Abstract
Asthma is the most common chronic pediatric lung disease that has traditionally been defined as a syndrome of airway inflammation characterized by clinical symptoms of cough and wheeze. Highlighting the complex and heterogenous nature of asthma, this review summarizes recent advances in asthma classification that are based on pathobiology, and thereby directly addresses limitations of existent definitions of asthma. By reviewing and contrasting clinical and mechanistic features of adult and childhood asthma, the review summarizes key biomarkers that distinguish childhood asthma subtypes. While atopy and its severity are important features of childhood asthma, there is evidence to support the existence of a childhood asthma endotype distinct from the atopic endotype. Although biomarkers of non-atopic asthma are an area of future research, we summarize a clinical approach that includes existing measures of airway-specific and systemic measures of atopy, co-existing morbidities, and disease severity and control, in the definition of childhood asthma, to empower health care providers to better characterize asthma disease burden in children. Identification of biomarkers of non-atopic asthma and of the contribution of genetics and epigenetics to pediatric asthma burden remain a research need, which can potentially allow delivery of precision medicine to pediatric asthma.
Keywords: Asthma, Lung, Pulmonology
Introduction
Asthma is the most common chronic lung disease of childhood with an estimated 6 million children affected in the United States (1). Asthma has long been proposed to be a heterogenous syndrome of airway hyper-responsiveness in response to varied triggers with differing pathobiology (2), that presents clinically with cough, wheeze, and shortness of breath (3). The most common, and therefore the better understood prototype, is the disease associated with T helper 2 cell-mediated allergic sensitization, now known as the T2-hi asthma (4). Advances in the management of T2-hi asthma (5, 6) has, in turn, identified the subset of “difficult-to-control” or “severe” asthma that is poorly responsive to currently available therapies, which are most effective against T2 pattern of inflammation (7). Thus, heterogeneity in disease presentation in the advent of personalized medicine has again placed the spotlight on the need for more a nuanced definition of asthma. In this review, we discuss recent advances in our understanding of the pathobiology of asthma, which directly inform the need for better disease definition(s).
1. Asthma Definition- evolution over time
The complex and diverse nature of asthma has led to its classification as a syndrome or a constellation of symptoms and signs rather than a single diagnosis (2, 3, 8). Initial attempts to subclassify the syndrome included definitions such as “extrinsic” (due to external exposures, such as allergens) and “intrinsic” (due to non-allergic causes) asthma (9). Subsequent classifications were based on disease severity defined by symptom frequency (10) and clinical characteristics such as exercise-induced asthma or cough-variant asthma (3). None of these definitions fully addressed the variability of the syndrome.
To broaden the scope of disease explained by the definition, in 2007, the National Heart Lung and Blood Institute (NHLBI) Guidelines for the Diagnosis and Management of Asthma, defined asthma as,
“a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role: in particular, mast cells, eosinophils, T lymphocytes, macrophages, neutrophils, and epithelial cells. In susceptible individuals, this inflammation causes recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, particularly at night or in the early morning. These episodes are usually associated with widespread but variable airflow obstruction that is often reversible either spontaneously or with treatment. The inflammation also causes an associated increase in the existing bronchial hyperresponsiveness to a variety of stimuli. Reversibility of airflow limitation may be incomplete in some patients with asthma” (10).
More recently, in 2019, Global Initiative for Asthma (GINA) report, defined asthma as “a heterogenous disease, usually characterized by chronic airway inflammation defined by the history of respiratory symptoms such as wheeze, shortness of breath, chest tightness, and cough that vary over time and in intensity, together with variable expiratory airflow limitations” (11).
While these umbrella definitions of asthma account for the varied disease presentation, they are limited in defining early childhood asthma, since objective measures of airflow obstruction included in these definitions, such as spirometry, cannot be reliably performed prior to 5 years of age. These limitations have been addressed by the Asthma Predictive Index (API), a validated clinical tool comprised of major (parental history of asthma or eczema) and minor (eosinophilia, wheezing without colds, and allergic rhinitis) clinical criteria (12) which was later modified (mAPI) to include more stringent criteria, such as more than 4 episodes of wheezing, and sensitization to aeroallergens as well as to milk, egg, and peanut as minor criteria (13). The validity of this tool in predicting asthma was confirmed in a longitudinal study where a positive API and persistent wheezing at age three years was associated with increased asthma risk at age seven, objectively confirmed by lung function testing (14). Thus, the mAPI highlights the need for a more nuanced umbrella asthma definition inclusive of criteria for preschool children, since early asthma diagnosis allows for early classification of severity and control, which in turn informs initiation of appropriate treatment and therefore prevention of disease progression.
