Abstract
Arterial switch operations (ASO) are live-saving procedures performed on neonates to treat transposition of the great arteries (TGA). However, future operations on the neoaorta may be required due to dilation. We present a case of a 25-year-old female who presented with dilation of her neoaorta and required a David procedure. Her previous ASO resulted in an anterior lie of the pulmonary artery in front of the neoaorta, with both coronary arteries coming off anteriorly. We describe our approach to performing a David procedure on this patient with this unique anatomy.
Introduction
An arterial switch operation (ASO) is a lifesaving procedure performed at birth to treat D-transposition of the great arteries (TGA).1 However, long term complications from an ASO can include main pulmonary artery or branch stenosis, pulmonary regurgitation, neoaortic stenosis/regurgitation, and other complications.2 Ascending aortic aneurysms have been treated for over two decades with excellent outcomes using a valve-sparing root procedure, the David procedure.3 Here, we present the use of the David procedure for dilation of a neoaortic root with concomitant aortic valve insufficiency on a 25-year-old female status post neonatal arterial switch operation.
Clinical Summary
This case report does not require IRB review at our institution and the patient has given consent for their case to be used for educational purposes. Our patient, a 25-year-old female, was born with D-transposition of the great vessels (TGA) and a ventricular septal defect (VSD). She was treated at an outside institution with an arterial switch operation (ASO) and closure of her VSD at the age of 30 hours. The patient thrived for approximately two decades. She then had septic shock with pneumonia at age 22 complicated by complete heart block requiring implantation of a permanent pacemaker. She presented to our institution at age 23 with moderate dilation of her neoaorta at 4.4 cm and mild neoaortic regurgitation as diagnosed by CT angiogram and echocardiogram, respectively. The aneurysmal dilation was present in the neoaorta at the level of the aortic sinus measuring 46 mm × 