Abstract
Introduction
The phase III KEYNOTE-048 trial showed that the programmed death receptor 1 (PD-1) inhibitor pembrolizumab, in the combined positive score (CPS) ≥ 1 population and combined with platinum + 5-fluorouracil in the total population, improves survival over cetuximab + platinum + 5-fluorouracil in recurrent or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). We evaluated the cost-effectiveness of pembrolizumab as monotherapy in the CPS ≥ 1 population or combined with platinum + 5-fluorouracil in the total population versus cetuximab + platinum + 5-fluorouracil from the social security perspective in Argentina.
Methods
A partitioned survival model projected costs and outcomes over 20 years with 3% annual discounting. Health state occupancy was modeled using KEYNOTE-048 Kaplan–Meier curves until the final analysis data cutoff, followed by parametric extrapolations guided by statistical criteria. Costs for initial and subsequent treatments, disease and adverse events management, and terminal care were included (AR $74.00 = 1 USD). Time-on-treatment and EuroQol five-dimension scores were taken from KEYNOTE-048. Utilities were derived using an Argentina-specific algorithm.
Results
With pembrolizumab monotherapy, patients accrued 1.1040 additional life-years and 0.8768 additional quality-adjusted life-years (QALYs), for incremental cost-effectiveness ratios (ICERs) of AR $135,801/life-year and AR $170,985/QALY gained over cetuximab + platinum + 5-fluorouracil. Additional life-years and QALYs gained with pembrolizumab combination therapy versus cetuximab + platinum + 5-fluorouracil were 1.3296 and 1.0536, respectively (ICERs of AR $680,143/life-year and AR $858,306/QALY). Considering a threshold of AR $1,676,122/QALY gained, pembrolizumab monotherapy and combination therapy had an 88.0% and a 77.1% probability of being cost-effective, respectively.
Conclusion
Pembrolizumab either as monotherapy or in combination with chemotherapy offers substantial survival gains for patients with R/M HNSCC at small additional costs, making it a cost-effective treatment versus cetuximab + platinum + 5-FU in Argentina.
Keywords: Argentina, Cetuximab, Cost-effectiveness, Head and neck squamous cell carcinoma, Latin America, Pembrolizumab
Key Summary Points
| Why carry out this study? |
| KEYNOTE-048 showed that the programmed death receptor 1 inhibitor pembrolizumab improves survival in recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC). |
| We assessed whether pembrolizumab is cost-effective over cetuximab + platinum + 5-fluorouracil in Argentina, as monotherapy in patients with combined positive score (CPS) ≥1, and in combination with platinum + 5-fluorouracil in the total population. |
| What was learned from the study? |
| Pembrolizumab monotherapy and combination therapy cost AR $170,985 (2311 USD) and AR $858,306 (11599 USD) per QALY gained, respectively, making pembrolizumab a cost-effective option in Argentina. |
| The results support access to pembrolizumab for patients with R/M HNSCC in Argentina. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.13705768.
Introduction
Head and neck squamous cell carcinoma (HNSCC) refers to tumors arising from the mucosa of the upper aerodigestive tract and accounts for roughly 90% of tumors occurring in the region of the head and neck [1, 2]. HNSCCs are relatively common and more often found in men in whom the incidence is two to four times higher than among women [3, 4]. In 2018, 3357 Argentinians were diagnosed with HNSCC, 9.88 cases per 100,000 men and 2.43 cases per 100,000 women [4].
A minority of patients with HNSCC (< 20%) are initially diagnosed with metastatic HNSCC disease [5]. Many patients are diagnosed with locally advanced disease but recurrence occurs within 1 year for 30–45% of patients [6–12]. Patients with localized HNSCC (stage I–II) are more likely to be cured, but the disease nonetheless recurs in 5–10% of patients [3, 13].
The long-term prognosis of patients with recurrent or metastatic (R/M) HNSCC is poor. Historically, first-line treatment of R/M HNSCC consisted of platinum-based chemotherapy (cisplatin or carboplatin) in combination with 5-fluorouracil (5-FU) or a taxane (docetaxel or paclitaxel). These platinum-based combination regimens resulted in median overall survival (OS) ranging from 5.0 to 8.7 months across trials [14, 15]. Most recent evidence from prospective clinical trial data reported an improvement in the median OS to 10.1 months for the targeted systemic therapy cetuximab combined with platinum and 5-FU chemotherapy and thereby defined a new standard of care in first-line treatment of R/M HNSCC according to clinical guidelines [16–18].
The immune system plays a major role in regulating the growth of cancer [19]. Evidence suggests that some types of inflammatory signaling induced in a tumor may create an immunosuppressive environment that allows tumors to evade the immune response [19]. The programmed cell death-ligand 1 (PD-1) pathway is an immune control checkpoint that may be engaged by tumor cells to inhibit active T cell immune surveillance [20–22]. Pembrolizumab is a selective humanized monoclonal antibody designed to block the interaction between PD-1 and its ligands, PD-L1 and PD-L2. By inhibiting the PD-1 receptor from binding to its ligands, pembrolizumab reactivates tumor-specific cytotoxic T lymphocytes in the tumor microenvironment [22].
PD-1 inhibitors have demonstrated effective antitumor activity with manageable safety in patients with R/M HNSCC [23–27]. In the phase 3 KEYNOTE-048 study, the efficacy and safety of pembrolizumab as monotherapy and in combination with chemotherapy (5-FU plus carboplatin or cisplatin) compared with cetuximab in combination with chemotherapy were evaluated in participants with previously untreated R/M HNSCC with PD-L1 combined positive score (CPS; defined as the number of PD-L1-positive tumor and immune cells divided by the total number of tumor cells × 100) ≥ 20 and CPS ≥ 1 and in the total population [28]. The primary endpoints were OS and progression-free survival (PFS). At the second interim analysis, pembrolizumab monotherapy improved OS compared with cetuximab plus chemotherapy in participants with CPS ≥ 20 [hazard ratio (HR) 0.61; 95% confidence interval (CI) 0.45, 0.83] and CPS ≥ 1 (HR 0.78; 95% CI 0.64, 0.96). Pembrolizumab plus chemotherapy improved OS compared with cetuximab plus chemotherapy in the total population (HR 0.77; 95% CI 0.63, 0.93) at the second interim analysis and in the CPS ≥ 20 (HR 0.60; 95% CI 0.45, 0.82) and CPS ≥ 1 (HR 0.65; 95% CI 0.53, 0.80) populations at the final analysis. Pembrolizumab demonstrated favorable safety compared with cetuximab plus chemotherapy, and pembrolizumab plus chemotherapy showed comparable safety with cetuximab plus chemotherapy; the incidence of grade ≥ 3 adverse events was lower with pembrolizumab and similar with pembrolizumab plus chemotherapy as compared to cetuximab plus chemotherapy.
These results of the KEYNOTE-048 trial were the basis for evaluating the cost-effectiveness of pembrolizumab for the first-line treatment of R/M HNSCC in Argentina, either as monotherapy for patients with CPS ≥ 1 or in combination with chemotherapy in the total population. This evaluation can help Argentinian health authorities and payers make informed decisions about funding and reimbursement of pembrolizumab for these patients.