2. Importance of a precise yet comprehensive asthma definition
A precise but comprehensive definition of asthma is important since it directly informs clinical care and research. In addition to highlighting the role of inflammation, the 2007 NHLBI definition introduced severity and control classifications (10). However, in 2010, The World Health Organization (WHO) identified the lack of standardized use of asthma severity and control classification with the terms being used interchangeably (15). They also highlighted the need for a uniform definition for severe asthma, differentiating treatment-resistant severe asthma from difficult-to-treat severe asthma (15), based on the high doses of inhaled corticosteroids (ICS) and systemic corticosteroids required to achieve asthma control (16).
Similar to the clinical realm, issues with asthma definitions also exist in the research setting, where different studies have used differing definitions of asthma. A comprehensive review of birth cohort definitions of “childhood asthma” found 60 different asthma definitions among 122 studies with over 50% using a definition based on a doctor’s diagnosis of asthma with or without other symptoms, medication use, or any time constraint (17). Depending on the asthma definition used, that prevalence estimates of asthma varied between 15.1% and 51.1% (17).
There are several factors that make defining asthma difficult. Inflammation is a major component of the disease and heterogeneity in immune responses underlie clinical heterogeneity but are poorly understood. The presence of asthma masqueraders (i.e., rhinosinusitis, gastroesophageal reflux (GERD), laryngotracheomalacia, congenital airway anomalies, and aspiration) (18) as well as common comorbidities such as obesity, eczema, bronchopulmonary dysplasia (BPD) and sleep-disordered breathing (SDB), that exacerbate asthma symptoms (19), also complicate defining asthma.
3a. Phenotypes facilitate asthma definitions: Endotyping
Defining asthma based on distinct etiologies and their associated clinical biomarkers has garnered renewed interest in light of the need for precision medicine. This approach has been informed by the availability of targeted therapies, an improved understanding of the structural and immune alterations in the asthmatic lung (20, 21), as well as the contribution of genetic and epigenetic mechanisms to asthma (22, 23).
Unbiased comprehensive phenotyping using hypothesis-free analysis of extremely large datasets (i.e., “big data”) to elucidate novel relationships between clinical, environmental and biological characteristics, has begun to address the need for a precise disease definition. Using this methodology, the NHLBI-sponsored Severe Asthma Research Program (SARP) made substantial inroads into asthma categorization among adults. Taking into account over six-hundred clinical and biochemical variables grouped into 34 groups (24), SARP identified five clusters of patients (25). As summarized in Table 1, 3 of the 5 clusters included individuals who had early-onset asthma, emphasizing the important contribution of childhood asthma to adult disease burden. Cluster 3 was distinguished by obesity, highlighting the importance of including co-existent morbidities in disease phenotyping. Although the five clusters did not differ with regards to fractional exhaled nitric oxide (FeNO), a measure of allergic airway inflammation, their serum IgE correlated with their atopic status, but not with blood or sputum eosinophils, highlighting the importance of biomarkers and the biologic compartment being investigated in the context of asthma. Together, this deeper characterization of asthma phenotypes led to coining of the term “endotypes” (26), defined as a subtype of a condition distinguished by its pathophysiology (26). Similar analyses have subsequently been conducted on pediatric severe asthma databases and highlight key differences from adult severe asthma.
Table 1.
Clinical Characteristics of Asthma Clusters in Adults*
| Cluster | Summary of Clinical Characteristics |
|---|---|
|
| |
| Younger individuals | |
| Predominantly Females | |
| Childhood onset/atopic asthma | |
| Cluster 1 | Normal lung function |
| Few exacerbations | |
| Frequent symptoms and rescue bronchodilator use | |
|
| |
| Older Individuals | |
| Predominantly females | |
| Cluster 2 | Childhood onset/atopic asthma |
| Normal lung function | |
| More controller medications | |
|
| |
| Older individuals | |
| Predominantly females | |
| Cluster 3 | Late-onset asthma |
| High Body mass index (BMI) | |
| Baseline lung function deficits | |
| Multiple controller medications | |
| Frequent symptoms and oral corticosteroid use | |
|
| |
| Males and Females | |
| Childhood onset/atopic asthma | |
| Cluster 4 | Severe Asthma |
| Severe lung function deficits | |
|
| |
| Males and Females | |
| Late-onset asthma | |
| Cluster 5 | Severe Asthma |
| Lowest lung function, least reversibility | |
| Multiple controller medications | |
| Frequent symptoms and oral corticosteroid use | |
Summarized from Reference 25 (Moore W. C., et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 181, 315-323 (2010))
Table 3.
