Abstract
As vaccine formulations have progressed from including live or attenuated strains of pathogenic components for enhanced safety, developing new adjuvants to more effectively generate adaptive immune responses has become necessary. In this context, polymeric nanoparticles have emerged as a promising platform with multiple advantages, including the dual capability of adjuvant and delivery vehicle, administration via multiple routes, induction of rapid and long-lived immunity, greater shelf-life at elevated temperatures, and enhanced patient compliance. This comprehensive review describes advances in nanoparticle-based vaccines (i.e., nanovaccines) with a particular focus on polymeric particles as adjuvants and delivery vehicles. Examples of the nanovaccine approach in respiratory infections, biodefense, and cancer are discussed.
1. Introduction
Vaccination plays a key role in preventive medicine by protecting individuals against harmful bacterial and viral diseases as well as for cancer immunotherapy. Recently, vaccine formulations have shifted away from whole bacteria or their lysates and inactivated viral particles towards highly purified recombinant protein antigens (Aoshi 2017; Leroux-Roels 2010). While these purified antigens allow for enhanced safety and targeting of the immune system towards specific epitopes, they are often poorly immunogenic compared to their live or attenuated counterparts (Aoshi 2017; Leroux-Roels 2010). Thus, adjuvants, or components that enhance the immune response, are an important consideration in modern vaccine design.
Adjuvants fulfill a wide variety of functions within vaccine formulations, with an overall goal to induce a potent immune response capable of providing long-term protection against future exposures (Montomoli et al. 2011). Adjuvants may act by directly stimulating immune cells via pattern recognition receptors or modulating the immune response to prioritize humoral or cell-mediated immunity (Montomoli et al. 2011; Coffman et al. 2010; Garlapati et al. 2009). Similarly, adjuvants may also be designed to overcome specific immune defects, such as immunosenescence in older adults, to improve vaccine efficacy (Leroux-Roels 2010; Montomoli et al. 2011). Another aspect of augmenting vaccine efficacy is through patient compliance. Adjuvants may not only provide immune stimulation but function as delivery vehicles capable of sustaining antigen release. The ability to enhance delivery and provide an antigenic depot allows for a reduction in doses, or the number of immunizations required, thereby enhancing patient compliance (Montomoli et al. 2011; Coffman et al. 2010). Furthermore, adjuvant vehicles may increase vaccine stability and shelf-life, allowing for a cost-effective vaccine to be deployed widely (Chen and Kristensen 2009). Thus, while adjuvants enable a wide variety of functions within vaccine formulations, multiple aspects must be considered when selecting the most appropriate adjuvant(s) for each vaccine application.
The focus of this chapter is on polymeric nanoparticle-based adjuvants, which provide multiple competitive advantages in the rational design of vaccines. By rationally selecting/designing polymers based on their physicochemical properties, and considering antigen and vaccine regimen, it is possible to modulate appropriate immune responses for specific diseases. We begin with a brief overview of the mechanisms of humoral- and cell-mediated immunity. In subsequent sections, the various types of polymeric nanoparticles that have been studied for vaccine use will be summarized and the advantages of natural and synthetic polymers in modulating immune response phenotypes will be described. Finally, examples of nanoparticle-based vaccines (or nanovaccines) against multiple diseases as well as advances in manufacture/scale-up of nanoparticle commercialization and regulatory considerations will be discussed.
2. Mechanisms of Immunity
2.1. Humoral Immunity
Humoral immunity encompasses the functional capabilities of antibodies, complement cascade proteins, and antimicrobial peptides to eliminate extracellular and mucosal pathogens, signal innate immune cells, and enable immune protection. Antibodies have a wide range of functions including neutralizing virus and secreted toxins (McComb and Martchenko 2016; Klasse 2014), forming immune complexes to enhance complement activation, and binding to pathogens to promote cytolysis or phagocytosis by antigen-presenting cells (APCs) to activate CD4+ and CD8+ T cells (Wen et al. 2016).
Induction of antibody responses requires stimulation of B cells. B cells can initiate the production of T cell-independent antibodies in response to APC and T cell-derived cytokine stimulation or repetitive epitopes that cross-link B cell receptors (BCRs) (MacLennan et al. 2003). While antibodies produced this way can fix complement and are valuable in the early stages of an immune response, they have limited utility to meet the goals of vaccination due to their low affinity. These antibodies are not optimized for pathogen neutralization and the B cells that produce them are less likely to generate long-lived memory B cells and plasma cells.
Achieving protective and long-lived antibody production requires B cell enhancement by a subset of CD4+ T cells called follicular helper T cells (Tfh). B cells in the germinal centers (GCs) compete to interact with follicular dendritic cells (FDCs) that present antigens and Tfh cells. The cycling of B cell interactions with FDCs and Tfh cells leads to antibody isotype class switching and affinity maturation of the BCRs. Those B cells with higher affinity preferentially repeat this cycle, leading to the production of high affinity, class-switched antibodies. Tfh cell interactions also help signal B cells to leave GCs and differentiate into long-lived memory B cells and plasma cells.
2.2. Cell-Mediated Immunity
Cell-mediated immunity (CMI) involves activation of phagocytes [i.e., dendritic cells (DCs) and macrophages (MØs)], T helper (Th) cells, cytotoxic T lymphocytes (CTLs), and natural killer (NK) cells to eliminate pathogens. T cell activation requires interaction of the MHC: peptide complex on the surface of APCs with a complementary T cell receptor (TCR), signaling by other surface costimulatory receptor-ligand interactions, and cytokines provided by APCs. These cytokines are secreted as a consequence of APC interactions with the adjuvant during activation, allowing the adjuvant to indirectly determine the T cell phenotype (Wilson-Welder et al. 2009).
CMI is initiated by the internalization of antigen by DCs and MØs along with concomitant activation by the adjuvant; these APCs then migrate to the draining lymph nodes, present antigen to T cells in the context of MHC I and MHC II. Secretion of IL-4 and IL-2 promotes differentiation of naïve CD4+ T cells towards a Th2 phenotype, which is subsequently involved in the humoral response (Saravia et al. 2019). DCs secreting IL-12 induces naïve CD4+ T cell differentiation towards a Th1 phenotype and naïve CD8+ T cells towards a CTL phenotype (Kang et al. 2004). These Th1 CD4+ cells and CTLs then migrate to the site of vaccination/infection and interact with APCs capable of secreting IL-12, IFN-γ, TNF-α, and IL-2 (Vesely et al. 2011). Secreted IFN-γ activates MØs to enhance pathogen phagocytic ability, antimicrobial nitric oxide production, and antigen presentation to CD4+ and CD8+ T cells (Pennock et al. 2013). Additionally, differentiated CTLs have increased cytolytic capabilities through secretion of perforin and granzymes following cognate peptide-MHC I recognition on infected cells, which results in apoptosis (Annunziato et al. 2015).
3. Traditional Adjuvants
Currently, aluminum-based salts collectively referred to as ‘alum’, are one of the few adjuvants approved for human use. While alum has a long history of safe use in vaccines, it is far from a ‘universal adjuvant’ with a bias towards humoral immunity, a need for multiple doses, and incompatibility with many antigens (Gupta et al. 1995).
Mechanistically, alum promotes the recruitment of APCs, antigen uptake, and provides for a ‘depot effect.’ Antigen adsorbs onto alum and is slowly released into the surrounding microenvironment. These depots may persist for a significant amount of time and alum particles have been observed at the site of injection a year after administration (Gupta et al. 1995; Awate et al. 2013). Additionally, there is evidence that alum activates the innate immune system at the injection site. Research suggests alum binds to DC membrane lipids triggering the release of uric acid, which acts as a potent immunostimulant or damage-associated molecular pattern (DAMP) (Kool et al. 2008; Flach et al. 2011). Research has also found that alum may stimulate NLRP3 inflammasomes or induce apoptosis, which further activates immune responses (Quandt et al. 2015; Franchi and Núñez 2008).
Alum induces a strong Th2 response, thus it is effective for disease platforms that require a humoral response for clearance. However, alum falls short in inducing Th1 responses, a crucial response for clearing viral infections (Oleszycka et al. 2018; Igietseme et al. 2004). Additionally, alum is not a powerful immune stimulator; while not a significant handicap when working with killed or inactivated pathogens that provide a range of immunogenic components, this becomes a limitation when recombinant proteins are used for subunit vaccines. Typically, other immunogenic compounds must be co-administered in order to induce a protective response (McNeela and Mills 2001; Lavigne et al. 2004).
In addition to alum, monophosphoryl lipid A (MPLA) emulsions are also approved for human use (Reed et al. 2013). MPLA, a Toll-like receptor (TLR)-4 agonist, has been combined with a number of other TLR ligands to generate a wide range of adjuvant systems, many of which have undergone clinical testing (Table 1). These TLR ligands leverage specific pathways in the innate immune system rather than generalized inflammation. For example, the liposome adjuvant AS01 is a combination of MPLA and saponin QS-21. The resulting adjuvant system exhibits more balanced humoral and cellular responses than when either component is given alone (Didierlaurent et al. 2017).
Table 1.
List of adjuvants that have undergone clinical testing
| Adjuvant | Components | Formulation | Mechanism |
|---|---|---|---|
| Alhydrogel (aluminum hydroxide) | Alum | Aluminum adsorption | Depot effect, Recruitment |
| Alum (aluminum phosphate salts) | Alum | Aluminum adsorption | Depot effect, Recruitment |
| AS01 | MPL and QS-21 in liposomes AS01B/AS01E includes DOPC and cholesterol in phosphate buffered saline | Liposome | TLR-4, Recruitment |
| AS02 | MPL and QS-21 in liposomes | O/W emulsion | TLR-4 |
| AS03 | Oil-in-water emulsion with α-tocopherol | O/W emulsion | IRE1α |
| AS04 | MPL adsorbed onto aluminum hydroxide or aluminum phosphate | Aluminum adsorption | TLR-4 |
| AS15 | MPL, QS-21 and CpG 7909 | Liposome | TLR-4, TLR-9, Recruitment |
| AS25/AS50 | AS02 without QS-21 | O/W emulsion | TLR-4 |
| CAF01 | dimethyl dioctadecyl ammonium delivery vehicle | Liposome | Recruitment |
| CpG 7909 | Soluble | TLR-9 | |
| CRM197 | A modified diphtheria toxin | Soluble | Recruitment |
| dmLT | Proteins derived from E. coli enterotoxin | Soluble | TLR-4 |
| GLA-AF | Aqueous glucopyranosyl lipid adjuvant | O/W emulsion | TLR-4 |
| GLA-SE | Stable-oil-in-water-emulsion with glucopyranosyl lipid adjuvant | O/W emulsion | TLR-4 |
| GM-CSF | Soluble | Recruitment | |
| IC31 | Synthetic oligodeoxynuclotide and antibacterial peptide | Soluble | TLR-9 (Olafsdottir et al. 2009) |
| Type I interferon | Soluble | Recruitment | |
| IL-12 pDNA | Soluble | Recruitment (Jalah et al. 2012) | |
| IL-2 | Soluble | Recruitment (Nunberg et al. 1989) | |
| ISCOMATRIX | Saponin and lipid complex | Immunostimulatory complex | Recruitment (Pearse and Drane 2005) |
| IsdB | Recombinant S. aureus protein | Soluble component | TLR-7 (Wu et al. 2014; Bagnoli et al. 2015) |
| KLH carrier protein | Immunogenic carrier protein | Soluble component | Recruitment |
| LT Adjuvant patch | Transcutaneous immunostimulatory patch | External microneedle | Recruitment (Mkrtichyan et al. 2011) |
| Matrix-M1 | Saponins | Soluble component | Antigen uptake and Recruitment (Magnusson et al. 2013) |
| MF59 | Squalene based emulsion | O/W emulsion | MyD88, ASC |
| Montanide ISA | Incomplete Freund’s adjuvant in mannide monooleaste and mineral oil | W/O emulsion | Depot effect, Recruitment |
| MPL | O/W emulsion | TLR-4 | |
| MPL-SE | MPL plus glycerol, phosphatidylcholine and squalene | O/W emulsion | TLR-4 |
| poly-ICLC | Double stranded RNA complex | Soluble Component | TLR-3 (Saxena et al. 2019) |
| QS-21 (or OBI-821, OPT-821 or QS-DG all of which are equivalent derivatives or derived from different sources) | Saponins | Soluble component | Recruitment (Kensil and Kammer 2010) |
| rCTB | Cholera endotoxins | Soluble component | Recruitment (Isaka et al. 2004) |
While a complete review of all adjuvants lies beyond the scope of this chapter, it is sufficient to note that a select subset of adjuvants acts as TLR agonists. These agonists may be combined with other adjuvants/adjuvant systems or delivered via polymeric nanoparticles to further tailor the specific immune response elicited by the vaccine platform. Agonists to additional immune pathways, such as NOD-like receptors, C-type lectin receptors, folate receptors, and the STING pathway present more tools for fine-tuning the immune-enhancing capabilities of adjuvant systems (Garlapati et al. 2009; Chavez-Santoscoy et al. 2012; Narasimhan et al. 2016).
4. Polymer Nanoparticle Adjuvants
Polymer nanoparticles represent a promising adjuvant platform for a multitude of reasons (Fig. 1). One advantage of nanoparticle-based vaccines is that their biophysical and biochemical properties may be manipulated in order to enhance antigen uptake, processing, and presentation. Furthermore, rational selection of polymer chemistry (either natural or synthetic) may enhance antigen stability, influence release kinetics, and modulate the particular type of immune response. In this section, the overall physiochemical properties (including size, shape, charge, and hydrophobicity) of nanoparticle platforms will be described. In addition, detailed examples of the unique advantages of particle platforms (such as controlled release, co-encapsulation, and induction of CMI) will be summarized below.
Fig. 1.
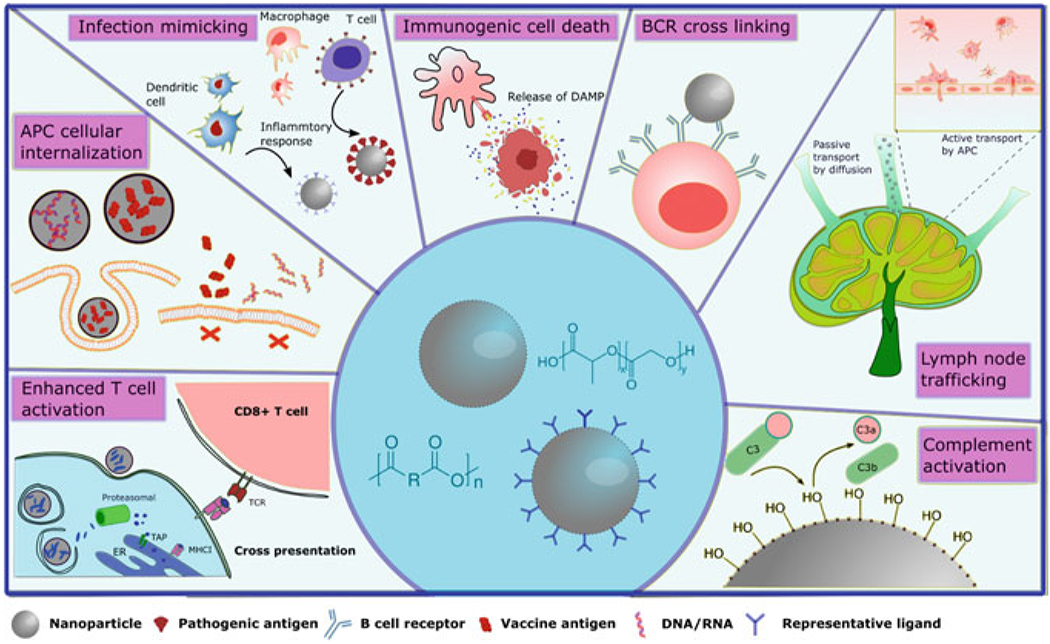
Schematic illustration of advantages provided by polymeric nanoparticle adjuvant systems for vaccination. Clockwise left to right: nanoparticle enhanced T cell activation, showing antigen cross-presentation; APC internalization of vaccine payload carried by nanoparticles versus free diffusion; nanoparticle-enabled infection mimicking inducing immune recognition and pro-inflammatory responses; immune activation by immunogenic cell death; nanoparticle-enabled B cell receptor (BCR) crosslinking; nanoparticle trafficking to lymph node; and complement activation by nanoadjuvants
4.1. Physiochemical Properties of Polymeric Nanoparticles
4.1.1. Size
Particle size plays a large role in the distribution and cellular internalization of vaccine formulations. Particles in the 20–200 nm range can successfully enter the lymphatic system within a few hours of administration (Bachmann and Jennings 2010). Particles with diameters of 200–500 nm cannot enter the lymphatic system directly but can be internalized by APCs and reach the lymphatic system within 24 hours (Bachmann and Jennings 2010). Particle size also affects the mechanism of internalization by APCs. Generally, sub-micron particles are internalized effectively by APCs and generate robust immune responses, while particles >10 μm cannot be internalized by APCs (Thomas et al. 2011; Ramirez et al. 2017). Particles with a diameter of 20–200 nm are internalized by endocytosis (Silva et al. 2013). In contrast, relatively larger particles (>0.5 μm) are internalized via micropinocytosis and phagocytosis by APCs (Silva et al. 2013).
4.1.2. Shape
While most studies focus on spherical particles, recent efforts have analyzed the effect of particle shape on immune responses. Particle shape influences cellular internalization, as it is suggested that rod-shaped particles may associate with and be internalized more readily by APCs than other particle geometries (He and Park 2016; Gratton et al. 2008). Experiments have shown improved cellular uptake of rod-shaped particles compared to spherical particles by DCs and MØs (Jindal 2017; Sharma et al. 2010; Banerjee et al. 2016). Ex vivo studies have shown that rod-shaped nanoparticles can remain with the gastrointestinal tract for longer periods of time relative to spherical particles (Zhao et al. 2017). In vivo studies demonstrated that longer rod-shaped nanoparticles circulated in the blood longer than short-rod and spherical nanoparticles (Zhao et al. 2017).
