Abstract
Introduction
Eribulin was approved in the United States (US) in 2010 for patients with metastatic breast cancer (MBC) who previously received at least two chemotherapeutic regimens, including anthracycline and taxane in the adjuvant or metastatic setting. With significant changes to the treatment landscape over the past decade, assessment of the real-world effectiveness of eribulin in clinical practice when used according to the approved US indication is valuable.
Methods
Patients with MBC were identified by community oncologists through a retrospective, multi-site patient chart review; de-identified data were abstracted into electronic case report forms. Eligible patients initiated eribulin consistent with approved US indication between 1 January 2011 and 31 December 2017. Clinical outcomes assessed included objective response rate (ORR), progression-free survival (PFS) and overall survival (OS) in all patients and those with triple negative breast cancer (TNBC).
Results
The analysis included 513 patients (median 59.0 years; 38.8% with Eastern Cooperative Oncology Group status ≥ 2). Eribulin was third-line therapy for 78.0% of patients, and fourth-line or later for the remainder. ORR was 54.4%, median PFS was 6.1 months (95% CI: 5.8, 6.6), and median OS was 10.6 months (95% CI 9.9, 11.7) in all patients. Among the 49.9% of patients with TNBC, ORR was 55.1%, median PFS was 5.8 months (95% CI 5.1, 6.4), and median OS was 9.8 months (95% CI 8.6, 11.0).
Conclusion
The current retrospective chart review study reinforces the clinical effectiveness of eribulin in patients with MBC, including those with TNBC, when used according to the approved US indication in real-world clinical practice.
Keywords: Eribulin, Metastatic breast cancer, Real-world, Triple negative
Key Summary Points
| Why carry out this study? |
| Eribulin mesylate was approved by the Food and Drug Administration ten years ago for the treatment of patients with metastatic breast cancer (MBC), who had previously received at least two chemotherapy regimens for MBC, including an anthracycline and a taxane in the adjuvant or metastatic setting. The treatment landscape for MBC evolved dramatically over the past decade, so up-to-date real-world effectiveness data of eribulin is valuable when used according to the approved US indication in clinical practice. |
| We sought to investigate clinical effectiveness of eribulin therapy in a large, diverse cohort of patients with MBC in real-world clinical practice, including a subgroup of patients with triple negative breast cancer (TNBC), treated in accordance with the approved indication for eribulin in the United States. |
| What was learned from the study? |
| In a cohort of 513 MBC patients treated with eribulin, ORR was 54.4%, median PFS was 6.1 months, and median OS was 10.6 months. Within the TNBC subtype, ORR was 55.5%, median PFS was 5.8 months, and median OS was 9.8 months. |
| The present study reinforces the clinical effectiveness of eribulin in a large, heterogeneous patient population in clinical practice over the past decade, when used consistent with the approved US indication in the overall MBC population as well as within the TNBC subtype. |
Digital Features
This article is published with digital features, including summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13415105.
Introduction
An estimated 168,292 women in the United States (US) currently live with metastatic breast cancer (MBC) [1]. The most common breast cancer molecular subtype is hormone receptor positive (HR+)/human epidermal growth factor receptor status negative (HER2−), followed by HR+/HER2+, HR−/HER2− (“triple negative” breast cancer, or TNBC) and HR−/HER2+ [2]. Although survival has significantly improved over the past decade due to advances in treatment, mortality for MBC remains high, and an estimated 42,170 patients in the US will die as a result of their disease in 2020 [3, 4]. Survival differs by subtype, with HR+/HER2− cases experiencing the best prognosis and TNBC the worst prognosis [5].
Eribulin mesylate, a novel nontaxane inhibitor of microtubule dynamics, was approved by the Food and Drug Administration (FDA) 10 years ago for the treatment of patients with MBC, who have previously received at least two chemotherapeutic regimens for MBC, including an anthracycline and a taxane in either the adjuvant or metastatic setting [6]. The approval of eribulin was based on the results of the EMBRACE trial, in which treatment with eribulin conferred an overall survival (OS) benefit of 2.5 months over treatment of physician’s choice in women with MBC who had received two to five prior lines of therapy [7].
In the years post-approval, the effectiveness of eribulin in real-world practice has been demonstrated in prospective observational studies and retrospective analyses reflecting different patient populations and diverse treatment settings worldwide [8–14]. However, the majority of the studies were conducted outside the US, where the approved indication differs, and none utilized eribulin in a manner exclusively consistent with the approved US indication. The treatment landscape for MBC has changed in the decade since the FDA approval of eribulin, with multiple new therapeutic options available for specific molecular subtypes of MBC. Given these advances in therapy, we sought to assess the real-world effectiveness of eribulin, for which the approved US indication is not receptor subtype-specific, in a contemporary clinical setting. We chose to focus on the TNBC subtype in particular, which has fewer treatment options. In this study, we investigated long-term outcomes of eribulin therapy in a large, diverse cohort of patients with MBC, including a subgroup of patients with TNBC, treated in accordance with the approved indication for eribulin in the US.