Severe Asthma Biomarkers
| Biomarker | Endotype | Scientific Reference |
|---|---|---|
| Elevated Eosinophils | T2-hi | (Teach et al. (38), Price et al. (58)) |
| High Total IgE | T2-hi | (Zoratti et al. (30)) |
| Presence of specific IgE or allergy skin prick testing to assess inhalant allergen sensitization | T2-hi | (Zoratti et al. (30)) |
| Elevated Fractional exhaled nitric oxide | T2-hi | (van der Valk et al. (59), van |
| (FeNO) | Vliet et al. (60), Teach et al. (38)) | |
| Elevated Neutrophils | T2-hi/T2-lo | (Teague et al. (27)) |
| Elevated Periostin | T2-hi | (Izuhara et al. (61)) |
Analysis of the pediatric SARP III cohort revealed a higher proportion of males relative to females and African Americans as compared to whites had severe asthma. Unlike the adults, the clusters were not differentiated based on obesity, airflow obstruction on pulmonary function testing, atopic sensitization, or markers of inflammation (27), highlighting the importance of phenotypic characterization specifically in children (28). The Asthma Phenotypes in the Inner City (APIC) study (29) addressed this gap in knowledge and investigated asthma phenotypes in 717 inner city children aged 6 to 17 years (65% African American and 28% Hispanic) living in 9 urban communities in the United States. Unlike SARP, which was observational in nature, the APIC study participants received optimized asthma management based upon NHLBI guidelines for a year, thereby decreasing the contribution of non-adherence to disease phenotyping. Three different analytic approaches of the APIC data to date have identified several distinct phenotypic features of severe asthma among urban children.
In the first analysis, using the same “clustering” analytic approach as SARP, Zoratti et al. categorized APIC children into 5 different asthma endotypes distinguished by the presence and degree of atopy, nasal symptoms, and pulmonary function (30) [Table 2]. Two groups, each representing 15% of the cohort, were relatively severe. The first group had the highest levels of asthma severity and exacerbations, and were highly atopic (median 14 of 22 positive skin tests and median sIgE of 616kU/L) with severe rhinitis and abnormal PFTs. The second severe group, also with high asthma symptomatology despite high doses of controller medications, had low to no atopy. Given the young age of the participants, these findings suggest that origins of severe asthma phenotypes likely begin in early childhood. This speculation was supported by cluster analysis of a high-risk urban cohort of infants, where the atopy-high/ exacerbation-prone with lung function deficits, and low/no-atopy highly symptomatic phenotypes, could be distinguished from transient wheezers and disease remitters as early as age 4 years (31). Therefore, while atopy and its intensity are important distinguishing features of childhood asthma, in keeping with the adult T2-lo endotype, there is a phenotype of childhood asthma that is quite dissociated from atopy.
Table 2.
Clinical Characteristics of Asthma Clusters in Children*
| Cluster | Summary of Clinical Characteristics |
|---|---|
|
| |
| Minimally symptomatic asthma and rhinitis | |
| Cluster A | Lowest levels of allergy and allergic inflammation |
| Normal pulmonary physiology | |
|
| |
| Cluster B | Highly symptomatic asthma (high step-level controller therapy) |
| Lower levels of allergy and allergic inflammation | |
| Mildly impaired pulmonary physiology | |
|
| |
| Minimally symptomatic asthma and rhinitis | |
| Cluster C | Intermediate levels of allergy and allergic inflammation |
| Mildly impaired pulmonary physiology | |
|
| |
| Minimally symptomatic asthma (intermediate step-level controller therapy) | |
| Cluster D | Symptomatic rhinitis |
| Higher levels of allergy and allergic inflammation | |
| Intermediately impaired pulmonary physiology | |
|
| |
| Cluster E | Highest levels of symptomatic asthma and rhinitis (high-level step treatment) |
| Highest levels of allergy and allergic inflammation | |
| Most impaired pulmonary physiology | |
Summarized from Reference 30. (Zoratti E. M., et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol. 138, 1016-1029 (2016).)
The second analysis, focused on distinguishing endotypes in the subset of “difficult to control” children on high-dose daily controller therapy (29) found spirometric (FEV1) bronchodilator responsiveness, Asthma Control Test score, rhinitis severity, and atopy, to be predictive of difficult-to-control pediatric asthma. Furthermore, the African American subset of APIC children (n=235) with difficult-to-control asthma had higher peripheral blood eosinophil and neutrophil counts and higher IL-5, IFN-γ and Th17 associated cytokines (CXCL-1, IL-17A, and IL-8), supporting a role of systemic immune dysregulation in difficult-to-control asthma that encompasses both T2 and non-T2 markers (32).
The third analytic approach focused on delineating the individual and cumulative contribution of eight key clinical factors previously linked to childhood asthma severity (atopic sensitization, atopic inflammation, pulmonary physiology, stress, obesity, vitamin D, environmental tobacco smoke (ETS) exposure, and rhinitis severity) (33). The authors found that these key factors explained only 53.4% of the asthma severity observed in children, with the atopic pathway, and the non-atopic ETS exposure pathway, being two distinct contributors to asthma severity (33). While these findings can be used to prioritize interventions to reduce disease severity and personalize asthma management, they also highlight the need for identification of additional factors that contribute to asthma severity.