Methods
Population
The target population for the economic evaluation is aligned with the patient population targeted by the KEYNOTE-048 trial: adult patients with histologically or cytologically confirmed RM/HNSCC and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 who have not received prior systemic therapy administered in the recurrent or metastatic setting. Patients enrolled in the trial had a median age of 61 years, 80% were male, 85% had CPS ≥ 1, and 43% had CPS ≥ 20 [28, 29].
Model Structure and Analysis
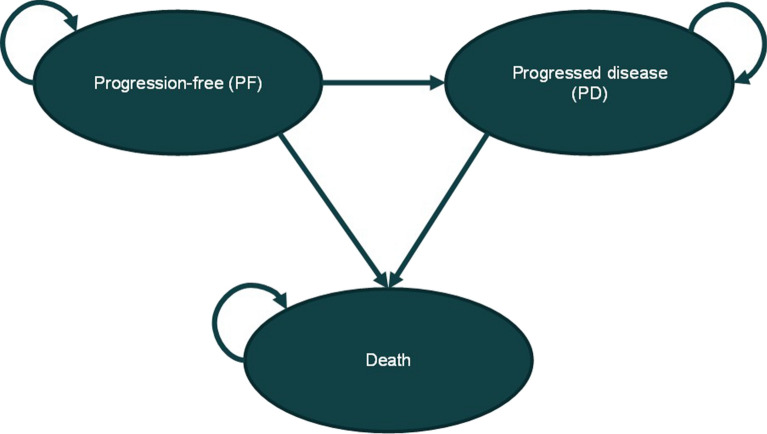
In accordance with the modelling paradigm in oncology, the effects of treatments are assessed in terms of delaying time to disease progression (PFS) and improving life-expectancy (OS). As such, a partitioned survival model with progression-free, progressive disease, and death as mutually exclusive health states was developed (Fig. 1). Health states are associated with variations in direct medical resource utilization, reflecting the different requirements of patients with and without disease progression, and health utility, assuming disease progression results in deterioration in health-related quality of life.
Fig. 1.
Model schematic
In line with the World Health Organization (WHO) [30] recommendations on economic evaluation of health technologies, both costs and outcomes are discounted at 3% annually over the analytical horizon of 20 years which approximates a lifetime perspective for the vast majority of patients. Two pembrolizumab regimens are compared to cetuximab in combination with platinum-based therapies: pembrolizumab as a monotherapy among patients with CPS ≥ 1 and pembrolizumab in combination with platinum-based therapies and 5-FU in the total population, regardless of CPS. The simulated cohorts enter the model in the progression-free health state and initiate treatment with the assigned first-line treatment option. At the end of each weekly cycle, patients may remain within the same health state, transition to the progressive disease state, or die. The model assumes disease progression is irreversible, meaning that once disease progression is noticed, a patient cannot return to the progression-free state.
The long-term costs and health outcomes of patients with R/M HNSCC are projected according to first-line treatment option received, and incremental cost-effectiveness ratios (ICERs) are assessed in terms of cost per quality-adjusted life-year (QALY) or life-year gained. A series of scenarios varying model parameters and settings across a range of plausible values were assessed in one-way sensitivity analyses. These assessed the impact on incremental costs per QALY gained when varying time horizon, discount rates for costs or for outcomes, patient age, weight and body surface area (BSA), assumptions with respect to vial sharing, medical care costs, rates of treatment-related adverse events, health state utility values and treatment-specific OS, PFS, and time-on-treatment. The robustness of the cost-effectiveness results was further assessed through a 1000-iteration probabilistic sensitivity analysis (PSA). Standard errors or variance–covariance matrices of the selected distributions were based on original data sources, where available, and otherwise were set at 20% of mean values (Table 1).
Table 2.
Drug costs
| Drug | Vials | Dosing | ||
|---|---|---|---|---|
| Size, mg | Unit costa, AR$ | Schedule | Cost per doseb, AR$ | |
| Pembrolizumab | 100 | 202,655.24 | 200 mg, Q3W (up to 35 cycles) | 405,310.48 |
| Cetuximab | 100 | 27,447.35 | Loading dose: 400 mg/m2 | 219,573.50 |
| 500 | 137,231.46 | Subsequent: 250 mg/m2 weekly | 137,231.46 | |
| Cisplatin | 10 | 453.14 | 100 mg/m2, Q3W (up to 6 cycles) | 7,635.09 |
| 50 | 1,940.84 | |||
| Carboplatin | 600 | 8,894.07 | AUC 5 mg/m2, Q3W (up to 6 cycles) | 8,894.07 |
| 5-FU | 500 | 368.74 | 1000 mg/m2 for four days, Q3W | 5,531.14 |
Q3W once every 3 weeks, 5-FU 5-fluorouracil, AUC area under curve
AR $74 = 1 USD = 0.84 euro. Ex-factory public prices from AlfaBeta, August 28, 2020
aEx-factory price (57% of public prices to exclude taxes and logistic costs not applicable to oncology medications)
bBased on an average patient with a body surface area of 1.81 m2
Table 1.
Model inputs
| Parameters | Patients with CPS ≥ 1 Pembrolizumab monotherapy versus cetuximab + platinum + 5-FU |
Total population Pembrolizumab combination versus cetuximab + platinum + 5-FU |
Estimation approach / data sources | Sensitivity analyses (PSAa distribution) | ||
|---|---|---|---|---|---|---|
| Intervention | Comparator | Intervention | Comparator | |||
| Effectiveness | Piecewise approach using KEYNOTE-048 data: KM curve followed by parametric extrapolation |
Alternative cut-off points and/or parametric distributions Survival distributional parameters (multinormal) |
||||
| PFS | Week-52 cut-off followed by: | |||||
| e(− 4.73) | e(− 4.21) | e(− 4.66) | e(− 3.98) | |||
| OS | Week-80 cut-off followed by: | |||||
| ll(-0.10; 4.60) | ll(0.25; 3.75) | ln(4.87; 0.64) | ln(3.79; 0.27) | |||
| Direct medical costs, AR$ |
Costs sourced from different public and private Argentinian institutions The incidence of TRAE was taken from KEYNOTE-048 trial datab Drug costs are based on ex-factory public prices from AlfaBeta, schedule recommendations and KEYNOTE-048 ToT data Treatment duration caped to disease progression and pembrolizumab given for a maximum of 2 years. (Table 2) |
DSA and PSA on: TRAE incidence (gamma) Resource usage (beta) Weekly resource costs (gamma) Terminal care resources (constrained beta) Patient weight and BSA (log-normal) Subsequent treatment duration (gamma) Allow for vial sharing |