Methods
A retrospective, multi-site patient chart review was conducted. Physicians invited to participate in the study were recruited from the Cardinal Health Oncology Provider Extended Network, a group of more than 7000 unique physicians in oncology and hematology practices from across the US. Physicians who were selected to participate in this study completed an initial feasibility request indicating that they treated > 2 MBC patients per month who met the study eligibility criteria. All participating physicians represented separate practices to ensure there was no duplication of patients. Prior to chart data abstraction, study materials (research protocol and pilot-tested electronic case report form [eCRF]) were approved by Western Institutional Review Board (IRB), an independent, central IRB. The IRB granted the study a waiver for obtaining informed consent from patients.
Physicians performed the data abstraction themselves. All abstracted data were de-identified in compliance with the Health Insurance Portability and Accountability Act. Physicians were asked to identify the earliest (over the time period 2011–2017) patient that met the selection criteria and randomly select patients forward in time until up to 20 eligible patients were identified. Starting with the earliest patient rather than those who had initiated eribulin therapy recently reduced the risk of incomplete follow-up for patients. The limit of 20 patients per provider was imposed to minimize potential bias from one single provider or practice. To be included in the study, a patient had to have a confirmed diagnosis of MBC and initiated eribulin treatment for MBC between 1 January 2011 and 31 December 2017. The latest index date of 31 December 2017 was selected to ensure that sufficient long-term follow-up data were available for all patients. Data collection occurred from February through May 2020, allowing for more than 2 years of follow-up for all patients.
The study was limited to female adults who were 18 years or older at the time of initiation of eribulin, and had received at least two chemotherapy regimens in the metastatic setting prior to treatment with eribulin. All patients had received both an anthracycline and a taxane therapy in either the adjuvant or metastatic setting. Patients receiving eribulin treatment for indications other than MBC, or patients receiving eribulin treatment for MBC in the context of a clinical trial, were excluded.
Completed eCRFs were reviewed independently for data quality and consistency, and to ensure no duplicate patient data. Queries and random data point validation were conducted. Patients for whom eCRFs could not be validated (or if the physician was non-responsive to requests for validation) were excluded from the analyses.
Outcomes assessed were objective response rate (ORR), clinical benefit rate (CBR), progression-free survival (PFS), and OS. Outcomes were assessed in all patients and separately for patients with TNBC.
Tumor response was abstracted as substantiated in the patient’s electronic health record by the physicians: complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). The ORR was calculated as: (CR + PR)/(all patients). The CBR was calculated as: (CR + PR + SD lasting more than 24 weeks)/(all patients). A baseline scan must have been completed prior to the initiation of treatment with eribulin (alternatively, if a scan was completed to assess disease response to the most recent prior line of therapy, that scan could be used).
Progression-free survival and OS were calculated using the Kaplan–Meier method. The index date was the date of eribulin initiation. For PFS analysis, patients who had not progressed were censored on their last date of eribulin therapy. For OS analysis, patients alive at the time of data cut-off were censored on their last recorded clinic visit date. Additionally, PFS at 3-, 6-, 9-, and 12-months and OS at 3-, 6-, 12-, 24- and 36-months with 95% confidence intervals (CIs) are reported. All analyses were conducted in SAS v9.4.
Results
A total of 513 eCRFs were included in the current analyses. Data were abstracted by 46 physicians, 69.6% of whom reported their primary specialty as hematology/oncology. The primary practice setting for the physicians was identified as private community practice by 54.4% of physicians, while 21.7% reported practicing at academic centers or affiliated teaching hospitals, 19.6% at private community practices owned by hospitals, and 4.4% as solo practitioners. The median number of years in practice for the physicians was 16 (range 4–30).
Demographics and Clinical Characteristics
Demographics for the overall cohort and the TNBC subgroup are shown in Table 1.
Table 1.