The APIC study examines specific clinical and lab-based variables in a population with high asthma morbidity. In addition, there have been a number of studies showing similar clusters of asthma phenotypes in other pediatric populations. In keeping with the distinguishing role of atopy in APIC participants (30), a cluster analysis of data from 315 school-aged children in France identified three independent clusters: Cluster 1 including asthmatics with severe exacerbations and multiple allergies, Cluster 2 including children with severe asthma with bronchial obstruction, and Cluster 3 including mild asthma (34). Using a similar analytic approach, Panico et al. reported four unique trajectories of asthma in a large population-based study of early childhood British children, which included a trajectory with both low levels of wheeze and atopic symptoms; one with low levels of wheeze but high prevalence of atopic symptoms; one with high prevalence of both wheeze and atopic symptoms; and one with high levels of wheeze but low levels of atopic symptoms (35). Work by Su et al noted that these patterns of distinct clusters, were present in a study of Taiwanese asthmatic children in a consortium-based study. In this study, five distinct phenotypes of childhood asthma could be characterized by either eosinophil-predominant or neutrophil-predominant inflammatory characteristics (36). Similarly, in a Turkish school aged population, asthma subtypes were identified as 5 clusters labeled as: difficult asthma, early-onset mild atopic, early-onset mild non-atopic, late-onset, and exacerbation-prone asthma (37).
In addition, mechanistic studies, as extensions of clinical trials, have contributed to asthma endotyping. For instance, while it is known that allergen exposures in sensitized individuals can trigger asthma exacerbations, findings from the Preventative Omalizumab or Step-up Therapy (PROSE) study suggested that atopic sensitization also renders individuals susceptible to asthma exacerbations triggered by rhinoviruses (38). Conversely, airway irritation by indoor airborne irritants, particularly environmental tobacco smoke (ETS), as well as outdoor exposures, including coal smoke, diesel exhaust particles (DEP), sulfur dioxide, and nitrogen dioxide are linked with incident asthma, exacerbations, and diminished lung function (39–41) via a distinct, non-atopic pathway (42–44). Similarly, high ambient ozone levels are associated with asthma morbidity, likely by causing oxidant-driven injury to the airways (45, 46). Furthermore, given the rapidly increasing marijuana and e-cigarette usage in teenage children (47), there is evidence of suggest that e-cigarettes contain numerous respirable toxicants that are associated with epithelial, innate immune, neutrophilic and mucous secretion responses partly overlapping with those seen in cigarette smokers, and likely distinct from the atopic pathway (48–50), while marijuana allergens, identified by allergy skin testing and serum allergen-specific IgE, have been associated with anaphylaxis (51).
3b. Phenotypes inform asthma management
In addition to defining asthma, phenotyping also contributes to determining medication responsiveness. Chang et al. replicated the previously described SARP pediatric asthma clusters and showed that the early-onset/severe-lung function cluster had the best response to fluticasone/salmeterol while the early-onset/comorbidity cluster had the least clinical response to the controller treatments (52). Even prior to coining of the term “endotype”, Szefler et al. identified a differential response to ICS and leukotriene receptor antagonist (LTRA) in 6 to 17 year-old children with mild to moderate persistent asthma based on markers of allergic airway inflammation (elevated FeNO, eosinophilia, and elevated IgE levels) (53). Subsequently, several studies used this analytic approach. In 2010, the Best Add-on Therapy Giving Effective Responses (BADGER) trial assessed the frequency of differential responses to three blinded step-up treatments (medium dose ICS vs low-dose ICS/LABA vs low dose ICS/LTRA) in 182 children ages 6 to 17 years with uncontrolled mild to moderate persistent asthma receiving low-dose ICS (54). Although the overall best step-up therapy for children with inadequate control on low dose ICS was LABA, in post-hoc analysis, the authors found differences in response to add-on therapy based on eczema history and race with LABA step-up therapy being preferentially effective in those without eczema (55). Similarly, the Individualized Therapy for Asthma in Toddlers (INFANT) study looked at treatment response to daily ICS, as needed ICS, and LTRA based on phenotyping in preschool aged children with mild persistent asthma and found that blood eosinophil count (300 cells/ul or greater) and aeroallergen sensitization predicted better response to daily ICS (28).
Phenotyping has also been utilized to evaluate medication responsiveness to biologics in pediatric asthma. For instance, while omalizumab improved asthma control and reduced seasonal asthma exacerbations in inner-city children and adolescents with allergic asthma, especially among those both sensitized and exposed to cockroach (5). In a post hoc analysis, participants with higher FeNO, blood eosinophils, and BMI were found more likely to benefit from omalizumab based on reduction of asthma exacerbations (56).
Together, these studies not only define the clinical and biochemical variables that contribute to asthma endotyping in children, they also conclusively demonstrate their utility in determining medication responsiveness. These findings provide a fundamental framework to address the need for a precise yet comprehensive definition of asthma in children.
