Abstract
The protonation state of the iron(IV) oxo (or ferryl) form of ascorbate peroxidase compound II (APX-II) is a subject of debate. It has been reported that this intermediate is best described as an iron(IV) hydroxide species. Neutron diffraction data obtained from putative APX-II crystals indicate a protonated oxygenic ligand at 1.88 Å from the heme iron. This finding, if correct, would be unprecedented. A basic iron(IV) oxo species has yet to be spectroscopically observed in a histidine-ligated heme enzyme. The importance of ferryl basicity lies in its connection to our fundamental understanding of C─H bond activation. Basic ferryl species have been proposed to facilitate the oxidation of inert C─H bonds, reactions that are unknown for histidine-ligated hemes enzymes. To provide further insight into the protonation status of APX-II, we examined the intermediate using a combination of Mössbauer and X-ray absorption spectroscopies. Our data indicate that APX-II is an iron(IV) oxo species with an Fe─O bond distance of 1.68 Å, a K-edge pre-edge absorption of 18 units, and Mössbauer parameters of ΔEq = 1.65 mm/s and δ = 0.03 mm/s.
Graphical Abstract

INTRODUCTION
This article reports on our efforts to characterize the protonation state of the iron(IV) oxo (or ferryl) intermediate in ascorbate peroxidase compound II (APX-II). The importance of ferryl protonation in APX-II lies in its connection to our understanding of the factors that promote and control metal–oxo-mediated C─H bond activation. Among heme proteins, only thiolate-ligated systems such as cytochrome P450 (P450) are known to activate C─H bonds.1-4 It has been argued that strong electron donation from P450s axial thiolate ligand serves a dual role, increasing the reactivity of the principal oxidant, compound I, toward C─H bonds while protecting the enzyme from oxidative damage.5-8
Strong electron donation from an axial thiolate ligand has been shown to result in a basic ferryl intermediate.5,6,9-11 Spectroscopic measurements indicate that P450 compound II is best described as an iron(IV) hydroxide species with a pKa of ~12.6 Metal–hydroxide pKa’s (or metal-oxo basicities) form a central component of our understanding of metal–oxo-mediated C─H bond activation,12-22 and the elevated value of this thermodynamic parameter in P450s suggests a role for the thiolate ligand in promoting C─H bond cleavage at biologically viable reduction potentials.6
Histidine-ligated peroxidases, such as ascorbate peroxidase, typically function through sequential one-electron oxidations of substrates.2,23 They are not known to activate C─H bonds. The histidine ligand is not as donating as the thiolate, and as a result, histidine-ligated compound II species are generally thought to be more electrophilic in nature. Consistent with this, there are no spectroscopic data to support the existence of iron(IV) hydroxide species in histidine-ligated heme proteins.24 Resonance Raman experiments on horseradish peroxidase compound II have shown an iron(IV) oxo stretch at pD 4, indicating that the pKa of HRP-II is ≤3.6, while Mössbauer, resonance Raman, and X-ray absorption spectroscopies have been used to place an upper limit of pKa ≤ 2.7 for myoglobin compound II.25,26
In light of the available data for histidine-ligated heme ferryl species, the recent assignment of an iron(IV) hydroxide center in APX-II attracted our attention.27 X-ray and neutron crystallographic characterization of APX-II in this report produced an Fe─O bond distance of ~1.88 Å with the neutron data revealing what appears to be a hydroxide ligand bound to the heme iron. Investigators have argued that the presence of the hydroxide ligand indicates that APX-II is best described as an iron(IV) hydroxide species. However, the reported Fe─O bond distance is reminiscent of the distances found in photoreduced crystals of other histidine-ligated compound II species.28-32
At the beginning of the century, with the advent of high-resolution crystal structures, a number of histidine-ligated compound II X-ray structures were purported to show long Fe─O bonds indicative of ferryl protonation. These long Fe─O bonds, however, were at odds with a number of spectroscopic measurements (some of which are outlined above) that pointed to iron(IV) oxo descriptions of these systems.24,28 Investigations of the discrepancy between X-ray and spectroscopic data led researchers to conclude that the radiation dosage delivered during high-resolution X-ray studies was sufficient to induce photoreduction of the heme cofactor.28,33,34 Thus, the ferryl centers within these crystals had been reduced to ferric or ferrous hydroxide species in the X-ray beam. Later efforts aimed at minimizing X-ray exposure times (multicrystal techniques and the use of the free electron laser) reported authentic iron(IV) oxo centers in these systems, consistent with spectroscopic data.33,35
Importantly, the 1.88 Å Fe─O distance reported for APX-II is too long to be associated with a histidine-ligated iron(IV) hydroxide intermediate, which should have an Fe─O distance of ~1.76 Å.11,24,28,36,37 The 1.88 Å bond distance reported in the neutron study is squarely in the range expected for a ferric hydroxide species.28 Observation of a ferric hydroxide species in an X-ray structure of a histidine-ligated compound II crystal would not be surprising given the problems associated with photoreduction in the X-ray beam. What is intriguing about the APX-II report, however, is that it also employed neutron diffraction techniques, which do not suffer from the effects of photoreduction.38 However, while the neutron data appear to show a hydroxide ligand bound to the heme iron, they do not reveal the oxidation state of the iron–hydroxide species within the crystal. In addition, while the authors of the APX-II study went to great lengths using microspectrophotometry and X-ray fluorescence techniques to confirm formation of an iron(IV) intermediate in APX-II crystals, it is not clear that these techniques were applied to the actual crystal used for neutron data collection as no control experiments were reported for this crystal.27
The existence of an iron(IV) hydroxide species in APX-II would be a clear outlier in peroxidase and globin chemistry. Given the central role of metal hydroxide pKa’s in metal–oxo-mediated C─H bond activation, it is important to understand if a histidine-ligated heme can support an iron(IV) hydroxide center. In the past, it has been shown that a mixture of spectroscopic methods can be used to accurately determine the protonation state of ferryl species. Here, we report on our efforts to characterize APX-II using Mössbauer and X-ray absorption spectroscopies.