42mm (Figure 1) with Yacoub Type A anatomy. The patient had pectus excavatum causing a mass effect on her right ventricle but remained asymptomatic (New York Heart Association class I). The patient was monitored for a year until the dilation of her neoaorta increased to 4.7 cm with her aortic valve regurgitation progressing to mild. After discussion of the complicating factors of the operation, she was interested in proceeding with a valve-sparing aortic root replacement to treat the root dilation and preserve the aortic valve. Preoperative CT scan confirmed her complex pulmonary anatomy with the pulmonary trunk and right main pulmonary artery overlying the neoaorta secondary to the Lecompte maneuver performed during her previous ASO (Figure 1).
Figure 1.
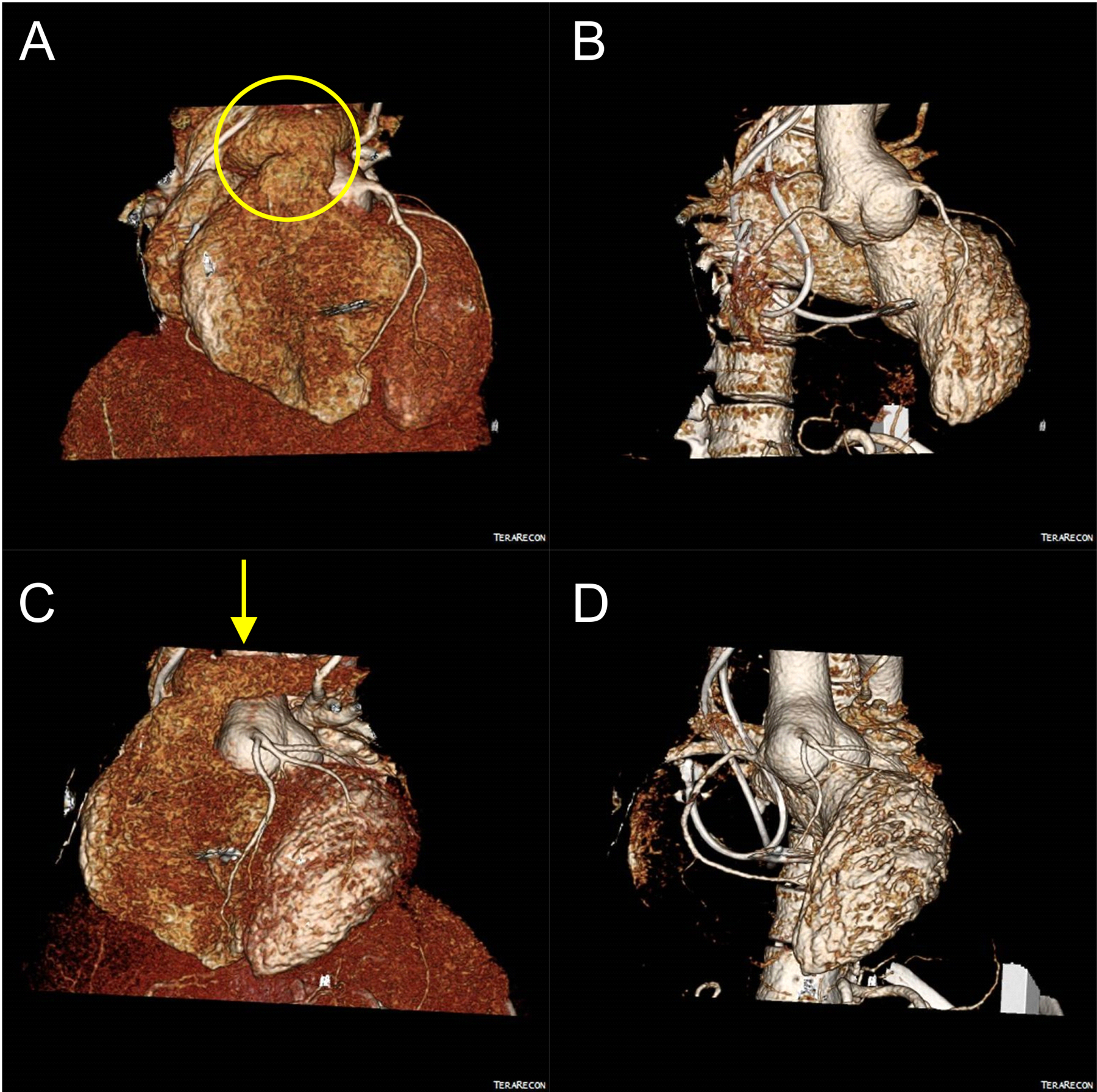
ECG-Gated CT Angiography with 3D postprocessing is shown for images pre-surgery. 3D volume rendered CTA images from multiple orientations are shown in A)-D). The overlying pulmonary artery is shown with a yellow oval (A) and the location of the incision of the left pulmonary artery is shown with a yellow arrow (C). Reconstructed images with the right ventricle removed are shown in B) and D) to view the aortic root aneurysm.
After dissecting the mediastinal structures below the sternum, we performed a redo sternotomy. The patient’s pectus excavatum made the sternotomy somewhat challenging. After successful reentry, the aorta was identified below the innominate vein. The pulmonary arterial bifurcation was dissected off the aorta posteriorly and ensnared with a vessel loop. The distal ascending aorta and right atrium were cannulated as well as a retrograde coronary sinus cardioplegia catheter and a left ventricular vent inserted. After initiation of cardiopulmonary bypass, the main pulmonary artery and branches were completely dissected off of the aorta. To expose the proximal ascending aorta, the left main pulmonary artery was divided at the bifurcation. The divided pulmonary arteries were retracted laterally to allow access to the aortic root. The aorta was cross clamped and cardioplegia was administered antegrade as well as retrograde.