||||
| Weekly costs | ||||||
| Pre-progression, year 1 | 6,343.32 | 6,343.32 | 6,343.32 | 6,343.32 | ||
| Pre-progression, after year 1 | 5,779.19 | 5,779.19 | 5,779.19 | 5,779.19 | ||
| Post-progression | 4,327.36 | 4,327.36 | 4,327.36 | 4,327.36 | ||
| One-time cost | ||||||
| Disease progression | 37,804.12 | 37,804.12 | 37,804.12 | 37,804.12 | ||
| Terminal care | 185,909.98 | 185,909.98 | 185,909.98 | 185,909.98 | ||
| Drug administration per dose | 7,877.87 | 18,980.95 | 18,980.95 | 18,980.95 | ||
| TRAEs, weekly | 11.70 | 633.16 | 767.17 | 633.16 | ||
| 1L treatment, weekly average | ||||||
| First 3-week cyclec | 135,103.49 | 169,310.41 | 139,735.10 | 169,310.41 | ||
| Subsequent 3-week cycles | ||||||
| With platinum + 5-FU | NA | 141,863.06 | 139,735.10 | 141,863.06 | ||
| Without platinum + 5-FUd | 135,103.49 | 137,231.46 | 135,103.49 | 137,231.46 | ||
| Subsequent treatmentse | 1,076,761.67 | 1,691,770.64 | 1,316,616.83 | 1,578,559.19 | ||
| Utilities | Utilities derived through a linear mixed regression of EQ-5D-3L scores from KEYNOTE-048 and using an Argentina algorithm |
Alternative utility values DSA and PSA (normal) |
||||
| Health state utilities | ||||||
| PFS | 0.8300 | 0.8300 | 0.8300 | 0.8300 | ||
| PD | 0.7900 | 0.7900 | 0.7900 | 0.7900 | ||
| Disutilities | ||||||
| TRAE Grade 3–5 | 0.0300 | 0.0300 | 0.0300 | 0.0300 | ||
| Days prior to death | ||||||
| 90–180 | 0.0423 | 0.0423 | 0.0423 | 0.0423 | ||
| 60–90 | 0.1123 | 0.1123 | 0.1123 | 0.1123 | ||
| 30–60 | 0.1123 | 0.1123 | 0.1123 | 0.1123 | ||
| 0–30 | 0.2302 | 0.2302 | 0.2302 | 0.2302 | ||
CPS combined positive score, EQ-5D-3L EuroQol five-dimension three-level, e exponential, ll log-logistic, ln log-normal, NA not applicable, OS overall survival, PFS progression-free survival, PSA probabilistic sensitivity analysis, ToT time-on-treatment, TRAE treatment-related adverse events, 1L first line, 5-FU 5-fluorouracil
aThe variability of the selected distributions was based on standard errors or variance–covariance from original data sources. For costs, the standard errors were assumed to be equal to 20% of the base-case values. Other elements of the PSA (distribution) include patients age (gamma), gender distribution (beta), and survival distributional time-on-treatment parameters (multinormal)
bTRAEs grade 3–5 occurring in at least 5% of patients (any severity) in at least one treatment arm were included
cPatient in the comparator arm received a loading dose of cetuximab the first week. For all other drugs, the dosing regimens are equal for first and subsequent doses
dPatients in pembrolizumab combination therapy arm or in the comparator arm received platinum (42.13% cisplatin and 57.87% carboplatin) + 5-FU for up to six 3-week cycles
eSubsequent treatment costs are inclusive of the cost associated with the management of treatment-related adverse events AR $74 = 1 USD = 0.84 euro. Ex-factory public prices from AlfaBeta, August 28, 2020
Health State Occupation
Disease progression was modeled according to progressions determined by an independent review committee (IRC) during the trial. This data was preferred over investigator-assessed (INV) progressions in accordance with regulatory authorities indicating that IRC assessment minimizes bias in radiographic interpretation [31]. The trial data for the analysis was from randomization until disease progression or death, or until the date of censoring. Upon discontinuation, patients were censored at the time of visit or, in the case of missing follow-up data, at the date of the last recorded follow-up visit. Progression-free and OS projections were guided by statistical goodness-of-fit criteria (AIC and the BIC), visual inspection, and plausibility of long-term extrapolation.
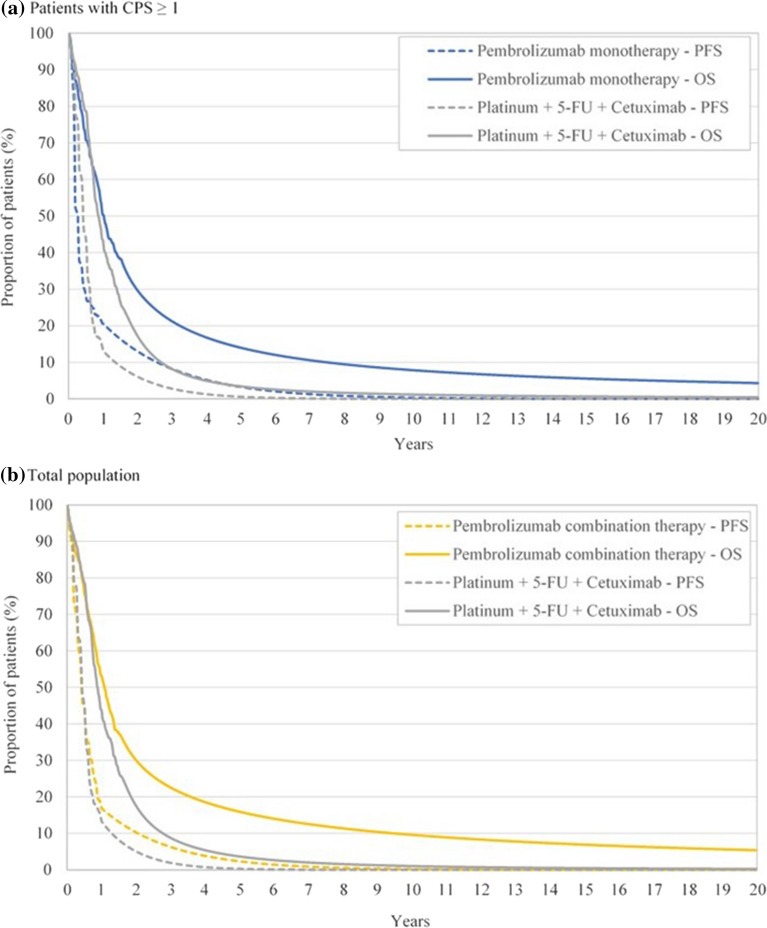
Health state occupancy was determined on the basis of KEYNOTE-048 trial data using a piecewise modeling approach comprising of the Kaplan–Meier curves used until the final analysis data cutoff followed by parametric extrapolation of individual patient-level data until the end of the analytical horizon, 20 years in the present case (Table 1). The inspection of hazards suggested week 25 or week 52 and week 45 or week 80 as potential cutoff points for modelling PFS and OS, respectively. For PFS, the 52-week cutoff point was selected to utilize the most trial data which also provides the most meaningful fit. The exponential curve was used to extrapolate beyond this cutoff point as this resulted in the best fit based on the AIC and the BIC and also as it is the standard model to use in a piecewise approach [32]. For OS, the 80-week cutoff was taken as it allowed greater utilization of the available Kaplan–Meier data and gave a much better fit allowing for a more meaningful extrapolation when using the log-logistic function for patients with CPS ≥ 1 and a log-normal for the total population. The resulting projections of PFS and OS are shown in Fig. 2.
Fig. 2.
Predicted long-term outcomes under base-case parametric distribution assumptions. CPS combined positive score, OS overall survival, PFS progression-free survival, 5-FU 5-fluorouracil. AR $74 = 1 USD = 0.84 euro
Treatment-related adverse events occurring in at least 5% of patients in at least one treatment arm were included. Medical costs and disutilities were ascribed for those having a meaningful impact on patient well-being as denoted by a Common Terminology Criteria for Adverse Events (CTCAE version 4.0) grade 3 or above [33].
