Abstract
Immune checkpoint inhibitors (ICIs) have shown significant efficacy in various solid tumors, but only a small subgroup of patients benefit from them because of immune resistance. Oncorine (formerly H101), a recombinant human adenovirus type 5, has direct anticancer properties and enhances cell‐mediated immune responses. At present, few studies on the role of Oncorine in reversing resistance to ICIs have been reported. Here, we present a case with recurrent non‐small cell lung cancer (NSCLC). The patient developed resistance to nivolumab therapy. After trying immunotherapy plus chemotherapy or antiangiogenesis therapy, the patient only obtained a transient response. The patient then received experimental treatment with Oncorine together with nivolumab and anlotinib. She experienced symptomatic improvement with a performance status score of 1, and achieved stable disease despite partial lung tissue necrosis. This was a successful exploration of oncolytic viruses reversing immune resistance.
Keywords: immune resistance, nivolumab, non‐small cell lung cancer, Oncorine
Our patient with recurrent NSCLC experienced resistance to ICI (nivolumab), and was subsequently treated with nivolumab plus anlotinib with a transient response, followed by the combination of oncolytic virus (Oncorine) at the time of progression with obvious disease control. This was a successful exploration of oncolytic viruses reversing immune resistance.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have shown significant efficacy in various solid tumors, but only a small subgroup of patients benefit from them because of immune resistance. 1 , 2 , 3 Oncorine (formerly H101), a recombinant human adenovirus type 5, is the first China Food and Drug Administration approved oncolytic virus product for cancer therapy. 4 Recent clinical and preclinical trials have revealed that oncolytic viruses enhance the effectiveness of other antititumor therapies, but without more side effects. 5 However, there are few reports on the role of oncolytic viruses in reversing resistance to ICIs. Here, we present a case with recurrent non‐small cell lung cancer (NSCLC) who developed resistance to nivolumab therapy. After the patient received experimental treatment with Oncorine together with nivolumab and anlotinib, she achieved stable disease.
CASE REPORT
In January 2015, a 57‐year‐old Chinese female was diagnosed with right NSCLC and subsequently underwent radical resection. Pathological examination revealed a well‐to‐moderately differentiated mucinous adenocarcinoma (stage IIB, T3N0M0). She refused postoperative adjuvant chemotherapy and was discharged.
In July 2017, she was admitted to our hospital due to an aggravated cough with white sputum. Chest computed tomography (CT) scan showed an increase in the size and number of the ground‐glass nodules (GGNs) in both lungs and enlarged lymph nodes in the mediastinum (Figure 1(a)). CT‐guided percutaneous needle biopsy confirmed the recurrence of lung mucinous adenocarcinoma (stage IIB) (Figure 2(a)). Next generation sequencing (NGS) with the Illumina Hiseq platform revealed a KRAS mutation. Programmed death ligand 1 (PD‐L1) immunohistochemical staining reported a tumor proportion score of 1%–49% (Figure 2(b)). The patient received platinum‐containing chemotherapy and antiangiogenic therapy for about 1.5 years, but CT imaging in February 2019 demonstrated further disease progression (Figure 1(b)).
FIGURE 1.
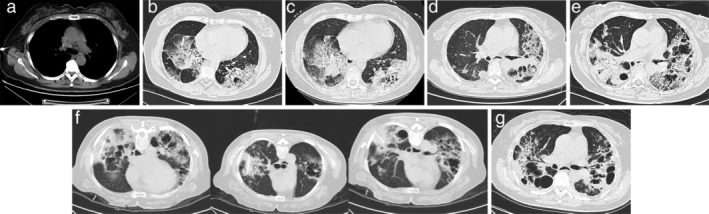
Chest computed tomography (CT) imaging of the patient. (a) Chest CT on July 19, 2017 showed an increase in the size and number of ground‐glass nodules (GGNs) in both lungs and enlarged lymph nodes in the mediastinum. (b) Chest CT on February 18, 2019, (c) April 12, 2019, (d) November 26, 2019, and (e) June 4, 2020 demonstrated further disease progression. (f) On June 9, 2020, a total of 5.0 × 1011 virus particles every six weeks (one cycle) were injected into three lesions of the patient, respectively. (g) Chest CT on December 16, 2020 revealed that the patient had achieved stable disease despite partial lung tissue necrosis
FIGURE 2.
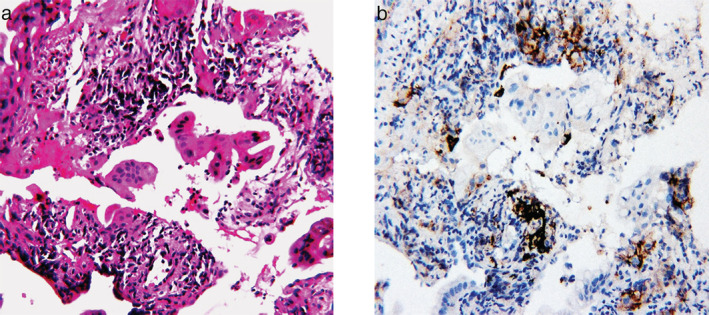
Microscopic examination. (a) Hematoxylin and eosin staining was consistent with adenocarcinoma. (b) Programmed death ligand 1 (PD‐L1) immunohistochemical staining of lung adenocarcinoma specimens with a tumor proportion score of 1%–49%
Considering the disease progression and recent approval of nivolumab (Opdivo) for NSCLC in China, the patient commenced this drug at a dose of 200 mg intravenously every two weeks from February 20, 2019. Two months later in April 2019, her CT imaging demonstrated further disease progression (Figure 1(c)), indicating that the patient had not benefited from nivolumab monotherapy. From April 29, 2019, the patient received combined immunotherapy including nivolumab, chemotherapy and bevacizumab, and the lesions remained stable only for several months. CT imaging showed disease progression (Figure 1(d)), and symptoms of cough, sputum, chest tightness and shortness of breath were aggravated. The patient had a performance status score of 4 and was no longer able to toleratechemotherapy. On January 14, 2020, the patient received nivolumab (200 mg, day 1, every three weeks) plus anlotinib (Focus V; one cycle of 12 mg daily for 14 days, discontinued for seven days, and repeated every 21 days) as maintenance therapy. After a brief period of disease control, her CT imaging on June 4 demonstrated further disease progression (Figure 1(e)), after which all treatment regimens were discontinued.