The aorta was divided and the coronary buttons were isolated. The non-coronary sinus was excised. Care was taken to leave 5–7mm of aorta along the aortic valve. Due to the previous ASO, the right coronary artery (RCA) was coming off the conventional “noncoronary cusp” and the left main coronary artery (LMCA) was coming off of the conventional “right coronary cusp.” A 30mm Dacron Gelweave Valsalva graft (Terumo Ann Arbor, MI) was chosen for the root replacement. A total of 12 horizontal mattress Ethibond (Ethicon Inc. Somerville, NJ) sutures were placed below the aortic valve circumferentially. The sutures were placed through the Valsalva graft and tied down. The top of each commissure was resuspended with 5–0 pledgeted prolene (Ethicon Inc.) sutures through the graft. Each cusp was resuspended to the Valsalva graft with running 4–0 prolene suture. The LMCA was anastomosed with running 5–0 prolene to the Valsalva graft in the conventional “right coronary cusp.” Next, the RCA anastomosis to the Valsalva graft was performed in the conventional “non coronary cusp” with 5–0 prolene suture. The patient was rewarmed from 30 degrees and the distal aortic anastomosis was performed with running 4–0 prolene suture after trimming the Valsalva graft to the appropriate length. Finally, the pulmonary arteries were mobilized to ensure no tension and reconstructed with 5–0 prolene suture. After removal of the cross clamp, the heart regained normal function and was easily weaned from bypass. The patient had an uneventful post-op course was discharged home on post-op day 6. The patient’s CTA following surgery is shown in Figure 2. At her follow-up 7 months later, the patient had recovered well with only mild aortic regurgitation. She remains healthy with only mild aortic regurgitation at her one and two year follow-ups.
Figure 2.
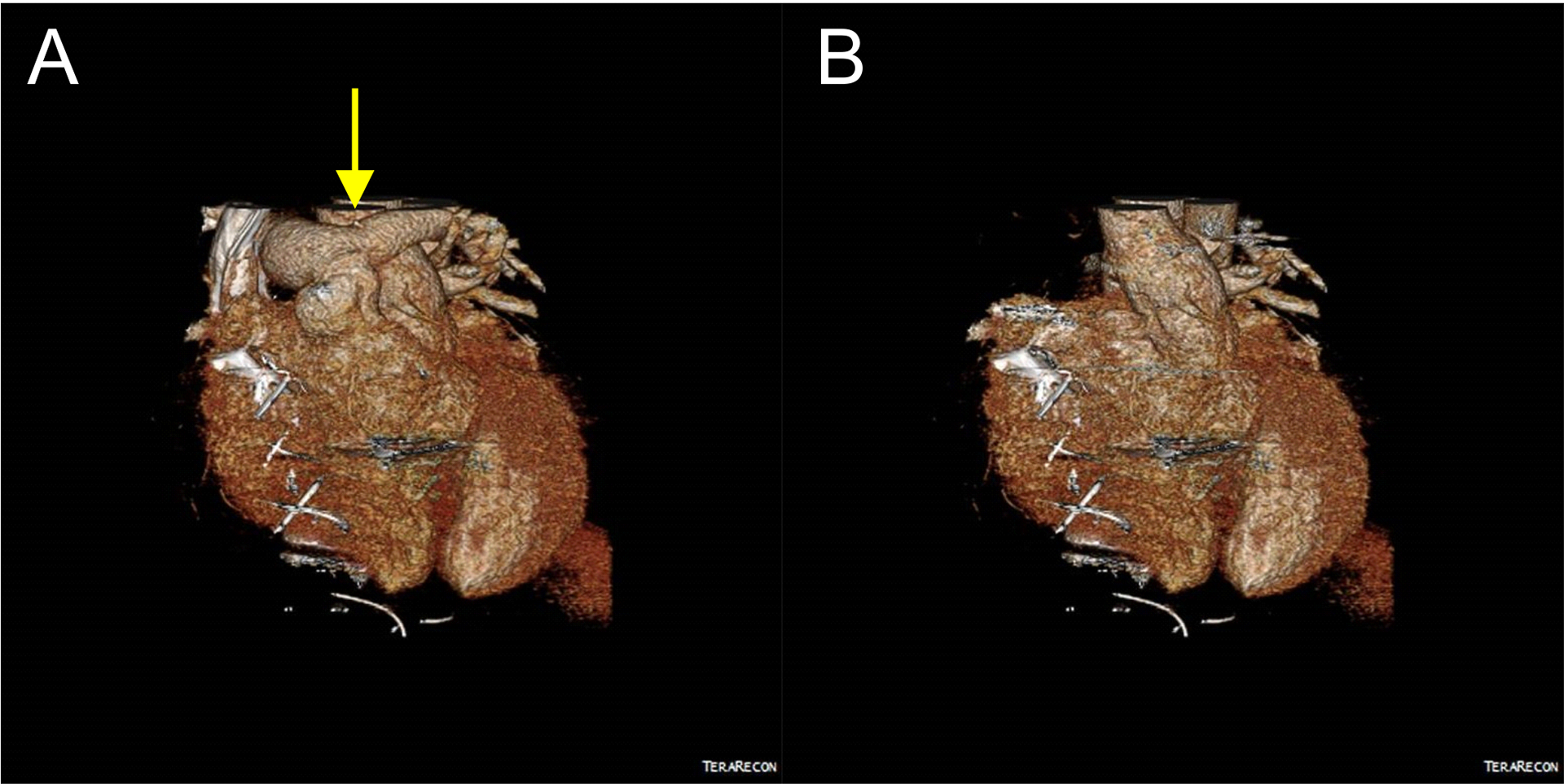
CT Angiography of the chest was performed post-surgery. The location of the left pulmonary artery incision is shown with a yellow arrow (A). A reconstructed image of the pulmonary artery removed to view the reconstructed aortic root is shown (B).