Health Utility
Life-years were adjusted for quality of life using patient-level EuroQol five-dimension three-level (EQ-5D-3L) responses collected from KEYNOTE-048, with utilities obtained from an Argentina algorithm [34]. Health utility values were modelled according to health state, presence of treatment-related adverse events of grade ≥ 3, and proximity to death using a fixed effect linear mixed regression model accounting for ECOG score, with the same weights applied to each treatment arm (Table 1).
Costs
Direct medical costs for drug acquisition and administration of first-line and subsequent treatments, disease management, terminal care, and management of adverse events were included from the perspective of the Argentinian social security system (Table 1). All medical resource mean unit costs were obtained from different private and public Argentinian institutions [35, 36]. All costs were expressed in 2020 Argentinian pesos (August 28, 2020, AR $74.00 = 1 USD = 0.84 euro [37]). Costs for drug acquisition and their administration were ascribed at the beginning of each cycle of drug administration. Conversely, for weekly resources such as routine disease management, the Simpson’s within-cycle correction 1/3 rule was applied to reflect the timing of cost occurrence more accurately. The costs of each first-line treatment regimen were assessed on the basis of the number of patients on treatment estimated from the KEYNOTE-048 data. Treatment dosages were in accordance with schedule recommendations and KEYNOTE-048 trial protocol. Drugs were subject to their respective capped durations, and therapies were discontinued upon disease progression. Pembrolizumab was given at 200 mg fixed dose intravenously every 3 weeks (Q3W) for a maximum of 2 years. As a combination therapy, pembrolizumab-treated patients also received either cisplatin (100 mg/m2) or carboplatin [area under the curve (AUC) 5 mg/m2] and 5-FU (1000 mg/m2 per day for four consecutive days) Q3W for a maximum of six cycles. Patients in the comparator arm received cetuximab (400 mg/m2 as initial loading dose and 250 mg/m2 thereafter) intravenously administered weekly plus either cisplatin (100 mg/m2) or carboplatin (AUC 5 mg/m2) and 5-FU (1000 mg/m2 per day for four consecutive days) Q3W for a maximum of six cycles. On the basis of the KEYNOTE-048 data, regardless of treatment regimen, 42% of platinum-based chemotherapies included cisplatin and the remaining included carboplatin. Calculations assumed no vial sharing and were based on the mix of vials that minimized acquisition costs, considering a patient with an average BSA of 1.81 m2 as assessed by an expert input panel in Argentina. The costs of therapy infusions required at each treatment administration were included. The average costs from different public and private Argentinian institutions were used [35, 38–40]. A cost of AR $7877.87 (106.46 USD; 89.42 euro) was applied for the infusion of pembrolizumab monotherapy or for the infusion of cetuximab for days the chemotherapy regimens were not also administered. For infusions of pembrolizumab or cetuximab with platinum + 5-FU, a longer infusion cost of AR $18,980.95 (256.50 USD; 215.46 euro) was applied (Table 1).
Subsequent treatments included any systemic therapy administered after first-line treatment with pembrolizumab or the comparators. A one-off cost was applied at disease progression (the front-loading approach reasonably approximates the gradual accrual of costs over time as patients progress through the clinical pathway). The cost of subsequent treatments was determined according to the expected treatment mix in Argentina, as assessed by local expert input panel, the mean time on treatments, derived from KEYNOTE-048, and the drug prices obtained from AlfaBeta [41] (Table 1).
Medical management costs were linked to health state membership and transitions. The costs were mainly sourced from different public and private Argentinian institutions [35, 38–40]. Routine disease management consisted of procedures for follow-up care and monitoring of patients with HNSCC. This includes scans, blood tests, and consultations with healthcare practitioners to evaluate disease activity which was sourced from the American Cancer Society Head and Neck Cancer Survivorship Care Guideline [42]. Nutritional support was factored in during the first year free of disease progression whereas the other resource types were accrued each year. Resource unit cost and expected frequency use led to weekly cost in the first and subsequent years of AR $6343.32 (85.72 USD; 72.01 euro) and AR $5779.19 (78.10 USD; 65.60 euro) in the progression-free state, respectively, and of AR $4327.36 (58.48 USD; 49.12 euro) in the progressive disease state. The cost for oncologist visits and CT scan monitoring at disease progression (AR $37,804.12; 510.87 USD; 429.13 euro) and for terminal care (AR $185,909.98; 2512.30 USD; 2110.33 euro) in the weeks or months prior to death were included. The one-off costs were applied across the model time horizon based on the health state occupancy dynamic between model cycles to reflect the timing of transitions. Finally, the model included the weekly cost of managing treatment-related AE based on the incidence observed in each KEYNOTE-048 trial arm (Table 1). Probabilities for pembrolizumab interventions to be cost-effective were calculated on the basis of a willingness-to-pay threshold of three times the gross domestic product (GDP) per capita for each QALY gained (AR$1,676,122; 27,290 USD; 24,726 euro), in accordance with the World Health Organization’s Choosing Interventions that are Cost-Effective (WHO-CHOICE) project [43, 44].
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors; this study consequently did not require ethical approval. The authors had permission to access the databases needed to perform this study.
Results
Base-Case Analyses
Treatment with pembrolizumab, as a monotherapy among patients with CPS ≥ 1, or in combination with chemotherapy in the total population regardless of PD-L1 expression, resulted in life-year and QALY gains at small additional cost in comparison with cetuximab plus chemotherapy. In both populations, patients treated with pembrolizumab were projected to live longer as compared to cetuximab plus chemotherapy (Fig. 2). For example, 7.83% of patients with CPS ≥ 1 who received first-line pembrolizumab monotherapy survived at least 10 year versus 1.20% among those who received cetuximab plus chemotherapy. Among all patients, 9.58% of those treated with pembrolizumab combination therapy were alive after 10 years as compared to 1.03% of those who received cetuximab plus chemotherapy. Treating patients with CPS ≥ 1 with pembrolizumab monotherapy resulted in 0.8768 QALY gained and in incremental costs of AR $149,921 (2026 USD; 1702 euro) over cetuximab plus chemotherapy (QALY 1.9504 vs. 1.0736; total costs AR $5,867,976 vs. AR $5,718,055; Table 3). In the total population, pembrolizumab combination therapy resulted in 1.0536 QALY gained and in incremental costs of AR $904,298 (12,220 USD; 10,265 euro) over cetuximab plus chemotherapy (QALY 2.1339 vs. 1.0803; total costs AR $6,664,560 vs. AR $5,760,262; Table 3). The resulting ICERs for pembrolizumab monotherapy and pembrolizumab combination therapy versus cetuximab plus chemotherapy were AR $170,985 (2311 USD; 1,941 euro) and AR $858,306 (11,599 USD; 9743 euro) per QALY gained, and AR $135,801 (1835 USD; 1542 euro) and AR $680,143 (9191 USD; 7721 euro) per life-year gained, respectively (Table 3).
Table 3.