Patient demographics at the initiation of eribulin
| All | TNBC | |
|---|---|---|
| n = 513 | n = 256 | |
| Age | ||
| Mean, SD | 58.7 (10.9) | 56.3 (10.5) |
| Median, IQR | 59.0 (15.0) | 57.0 (16.0) |
| < 65 (n, %) | 344 (67.1) | 191 (74.6) |
| ≥ 65 (n, %) | 169 (32.9) | 65 (25.4) |
| Race (n, %) | ||
| Caucasian | 333 (64.9) | 158 (61.7) |
| African American | 135 (26.3) | 83 (32.4) |
| Asian | 41 (8.0) | 13 (5.1) |
| Native Hawaiian or Other Pacific Islander | 1 (0.2) | 0 (0.0) |
| American Indian or Alaska Native | 1 (0.2) | 1 (0.4) |
| Other | 2 (0.4) | 1 (0.4) |
| Ethnicity (n, %) | ||
| Hispanic/Latino | 54 (10.5) | 23 (9.0) |
| Non-Hispanic/Non-Latino | 459 (89.5) | 233 (91.0) |
| Payer (n, %) | ||
| Medicare | 108 (21.1) | 39 (15.2) |
| Medicare Advantage/Supplement | 111 (21.6) | 41 (16.0) |
| Medicaid | 81 (15.8) | 55 (21.5) |
| Medicare/Medicaid (dual eligible) | 11 (2.1) | 1 (0.4) |
| Commercial | 220 (42.9) | 117 (45.7) |
| Military health insurance | 3 (0.6) | 3 (1.2) |
| Unknown | 4 (0.8) | 2 (0.8) |
| Region of patient primary residence (n, %) | ||
| Northeast | 114 (22.2) | 48 (18.8) |
| Midwest | 117 (22.8) | 41 (16.0) |
| South | 172 (33.5) | 105 (41.0) |
| West | 110 (21.4) | 62 (24.2) |
IQR interquartile range, SD standard deviation, TNBC triple negative breast cancer
The median age at initiation of eribulin therapy in the overall patient population was 59.0 years (range 26–86); 32.9% were ≥ 65 years old. Most patients were Caucasian (64.9%) and non-Hispanic (89.5%); African American patients comprised 26.3% of the study population. Demographics in the TNBC subgroup were largely similar to those in the overall cohort.
Table 2 describes the clinical characteristics of the patients at the time of initiation of eribulin, for both the overall population and the TNBC subgroup. Eribulin was most commonly used in the third line (78.0%), with the remainder treated in the fourth to seventh line. The majority of patients (61.0%) had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 at the initiation of eribulin. Overall, 45.4% of patients were HR+/HER2−, and 49.9% had TNBC. Within the TNBC subgroup, most patients received eribulin for third-line therapy (87.9%). Approximately two-thirds of the patients with TNBC had an ECOG performance status of 0 or 1. In both the overall cohort and the TNBC subgroup, > 90% of patients had visceral metastatic disease at the time of eribulin initiation. The most common sites of metastases were lung and liver (66.7% and 57.3% respectively).
Table 2.
Patient clinical characteristics at the initiation of eribulin
| All | TNBC | |
|---|---|---|
| n = 513 | n = 256 | |
| Line of therapy at initiation of eribulin (n, %) | ||
| 3 | 400 (78.0) | 225 (87.9) |
| 4 | 77 (15.0) | 25 (9.8) |
| ≥ 5 | 36 (7.0) | 6 (2.3) |
| ECOG-PS Categorical (n, %) | ||
| 0/1 | 313 (61.0) | 168 (65.6) |
| ≥ 2 | 199 (38.8) | 88 (34.4) |
| Unknown | 1 (0.2) | 0 (0.0) |
| Sites of metastases at initiation of eribulin (n, %) | ||
| Adrenal gland | 63 (12.3) | 26 (10.2) |
| Brain | 22 (4.3) | 15 (5.9) |
| Local lymph node(s) | 56 (10.9) | 40 (15.6) |
| Gastrointestinal system | 10 (2.0) | 1 (0.4) |
| Genitourinary system | 10 (2.0) | 4 (1.6) |
| Ovary | 14 (2.7) | 5 (2.0) |
| Liver | 294 (57.3) | 159 (62.1) |
| Lung | 342 (66.7) | 179 (69.9) |
| Lytic or mixed lytic-blastic bone | 99 (19.2) | 33 (12.9) |
| Pleura, pericardial, and/or peritoneal cavity | 28 (5.5) | 16 (6.3) |
| Othera | 4 (0.8) | 3 (1.2) |
| Duration of follow-up from metastatic diagnosisb, months | ||
| Mean, STD | 32.5 (16.8) | 28.5 (16.0) |
| Median, IQR | 29.9 (22.7) | 25.9 (20.6) |
ECOG-PS Eastern Cooperative Oncology Group performance status, IQR interquartile range, STD standard deviation, TNBC triple negative breast cancer
aIncluding chest wall and those with missing site information
bUntil last visit with provider or at clinic
Duration of Eribulin Therapy
At the time of data cut-off, 497 (96.9%) patients in the overall cohort and 248 (96.9%) patients in the TNBC subgroup had discontinued eribulin. Among all patients who discontinued, median duration of eribulin therapy was 5.5 months (range 0.03–21.5) overall, and 5.4 months (range 1.2–21.5) in the TNBC subgroup. Among patients who discontinued treatment with eribulin, the majority did so due to disease progression in both the overall cohort (78.1%) and the TNBC subgroup (84.3%). Other reasons for discontinuation in the overall cohort and TNBC subgroup included decline in performance status (4.0% and 2.4%), referral to hospice (5.0% and 6.1%), palliative care (3.4% and 0.4%), completion of the scheduled duration of eribulin therapy (4.8% and 4.8%), patient choice (2.2% and 1.2%), and other reasons (1.4% and 0.4%). Toxicity/intolerability was reported as the reason for discontinuation in 1.0% of patients overall and 0.4% of TNBC patients.