4. Practical Application of Endotyping in Clinical Asthma Management
Based on the above discussion on endotyping, we propose the following approach for defining asthma in an individual patient in the clinic setting [Figure 1]. The utility of classifying asthma severity and control is well known (10). The role of comorbidities and asthma masqueraders has also been discussed (18). Given the role of biomarkers in endotyping, we encourage providers to include inflammatory biomarkers such as blood and sputum eosinophils, serum IgE, and FeNO as part of routine asthma care to guide definition and thereby therapeutic management of pediatric asthma [Table 3]. Blood eosinophilia has been shown to be predictive of asthma exacerbations (57, 58). In addition, FeNO may serve as a predictor of asthma exacerbations (59, 60). Serum periostin is a relatively new biomarker that correlates with other biomarkers of T2 inflammation (including eosinophil count, FeNO, and total IgE) (61). However, its usefulness in childhood asthma is not well established since periostin is elevated at baseline due to high bone turnover in children and does not always correlate with blood eosinophils or FeNO (62, 63). While the T2 biomarkers are better understood, the role of neutrophils in childhood asthma continues to need exploration. SARP cluster analysis, as previously discussed, identified an inverse correlation between sputum neutrophil count and lung function despite maximal therapies (27). However, neutrophilic airway inflammation has been associated with better lung function in children with severe therapy-resistant asthma (64). In addition to biomarkers in peripheral blood, although there are no standardized protocols for use of bronchoscopy and bronchoalveolar lavage (BAL), they may have a role in the classification and management of pediatric asthma. Bronchoscopy and BAL can assess for asthma masqueraders (discussed in Section 2), identify anatomical abnormalities and subacute infections, and better assess airway inflammation using BAL cell counts and biopsy (65). The role of bronchoscopy is asthma is also pertinent because blood eosinophil count and airway inflammation do not always concur, and may not be reliable in characterizing airway inflammation (66). Moreover, these biomarkers are mainly for T2-hi asthma and those for T2-lo asthma continue to need more research.
Figure 1. Approach for defining asthma for children.
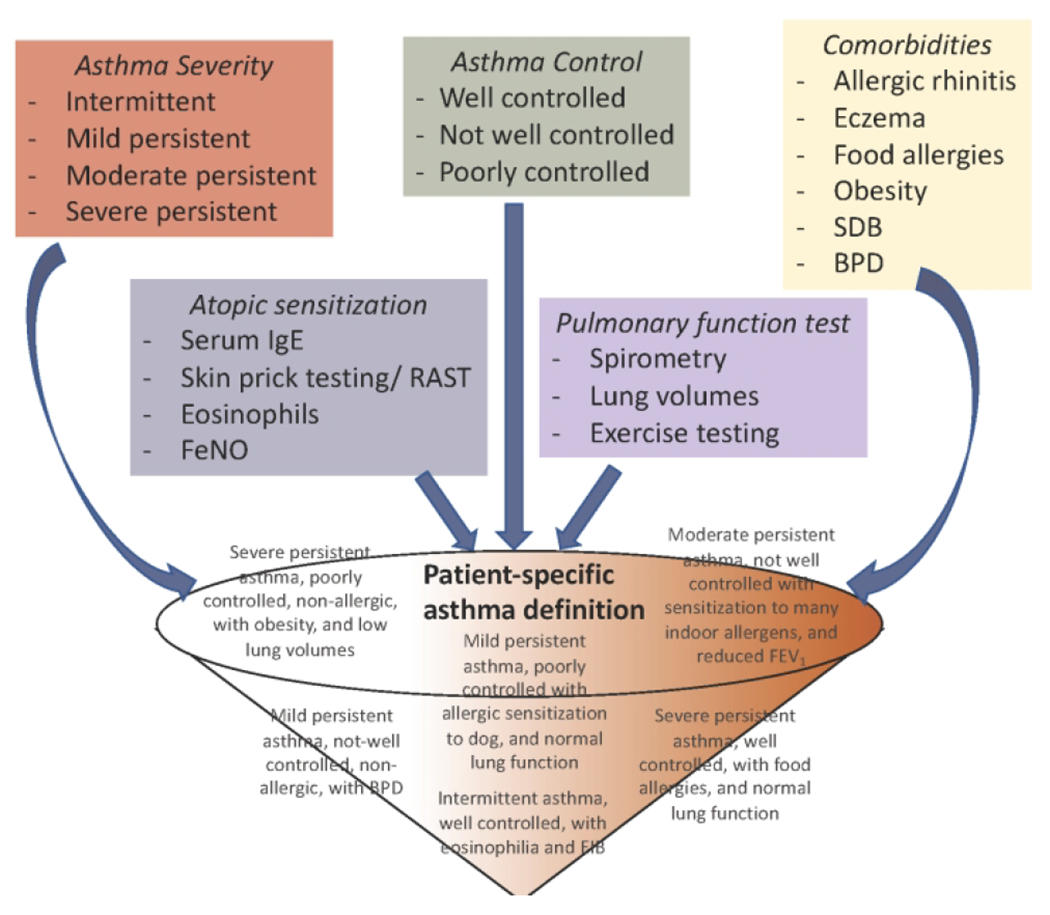
We propose the use of the following approach for defining asthma in children. Health care providers have the tools at hand to define asthma severity (red box) and control (green box), as per the NHLBI guidelines, based on symptom frequency and medication use. In addition, the providers should identify presence of comorbidities (yellow box) that have been associated with asthma burden, define the atopic status of the patient (blue box), and the pattern of pulmonary function testing (purple box). Together, these details can be funneled into personalized definition of asthma for any given patient. Examples of asthma definitions for individual patients using this approach are included in grey text within the funnel.