Base-case cost-effectiveness results: pembrolizumab interventions versus KEYNOTE-048 trial comparator cetuximab + platinum + 5-FU
| Costs and outcomes | Patient with CPS ≥ 1 Pembrolizumab monotherapy versus cetuximab + platinum + 5-FU |
Total population Pembrolizumab combination versus cetuximab + platinum + 5-FU |
||||
|---|---|---|---|---|---|---|
| Comparator | Intervention | Incremental | Comparator | Intervention | Incremental | |
| Costs, AR$ | ||||||
| Treatments | 5,149,867 | 5,045,882 | − 103,985 | 5,189,755 | 5,791,572 | 601,817 |
| Drug acquisition | 3,808,172 | 4,224,319 | 416,147 | 3,933,060 | 4,669,354 | 736,295 |
| Drug administration | 94,052 | 82,107 | − 11,945 | 94,836 | 215,432 | 120,595 |
| Subsequent treatment | 1,231,081 | 739,112 | − 491,969 | 1,144,727 | 882,017 | − 262,709 |
| Adverse event | 16,562 | 344 | − 16,218 | 17,132 | 24,768 | 7636 |
| Disease management | 568,188 | 822,095 | 253,907 | 570,508 | 872,988 | 302,481 |
| Pre-progression | 206,851 | 259,415 | 52,564 | 200,068 | 258,383 | 58,316 |
| Disease progression | 24,268 | 24,149 | − 119 | 24,207 | 24,183 | − 25 |
| Post-progression | 158,109 | 365,631 | 207,522 | 165,580 | 421,278 | 255,697 |
| Terminal care | 178,960 | 172,901 | − 6059 | 180,652 | 169,145 | − 11,508 |
| Total | 5,718,055 | 5,867,977 | 149,922 | 5,760,262 | 6,664,560 | 904,298 |
| Outcomes | ||||||
| Life-years | 1.3439 | 2.4479 | 1.1040 | 1.3538 | 2.6834 | 1.3296 |
| Quality-adjusted life years | 1.0736 | 1.9504 | 0.8768 | 1.0803 | 2.1339 | 1.0536 |
| Progression-free | 0.5317 | 0.6823 | 0.1507 | 0.5123 | 0.6725 | 0.1602 |
| Progressive disease | 0.5519 | 1.2762 | 0.7243 | 0.5780 | 1.4705 | 0.8925 |
| Adverse events | − 0.0010 | 0.0000 | 0.0010 | − 0.0010 | − 0.0010 | 0.0000 |
| Time-to-death disutilities | − 0.0090 | -0.0081 | 0.0009 | − 0.0089 | − 0.0081 | 0.0008 |
| Cost-effectiveness ratio, AR$ | ||||||
| Per life year | 135,801 | 680,143 | ||||
| Per QALY | 170,985 | 858,306 | ||||
Ex-factory public prices from AlfaBeta, August 28, 2020
CPS combined positive score, 5-FU 5-fluorouracil, QALY quality-adjusted life-year AR $74 = 1 USD = 0.84 euro
Sensitivity Analyses
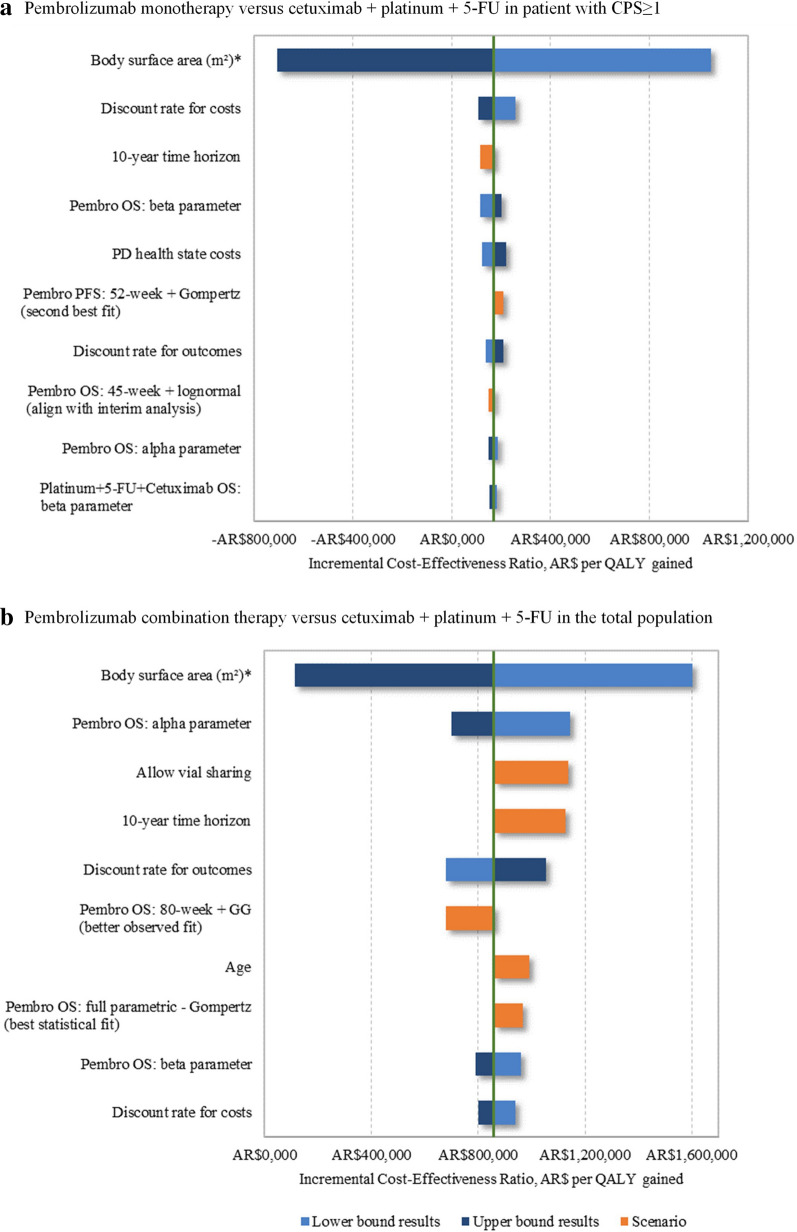
The tornado diagrams in Fig. 3 show the incremental costs per QALY gained for pembrolizumab monotherapy in patients with CPS ≥ 1 (Fig. 3a) and in the total population (Fig. 3b) obtained over comparator cetuximab plus chemotherapy when varying key model parameters and setting in one-way deterministic sensitivity analyses and scenarios. Across the one-way deterministic sensitivity analysis and scenarios, pembrolizumab monotherapy compared to cetuximab plus chemotherapy for patients with CPS ≥ 1 ranged from being dominant (more effective, less costly) to having a ICER of AR $1,049,280 (14,179 USD; 11,911 euro) per QALY gained when variation of the BSA was implemented. In all patients, the incremental cost per QALY for pembrolizumab combination therapy compared to cetuximab plus chemotherapy ranged from AR $114,393 (1546 USD; 1299 euro) to AR $1,602,095 (21,650 USD; 18,186 euro) also when modifying the BSA. The ICERs were robust to variations in other parameters and settings such as vial sharing, treatment durations, time horizon, and extrapolation of PFS and OS.
Fig. 3.