Following discontinuation of eribulin, 175 (34.1%) of all patients and 99 (38.7%) of TNBC patients initiated another line of therapy. Among patients who initiated another line of treatment, the median time to next treatment was 8.1 months (range 1.6–22.4) overall and 8.3 months (range 2.1–22.4) in the TNBC subgroup.
Disease Response
In the overall population, ORR was 54.4% (95% CI 50.1–58.7%) (Fig. 1). The CBR was 52.3% (95% CI 52.4–61.0%). A total of 41 patients (8.0%) had a CR, 238 (46.4%) had a PR, 88 (17.2%) had SD and 146 (28.5%) had PD. The time to best response among patients with a CR or PR was a median of 3.0 months [interquartile range (IQR) 2.2, 4.1]. The duration of best response among the 261 patients with a CR or PR and known dates of initial response and progression was a median of 4.5 months (IQR 2.9, 7.1).
Fig. 1.
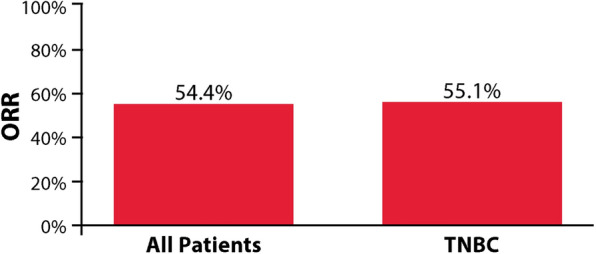
ORR to eribulin treatment in all patients and the TNBC subgroup
For patients in the TNBC subgroup, ORR was 55.1% (95% CI 48.8–61.2%) (Fig. 1). The CBR was 57.4% (95% CI: 51.1–63.5%). A total of 15 (5.9%) patients with TNBC had a CR, 126 (49.2%) had a PR, 46 (18.0%) had SD and 69 (27.0%) had PD. The time to best response for patients with a CR or PR was a median of 2.8 months (IQR 2.1, 3.9), and the duration of best response for the 132 patients with a CR or PR and known dates of initial response and progression was a median of 4.2 months (IQR 2.8, 6.6).
PFS
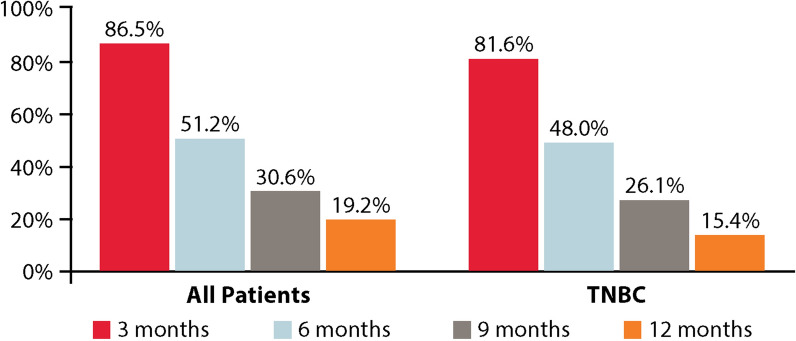
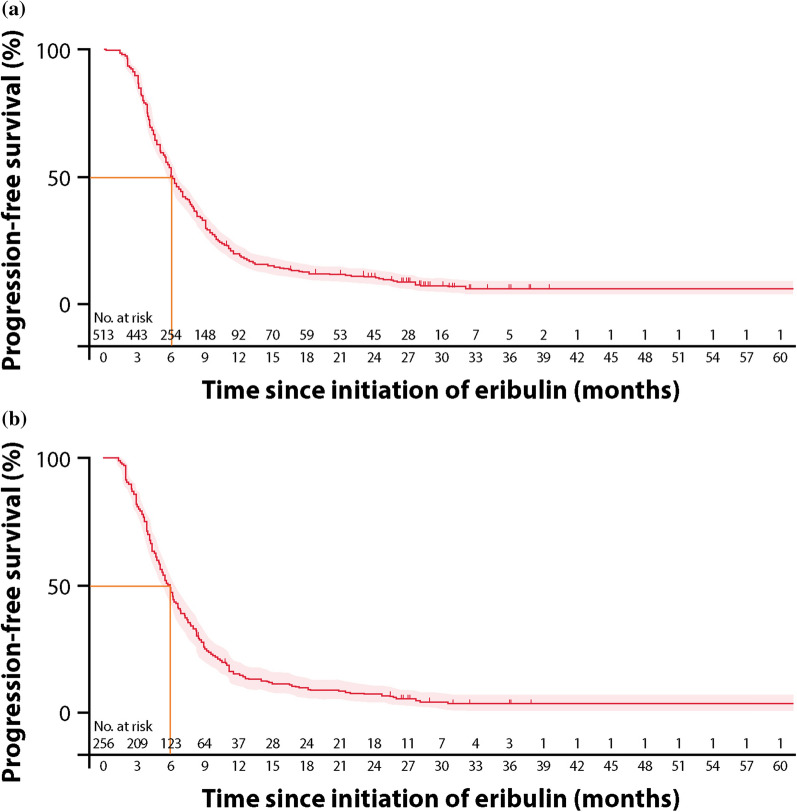
