Abstract
Introduction
The COVID-19 pandemic has disrupted healthcare services, reducing opportunities to conduct routine hepatitis C virus antibody screening, clinical care, and treatment. Therefore, people living with undiagnosed hepatitis C virus during the pandemic may later become identified at more advanced stages of the disease, leading to higher morbidity and mortality rates. Further, unidentified hepatitis C virus–infected individuals may continue to unknowingly transmit the virus to others.
Methods
To assess the impact of the COVID-19 pandemic, data were evaluated from a large national reference clinical laboratory and from national estimates of dispensed prescriptions for hepatitis C virus treatment. Investigators estimated the average number of hepatitis C virus antibody tests, hepatitis C virus antibody–positive test results, and hepatitis C virus RNA–positive test results by month in January–July for 2018 and 2019, compared with the same months in 2020. To assess the impact of hepatitis C virus treatment, dispensed hepatitis C virus direct-acting antiretroviral medications were examined for the same time periods. Statistical analyses of trends were performed using negative binomial models.
Results
Compared with the 2018 and 2019 months, hepatitis C virus antibody testing volume decreased 59% during April 2020 and rebounded to a 6% reduction in July 2020. The number of hepatitis C virus RNA–positive results fell by 62% in March 2020 and remained 39% below the baseline by July 2020. For hepatitis C virus treatment, prescriptions decreased 43% in May, 37% in June, and 38% in July relative to the corresponding months in 2018 and 2019.
Conclusions
During the COVID-19 pandemic, continued public health messaging, interventions and outreach programs to restore hepatitis C virus testing and treatment to prepandemic levels, and maintenance of public health efforts to eliminate hepatitis C infections remain important.
INTRODUCTION
Hepatitis C virus (HCV) infection is the most commonly reported bloodborne infection in the U.S. and is a leading cause of liver-related morbidity and mortality.1 , 2 An estimated 2.4 million adults are living with HCV infection in the U.S., and hepatitis C was reported as the underlying or contributing cause for 15,713 deaths in 2018.1 Approximately 70% of adults with acute HCV develop chronic HCV infection, and if untreated, 1 in 4 of these individuals will die prematurely from HCV-associated complications, such as liver failure and hepatocellular carcinoma.2 During 2015–2018, only 61% (95% CI=46, 74) of people diagnosed with hepatitis C reported having ever been told they were infected, suggesting a gap in care.3
Healthcare screenings are essential for early identification of disease before severe morbidity or mortality occur. Hepatitis C testing is the first step in linking people with HCV infection to care and curative treatment. In 2012, the Centers for Disease Control and Prevention (CDC) recommended a 1-time HCV antibody test for all individuals born during 1945–1965 (baby boomers).4 In 2013, the U.S. Preventive Services Task Force followed with recommendations for hepatitis C testing of those at high risk for infection, such as injection drug users, and a 1-time test for all adults born during 1945–1965.5 More recently, in response to increasing rates of acute HCV among young adults, including reproductive-aged people, the U.S. Preventive Services Task Force recommends screening for HCV infection in adults (including pregnant women) aged 18–79 years,6 and CDC recommends hepatitis C testing at least once per lifetime, in settings with a prevalence of HCV infection ≥0.1%, for all adults aged ≥18 years and for all pregnant women during each pregnancy.7
Fortunately, an effective and curative treatment is available.8 Direct-acting antiretroviral (DAA) therapies, first introduced in 2013, have improved hepatitis C treatment success and reduced adverse drug events relative to interferon therapies.9 , 10 Improvements have also been observed in the cascade of care among patients when restrictions to effective pharmaceuticals based on risk behavioral profiles have been removed. As a result, WHO established ambitious targets for hepatitis C elimination by 2030.11
During 2020, the coronavirus disease 2019 (COVID-19) pandemic has impacted the practice of medicine.12 , 13 CDC released guidance on delaying nonessential procedures and postponing routine clinical visits as part of initial mitigation strategies for the COVID-19 pandemic.14 As part of this guidance, patients have been advised to utilize telemedicine, patient portals, phone, and e-mail for communication with their healthcare provider and to limit in-person visits.15
Deferral of healthcare services during the pandemic may lead to delays in diagnosis and treatment, leaving people living with hepatitis C unaware of their disease status and vulnerable to progression to advanced liver disease, including cirrhosis and hepatocellular carcinoma, as well as potential spread of the virus.16 Changes are examined in provided healthcare services during the COVID-19 pandemic relative to prior years with a focus on HCV antibody testing and positivity, HCV RNA positivity, and hepatitis C drug treatment prescriptions.