Tornado diagram showing the variation in ICER value based upon one-way deterministic parameters changes and scenarios. a Pembrolizumab monotherapy versus Cetuximab + Platinum + 5-FU in patient with CPS ≥ 1 and b Pembrolizumab combination therapy versus Cetuximab + Platinum + 5-FU in the total population. CPS combined positive score, GG generalized gamma, mg milligram, m meter, OS overall survival, Pembro pembrolizumab, PFS progression-free survival, QALY quality-adjusted life-year, 1L first line, 5-FU 5-fluorouracil. AR $74 = 1 USD = 0.84 euro
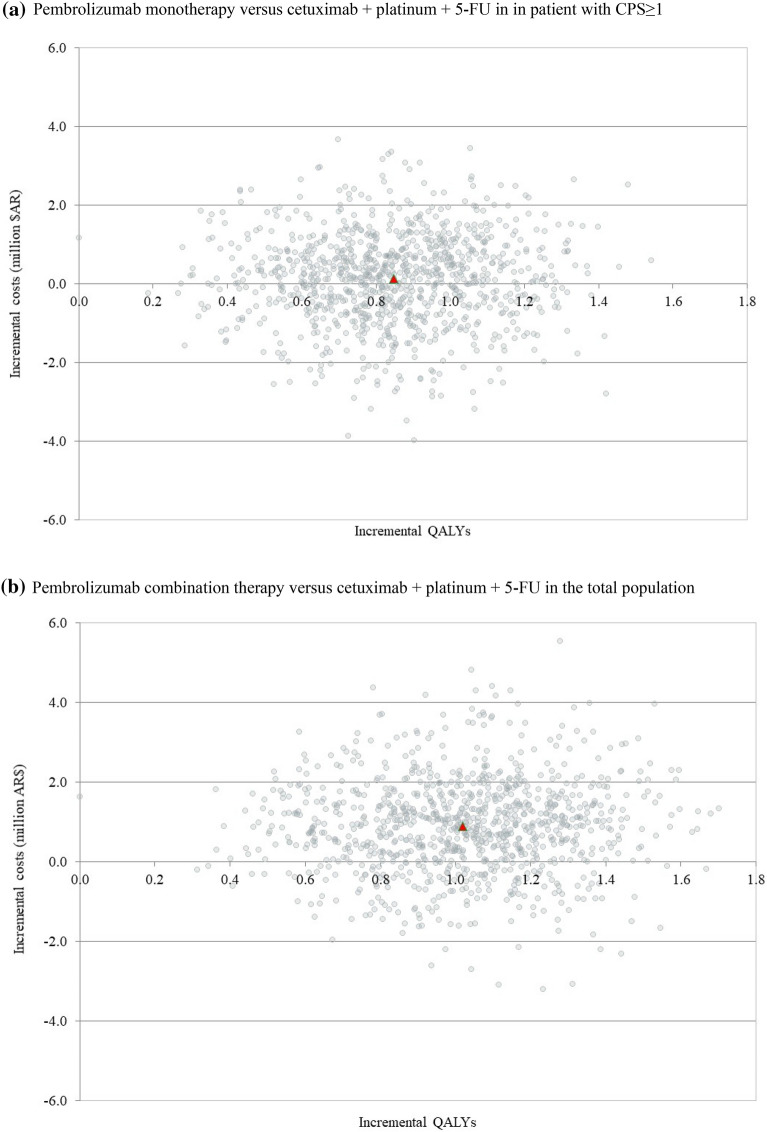
The results of the probabilistic analysis are presented graphically on the cost-effectiveness planes in Fig. 4. Across the 1000 iterations, both pembrolizumab monotherapy in patients with CPS ≥ 1 and pembrolizumab combination therapy in the total population were consistently more effective than the cetuximab plus chemotherapy, and also often resulted in lower costs (Fig. 4a and Fig. 4b, respectively). Considering a willingness-to-pay threshold of AR $1,676,122 (27,290 USD; 24,726 euro) [43] per QALY in Argentina, pembrolizumab monotherapy and pembrolizumab combination therapy were cost-effective vs. comparator in 88.0% and 77.1% of scenarios, respectively.
Fig. 4.
Cost-effectiveness plane for pembrolizumab regimens compared to cetuximab + platinum + 5-fluorouacil. CPS combined positive score, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year. AR $74 = 1 USD = 0.84 euro
Discussion
Historically, the long-term prognosis of patients with R/M HNSCC has been poor, highlighting the high unmet need to improve survival while maintaining health-related quality-of-life. Platinum-based chemotherapies have been the standard treatments [14, 15] for these patients until their combination with cetuximab was shown beneficial [18], making cetuximab plus chemotherapy the new standard first-line systemic treatment in R/M HNSCC [16]. This treatment combination offers a median OS of approximately 10 months but is associated with substantial toxicity [18]. In KEYNOTE-048, pembrolizumab monotherapy demonstrated a statistically significant and clinically meaningful improvement in OS in the CPS ≥ 1 and CPS ≥ 20 populations, and pembrolizumab plus chemotherapy demonstrated a statistically significant and clinically meaningful improvement in the overall, CPS ≥ 1, and CPS ≥ 20 populations in comparison with cetuximab plus chemotherapy [28]. The safety profile was favorable with pembrolizumab monotherapy compared with cetuximab plus chemotherapy and similar in the pembrolizumab plus chemotherapy and cetuximab plus chemotherapy groups. To support Argentinian health authorities and payers making informed decisions about funding and reimbursement of pembrolizumab for these patients, we assessed the cost-effectiveness of pembrolizumab versus cetuximab plus chemotherapy, as a monotherapy among PD-L1 expressers (CPS ≥ 1) and in combination with platinum + 5-FU in all patients, irrespective of CPS, from the perspective of the social security system in Argentina. In accordance with findings in other settings such as the People’s Republic of China or the USA [45, 46], we found that pembrolizumab regimens were cost-effective interventions in Argentina over the trial comparator. Modest incremental expenditures from social security would enable, indeed, considerable survival gains compared to the current standard of care in Argentina. Importantly, the increased time on treatment observed in the intervention arms of the KEYNOTE-048 trial supports pembrolizumab as a more tolerated and effective intervention as opposed to current therapies. The small additional costs for pembrolizumab regimens over the standard of care trial comparator are economically justified by the health benefits they confer.
According to the WHO-CHOICE project [44], interventions with an ICER below three times the GDP per capita for each QALY gained (AR $1,676,122; 27,290 USD; 24,726 euro) [43] should be considered cost-effective and those costing less than the GDP per capita (AR $558,707 (9097 USD; 8242 euro) [43] should be viewed as highly cost-effective. Accordingly, under base-case assumptions, pembrolizumab monotherapy was found to be highly cost-effective versus cetuximab plus chemotherapy in patients expressing PD-L1 CPS ≥ 1 with an ICER of AR $170,985 (2311 USD; 1941 euro). In the total population, pembrolizumab combination therapy was also cost-effective with a base-case ICER under twice the GDP per capita at AR $858,306 (11,599 USD; 9743 euro). These conclusions were highly robust to deterministic changes in key model parameters as well as in scenarios changing the vial sharing assumption, the time horizon, or the modelling approach for projecting long-term PFS and OS. While patients’ BSA (affecting drug acquisition costs) impacted the ICERs, more importantly, pembrolizumab regimens remained cost-effective with an incremental cost per QALY gained of AR $1,049,280 (14,179 USD; $11,911 euro), below twice the GDP per capita. In the PSAs, considering this willingness-to-pay threshold, pembrolizumab monotherapy and pembrolizumab combination therapy were cost-effective versus comparator in the majority (88.0% and 77.1%, respectively) of probabilistic variations of key model parameters, highlighting the economic attractiveness of the treatment options. Moreover, in both comparisons, probabilistic variations of key model parameters found that pembrolizumab regimens are likely to be economically dominant (more effective, and cost-saving) versus cetuximab plus chemotherapy, representing 42.3% and 20.0% of iterations for pembrolizumab monotherapy and pembrolizumab combination therapy, respectively.