RESULTS AND DISCUSSION
Stopped-flow UV–vis measurements revealed that the reaction of ferric APX with the two-electron oxidant m-chloroperbenzoic acid generates APX compound I (APX-I, an iron(IV) oxo porphyrin radical species). In the absence of substrate, APX-I subsequently decays through oxidation of a protein residue (presumably Trp179, although the location of the protein radical has not been verified) to generate a cytochrome c peroxidase (CCP) compound ES-like species,23 which has been referred to in the literature (and we will continue to refer to here) as APX-II. Figure 1 shows the UV–vis spectra of ferric APX and AXP-II. The spectra are similar to those reported previously.27,39
Figure 1.
UV–vis spectra of ferric APX (black) and APX-II (red) in 50 mM potassium phosphate, pH 7. Ferric APX displays a Soret maximum at 405 nm and Q bands at 509 and 544 nm. APX-II has a Soret maximum at 418 nm and Q bands at 528 and 559 nm.
We note in passing that the term ES-like intermediate indicates that the enzyme/protein intermediate remains as a whole two oxidizing equivalents above the ferric resting state, but one of the oxidizing equivalents of compound I has moved from the heme-cofactor, off of the porphyrin and on to an oxidizable residue within the protein framework.6,40 The electronic coupling between the iron(IV) center and the protein radical in these ES-like intermediates is typically nonexistent or very weak.6,23,40-44 In CCP-ES, for example, the exchange coupling is 1–2 orders of magnitude weaker than it is in horseradish peroxidase compound I.40 As a result of these very weak couplings, the chemistry and spectroscopic signatures of the iron(IV) centers in these ES-like systems are typically indistinguishable from authentic compound II species. When the heme-cofactor is the focal point of discussion, as in the current paper, these compound ES-like species are frequently referred to simply as compound II intermediates.6,27
Using insights gained from our stopped-flow investigations, APX-II samples (~3 mM, ~90% yield) for Mössbauer and X-ray absorption spectroscopies were prepared by mixing ferric enzyme with 5 equiv of m-CPBA. The reaction mixture was aged for several seconds to allow for maximum APX-II formation, and the reaction was then quenched by spraying into liquid ethane (90 K). The liquid ethane was subsequently removed at −80 °C, and the sample was then packed into Mössbauer and XAS sample holders at 77 K.
Mössbauer spectroscopy is an excellent tool for determining the oxidation state and the local bonding environment of the iron atom at the center of the heme cofactor. The isomer shift, δ, provides insight into the oxidation state, while the quadrupole splitting, ΔEq, can be used to track the protonation state of the ferryl oxygen. We have shown that ΔEq increases significantly upon ferryl protonation. In P450-II, for example, ΔEq increases from 1.30 to 2.02 mm/s when the ferryl oxygen is protonated,6 and similar results were observed for the thiolate-ligated chloroperoxidase compound II.45
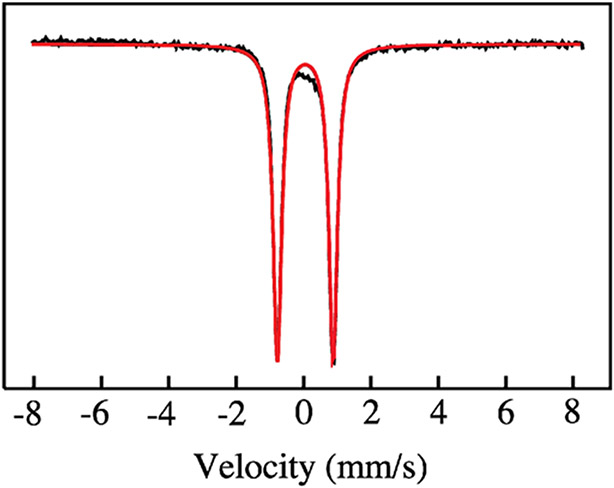
Figure 2 shows the Mössbauer spectrum of APX-II (pH 7, 4.2 K). As expected, it consists of a single quadrupole doublet with an isomer shift of δ = 0.03 mm/s and a quadrupole splitting of ΔEq = 1.65 mm/s. These values are typical of those found for histidine-ligated iron(IV) oxo porphyrin species.24 Myoglobin compound II (Mb-II) has values of δ = 0.07 mm/s and ΔEq = 1.58 mm/s at pH 3.9,26 while horseradish peroxidase compound II (HRP-II) has values of δ = 0.03 mm/s and ΔEq = 1.61 mm/s at pH 6.9.46 X-ray absorption and resonance Raman measurements have shown that Mb-II and HRP-II are authentic iron(IV) oxo species.25,26,47 The similarity between the Mössbauer parameters of APX-II and those found for Mb-II and HRP-II suggests that APX-II is an iron(IV) oxo species at pH 7.
Figure 2.
Mössbauer spectrum (4.2 K, 50 mT) of APX-II EXAFS sample (50 mM potassium phosphate, pH 7). APX-II was generated in ~90% yield. Raw data are shown in black. Fit is shown in red. Parameters obtained from fitting are ΔEq = 1.65 mm/s and δ = 0.03 mm/s.