Putting this together, given the details already available in the clinical setting, we encourage clinicians to uniquely define asthma for each child based on 1) the age of onset (early vs. late onset), 2) the severity and control of disease (as per the NHLBI guidelines as intermittent, mild, moderate or severe persistent), 3) the predominant form of immune response (allergic vs. non-allergic) 4) the inciting trigger (exercise induced vs. viral induced), 5) the pattern of pulmonary function deficits and 6) the presence of comorbidities. Some examples of this comprehensive definition for an individual child including severity, control, allergen sensitization, comorbidity and pulmonary function are included in grey font in Figure 1. We anticipate that defining the patient’s asthma in this manner will empower the clinicians to understand the disease better, which they can they utilize to educate the caregivers regarding potential times for exacerbations/loss of control as well as on the role of controller medications. Endotyping of disease will also allow early identification of “responders” and “non-responders”. Rather than classifying the non-responders as severe or difficult-to-treat asthma, further endotyping will provide a detailed clinical picture of these children.
5. Future directions
The progress made in defining the asthma endotypes in the past decade provide a fertile ground for future advances. We discuss some of these areas of research that will further assist in better defining childhood asthma.
One major issue is the variability in the definition used in epidemiological and mechanistic studies in the field of pediatric asthma. Along these lines, there is need for additional detail on clinical phenotyping of participants included in research studies. As seen with SARP (24, 67), the same variables collected in children and adults led to differing groups of clinical variables (67), although the pediatric SARP clusters could be replicated in the Childhood Asthma Research and Education (CARE) cohort and correlated with medication responsiveness (52). Given the existent variability in defining the disease, studies such as SARP and APIC studies, along with the recent GINA and NHLBI guidelines, provide an excellent framework of variables to be included to facilitate a more nuanced disease definition. By including these variables, clinical and mechanistic research studies will lead the field in comprehensively defining their study participants, an approach that will also allow better contextualizing of the research findings in the clinic. To facilitate this approach, the Agency for Healthcare Research and Quality (AHRQ) recently put out a list of harmonized measures that represent a minimum set of outcomes that are relevant in asthma research and clinical practice (68).
The second aspect that needs to be considered is inclusion of all races and ethnicities in future asthma studies to facilitate defining disease and genetic risk that may be race/ ethnicity-specific. It is well known that genetic studies are skewed towards greater inclusion of whites. For studies on diseases such as childhood asthma, there is an urgent need for inclusion of those most afflicted by disease. While the Study of African Americans, Asthma, Genes and Environment (SAGE)(69, 70) have addressed these limitations to some extent, much wider inclusion with larger sample sizes are needed to truly define the gene by environment interaction specific to African American children. Furthermore, while SARP included mainly Whites and non-Hispanic African Americans, there is need for greater inclusion of Hispanics in all such population-based studies. Hispanics comprise over 10% of the US population, and track closely with African Americans with regards to the asthma burden (1). In fact, Puerto Ricans are a subset in whom asthma burden is higher than that in African Americans (1). While the Genes-Environments and Admixture in Latino Americans (GALA) studies continue to address some of these limitations (69, 70), larger population-based studies will inclusion of the different Hispanic heritage groups will further add granularity to the contributions of genetic variants to disease morbidity. As we quantify the contribution of genetics and epigenetics to childhood asthma disease burden, the goal will be to identify at-risk alleles in all ancestries, thereby allowing race/ethnicity specific endotyping of disease. Furthermore, increasing admixing between populations additionally reinforces the need for inclusion of race-specific and race-agnostic genetic risk alleles in asthma endotyping.
The third aspect that needs to be addressed is indentification of additional biochemical measures beyond the known T2 biologic variables. Given the improved understanding of the role of neutrophils in severe/ difficult to control asthma, their proportion in addition to downstream measures of inflammation should be considered as biomarkers for asthma, specifically for non-allergic asthma. Along the same lines, there are multiplexed panels to study T helper cells and their corresponding cytokines. Inclusion of these details, in addition to T2 markers, will allow an improved and detailed understanding of the immune profiles associated with the asthma being described in the study. Lastly, inclusion of airway-specific measures, in addition to systemic measures, to define the airway and systemic immune phenotypes of childhood asthma, is a much-needed detail that will contribute to our understanding of the disease and thereby a cleaner definition.