METHODS
Study Sample
Clinical laboratory results were extracted from the database of Quest Diagnostics, a large clinical laboratory test provider throughout the U.S. Deidentified person-level data from HCV antibody immunoassay testing and RNA diagnostic tests ordered January 1, 2018–August 8, 2020, were included. Patient age and sex were included in the analysis, when available. Data extracted from Quest Diagnostics were determined not to be individually identifiable.
All specimens with positive antibody test results reflex to HCV RNA quantitative testing, used to diagnose current infection. HCV qualitative immunoglobulin G antibody testing was performed using the U.S. Food and Drug Administration–cleared, automated VITROS ECi Immunodiagnostic System (Ortho Clinical Diagnostics). The Food and Drug Administration–cleared HCV RNA test methods included the COBAS AmpliPrep/COBAS TaqMan HCV v2.0 (Quantitative) method and COBAS HCV Quantitative nucleic acid test for use on the COBAS 6800/6880 systems (both from Roche Diagnostics).
Measures
For each year from 2018 to 2020, the total number of HCV antibody tests, HCV antibody–positive test results, and HCV RNA–positive test results were calculated by month from January through July. All specimens that produced an HCV antibody–positive test result were reflexed to RNA testing. The average number of HCV antibody tests, HCV antibody–positive test results, and HCV RNA tests and positive results was calculated by month from January through July for 2018 and 2019 to compare with the same time during 2020. Percentage change was calculated by month between the 2018 and 2019 monthly average and the corresponding month in 2020. For the HCV antibody tests, comparison was provided by week for the same period, January–July, to provide temporal granularity. Repeat laboratory testing within a calendar month was highly unlikely, and such duplicate events were not excluded. This activity was determined by CDC to be research, as defined in 46.102(l), but did not involve human subjects, as defined in 46.102(e)(1)(ii).
To evaluate the impact of the pandemic on hepatitis C treatment prescriptions, hepatitis C DAA medications (Appendix 1, available online) dispensed from January 2018 to July 2020 were examined. New and refilled pharmacy transactions from the IQVIA National Prescription Audit Extended Insights database include outpatient medications dispensed through retail pharmacies, mail-order prescriptions, and the vast majority of prescriptions dispensed in pharmacies serving long-term care facilities. The IQVIA pharmacy transaction data were estimated to cover 92% of all outpatient retail prescription fills and 78% of all mail-order prescription fills in all 50 U.S. states and the District of Columbia and were weighted to be nationally representative of all dispensed prescriptions in these channels. The total volume of dispensed prescriptions included new and refilled prescriptions.
For each year from 2018 to 2020, the total number of dispensed prescriptions was calculated by month, January–July. The average number of dispensed prescriptions was also estimated by month, January–July, for 2018 and 2019 to compare with the same time in 2020. Percentage change was calculated by month, January–July, between the 2018 and 2019 monthly average and the corresponding month in 2020. Multiple prescriptions within a calendar month were not excluded because these would be unlikely events.
Statistical Analysis
Trends in all outcomes were tested for deviations in counts that occurred during the pandemic period. For the weekly HCV antibody test counts, negative binomial models were used and included terms for year, week, and pandemic period. For the monthly outcomes, negative binomial models were used that included terms for year, month, and pandemic period. All statistical analyses were performed using SAS, version 9.4.
RESULTS
The analysis included 12,309,475 HCV antibody test results and 326,603 HCV RNA test results. Limiting HCV antibody testing to March–July for 2018–2020, the study included 5,587,943 results (Table 1 ). Majority of the antibody testing occurred among those aged 20–84 years: 95.6% for 2018, 95.4% for 2019, and 95.5% for 2020. HCV antibody testing shifted from 2018 and 2019 to 2020 toward a higher percentage of patients aged 20–39 years (39.2% in 2018 and 2019 to 43.1% in 2020) and toward a corresponding lower percentage of patients aged 40–84 years (56.4% in 2018 and 2019 to 52.4% in 2020). There was a shift toward a higher percentage of female patients (56.5% in 2018 and 2019 to 58.4% in 2020).
Table 1.