X-ray absorption measurements can also provide insight into the protonation state of the ferryl moiety. Both the extended X-ray absorption fine structure (EXAFS) and the pre-edge regions contain information about the Fe─O bond distance, which is directly related to the ferryl protonation state. Iron(IV) oxo species possess a double bond between the iron and the oxygen atoms, resulting in a short internuclear Fe─O distance that is typically on the order of ~1.65 Å. This short Fe─O bond creates an asymmetric ligand field that increases the intensity of formally forbidden Fe1s–>3d pre-edge transitions through 4p mixing.48 Protonation of the ferryl moiety reduces the Fe─O bond order, increasing the Fe─O bond length and diminishing the intensity of the pre-edge.
In what follows we report both pre-edge and EXAFS data for APX-II. We note that all XAS samples were examined by Mössbauer spectroscopy prior to XAS data collection, allowing us to confirm preparation of compound II intermediate. In addition, to minimize the effects of photoreduction during data collection, XAS samples were moved in the X-ray beam so that unexposed portions of the sample were examined for each set of scans. The effects of photoreduction were then monitored by comparing the first and second scans. Data sets used for final analyses were comprised of first scans. Experiments were reproduced using two different preparations of APX-II.
Figure 3 shows normalized pre-edge data for APX-II and for the oxo and hydroxide forms of myoglobin (Mb) and cytochrome P450. For P450, data for both the iron(IV) oxo and the iron(IV) hydroxide forms are shown.6 For Mb, data are presented for the iron(IV) oxo and iron(III) hydroxide forms.26 (Recall that Mb does not support an iron(IV) hydroxide state.)26 We first draw the reader’s attention to the P450 data, which are shown in blue. P450-II has been shown to exist as an iron(IV) hydroxide species with pKa ≈ 12. The transition of P450-II through the iron(IV) hydroxide pKa has been tracked with UV–vis, EXAFS, and Mössbauer spectroscopies.6 At pH 9, the Fe─O distance in P450-II is 1.84 Å. As the pH increases and the iron(IV) hydroxide species deprotonates, the Fe─O distance shortens to 1.68 Å.6
Figure 3.
Pre-edge analysis. Oxo species (Mb-II, P450-II, and APX-II) are shown with solid lines. Hydroxide species (ferric myoglobin and P450-II) are shown with dashed lines. Pre-edge intensities/areas and Fe─O bond distances are listed for each species.
The P450 data in Figure 3 show nicely how the pre-edge intensity can be used as an indicator of the Fe─O distance and protonation state. Pre-edge data for P450-II above (blue line) and below (blue dashed line) the iron(IV) hydroxide pKa are shown. The unprotonated form of P450-II has an intense pre-edge feature that weakens significantly upon protonation of the ferryl moiety. A similar behavior can be seen with Mb. The Mb data are shown in black. Mb-II, which possesses a short Fe─O bond of 1.66 Å, has an intense pre-edge feature (black line), while the ferric hydroxide form of Mb with an Fe─O distance of 1.86 Å has a much weaker pre-edge feature (black dashed line).26 The Mb and P450 data suggest that the pre-edge intensity of APX-II can be used to assign the protonation state of the ferryl moiety. The data for APX-II show a pre-edge feature (red line) that is very similar to those obtained for the iron(IV) oxo forms of P450 and Mb.
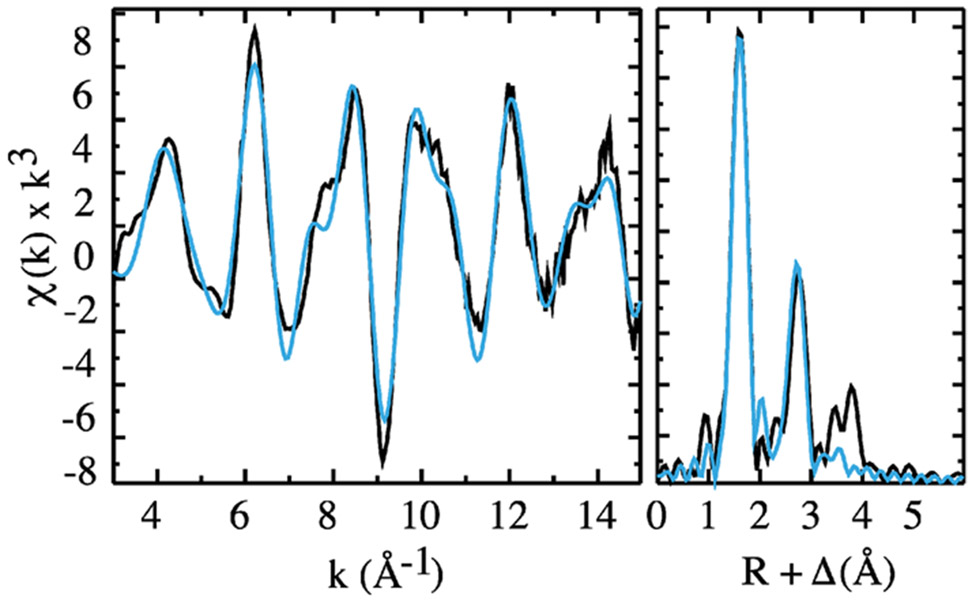
The pre-edge data suggest that APX-II is an iron(IV) oxo species with a short ~1.65 Å Fe─O bond. However, precise bond distances are not readily obtained from analysis of pre-edge resonances. To obtain the Fe─O distance in APX-II, we collected and analyzed X-ray absorption data in the fine structure region. Figure 4 shows the raw and Fourier-transformed (FT) EXAFS data for APX-II.
Figure 4.
APX-II EXAFS data (left) and Fourier transform (right). Black lines show experimental data. Blue lines show the best fit.