Comment
Aortic switch operations (ASO) are critical to survival of neonates with transposition of the great arteries. However, one uncommon, late complication of these procedures is dilation of the neo-aortic root.4 Valve-sparing root replacement (David procedure) can be used for the treatment of aortic root aneurysms when the aortic valve is salvageable. Herein, we present the performance of a David procedure to correct a neo-aortic aneurysm in a 25-year-old female who had undergone a neonatal arterial switch operation. The resultant anatomy with an anterior pulmonary artery and bifurcation overlying the aorta added some complexity to the operation. It may be worth noting that spiral anastomosis (physiological spiral arrangement reconstructed) has been shown to have less abnormal cardiac physiology compared to the Lecompte technique.5 Mainly, there is better perfusion to the left lung, closer to normal anatomical position of the great arteries, and closer to normal aortic arch angle.5 Second, any future aortic operation is compounded by use of the Lecompte technique. A spiral anastomosis may allow for better cardiac physiology and allows for a more accessible aortic approach if necessary in the future. Dissection of the coronary arteries is somewhat more challenging due to the previous reimplantation and location behind the right ventricular outflow tract. Meanwhile, the reconstruction of the coronary arteries to the new Valsalva graft is easier than a tradition David procedure since both the RCA and LMCA lie anteriorly and can be visualized easily. Valve sparing aortic root operations are safe and feasible following neonatal arterial switch operations.
Funding:
AKN (NIH F30CA236370), GA (NIH R01HL126668)
Footnotes
Conflicts of Interest: None
Data Availability: All data is available within submission.
References
- 1.Castaneda AR, Norwood WI, Jonas RA, Colon SD, Sanders SP, Lang P. Transposition of the great arteries and intact ventricular septum: anatomical repair in the neonate. Ann Thorac Surg. 1984;38(5):438–443. [DOI] [PubMed] [Google Scholar]
- 2.Khairy P, Clair M, Fernandes SM, et al. Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation. 2013;127(3):331–339. [DOI] [PubMed] [Google Scholar]
- 3.David TE, Ivanov J, Armstrong S, Feindel CM, Webb GD. Aortic valve-sparing operations in patients with aneurysms of the aortic root or ascending aorta. Ann Thorac Surg. 2002;74(5):S1758–1761; discussion S1792–1759. [DOI] [PubMed] [Google Scholar]
- 4.Hutter PA, Thomeer BJ, Jansen P, et al. Fate of the aortic root after arterial switch operation. Eur J Cardiothorac Surg. 2001;20(1):82–88. [DOI] [PubMed] [Google Scholar]
- 5.Rickers C, Kheradvar A, Sievers HH, et al. Is the Lecompte technique the last word on transposition of the great arteries repair for all patients? A magnetic resonance imaging study including a spiral technique two decades postoperatively. Interact Cardiovasc Thorac Surg. 2016;22(6):817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]


