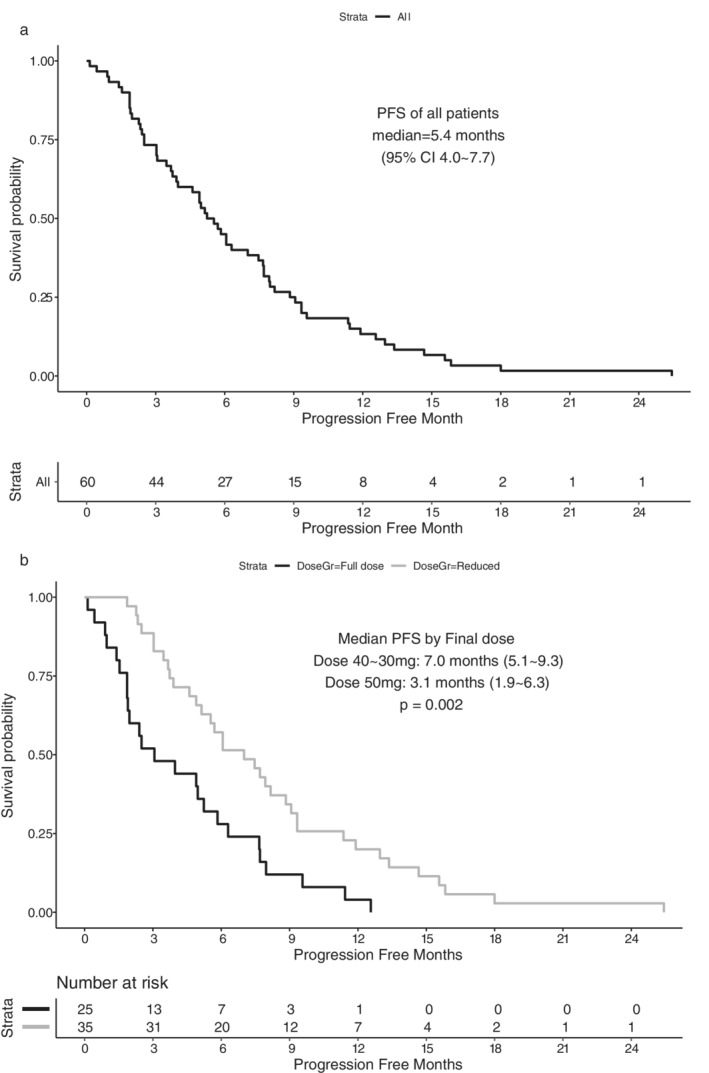
(a) Progression‐free survival (PFS) of 60 patients treated with afatinib and (b) PFS according to the final dose of afatinib
During the median follow‐up of 89.0 months (range 78.9–112.3 months), 59 patients died, and one was still alive as of February 2021. The median OS was 10.1 (95% CI: 8.5 to 13.6) months from the commencement of afatinib. The OS of patients with dose reduction was also significantly longer (median 13.5, 95% CI: 10.2 to 18.6) than that of those without dose reduction (median 8.1, 95% CI: 5.0 to 12.9, log rank p = 0.018, Figure 2(a) and (b)).
FIGURE 2.
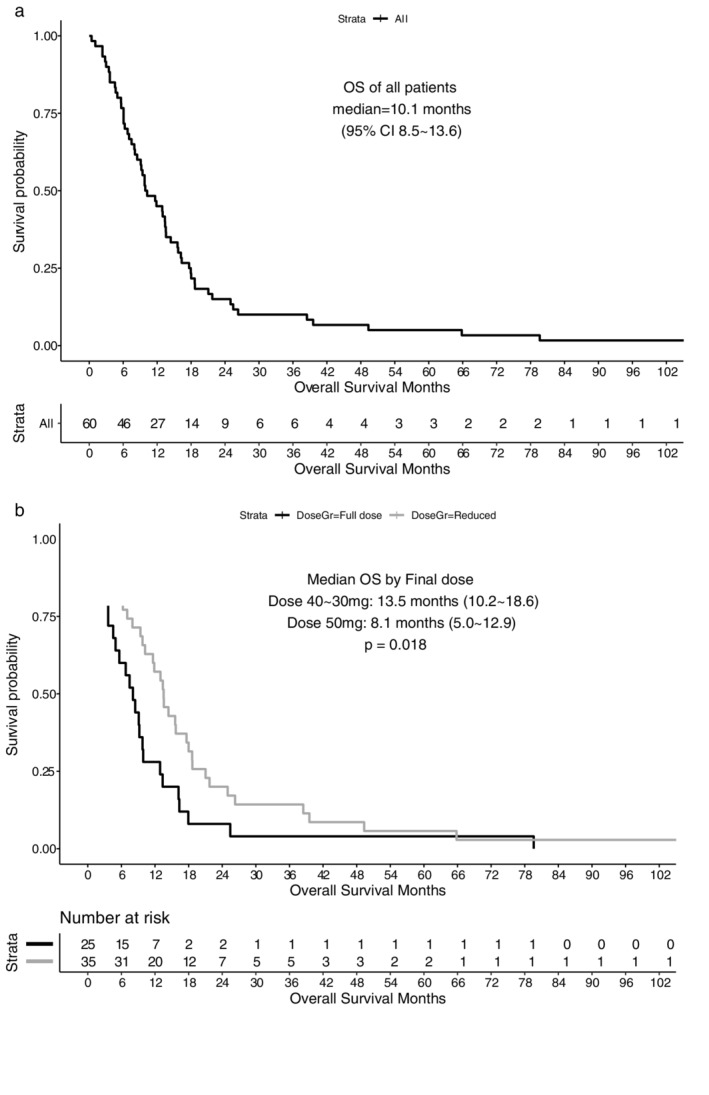
(a) Overall survival (OS) of 60 patients treated with afatinib and (b) OS according to final dose of afatinib
Safety
The most frequently experienced AE with afatinib was diarrhea in 59 patients (98.3%), followed by skin rash in 46 (76.7%), paronychia in 39 (65.0%), and mucositis in 39 (65.0%). Furthermore, ≥grade 3 diarrhea was experienced by 10 (16.7%) patients, paronychia and mucositis by four (6.7%) each, and skin rash by two (3.3%, Table 2).
TABLE 2.
Adverse events (AEs) according to common terminology criteria of AEs (CTCAE)
| Grade/number (%) | 1 | 2 | 3 | 4 | Any grade | ≥ grade 3 |
|---|---|---|---|---|---|---|
| Skin eruption | 26 (43.3) | 18 (30.0) | 2 (3.3) | (76.7) | (3.3) | |
| Mucositis | 23 (38.3) | 12 (20.0) | 4 (6.7) | (65.0) | (6.7) | |
| Paronychia | 24 (40.0) | 11 (18.3) | 4 (6.7) | (65.0) | (6.7) | |
| Diarrhea | 28 (46.7) | 21 (35.0) | 10 (16.7) | (98.3) | (16.7) | |
| Fatigue | 14 (23.3) | 2 (3.3) | (26.6) | |||
| Anorexia | 22 (36.7) | 3 (5.0) | (41.7) | |||
| Interstitial pneumonitis | 1 (1.7) | (1.7) | (1.7) |
DISCUSSION