Table 1 shows the results obtained from fitting the raw EXAFS data. Fitting was performed with EXAFSPAK, which is freely available at https://www-ssrl.slac.stanford.edu/exafspak.html, using phases and amplitudes generated with FEFF 8.x39.49 Fits included first- and second-shell atoms and one multiple scattering component. The first shell was modeled with two components: one representing five nitrogen atoms (four from the porphyrin and one from the histidine ligand) and the other representing a single oxygen scatter. The second shell was modeled with atoms from the porphyrin ligand: 8 α carbons, 4 meso carbons, and 16 Fe─Cα─N─Fe multiple scattering paths. Fits of the APX-II EXAFS data yielded an Fe─O distance of 1.68 Å, indicating that APX-II is an oxo species. Fits that excluded the short Fe─O scattering path had significantly greater errors.
Table 1.
EXAFS Fitting Results for Raw and Fourier-Filtered APX-II Dataa
| Fe─N |
Fe─O |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | R | σ 2 | N | R | σ 2 | E 0 | errorb | |
| raw data | 5 | 1.99 | 0.002 | 1 | 1.68 | 0.004 | −15 | 0.277 |
| 5 | 2.04 | 0.006 | 1 | 1.96 | −0.002 | −12 | 0.357 | |
| 5 | 1.99 | 0.002 | 0 | −17 | 0.365 | |||
| 6 | 1.99 | 0.003 | 0 | −16 | 0.402 | |||
| Fourier-filtered data | 5 | 1.99 | 0.003 | 1 | 1.68 | 0.003 | −16 | 0.23 |
| 5 | 1.99 | 0.002 | 0 | −18 | 1.01 | |||
| 5 | 2.03 | 0.005 | 1 | 1.95 | −0.001 | −15 | 1.15 | |
| 6 | 1.99 | 0.003 | 0 | −18 | 1.32 | |||
| 5 | 1.99 | 0.002 | 1 | 1.88 | 0.003 | −21 | 1.53 | |
Data were fit over the region k = 3–15 Å−1. Coordination number N, interatomic distance R (Å), mean square deviation in R (the Debye–Waller factor), σ2 (Å2), and the threshold energy shift E0 (eV).
The fit errors listed for the raw data come from the weighted F factor which is defined as [Σk6(χexptl – χcalcd)2/Σk6 χ2exptl]1/2. EXAFSPAK does not report a weighted F factor for fits of Fourier-filtered data. For fits of the Fourier-filtered data, the errors listed are the sum of the squares between the calculated and the Fourier-filtered data divided by 100. Best fits are shown in bold. Alternative fits with different coordination numbers are shown also. Coordination numbers, N, were constrained during the fits. Distance and Debye–Waller values shown in italic were constrained during the fit.
The highly symmetric nature of the porphyrin macrocycle leads to the shell structure observed in the FT EXAFS. Since all of the shells shown in the FT contribute to the raw EXAFS data, it can be difficult to see by eye the need for a single scattering component in fits of the total data. In an effort to make the assignment of APX-II as an iron(IV) oxo species as clear as possible (to expert and nonexpert alike), we included fitting analysis for the EXAFS data obtained by Fourier filtering the first shell of the APX-II data.
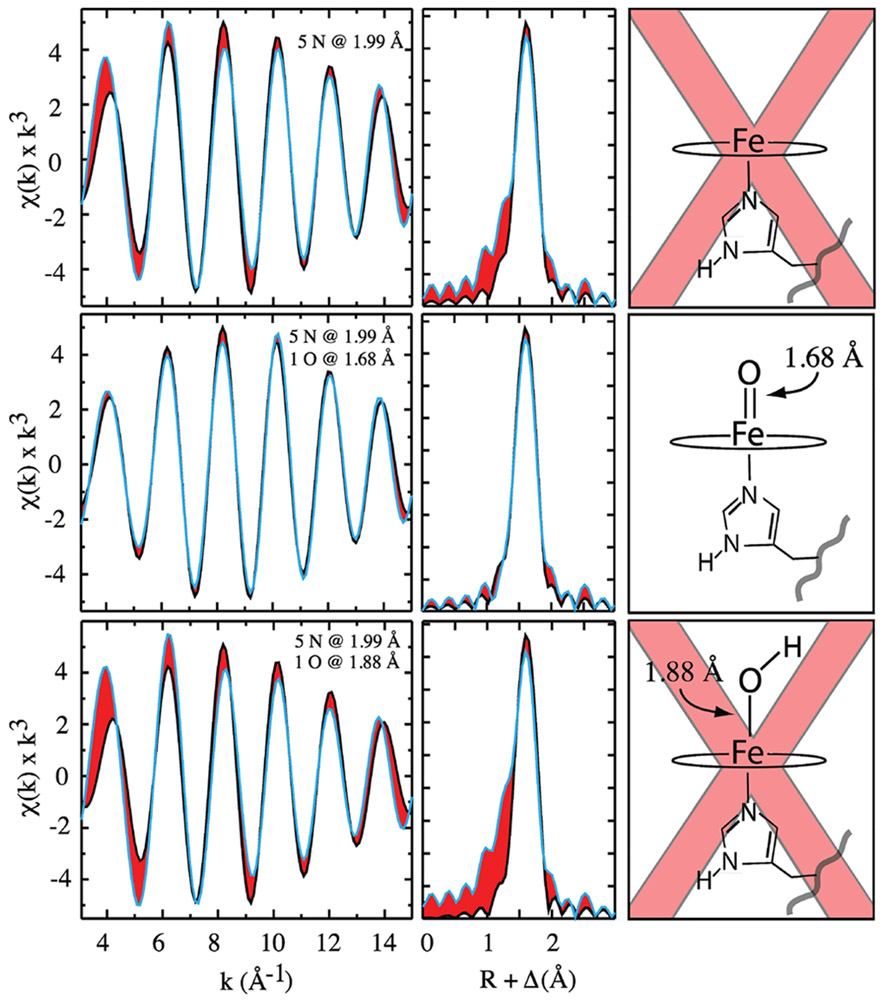
The Fourier-filtered (FF) EXAFS can be seen in Figure 5. The FF EXAFS data are shown in black; fits are shown in blue, and the difference between the two is shaded/highlighted in red. To obtain the FF EXAFS a window was placed around the first shell of the R-space data, and all values outside this window were set to zero. The windowed R-space data was then back transformed to k space to yield the FF EXAFS, which contains only contributions from the first-shell scatters. Fits of the first-shell FF EXAFS data with and without a short Fe─O scattering path suggest that APX-II is an iron(IV) oxo species.
