Dear Editor
The COVID-19 pandemic has proven to be devastating for all the countries.1 The treatment has been mostly limited to symptomatic and supportive care. Various drugs have been repurposed and tried for their potential role in treatment of COVID-19.2 At the same time, management of the adverse reactions secondary to these new and experimental drugs has been a matter of concern. In this letter, we discuss the literature available on methemoglobinemia in patients suffering from COVID-19.
In total, we found eight cases of methemoglobinemia and COVID-19 reported till October 30, 2020 (Table 1 ). Largest case series has been reported by Naymagon et al. consisting of 3 patient data.3 Table 1 shows that all patients were male, mostly middle aged. Maximum methemoglobin level of 30% or more was reported in 2 patients.3 , 4 Except one, all of the other seven patients did receive hydroxychloroquine (HCQ) as a part of treatment strategy for COVID-19 patients.5 Almost all patients were critically ill and required intensive level of care. Intravascular hemolysis in addition to methemoglobinemia was noted in four patients.4, 5, 6, 7 Except the case reported by Palmer et al., all other cases required treatment with one or more antioxidants namely methylene blue, ascorbic acid, vitamin B12 and red blood cell exchange.3, 4, 5, 6, 7, 8 Outcome of the patients of this study group was variable, four patients were successfully discharged after recovery, three patients were still admitted while case reporting, and one patient succumbed to his illness.
TABLE 1.
Descriptive analysis of reported cases of methemoglobinemia in patients with COVID-19.
| Age/Sex | Peak Meth Hb level | Medications for COVID-19 pneumonia | Treatment for methemoglobinemia | Hospital course | Outcome | |
|---|---|---|---|---|---|---|
| Naymagon et al.3 | 50/Male | 10.6% | Hydroxychloroquine, Azithromycin, Ceftriaxone | Methylene blue Ascorbic acid | Patient required intensive care, was intubated and required vasopressors | MethHb levels normalized by Day 11 of hospitalization, patient got extubated, was still hospitalized |
| Naymagon et al.3 | 52/M | >30% | Hydroxychloroquine, Azithromycin, Cefepime, Cancomycin | Methylene blue Ascorbic acid Red cell exchange | Patient required intensive care, was intubated, required vasopressors, developed ARF mandating renal replacement therapy | Improved clinically with a complete normalization of Met‐Hb level. Patient remains critically ill, ventilated and on vasopressors (Still hospitalized at the time of write up of case) |
| Naymagon et al.3 | 54/M | 18.8% | Hydroxychloroquine, Azithromycin | Methylene blue | Patient's laboratory suggested worsening hemolysis once started on methylene blue. Patient's Met‐Hb worsened from 13.6% to 18.8%. A new diagnosis of G6PD deficiency was found concurrent to methemoglobinemia. Direct antiglobulin test was negative. | The patient died shortly after admission. |
| Faisal et al.8 | 74/M | 15.9% | Azithromycin, Hydroxychloroquine, Lopinavir-ritonavir, Ribavirin, Tocilizumab | Intravenous hydroxocobalamin Methylene blue Ascorbic acid Red cell exchange | Patient required prolonged intensive care (4 weeks), was intubated, developed ARF requiring renal replacement therapy | After prolonged ICU course, patient recovered, got extubated, RRT frequency went down and was discharged to rehabilitation. |
| Palmer et al.5 | 62/M | 6.5% | Amoxicillin/clavulanic acid, Folic acid | None | Patient required high flow oxygen and renal replacement therapy. Direct antiglobulin test was negative G6DP assay confirmed G6DP deficiency | Prolonged duration of stay, was discharged after 22 days of hospital stay. |
| Lim et al.6 | 39/M | 14.8% | Hydroxychloroquine | Ascorbic acid Red cell exchange | Patient required high flow oxygen and renal replacement therapy G6DP assay confirmed G6DP deficiency | Improved, discharged home |
| Choo et al.4 | 52/M | 30% | Hydroxychloroquine, Azithromycin | Methylene blue Ascorbic acid Red cell exchange | Patient required intensive care, was intubated, required vasopressors, renal replacement therapy. There was a rapid fall in hemoglobin requiring 12 units of PRBCs support | Hemolysis resolved; methemoglobin levels improved. The patient was on vasopressors at the time writing up of case. |
| Kuipers et al.7 | 56/M | 9.1 | Hydroxychloroquine | Ascorbic acid | Patient required intensive care, was intubated. G6DP assay confirmed G6DP deficiency. There was a rapid fall in hemoglobin requiring 3 units of PRBCs support | Improved, discharged home |
Methemoglobin is formed when iron in hemoglobin gets oxidized from ferrous [Fe2+] state to the ferric [Fe3+] state. Drugs including HCQ have the potential to initiate this reaction by reducing free O2 to the free radical O2-. This in turn oxidizes hemoglobin to methemoglobin. Various drugs have variable potency to accelerate this oxidation reaction from 100 to 1000 times. Similarly, the body tries to keep the methemoglobin level in the blood to a minimum via cytochrome-b5 reductase mediated reduction process which requires NADH. In the event of excessive methemoglobin production, the body's normal homeostatic mechanism is not able to nullify the overproduction of methemoglobin which ultimately leads to methemoglobinemia.