Currently, there are many first‐line treatment options for locally advanced or metastatic NSCLC with activating EGFR mutations. These options include first‐generation EGFR TKIs (gefitinib or erlotinib), second‐generation TKIs (afatinib, dacomitinib), and a third‐generation TKI (osimertinib). 3 , 16 Osimertinib has shown superior PFS with a lower rate of serious AEs than the first‐generation EGFR TKIs. 17 However, a subsequent analysis did not demonstrate a superior OS in an Asian subgroup, 18 and no direct comparison has been made between osimertinib and second‐generation EGFR TKIs. Currently, another third‐generation TKI, lazertinib, is being developed to overcome TKI resistance in patients with the EGFR T790M mutation. 16
Osimertinib is currently not reimbursed by health insurance in many countries as first‐line treatment. Thus, in real world practice, first‐ or second‐generation EGFR TKIs are still used as first‐line treatment for NSCLC with EGFR mutations. Second‐generation EGFR TKIs were developed to combat the inevitable acquired resistance after treatment with first‐generation EGFR TKIs. 19 However, initial studies using second‐generation EGFR TKIs for patients with molecularly heterogeneous NSCLC who progressed after first‐generation EGFR TKIs failed to show an OS benefit. 9 , 20 In the Lux‐Lung 1 trial, afatinib improved the PFS and QOL, but no benefit was observed in OS. 9
Although further development of afatinib as second‐line treatment was stopped, the global expanded access program was initiated under the title NPU in 2011. Among the globally enrolled 3966 heavily pretreated patients with NSCLC from 41 countries, the median time to treatment failure (TTF) was 4.4 months and the ORR was 23.4%. 10 In Korea, 332 patients were enrolled in this program and the TTF and ORR were reported as 3.3 months and 27.4%, respectively. 11
The Lux‐Lung 4 study 21 was a phase II trial of afatinib in patients with advanced NSCLC who progressed after prior treatment with erlotinib or gefitinib, which is similar to our study. In the Lux‐Lung 4 trial, the PFS and ORR were 4.4 months and 8.2%, respectively, whereas in the present study, the values were 5.4 months and 21.7%, respectively.