Figure 5.
Analysis of Fourier-filtered EXAFS data. Fourier-filtered data are shown in black, and best fits are in blue. Areas shaded in red highlight errors in the fit. (Top) Effort to model the data without an oxygenic ligand, leading to significant error in the fit. (Middle) Fit that includes a short 1.68 Å Fe─O scattering path: there is good agreement between the data and the fit. (Bottom) Effort to model the Fe─O scattering path with a longer distance consistent with a hydroxide ligand. Importantly, it was not possible to obtain acceptable fits with a hydroxide scattering path. Longer Fe─O scattering paths optimized either to the shorter 1.68 Å oxo distance or to a distance that was not resolvable from the first-shell nitrogen atoms. To model a hydroxide ligand, it was necessary to restrain the Fe─O distance. In the fit shown in the bottom panel, the Fe─O distance was restrained to the value (1.88 Å) reported in the neutron diffraction study. Fit parameters are listed in Table 1.
The top panel of Figure 5 shows the results when the first shell is modeled with only five nitrogen atoms (i.e., without an oxygenic ligand). There is significant error (shaded red) in the EXAFS fit. This difference is accentuated in the R-space data. The middle panel shows the results when a short Fe─O scattering path is added to the first shell. In this case, there is good agreement between the fit and the EXAFS/FT data. The bottom panel shows an effort to model the Fe─O scattering path with a longer distance consistent with a hydroxide ligand.
It was not possible to obtain acceptable fits with a hydroxide scattering path. The EXAFS data are inconsistent with such a species. Longer Fe─O scattering paths optimized either to the shorter 1.68 Å oxo distance or to a distance that was not resolvable from the first-shell nitrogen atoms. To model a hydroxide ligand, it was necessary to restrain the Fe─O distance. For illustrative purposes, we show a fit with the Fe─O distance locked to the 1.88 Å reported in the neutron diffraction study (Figure 5).
The Mössbauer and X-ray absorption data detailed above indicate that APX-II is an iron(IV) oxo species at pH 7. Using UV–vis spectroscopy, we determined that the ferryl oxygen remains unprotonated down to at least pH 5.5, indicating that the pKa of APX-II is ≤4.5. The major UV–vis absorption band of heme proteins (the Soret band) is sensitive to changes in axial ligation. Protonation of the ferryl oxygen reduces the Fe─O bond order, increasing the Fe─O bond length. In P450-II, this has been shown to result in a significant change in the Soret spectrum: λmax shifts from 437 to 426 nm.6 Protonation of the ferryl oxygen also results in considerable changes in the Q-band region.6 Using a stopped-flow apparatus, we prepared APX-II at pH 7 by mixing ferric enzyme with m-CPBA and with a second mix jumped the intermediate to pH 5.5. The UV–vis spectra of APX-II obtained at pH 5.5 and 7 are essentially identical with no change in the Soret maximum or Q-band region, indicating no change in protonation state (Supporting Information). Assuming that the spectrum of a 90:10 oxo/hydroxide distribution would be distinguishable from that of the iron(IV) oxo species, we can place an upper limit (≤4.5) on the pKa of APX-II.
CONCLUSIONS
We utilized Mössbauer and XAS spectroscopies to examine the protonation state of APX-II. While this combination of techniques cannot observe protons directly, it does allow us to determine the composition and oxidation state of the sample and provides a structural metric (the Fe─O bond distance) that is intimately related to the protonation state of the ferryl oxygen.
Our experiments indicate that APX-II is not protonated. The Mössbauer parameters, Fe K-edge pre-edge intensity, and Fe─O distance obtained from our measurements indicate that APX-II is best described as an iron(IV) oxo species at pH 7. These results coupled with pH jump stopped-flow UV–vis spectroscopic measurements set an upper limit (pKa ≤ 4.5) on the APX-II pKa.
APX, thus, appears to be a typical histidine-ligated peroxidase, possessing a compound II species with an acidic pKa, in contrast to the much more basic P450-II (pKa ≈ 12.0). We argued that the elevated pKa in P450-II is important for understanding how C─H bond activation is catalyzed in biological systems and that tuning the iron(IV) hydroxide pKa is one aspect of how Nature controls the functionality of heme enzymes.6,8
METHODS
Protein Expression and Purification.
Soybean ascorbate peroxidase was expressed in E. coli BL21 (DE3) cells for 36 h in 2XYT media at 30 °C using ampicillin and IPTG induction. 57Fe APX was expressed in M9 minimal media for 48 h with isotopic iron (FeCl3) and induced with IPTG. Cells were harvested by centrifugation and lysed through sonication. Cell debris was removed through centrifugation before lysates were subjected to ammonium sulfate fractionation, where APX was found in the 40–50% ammonium sulfate fraction. This fraction was washed excessively with distilled water using an Amicon stirred cell and centrifuged at 20 000g to remove a large portion of the protein content which precipitated from the low ionic strength, leaving a clarified red solution. The solution was buffer exchanged into 50 mM Tris-HCl pH 6.4, filtered, and loaded onto a Q-sepharose Fast Flow anionic exchange column. The column was washed with 50 mM Tris-HCl pH 6.4 and eluted with 250 mM KCl in the same buffer. All eluted fractions were collected and washed with distilled water via stirred cell buffer exchange, centrifuged to remove precipitated proteins, and then loaded onto a Q-Sepharose high-performance column. This was eluted with a gradient from 0 to 250 mM KCl in 50 mM Tris-HCl pH 6.4 after washing with multiple column volumes of the KCl-free buffer. Fractions with high Rz values (≥1.6) were combined and loaded onto a size exclusion column, run in 50 mM potassium phosphate buffer, pH 7. Fractions were kept with Rz above 2, yielding final protein at Rz 2–2.2. Purified enzyme was frozen in liquid nitrogen for long-term storage and thawed immediately before sample preparation.