4.1.3. Surface Charge
Both anionic and cationic particles are efficiently internalized by APCs, promoting induction of robust immunity (Thomas et al. 2011; Ramirez et al. 2017; Carrillo-Conde et al. 2012). However, intracellular tracking experiments show that internalized cationic nanoparticles escape from lysosomes in contrast to neutral and anionic particles that tend to localize within lysosomes (Yue et al. 2011). Therefore, particle charge can impact the ability of particles to influence induced immune response phenotype(s) by influencing the activation of APCs and targeting antigen to specific immune response pathways, which may mimic that of intracellular pathogens.
4.1.4. Hydrophobicity
Hydrophobicity has been suggested to act as a DAMP for activating innate immunity (Moyano et al. 2012). During host cell disruption, hydrophobic cellular materials are exposed to the external environment and trigger an innate immune response. It is hypothesized that increased surface hydrophobicity of nanoparticles may promote their interactions with hydrophobic components of cell surfaces to result in enhanced cellular uptake (Shima et al. 2013). In fact, hydrophobic polymeric particles synthesized with higher molecular weights tend to be phagocytosed more than their hydrophilic counterparts (Thomas et al. 2011; Shima et al. 2013; Goodman et al. 2014).
4.2. Unique Advantages of Nanoparticle Vaccines
4.2.1. Controlled Release of Antigens
Polymeric particles can be designed to enable tunable release kinetics of antigens (Kumari et al. 2010). Depending on the synthesis method used, polymeric nanoparticles can be synthesized as nanospheres or nanocapsules. Nanospheres are comprised of a spherical matrix of polymer into which the antigen is physically entrapped. In nanocapsules, antigen is contained within a cavity surrounded by a polymer shell. Antigen can be loaded within the particles or onto their surface to manipulate release and subsequent immune effects. Unlike other delivery systems, polymeric nanoparticles are capable of encapsulating both hydrophilic and hydrophobic antigens (Kumari et al. 2010). Numerous factors contribute to antigen release rate from particles. For example, polymer hydrophobicity determines erosion kinetics, which in turn, determines antigen release kinetics, with hydrophobic surface eroding polymers exhibiting much slower release rates (Lopac et al. 2009). Combining physiochemical properties with chemistry allows for fine-tuned control. For example, short-rod nanoparticles exhibit a higher specific surface area than long-rod and spherical nanoparticles. When produced with a surface eroding polymer, the short-rod particles display shorter degradation time scales than their long-rod and spherical counterparts (Zhao et al. 2017).
4.2.2. Encapsulation of Co-adjuvants
The ability to encapsulate antigenic payloads into polymeric nanoparticles has been extensively shown to enhance the antibody response, allowing for both dose sparing strategies and reduced vaccination schedules (Huntimer et al. 2013 a; Bershteyn et al. 2012). The physiochemical properties of polymeric nanosystems encompass both immunostimulatory adjuvant-like properties of the polymer and its chemical structure. The interplay between these two aspects leads to increased nanoparticle internalization by APCs, which enhances antigen uptake (Ulery et al. 2009). It has been demonstrated that some polymeric nanoparticle components are able to signal these pattern recognition receptors (PRRs) directly (Tamayo et al. 2010; Locatelli et al. 2017), or incorporate PRR stimulants (Phanse et al. 2017; Liu et al. 2018), to enhance antigenicity. By targeting various APC types with the right immunostimulatory signals through their physiochemical properties or by the inclusion of co-adjuvants (via co-administering small molecule adjuvants, co-encapsulation of adjuvant with antigen, or surface conjugation), it is possible to further enhance the magnitude of antibody responses, including generating neutralizing antibodies, as has been demonstrated for influenza and Ebola (Zacharias et al. 2018; Yang et al. 2017). The synergy in co-administration of multiple adjuvants in synthetic nanoparticle vaccines generates both increased and longer-lived humoral immune responses than when either adjuvant is used alone (Ulery et al. 2011; Stieneker et al. 1995; Wagner et al. 2019). It was demonstrated that co-administration of polymeric nanoparticles encapsulating TLR-4 and TLR-7 agonists induced neutralizing antibodies to hemagglutinin that were significantly higher in titer than those induced by either TLR agonist alone, and which persisted for over 1.5 years following immunization (Kasturi et al. 2011).
4.2.3. Cell-Mediated Immunity
Conventional live-attenuated vaccines or inactivated vaccines provide limited control over specific types of immune response as their biochemical properties are largely determined by the conserved nature of the pathogen itself. Additionally, vaccines using alum- or emulsion-based adjuvant strategies generally fall into generating either dominant humoral immunity or cause severe adverse reactions (Bhardwaj et al. 2020). To generate CMI, co-delivery of antigen mediated by both the MHC I and MHC II presentation pathways is key. Subunit antigens are generally poor inducers of CD8+ T cells by themselves. Multiple polymeric nanoparticle formulations have demonstrated the ability to induce CD8+ T cell responses (Huntimer et al. 2014; Zhang et al. 2011). This can be achieved through targeting of the nanoparticles to APCs, where the ability to activate these cells through both MHC II and MHC I presentation pathways can activate CD4+ and CD8+ T cells, respectively, and further allow CD4+ T cells to secrete pro-inflammatory cytokines (e.g., IFN-γ) that enhance CTL responses, as well as by activating pathogen killing immune responses by innate immune cells. Mechanisms of nanoparticle adjuvanticity, including increased cellular uptake, enhanced cross-presentation, and immunogenic cell death (ICD) have been demonstrated to contribute to the induction of T cell activation (Lu et al. 2017; Urbanavicius et al. 2018). Studies have also shown induction of CMI by controlling nanoparticle polymer chemistry, hydrophobicity, size, charge, and pH responsiveness (Goodman et al. 2014; Gu et al. 2019; Rayahin et al. 2015; Luo et al. 2017). In addition, polymeric nanoparticles can incorporate co-adjuvants (as described above), which can further enhance CD4+ and CD8+ T cell responses (Zhang et al. 2011; De Titta et al. 2013; Hamdy et al. 2007).
5. Types of Polymeric Nanoparticle Adjuvants
Polymers are attractive materials for particle synthesis because of their biocompatibility, biodegradability, and low toxicity (Peres et al. 2017). Although inorganic particles such as gold, carbon, or silica have shown promising results as adjuvants, concerns over the potential risks of utilizing non-biodegradable materials remain (Pati et al. 2018; Tao and Gill 2015; Niikura et al. 2013; Kim et al. 2014). We next discuss both natural and synthetic polymers utilized for particle-based adjuvants, as well as hybrid polymer systems. This information is also summarized in Table 2.
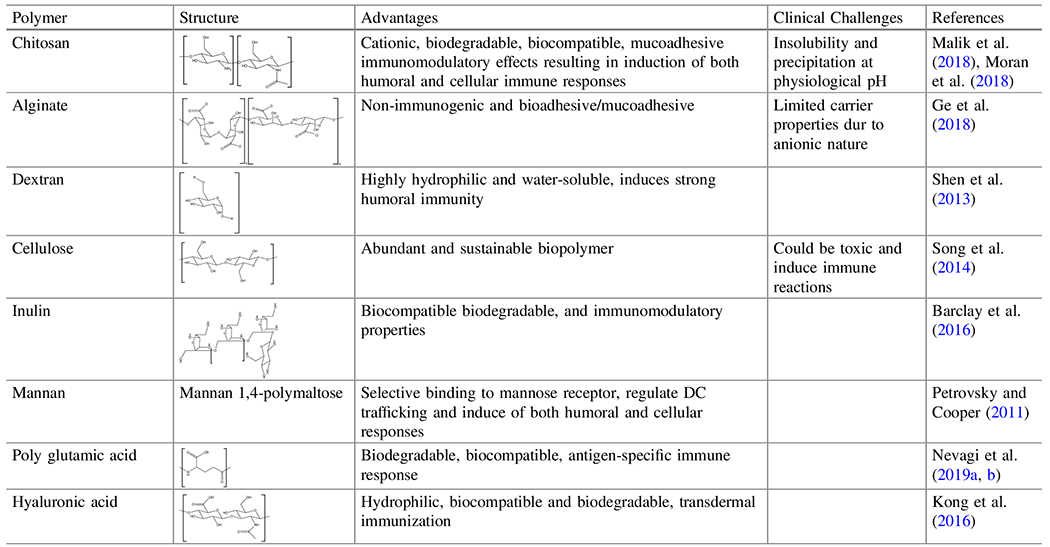
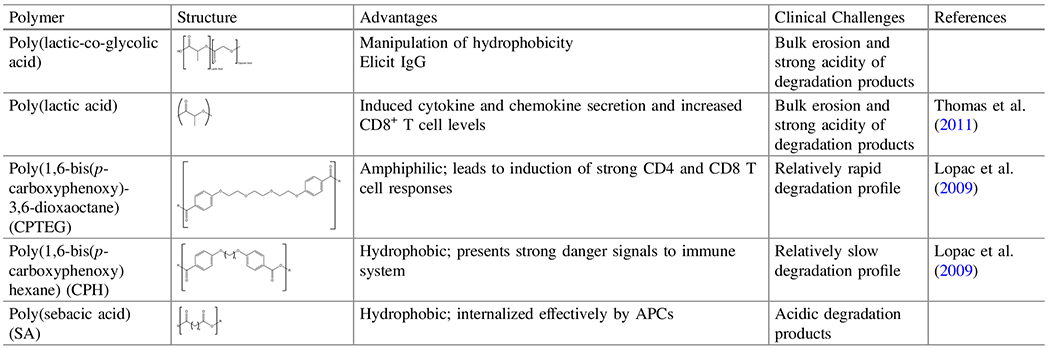
Table 2.
Summary of polymer chemistries and characteristics
|
|
The table shows the molecular structures of polymers discussed in this review and their advantages and clinical challenges
5.1. Natural Polymers
Natural polymers, obtained from renewable, sustainable, and natural resources, are considered as non-toxic, biocompatible, and biodegradable materials. Chitosan, alginate, dextran, hyaluronic acid, and inulin are the most common examples of natural polymers that are used as adjuvants in vaccine formulations (Torres et al. 2019; Bose et al. 2019; Guo et al. 2019; Nevagi et al. 2019a, b). Some of these natural polymers, such as β-glucans, are known to inherently target APCs and induce cellular and humoral immune responses, while some others, such as chitosan or alginate, are known for their mucoadhesive properties which may increase retention time (Petrovsky and Cooper 2011). Despite these advantages, natural polymers provide limited immune modulation compared to their synthetic counterparts (Guo et al. 2019). Nevertheless, the presence of specific chemical groups in their structure (i.e., amine or carboxyl groups) along with the anionic or cationic nature of these polymers enable different functionalities via alternative chemical and/or physical modifications. Thus, natural polymers can be modified by targeting ligands and receptors or loaded with antigens and other active agents to provide an adjuvanting effect and enhance immune response. Antigens and other active agents, such as nucleic acids, can be loaded via physical encapsulation, chemical adsorption, or electrostatic interactions to obtain natural polymer-based adjuvants and vaccine carriers in the forms of micro/nanoparticles, nanogels, nanofibers, and hydrogel capsules/nanoparticles. Numerous methods can be used to obtain these formulations, including solvent evaporation, emulsification-solvent diffusion, nanoprecipitation (Rao and Geckeler 2011), self-assembly, electrostatic complexation, ionic gelation, chemical crosslinking, template-assisted nanofabrication (Ferreira et al. 2013), electrospinning, (Jahantigh et al. 2014) or layer-by-layer assembly (Highton et al. 2015). These approaches are mainly used to provide high antigen loading and stability, which in turn improve delivery and immune response stimulation (Highton et al. 2015; Leleux and Roy 2013). Additionally, natural polymers can be used in combination with other synthetic or inorganic materials (i.e., synthetic polymers or inorganic particles) to develop hybrid systems with improved properties. These overall features of natural polymers make them attractive candidates as vaccine adjuvants (Han et al. 2018). We next describe some well-studied natural polymers that have been used in nanoparticle-based vaccine formulations.
5.1.1. Chitosan
Chitosan, obtained through deacetylation of chitin in shrimp and crustaceans, is a natural polymer commonly studied as a vaccine adjuvant. Highly acetylated chitosan is cationic in nature and is generally considered to be biodegradable, biocompatible and non-toxic. The main hurdle with chitosan is its insoluble nature at physiological pH. This may be addressed by additional deacetylation or chemical modification of amino moieties to obtain soluble derivatives of chitosan (i.e., trimethyl chitosan) (Moran et al. 2018).
Chitosan and its derivatives demonstrate inherent adjuvanting properties and immunomodulatory effects resulting in induction of both humoral and cellular immune responses (Nevagi et al. 2019a, b; Bose et al. 2019; Malik et al. 2018; Moran et al. 2018). Chitosan-based formulations have been reported to interact with various receptors on APCs (e.g., Dectin-1) and induce different signaling pathways involving NLRP3 inflammasome or cGAS-STING activation. These interactions trigger a cascade of different cellular events such as dendritic cell activation via type I interferon (IFN) resulting in secretion of chemokines (e.g., CXCL10/IP-10) and/or cytokines (e.g., TNF-α, IL-12, IL-4, and IL-10) and promoting Th1/Th2 immune response (Lampe et al. 2019). In addition, it has also been demonstrated that chitosan-based formulations can activate MØs (Malik et al. 2018; Moran et al. 2018), promote DC maturation, and enhance IgG and IgA antibody titers (Sui et al. 2010).
Chitosan and its derivatives can be used as nanoparticle adjuvants loaded with specific proteins, antigens, or active agents via ionic gelation. A study demonstrated that a chitosan nanoparticle-based Rift Valley Fever Virus vaccine formulation enhanced humoral and cellular immune responses by increasing cytokine secretion compared to its alum based counterparts (El-Sissi et al. 2020). Another study reported that a chitosan nanoparticle-based pneumococcal conjugate vaccine formulation provided enhanced immune responses by increasing IgG1, IgG2a, IgG2b, and IgG3 antibody titers compared to liposome nanoparticle-based formulations (Haryono et al. 2017). In classical approaches, chitosan nanoparticle formulations encapsulating proteins acted as vaccine adjuvants by boosting cytokine secretion, promoting Th1/Th2 responses, increasing natural killer cells activity and humoral/cellular immune response (Wen et al. 2011).
Chitosan based nanovaccine formulations have demonstrated mucoadhesive and adjuvanting properties (Xia et al. 2015). Considering this, a study developed chitosan and trimethyl chitosan adjuvants for intranasal H5N1 influenza vaccines which resulted in significant antibody responses. They also reported that the formulation with trimethyl chitosan demonstrated no clinical signs post-challenge (Mann et al. 2014). With this perspective, another study intranasally administered early secreted antigenic target 6-kDa protein (ESAT-6) encapsulated in trimethyl chitosan (TMC) nanoparticles which induced IgG against Mycobacterium tuberculosis (Amini et al. 2017). As a new approach, a study used mast cell activator C48/80 in combination with chitosan nanoparticles as an alternative vaccine adjuvant for Hepatitis B via the nasal route (Bento et al. 2019). Finally, encapsulation of antigens in chitosan-based vaccine formulations also provides stability and antigenicity in the gastrointestinal tract following oral immunization (Farhadian et al. 2015).
5.1.2. Alginate
Alginate is a biodegradable, biocompatible, muchoadhesive, natural polymer, and is commonly used as an adjuvant in vaccine formulations. The nanoparticle form of hydrophilic alginate provides efficient antigen release and improved immunogenicity particularly via nasal and oral administration (Guo et al. 2019; Nevagi et al. 2019a; Sarei et al. 2013). It has been reported that alginate incorporated influenza vaccine formulations induced cytokine production, increased antibody titers, activated DCs, and enhanced cellular immunity (Ge et al. 2018; Dehghan et al. 2018). Negatively charged alginate was also used to coat chitosan nanoparticles loaded with inactivated influenza virus to modulate immunostimulatory properties. Intranasal administration of this vaccine formulation resulted in an increased Th1 immune response (Mosafer et al. 2019). Similar strategies have also been used to develop alginate-based vaccines for hepatitis B and A providing improved humoral and cellular immune responses (AbdelAllah et al. 2016; AbdelAllah et al. 2020).
5.1.3. Dextran
Dextran and its derivatives [e.g., diethylaminoethyl (DEAE)-dextran or acetylated dextran (Ac-DEX)] are hydrophilic and water-soluble natural polymers, demonstrating adjuvant properties and providing robust immune responses (Moreno-Mendieta et al. 2017; Bachelder et al. 2017). It was reported that dextran nanoparticles incorporating ovalbumin and lipopolysaccharide promoted mannose receptor-dependent APC stimulation, inducing both cellular and humoral immune responses in mice (Shen et al. 2013; De Geest et al. 2012). In a recent study, ovalbumin encapsulated Ac-DEX microparticles demonstrated controlled release of active cargo accompanied by efficient antibody and cytokine production (Chen et al. 2018a). The same group also developed Ac-DEX, matrix protein 2, and 3′3′-cyclic GMP-AMP (cGAMP)-based vaccine formulation and reported enhanced humoral and cellular responses resulting in protection against lethal influenza (Chen et al. 2018b). In a follow-up study, the same group encapsulated STING agonist cGAMP and soluble Toll-like receptor 7/8 (TLR7/8) agonist resiquimod (R848) into Ac-DEX microparticles that enabled enhanced cellular and humoral responses (Collier et al. 2018). Similar approaches with Ac-DEX and cGAMP were also used by the same group to further support their findings (Junkins et al. 2018). Another group also used Ac-DEX to encapsulate TLR-7 agonist imiquimod and deliver it to intracellular TLR-7 receptors resulting in an increase of IL-1β, IL-6, and TNF-α (Bachelder et al. 2010). Chemical conjugation of dextran with type B CpG DNA, a TLR-9 agonist, was also conducted resulting in enhanced APC internalization, improved CpG accumulation in lymph node, and improved CD8+ T cell responses in mice (Zhang et al. 2017a).
5.1.4. Hyaluronic Acid
Hyaluronic acid, a hydrophilic, biocompatible, and biodegradable natural mucopolysaccharide (Ekici et al. 2011) can be used as a adjuvant stabilizer and delivery vehicle to be applied via the transdermal route due to its involvement in skin extracellular matrix and ability to penetrate the skin due to its small size (Nashchekina and Raydan 2018; Bussio et al. 2019). Hyaluronic acid also improves solubility of adjuvants or antigens, such as MPLA and alum adjuvanted hepatitis B vaccine, enhancing both cellular and humoral immune responses (Moon et al. 2015).