Conclusions
In summary, adoption of endotyping of asthma in children will allow for a precise yet comprehensive definition of pediatric asthma for an individual patient as well as for populations, which will directly inform disease management as well as research. As future multi-omics approaches better define the contribution of genetics and epigenetics to childhood asthma, we anticipate that asthma endotyping will include details such as those that currently exist in the world of oncology where at the time of diagnosis, a malignancy is phenotyped with genetic, molecular, and biomarker details that directly guide targeted therapy. By applying these details into defining asthma in children, the field can more directly inform both development of new therapies and repurposing of available medications.
Impact Statement:
This review highlights asthma as a complex and heterogenous disease and discusses recent advances in the understanding of pathobiology of asthma to demonstrate need for more nuanced definitions of asthma.
We review current knowledge of asthma phenotypes and endotypes and put forth an approach to endotyping asthma that may be useful for defining asthma for clinical care as well as for future research studies in the realm of personalized medicine for asthma.
Acknowledgments
Financial Support: The authors DR and MDC are supported by NIH.
Footnotes
Disclosure: The authors have no disclosures.
Category of Study: Review
References:
- 1. https://www.cdc.gov/vitalsigns/childhood-asthma/index.html.
- 2.Hopkin JM The diagnosis of asthma, a clinical syndrome. Thorax. 67, 660–662 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Wenzel SE Complex phenotypes in asthma: current definitions. Pulm Pharmacol Ther. 26, 710–715 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Wenzel SE Emergence of Biomolecular Pathways to Define Novel Asthma Phenotypes. Type-2 Immunity and Beyond. Am J Respir Cell Mol Biol. 55, 1–4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busse WW, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 364, 1005–1015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortega HG, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 371, 1198–1207 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Chung KF, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 43, 343–373 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Hunt J If it smells like a duck, it might be an asthma subphenotype. Am J Respir Crit Care Med. 175, 975–976 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Rackemann FM A working classification of asthma. Am J Med. 3, 601–606 (1947). [DOI] [PubMed] [Google Scholar]
- 10.Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Institute of Health: National Heart, Lung, and Blood Institute.(2007) [Google Scholar]
- 11.https://ginasthma.org/reports/ (2019)
- 12.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 162, 1403–1406 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Guilbert TW, et al. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials. 25, 286–310 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Amin P, et al. Optimum predictors of childhood asthma: persistent wheeze or the Asthma Predictive Index? J Allergy Clin Immunol Pract. 2, 709–715 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bousquet J, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 126, 926–938 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Bell MC, Busse WW Severe asthma: an expanding and mounting clinical challenge. J Allergy Clin Immunol Pract. 1, 110–121; (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Wonderen KE, et al. Different definitions in childhood asthma: how dependable is the dependent variable? Eur Respir J. 36, 48–56 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Barsky EE, Giancola LM, Baxi SN, Gaffin JM A Practical Approach to Severe Asthma in Children. Ann Am Thorac Soc. 15, 399–408 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rastogi D, Liu A. Severe Asthma in Childhood: Special Considerations. In Difficult to Treat Asthma: Clinical Essentials. (Khurana S, Holguin F (eds) (Humana Press; 2020) [Google Scholar]
- 20.Gibson PG, Simpson JL The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 64, 728–735 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Postma DS, Rabe KF The Asthma-COPD Overlap Syndrome. N Engl J Med. 373, 1241–1249 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Holgate ST Pathogenesis of asthma. Clin Exp Allergy. 38, 872–897 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Holgate ST, et al. Asthma. Nat Rev Dis Primers. 1, 15025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore WC, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 119, 405–413 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore WC, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 181, 315–323 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotvall J, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 127, 355–360 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Teague WG, et al. Baseline Features of the Severe Asthma Research Program (SARP III) Cohort: Differences with Age. J Allergy Clin Immunol Pract. 6, 545–554 e544 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzpatrick AM, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol. 138, 1608–1618 e1612 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pongracic JA, et al. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J Allergy Clin Immunol. 138, 1030–1041 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoratti EM, et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol. 138, 1016–1029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy MB, Liu AH, Robinson JL, Klinnert MD Recurrent wheeze phenotypes in poor urban preschool-age children. J Allergy Clin Immunol Pract. (2018). [DOI] [PubMed] [Google Scholar]