At the time of data cut-off, 402 (78.4%) patients, including 219 (85.5%) in the TNBC subgroup, had progressed on eribulin therapy. The landmark PFS is presented in Fig. 2. At the 6-month landmark, 262 patients (51.2%, 95% CI 46.8–55.5%) were alive and progression-free in the overall population, and, at the 12-month landmark, 94 patients (19.2%, 95% CI 15.8–22.8%) were alive and progression-free (Fig. 2). Progression-free survival as estimated using the Kaplan–Meier method is presented in Fig. 3. The median PFS of patients in the overall population was 6.1 months (95% CI 5.8–6.6) (Fig. 3a).
Fig. 2.
PFS at 3, 6, 9, and 12 months in all patients and the TNBC subgroup
Fig. 3.
Kaplan–Meier PFS from initiation of eribulin in a all patients and b the TNBC subgroup
In the TNBC subgroup, 125 patients (48.0%, 95% CI 41.8–54.0%) were alive and progression-free at the 6-month landmark, and 38 (15.4%, 95% CI 11.3–20.2%) were alive and progression-free at the 12-month landmark (Fig. 2). The median PFS of patients in the TNBC subgroup was 5.8 months (95% CI 5.1–6.4) (Fig. 3b).
OS
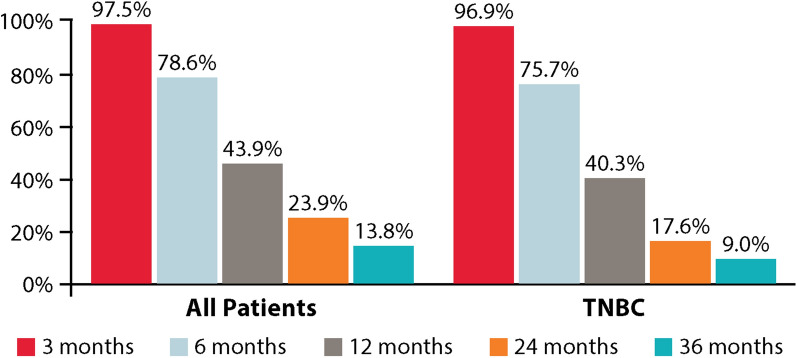
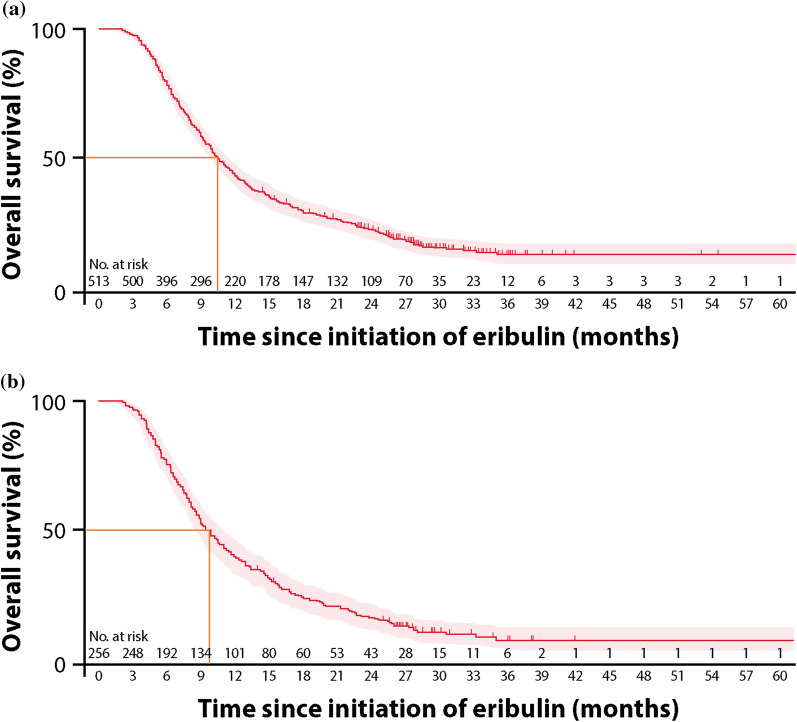
At the time of data cut-off, 415 (80.9%) patients overall and 222 (86.7%) in the TNBC subgroup were deceased. The landmark OS is presented in Fig. 4. At the 12-month landmark, 43.9% (95% CI 39.6–48.2%) of patients were alive in the overall population, while at the 24-month landmark, 23.9% (95% CI 20.2–27.7%) were alive (Fig. 4). A Kaplan–Meier graph for OS is presented in Fig. 5. The median OS of patients in the overall population was 10.6 months (95% CI 9.9–11.7) (Fig. 5a).