Demographics of Persons Who Received HCV Antibody Testing and HCV Treatment Prescriptions
| Variables | HCV antibody testing by year, age, sex |
HCV treatment prescriptions by year, age, sex |
||||
|---|---|---|---|---|---|---|
| 2018 (March‒July), | 2019 (March‒July), | 2020 (March‒July), | 2018 (January‒July), | 2019 (January‒July), | 2020 (January‒July), | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Age, years | ||||||
| Unknown | 1,493 (0.1) | 1,253 (0.1) | 737 (0.1) | — | — | — |
| 0‒19 | 78,639 (3.8) | 82,813 (4.0) | 56,797 (3.9) | 470 (0.3) | 482 (0.3) | 605 (0.5) |
| 20‒39 | 789,686 (37.8) | 831,065 (40.6) | 625,175 (43.1) | 26,240 (14.1) | 31,963 (19.0) | 31,968 (24.1) |
| 40‒59 | 693,239 (33.2) | 659,700 (32.2) | 452,357 (31.2) | 75,091 (40.4) | 65,798 (39.0) | 50,729 (38.2) |
| 60‒84 | 514,717 (24.6) | 463,625 (22.6) | 308,215 (21.2) | 83,392 (44.9) | 69,730 (41.4) | 49,126 (37.0) |
| ≥85 | 10,742 (0.5) | 10,096 (0.5) | 7,594 (0.5) | 518 (0.3) | 542 (0.3) | 292 (0.2) |
| Sex | ||||||
| Female | 1,180,244 (56.5) | 1,156,144 (56.4) | 847,386 (58.4) | 70,828 (38.2) | 64,836 (38.5) | 51,200 (38.6) |
| Male | 902,867 (43.2) | 886,914 (43.3) | 599,938 (41.4) | 114,467 (61.8) | 103,390 (61.5) | 81,460 (61.4) |
| Unknown | 5,405 (0.3) | 5,494 (0.3) | 3,551 (0.2) | — | — | — |
| Total | 2,088,516 (100.0) | 2,048,552 (100.0) | 1,450,875 (100.0) | 185,295 (100.0) | 168,226 (100.0) | 132,660 (100.0) |
HCV, Hepatitis C virus.
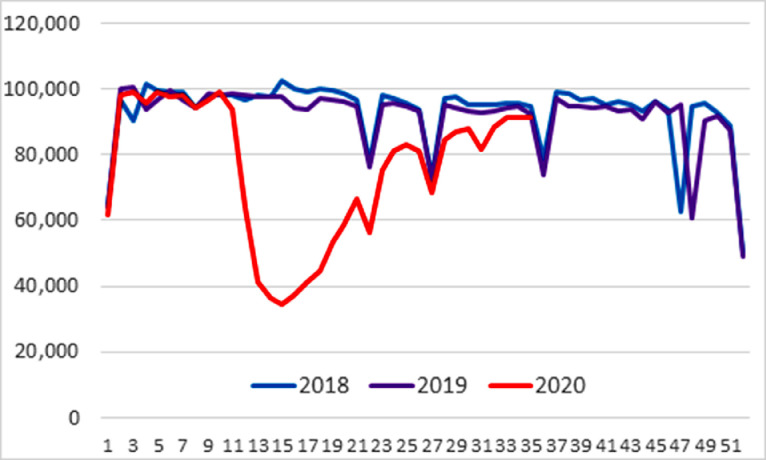
Figure 1 shows the weekly HCV antibody test volume. Related to the COVID-19 pandemic, test volume began decreasing in mid-March 2020. At the lowest levels of testing in 2020, the weekly test volume declined by 65.6% in the second week of April relative to the corresponding week in 2018 and 2019. In July, the relative decline (range=5.6%–11.8%) was substantially smaller than observed during the earlier months, March–June. The decrease in HCV antibody testing during the pandemic period was statistically significant (b= −0.440; 95% CI= −0.580, −0.310; p<0.001). In addition, the number of HCV RNA–positive tests declined approximately 62.3% in April 2020 and remained relatively low through July, compared with 2018 and 2019. HCV RNA–positive tests were already approximately 14% lower in January and February 2020 (prepandemic) than in the same months in the 2 prior years (Table 2 ). The decrease in HCV antibody–positive results during the pandemic period was statistically significant (b= −0.410; 95% CI= −0.580, −0.240; p<0.001). This decline, when combined with the observed HCV antibody–positive result volume reduction in subsequent months relative to the 2 prior years, yielded a similar pattern of HCV RNA–positive volume decline as the HCV antibody–positive decrease for March–July 2020, relative to 2018 and 2019. Specifically, the decline in HCV RNA–positive tests declined approximately 40% in June and July, relative to 2018 and 2019, which reflects the combination of the 27% decrease for HCV antibody–positive tests in June and July 2020, relative to 2018 and 2019, and the underlying approximately 14% reduction observed prepandemic in January and February 2020, relative to the same time periods in 2018 and 2019. The decrease in HCV RNA–positive results during the pandemic period was statistically significant (b= −0.450; 95% CI= −0.600, −0.290; p<0.001).
Figure 1.