5.1.5. Inulin
Inulin is another biocompatible and biodegradable natural biopolymer demonstrating vaccine adjuvant properties (Barclay et al. 2016; Cooper et al. 2013; Cooper and Petrovsky 2011; Cooper et al. 2015; Kumar and Tummala 2013). Acetylated inulin (Ace-IN) is most commonly used in vaccine formulations and has been shown to trigger cytokine secretion, DC maturation, antibody titer production (Rajput et al. 2018). An Ace-IN-based pathogen-mimicking vaccine delivery system was reported to improve antigen delivery to APCs and simultaneously activate TLR-4 on APCs (Kumar et al. 2017; Kumar et al. 2016).
5.1.6. DNA
Nucleic acids or DNA-based nanomaterials have also been used as vaccine adjuvants through functionalization with short immunogenic sequences. For example, it was reported that CpG-containing dendrimer like-DNA showed shape and size-dependent immunostimulatory activity (Mohri et al. 2015; Mohri et al. 2014). Differently, self-assembled single-stranded DNA sequences (e.g., immune nanoflowers) as well as DNA hydrogels have also been developed to provide cytokine secretion and antigen delivery (Zhang et al. 2015; Ishii-Mizuno et al. 2017). Recently, DNA origami-based approaches have been developed to provide immune stimulation based on different origami geometries and shapes (Hong et al. 2017; Bila et al. 2019; Bastings et al. 2018). It was reported that compact shapes possessing low-aspect ratios are efficiently internalized while non-compact shapes remained on the cell surface, demonstrating the ability to affect DC uptake and activation by rational design (Bastings et al. 2018).
5.1.7. Other Natural Polymers
Cellulose-based materials have also been used in vaccine formulations as adjuvants for proteins, antigens, or DNA in the form of particles, nanowires, or nanofibers to enhance the immune response via increasing the secretion of pro-inflammatory cytokines (Song et al. 2014; Bin et al. 2018; Catalan et al. 2010; Čolić et al. 2015; Tomić et al. 2016; Vartiainen et al. 2011; Pereira et al. 2013).
Antigen-containing natural, biodegradable, biocompatible, and non-toxic anionic poly(glutamic acid), γ-PGA, nanoparticles have also shown significant antigen-specific immune responses by activation of CD8+ T cells (Uto et al. 2013) and human monocyte-derived DCs (Broos et al. 2010). The γ-PGA particles are generally modified with L-PAE and are potential antigen carriers with excellent adjuvant properties via TLR-4, activation enhancing both humoral and cellular immunity (Uto et al. 2015; Ikeda et al. 2018).
Other biopolymers demonstrating adjuvant effects in vaccine formulations are mannan (Apostolopoulos et al. 2013), lentinan (Zhang et al. 2017b), zymosan (Ainai et al. 2006), pullulan (Singh et al. 2017), and carrageenan (Zhang et al. 2010) which all provided immunomodulation via of different signaling pathways that enhanced humoral and cellular immune responses.
5.2. Synthetic Polymers
Synthetic polymers serve as a promising vaccine adjuvant materials because of their biocompatibility, biodegradability, and low cost. The following sections will discuss polymeric platforms such as polyesters, polyanhydrides, and synthetic micelles.
5.2.1. Polyesters
Numerous polyesters have been studied for nanoparticle vaccines; however, poly (lactic-co-glycolic acid) (PLGA) is the most well-studied because it is biodegradable and exhibits high biocompatibility (Silva et al. 2016; Danhier et al. 2012). Due to a favorable safety profile, PLGA has received FDA approval for various biomedical applications, including sutures and drug and vaccine delivery (Silva et al. 2016; Danhier et al. 2012). The release rate of the cargo and hydrophobicity of PLGA particles can be directly manipulated by varying the ratio of lactic acid to glycolic acid monomers (Allahyari and Mohit 2016). PLGA particles release encapsulated material through a bulk erosion mechanism, resulting in burst-like release profiles. PLGA particles with higher amounts of glycolic acid result in a larger and more rapid burst release of antigen (Thomas et al. 2011; Allahyari and Mohit 2016).
When considering the effects of PLGA particles on the immune system, PLGA particles have shown success in presenting antigen to stimulate both humoral and cellular immunity (Nicolete et al. 2011). Multiple studies have shown successful internalization of PLGA particles by DCs and MØs (Danhier et al. 2012; Semete et al. 2010; Liu et al. 2015). Following internalization by DCs, PLGA particles release antigen for presentation through the MHC I pathway to elicit CD8+ T cell responses (Liu et al. 2015). Studies have shown PLGA enhanced numbers of CD4+ and CD8+ T cells in the spleen when compared to administration of antigen alone (Liu et al. 2015). These findings were also supported by other studies that showed activation of CD4+ and CD8+ T cells by antigen-encapsulated in PLGA nanoparticles (Demento et al. 2012).
PLGA particles also result in enhanced cytokine secretion profiles from DCs. Hepatitis B antigen-encapsulated into PLGA particles showed a significant increase in IL-2 and IFN-γ when compared to antigen alone (Thomas et al. 2011). Antigen-encapsulated PLGA particles also induced higher amounts of IL-1β, IL-6, and IFN-α (Demento et al. 2012). Mice immunized with Bordetella pertussis encapsulated in PLGA particles showed a significant enhancement of IFN-γ and IL-17, suggesting a mixed Th1 and Th17 immune response (Li et al. 2016).
With respect to humoral immunity, PLGA nanoparticles have shown efficacy at eliciting significant levels of systemic IgG and mucosal IgA. Mice injected with OVA-loaded PLGA particles reached a peak concentration of antigen-specific IgG antibodies at week 6 post-immunization that was maintained for 13 weeks (Demento et al. 2012). Orally administered PLGA particles co-loaded with OVA and the immunostimulant, MPLA, maintained an 8-fold increase in IgG titers for 5 weeks when compared to OVA alone (Sarti et al. 2011). PLGA nanoparticles loaded with OVA and CpG elicited enhanced production of OVA-specific IgG2a antibodies when compared to OVA and CpG alone (Joshi et al. 2013). In addition, enhanced titers of mucosal IgA were observed upon administration of hepatitis B antigen-encapsulated in PLGA particles (Thomas et al. 2011).
5.2.2. Polyanhydrides
Polyanhydrides are another class of biodegradable polymers with high biocompatibility (Huntimer et al. 2013b). This class of polymers has been approved for human use in medical controlled release products such as Gliadel® and Septacin (Roy et al. 2016; Wafa et al. 2019). Polyanhydrides degrade by surface erosion into non-toxic and easily metabolized carboxylic by-products (Lopac et al. 2009). During erosion, polyanhydride particles degrade only at the surface and exclude water from the bulk material, in contrast to the bulk erosion exhibited by polyesters (Silva et al. 2013). This surface erosion phenomenon contributes to tunable and sustained release profiles of encapsulated payloads. Polyanhydride hydrophobicity (and antigen release kinetics) can be directly manipulated by varying the ratio of its copolymer constituents. For example, copolymers rich in 1,6-bis(p-carboxyphenoxy)hexane (CPH) are largely hydrophobic and release antigen over a period of months (Kipper et al. 2002). In contrast, copolymers rich in 1,8-bis(p-carboxyphenoxy)-3,6-dioxaoctane (CPTEG) or sebacic anhydride (SA) degrade more quickly (ca. days-weeks).
Polyanhydride particles are also advantageous in terms of their enhanced antigen stability (Determan et al. 2006; Carrillo-Conde et al. 2010; Haughney et al. 2013; Ross et al. 2014; Vela Ramirez et al. 2016; McGill et al. 2018), sustained antigen release, and activation of B cells and T cells (inherent adjuvanticity), all of which lead to induction of robust humoral and cell-mediated immunity (Vela Ramirez et al. 2016; Huntimer et al. 2013a, 2014; Ramirez et al. 2014; Vela Ramirez 2015). Another significant benefit of polyanhydride nanovaccines is their superior thermal stability at room temperature for extended periods of time (Shen et al. 2001; Petersen et al. 2011; Wagner-Muñiz et al. 2018).
Polyanhydride particles are internalized by and activate APCs (Ulery et al. 2009). CPH, CPTEG, and SA-based particles have been shown to be internalized by bone marrow-derived DCs and MØs (Wafa et al. 2019; Torres et al. 2011). Generally, hydrophobic particles are internalized more readily by APCs (Ramirez et al. 2017; Petersen et al. 2011; Phanse et al. 2016). DCs or MØs exposed to polyanhydride particles upregulated MHC I and II, costimulatory molecules such as CD40, CD80, and CD86, and cytokine secretion (e.g., IL-6, IL-12p70, IL-1β, TNF-α) (Wafa et al. 2019; Torres et al. 2011; Phanse et al. 2016; Wafa et al. 2017).
Polyanhydride nanovaccines have been shown to induce robust humoral and cell-mediated immunity. For example, it was shown that a single dose of a polyanhydride nanovaccine with a 64-fold less dose of antigen as compared to soluble antigen induced similar antibody titers (Huntimer et al. 2013a). Studies have demonstrated the successful formation of GCs by a single administration of CPTEG:CPH nanoparticles (Vela Ramirez et al. 2016). Polyanhydride nanovaccines have been shown to induce sustained levels of both antigen-specific IgG and IgA antibodies (Zacharias et al. 2018; Wafa et al. 2017; Haughney et al. 2014). Finally, a single dose of polyanhydride nanovaccine was able to induce neutralizing antibodies against influenza virus in mice and pigs (Ross et al. 2015; Dhakal et al. 2017a, b; 2019). Polyanhydride nanovaccine formulations have been shown to promote both CD4+ and CD8+ T cell responses (Zacharias et al. 2018; Huntimer et al. 2013a, 2014). Following intranasal immunization, these studies demonstrated the induction of tissue-resident memory phenotypes that are believed to be important in conferring heterosubtypic protective immunity against influenza virus infection (Zacharias et al. 2018).
Another advantage of polyanhydride particles is their easily functionalized surfaces. Studies have shown that attachment of sugars to polyanhydride nanoparticles assist in targeting CLRs on APCs (Carrillo-Conde et al. 2012). Functionalization of polyanhydride particles with di-mannose significantly enhanced particle internalization by DCs (Ramirez et al. 2014; Phanse et al. 2013). In addition, these di-mannose functionalized particles upregulated MHC II, CD86, and CD40 expression on DCs (Carrillo-Conde et al. 2011). Further studies determined that di-mannose functionalized polyanhydride particles also upregulated CD206, a CLR, on bone marrow-derived DCs (Ramirez et al. 2014). Functionalized nanoparticles were also shown to elevate IL-6 and TNF-α secretion from DCs (Carrillo-Conde et al. 2011).
5.2.3. Micelles
Synthetic polymers may also be used to produce micelles. Micelles contain a hydrophobic core (for holding payloads) and a hydrophilic shell/corona. Similar to polymeric particles, micelles are capable of delivering antigen while maintaining adjuvant-like properties (Trent et al. 2015).
Micelles can be synthesized from di-block or multi-block copolymers. Typical polymers used to form the hydrophilic shell include polyethylene glycol (PEG) and polyethylene oxide (PEO). Polymers used to form the hydrophobic core include poly(L-amino acids), polyesters, and phospholipids. Phospholipids can be produced through both synthetic and natural means. When exposed to block-selective solvents, block copolymers undergo spontaneous self-assembly to form micelles (Abetz 2005). The specific shape of the micelle is determined by the type of block copolymer used and interactions between the micelle core and block-selective solvent (Abetz 2005).
In vivo studies have shown that administration of antigen-loaded micelles elicits an immune response similar to antigen administered with a TLR-2 ligand (Trent et al. 2015). In addition, it was shown that the adjuvanticity of the micelle-peptide formulation was directly related to the delivery of the peptide as opposed to induction of cytokines or costimulatory molecule upregulation. Micelles, which are typically less than 100 nm, are successful at delivering antigen to DCs (Trent et al. 2015). Due to their relatively small size, micelles are capable of both interacting with DCs at the site of delivery and transiting lymphatic vessels directly to the lymph nodes (Facciolà et al. 2019).
A family of amphiphilic pentablock copolymers with controlled architectures possessing different cationic end blocks has been reported as potential vaccine carriers for subunit vaccines (Adams et al. 2014, 2015, 2019). Particularly, a pentablock copolymer, composed of a temperature-responsive Pluronic F127 middle block and two pH-responsive poly(diethyl aminoethyl methacrylate) end blocks, demonstrated temperature and pH-responsive micellization and gelation which forms a depot for controlled delivery of proteins and genes (Adams et al. 2014, 2015, 2019). The amphiphilic central block, Pluronic F127, is an effective vaccine adjuvant promoting cellular entry and contributes to gene delivery while the cationic outer blocks spontaneously condense negatively charged DNA via electrostatic interactions for sustained combinational therapy. This polymer was also further modified with mannose to enhance interactions with CD206, improving its adjuvanting properties for DNA-based vaccine delivery (Adams et al. 2014). In addition, this pentablock polymer demonstrated in vivo persistence at the injection site for over 50 days providing sustained protein release and five-fold enhancement in the antigen-specific antibody titer compared to soluble protein alone (Adams et al. 2015). Recently, the high biocompatibility of these injectable Pluronic-PDEAEM micelle adjuvants was demonstrated, further enhancing their potential as components of next-generation vaccines (Adams et al. 2019).
5.3. Hybrid Polymer Adjuvants
Hybrid systems composed of different classes of materials, such as natural/synthetic polymers and lipids have been developed as adjuvants for vaccine formulations. Using PEG as surfactant is one of the most commonly applied approaches enhancing the stability and stealth properties of the systems. To increase mucoadhesive and mucopenetrating properties of vaccines via nasal administration, a study modified the surface of chitosan-based nano-emulsions with PEG (Di Cola et al. 2019). Another common approach is to synergistically use carbohydrates to coat synthetic polymer surfaces to enhance cellular uptake. For example, mannosylated poly(beta-amino esters) (PBAEs), free of inflammatory co-adjuvants, provided enhanced gene delivery resulting in improved APC activation and immune response (Jones et al. 2015). In another example, alginate was used to form cationic nanogels disulfide cross-linked with polyethylenimine (PEI) for delivery of OVA. It was reported that the OVA-containing nanogels significantly improved the induction of tumor-specific CD8+ T cells and antigen-specific antibody production (Li et al. 2013).
The cationic nature of chitosan and its derivatives can be used to coat synthetic polymer particles to increase antigen loading efficiency and/or adjuvanticity (Wusiman et al. 2019). For example, PLGA nanoparticles, loaded with Heptatis B surface antigen (HBsAg) and coated with trimethyl chitosan (TMC), were reported to provide efficient absorption and increase antibody titers via nasal route (Pawar et al. 2010). Similarly, chitosan-coated PLA particles enhanced antigen adsorption capacity and macrophage uptake resulting upregulation of MHC I and MHC II and secretion of pro-inflammatory cytokines (Chen et al. 2014). In comparison to a commercially available vaccine, another study demonstrated that coating poly-ε-caprolactone (PCL) with chitosan resulted in elevated IgG responses against HBsAg while avoiding the induction of IgE (Jesus et al. 2018). Furthermore, the nanoparticle formulation induced CMI against HBsAg leading to IL-17 and IFN-γ secretion. Along these lines, B and T cell epitope-containing peptides were coupled with PGA and mixed with TMC to produce self-adjuvanting nanovaccines (Nevagi et al. 2019b).
Hybrid polymer systems can also combine disparate polymer systems to achieve synergistic immune responses for a wide range of pathogens and delivery routes. In a recent study, an increased humoral immune response upon oral delivery was achieved by developing a hybrid delivery system in which self-assembled lipopetides nanoparticles were coated with alginate and TMC layers via electrostatic interactions (Bartlett et al. 2020). Similarly, a cationic liposome and natural hyaluronic acid-based hybrid nanoparticle system was shown to provide improved stability and antigen release characteristics that significantly enhanced the serum IgG response to Y. pestis F1-V following intranasal administration (Fan et al. 2015). In another study, liposome-PEG-PEI complex-based adjuvant demonstrated enhanced uptake, expression of surface markers, induction of pro-inflammatory cytokines, and antigen presentation (Chen et al. 2012). It was also shown that intranasal immunization with chitosan-coated, lipid-polymer hybrid nanoparticles enhanced mucosal immune responses via induction of both humoral and cell mediated immunity (Rose et al. 2018). Liposomes, modified with pH-sensitive polymers, could also serve as a delivery system for the induction of antigen-specific CD8+ T cells. For example, inclusion of cationic lipids into polymer-modified liposomes promoted costimulatory molecule expression and secretion of IL-12 and TNF-α from DCs and induced antigen-specific secretion of IFN-γ from splenocytes (Yoshizaki et al. 2017).
6. Polymeric Nanovaccines Against Diseases
Polymeric nanovaccines have demonstrated great potential as vaccine adjuvants against both viral and bacterial pathogens. In this section, we include examples of the use of polymeric nanovaccines against infectious diseases, biodefense pathogens, and cancer. These examples demonstrate how nanovaccines can be tailored to be effective countermeasures against each of these significant health challenges.
6.1. Infectious Diseases
Infectious diseases such as influenza, coronavirus disease, and infections caused by respiratory syncytial virus (RSV) continue to pose great threats to public health. These pathogens are often highly contagious or can be transmitted from natural reservoirs, which makes disease eradication extremely difficult. Their high mutation rate and variety of subtypes render traditional inactivated or live-attenuated vaccines unlikely to provide long-term protection to susceptible populations that vary by age, immune heritage, and underlying health conditions. The COVID-19 pandemic has underlined the need for development of novel vaccination strategies capable of inducing long-lasting, heterosubtypic (i.e., cross-protective) immunity. In this regard, nanovaccines demonstrate unique advantages because of their flexibility with respect to administration routes, ability to co-deliver multiple payloads, enhanced thermal stability, and induction of rapid and long-lived immunity in a single dose, as described below.