Fig. 4.
OS at 3, 6, 12, 24, and 36 months in all patients and the TNBC subgroup
Fig. 5.
Kaplan–Meier OS from initiation of eribulin in a all patients and b the TNBC subgroup
In the TNBC subgroup, 40.3% (95% CI 34.3–46.3%) were alive at the 12-month landmark, and 17.6% (95% CI 13.1–22.6%) were alive at the 24-month landmark (Fig. 4). The median OS of patients in the TNBC subgroup was 9.8 months (95% CI 8.6–11.0) (Fig. 5b).
Discussion
With over 500 patients treated with eribulin in accordance with the approved US indication, our study represents the largest real-world retrospective study of eribulin conducted in a US population to date. Nearly 80% of the patients in our study received eribulin as third-line treatment, with the remainder receiving the drug in later lines of therapy. Some key differences between our patient population and that of the EMBRACE trial include racial diversity (26.3% African American in this study vs. 4% in EMBRACE); performance status (38.8% with ECOG status ≥ 2 in this study, vs. 8% with ECOG status = 2 in EMBRACE); and the proportion of patients with a diagnosis of TNBC (49.9% in this study vs. 19% in EMBRACE). Despite these differences, clinical benefit was still observed (ORR was 54.4% in this study vs. 12% in EMBRACE, and CBR was 56.7% in this study vs. 23% in EMBRACE). Time to event outcomes like PFS and OS were also similar with those observed in EMBRACE.
Less than 5% of cancer patients participate in clinical trials, and those that do tend to be younger, healthier, and less diverse than the patient population as a whole [15]. Furthermore, it has been shown that cancer clinical trial participants have better survival than patients in the real world for the first year following diagnosis, a finding attributed in part to the strict eligibility criteria for most trials that screen out patients with a poorer prognosis [16]. Therefore, it is valuable to see if the survival benefit observed in the EMBRACE clinical trial can be consistently observed in real-world patients treated with eribulin. The results of our study support the effectiveness of eribulin in contemporary clinical practice, and the continuing relevance of eribulin as a treatment option in the current therapeutic landscape.
The mean duration of eribulin therapy reported in this study (5.5 months) was consistent with the median PFS observed. This duration of therapy is also comparable to what has been reported in other recent real-world studies of eribulin conducted in the US: a mean 5.3 months treatment duration was observed for TNBC patients treated with eribulin in the third-line or later, and a mean 2.7–3.6 months was observed in HR+/HER2− patients who received eribulin treatment in third-line or later after a CDK 4/6 inhibitor [13, 14]. Similarly, another study found the median time to next treatment across all single agents in third-line or later therapy for MBC was 4.6 months [17]. This suggests that the treatment duration in our cohort accurately reflects real-world treatment within the MBC patient population.