UV–vis Spectroscopy of APX-II.
Ferric APX (11 μM) was reacted with 5 equiv of m-CPBA in 50 mM potassium phosphate pH 7 buffer. Spectra were recorded after maximum APX-II formation using a Cary-50 UV–vis spectrophotometer.
Preparation of Samples.
APX-II Mössbauer/XAS samples (~3 mM, ~90% yield) were made through reacting ferric APX with 5 equiv of m-CPBA. The reaction was aged for several seconds and then rapidly quenched in liquid ethane. Mixing was performed by hand. Ethane was removed quickly at −80 °C, and samples were stored at 77 K.
Mössbauer Spectroscopy.
Data were collected using a spectrometer from WEB research in constant acceleration mode using transmission geometry and a 50 mT magnetic field applied parallel to the γ-beam. Spectra were recorded at 4.2 K using a Janis SVT400 cryostat. Isomer shifts were calibrated using the centroid of the spectrum of a foil of α-Fe at room temperature. Data analyses were performed using the program WMOSS from WEB research.
X-ray Absorption Spectroscopy (XAS).
XAS data were collected in fluorescence mode at 10 K with a 30-element germanium detector (SSRL, BL7-3) using a Si(220) Phi = 0° double monochromator with a 9.5 keV cutoff for harmonic rejection. Mounted samples were moved in the beam after each scan to an unexposed region of the sample to minimize the effects of photoreduction. Thirty-six first scans were included in the APX-II EXAFS data set. Fitting of EXAFS and pre-edge data was performed using EXAFSPAK.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the NIH (R01-GM101390). We thank M. Latimer and E. Nelson for onsite assistance at the synchrotron. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program was supported by the DOE Office of Biological and Environmental Research and by the National Institutes of Health, National Institute of General Medical Sciences (including P41GM103393).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c09108.
Iron K-edge X-ray absorption spectra for ferric APX and APX-II; EPR spectrum of APX-II sample; UV–vis data from APX-II pH jump experiment (PDF)
The authors declare no competing financial interest.
Contributor Information
Aaron P. Ledray, Department of Molecular Biology and Biochemistry, University of California, Irvine, California 92697, United States.
Courtney M. Krest, Roach & Associates, Limited Liability Company, Seymour, Wisconsin 54942, United States
Timothy H. Yosca, Department of Chemistry and Department of Molecular Biology and Biochemistry, University of California, Irvine, California 92697, United States
Kaustuv Mittra, Department of Chemistry and Department of Molecular Biology and Biochemistry, University of California, Irvine, California 92697, United States.
Michael T. Green, Department of Chemistry and Department of Molecular Biology and Biochemistry, University of California, Irvine, California 92697, United States.
REFERENCES
- (1).Cytochrome P450: Structure, Mechanism, and Biochemistry, 3rd ed.; Ortiz de Montellano PR, Ed.; Kluwer Academic/Plenum Publishers: New York, 2005. [Google Scholar]
- (2).van Rantwijk F; Sheldon RA Selective Oxygen Transfer Catalysed by Heme Peroxidases: Synthetic and Mechanistic Aspects. Curr. Opin. Biotechnol 2000, 11 (6), 554–564. [DOI] [PubMed] [Google Scholar]
- (3).Green MT C-H Bond Activation in Heme Proteins: The Role of Thiolate Ligation in Cytochrome P450. Curr. Opin. Chem. Biol 2009, 13 (1), 84–88. [DOI] [PubMed] [Google Scholar]
- (4).Peter S; Kinne M; Wang X; Ullrich R; Kayser G; Groves JT; Hofrichter M Selective Hydroxylation of Alkanes by an Extracellular Fungal Peroxygenase. FEBS J. 2011, 278 (19), 3667–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Green MT; Dawson JH; Gray HB Oxoiron(IV) in Chloroperoxidase Compound II Is Basic: Implications for P450 Chemistry. Science 2004, 304 (5677), 1653–1656. [DOI] [PubMed] [Google Scholar]
- (6).Yosca TH; Rittle J; Krest CM; Onderko EL; Silakov A; Calixto JC; Behan RK; Green MT Iron(IV)hydroxide pK(a) and the Role of Thiolate Ligation in C-H Bond Activation by Cytochrome P450. Science 2013, 342 (6160), 825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Krest CM; Silakov A; Rittle J; Yosca TH; Onderko EL; Calixto JC; Green MT Significantly Shorter Fe-S Bond in Cytochrome P450-I Is Consistent with Greater Reactivity Relative to Chloroperoxidase. Nat. Chem 2015, 7 (9), 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Yosca TH; Ledray AP; Ngo J; Green MT A New Look at the Role of Thiolate Ligation in Cytochrome P450. J. Biol. Inorg. Chem 2017, 22 (2–3), 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Wang X; Ullrich R; Hofrichter M; Groves JT Heme-Thiolate Ferryl of Aromatic Peroxygenase Is Basic and Reactive. Proc. Natl. Acad. Sci. U. S. A 2015, 112 (12), 3686–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Behan RK; Hoffart LM; Stone KL; Krebs C; Green MT Evidence for Basic Ferryls in Cytochromes P450. J. Am. Chem. Soc 2006, 128 (35), 11471–11474. [DOI] [PubMed] [Google Scholar]