6.1.1. Influenza
Influenza remains a serious source of morbidity and mortality worldwide despite decades of research towards developing a vaccine for both seasonal and pandemic strains. Typical ‘flu shots’ can successfully build strain-specific humoral immunity, but fail to initiate robust CMI necessary for clearing heterologous viral infections (Ho et al. 2011; Muruganandah et al. 2018). The performance of seasonal vaccinations is affected by the high mutation rate of influenza strains, with tetravalent vaccines rarely reaching 50% efficacy, compounded with poor immune responses in at-risk populations, immunosenescence in older adults and immunocompromised individuals (Demicheli et al. 2018; Ohmit et al. 2013; Della Bella et al. 2007). In addition to immunological shortcomings, current vaccines are also limited by manufacturing capacity, antigenic changes (i.e., glycosylation) and specificity associated with 70-year old egg-based technologies, and dependence on a cold chain for both storage and distribution (Wu et al. 2017; Zost et al. 2017).
Polymeric nanovaccines present a powerful alternative to current influenza vaccinations. Acting in a dual capacity as adjuvants and delivery systems for diverse payloads (influenza virus antigens such as hemagglutinin, nucleoprotein, neuraminidase, etc. and additional co-adjuvants), polymeric nanovaccines can deliver multiple formulations of vaccines tailored to either specific age groups, or particular patient needs (Ross et al. 2019). These nanovaccines benefit from rapid and large-scale production, delivering a thermostable product that is cost-effective to produce, stockpile, and deploy (Kelly et al. 2020). To this end, polymers composed of polysaccharides, polyesters, and polyanhydrides have been used to formulate nanoparticles to deliver influenza virus antigens (Ross et al. 2015; Dhakal et al. 2017a, b; Renu et al. 2020). These versatile nanovaccines have been successfully delivered intranasally, intramuscularly, and subcutaneously. For respiratory pathogens, such as influenza virus, intranasal immunizations with nanovaccines have been shown to generate local tissue-resident T cell immunity, which leads to protection against heterologous challenge. This is a distinct advantage over current influenza vaccines (Lau et al. 2012), setting the stage for a “universal flu vaccine”. Intranasal vaccinations mimic both the natural site and route of infection, leveraging the natural immune response to protect the body from future infections (Ross et al. 2015).
6.1.2. Respiratory Syncytial Virus
Human respiratory syncytial virus (RSV) infects epithelial cells of the lungs to cause potentially severe upper and lower respiratory infections in newborns, young children, immunosuppressed, and the aged with no approved vaccines available (Gilbert et al. 2018; Tang et al. 2019; Swanson et al. 2020). RSV has multiple surface proteins (attachment glycoprotein (G) and fusion (F) glycoprotein) which are necessary for viral cell attachment and fusion to epithelial cells for successful infection. These proteins are viable vaccine targets due to their necessity for infection and multiple epitopes for neutralization (Swanson et al. 2020). However, these proteins are poorly immunogenic, complicating the formulation of a successful vaccine (McGill et al. 2017). Although the FDA has approved a monoclonal antibody, palivizumab, solely as a preventative treatment reserved for high-risk infants, the use of palivizumab can promote the escape of mutant RSV strains resistant to neutralization (Gilbert et al. 2018).
Multiple studies have shown robust immune response in mice and rats promoted by F protein encapsulated polyanhydride nanoparticles (Gilbert et al. 2018; Stephens et al. 2019, 2020). Other studies have shown the success of co-encapsulating G and F proteins into polyanhydride nanoparticles and administered to neonatal calves to induce protection from bovine respiratory syncytial virus (BRSV) (McGill et al. 2017, 2018). Calves serve as an excellent animal model because BRSV infection in calves mimics RSV infection in humans (McGill et al. 2017). All of these studies attest to the strong potential of RSV antigen-based nanovaccines as a viable approach to protect against RSV infections.
6.1.3. Coronavirus
Coronaviruses are responsible for multiple respiratory infections, including severe acute respiratory syndrome (SARS-CoV), Middle East Respiratory Syndrome (MERS-CoV), and most recently, SARS-CoV-2, which causes coronavirus disease (COVID-19) (Coleman and Frieman 2014; Rothan and Byrareddy 2020). These infections are responsible for severe complications in older adults and the immune-compromised. Coronaviruses are characterized by surface spike proteins responsible for viral entry (Coleman et al. 2014). The spike protein also serves as a promising target for vaccines. Due to the contagious nature of SARS-CoV-2 transmission between humans, there is an immediate need to develop a safe and effective vaccine.
Recent studies evaluating intranasal delivery of SARS-CoV S DNA encapsulated within polyethylimine nanoparticles demonstrated induction of antigen-specific humoral and cellular immune responses in mice (Shim et al. 2010). The DNA-containing nanoparticles induced enhanced production of IgA as well as CD80, CD86, and MHC II expression on DCs that likely contributed to an increase in polyfunctional CD4+ and CD8+ T cells. These studies present a promising avenue to combat coronaviruses, including the development of a COVID-19 nanovaccine.
6.1.4. Pneumonia
Streptococcus pneumoniae is the biological agent that causes pneumococcal pneumonia. Pneumococcal infections are high-risk to both young and aged populations, and among the deadliest in children under the age of five. The capsular polysaccharide of this organism is highly variable, accounting for 98 serotypes, making it difficult to develop a ‘universal’ vaccine (Masomian et al. 2020). Although there currently are three licensed vaccines for pneumococcal pneumonia, namely PPV23, PCV10, and PCV13, their introduction has inadvertently increased disease prevalence caused by strains expressing non-vaccine serotypes. This has led to renewed interest in developing a vaccine that more broadly protects individuals (Masomian et al. 2020). Recent approaches include the pneumococcal surface protein A (PspA) for which there are only three families representing six clades (Piao et al. 2014). Strains expressing family 1 of 2 PspA represent approximately 96% of clinical isolates. For this reason, vaccines incorporating PspA proteins may overcome the challenges posed by the use of capsular vaccines. In this regard, polyanhydride nanoparticles encapsulating PspA induced high titer antigen-specific IgG and demonstrated the ability to protect mice from pneumococcal challenge (Wagner-Muñiz et al. 2018). Compared to PspA alone, this vaccine formulation provided a high level of protection using a 25-fold dose reduction and was shelf-stable for at least 60 days at room temperature. Chitosan nanoparticles have also been used to intranasally deliver DNA encoding pneumococcal PsaA, demonstrating the ability to induce mucosal and systemic antibody responses to PsaA, as well as reduced bacterial burden in the nasopharynx of challenged mice (Xu et al. 2011). Chitosan-based nanoparticles containing PspA have been shown to induce antigen-specific IgA and IgG as well as CMI; following challenge, mice were protected against otitis media and against intraperitoneal challenge with both serotypes 3 and 14 (Xu et al. 2015).
6.2. Biodefense Pathogens
Developing new vaccines to counteract biodefense pathogens is a high priority. Of these pathogens, tier 1 select agents pose the greatest risk to military and civilian populations. These include numerous toxins, viral and bacterial organisms, including Yersinia pestis, Bacillus anthracis, and Ebola virus. Polymeric nanoparticles represent an attractive vaccine platform against these deadly biological agents. Subunit vaccines are ideal biodefense vaccines due to concerns of adverse reactions or incomplete inactivation of dangerous biological organisms. However, subunit antigens are generally poorly immunogenic. Hence, the physiochemical characteristics of nanoparticles enable the design of vaccine formulations that can adjuvant subunit antigens and induce protective immune responses through activating multiple arms of adaptive immunity. The following is a brief overview of nanovaccine development against multiple biodefense pathogens.
6.2.1. Pneumonic Plague
Yersinia pestis is a tier 1 select agent that causes plague, which can manifest as pneumonic, bubonic, and septicemic forms (Riedel 2005). F1-V, a fusion protein of the F1 capsular and the V antigen (part of the type III secretion appendage) of Y. pestis, has been investigated as a subunit vaccine candidate. Our laboratory has previously reported on polyanhydride nanoparticles encapsulating F1-V that induced high avidity IgG1 responses in immunized mice, which persisted over 280 days post-immunization and protected vaccinated mice against lethal challenge (Ulery et al. 2011). We have also reported on a combination adjuvant-based nanovaccine containing F1-V encapsulated into polyanhydride nanoparticles co-adjuvanted with cyclic di-GMP (cdG), which also protected mice against lethal challenge with Y. pestis CO92 in as early as 14 days post-immunization (Wagner et al. 2019).
6.2.2. Anthrax
B. anthracis is a tier 1 select agent that is the causative agent of anthrax. This organism can form durable spores that can infect humans through multiple routes, including inhalation, subcutaneous, and gastrointestinal (WHO 2008). Studies using polyanhydride nanoparticles encapsulating the B. anthracis protective antigen (PA) demonstrated the ability to maintain the structural stability of encapsulated PA (Petersen et al. 2012). The conformational stability of PA is important for generating toxin-neutralizing antibody responses. This stability was maintained for at least four months following storage at temperatures up to 40 °C (Petersen et al. 2012). Additionally, PLGA nanoparticles encapsulating domain IV of PA have been shown to induce a mixed Th1/Th2 immune response as evidenced by the in vitro secretion of IL-1 and IFN-γ (Manish et al. 2013).
6.2.3. Ebola Virus
Ebola virus is a tier 1 select agent that causes Ebola virus disease. This disease is zoonotic, and can be transmitted from person-to-person via direct contact, infected bodily fluids, or contaminated fomites (Jacob et al. 2020). Polymeric nanoparticles have been used as a DNA vaccine adjuvant and delivery platform against Ebola (Yang et al. 2017). Ebola DNA (EboDNA) was coated onto cationic PLGA-poly-L-lysine/poly-γ-glutamic acid nanoparticles and administered to mice using a microneedle patch or through intramuscular injection. The microneedles dissolve within five minutes, allowing the particles to disseminate and induce immune responses, resulting in an enhanced magnitude of neutralizing IgG antibodies to Ebola virus.
6.2.4. Brucella abortus
Brucella abortus is a select agent that causes brucellosis. Its primary host species are cattle, and it is capable of infecting humans as an incidental host, usually through direct contact or consumption of animal products such as non-pasteurized dairy products (De Figueiredo et al. 2015). PLGA nanoparticles have been investigated as a vaccine platform against B. abortus (Singh et al. 2015). Nanoparticles encapsulating rL7/L12 ribosomal protein demonstrated the ability to induce high IgG1 antibody responses as well as secretion of IFN-γ from lymphocytes following ex vivo stimulation. This resulted in protection from bacterial challenge, as demonstrated by decreased bacterial load in the spleen.
6.3. Cancer
Both therapeutic and prophylactic cancer vaccines need immune-stimulatory adjuvants that can break tolerance and induce humoral and cell-mediated immune responses to tumor-associated antigens (TAAs). To overcome the challenges of immune dysfunction associated with the tumor microenvironment, various small molecule adjuvants or nano-adjuvanted therapies have been tested in clinical and preclinical settings. The first examples of FDA-approved or late-stage clinical nanotherapeutic endeavors were liposome-based platforms. The applications of nano-enabled therapies have been pioneered by liposome-based drug delivery systems, as discussed by excellent reviews (Shi et al. 2017; Wicki et al. 2015; Nam et al. 2019). Here, we focus on translational examples of adjuvant molecules and polymeric nanoparticulate systems with the potential for cancer vaccines.
6.3.1. Breast Cancer
Breast cancer is the most common malignancy worldwide. There are many recently concluded or ongoing clinical trials for the application of granulocyte-monocyte colony-stimulating factor (GM-CSF), one of the most widely used cytokine-based adjuvants, in vaccines for breast cancer patients. Many of these efforts are focused on the HER2 antigen that is expressed in about 15–30% of invasive breast cancer. In a series of clinical trials with HER2 derived peptide, the GM-CST adjuvanted vaccine was used as an adjunct therapy to prevent tumor recurrence after the completion of standard-of-care therapies. These vaccines, incorporating HER2 peptides E75, GP2, AE37, augmented CD8+ or CD4+ T lymphocyte responses and demonstrated acceptable safety and tolerability (Clifton et al. 2015). All three vaccines were advanced into phase II or III clinical investigations and revealed increased but not significant disease-free 5-year survival compared to control patients (Mittendorf et al. 2014, 2016a, b).
Nanogel delivery vehicles formulated with adjuvants have been designed to aid HER2 vaccine immunotherapy (Neamtu et al. 2017). The nanogels release polypeptide payload upon swelling in water and induce improved T cell activation via enhanced cellular internalization and cytosolic antigen presentation (Gu et al. 1998). In phase I clinical trial, self-assembled amphiphilic cholesteryl pullulan nanogels encapsulating truncated HER2 antigen were formulated with GM-CSF or OK-432 adjuvant. It was demonstrated that five out of nine patients with various types of solid tumors were characterized with HER2-specific CD8+/CD4+ T cell responses (Kitano et al. 2006). A similar nanogel was used in another phase I clinical trial characterizing humoral immunity. In 14 of the 15 patients studied, HER2-specific IgG antibodies were measurable in patient sera (Kageyama et al. 2008).
More nanovaccine formulations are being tested for breast cancer in preclinical animal models (Allahverdiyev et al. 2018). One example describes the activation of DCs and the induction of tumor-specific CD8+ T cells following administration of CpG-coated PLGA nanoparticles loaded with tumor antigen (Kokate et al. 2016). This functional co-delivery system (i.e., tumor antigen and TLR agonist) was suggested to specifically interact with DCs in a bacteriomimetic manner (Kokate et al. 2015). The multifunctional immunostimulatory capability of these nanoformulations distinguishes them from conventional vaccination.
6.3.2. Lung Cancer
Liposome-based lung cancer vaccines have been advanced into clinical study. Tecemotide (L-BLP25) is a MUC1 glycoprotein immunotherapy liposomal vaccine combined with MPLA that is capable of inducing antigen-specific T cell responses (Mehta et al. 2012). The vaccine formulation was proven to induce a dominant Th1 response and CTL specific to MUC1. Multiple phase II clinical studies were carried out with systemic analysis of disease prognosis from late-stage unresectable non-small lung cancer (NSCLC) patients (Butts et al. 2005, 2010, 2011; Wu et al. 2011). Although phase III trials concluded similar performance after L-BLP25 administration as placebo, in contrast to the mild adverse reactions and improved median survival observed in phase II trials (Butts et al. 2014). Thus, more liposomal vaccine studies are worth pursuing depending upon the stage of cancer relative to the treatment regimen employed. In a recent example using a murine model, hyaluronic acid nanoparticles loaded with microRNA-125b were used to reprogram M2 phenotype MØs in a NSCLC model. These particles were previously shown to target CD44+ MØs and modified to enable negatively charged nucleotide encapsulation (Jain et al. 2015). Nanoparticle accumulation in lung tissue was confirmed. Repolarization of tumor-associated MØs to M1 phenotype was demonstrated by altered surface biomarker expression.
6.3.3. Pancreatic Cancer
Pancreatic cancer (PC) is characterized by a dense stromal barrier and dysregulated immune cells, causing resistance to traditional therapies and immunomodulation. Immunoadjuvant nanoparticulate systems have been applied to counter such barriers and sensitize PC for immunotherapy. Lu et al. prepared a lipid bilayer encapsulating mesoporous silica nanoparticles that co-deliver an immunogenic cell death (ICD) inducing drug and indoleamine 2,3-dioxygenase (IDO) pathway inhibitor to induce anti-tumor immunity (Lu et al. 2017). The nano-delivery formulation was proposed to prolong ICD potency and simultaneously reverse immunosuppression mediated by the IDO signaling pathway. Improved survival of tumor-bearing mice was demonstrated along with increased numbers of tumor-infiltrating immune cells. Shen et al. reported on the design of lipid-protamine nanoparticles loaded with DNA encoding trap proteins for IL-10 and CXCL12, two major proteins that contribute to the tumor immunosuppression (Shen et al. 2018). The perivascular delivery of nanoparticles induced local expression of trap proteins and reversal of immunosuppression, also indicated by increased numbers of tumor-infiltrating DCs, CD8+ T cells, and NK cells. This nanoformulation showed improved survival in a KPC murine model, further underlining the potential of nano-therapies to treat PC.
6.3.4. Brain Cancer
Glioblastoma multiforme (GBM) is the most common and deadly glioma with an incidence rate of 4–5 per 100,000 persons and a dismal 5% five-year survival rate (Ostrom et al. 2014). While surgical removal of the tumor can delay disease progression, residual cancerous cells often result in tumor reoccurrence. Attempts to address these cells with systemically administered chemotherapeutics have been limited by bioavailability across the blood–brain barrier (BBB), short circulation half-life, and toxic off-target effects, leading to brief and irregular dosage at the tumor site (Wait et al. 2015). The Stupp method combines resection, external beam radiation, and systemically administered temozolomine (TMZ), and has become the standard of care for GBM. However, mean survival of patients was about 14 weeks as reported in 2005 (Stupp et al. 2005).
To overcome these limitations, a local sustained release platform was developed using polyanhydride (CPP:SA) wafers loaded with carmustine (Tamargo et al. 1993). This product, known as the GLIADEL® wafer, is placed by the surgeon directly into the tumor bed post-resection. The surface eroding properties of the wafer protect carmustine from degradation and produce a sustained release, while direct placement in the intracerebral cavity circumvents the BBB and avoids systemic, off-target toxicity (Tamargo et al. 1993; Brem et al. 1994). These advantages have borne out in clinical trials where carmustine-loaded wafers increase mean survival of both recurrent and newly diagnosed high-grade glioma patients, either as a monotherapy or in combination therapy (Wait et al. 2015).
While the GLIADEL® wafer is a great example of a polymer-based drug delivery system, it has some limitations. First, the wafer must be placed directly in the brain, limiting its application to immediate use after surgery. To address this, nanoparticles capable of crossing the BBB and delivering carmustine to target locations are necessary (Wadajkar et al. 2017). Second, the GLIADEL® wafer does not enhance the immune response to GBM or contribute to long-term protective immunity. Several nanoformulations target DCs to enhance antigen presentation and CTL activation, leading to successful treatment in glioma animal models. Poly (β-L-malic acid) based nanoscale immunoconjugates (NICs) covalently linked to anti-PD-1 and anti-CLTA-4 monoclonal antibodies have been evaluated for their ability to deliver checkpoint therapy across the BBB. In rats treated with the NICs, there was an increase of CTLs, NK cells, and M1 MØs in the tumor accompanied by a decrease in TREG cells, and an increase in the length of survival (Galstyan et al. 2019). These studies demonstrated the feasibility of delivering nanoscale immunodrugs across the BBB to treat brain tumors and other neurodegenerative diseases.