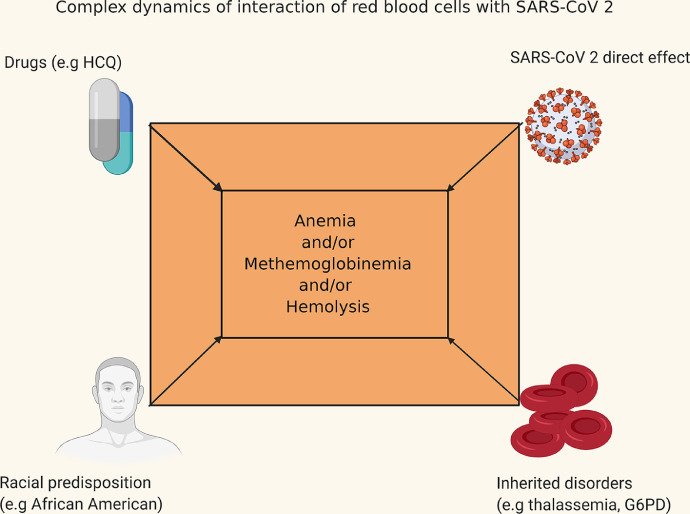
The study shows that management of COVID-19 can get complicated due to unforeseen reasons, and hence, high vigilance is of the utmost importance (Fig. 1). Drugs like HCQ, azithromycin, etc., could potentially lead to fatal methemoglobinemia and/or intra vascular hemolysis leading to higher mortality. Other factors like congenital disorders (e.g., thalassemia, sickle cell disease, and hereditary spherocytosis) can potentially worsen the anemia due to their various pathophysiological mechanisms.9, 10, 11, 12 In cases with co-existent G6PD deficiency, methylene blue use could be detrimental as it worsens the hemolysis.4 In such scenarios, use of ascorbic acid and vitamin B 12 has been shown to be beneficial.13 , 14
figure 1.
Possible interactions in patients with COVID-19 contributing to methemoglobinemia.
To conclude, though use of HCQ has gone down significantly especially in developed countries, it is still a prevalent drug in use in developing nations and knowledge about the associated side effects could be lifesaving.
Footnotes
The authors have no conflicts of interest or sources of funding to declare.
References
- 1.Karunakaran P., Nampoothiri R.V., Sahu K.K. Managing blood disorders during the Covid-19 pandemic: current pharmacological insights. Expert Rev Clin Pharmacol. 2020 Oct 22 doi: 10.1080/17512433.2020.1841633. [DOI] [PubMed] [Google Scholar]
- 2.Babayeva M., Loewy Z. Repurposing Drugs for COVID-19: pharmacokinetics and Pharmacogenomics of Chloroquine and Hydroxychloroquine. Pharmgenomics Pers Med. 2020;13:531–542. doi: 10.2147/PGPM.S275964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naymagon L., Berwick S., Kessler A. The emergence of methemoglobinemia amidst the COVID-19 pandemic. Am. J. Hematol. 2020;95(8):E196–E197. doi: 10.1002/ajh.25868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choo S.Y. Rapidly rising methemoglobinemia in a patient with severe COVID-19 treated successfully with red cell exchange transfusion. Ther Apher Dial. 2020 Oct 11 doi: 10.1111/1744-9987.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer K., Dick J., French W. Methemoglobinemia in Patient with G6PD deficiency and SARS-CoV-2 infection. Emerging Infect. Dis. 2020;26(9):2279–2281. doi: 10.3201/eid2609.202353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim S, Bhatia K, Lee Y. Hemolytic anemia and methemoglobinemia due to hydroxychloroquine use for COVID-19 treatment in a glucose-6-phosphate dehydrogenase-deficient patient. Chest. 2020; 158(4): A558–A559. doi: 10.1016/j.chest.2020.08.527. [DOI]
- 7.Kuipers M.T., van Zwieten R., Heijmans J. Glucose-6-phosphate dehydrogenase deficiency-associated hemolysis and methemoglobinemia in a COVID-19 patient treated with chloroquine. Am J Hematol. 2020;95(8):E194–E196. doi: 10.1002/ajh.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faisal H., Bloom A., Gaber A.O. Unexplained methemoglobinemia in coronavirus disease 2019: a case report. A&A Practice. 2020;14(9):e01287. doi: 10.1213/XAA.0000000000001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Franceschi L., Costa E., Dima F. Acute hemolysis by hydroxycloroquine was observed in G6PD-deficient patient with severe COVD-19 related lung injury. Eur J Intern Med. 2020;77:136–137. doi: 10.1016/j.ejim.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maillart E., Leemans S., Van Noten H. A case report of serious haemolysis in a glucose-6-phosphate dehydrogenase-deficient COVID-19 patient receiving hydroxychloroquine. Infect Dis (Lond) 2020;52(9):659–661. doi: 10.1080/23744235.2020.1774644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beauverd Y., Adam Y., Assouline B. COVID-19 infection and treatment with hydroxychloroquine cause severe haemolysis crisis in a patient with glucose-6-phosphate dehydrogenase deficiency. Eur J Haematol. 2020;105(3):357–359. doi: 10.1111/ejh.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Severance T.S., Rahim M.Q., French J., 2nd . Pediatr Blood Cancer. 2020 Jul 25. COVID-19 and hereditary spherocytosis: a recipe for hemolysis; p. e28548. [DOI] [PubMed] [Google Scholar]
- 13.Sahu K.K., Mishra A.K., Lal A. Closing the saturation gap: a ten-year retrospective experience with methemoglobinemia. Intern Emerg Med. 2020;15(6):1109–1112. doi: 10.1007/s11739-020-02332-0. [DOI] [PubMed] [Google Scholar]
- 14.Dhibar D.P., Sahu K.K., Jain S. Methemoglobinemia in a case of paint thinner intoxication, treated successfully with Vitamin C. J Emerg Med. 2018;54(2):221–224. doi: 10.1016/j.jemermed.2017.10.035. [DOI] [PubMed] [Google Scholar]