HCV antibody test volume, 2018 through 2020, by MMWR week.
Note: Sporadic dips reflect national holiday weeks. The MMWR week is the week of the epidemiologic year for which the NNDSS disease report is assigned by the reporting local or state health department for the purposes of MMWR disease incidence reporting and publishing. Values for MMWR week range from 1 to 53, although most years consist of 52 weeks.
HCV, Hepatitis C virus; MMWR, Morbidity and Mortality Weekly Report; NNDSS, National Notifiable Diseases Surveillance System.
Table 2.
HCV Laboratory Testing and HCV Treatment Prescriptions, January‒July, 2018‒2020
| Months | 2018 | 2019 | 2020 | Baseline average (2018 and 2019) | 2020 compared with baseline, percent change |
|---|---|---|---|---|---|
| Antibody testing | |||||
| January | 406,102 | 420,084 | 419,326 | 413,093 | 1.5 |
| February | 392,345 | 388,853 | 396,350 | 390,599 | 1.5 |
| March | 436,998 | 421,331 | 311,510 | 429,165 | −27.4 |
| April | 412,803 | 414,479 | 168,126 | 413,641 | −59.4 |
| May | 431,880 | 419,572 | 248,999 | 425,726 | −41.5 |
| June | 411,760 | 389,315 | 345,964 | 400,538 | −13.6 |
| July | 395,075 | 403,855 | 376,276 | 399,465 | −5.8 |
| Antibody positives | |||||
| January | 22,910 | 23,451 | 22,263 | 23,181 | −4.0 |
| February | 21,470 | 21,760 | 21,464 | 21,615 | −0.7 |
| March | 24,805 | 23,622 | 17,744 | 24,214 | −26.7 |
| April | 23,831 | 23,106 | 10,186 | 23,469 | −56.6 |
| May | 24,466 | 23,493 | 13,137 | 23,980 | −45.2 |
| June | 22,714 | 21,471 | 15,907 | 22,093 | −28.0 |
| July | 21,782 | 22,273 | 16,330 | 22,028 | −25.9 |
| RNA positives | |||||
| January | 13,272 | 13,126 | 11,164 | 13,199 | −15.4 |
| February | 12,343 | 12,147 | 10,628 | 12,245 | −13.2 |
| March | 14,261 | 13,175 | 8,637 | 13,718 | −37.0 |
| April | 13,674 | 12,720 | 4,980 | 13,197 | −62.3 |
| May | 13,928 | 12,895 | 6,152 | 13,412 | −54.1 |
| June | 12,872 | 11,654 | 7,215 | 12,263 | −41.2 |
| July | 12,336 | 11,918 | 7,345 | 12,127 | −39.4 |
| Prescriptions | |||||
| January | 26,755 | 22,533 | 21,032 | 24,644 | −14.7 |
| February | 25,138 | 22,266 | 20,894 | 23,702 | −11.8 |
| March | 28,196 | 24,148 | 24,389 | 26,172 | −6.8 |
| April | 27,314 | 25,887 | 20,568 | 26,601 | −22.7 |
| May | 28,366 | 26,008 | 15,455 | 27,187 | −43.2 |
| June | 24,955 | 22,870 | 15,081 | 23,913 | −36.9 |
| July | 25,046 | 24,858 | 15,399 | 24,952 | −38.3 |
Note: All Quest Diagnostic specimens with HCV antibody–positive results are reflexed to RNA testing; therefore, the number of RNA tests have been excluded from this study because it would be redundant information with respect to HCV antibody–positive results.
HCV, Hepatitis C virus.
Among all DAA prescriptions during January–July, the percentage dispensed to patients aged 60–84 years decreased by year: 44.9% in 2018, 41.4% in 2019, and 37.0% in 2020. By contrast, of all dispensed DAA treatment prescriptions, those for patients aged 20–39 years increased: 14.1% for 2018, 19.0% for 2019, and 24.1% for 2020 (Table 1). In each of the 3 years, a greater proportion (61.4%–61.8%) of DAA prescriptions was consistently dispensed to male than to female patients.
The volume of treatment prescriptions dispensed January–July decreased by 9.3% from 2018 (185,770) to 2019 (168,570) (Table 2). In 2019, the volume of dispensed DAAs was less than in 2018, but the monthly dispensing trends were similar. February of each year was a low point (25,138 in 2018; 22,266 in 2019) and May was a high point (28,366 in 2018; 26,008 in 2019). The decrease in prescriptions during the pandemic period was statistically significant (b= −0.280; 95% CI= −0.410, −0.015; p<0.001).