7. Manufacturing, Scale-up, and Regulatory Considerations
While nanoparticle adjuvants clearly demonstrate immunological and delivery benefits, it is important to consider all aspects of vaccine development for translation from research and development to commercialization. A crucial aspect of vaccine development is the ability to maintain stability and viability over long periods of time and/or variable temperatures. Exposure of vaccines to elevated temperatures may result in denaturation of proteins and loss of potency (Kumru et al. 2014; Lloyd and Cheyne 2017). Similarly, exposure to freezing temperatures can be just as destructive, inducing agglomeration of adjuvants and precipitation (Kumru et al. 2014; Lloyd and Cheyne 2017; Hanson et al. 2017). Thus, the vaccine cold chain, a network of maintaining refrigerated temperatures (2–8 °C) through manufacturing, storage, and distribution, is required for most vaccines.
The vaccine cold chain, while largely successful, is not without its challenges. Power outages, failing, and outdated equipment, as well as transportation delays, may result in exposure to increased temperatures (Chen and Kristensen 2009). Traditionally, vaccine vial monitors indicate storage exceeded elevated temperatures, which consequently leads to vaccine wastage (Chen and Kristensen 2009; Kumru et al. 2014; Lloyd and Cheyne 2017). However, freeze exposure is often less obvious and undetected, despite an estimate of at least 75% of vaccine shipments being exposed to freezing temperatures (Chen and Kristensen 2009). In addition, maintaining the vaccine cold chain is increasingly expensive, accounting for up to 80% of the cost of each dose (Chen and Kristensen 2009; Lee et al. 2017). As such, countries in need of basic vaccines often lack the resources or infrastructure to be able to successfully maintain the cold chain (Chen and Kristensen 2009; Lee et al. 2017).
Recently, researchers have turned their focus to designing thermostable vaccines through the use of particle adjuvants and delivery vehicles. Although liquid formulations have thus far been preferred due to ease of manufacturing and packaging, dry formulations via spray drying are an increasingly appealing option to enhance vaccine thermostability. As an example, Saboo et al. demonstrated that a spray-dried virus-like particle (VLP) vaccine maintained stability and immunogenicity after storage at 37 °C for up to 14 months (Saboo et al. 2016). Similarly, adsorption to or encapsulation of vaccines within polymer matrices may enhance vaccine thermostability. Recently, a vaccine for Ebola virus coated onto PLGA particles was found to maintain stability for six weeks at 37 °C (Yang et al. 2017). Additionally, PLGA particles have been demonstrated to preserve the stability of encapsulated inactivated poliovirus (Tzeng et al. 2016). Furthermore, polyanhydride nanoadjuvants have demonstrated the ability to maintain the stability of multiple subunit vaccine antigens (Carrillo-Conde et al. 2010; Haughney et al. 2013; Ross et al. 2014). In contrast to alum, Petersen et al. showed encapsulation into polyanhydride nanoparticles preserved PA from B. anthracis over a wide range of temperatures (−20 to 40 °C) for up to four months (Petersen et al. 2012). Thus, nanoadjuvants are a promising platform to enhance the thermostability of vaccines and eliminate the vaccine cold chain.
Despite their improved thermostability, commercial manufacturing of nanovaccines includes many challenges. Current vaccine production facilities are highly specific to a single, unique product and new manufacturing facilities would be needed (Hosangadi et al. 2020). Although this may require significant capital upfront, many nanovaccines offer a “plug-and-play” platform approach which lends flexibility to the manufacturing process of different vaccine formulations (Hosangadi et al. 2020). In addition, current dry vaccines utilize lyophilization, a lengthy, high energy, and expensive process which requires reconstitution before administration (Amorij et al. 2008; Clements and Wesselingh 2005; McAdams et al. 2012; Plotkin et al. 2017). High throughput, cost-effective production of nanovaccines could be achieved via spray drying (McAdams et al. 2012; Jin and Tsao 2013). However, unlike the food and pharmaceutical industries, current good manufacturing practices (cGMP) are not yet established for vaccine production (McAdams et al. 2012; Jin and Tsao 2013). Thus, further exploration is needed to translate nanovaccines from laboratory benchtop to commercial scale.
Commonly employed techniques for nanoparticle synthesis include emulsion-based methods, nanoprecipitation, salting out, microfluidics, and spray drying (Paliwal et al. 2014; Sosnik and Seremeta 2015). Benchtop methods such as single and double emulsion methods, as well as nanoprecipitation, have traditionally been widely employed in the design of polymeric nanoparticles encapsulating a wide variety of payloads, however, there are issues with its scalability, including batch-to-batch variability and high cost due to the requirement of large solvent volumes, and extensive drying following synthesis to ensure loss of solvent, all of which can become costly on the material and processing end and require large containers to accommodate such volumes (Martínez Rivas et al. 2017; Truong-Dinh Tran et al. 2016). These limitations, as well as investor skepticism about the feasibility of scaling up emulsion-based methods and nanoprecipitation, have made it difficult to scale-up for commercial use.
As mentioned above, spray drying is an alternative method for nanoparticle synthesis that is gaining interest for large-scale production. This method is used to synthesize polymeric micro- and nanoparticles encapsulating proteins in a variety of polymer chemistries, including the widely used PLGA (Brenza et al. 2015; Arpagaus 2019; Sharma et al. 2015), but also for emulsions and micelles. Spray drying is a method of forming particles through atomization of a liquid containing polymer and protein, either co-dissolved in solution, emulsified, or as a solid/oil suspension. The main advantages of spray drying over conventional polymeric nanoparticle syntheses are that it is rapid, continuous, low energy, easily scalable, has high ease of operation, is versatile to multiple formulations, single-step, and does not require any purification or further processing (Sosnik and Seremeta 2015; Arpagaus et al. 2018). In addition, multiple studies have documented high yields using this process, capable of achieving yields well above 50% (Schmid et al. 2011; Draheim et al. 2015; Li et al. 2010; Shi et al. 2020).
Currently, the ability to spray dry nanoparticles on a commercial scale is limited. For benchtop nanoparticle synthesis, the Buchi Nano Spray Dryer B-90 HP is capable of spray drying particles ≥ 200 nm by atomizing particles via ultrasonic vibrating nozzle and collected via an electrical precipitator (Wisniewski 2015). However, translation of this technology to pilot and eventually commercial scale is challenging as industrial-scale spray dryers of this nature do not currently exist. That being said, microparticle spray drying is a much more developed field of study with technology established to commercial scale. Several options for laboratory benchtop models exist, including the Büchi B-290 Mini Spray Dryer which atomizes particles via a two-fluid nozzle and collects particles with a cyclone. In addition, Büchi Corporation published a report on the scalability from the B-290 Micro Spray Dryer to the pilot plant spray dryer Niro MOBILE (Arpagaus and Schwartzbach 2008). A previous study demonstrated the scalability of influenza virus-derived hemagglutinin microparticle (5–10 μm) formulation from the B-290 to the pilot-scale spray dryer BLD-1 (0.5–30 μm) (Zhu et al. 2014). Therefore, the avenue exists to scale the production of smaller sized microparticles, which is required by the United States Food and Drug Administration (FDA), and there is a great need for research into developing similar pathways for spray dryers that can produce nanoparticles on a pilot-scale and beyond.
It is also important to partner with regulatory agencies to more carefully understand scale-up and manufacturing of nanovaccines. Whereas much effort has been placed toward the research and development of nanovaccines, regulatory guidance and processes for nanoparticles are still evolving. Indeed, any changes in vaccine manufacturing induce new regulatory requirements. Often, even small changes require re-validation of clinical trials before licensing the vaccine product (Plotkin et al. 2017; Hua et al. 2018; Zehrung et al. 2017), demonstrating a need for standardized rapid, in vitro assays that can ensure nanoparticle safety and efficacy (Hua et al. 2018). Similarly, new administration devices will also need to meet regulatory agency requirements and may need different pathways for approval (Zehrung et al. 2017).
Although commercialization of nanovaccines presents some challenges, it is important to realize the full impact nanovaccines may have on preserving health worldwide. In recent years, pandemics have highlighted a need for preparedness. As mentioned, the “plug-and-play” manufacturing of nanovaccines offers flexibility, allowing facilities to be easily modified to meet urgent needs (Hosangadi et al. 2020). Needle-free administration would enable the ability to self-vaccinate without highly trained healthcare professionals, providing a cost-effective method for rapid vaccination (Hosangadi et al. 2020; Jin and Tsao 2013; Zehrung et al. 2017; Lee et al. 2015). As an example, the ability to self-administer an influenza vaccine has been reported to increase the intent to vaccinate by approximately 20%, and reduce societal costs by $2.6 billion US dollars (Lee et al. 2015). Furthermore, the enhanced thermostability of nanovaccines would allow for large, inexpensive stockpiles ready for deployment in critical public health crises, such as pandemics (Hosangadi et al. 2020; Amorij et al. 2008; McAdams et al. 2012; Lee et al. 2015).
8. Conclusions and Perspectives
Nanovaccines represent a promising platform for many of today’s vaccines and for future vaccines. In particular, polymeric adjuvants offer multiple advantages in designing novel vaccines to help counter emerging and re-emerging pathogens (including ones that can cause pandemics), non-communicative diseases such as cancer, and biodefense pathogens in both humans and animals. As described in this review, polymeric nanovaccines can reduce the dose and number of immunizations for many vaccines, provide rapid and long-lived immunity in a single dose, activate humoral and cell-mediated immunity, provide for room temperature storage for long periods of time, and enhanced patient compliance. Many of these advantages also translate very effectively to the design and development of novel vaccines for livestock and companion animal vaccines. With the advent of the data science revolution, new approaches can be developed for rational design of novel nanovaccine formulations using artificial intelligence and data analytics. Challenges that still need to be overcome include translation from lab to clinic, scale-up/manufacturing/costs, and regulatory approvals. Altogether, polymeric nanovaccines represent a platform technology with great promise to enhance public health and pandemic preparedness.
Acknowledgements
The authors acknowledge partial financial support from the National Institutes of Health (R01 AI111466-01, R01 AI127565-01A1, R01 AI141196-01, U01 CA213862-01, and R01 HD095880-01A1) and the Nanovaccine Institute. B.N. acknowledges the Vlasta Klima Balloun Faculty Chair and S.K.M. is grateful to the Carol Vohs Johnson Chair.
Footnotes
Disclosures Balaji Narasimhan and Michael Wannemuehler are co-founders of ImmunoNanoMed Inc., a start-up with business interests in the development of nano-based vaccines against infectious diseases. Narasimhan also has a financial interest in Degimflex LLC. Surya Mallapragada is a co-founder of Degimflex LLC., a start-up with business interests in the development of flexible degradable electronic films for biomedical applications. She also has a financial interest in ImmunoNanoMed Inc.
Contributor Information
Elizabeth A. Grego, Departments of Chemical and Biological Engineering, Iowa State University, Ames, IA 50011, USA
Alaric C. Siddoway, Departments of Chemical and Biological Engineering, Iowa State University, Ames, IA 50011, USA
Metin Uz, Departments of Chemical and Biological Engineering, Iowa State University, Ames, IA 50011, USA; Departments of Nanovaccine Institute, Iowa State University, Ames, IA 50011, USA.
Luman Liu, Departments of Chemical and Biological Engineering, Iowa State University, Ames, IA 50011, USA.
John C. Christiansen, Departments of Veterinary Microbiology and Preventive Medicine, Iowa State University, Ames, IA 50011, USA
Kathleen A. Ross, Departments of Nanovaccine Institute, Iowa State University, Ames, IA 50011, USA
Sean M. Kelly, Departments of Chemical and Biological Engineering, Iowa State University, Ames, IA 50011, USA
Surya K. Mallapragada, Departments of Chemical and Biological Engineering, Iowa State University, Ames, IA 50011, USA; Departments of Nanovaccine Institute, Iowa State University, Ames, IA 50011, USA
Michael J. Wannemuehler, Departments of Veterinary Microbiology and Preventive Medicine, Iowa State University, Ames, IA 50011, USA; Departments of Nanovaccine Institute, Iowa State University, Ames, IA 50011, USA
Balaji Narasimhan, Departments of Chemical and Biological Engineering, Iowa State University, Ames, IA 50011, USA; Departments of Nanovaccine Institute, Iowa State University, Ames, IA 50011, USA.
References
- AbdelAllah NH, Abdeltawab NF, Boseila AA, Amin MA (2016) Chitosan and sodium alginate combinations are alternative, efficient, and safe natural adjuvant systems for hepatitis B vaccine in mouse model. Evidence-Based Complement Altern Med 2016:7659684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AbdelAllah NH, Gaber Y, Rashed ME et al. (2020) Alginate-coated chitosan nanoparticles act as effective adjuvant for hepatitis A vaccine in mice. Int J Biol Macromol 152:904–912 [DOI] [PubMed] [Google Scholar]
- Abetz V (2005) Block copolymers I. Springer-Verlag, Berlin Heidelberg [Google Scholar]
- Adams JR, Goswami M, Pohl NLB, Mallapragada SK (2014) Synthesis and functionalization of virus-mimicking cationic block copolymers with pathogen-associated carbohydrates as potential vaccine adjuvants. RSC Adv 4:15655–15663 [Google Scholar]
- Adams JR, Haughney SL, Mallapragada SK (2015) Effective polymer adjuvants for sustained delivery of protein subunit vaccines. Acta Biomater 14:104–114 [DOI] [PubMed] [Google Scholar]
- Adams JR, Senapati S, Haughney SL et al. (2019) Safety and biocompatibility of injectable vaccine adjuvants composed of thermogelling block copolymer gels. J Biomed Mater Res—Part A 107:1754–1762 [DOI] [PubMed] [Google Scholar]
- Ainai A, Ichinohe T, Tamura S et al. (2006) Zymosan enhances the mucosal adjuvant activity of poly(I:C) in a nasal influenza vaccine. Antivir Ther 55:52–55 [DOI] [PubMed] [Google Scholar]
- Allahverdiyev A, Tari G, Bagirova M, Abamo ES (2018) Current approaches in development of immunotherapeutic vaccines for breast cancer. J Breast Cancer 21:343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahyari M, Mohit E (2016) Peptide/protein vaccine delivery system based on PLGA particles. Hum Vaccines Immunother 12:806–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini Y, Tebianian M, Mosavari N et al. (2017) Development of an effective delivery system for intranasal immunization against Mycobacterium tuberculosis ESAT-6 antigen. Artif Cells, Nanomed Biotechnol 45:291–296 [DOI] [PubMed] [Google Scholar]
- Amorij JP, Huckriede A, Wilschut J et al. (2008) Development of stable influenza vaccine powder formulations: challenges and possibilities. Pharm Res 25:1256–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato F, Romagnani C, Romagnani S (2015) The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol 135:626–635 [DOI] [PubMed] [Google Scholar]
- Aoshi T (2017) Modes of action for mucosal vaccine adjuvants. Viral Immunol 30:463–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolopoulos V, Thalhammer T, Tzakos AG, Stojanovska L (2013) Targeting antigens to dendritic cell receptors for vaccine development. J Drug Deliv 2013:1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpagaus C (2019) PLA/PLGA nanoparticles prepared by nano spray drying. J Pharm Investig 49:405–426 [Google Scholar]
- Arpagaus C, Schwartzbach H (2008) Scale-up from bench-top research to laboratory production. Büschi Inf Bull 8 [Google Scholar]
- Arpagaus C, Collenberg A, Rütti D et al. (2018) Nano spray drying for encapsulation of pharmaceuticals. Int J Pharm 546:194–214 [DOI] [PubMed] [Google Scholar]
- Awate S, Babiuk LA, Mutwiri G (2013) Mechanisms of action of adjuvants. Front Immunol 4:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelder EM, Beaudette TT, Broaders KE et al. (2010) In vitro analysis of acetalated dextran microparticles as a potent delivery platform for vaccine adjuvants. Mol Pharm 7:826–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelder EM, Pino EN, Ainslie KM (2017) Acetalated dextran: a tunable and acid-labile biopolymer with facile synthesis and a range of applications. Chem Rev 117:1915–1926 [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Jennings GT (2010) Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 10:787–796 [DOI] [PubMed] [Google Scholar]
- Bagnoli F, Fontana MR, Soldaini E et al. (2015) Vaccine composition formulated with a novel TLR7-dependent adjuvant induces high and broad protection against Staphylococcus aureus. Proc Natl Acad Sci U S A 112:3680–3685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Qi J, Gogoi R et al. (2016) Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Control Release 238:176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay TG, Rajapaksha H, Thilagam A et al. (2016) Physical characterization and in silico modeling of inulin polymer conformation during vaccine adjuvant particle formation. Carbohydr Polym 143:108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett S, Eichenberger RM, Nevagi RJ et al. (2020) Lipopeptide-based oral vaccine against hookworm infection. J Infect Dis 221:934–942 [DOI] [PubMed] [Google Scholar]
- Bastings MMC, Anastassacos FM, Ponnuswamy N et al. (2018) Modulation of the cellular uptake of DNA origami through control over mass and shape. Nano Lett 18:3557–3564 [DOI] [PubMed] [Google Scholar]