OS was 10.8 months in the Lux‐Lung‐1 trial, 9 in which afatinib was used as third‐ or fourth‐line treatment. The OS of patients in this analysis was 10.1 months. As shown in Table 1, the patients in this analysis were heavily treated before enrolling in this program. In this study, afatinib was used as fourth‐ or fifth‐line treatment in most patients, and more than half were ineligible for further treatment as defined in the inclusion criteria.
First‐line afatinib at a daily dose of 40 mg was compared with platinum doublets in two global phase III trials 22 , 23 and, based on the results, this dose was approved as first‐line treatment for EGFR mutant NSCLC by the US Food and Drug Administration in 2013. Our current data also supports the 40 mg daily dose, as we observed a significantly superior PFS and OS in the reduced dose (30–40 mg) group than in the 50 mg group. Further dose adjustment is warranted because doses below 40 mg daily do not shorten the PFS and decrease the drug‐related AEs. 24
In this analysis, the most common AE was diarrhea, followed by skin rash, paronychia, and mucositis. Despite a similar AE rate to that in the Lux‐Lung 2 trial, the rate of severe AEs in our study was lower, 25 where the rates of grade 3 diarrhea and skin rash were 22% and 28%, respectively, with a 50 mg daily dose, and both rates were 7% with the 40 mg daily dose. In this study, grade 3 diarrhea was observed in 16.7% of patients, whereas grade 3 skin rash occurred only in 3.3%. The less severe toxicities might be attributable to the anticipatory control of AEs achieved by prescribing doxycycline or loperamide.
DILD is the most serious AE associated with EGFR TKIs, whereas hematological and neurological toxicities are rare. With gefitinib, a 2.6% incidence of DILD and 0.5% death rate were observed in the IPASS study. 5 The incidence and mortality were 5.3% and 0.8%, respectively, in the North‐East Japan study group data, 4 and approximately 1% of patients receiving erlotinib experienced DILD in both the Optimal 6 and EURTAC 26 studies. In the Lux‐Lung 3 trial, the incidence rate of DILD was 1% in patients taking afatinib 22 and we observed DILD in one (1.7%) patient, which was similar to the incidence observed in the previous studies.
The drug discontinuation rate was 6.9% for gefitinib in the IPASS study, 5 whereas it was 6%–13% for erlotinib in the EURTAC study, 26 8% for afatinib in the Lux‐Lung 3 study, 22 and 11% for afatinib in the Korean NPU data. 11 Because 6.7% of our patients stopped afatinib because of AEs, there was no significant difference in the discontinuation rate compared with previous studies.
In conclusion, the subgroup analysis showed a similar efficacy to that reported by the global data without unexpected AEs. Afatinib demonstrated a better PFS and OS with the 40 mg or lower dose than with the 50 mg daily dose in patients with NSCLC after the failure of prior gefitinib or erlotinib administration, without deceasing the treatment efficacy. Our data support the currently approved dose of afatinib as first‐line treatment for NSCLC.
ACKNOWLEDGMENTS
We would like to thank to Editage (www.editage.co.kr) for English language editing.
Choi H, Lee J‐K, Oh H‐J, et al. Efficacy and dose of afatinib in patients with non‐small cell lung cancer after failure of prior gefitinib or erlotinib treatment. Thorac Cancer. 2021;12:1598–1604. 10.1111/1759-7714.13957
Contributor Information
Hayoung Choi, Email: hychoimd@gmail.com.
Jae‐Kyeong Lee, Email: oceanbeachsf@naver.com.
Hyung‐Joo Oh, Email: ohj4250@naver.com.
Min‐Seok Kim, Email: kmin-suk@hanmail.net.
Bo Gun Kho, Email: imdrkbg@gmail.com.
Cheol Kyu Park, Email: ckpark214@jnu.ac.kr.
In‐Jae Oh, Email: droij@chonnam.ac.kr.
Young‐Chul Kim, Email: kyc0923@jnu.ac.kr.