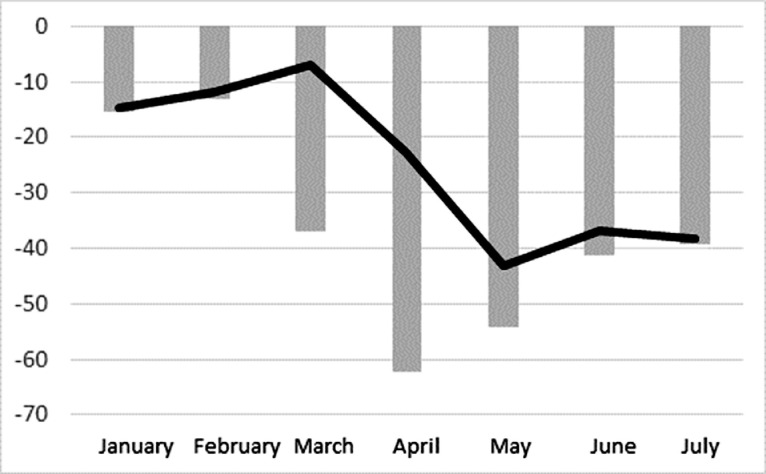
During January–March 2020, the trends of dispensed DAAs were similar to 2018 and 2019, although the volume in 2020 was 11.0% less than the average in the previous 2 years (66,315 vs 74,518). The volume of dispensed DAAs decreased 22.7% in April 2020 compared with the April average in 2018 and 2019 (20,568 vs 26,601) and continued to decrease by 39.6% in May–July 2020 compared with the average for the same months in 2018 and 2019 (45,935 vs 76,052). When volumes of dispensed DAAs were compared with HCV RNA tests, the declines in both tests and prescriptions were similar over the prepandemic months of January and February 2020 and the pandemic months of May–July (Figure 2 ). However, HCV RNA testing declined substantially more in March and April, with the biggest decline in HCV RNA–positive tests in April and the biggest decline for dispensed DAAs a month later in May.
Figure 2.
Monthly change in HCV RNA–positive test results and HCV treatment prescriptions in 2020 versus a combined 2018 and 2019 baseline.
Note: Gray bars represent percent change in HCV RNA–positive test results in 2020 versus a combined 2018 and 2019 baseline. Black line represents percent change in HCV treatment prescriptions in 2020 versus a combined 2018 and 2019 baseline.
HCV, Hepatitis C virus.
DISCUSSION
Reduction in hepatitis C testing and treatment during the COVID-19 pandemic has the potential to increase HCV-associated morbidity and mortality. Much of the focus of hepatitis C testing efforts has been in emergency department and primary care settings.16 During the early months of the COVID-19 pandemic, most nonurgent health care was suspended or delayed, and emergency department visits decreased.17 Although rates of outpatient visits started to decline in mid-March and began to increase again in May 2020, they remained substantially below prepandemic numbers from 2018 and 2019.14 , 18 The level of HCV antibody testing substantially declined and nearly returned to prepandemic levels by late July. In contrast, HCV antibody–positive results, HCV RNA–positive results, and prescriptions dispensed did not return to prepandemic levels and continued to be low relative to 2018 and 2019 measures. This sharper decline in HCV antibody–positive and RNA-positive results than those observed through HCV antibody testing in June and July suggests that many patients who returned for testing in June and July might have been lower-risk individuals, implying a potential significant gap in higher-risk patients.
Supporting these findings, a study reported that declines in HCV antibody testing and new patient identification declined sharply in the 3.5 months after March 16, 2020.19 Hospital-wide testing and new patient identification fell by 49.6% and 42.1%, respectively. As expected, ambulatory-only testing and new patient identification fell more sharply, by 71.9% and 63.3%.
Consequences of delays in identification of hepatitis C–infected individuals and their subsequent receipt of curative treatment will increase potential transmission of HCV infection to others and, in the long term, increase the risk of disease progression of untreated individuals.16 Patients with advanced liver disease because of HCV infection are at risk for death and frequently need hepatic transplantation; however, effective antiviral therapy can avert these severe outcomes.20 Future studies could examine how many patients who likely missed screening opportunities during the pandemic will develop HCV-associated complications, including advanced liver disease, liver failure, need for liver transplantation, or liver cancer.