- Bento D, Jesus S, Lebre F et al. (2019) Chitosan plus compound 48/80: formulation and preliminary evaluation as a hepatitis B vaccine adjuvant. Pharmaceutics 11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershteyn A, Hanson MC, Crespo MP et al. (2012) Robust IgG responses to nanograms of antigen using a biomimetic lipid-coated particle vaccine. J Control Release 157:354–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj P, Bhatia E, Sharma S et al. (2020) Advancements in prophylactic and therapeutic nanovaccines. Acta Biomater 108:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bila H, Kurisinkal EE, Bastings MMC (2019) Engineering a stable future for DNA-origami as a biomaterial. Biomater Sci 7:532–541 [DOI] [PubMed] [Google Scholar]
- Bin Lee H, Yoon SY, Singh B et al. (2018) Oral immunization of FMDV vaccine using pH-sensitive and mucoadhesive thiolated cellulose acetate phthalate microparticles. Tissue Eng Regen Med 15:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose RJC, Kim M, Chang JH et al. (2019) Biodegradable polymers for modern vaccine development. J Ind Eng Chem 77:12–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem H, Tamargo RJ, Olivi A et al. (1994) Biodegradable polymers for controlled delivery of chemotherapy with and without radiation therapy in the monkey brain. J Neurosurg 80:283–290 [DOI] [PubMed] [Google Scholar]
- Brenza TM, Petersen LK, Zhang Y et al. (2015) Pulmonary biodistribution and cellular uptake of intranasally administered monodisperse particles. Pharm Res 32:1368–1382 [DOI] [PubMed] [Google Scholar]
- Broos S, Lundberg K, Akagi T et al. (2010) Immunomodulatory nanoparticles as adjuvants and allergen-delivery system to human dendritic cells: implications for specific immunotherapy. Vaccine 28:5075–5085 [DOI] [PubMed] [Google Scholar]
- Bussio JI, Molina-Perea C, González-Aramundiz JV (2019) Hyaluronic acid nanocapsules as a platform for needle-free vaccination. Pharmaceutics 11:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts C, Murray N, Maksymiuk A et al. (2005) Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol 23:6674–6681 [DOI] [PubMed] [Google Scholar]
- Butts C, Murray RN, Smith C et al. (2010) A multicenter open-label study to assess the safety of a new formulation of BLP25 liposome vaccine in patients with unresectable stage III non-small-cell lung cancer. Clin Lung Cancer 11:391–395 [DOI] [PubMed] [Google Scholar]
- Butts C, Maksymiuk A, Goss G et al. (2011) Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): Phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol 137:1337–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts C, Socinski MA, Mitchell PL et al. (2014) Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 15:59–68 [DOI] [PubMed] [Google Scholar]
- Carrillo-Conde B, Schiltz E, Yu J et al. (2010) Encapsulation into amphiphilic polyanhydride microparticles stabilizes Yersinia pestis antigens. Acta Biomater 6:3110–3119 [DOI] [PubMed] [Google Scholar]
- Carrillo-Conde B, Song EH, Chavez-Santoscoy A et al. (2011) Mannose-functionalized “Pathogen-like” polyanhydride nanoparticles target C-type lectin receptors on dendritic cells. Mol Pharm 8:1877–1886 [DOI] [PubMed] [Google Scholar]
- Carrillo-Conde BR, Roychoudhury R, Chavez-Santoscoy AV et al. (2012) High-throughput synthesis of carbohydrates and functionalization of polyanhydride nanoparticles. J Vis Exp. 10.3791/3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan J, Ilves M, Jarventaus H et al. (2010) Genotoxic and immunotoxic effects of cellulose nanocrystals in vitro. Environ Mol Mutagen 405:391–405 [DOI] [PubMed] [Google Scholar]
- Chavez-Santoscoy AV, Roychoudhury R, Pohl NLB et al. (2012) Tailoring the immune response by targeting C-type lectin receptors on alveolar macrophages using “pathogen-like” amphiphilic polyanhydride nanoparticles. Biomaterials 33:4762–4772 [DOI] [PubMed] [Google Scholar]
- Chen D, Kristensen D (2009) Opportunities and challenges of developing thermostable vaccines. Expert Rev Vaccines 8:547–557 [DOI] [PubMed] [Google Scholar]
- Chen CH, Lin YL, Liu YK et al. (2012) Liposome-based polymer complex as a novel adjuvant: enhancement of specifc antibody production and isotype switch. Int J Nanomed 7:607–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liu Y, Wang L et al. (2014) Enhanced humoral and cell-mediated immune responses generated by cationic polymer-coated PLA microspheres with adsorbed HBsAg. Mol Pharm 11:1772–1784 [DOI] [PubMed] [Google Scholar]
- Chen N, Johnson MM, Collier MA et al. (2018a) Tunable degradation of acetalated dextran microparticles enables controlled vaccine adjuvant and antigen delivery to modulate adaptive immune responses. J Control Release 273:147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Gallovic MD, Tiet P et al. (2018b) Investigation of tunable acetalated dextran microparticle platform to optimize M2e-based influenza vaccine efficacy. J Control Release 289:114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements CJ, Wesselingh SL (2005) Vaccine presentations and delivery technologies—what does the future hold? Expert Rev Vaccines 4:281–287 [DOI] [PubMed] [Google Scholar]
- Clifton GT, Mittendorf EA, Peoples GE (2015) Adjuvant HER2/neu peptide cancer vaccines in breast cancer. Immunotherapy 7:1159–1168 [DOI] [PubMed] [Google Scholar]
- Coffman RL, Sher A, Seder RA (2010) Vaccine adjuvants: putting innate immunity to work. Immunity 33:492–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CM, Frieman MB (2014) Coronaviruses: important emerging human pathogens. J Virol 88:5209–5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CM, Liu YV, Mu H et al. (2014) Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine 32:3169–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čolić M, Mihajlović D, Mathew A et al. (2015) Cytocompatibility and immunomodulatory properties of wood based nanofibrillated cellulose. Cellulose 22:763–778 [Google Scholar]
- Collier MA, Junkins RD, Gallovic MD et al. (2018) Acetalated dextran microparticles for codelivery of STING and TLR7/8 agonists. Mol Pharm 15:4933–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PD, Petrovsky N (2011) Delta inulin: a novel, immunologically active, stable packing structure comprising β-D-[2 → 1] poly(fructo-furanosyl) αd-glucose polymers. Glycobiology 21:595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PD, Barclay TG, Ginic-Markovic M, Petrovsky N (2013) The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology 23:1164–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PD, Rajapaksha KH, Barclay TG et al. (2015) Inulin crystal initiation via a glucose-fructose cross-link of adjacent polymer chains: atomic force microscopy and static molecular modelling. Carbohydr Polym 117:964–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhier F, Ansorena E, Silva JM et al. (2012) PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161:505–522 [DOI] [PubMed] [Google Scholar]
- De Figueiredo P, Ficht TA, Rice-Ficht A et al. (2015) Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. Am J Pathol 185:1505–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geest BG, Willart MA, Lambrecht BN et al. (2012) Surface-engineered polyelectrolyte multilayer capsules: synthetic vaccines mimicking microbial structure and function. Angew Chemie—Int Ed 51:3862–3866 [DOI] [PubMed] [Google Scholar]
- De Titta A, Ballester M, Julier Z et al. (2013) Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc Natl Acad Sci U S A 110:19902–19907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan S, Kheiri MT, Abnous K et al. (2018) Preparation, characterization and immunological evaluation of alginate nanoparticles loaded with whole inactivated influenza virus: dry powder formulation for nasal immunization in rabbits. Microb Pathog 115:74–85 [DOI] [PubMed] [Google Scholar]
- Della Bella S, Bierti L, Presicce P et al. (2007) Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol 122:220–228 [DOI] [PubMed] [Google Scholar]
- Demento SL, Cui W, Criscione JM et al. (2012) Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 33:4957–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demicheli V, Jefferson T, Ferroni E, et al. (2018) Vaccines for preventing influenza in healthy adults (Review)—summary of findings for the main comparison. Cochrane Database Syst Rev 1–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Determan AS, Wilson JH, Kipper MJ et al. (2006) Protein stability in the presence of polymer degradation products: consequences for controlled release formulations. Biomaterials 27:3312–3320 [DOI] [PubMed] [Google Scholar]
- Dhakal S, Goodman J, Bondra K et al. (2017a) Polyanhydride nanovaccine against swine influenza virus in pigs. Vaccine 35:1124–1131 [DOI] [PubMed] [Google Scholar]
- Dhakal S, Hiremath J, Bondra K et al. (2017b) Biodegradable nanoparticle delivery of inactivated swine influenza virus vaccine provides heterologous cell-mediated immune response in pigs. J Control Release 247:194–205 [DOI] [PubMed] [Google Scholar]
- Dhakal S, Ghimire S, Renu S et al. (2019) Evaluation of CpG-ODN-adjuvanted polyanhydride-based intranasal influenza nanovaccine in pigs. Vet Microbiol 237:108401. [DOI] [PubMed] [Google Scholar]
- Di Cola E, Cantu L, Brocca P et al. (2019) Novel O/W nanoemulsions for nasal administration: structural hints in the selection of performing vehicles with enhanced mucopenetration. Colloids Surf B Biointerfaces 183:110439. [DOI] [PubMed] [Google Scholar]
- Didierlaurent AM, Laupèze B, Di Pasquale A et al. (2017) Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines 16:55–63 [DOI] [PubMed] [Google Scholar]
- Draheim C, de Crécy F, Hansen S et al. (2015) A design of experiment study of nanoprecipitation and nano spray drying as processes to prepare PLGA nano- and microparticles with defined sizes and size distributions. Pharm Res 32:2609–2624 [DOI] [PubMed] [Google Scholar]
- Ekici S, Ilgin P, Butun S, Sahiner N (2011) Hyaluronic acid hydrogel particles with tunable charges as potential drug delivery devices. Carbohydr Polym 84:1306–1313 [Google Scholar]
- El-Sissi AF, Mohamed FH, Danial NM et al. (2020) Chitosan and chitosan nanoparticles as adjuvant in local Rift Valley Fever inactivated vaccine. 3 Biotech 10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciolà A, Visalli G, Laganà P et al. (2019) The new era of vaccines: the “nanovaccinology”. Eur Rev Med Pharmacol Sci 23:7163–7182 [DOI] [PubMed] [Google Scholar]
- Fan Y, Sahdev P, Ochyl LJ et al. (2015) Cationic liposome-hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. J Control Release 208:121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadian A, Dounighi NM, Avadi M (2015) Enteric trimethyl chitosan nanoparticles containing hepatitis B surface antigen for oral delivery. Hum Vaccines Immunother 11:2811–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SA, Gama FM, Vilanova M (2013) Polymeric nanogels as vaccine delivery systems. Nanomed Nanotechnol Biol Med 9:159–173 [DOI] [PubMed] [Google Scholar]
- Flach TL, Ng G, Hari A et al. (2011) Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med 17:479–487 [DOI] [PubMed] [Google Scholar]
- Franchi L, Núñez G (2008) The Nlrp3 inflammasome is critical for aluminum hydroxide-mediated IL-1β secretion but dispensable for adjuvant activity. Eur J Immunol 38:2085–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galstyan A, Markman JL, Shatalova ES et al. (2019) Blood–brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat Commun 10:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlapati S, Facci M, Polewicz M et al. (2009) Strategies to link innate and adaptive immunity when designing vaccine adjuvants. Vet Immunol Immunopathol 128:184–191 [DOI] [PubMed] [Google Scholar]
- Ge F, Zhu L, Yang L et al. (2018) The soluble and particulate form of alginates positively regulate immune response. Iran J Immunol 15:228–238 [DOI] [PubMed] [Google Scholar]
- Gilbert BE, Patel N, Lu H et al. (2018) Respiratory syncytial virus fusion nanoparticle vaccine immune responses target multiple neutralizing epitopes that contribute to protection against wild-type and palivizumab-resistant mutant virus challenge. Vaccine 36:8069–8078 [DOI] [PubMed] [Google Scholar]
- Goodman JT, Ramirez JEVV, Boggiatto PM et al. (2014) Nanoparticle chemistry and functionalization differentially regulates dendritic cell-nanoparticle interactions and triggers dendritic cell maturation. Part Part Syst Charact 31:1269–1280 [Google Scholar]
- Gratton SEA, Ropp PA, Pohlhaus PD et al. (2008) The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA 105:11613–11618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu XG, Schmitt M, Hiasa A et al. (1998) A novel hydrophobized polysaccharide/oncoprotein complex vaccine induces in vitro and in vivo cellular and humoral immune responses against HER2- expressing murine sarcomas. Cancer Res 58:3385–3390 [PubMed] [Google Scholar]
- Gu P, Wusiman A, Zhang Y et al. (2019) Rational design of PLGA nanoparticle vaccine delivery systems to improve immune responses. Mol Pharm 16:5000–5012 [DOI] [PubMed] [Google Scholar]
- Guo SH, Fu DW, Utupova A et al. (2019) Applications of polymer-based nanoparticles in vaccine field. Nanotechnol Rev 8:143–155 [Google Scholar]
- Gupta RK, Rost BE, Relyveld E, Siber GR (1995) Adjuvant properties of aluminum and calcium compounds. Pharm Biotechnol 6:229–248 [DOI] [PubMed] [Google Scholar]
- Hamdy S, Elamanchili P, Alshamsan A et al. (2007) Responses by codelivery of ovalbumin and toll-like receptor ligand monophosphoryl lipid A in poly (D, L-lactic-co-glycolic acid) nanoparticles. J Biomed Mater Res A 81:652–662 [DOI] [PubMed] [Google Scholar]
- Han J, Zhao D, Li D et al. (2018) Polymer-based nanomaterials and applications for vaccines and drugs. Polymers (Basel) 10:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson CM, George AM, Sawadogo A, Schreiber B (2017) Is freezing in the vaccine cold chain an ongoing issue? A literature review. Vaccine 35:2127–2133 [DOI] [PubMed] [Google Scholar]
- Haryono A, Salsabila K, Restu WK et al. (2017) Effect of chitosan and liposome nanoparticles as adjuvant codelivery on the immunoglobulin G subclass distribution in a mouse model. J Immunol Res 2017:9125048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughney SL, Petersen LK, Schoofs AD et al. (2013) Retention of structure, antigenicity, and biological function of pneumococcal surface protein A (PspA) released from polyanhydride nanoparticles. Acta Biomater 9:8262–8271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughney SL, Ross KA, Boggiatto PM et al. (2014) Effect of nanovaccine chemistry on humoral immune response kinetics and maturation. Nanoscale 6:13770–13778 [DOI] [PubMed] [Google Scholar]
- He Y, Park K (2016) Effects of the microparticle shape on cellular uptake. Mol Pharm 13:2164–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highton AJ, Kojarunchitt T, Girardin A et al. (2015) Chitosan hydrogel vaccine generates protective CD8 T cell memory against mouse melanoma. Immunol Cell Biol 93:634–640 [DOI] [PubMed] [Google Scholar]
- Ho AWS, Prabhu N, Betts RJ et al. (2011) Lung CD103 + dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC Class I for presentation to CD8 T cells. J Immunol 187:6011–6021 [DOI] [PubMed] [Google Scholar]
- Hong F, Zhang F, Liu Y, Yan H (2017) DNA origami: Scaffolds for creating higher order structures. Chem Rev 117:12584–12640 [DOI] [PubMed] [Google Scholar]
- Hosangadi D, Warmbrod KL, Martin EK et al. (2020) Enabling emergency mass vaccination: innovations in manufacturing and administration during a pandemic. Vaccine 38:4167–4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S, de Matos MBC, Metselaar JM, Storm G (2018) Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front Pharmacol 9:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntimer L, Wilson Welder JH, Ross K, et al. (2013) Single immunization with a suboptimal antigen dose encapsulated into polyanhydride microparticles promotes high titer and avid antibody responses. J Biomed Mater Res—Part B Appl Biomater 101 B:91–98 [DOI] [PubMed] [Google Scholar]
- Huntimer L, Ramer-Tait AE, Petersen LK et al. (2013b) Evaluation of biocompatibility and administration site reactogenicity of polyanhydride-particle-based platform for vaccine delivery. Adv Healthc Mater 2:369–378 [DOI] [PubMed] [Google Scholar]
- Huntimer LM, Ross KA, Darling RJ et al. (2014) Polyanhydride nanovaccine platform enhances antigen-specific cytotoxic T cell responses. Technology 2:171–175 [Google Scholar]
- Igietseme JU, Eko FO, He Q, Black CM (2004) Antibody regulation of T-cell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev Vaccines 3:23–34 [DOI] [PubMed] [Google Scholar]
- Ikeda M, Akagi T, Yasuoka T et al. (2018) Characterization and analytical development for amphiphilic poly(γ-glutamic acid) as raw material of nanoparticle adjuvants. J Pharm Biomed Anal 150:460–468 [DOI] [PubMed] [Google Scholar]
- Isaka M, Komiya T, Takahashi M et al. (2004) Recombinant cholera toxin B subunit (rCTB) as a mucosal adjuvant enhances induction of diphtheria and tetanus antitoxin antibodies in mice by intranasal administration with diphtheria-pertussis-tetanus (DPT) combination vaccine. Vaccine 22:3061–3068 [DOI] [PubMed] [Google Scholar]
- Ishii-Mizuno Y, Umeki Y, Onuki Y et al. (2017) Improved sustained release of antigen from immunostimulatory DNA hydrogel by electrostatic interaction with chitosan. Int J Pharm 516:392–400 [DOI] [PubMed] [Google Scholar]
- Jacob ST, Crozier I, Fischer WA et al. (2020) Ebola virus disease. Nat Rev Dis Prim 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahantigh D, Saadati M, Fasihi Ramandi M et al. (2014) Novel intranasal vaccine delivery system by chitosan nanofibrous membrane containing N-terminal region of IpaD antigen as a nasal Shigellosis vaccine, studies in Guinea pigs. J Drug Deliv Sci Technol 24:33–39 [Google Scholar]
- Jain S, Tran TH, Amiji M (2015) Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials 61:162–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalah R, Patel V, Kulkarni V et al. (2012) IL-12 DNA as molecular vaccine adjuvant increases the cytotoxic T cell responses and breadth of humoral immune responses in SIV DNA vaccinated macaques. Hum Vaccines Immunother 8:1620–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesus S, Soares E, Borchard G, Borges O (2018) Adjuvant activity of poly-ϵ-caprolactone/chitosan nanoparticles characterized by mast cell activation and IFN-γ and IL-17 production. Mol Pharm 15:72–82 [DOI] [PubMed] [Google Scholar]