- (11).Stone KL; Behan RK; Green MT Resonance Raman Spectroscopy of Chloroperoxidase Compound II Provides Direct Evidence for the Existence of an Iron(IV)-Hydroxide. Proc. Natl. Acad. Sci. U. S. A 2006, 103 (33), 12307–12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Sacramento JJD; Goldberg DP Factors Affecting Hydrogen Atom Transfer Reactivity of Metal-Oxo Porphyrinoid Complexes. Acc. Chem. Res 2018, 51 (11), 2641–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Prokop KA; de Visser SP; Goldberg DP Unprecedented Rate Enhancements of Hydrogen-Atom Transfer to a Manganese(V)-Oxo Corrolazine Complex. Angew. Chem., Int. Ed 2010, 49 (30), 5091–5095. [DOI] [PubMed] [Google Scholar]
- (14).Borovik AS Role of Metal-Oxo Complexes in the Cleavage of C-H Bonds. Chem. Soc. Rev 2011, 40 (4), 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Parsell TH; Yang MY; Borovik AS C-H Bond Cleavage with Reductants: Re-Investigating the Reactivity of Monomeric Mn-III/IV-Oxo Complexes and the Role of Oxo Ligand Basicity. J. Am. Chem. Soc 2009, 131 (8), 2762–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Donoghue PJ; Tehranchi J; Cramer CJ; Sarangi R; Solomon EI; Tolman WB Rapid C-H Bond Activation by a Monocopper(III)-Hydroxide Complex. J. Am. Chem. Soc 2011, 133 (44), 17602–17605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Nieto I; Ding F; Bontchev RP; Wang HB; Smith JM Thermodynamics of Hydrogen Atom Transfer to a High-Valent Iron Imido Complex. J. Am. Chem. Soc 2008, 130 (9), 2716–2717. [DOI] [PubMed] [Google Scholar]
- (18).Sastri CV; Lee J; Oh K; Lee YJ; Lee J; Jackson TA; Ray K; Hirao H; Shin W; Halfen JA; Kim J; Que L; Shaik S; Nam W Axial Ligand Tuning of a Nonheme Iron(IV)-Oxo Unit for Hydrogen Atom Abstraction. Proc. Natl. Acad. Sci. U. S. A 2007, 104 (49), 19181–19186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Bim D; Maldonado-Dominguez M; Rulisek L; Srnec M Beyond the Classical Thermodynamic Contributions to Hydrogen Atom Abstraction Reactivity. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (44), E10287–E10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Xue GQ; De Hont R; Munck E; Que L Million-Fold Activation of the [Fe-2(mu-O)(2)] Diamond Core for C-H Bond Cleavage. Nat. Chem 2010, 2 (5), 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Darcy JW; Koronkiewicz B; Parada GA; Mayer JM A Continuum of Proton-Coupled Electron Transfer Reactivity. Acc. Chem. Res 2018, 51 (10), 2391–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Gardner KA; Mayer JM Understanding C-H Bond Oxidations - H-Center-Dot and H- Transfer in the Oxidation of Toluene by Permanganate. Science 1995, 269 (5232), 1849–1851. [DOI] [PubMed] [Google Scholar]
- (23).Dunford HB Heme Peroxidases; Wiley-VCH, John Wiley and Sons: New York, 1999. [Google Scholar]
- (24).Behan RK; Green MT On the Status of Ferryl Protonation. J. Inorg. Biochem 2006, 100 (4), 448–459. [DOI] [PubMed] [Google Scholar]
- (25).Sitter AJ; Reczek CM; Terner J Heme-Linked Ionization of Horseradish Peroxidase Compound II Monitored by the Resonance Raman Fe(IV)=0 Stretching Vibration. J. Biol. Chem 1985, 260, 7515–7522. [PubMed] [Google Scholar]
- (26).Yosca TH; Behan RK; Krest CM; Onderko EL; Langston MC; Green MT Setting an Upper Limit on the Myoglobin Iron(IV)hydroxide pK(a): Insight into Axial Ligand Tuning in Heme Protein Catalysis. J. Am. Chem. Soc 2014, 136 (25), 9124–9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Kwon H; Basran J; Casadei CM; Fielding AJ; Schrader TE; Ostermann A; Devos JM; Aller P; Blakeley MP; Moody PC; Raven EL Direct Visualization of a Fe(IV)-OH Intermediate in a Heme Enzyme. Nat. Commun 2016, 7, 13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Green MT Application of Badger’s Rule to Heme and Non-Heme Iron-Oxygen Bonds: An Examination of Ferryl Protonation States. J. Am. Chem. Soc 2006, 128 (6), 1902–1906. [DOI] [PubMed] [Google Scholar]
- (29).Nilsson K; Hersleth HP; Rod TH; Andersson KK; Ryde U The Protonation Status of Compound II in Myoglobin, Studied by a Combination of Experimental Data and Quantum Chemical Calculations: Quantum Refinement. Biophys. J 2004, 87 (5), 3437–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Berglund GI; Carlsson GH; Smith AT; Szoke H; Henriksen A; Hajdu J The Catalytic Pathway of Horseradish Peroxidase at High Resolution. Nature 2002, 417 (6887), 463–468. [DOI] [PubMed] [Google Scholar]