REFERENCES
- 1. Choi CM, Kim HC, Jung CY, Cho DG, Jeon JH, Lee JE, et al. Report of the Korean Association of Lung Cancer Registry (KALC‐R), 2014. Cancer Res Treat. 2019;51(4):1400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non–small‐cell lung cancer. N Engl J Med. 2002;346(2):92–8. [DOI] [PubMed] [Google Scholar]
- 3. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology, Non‐Small Cell Cancer: NCCN. 2021. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 4. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non–small‐cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. [DOI] [PubMed] [Google Scholar]
- 5. Mok TS, Wu Y‐L, Thongprasert S, Yang C‐H, Chu D‐T, Saijo N, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. [DOI] [PubMed] [Google Scholar]
- 6. Zhou C, Wu Y‐L, Chen G, Feng J, Liu X‐Q, Wang C, et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): a multicentre, open‐label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–42. [DOI] [PubMed] [Google Scholar]
- 7. Kosaka T, Yatabe Y, Endoh H, Yoshida K, Hida T, Tsuboi M, et al. Analysis of epidermal growth factor receptor gene mutation in patients with non‐small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res. 2006;12(19):5764–9. [DOI] [PubMed] [Google Scholar]
- 8. Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller VA, Hirsh V, Cadranel J, Chen Y‐M, Park K, Kim S‐W, et al. Afatinib versus placebo for patients with advanced, metastatic non‐small‐cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX‐lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13(5):528–38. [DOI] [PubMed] [Google Scholar]
- 10. Cappuzzo F, Soo R, Hochmair M, Schuler M, Lam KC, Stehle G, et al. Global named patient use program of afatinib in advanced non‐small‐cell lung carcinoma patients who progressed following prior therapies. Future Oncol. 2018;14(15):1477–86. [DOI] [PubMed] [Google Scholar]
- 11. Choi MK, Ahn JS, Kim YC, Cho BC, Oh IJ, Kim SW, et al. Afatinib in heavily pretreated advanced NSCLC patients who progressed following prior gefitinib or erlotinib: compassionate use program in Korea. Lung Cancer. 2018;119:36–41. [DOI] [PubMed] [Google Scholar]
- 12. Yoon SH, Choi YD, Oh IJ, Kim KS, Choi H, Chang J, et al. Peptide nucleic acid clamping versus direct sequencing for the detection of EGFR gene mutation in patients with non‐small cell lung cancer. Cancer Res Treat. 2015;47(4):661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 14. R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 15. Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Control Clin. Trials. 1996;17(4):343–6. [DOI] [PubMed] [Google Scholar]
- 16. Ahn MJ, Han JY, Lee KH, Kim SW, Kim DW, Lee YG, et al. Lazertinib in patients with EGFR mutation‐positive advanced non‐small‐cell lung cancer: results from the dose escalation and dose expansion parts of a first‐in‐human, open‐label, multicentre, phase 1‐2 study. Lancet Oncol. 2019;20(12):1681–90. [DOI] [PubMed] [Google Scholar]
- 17. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med. 2018;378(2):113–25. [DOI] [PubMed] [Google Scholar]
- 18. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with Osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. [DOI] [PubMed] [Google Scholar]
- 19. Pao W, Chmielecki J. Rational, biologically based treatment of EGFR‐mutant non‐small‐cell lung cancer. Nat Rev Cancer. 2010;10(11):760–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reckamp KL, Giaccone G, Camidge DR, Gadgeel SM, Khuri FR, Engelman JA, et al. A phase 2 trial of dacomitinib (PF‐00299804), an oral, irreversible pan‐HER (human epidermal growth factor receptor) inhibitor, in patients with advanced non‐small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer. 2014;120(8):1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katakami N, Atagi S, Goto K, Hida T, Horai T, Inoue A, et al. LUX‐lung 4: a phase II trial of afatinib in patients with advanced non‐small‐cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31(27):3335–41. [DOI] [PubMed] [Google Scholar]
- 22. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–34. [DOI] [PubMed] [Google Scholar]
- 23. Wu Y‐L, Zhou C, Hu C‐P, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐lung 6): an open‐label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–22. [DOI] [PubMed] [Google Scholar]
- 24. Yang JC, Sequist LV, Zhou C, Schuler M, Geater SL, Mok T, et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation‐positive lung adenocarcinoma: post hoc analyses of the randomized LUX‐lung 3 and 6 trials. Ann Oncol. 2016;27(11):2103–10. [DOI] [PubMed] [Google Scholar]
- 25. Yang JC‐H, Shih J‐Y, Su W‐C, Hsia T‐C, Tsai C‐M, Ou S‐HI, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX‐lung 2): a phase 2 trial. Lancet Oncol. 2012;13(5):539–48. [DOI] [PubMed] [Google Scholar]
- 26. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): a multicentre, open‐label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. [DOI] [PubMed] [Google Scholar]