As with any cost-effectiveness assessment, our analyses might have certain limitations. First, we conservatively modelled time-on-treatment on the basis of the clinical trial results. In the pembrolizumab intervention arms as well as in the comparator arms of the KEYNOTE-048 study, some patients received treatment beyond disease progression; however, in clinical practice, therapies are commonly administered on a treat-to-progression basis. Second, only treatment-related AEs with higher than 5% incidence at grade 3 or above were included as events that are most common and associated with hospitalizations. Nonetheless, limiting the AEs in the model could underestimate the impact of AEs. While pembrolizumab has a more favorable tolerability profile than the cetuximab plus chemotherapy, this has no impact for the pembrolizumab combination regimen which had a similar safety profile to cetuximab plus chemotherapy. It should be noted, however, that the impact of AEs on the results is marginal, as treatment-related AEs contributed to less than 1% of the total costs for both pembrolizumab treatment regimens and comparator, and that related disutilities were small. Third, extrapolation of OS curves from short-term clinical trials is always subject to uncertainty and therefore should be validated against long-term data as they become available. Modifying the assumptions about OS extrapolation, however, did not alter the conclusion about cost-effectiveness of pembrolizumab.
Our study provides a valid and informative economic assessment of pembrolizumab treatment regimens that can support healthcare payers in making decisions with respect to funding and reimbursing of the therapy for patients with R/M HNSCC in need of effective treatments. We used a three-health-state partitioned survival model structure that has been validated and extensively applied in technology appraisals in oncology, including HNSCC [47, 48]. Progression-free and progressive disease state occupancy was modelled using patient-level survival data from the KEYNOTE-048 clinical trial, therefore ensuring a high degree of internal validity and proper representation of the clinical trial results. Quality-of-life decrements associated with disease progression, treatment-related adverse events, and proximity to death were derived from data collected in the KEYNOTE-048 trial and mapped to the Argentinian settings through local health utility values. Therefore, the utility and disutility values used in the model are considered representative of the target population for pembrolizumab. The time on treatment was also fully informed by the trial as the maturity of the Kaplan–Meier data covered the total duration of treatment. Finally, the wide range of scenario analyses explored in the parametric and structural assumptions of the economic analysis provided a clear and comprehensive assessment of assumptions potentially influencing the model results, allowing an accurate depiction of the robustness of the study conclusions.
Conclusions
Pembrolizumab for the first-line treatment of R/M HNSCC, both as a monotherapy for patients with CPS ≥ 1 or in combination with chemotherapy in all patients, demonstrates substantial clinical benefits in terms of OS and QALYs gained compared with cetuximab plus chemotherapy, at a small additional cost. Across a range of sensitivity analyses and scenarios, pembrolizumab regimen remained cost-effective with ICERs well below the commonly accepted willingness-to-pay threshold in Argentina.
Acknowledgments
Funding
Sponsorship for this study and the journal’s Rapid Service and Open Access Fees were funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing, Editorial, and Other Assistance
The authors would like to thank Martin Sénécal (Complete Health Economics Outcomes Research Solutions) for providing editorial and overall assistance for the write-up and development of this article, as well as Pablo Andre (MSD Argentina), Hernán Barugel (MSD Argentina), Roberto Gomez (MSD Argentina), Karina Mendez (MSD Argentina), Raúl Soler (MSD Argentina), Katia Wendt (MSD Panama), Cristina Okuyama (Merck & Co., Inc., Kenilworth, NJ USA), Alfredo Caparros (MSD Argentina), Miguel Korte (Merck & Co., Inc., Kenilworth, NJ USA), Jaime Enrique Ruiz (MSD Colombia), Christopher Black (Merck & Co., Inc., Kenilworth, NJ USA), Homero Monsanto (Merck & Co., Inc., Kenilworth, NJ USA), José Salvador Velazco (MSD Mexico), Fernando Zuelgaray (MSD Argentina) for their editorial and scientific peer review, Maggie Hodgson (Merck & Co., Inc., Kenilworth, NJ USA) for providing scientific publication advice, and Ana Canchola Mendez (Merck & Co., Inc., Kenilworth, NJ USA), Camille Simpson (Merck & Co., Inc., Kenilworth, NJ USA), Tyrone Brewer (Merck & Co., Inc., Kenilworth, NJ USA) for their project implementation and funding assistance. MSD provided funding to support the write up and the development of this article.
Disclosures
Victoria Wurcel, Juan Ignacio Altuna, and Fernando Carabajal are employees of MSD Argentina, Buenos Aires, Argentina. Diana Chirovsky and Rebekah Borse are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth NJ, USA. Jyotika Gandhi is an employee of CHEORS, North Wales, PA, USA.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors; this study consequently did not require ethical approval. The authors had permission to access the databases needed to perform this study.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to its proprietary nature. Further information about the data and conditions for access can be obtained from the corresponding author.
References
- 1.Head and Neck Cancers-National Cancer Institute. 2020. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet Accessed 30 Oct 2020.
- 2.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans—Painting, Firefighting, and Shiftwork [Internet]. Vol. 98. Lyon, France; 2010. Available from: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono98.pdf. Accessed 29 Oct 2020. [PMC free article] [PubMed]
- 3.Union for International Cancer Control Public Health. Locally advanced squamous carcinoma of the head and neck-Executive Summary. https://www.who.int/selection_medicines/committees/expert/20/applications/HeadNeck.pdf. 2014. https://www.who.int/selection_medicines/committees/expert/20/applications/HeadNeck.pdf. Accessed 24 Nov 2020.
- 4.International Agency for Research on Cancer—Globocan 2018. Global cancer statistics [Internet]. 2018 [cited 2020 Nov 24]. Available from: https://gco.iarc.fr/today. Accessed 24 Nov 2020.
- 5.National Cancer Institute—Surveillance, Epidemiology and ERP. Laryngeal Cancer—Cancer Stat Facts [Internet]. Available from: https://seer.cancer.gov/statfacts/html/laryn.html. Accessed 30 Oct 2020.