- Jin T, Tsao E (2013) Powder vaccines for pulmonary delivery. In: Flower D, Perrie Y (eds) Immunomic discovery of adjvuants and candidate subunit vaccines. Springer Science + Business Media, New York [Google Scholar]
- Jindal AB (2017) The effect of particle shape on cellular interaction and drug delivery applications of micro- and nanoparticles. Int J Pharm 532:450–465 [DOI] [PubMed] [Google Scholar]
- Jones CH, Chen M, Ravikrishnan A et al. (2015) Mannosylated poly(beta-amino esters) for targeted antigen presenting cell immune modulation. Biomaterials 37:333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi VB, Geary SM, Salem AK (2013) Biodegradable particles as vaccine delivery systems: size matters. AAPS J 15:85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junkins RD, Gallovic MD, Johnson BM et al. (2018) A robust microparticle platform for a STING-targeted adjuvant that enhances both humoral and cellular immunity during vaccination. J Control Release 270:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama S, Kitano S, Hirayama M et al. (2008) Humoral immune responses in patients vaccinated with 1-146 HER2 protein complexed with cholesteryl pullulan nanogel. Cancer Sci 99:601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang I, Hong MS, Nolasco H et al. (2004) Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol 173:673–681 [DOI] [PubMed] [Google Scholar]
- Kasturi SP, Skountzou I, Albrecht RA et al. (2011) Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470:543–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SM, Mitra A, Mathur S, Narasimhan B (2020) Synthesis and characterization of rapidly degrading polyanhydrides as vaccine adjuvants. ACS Biomater Sci Eng 6:265–276 [DOI] [PubMed] [Google Scholar]
- Kensil CR, Kammer R (2010) Expert opinion on investigational drugs: foreword. Expert Opin Investig Drugs 19:1475–1482 [DOI] [PubMed] [Google Scholar]
- Kim MG, Park JY, Shon Y et al. (2014) Nanotechnology and vaccine development. Asian J Pharm Sci 9:227–235 [Google Scholar]
- Kipper MJ, Shen E, Determan A, Narasimhan B (2002) Design of an injectable system based on bioerodible polyanhydride microspheres for sustained drug delivery. Biomaterials 23:4405–4412 [DOI] [PubMed] [Google Scholar]
- Kitano S, Kageyama S, Nagata Y et al. (2006) HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin Cancer Res 12:7397–7405 [DOI] [PubMed] [Google Scholar]
- Klasse PJ (2014) Neutralization of virus infectivity by antibodies: old problems in new perspectives. Adv Biol 2014:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokate RA, Thamake SI, Chaudhary P et al. (2015) Enhancement of anti-tumor effect of particulate vaccine delivery system by “bacteriomimetic” CpG functionalization of poly-lactic-co-glycolic acid nanoparticles. Nanomedicine 10:915–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokate RA, Chaudhary P, Sun X et al. (2016) Rationalizing the use of functionalized poly-lactic-co-glycolic acid nanoparticles for dendritic cell-based targeted anticancer therapy. Nanomedicine 11:479–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong WH, Sung DK, Kim H et al. (2016) Self-adjuvanted hyaluronate—antigenic peptide conjugate for transdermal treatment of muscular dystrophy. Biomaterials 81:93–103 [DOI] [PubMed] [Google Scholar]
- Kool M, Soullié T, Van Nimwegen M et al. (2008) Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med 205:869–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Tummala H (2013) Development of soluble inulin microparticles as a potent and safe vaccine adjuvant and delivery system. Mol Pharm 10:1845–1853 [DOI] [PubMed] [Google Scholar]
- Kumar S, Kesharwani SS, Kuppast B et al. (2016) Discovery of inulin acetate as a novel immune-active polymer and vaccine adjuvant: synthesis, material characterization, and biological evaluation as a toll-like receptor-4 agonist. J Mater Chem B 4:7950–7960 [DOI] [PubMed] [Google Scholar]
- Kumar S, Kesharwani SS, Kuppast B et al. (2017) Pathogen-mimicking vaccine delivery system designed with a bioactive polymer (inulin acetate) for robust humoral and cellular immune responses. J Control Release 261:263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A, Yadav SK, Yadav SC (2010) Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces 75:1–18 [DOI] [PubMed] [Google Scholar]
- Kumru OS, Joshi SB, Smith DE et al. (2014) Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals 42:237–259 [DOI] [PubMed] [Google Scholar]
- Lampe AT, Farris EJ, Ballweg MD et al. (2019) Non-complexed chitosan acts as an adjuvant in an influenza A virus protein vaccine. J Immunol 202(139):14 [Google Scholar]
- Lau Y-F, Wright AR, Subbarao K (2012) The contribution of systemic and pulmonary immune effectors to vaccine-induced protection from H5N1 influenza virus infection. J Virol 86:5089–5098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne MV, Castro M, Andino J, Manghi M (2004) Alternative diphtheria, tetanus and whooping cough immunization schedule to evoke a Th2 tetanus and a Th1 pertussis immune response. Microbes Infect 6:481–484 [DOI] [PubMed] [Google Scholar]
- Lee BY, Bartsch SM, Mvundura M et al. (2015) An economic model assessing the value of microneedle patch delivery of the seasonal influenza vaccine. Vaccine 33:4727–4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BY, Wedlock PT, Haidari LA et al. (2017) Economic impact of thermostable vaccines. Vaccine 35:3135–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leleux J, Roy K (2013) Micro and nanoparticle-based delivery systems for vaccine immunotherapy: an immunological and materials perspective. Adv Healthc Mater 2:72–94 [DOI] [PubMed] [Google Scholar]
- Leroux-Roels G (2010) Unmet needs in modern vaccinology. Adjuvants to improve the immune response. Vaccine 28:C25–C36 [DOI] [PubMed] [Google Scholar]
- Li X, Anton N, Arpagaus C et al. (2010) Nanoparticles by spray drying using innovative new technology: the Büchi nano spray dryer B-90. J Control Release 147:304–310 [DOI] [PubMed] [Google Scholar]
- Li P, Luo Z, Liu P et al. (2013) Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. J Control Release 168:271–279 [DOI] [PubMed] [Google Scholar]
- Li P, Asokanathan C, Liu F et al. (2016) PLGA nano/micro particles encapsulated with pertussis toxoid (PTd) enhances Th1/Th17 immune response in a murine model. Int J Pharm 513:183–190 [DOI] [PubMed] [Google Scholar]
- Liu Q, Chen X, Jia J et al. (2015) PH-Responsive poly(D, L-lactic-co-glycolic acid) nanoparticles with rapid antigen release behavior promote immune response. ACS Nano 9:4925–4938 [DOI] [PubMed] [Google Scholar]
- Liu C, Chu X, Yan M et al. (2018) Encapsulation of Poly I: C and the natural phosphodiester CpG ODN enhanced the efficacy of a hyaluronic acid-modified cationic lipid-PLGA hybrid nanoparticle vaccine in TC-1-grafted tumors. Int J Pharm 553:327–337 [DOI] [PubMed] [Google Scholar]
- Lloyd J, Cheyne J (2017) The origins of the vaccine cold chain and a glimpse of the future. Vaccine 35:2115–2120 [DOI] [PubMed] [Google Scholar]
- Locatelli L, Cadamuro M, Spirli C et al. (2017) A STING-activating nanovaccine for cancer immunotherapy. Hepatology 63:965–982 [Google Scholar]
- Lopac SK, Torres MP, Wilson-Welder JH et al. (2009) Effect of polymer chemistry and fabrication method on protein release and stability from polyanhydride microspheres. J Biomed Mater Res—Part B Appl Biomater 91:938–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Liu X, Liao YP et al. (2017) Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat Commun 8:1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Wang H, Wang Z et al. (2017) A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol 12:648–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan ICM, Toellner K-M, Cunningham AF et al. (2003) Extrafollicular antibody responses. Immunol Rev 194:8–18 [DOI] [PubMed] [Google Scholar]
- Magnusson SE, Reimer JM, Karlsson KH et al. (2013) Immune enhancing properties of the novel Matrix-M™ adjuvant leads to potentiated immune responses to an influenza vaccine in mice. Vaccine 31:1725–1733 [DOI] [PubMed] [Google Scholar]
- Malik A, Gupta M, Gupta V et al. (2018) Novel application of trimethyl chitosan as an adjuvant in vaccine delivery. Int J Nanomedicine 13:7959–7970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manish M, Rahi A, Kaur M et al. (2013) A single-dose PLGA encapsulated protective antigen domain 4 nanoformulation protects mice against Bacillus anthracis spore challenge. PLoS ONE 8:e61885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann AJ, Noulin N, Catchpole A et al. (2014) Intranasal H5N1 vaccines, adjuvanted with chitosan derivatives, protect ferrets against highly pathogenic influenza intranasal and intratracheal challenge. PLoS ONE 9:e93761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez Rivas CJ, Tarhini M, Badri W et al. (2017) Nanoprecipitation process: from encapsulation to drug delivery. Int J Pharm 532:66–81 [DOI] [PubMed] [Google Scholar]
- Masomian M, Ahmad Z, Gew LT (2020) Development of next generation Streptococcus pneumoniae vaccines conferring broad protection. Vaccines 8:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams D, Chen D, Kristensen D (2012) Spray drying and vaccine stabilization. Expert Rev Vaccines 11:1211–1219 [DOI] [PubMed] [Google Scholar]
- McComb RC, Martchenko M (2016) Neutralizing antibody and functional mapping of Bacillus anthracis protective antigen—the first step toward a rationally designed anthrax vaccine. Vaccine 34:13–19 [DOI] [PubMed] [Google Scholar]
- McGill JL, Kelly S, Narasimhan B, et al. (2017) Efficacy and immunogenicity of a novel polyanhydride nanovaccine for use against RSV infection in the neonate. J Immunol 198:225.4 LP-225.4 [Google Scholar]
- McGill JLL, Kelly SMM, Kumar P et al. (2018) Efficacy of mucosal polyanhydride nanovaccine against respiratory syncytial virus infection in the neonatal calf. Sci Rep 8:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeela EA, Mills KHG (2001) Manipulating the immune system: humoral versus cell-mediated immunity. Adv Drug Deliv Rev 51:43–54 [DOI] [PubMed] [Google Scholar]
- Mehta NR, Wurz GT, Burich RA et al. (2012) L-BLP25 vaccine plus letrozole induces a T H1 immune response and has additive antitumor activity in MUC1-expressing mammary tumors in mice. Clin Cancer Res 18:2861–2871 [DOI] [PubMed] [Google Scholar]
- Mittendorf EA, Clifton GT, Holmes JP et al. (2014) Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol 25:1735–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorf EA, Ardavanis A, Symanowski J et al. (2016a) Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann Oncol 27:1241–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorf EA, Ardavanis A, Litton JK et al. (2016b) Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget 7:66192–66201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkrtichyan M, Ghochikyan A, Movsesyan N et al. (2011) Immunostimulant adjuvant patch enhances humoral and cellular immune responses to DNA immunization. Bone 23:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri K, Nishikawa M, Takahashi Y, Takakura Y (2014) DNA nanotechnology-based development of delivery systems for bioactive compounds. Eur J Pharm Sci 58:26–33 [DOI] [PubMed] [Google Scholar]
- Mohri K, Kusuki E, Ohtsuki S et al. (2015) Self-assembling DNA dendrimer for effective delivery of immunostimulatory CpG DNA to immune cells. Biomacromol 16:1095–1101 [DOI] [PubMed] [Google Scholar]
- Montomoli E, Piccirella S, Khadang B et al. (2011) Current adjuvants and new perspectives in vaccine formulation. Expert Rev Vaccines 10:1053–1061 [DOI] [PubMed] [Google Scholar]
- Moon S, Shin EC, Noh YW, Lim YT (2015) Evaluation of hyaluronic acid-based combination adjuvant containing monophosphoryl lipid A and aluminum salt for hepatitis B vaccine. Vaccine 33:4762–4769 [DOI] [PubMed] [Google Scholar]
- Moran HBT, Turley JL, Andersson M, Lavelle EC (2018) Immunomodulatory properties of chitosan polymers. Biomaterials 184:1–9 [DOI] [PubMed] [Google Scholar]
- Moreno-Mendieta S, Guillén D, Hernández-Pando R et al. (2017) Potential of glucans as vaccine adjuvants: a review of the α-glucans case. Carbohydr Polym 165:103–114 [DOI] [PubMed] [Google Scholar]
- Mosafer J, Sabbaghi AH, Badiee A et al. (2019) Preparation, characterization and in vivo evaluation of alginate-coated chitosan and trimethylchitosan nanoparticles loaded with PR8 influenza virus for nasal immunization. Asian J Pharm Sci 14:216–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano DF, Goldsmith M, Solfiell DJ et al. (2012) Nanoparticle hydrophobicity dictates immune response. J Am Chem Soc 134:3965–3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruganandah V, Sathkumara HD, Navarro S, Kupz A (2018) A systematic review: the role of resident memory T cells in infectious diseases and their relevance for vaccine development. Front Immunol 9:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam J, Son S, Park KS et al. (2019) Cancer nanomedicine for combination cancer immunotherapy. Nat Rev Mater 4:398–414 [Google Scholar]
- Narasimhan B, Goodman JT, Vela Ramirez JE (2016) Rational design of targeted next-generation carriers for drug and vaccine delivery. Annu Rev Biomed Eng 18:25–49 [DOI] [PubMed] [Google Scholar]
- Nashchekina YA, Raydan M (2018) Noninvasive penetration of 5 nm hyaluronic acid molecules across the epidermal barrier (in vitro) and its interaction with human skin cells. Skin Res Technol 24:129–134 [DOI] [PubMed] [Google Scholar]
- Neamtu I, Rusu AG, Diaconu A et al. (2017) Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv 24:539–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevagi RJ, Skwarczynski M, Toth I (2019a) Polymers for subunit vaccine delivery. Eur Polym J 114:397–410 [Google Scholar]
- Nevagi RJ, Dai W, Khalil ZG et al. (2019b) Self-assembly of trimethyl chitosan and poly(anionic amino acid)-peptide antigen conjugate to produce a potent self-adjuvanting nanovaccine delivery system. Bioorganic Med Chem 27:3082–3088 [DOI] [PubMed] [Google Scholar]
- Nicolete R, Santos DFD, Faccioli LH (2011) The uptake of PLGA micro or nanoparticles by macrophages provokes distinct in vitro inflammatory response. Int Immunopharmacol 11:1557–1563 [DOI] [PubMed] [Google Scholar]
- Niikura K, Matsunaga T, Suzuki T et al. (2013) Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano 7:3926–3938 [DOI] [PubMed] [Google Scholar]
- Nunberg JH, Doyle MV, York SM, York CJ (1989) Interleukin 2 acts as an adjuvant to increase the potency of inactivated rabies virus vaccine. Proc Natl Acad Sci U S A 86:4240–4243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmit SE, Petrie JG, Malosh RE et al. (2013) Influenza vaccine effectiveness in the community and the household. Clin Infect Dis 56:1363–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsdottir TA, Lingnau K, Nagy E, Jonsdottir I (2009) IC31®, a two-component novel adjuvant mixed with a conjugate vaccine enhances protective immunity against pneumococcal disease in neonatal mice. Scand J Immunol 69:194–202 [DOI] [PubMed] [Google Scholar]
- Oleszycka E, McCluskey S, Sharp FA et al. (2018) The vaccine adjuvant alum promotes IL-10 production that suppresses Th1 responses. Eur J Immunol 48:705–715 [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Bauchet L, Davis FG et al. (2014) The epidemiology of glioma in adults: a state of the science review. Neuro Oncol 16:896–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal R, Babu RJ, Palakurthi S (2014) Nanomedicine scale-up technologies: feasibilities and challenges. Ageing Int 15:1527–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati R, Shevtsov M, Sonawane A (2018) Nanoparticle vaccines against infectious diseases. Front Immunol 9:2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar D, Goyal AK, Mangal S et al. (2010) Evaluation of mucoadhesive PLGA microparticles for nasal immunization. AAPS J 12:130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse MJ, Drane D (2005) ISCOMATRIX® adjuvant for antigen delivery. Adv Drug Deliv Rev 57:465–474 [DOI] [PubMed] [Google Scholar]
- Pennock ND, White JT, Cross EW et al. (2013) T cell responses: naive to memory and everything in between. AJP Adv Physiol Educ 37:273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MM, Raposo NRB, Brayner R et al. (2013) Cytotoxicity and expression of genes involved in the cellular stress response and apoptosis in mammalian fibroblast exposed to cotton cellulose nanofibers. Nanotechnology 24:075103. [DOI] [PubMed] [Google Scholar]
- Peres C, Matos AI, Conniot J et al. (2017) Poly(lactic acid)-based particulate systems are promising tools for immune modulation. Acta Biomater 48:41–57 [DOI] [PubMed] [Google Scholar]
- Petersen LK, Ramer-Tait AE, Broderick SR et al. (2011) Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants. Biomaterials 32:6815–6822 [DOI] [PubMed] [Google Scholar]
- Petersen LK, Phanse Y, Ramer-Tait AE et al. (2012) Amphiphilic polyanhydride nanoparticles stabilize bacillus anthracis protective antigen. Mol Pharm 9:874–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky N, Cooper PD (2011) Carbohydrate-based immune adjuvants. Expert Rev Vaccines 10:523–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phanse Y, Carrillo-Conde BR, Ramer-Tait AE et al. (2013) Functionalization of polyanhydride microparticles with di-mannose influences uptake by and intracellular fate within dendritic cells. Acta Biomater 9:8902–8909 [DOI] [PubMed] [Google Scholar]
- Phanse Y, Lueth P, Ramer-Tait AE et al. (2016) Cellular internalization mechanisms of polyanhydride particles: implications for rational design of drug delivery vehicles. J Biomed Nanotechnol 12:1544–1552 [DOI] [PubMed] [Google Scholar]
- Phanse Y, Carrillo-Conde BR, Ramer-Tait AE et al. (2017) Functionalization promotes pathogen-mimicking characteristics of polyanhydride nanoparticle adjuvants. J Biomed Mater Res—Part A 105:2762–2771 [DOI] [PubMed] [Google Scholar]
- Piao Z, Akeda Y, Takeuchi D et al. (2014) Protective properties of a fusion pneumococcal surface protein A (PspA) vaccine against pneumococcal challenge by five different PspA clades in mice. Vaccine 32:5607–5613 [DOI] [PubMed] [Google Scholar]
- Plotkin S, Robinson JM, Cunningham G et al. (2017) The complexity and cost of vaccine manufacturing—an overview. Vaccine 35:4064–4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt D, Rothe K, Baerwald C, Rossol M (2015) GPRC6A mediates alum-induced Nlrp3 inflammasome activation but limits Th2 type antibody responses. Sci Rep 5:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput MKS, Kesharwani SS, Kumar S et al. (2018) Dendritic cell-targeted nanovaccine delivery system prepared with an immune-active polymer. ACS Appl Mater Interfaces 10:27589–27602 [DOI] [PubMed] [Google Scholar]