A delay is expected between hepatitis C diagnosis (i.e., a positive HCV RNA result) and subsequent HCV antiviral treatment administration.21 The reduction in prescriptions may have continued beyond the time that HCV screening has returned to near previous levels, owing to the time for patient referral to specialists and appointment scheduling, prescription authorization, and patient delays in seeking and obtaining appointments and authorizations.22 Factors influencing prescription administration delays include insurance coverage,23 , 24 racial and ethnic disparities,25 , 26 and substance use.27 Nevertheless, delays in diagnosis may result in significant delays in treatments. The lower number of HCV diagnoses (HCV RNA–positive test results) during the pandemic, extending at least through July 2020, may result in extended decreases in prescribed treatments. Further study is needed to evaluate the duration of the declines noted in this study and health disparities, particularly for those in historically underserved communities with more limited access to health care. Also worthy of study is how much the role of telemedicine during the pandemic has affected patients obtaining prescriptions, including the duration of each treatment prescription/refill.
An important observation is that over time, laboratory testing and treatment is shifting toward younger patients, aged 20–39 years (i.e., those born 1980 through 2000), born after the baby boomer birth cohort. This shift toward younger patients predates recent changes in HCV testing guidelines7 , 8 and likely reflects greater awareness of both the known risk behaviors associated with HCV acquisition and the recent injection opioid epidemic among young adults. Nevertheless, the shift toward younger adult patients for both testing and treatment is extremely encouraging in that the new guidelines are targeted largely at younger age groups.
Groups with high prevalence of chronic HCV infection, including people with substance use disorder who inject drugs, people with HIV, and incarcerated populations, are also likely to be severely affected by the COVID-19 pandemic and disruptions in the continuum of care, given the already existing challenges with linkage to care for these vulnerable populations.28, 29, 30 Key factors contributing to patients missing appropriate medical care during the pandemic include type of employment, (e.g., lower-wage jobs and lost employment),31 living circumstances, (e.g., population and household density and inability to work from home), and reduced access to healthcare services,32 factors disproportionately affecting non-Hispanic Black and Hispanic communities.33 This evidence suggests the need to expand HCV treatment with DAAs to populations at risk for adverse outcomes, such as those with lower socioeconomic means.
Volume of HCV antibody testing was evaluated at a national level; subanalyses on testing in specific populations were not conducted. However, based on national surveys and assessments, HCV testing trends among groups with high hepatitis C prevalence, such as people with substance use disorder who inject drugs, people born during 1945–1965 (baby boomers), and non-Hispanic Black individuals, likely follow the national testing trends.25 People with substance use disorder who inject drugs are further impacted by a decrease in syringe service providers, including HCV testing, harm reduction (e.g., sterile injection equipment), and linkage to care services (e.g., curative treatment).26 In addition, continued vigilance is needed in testing people born during 1945–1965, who are at the highest risk for dying of hepatitis C–related causes owing to increasing likelihood of having had HCV infection for a longer time. Lack of hepatitis C testing and treatment also has the risk of further widening racial/ethnic disparities and further impacting non-Hispanic Black and American Indian/Alaska Native populations, which are both disproportionately impacted by the COVID-19 pandemic.31 , 32 Thus, decreases in testing and treatment in vulnerable communities and among those with high hepatitis C prevalence have the risk of increasing HCV transmission among untreated people unaware of their status, further increasing hepatitis C incidence and prevalence in the U.S. and moving us further from the hepatitis C elimination goals.
There were several strengths of this study, including (1) distinctive yet complementary views of data from clinical laboratory testing and prescription data and (2) broad geographic coverage and scale provided by each data set. The reported 180,321 cases of reported HCV RNA positives per year (average of first half-years of 2018 and 2019, doubled for estimated full year) listed in Table 1 are 128% of CDC-reported cases of acute and chronic HCV in the U.S. (2018).34 This 128% figure recognizes both the scale of the clinical laboratory test results and under-reporting by public health departments to CDC.
Limitations
Limitations are noted. First, generalizability is limited because the information reported does not represent all HCV laboratory testing in the U.S. If race and ethnicity designation were available, the information may have provided additional information about likely disparities in health care. Second, concurrent existing prescriptions for those who fill DAA prescriptions were unavailable. Third, DAA prescriptions were limited to outpatient retail dispensing and may not include other ways patients can access medications, including pharmaceutical-sponsored patient assistance programs.35
CONCLUSIONS
This paper reports missed hepatitis C testing and treatment opportunities during the COVID-19 pandemic that may have severe consequences for those with hepatitis C and those at risk of infection (e.g., exposure to virus through contact with people who are undiagnosed or untreated). Healthcare systems design should consider new approaches that carefully balance public health strategies that deploy limited resources wisely.36 , 37 This should involve improved access to healthcare services, especially among those who are uninsured and underinsured; community engagement and outreach; and expansion of telemedicine, including safe laboratory testing options. Generally speaking, healthcare systems should focus on both the COVID-19 pandemic and the unintended consequences of delayed diagnosis and treatment for other non–COVID-19 diseases. This may include developing novel public health messaging, interventions, and outreach programs for specific populations that may have lost access to effective prevention programs designed to detect and cure diseases’ earlier stages.