- (31).Hersleth HP; Dalhus B; Gorbitz CH; Andersson KK An Iron Hydroxide Moiety in the 1.35 A Resolution Structure of Hydrogen Peroxide Derived Myoglobin Compound II at pH 5.2. J. Biol. Inorg. Chem 2002, 7 (3), 299–304. [DOI] [PubMed] [Google Scholar]
- (32).Bonagura CA; Bhaskar B; Shimizu H; Li H; Sundaramoorthy M; McRee DE; Goodin DB; Poulos TL High-Resolution Crystal Structures and Spectroscopy of Native and Compound I Cytochrome c Peroxidase. Biochemistry 2003, 42 (19), 5600–5608. [DOI] [PubMed] [Google Scholar]
- (33).Meharenna YT; Doukov T; Li H; Soltis SM; Poulos TL Crystallographic and Single-Crystal Spectral Analysis of the Peroxidase Ferryl Intermediate. Biochemistry 2010, 49 (14), 2984–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Beitlich T; Kuhnel K; Schulze-Briese C; Shoeman RL; Schlichting I Cryoradiolytic Reduction of Crystalline Heme Proteins: Analysis by UV-Vis Spectroscopy and X-ray Crystallography. J. Synchrotron Radiat 2007, 14, 11–23. [DOI] [PubMed] [Google Scholar]
- (35).Chreifi G; Baxter EL; Doukov T; Cohen AE; McPhillips SE; Song J; Meharenna YT; Soltis SM; Poulos TL Crystal Structure of the Pristine Peroxidase Ferryl Center and Its Relevance to Proton-Coupled Electron Transfer. Proc. Natl. Acad. Sci. U. S. A 2016, 113 (5), 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Moody PCE; Raven EL The Nature and Reactivity of Ferryl Heme in Compounds I and II. Acc. Chem. Res 2018, 51 (2), 427–435. [DOI] [PubMed] [Google Scholar]
- (37).Gumiero A; Metcalfe CL; Pearson AR; Raven EL; Moody PCE Nature of the Ferryl Heme in Compounds I and II. J. Biol. Chem 2011, 286 (2), 1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).O’Dell WB; Bodenheimer AM; Meilleur F Neutron Protein Crystallography: A Complementary Tool for Locating Hydrogens in Proteins. Arch. Biochem. Biophys 2016, 602, 48–60. [DOI] [PubMed] [Google Scholar]
- (39).Lad L; Mewies M; Raven EL Substrate Binding and Catalytic Mechanism in Ascorbate Peroxidase: Evidence for Two Ascorbate Binding Sites. Biochemistry 2002, 41 (46), 13774–13781. [DOI] [PubMed] [Google Scholar]
- (40).Houseman ALP; Doan PE; Goodwin DB; Hoffman BM Comprehensive Explanation of the Anomalous Epr-Spectra of Wild-Type and Mutant Cytochrome-C Peroxidase Compound-ES. Biochemistry 1993, 32 (16), 4430–4443. [DOI] [PubMed] [Google Scholar]
- (41).Ho PS; Hoffman BM; Kang CH; Margoliash E Control of the Transfer of Oxidizing Equivalents between Heme Iron and Free-Radical Site in Yeast Cytochrome-C Peroxidase. J. Biol. Chem 1983, 258 (7), 4356–4363. [PubMed] [Google Scholar]
- (42).Schunemann V; Lendzian F; Jung C; Contzen J; Barra AL; Sligar SG; Trautwein AX Tyrosine Radical Formation in the Reaction of Wild Type and Mutant Cytochrome P450cam with Peroxy Acids - A Multifrequency EPR Study of Intermediates on the Millisecond Time Scale. J. Biol. Chem 2004, 279 (12), 10919–10930. [DOI] [PubMed] [Google Scholar]
- (43).Schunemann V; Jung C; Lendzian F; Barra AL; Teschner T; Trautwein AX Mössbauer- and EPR-Snapshots of an Enzymatic Reaction: The Cytochrome P450 Reaction Cycle. Hyperfine Interact. 2004, 156/157 (1-4), 247–256. [Google Scholar]
- (44).Coulson AFW; Erman JE; Yonetani T Studies on Cytochrome-C Peroxidase. XVII. Stoichiometry and Mechanism of Reaction of Compound ES with Donors. J. Biol. Chem 1971, 246 (4), 917–924. [PubMed] [Google Scholar]
- (45).Stone KL; Hoffart LM; Behan RK; Krebs C; Green MT Evidence for Two Ferryl Species in Chloroperoxidase Compound II. J. Am. Chem. Soc 2006, 128 (18), 6147–6153. [DOI] [PubMed] [Google Scholar]
- (46).Schulz CE; Rutter R; Sage JT; Debrunner PG; Hager LP Mössbauer and Electron-Paramagnetic Resonance Studies of Horseradish-Peroxidase and Its Catalytic Intermediates. Biochemistry 1984, 23 (20), 4743–4754. [DOI] [PubMed] [Google Scholar]
- (47).Penner-Hahn JE; Smith Eble K; McMurry TJ; Renner M; Balch AL; Groves JT; Dawson JH; Hodgson KO Structural Characterization of Horseradish-Peroxidase Using EXAFS Spectroscopy - Evidence for Fe = O Ligation in Compound-I and Compound-II. J. Am. Chem. Soc 1986, 108 (24), 7819–7825. [DOI] [PubMed] [Google Scholar]
- (48).Shulman RG; Yafet Y; Eisenberger P; Blumberg WE Observation and Interpretation of X-Ray Absorption Edges in Iron Compounds and Proteins. Proc. Natl. Acad. Sci. U. S. A 1976, 73 (5), 1384–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Ankudinov AL; Ravel B; Rehr JJ; Conradson SD Real-Space Multiple-Scattering Calculation and Interpretation of X-ray-Absorption Near-Edge Structure. Phys. Rev. B: Condens. Matter Mater. Phys 1998, 58 (12), 7565–7576. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.