Among the multiple real-world studies conducted through different regions over the years, none specified the use of eribulin in accordance with the US label [6]. One systematic review and pooled analysis comparing outcomes with eribulin in real-world studies to those observed in 2 phase 3 trials (EMBRACE and Study 301 [18]) found that ORR and CBR were better in the real world studies (20.1% and 46.3% across 11 retrospective studies, vs. 14.9% and 30.9% in the clinical trials) [19]. Progression-free survival was similar in the real-world studies (median 3.84 months across 10 retrospective studies vs. 4.0 months in the clinical trials) and OS was shorter (median 9.71 months across 8 retrospective studies vs. 15.2 months in the clinical trials) [19]. A second systematic review of 34 studies of effectiveness outcomes with eribulin in patients with locally advanced breast cancer or MBC found that median PFS ranged from 2.3 to 14.7 months, while median OS ranged from 6.9 to 28.0 months. Despite the variability in outcomes, this systematic review supports the effectiveness of eribulin for the treatment of MBC in real-world practice [20]. Our study also found that outcomes in the TNBC subgroup were similar to those in the overall cohort, even though TNBC is associated with a more aggressive pathology and worse survival [21]. This finding is consistent with a prior retrospective, real-world US study in a TNBC population in which we observed a median OS of 14.7 months among patients receiving eribulin third-line or later [13], although patients in this study may not have received eribulin strictly per the US approved indication. These are promising results in a patient population where there is significant unmet need and few approved treatment options.
This study has several strengths. First, its robust size (> 500 patients) and long duration of follow-up allowed for greater precision in estimates of PFS and OS. Second, restriction of eligibility to patients treated with eribulin following at least two chemotherapy regimens, with prior therapies including both an anthracycline and a taxane therapy in adjuvant or metastatic setting provided an opportunity for assessment of real-world outcomes when use was consistent with the approved US indication, in contrast to other real-world studies where eribulin was used across all lines of therapy. Finally, the inclusion of patients from community and academic practices in all geographic locations of the US resulted in a heterogeneous patient population (in terms of age, race, and functional status) more representative of the overall MBC patient population. A significant proportion of the patients in our study had factors associated with poor prognosis; half were of the TNBC subtype and nearly 40% had an ECOG performance status of 2 or greater. This supports the generalizability of the effectiveness of eribulin across a broader spectrum of patients not well-represented in clinical trials.
Limitations of this study include its retrospective design and the potential for provider and patient selection bias. Additionally, the physician-reported response rates may be overestimated as standardized assessment schedules, and criteria might not have been routinely used outside of clinical trials. Third, this observational study was not designed to compare the real-world effectiveness of eribulin versus other agents, so no control group of non-eribulin treated patients was included. Finally, detailed safety data were not collected in this study, so specific information on adverse events in our patient population is not available.
Conclusion
In recent years, it has become increasingly apparent that cancer clinical trials may not be as representative of the broader patient experience, as they accrue younger, healthier, and less diverse patients. Our real-world cohort was racially diverse, included patients with poor functional status, and half of the patients had TNBC. Despite these patient characteristics, which are generally associated with worse outcomes, results from the current analyses indicate that both response rate and survival with eribulin therapy supports the clinical outcomes observed within the eribulin arm in the pivotal clinical trial. This retrospective chart review study reinforces the clinical effectiveness of eribulin in a large, diverse cohort of MBC patients including the TNBC subtype, when used according to the approved US indication in clinical practice.
Acknowledgements
The authors would like to thank Talia Miller, MSW, MPH, Hsing-Ting Yu, MPH, and JaLyna Laney, RN (full‐time employees of Cardinal Health), for their contribution in data collection and clinical data quality review.
Funding
This research was sponsored by Eisai Inc., and conducted by Cardinal Health. Eisai Inc. will fund the journal’s Rapid Service and Open Access Fees.
Medical Writing and Editorial Assistance
The authors acknowledge Marjorie Zettler, PhD, MPH, who provided writing and editorial assistance on the preparation of the manuscript (full-time employee of Cardinal Health).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Conception/design: SSM, JKK, JZ, DL, BAF. Provision of study material or patients: JKK. Collection and/or assembly of data: JKK, DL. Data analysis and interpretation: SSM, JKK, JZ, DL, BAF. Manuscript writing: SSM, JKK, JZ, BAF. Final approval of manuscript: SSM, JKK, JZ, DL, BAF.
Disclosures
SSM: Eisai Inc. (consulting fees), Celgene Corporation (consulting fees), Pfizer (research funding paid directly to my institution), Genentech (research funding directly to my institution). JKK: Cardinal Health Specialty Solutions (employee). JZ: Eisai Inc. (employee). DL: Cardinal Health Specialty Solutions (employee). BAF: Cardinal Health Specialty Solutions (employee).
Compliance with Ethics Guidelines
Prior to chart data abstraction, study materials (research protocol and pilot-tested electronic case report form [eCRF]) were approved by Western Institutional Review Board (IRB), an independent, central IRB. The IRB granted the study a waiver for obtaining informed consent from patients.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available as they will not be deposited.
Footnotes
The original online version of this article was revised due to update in Table 2.