ACKNOWLEDGMENTS
The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention or the authors’ affiliated institutions.
HWK, WAM III, and XH are employees of and own stock in Quest Diagnostics. No other financial disclosures were reported.
Footnotes
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2021.03.011.
Appendix. SUPPLEMENTAL MATERIAL
REFERENCES
- 1.Centers for Disease for Control and Prevention; August 7, 2020. 2018 Surveillance report.https://www.cdc.gov/hepatitis/statistics/2018surveillance/index.htm Updated. [Google Scholar]
- 2.Seo S, Silverberg MJ, Hurley LB. Prevalence of spontaneous clearance of hepatitis C virus infection doubled from 1998 to 2017. Clin Gastroenterol Hepatol. 2020;18(2):511–513. doi: 10.1016/j.cgh.2019.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryerson AB, Schillie S, Barker LK, Kupronis BA, Wester C. Vital signs: newly reported acute and chronic hepatitis C cases - United States, 2009-2018. MMWR Morb Mortal Wkly Rep. 2020;69(14):399–404. doi: 10.15585/mmwr.mm6914a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colussi G, Donnini D, Brizzi RF. Sustained virologic response to direct-acting antiviral agents predicts better outcomes in hepatitis C virus-infected patients: a retrospective study. World J Gastroenterol. 2019;25(40):6094–6106. doi: 10.3748/wjg.v25.i40.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith BD, Morgan RL, Beckett GA. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. [published correction appears in MMWR Recomm Rep. 2012;61(43):886] MMWR Recomm Rep. 2012;61(RR-4):1–32. www.cdc.gov/mmwr/preview/mmwrhtml/rr6104a1.htm Updated. [PubMed] [Google Scholar]
- 6.Moyer VA. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349–357. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Preventive Services Task Force Screening for hepatitis C virus infection in adolescents and adults: screening: U.S. Preventative Services Task Force recommendations statement. JAMA. 2020;323(10):970–975. doi: 10.1001/jama.2020.1123. [DOI] [PubMed] [Google Scholar]
- 8.Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB. CDC recommendations for hepatitis C screening among adults — United States, 2020. MMWR Recomm Rep. 2020;69(2):1–17. doi: 10.15585/mmwr.rr6902a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowdley KV, Gordon SC, Reddy KR. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 10.Lawitz E, Mangia A, Wyles D. Vol. 368. 2013. Sofosbuvir for previously untreated chronic hepatitis C infection; pp. 1878–1887. (N Engl J Med). [DOI] [PubMed] [Google Scholar]
- 11.WHO . WHO; Geneva, Switzerland: 2013. Global policy report on the prevention and control of viral hepatitis in WHO member states.http://apps.who.int/iris/bitstream/10665/85397/1/9789241564632_eng.pdf Published. [Google Scholar]
- 12.Rosenbaum L. The untold toll - the pandemic's effects on patients without COVID-19. N Engl J Med. 2020;382(24):2368–2371. doi: 10.1056/NEJMms2009984. [DOI] [PubMed] [Google Scholar]
- 13.Czeisler MÉ, Marynak K, Clarke KEN. Delay or avoidance of medical care because of COVID-19–related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250–1257. doi: 10.15585/mmwr.mm6936a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Healthcare facilities: managing operations during the COVID-19 pandemic. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-hcf.html#outpatient-ambulatory. Accessed August 18, 2020.