As the patient and her family strongly requested further antitumor therapy, a final decision was made to volunteer the patient for experimental treatment with oncolytic viruses in addition to combination therapy with nivolumab and amlotinib on June 9. The doses of nivolumab and amlotinib were the same as previously. Before the instigation of combination therapy with nivolumab and amlotinib, Oncorine (Sunway Biotech) was administered. A total of 5.0 × 1011 virus particles every six weeks (one cycle) were injected into three lesions, respectively (Figure 1(f)). After the intratumor injection of Oncorine, the patient developed fever which was controlled after antipyretic treatment. The patient received four cycles of treatment with Oncorine, and CT imaging on December 16 revealed that the patient had achieved stable disease despite partial lung tissue necrosis (Figure 1(g)). The performance status score of the patient was 1 on January 7, 2021. The long‐term management plan was to continue with the combination therapy of nivolumab and amlotinib with careful follow‐up.
DISCUSSION
Our patient with recurrent NSCLC experienced resistance to an ICI (nivolumab), and was then treated with nivolumab plus anlotinib with a transient response, followed by the combination of oncolytic virus (Oncorine) at the time of progression with obvious disease control. To our knowledge, this is the first case report describing the reversal of immune resistance by oncolytic viruses in NSCLC.
Oncolytic virotherapy is a promising therapy in cancer treatment and is being increasingly used in the clinic with favorable results. A preclinical study showed that the combination therapy of oncolytic viruses and PD‐L1 blockade could significantly reduce tumor burden and achieve better survival compared to monotherapy. 6 A phase Ib clinical trial on advanced melanoma suggested that talimogene laherparepvec (T‐VEC; first Food And Drug Administration‐approved oncolytic immunotherapy) in combination with ipilimumab appeared to have greater efficacy than either T‐VEC or ipilimumab monotherapy. 7 In November 2005, Oncorine combined with chemotherapy was approved for the treatment of nasopharyngeal carcinoma in China. 8 Considering the disease progression, the patient received experimental treatment of Oncorine together with nivolumab and amlotinib. After four cycles of intratumoral Oncorine administration, the patient achieved stable disease with a performance status score of 1. Our study revealed that Oncorine might reverse resistance to ICIs in a patient with recurrent NSCLC. Our patient tolerated Oncorine well. The main adverse effect was fever which was controlled after antipyretic treatment. However, partial lung tissue necrosis was found on CT imaging. It is not certain whether the lung tissue necrosis was caused by the disease itself or by oncolytic viruses, and this requires more clinical data to provide an explanation.
This was a successful exploration of oncolytic viruses reversing immune resistance in the patient in the study. However, further investigations are necessary to validate the efficacy and safety of oncolytic viruses combined with immunotherapy in patients with recurrent NSCLC.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Zhang Q‐N, Li Y, Zhao Q, et al. Recombinant human adenovirus type 5 (Oncorine) reverses resistance to immune checkpoint inhibitor in a patient with recurrent non‐small cell lung cancer: A case report. Thorac Cancer. 2021;12:1617–1619. 10.1111/1759-7714.13947
Contributor Information
Li‐Yun Miao, Email: liyunmiao462@163.com.
Yu‐Jie Zhou, Email: yujiezhoum@163.com.
REFERENCES
- 1. Das S, Johnson DB. Immune‐related adverse events and anti‐tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pike LRG, Bang A, Mahal BA, Taylor A, Krishnan M, Spektor A, et al. The impact of radiation therapy on lymphocyte count and survival in metastatic cancer patients receiving PD‐1 immune checkpoint inhibitors. Int J Radiat Oncol Biol Phys. 2019;103(1):142–51. [DOI] [PubMed] [Google Scholar]
- 3. Song Y, Fu Y, Xie Q, Zhu B, Wang J, Zhang B. Anti‐angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Front Immunol. 2020;11:1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He CB, Lin XJ. Inflammation scores predict the survival of patients with hepatocellular carcinoma who were treated with transarterial chemoembolization and recombinant human type‐5 adenovirus H101. PLoS One. 2017;12(3):e0174769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beljanski V, Hiscott J. The use of oncolytic viruses to overcome lung cancer drug resistance. Curr Opin Virol. 2012;2(5):629–35. [DOI] [PubMed] [Google Scholar]
- 6. Liu Z, Ravindranathan R, Kalinski P, Guo ZS, Bartlett DL. Rational combination of oncolytic vaccinia virus and PD‐L1 blockade works synergistically to enhance therapeutic efficacy. Nat Commun. 2017;8:14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puzanov I, Milhem MM, Minor D, Hamid O, Li A, Chen L, et al. Talimogene Laherparepvec in combination with Ipilimumab in previously untreated, unresectable stage IIIB‐IV melanoma. J Clin Oncol. 2016;34(22):2619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98(5):298–300. [DOI] [PubMed] [Google Scholar]