- 6.Sivanandan R, Kaplan MJ, Lee KJ, et al. Long-term results of 100 consecutive comprehensive neck dissections: implications for selective neck dissections. Arch Otolaryngol Head Neck Surg. 2004;130(12):1369–1373. doi: 10.1001/archotol.130.12.1369. [DOI] [PubMed] [Google Scholar]
- 7.Cooper JS, Zhang Q, Pajak TF, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84(5):1198–1205. doi: 10.1016/j.ijrobp.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 9.Pignon JP, le Maître A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Kolli VR, Datta RV, Orner JB, Hicks WL, Loree TR. Archives of otolaryngology-head and neck surgery. London: American Medical Association; 2000. The role of supraomohyoid neck dissection in patients with positive nodes; pp. 413–416. [DOI] [PubMed] [Google Scholar]
- 11.Horiot JC, Le Fur R, Nuyen T, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomized trial of the EORTC cooperative group of radiotherapy. Radiother Oncol. 1992;25(4):231–241. doi: 10.1016/0167-8140(92)90242-M. [DOI] [PubMed] [Google Scholar]
- 12.Hosal AS, Carrau RL, Johnson JT, Myers EN. Selective neck dissection in the management of the clinically node-negative neck. Laryngoscope. 2000;110(12):2037–2040. doi: 10.1097/00005537-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 13.SEER*Explorer Application. 2020. https://seer.cancer.gov/explorer/application.html?site=1&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&race=1&age_range=1&hdn_stage=101&rate_type=2&advopt_precision=1&advopt_display=2. Accessed 30 Oct 2020.
- 14.Forastiere AA, Metch B, Schuller DE, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. J Clin Oncol. 1992;10(8):1245–1251. doi: 10.1200/JCO.1992.10.8.1245. [DOI] [PubMed] [Google Scholar]
- 15.Gibson MK, Li Y, Murphy B, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23(15):3562–3567. doi: 10.1200/JCO.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 16.Grégoire V, Lefebvre JL, Licitra L, Felip E. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(SUPPL. 5):v184–v186. doi: 10.1093/annonc/mdq185. [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Head and Neck Cancer (version 2.2018). 2018. http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed 24 Nov 2020.
- 18.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 19.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25(16):2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 20.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vademecum. Pembrolizumab. 2020. https://www.vademecum.es/principios-activos-pembrolizumab-l01xc18. Accessed 4 Nov 2020.
- 23.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35(14):1542–1549. doi: 10.1200/JCO.2016.70.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colevas AD, Bahleda R, Braiteh F, et al. Safety and clinical activity of atezolizumab in head and neck cancer: results from a phase I trial. Ann Oncol. 2018;29(11):2247–2253. doi: 10.1093/annonc/mdy411. [DOI] [PubMed] [Google Scholar]
- 26.Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 27.Siu LL, Even C, Mesía R, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. 2019;5(2):195–203. doi: 10.1001/jamaoncol.2018.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 29.National Institutes of Health—U.S. National Library of Medicine. A Study of Pembrolizumab (MK-3475) for First Line Treatment of Recurrent or Metastatic Squamous Cell Cancer of the Head and Neck (MK-3475-048/KEYNOTE-048) [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT02358031. Accessed 3 Nov 2020.
- 30.Tan-Torres ET, Baltussen RTA, Hutubessy R, Acahrya ADBE, Murray CJL. WHO guide to cost-effectiveness analysis. Geneva: WHO; 2003. [Google Scholar]
- 31.U.S. Food & Drug Administration. Clinical trial endpoints for the approval of cancer drugs and biologics—Guidance for industry. 2018. https://www.fda.gov/media/71195/download. Accessed 30 Oct 2020.
- 32.Latimer N. NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials-extrapolation with patient-level data report by the decision support unit. 2011. www.nicedsu.org.uk. Accessed 28 Aug 2020.
- 33.U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 [Internet]. 2009. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. Accessed 30 Oct 2020.
- 34.Augustovski FA, Irazola VE, Velazquez AP, Gibbons L, Craig BM. Argentine valuation of the EQ-5D health states. Value Heal. 2009;12(4):587–596. doi: 10.1111/j.1524-4733.2008.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ministerio de Salud. Gobierno de la Ciudad Autonoma de Buenos aires. 2020. https://documentosboletinoficial.buenosaires.gob.ar/publico/PE-RES-MSGC-MSGC-577-20-ANX.pdf. Accessed 25 Nov 2020.
- 36.Public Sector Nomenclators (HPGD, CABA) Social Security (PAMI, IAPOSS). 2020. https://www.boletinoficial.gob.ar/detalleAviso/primera/217123/20190923. Accessed 25 Nov 2020.
- 37.Banco Central de la República Argentina. Evolución de una moneda. 2020. http://www.bcra.gob.ar/PublicacionesEstadisticas/Evolucion_moneda.asp. Accessed 25 Nov 2020.
- 38.Instituto Autárquico Provincial de Obra Social. Tariff values and fixed value benefits—Year 2019 [Internet]. Available from: https://www.santafe.gov.ar/index.php/web/content/download/252775/1329102/file/ARANCELESPRACTICASMEDICASDISP78-19.pdf. Accessed 28 Aug 2020.
- 39.Programa de Asistencia Médica Integral—Instituto Nacional de Servicios Sociales para Jubilados. List of practices with relative units [Internet]. Available from: https://prestadores.pami.org.ar/bot_nomenclador_unico.php. Accessed 28 Aug 2020.
- 40.Programa de Asistencia Médica Integral - Instituto Nacional de Servicios Sociales para Jubilados. List of values for the relative units [Internet]. Available from: http://institucional.pami.org.ar/files/boletines_inssjp/RESOL-2019-9-INSSJP-SE-INSSJP.pdf. Accessed 28 Aug 2020.
- 41.Grupo Alfa Beta. 2020. http://www.alfabeta.net/vad/srv. Accessed 28 Aug 2020.
- 42.Cohen EEW, LaMonte SJ, Erb NL, et al. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016;66(3):203–239. doi: 10.3322/caac.21343. [DOI] [PubMed] [Google Scholar]
- 43.Ministerio de Economía. Portal de Datos Económicos - GDP Argentina Q1 2020 [Internet]. Available from: https://www.economia.gob.ar/datos/. Accessed 25 Nov 2020.
- 44.WHO . WHO-CHOICE methods. Geneva: WHO; 2017. [Google Scholar]
- 45.Lang Y, Dong D, Wu B. Pembrolizumab vs. the EXTREME regimen in recurrent or metastatic head and neck squamous cell carcinoma: a cost-effectiveness analysis. Clin Drug Investig. 2020;40:1137–1146. doi: 10.1007/s40261-020-00973-9. [DOI] [PubMed] [Google Scholar]
- 46.Zhou K, Li Y, Liao W, Zhang M, Bai L, Li Q. Pembrolizumab alone or with chemotherapy for squamous cell carcinoma of the head and neck: a cost-effectiveness analysis from Chinese perspective. Oral Oncol. 2020;1:107. doi: 10.1016/j.oraloncology.2020.104754. [DOI] [PubMed] [Google Scholar]
- 47.National Institute for Health and Care Excellence. Nivolumab for treating recurrent or metastatic squamous-cell carcinoma of the [ID971], head and neck after platinum-based chemotherapy. 2020. https://www.nice.org.uk/guidance/ta490/documents/committee-papers. Accessed 30 Oct 2020.
- 48.National Institute for Health and Care Excellence. Cetuximab for treating recurrent or metastatic squamous cell cancer of the head and neck. Guidance | NICE. 2020. https://www.nice.org.uk/guidance/ta473. Accessed 30 Oct 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to its proprietary nature. Further information about the data and conditions for access can be obtained from the corresponding author.