- 15.Fu B, Wang W, Shi X. Impact of delayed diagnosis time in estimating progression rates to hepatitis C virus-related cirrhosis and death. Stat Methods Med Res. 2015;24(6):693–710. doi: 10.1177/0962280211424667. [DOI] [PubMed] [Google Scholar]
- 16.Guss D, Sherigar J, Rosen P, Mohanty SR. Diagnosis and management of hepatitis C infection in primary care settings. J Gen Intern Med. 2018;33(4):551–557. doi: 10.1007/s11606-017-4280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephenson J. JAMA Health Forum. 2020. As outpatient visits rebound, COVID-19 pandemic's threat to outpatient care remains. In press. Online May 29. [DOI] [PubMed] [Google Scholar]
- 18.Hartnett KP, Kite-Powell A, DeVies J. Impact of the COVID-19 pandemic on emergency department visits - United States, January 1, 2019-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(23):699–704. doi: 10.15585/mmwr.mm6923e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sperring H, Ruiz-Mercado G, Schechter-Perkins EM. Impact of the 2020 COVID-19 pandemic on ambulatory hepatitis C testing. J Prim Care Community Health. 2020;11 doi: 10.1177/2150132720969554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beste LA, Green P, Berry K, Belperio P, Ioannou GN. Hepatitis C-related hepatocellular carcinoma incidence in the Veterans Health Administration after introduction of direct-acting antivirals. JAMA. 2020;324(10):1003–1005. doi: 10.1001/jama.2020.10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice DP, Ordoveza MA, Palmer AM, Wu GY, Chirch LM. Timing of treatment initiation of direct-acting antivirals for HIV/HCV coinfected and HCV monoinfected patients. AIDS Care. 2018;30(12):1507–1511. doi: 10.1080/09540121.2018.1499857. [DOI] [PubMed] [Google Scholar]
- 22.Spradling PR, Xing J, Rupp LB. Low uptake of direct-acting antiviral therapy among hepatitis C patients with advanced liver disease and access to care, 2014-2017. J Clin Gastroenterol. 2021;55(1):77–83. doi: 10.1097/MCG.0000000000001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo Re V, 3rd, Gowda C, Urick PN. Disparities in absolute denial of modern hepatitis C therapy by type of insurance. Clin Gastroenterol Hepatol. 2016;14(7):1035–1043. doi: 10.1016/j.cgh.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcus JL, Hurley LB, Chamberland S. Disparities in initiation of direct-acting antiviral agents for hepatitis C virus infection in an insured population. Public Health Rep. 2018;133(4):452–460. doi: 10.1177/0033354918772059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufman HW, Niles JK, Nash DB. Disparities in SARS-CoV-2 positivity rates: associations with race and ethnicity. Popul Health Manag. 2021;24(1):20–26. doi: 10.1089/pop.2020.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glick SN, Prohaska SM, LaKosky PA, Juarez AM, Corcorran MA, Des Jarlais DC. The impact of COVID-19 on syringe services programs in the United States. AIDS Behav. 2020;24(9):2466–2468. doi: 10.1007/s10461-020-02886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morano JP, Zelenev A, Lombard A, Marcus R, Gibson BA, Altice FL. Strategies for hepatitis C testing and linkage to care for vulnerable populations: point-of-care and standard HCV testing in a mobile medical clinic. J Community Health. 2014;39(5):922–934. doi: 10.1007/s10900-014-9932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kronfli N, Linthwaite B, Kouyoumdjian F. Interventions to increase testing, linkage to care and treatment of hepatitis C virus (HCV) infection among people in prisons: a systematic review. Int J Drug Policy. 2018;57:95–103. doi: 10.1016/j.drugpo.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Bajis S, Dore GJ, Hajarizadeh B, Cunningham EB, Maher L, Grebely J. Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: a systematic review. Int J Drug Policy. 2017;47:34–46. doi: 10.1016/j.drugpo.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Burgard SA, Lin KY. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am Behav Sci. 2013;57(8):1105–1127. doi: 10.1177/0002764213487347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94(8):666–668. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2594273/pdf/jnma00325-0024.pdf Both articles were accessed Accessed December 28, 2020. [PMC free article] [PubMed] [Google Scholar]
- 33.Selden TM, Berdahl TA. COVID-19 and racial/ethnic disparities in health risk, employment, and household composition. Health Aff (Millwood) 2020;39(9):1624–1632. doi: 10.1377/hlthaff.2020.00897. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention; August 28, 2020. Viral hepatitis surveillance report 2018 – Hepatitis C.https://www.cdc.gov/hepatitis/statistics/2018surveillance/HepC.htm Updated. [Google Scholar]
- 35.Ali S, Ur-Rehman T, Ali M. Improving access to the treatment of hepatitis C in low- and middle-income countries: evaluation of a patient assistance programme. Int J Clin Pharm. November 28, 2020 doi: 10.1007/s11096-020-01202-1. In press. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valdiserri R, Billioux A, Ntiri-Reid B, Canary L. HealthAffairs; Bethesda, MD: June 26, 2020. The uses of adversity: leveraging the COVID-19 response to eliminate viral hepatitis.https://www.healthaffairs.org/do/10.1377/hblog20200624.84875/full/ Published. [Google Scholar]
- 37.Siddiqui NJ, Andrulis DP, Chapman DA. National Collaborative for Health Equity; Washington, DC: June 26, 2020. The COVID-19 crisis: an opportunity to build a fairer, healthier nation.https://www.nationalcollaborative.org/the-covid-19-crisis-an-opportunity-to-build-a-fairer-healthier-nation/ Published. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.