Change history
4/7/2021
A Correction to this paper has been published: 10.1007/s12325-021-01683-0
Change history
5/16/2021
A Correction to this paper has been published: 10.1007/s12325-021-01780-0
References
- 1.Mariotto AB, Etzioni R, Hurlbert M, et al. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26(6):809–815. doi: 10.1158/1055-9965.EPI-16-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Cancer Stat Facts: Female Breast Cancer Subtypes. https://seer.cancer.gov/statfacts/html/breast-subtypes.html. Accessed 4 June 2020.
- 3.Caswell-Jin JL, Plevritis SK, Tian L, et al. Change in survival in metastatic breast cancer with treatment advances: meta-analysis and systematic review. JNCI Cancer Spectr. 2018;2(4):pky062. doi: 10.1093/jncics/pky062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Cancer Stat Facts: Female Breast Cancer. https://seer.cancer.gov/statfacts/html/breast.html Accessed 4 June 2020.
- 5.Howlader N, Cronin KA, Kurian AW, et al. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol Biomark Prev. 2018;27(6):619–626. doi: 10.1158/1055-9965.EPI-17-0627. [DOI] [PubMed] [Google Scholar]
- 6.Eisai Incorporated. Halaven (eribulin mesylate) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/201532lbl.pdf Revised November 2010. Accessed 9 July 2020.
- 7.Cortes J, O'Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 8.Adamo V, Ricciardi GRR, Giuffrida D, et al. Eribulin mesylate use as third-line therapy in patients with metastatic breast cancer (VESPRY): a prospective, multicentre, observational study. Ther Adv Med Oncol. 2019;11:1758835919895755. doi: 10.1177/1758835919895755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacot W, Heudel PE, Fraisse J, et al. Real-life activity of eribulin mesylate among metastatic breast cancer patients in the multicenter national observational ESME program. Int J Cancer. 2019;145(12):3359–3369. doi: 10.1002/ijc.32402. [DOI] [PubMed] [Google Scholar]
- 10.Inoue K, Takahashi M, Mukai H, et al. Effectiveness and safety of eribulin in Japanese patients with HER2-negative, advanced breast cancer: a 2-year post-marketing observational study in a real-world setting. Invest New Drugs. 2020;38(5):1540–1549. doi: 10.1007/s10637-019-00890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orditura M, Gravina A, Riccardi F, et al. Eribulin for metastatic breast cancer (MBC) treatment: a retrospective, multicenter study based in Campania, south Italy (Eri-001 trial) ESMO Open. 2017;2(2):e000176. doi: 10.1136/esmoopen-2017-000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barni S, Livraghi L, Morritti M, et al. Eribulin in the treatment of advanced breast cancer: real-world scenario from 39 Italian centers - ESEMPiO study. Future Oncol. 2019;15(1):33–44. doi: 10.2217/fon-2018-0324. [DOI] [PubMed] [Google Scholar]
- 13.Mougalian SS, Copher R, Kish JK, et al. Clinical benefit of treatment with eribulin mesylate for metastatic triple-negative breast cancer: Long-term outcomes of patients treated in the US community oncology setting. Cancer Med. 2018;7(9):4371–4378. doi: 10.1002/cam4.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mougalian SS, Feinberg BA, Wang E, et al. Observational study of clinical outcomes of eribulin mesylate in metastatic breast cancer after cyclin-dependent kinase 4/6 inhibitor therapy. Future Oncol. 2019;15(34):3935–3944. doi: 10.2217/fon-2019-0537. [DOI] [PubMed] [Google Scholar]
- 15.Unger JM, Cook E, Tai E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35:185–198. doi: 10.1200/EDBK_156686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unger JM, Barlow WE, Martin DP, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106(3):dju002. doi: 10.1093/jnci/dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinberg B, Kish J, Dokubo I, Wojtynek J, Gajra A, Lord K. Comparative effectiveness of palliative chemotherapy in metastatic breast cancer: a real-world evidence analysis. Oncologist. 2020;25(4):319–326. doi: 10.1634/theoncologist.2019-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman PA, Awada A, Twelves C, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015;33(6):594–601. doi: 10.1200/JCO.2013.52.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voutsadakis IA. A systematic review and pooled analysis of retrospective series of eribulin in metastatic breast cancer. Anticancer Drugs. 2017;28(5):557–564. doi: 10.1097/CAD.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 20.Chabot I, Zhao Q, Su Y. Systematic review of real-world effectiveness of eribulin for locally advanced or metastatic breast cancer. Curr Med Res Opin. 2020;36(12):2025–2036. doi: 10.1080/03007995.2020.1835853. [DOI] [PubMed] [Google Scholar]
- 21.Sharma P. Biology and management of patients with triple-negative breast cancer. Oncologist. 2016;21(9):1050–1062. doi: 10.1634/theoncologist.2016-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available as they will not be deposited.