- Ramirez JEV, Roychoudhury R, Habte HH et al. (2014) Carbohydrate-functionalized nanovaccines preserve HIV-1 antigen stability and activate antigen presenting cells. J Biomater Sci Ed 25:1387–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JEV, Boggiatto PM, Wannemuehler MJ, Narasimhan B (2017) Polyanhydride nanoparticle interactions with host serum proteins and their effects on bone marrow derived macrophage activation. ACS Biomater Sci Eng 3:160–168 [DOI] [PubMed] [Google Scholar]
- Rao JP, Geckeler KE (2011) Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci 36:887–913 [Google Scholar]
- Rayahin JE, Buhrman JS, Zhang Y et al. (2015) High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng 1:481–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SG, Orr MT, Fox CB (2013) Key roles of adjuvants in modern vaccines. Nat Med 19:1597–1608 [DOI] [PubMed] [Google Scholar]
- Renu S, Feliciano-Ruiz N, Ghimire S et al. (2020) Poly(I:C) augments inactivated influenza virus-chitosan nanovaccine induced cell mediated immune response in pigs vaccinated intranasally. Vet Microbiol 242:108611. [DOI] [PubMed] [Google Scholar]
- Riedel S (2005) Plague: From natural disease to bioterrorism. Baylor Univ Med Cent Proc 18:116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose F, Wern JE, Gavins F et al. (2018) A strong adjuvant based on glycol-chitosan-coated lipid-polymer hybrid nanoparticles potentiates mucosal immune responses against the recombinant Chlamydia trachomatis fusion antigen CTH522. J Control Release 271:88–97 [DOI] [PubMed] [Google Scholar]
- Ross KA, Loyd H, Wu W et al. (2014) Structural and antigenic stability of H5N1 hemagglutinin trimer upon release from polyanhydride nanoparticles. J Biomed Mater Res—Part A 102:4161–4168 [DOI] [PubMed] [Google Scholar]
- Ross KA, Lyod H, Wu W et al. (2015) Hemagglutinin-based polyanhydride nanovaccines against H5N1 infuenza elicit protective virus neutralizing titers and cell-mediated immunity. Int J Nanomedicine 10:229–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross K, Senapati S, Alley J et al. (2019) Single dose combination nanovaccine provides protection against influenza A virus in young and aged mice. Biomater Sci 7:809–821 [DOI] [PubMed] [Google Scholar]
- Rothan HA, Byrareddy SN (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy J, Adili R, Kulmacz R et al. (2016) Development of poly unsaturated fatty acid derivatives of aspirin for inhibition of platelet functions. J Pharmacol Exp Ther 359:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboo S, Tumban E, Peabody J et al. (2016) Optimized formulation of a thermostable spray-dried virus-like particle vaccine against human papillomavirus. Mol Pharm 13:1646–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravia J, Chapman NM, Chi H (2019) Helper T cell differentiation. Cell Mol Immunol 16:634–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarei F, Dounighi NM, Zolfagharian H et al. (2013) Alginate nanoparticles as a promising adjuvant and vaccine delivery system. Indian J Pharm Sci 75:442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarti F, Perera G, Hintzen F et al. (2011) In vivo evidence of oral vaccination with PLGA nanoparticles containing the immunostimulant monophosphoryl lipid A. Biomaterials 32:4052–4057 [DOI] [PubMed] [Google Scholar]
- Saxena M, Sabado RL, La Mar M et al. (2019) Poly-ICLC, a TLR3 agonist, induces transient innate immune responses in patients with treated HIV-infection: a randomized double-blinded placebo controlled trial. Front Immunol 10:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K, Arpagaus C, Friess W (2011) Evaluation of the nano spray dryer B-90 for pharmaceutical applications. Pharm Dev Technol 16:287–294 [DOI] [PubMed] [Google Scholar]
- Semete B, Booysen LIJ, Kalombo L et al. (2010) In vivo uptake and acute immune response to orally administered chitosan and PEG coated PLGA nanoparticles. Toxicol Appl Pharmacol 249:158–165 [DOI] [PubMed] [Google Scholar]
- Sharma G, Valenta DT, Altman Y et al. (2010) Polymer particle shape independently influences binding and internalization by macrophages. J Control Release 147:408–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Parmar A, Kori S, Sandhir R (2015) PLGA-based nanoparticles: a new paradigm in biomedical applications. Trends Anal Chem 80:30–40 [Google Scholar]
- Shen E, Pizsczek R, Dziadul B, Narasimhan B (2001) Microphase separation in bioerodible copolymers for drug delivery. Biomaterials 22:201–210 [DOI] [PubMed] [Google Scholar]
- Shen L, Higuchi T, Tubbe I et al. (2013) A trifunctional dextran-based nanovaccine targets and activates murine dendritic cells, and induces potent cellular and humoral immune responses in vivo. PLoS ONE 8:e80904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Li J, Liu Q et al. (2018) Local blockade of interleukin 10 and C-X-C Motif chemokinelLigand 12 with nano-delivery promotes antitumor response in murine cancers. ACS Nano 12:9830–9841 [DOI] [PubMed] [Google Scholar]
- Shi J, Kantoff PW, Wooster R, Farokhzad OC (2017) Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer 17:20–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N-Q, Zhou J, Walker J et al. (2020) Microencapsulation of luteinizing hormone-releasing hormone agonist in poly (lactic-co-glycolic acid) microspheres by spray-drying. Front Pharmacol 321:756–772 [DOI] [PubMed] [Google Scholar]
- Shim BS, Park SM, Quan JS et al. (2010) Intranasal immunization with plasmid DNA encoding spike protein of SARS-coronavirus/polyethylenimine nanoparticles elicits antigen-specific humoral and cellular immune responses. BMC Immunol 11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima F, Akagi T, Uto T, Akashi M (2013) Manipulating the antigen-specific immune response by the hydrophobicity of amphiphilic poly(γ-glutamic acid) nanoparticles. Biomaterials 34:9709–9716 [DOI] [PubMed] [Google Scholar]
- Silva JM, Videira M, Gaspar R et al. (2013) Immune system targeting by biodegradable nanoparticles for cancer vaccines. J Control Release 168:179–199 [DOI] [PubMed] [Google Scholar]
- Silva AL, Soema PC, Slutter B et al. (2016) PLGA particulate delivery systems for subunit vaccines: linking particle properties to immunogenicity. Hum Vaccin Immunother 12:1056–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Somani VK, Aggarwal S, Bhatnagar R (2015) PLGA (85: 15) nanoparticle based delivery of rL7/L12 ribosomal protein in mice protects against Brucella abortus 544 infection: a promising alternate to traditional adjuvants. Mol Immunol 68:272–279 [DOI] [PubMed] [Google Scholar]
- Singh RS, Kaur N, Rana V, Kennedy JF (2017) Pullulan: a novel molecule for biomedical applications. Carbohydr Polym 171:102–121 [DOI] [PubMed] [Google Scholar]
- Song Y, Zhou Y, Van Drunen Littel-Van Den Hurk S, Chen L (2014) Cellulose-based polyelectrolyte complex nanoparticles for DNA vaccine delivery. Biomater Sci 2:1440–1449 [DOI] [PubMed] [Google Scholar]
- Sosnik A, Seremeta KP (2015) Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv Colloid Interface Sci 223:40–54 [DOI] [PubMed] [Google Scholar]
- Stephens L, Ross KA, Hartwig SM et al. (2019) Robust immune response and protection generated by a polyanhydride-based nanoparticle vaccine utilizing the RSV prefusion F protein and/or M protein. J Immunol 202:139.11 LP-139.11 [Google Scholar]
- Stephens L, Ross KA, Hartwig SM et al. (2020) Polyanhydride-based RSV prefusion F nanoparticle vaccine establishes lasting protection mediated by humoral and cellular immune responses. J Immunol 204:166.3 LP-166.3 [Google Scholar]
- Stieneker F, Kersten G, Van BL et al. (1995) Comparison of 24 different adjuvants for inactivated HIV-2 split whole virus as antigen in mice. Induction of titres of binding antibodies and toxicity of the formulations. Vaccine 13:45–53 [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ et al. (2005) Radiotherapy plus concomitant and adjuvant Temozolomide for glioblastoma. N Engl J Med 352:987–996 [DOI] [PubMed] [Google Scholar]
- Sui Z, Chen Q, Fang F et al. (2010) Cross-protection against influenza virus infection by intranasal administration of M1-based vaccine with chitosan as an adjuvant. Vaccine 28:7690–7698 [DOI] [PubMed] [Google Scholar]
- Swanson KA, Rainho-Tomko JN, Williams ZP et al. (2020) A respiratory syncytial virus (RSV) F protein nanoparticle vaccine focuses antibody responses to a conserved neutralization domain. Sci Immunol 5:1–12 [DOI] [PubMed] [Google Scholar]
- Tamargo RJ, Myseros JS, Epstein JI et al. (1993) Interstitial chemotherapy of the 9L gliosarcoma: controlled release polymers for drug delivery in the brain. Cancer Res 53:329–333 [PubMed] [Google Scholar]
- Tamayo I, Irache JM, Mansilla C et al. (2010) Poly(Anhydride) nanoparticles act as active Th1 adjuvants through toll-like receptor exploitation. Clin Vaccine Immunol 17:1356–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A, Chen Z, Cox KS et al. (2019) A potent broadly neutralizing human RSV antibody targets conserved site IV of the fusion glycoprotein. Nat Commun 10:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Gill HS (2015) M2e-immobilized gold nanoparticles as influenza A vaccine: role of soluble M2e and longevity of protection. Vaccine 33:2307–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Rawat A, Hope-Weeks L, Ahsan F (2011) Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol Pharm 8:405–415 [DOI] [PubMed] [Google Scholar]
- Tomić S, Kokol V, Mihajlović D et al. (2016) Native cellulose nanofibrills induce immune tolerance in vitro by acting on dendritic cells. Sci Rep 6:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MP, Wilson-Welder JH, Lopac SK et al. (2011) Polyanhydride microparticles enhance dendritic cell antigen presentation and activation. Acta Biomater 7:2857–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres FG, Troncoso OP, Pisani A et al. (2019) natural polysaccharide nanomaterials: an overview of their immunological properties. Int J Mol Sci 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent A, Ulery BD, Black MJ et al. (2015) Peptide amphiphile micelles self-adjuvant group A Streptococcal vaccination. AAPS J 17:380–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong-Dinh Tran T, Ha-Lien Tran P, Tu Nguyen K, Tran V-T (2016) Nano-precipitation: preparation and application in the field of pharmacy. Curr Pharm Des 22:2997–3006 [DOI] [PubMed] [Google Scholar]
- Tzeng SY, Guarecuco R, McHugh KJ et al. (2016) Thermostabilization of inactivated polio vaccine in PLGA-based microspheres for pulsatile release. J Control Release 233:101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulery BD, Phanse Y, Sinha A et al. (2009) Polymer chemistry influences monocytic uptake of polyanhydride nanospheres. Pharm Res 26:683–690 [DOI] [PubMed] [Google Scholar]
- Ulery BD, Petersen LK, Phanse Y et al. (2011) Rational design of pathogen-mimicking amphiphilic materials as nanoadjuvants. Sci Rep 1:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanavicius D, Alvarez T, Such GK et al. (2018) The potential of nanoparticle vaccines as a treatment for cancer. Mol Immunol 98:2–7 [DOI] [PubMed] [Google Scholar]
- Uto T, Toyama M, Nishi Y et al. (2013) Uptake of biodegradable poly(γ-glutamic acid) nanoparticles and antigen presentation by dendritic cells in vivo. Results Immunol 3:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uto T, Akagi T, Akashi M, Baba M (2015) Induction of potent adaptive immunity by the novel polyion complex nanoparticles. Clin Vaccine Immunol 22:578–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen J, Pöhler T, Sirola K et al. (2011) Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 18:775–786 [Google Scholar]
- Vela Ramirez JE (2015) Design of polyanhydride-based targeted nanovaccines against HIV. ProQuest Diss Theses 259 [Google Scholar]
- Vela Ramirez JE, Tygrett LT, Hao J et al. (2016) Polyanhydride nanovaccines induce germinal center B cell formation and sustained serum antibody responses. J Biomed Nanotechnol 12:1303–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ (2011) Natural innate and adaptive immunity to cancer. Annu Rev Immunol 29:235–271 [DOI] [PubMed] [Google Scholar]
- Wadajkar AS, Dancy JG, Hersh DS et al. (2017) Tumor-targeted nanotherapeutics: overcoming treatment barriers for glioblastoma. Wiley Interdiscip Rev Nanomed Nanobiotechnol 9:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafa EI, Geary SM, Goodman JT et al. (2017) The effect of polyanhydride chemistry in particle-based cancer vaccines on the magnitude of the anti-tumor immune response. Acta Biomater 50:417–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafa EI, Geary SM, Ross KA et al. (2019) Single dose of a polyanhydride particle-based vaccine generates potent antigen-specific antitumor immune responses. J Pharmacol Exp Ther 370:855–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DA, Kelly SM, Petersen AC et al. (2019) Single-dose combination nanovaccine induces both rapid and long-lived protection against pneumonic plague. Acta Biomater 100:326–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner-Muñiz DA, Haughney SL, Kelly SM et al. (2018) Room temperature stable PspA-based nanovaccine induces protective immunity. Front Immunol 9:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wait SD, Prabhu RS, Burri SH et al. (2015) Polymeric drug delivery for the treatment of glioblastoma. Neuro Oncol 17:ii9–ii23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZS, Xu YL, Zou XT, Xu ZR (2011) Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Mar Drugs 9:1038–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Mu L, Shi Y (2016) Immunoregulatory functions of immune complexes in vaccine and therapy. EMBO Mol Med 8:1120–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2008) Anthrax in humans and animals. World Organ Anim Heal 219 [Google Scholar]
- Wicki A, Witzigmann D, Balasubramanian V, Huwyler J (2015) Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release 200:138–157 [DOI] [PubMed] [Google Scholar]
- Wilson-Welder JHH, Torres MPP, Kipper MJJ et al. (2009) Vaccine adjuvants: current challenges and future approaches. J Pharm Sci 98:1278–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski R (2015) Spray drying technology review. Int Conf Environ Syst 1–9 [Google Scholar]
- Wu YL, Park K, Soo RA et al. (2011) INSPIRE: a phase III study of the BLP25 liposome vaccine (L-BLP25) in Asian patients with unresectable stage III non-small cell lung cancer. BMC Cancer 11:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TYH, Singh M, Miller AT et al. (2014) Rational design of small molecules as vaccine adjuvants. Sci Transl Med 6:160. [DOI] [PubMed] [Google Scholar]
- Wu NC, Zost SJ, Thompson AJ et al. (2017) A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog 13:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wusiman A, Gu P, Liu Z et al. (2019) Cationic polymer modified PLGA nanoparticles encapsulating alhagi honey polysaccharides as a vaccine delivery system for ovalbumin to improve immune responses. Int J Nanomedicine 14:3221–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Fan Q, Hao D et al. (2015) Chitosan-based mucosal adjuvants: sunrise on the ocean. Vaccine 33:5997–6010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Dai W, Wang Z et al. (2011) Intranasal vaccination with chitosan-DNA nanoparticles expressing pneumococcal surface antigen A protects mice against nasopharyngeal colonization by Streptococcus pneumoniae. Clin Vaccine Immunol 18:75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JH, Dai WJ, Chen B, Fan XY (2015) Mucosal immunization with PsaA protein, using chitosan as a delivery system, increases protection against acute otitis media and invasive infection by Streptococcus pneumoniae. Scand J Immunol 81:177–185 [DOI] [PubMed] [Google Scholar]
- Yang HW, Ye L, Guo XD et al. (2017) Ebola vaccination using a DNA vaccine coated on PLGA-PLL/γPGA nanoparticles administered using a microneedle patch. Adv Healthc Mater 6 [DOI] [PubMed] [Google Scholar]
- Yoshizaki Y, Yuba E, Sakaguchi N et al. (2017) pH-sensitive polymer-modified liposome-based immunity-inducing system: effects of inclusion of cationic lipid and CpG-DNA. Biomaterials 141:272–283 [DOI] [PubMed] [Google Scholar]
- Yue ZG, Wei W, Lv PP et al. (2011) Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromol 12:2440–2446 [DOI] [PubMed] [Google Scholar]
- Zacharias ZR, Ross KA, Hornick EE et al. (2018) Polyanhydride nanovaccine induces robust pulmonary B and T cell immunity and confers protection against homologous and heterologous influenza A virus infections. Front Immunol 9:1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehrung D, Jarrahian C, Giersing B, Kristensen D (2017) Exploring new packaging and delivery options for the immunization supply chain. Vaccine 35:2265–2271 [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Tsai YC, Monie A et al. (2010) Carrageenan as an adjuvant to enhance peptide-based vaccine potency. Vaccine 28:5212–5219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Tongchusak S, Mizukami Y et al. (2011) Biomaterials induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials 32:3666–3678 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhu G, Mei L et al. (2015) Self-assembled DNA immunonanoflowers as multivalent CpG nanoagents. ACS Appl Mater Interfaces 7:24069–24074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, An M, Xi J, Liu H (2017a) Targeting CpG adjuvant to lymph node via dextran conjugate enhances antitumor immunotherapy. Bioconjug Chem 28:1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Hu M, Xu L et al. (2017b) Effect of edible fungal polysaccharides on improving influenza vaccine protection in mice. Food Agric Immunol 28:981–992 [Google Scholar]
- Zhao Y, Wang Y, Ran F et al. (2017) A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci Rep 7:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Shoji Y, McCray S et al. (2014) Stabilization of HAC1 influenza vaccine by spray drying: Formulation development and process scale-up. Pharm Res 31:3006–3018 [DOI] [PubMed] [Google Scholar]
- Zost SJ, Parkhouse K, Gumina ME et al. (2017) Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A 114:12578–12583 [DOI] [PMC free article] [PubMed] [Google Scholar]