- 32.Brown KR, et al. Endotypes of difficult-to-control asthma in inner-city African American children. PLoS One. 12, e0180778 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu AH, et al. Pathways through which asthma risk factors contribute to asthma severity in inner-city children. J Allergy Clin Immunol. 138, 1042–1050 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Just J, et al. Two novel, severe asthma phenotypes identified during childhood using a clustering approach. Eur Resp J. 40, 55–60 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Panico L, Stuart B, Bartley M, Kelly Y Asthma trajectories in early childhood: identifying modifiable factors. PLoS One. 9, e111922 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su MW, et al. Childhood asthma clusters reveal neutrophil-predominant phenotype with distinct gene expression. Allergy. 73, 2024–2032 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Deliu M, et al. Features of asthma which provide meaningful insights for understanding the disease heterogeneity. Clin Exp Allergy. 48, 39–47 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teach SJ, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 136, 1476–1485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian Z, et al. Respiratory responses to diverse indoor combustion air pollution sources. Indoor Air. 17, 135–142 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Patel MM, et al. Traffic-related particulate matter and acute respiratory symptoms among New York City area adolescents. Environ Health Perspect. 118, 1338–1343 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarnat SE, et al. Air pollution and acute respiratory response in a panel of asthmatic children along the U.S.-Mexico border. Environ Health Perspect. 120, 437–444 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanchongkittiphon W, et al. Indoor environmental exposures and exacerbation of asthma: an update to the 2000 review by the Institute of Medicine. Environ Health Perspect. 123, 6–20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dick S, et al. Associations between environmental exposures and asthma control and exacerbations in young children: a systematic review. BMJ Open. 4, e003827 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandt EB, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immmunol. 132, 1194–1204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delfino RJ, et al. Asthma morbidity and ambient air pollution: effect modification by residential traffic-related air pollution. Epidemiology. 25, 48–57 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Kheirbek I, et al. PM2.5 and ozone health impacts and disparities in New York City: sensitivity to spatial and temporal resolution. Air Qual Atmos Health. 6, 473–486 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bao W, et al. Changes in Electronic Cigarette Use Among Adults in the United States, 2014–2016. JAMA. 319, 2039–2041 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin EM, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol. 311, L135–144 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clapp PW, Jaspers I Electronic Cigarettes: Their Constituents and Potential Links to Asthma. Curr Allergy Asthma Rep. 17, 79 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reidel B, et al. E-Cigarette Use Causes a Unique Innate Immune Response in the Lung, Involving Increased Neutrophilic Activation and Altered Mucin Secretion. Am J Respir Crit Care Med. 197, 492–501 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Decuyper II, et al. Exploring the diagnosis and profile of cannabis allergy. J Allergy Clin Immunol Pract. (2018). [DOI] [PubMed] [Google Scholar]
- 52.Chang TS, et al. Childhood asthma clusters and response to therapy in clinical trials. J Allergy Clin Immunol. 133, 363–369 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szefler SJ, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 115, 233–242 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Lemanske RF Jr., et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 362, 975–985 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malka J, et al. Eczema and race as combined determinants for differential response to step-up asthma therapy. J Allergy Clin Immunol. 134, 483–485 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sorkness CA, et al. Reassessment of omalizumab-dosing strategies and pharmacodynamics in inner-city children and adolescents. J Allergy Clin Immunol Pract. 1, 163–171 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teach SJ, et al. Seasonal risk factors for asthma exacerbations among inner-city children. J Allergy Clin Immunol. 135, 1465–1473 e1465 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Price D, et al. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy. 9, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Valk RJ, et al. Daily exhaled nitric oxide measurements and asthma exacerbations in children. Allergy. 67, 265–271 (2012). [DOI] [PubMed] [Google Scholar]
- 60.van Vliet D, et al. Prediction of asthma exacerbations in children by innovative exhaled inflammatory markers: results of a longitudinal study. PLoS One. 10, e0119434 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Izuhara K, et al. Periostin: An emerging biomarker for allergic diseases. Allergy. 74, 2116–2128 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Inoue Y, et al. No increase in the serum periostin level is detected in elementary school-age children with allergic diseases. Allergol Int. 64, 289–290 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Konradsen JR, et al. Predicting asthma morbidity in children using proposed markers of Th2-type inflammation. Pediatr Allergy Immunol. 26, 772–779 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Andersson CK, et al. Intraepithelial neutrophils in pediatric severe asthma are associated with better lung function. J Allergy Clin Immunol. 139, 1819–1829 e1811 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Januska MN, et al. Bronchoscopy in severe childhood asthma: Irresponsible or irreplaceable? Pediatr Pulmonol. 55, 795–802 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ullmann N, et al. Blood eosinophil counts rarely reflect airway eosinophilia in children with severe asthma. Allergy. 68, 402–406 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Fitzpatrick AM, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immmunol. 127, 382–389 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gliklich RE, et al. Harmonized outcome measures for use in asthma patient registries and clinical practice. J Allergy Clin Immunol. 144, 671–681 e671 (2019). [DOI] [PubMed] [Google Scholar]
- 69.Nishimura KK, et al. Early-life air pollution and asthma risk in minority children. The GALA II and SAGE II studies. Am J Respir Crit Care Med. 188, 309–318 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thakur N, et al. Socioeconomic status and childhood asthma in urban minority youths. The GALA II and SAGE II studies. Am J Respir Crit Care Med. 188, 1202–1209 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


