Abstract
Transient receptor potential vanilloid 4 (TRPV4) channels are widely expressed in systemic tissues and can be activated by many stimuli. TRPV4, a Ca2+-permeable cation channel, plays an important role in the vasculature and is implicated in the regulation of cardiovascular homeostasis processes such as blood pressure, vascular remodeling, and pulmonary hypertension and edema. Within the vasculature, TRPV4 channels are expressed in smooth muscle cells, endothelial cells, and perivascular nerves. The activation of endothelial TRPV4 contributes to vasodilation involving nitric oxide, prostacyclin, and endothelial-derived hyperpolarizing factor pathways. TRPV4 activation also can directly cause vascular smooth muscle cell hyperpolarization and vasodilation. In addition, TRPV4 activation can evoke constriction in some specific vascular beds or under some pathological conditions. TRPV4 participates in the control of vascular permeability and vascular damage, particularly in the lung capillary endothelial barrier and lung injury. It also participates in vascular remodeling regulation mainly by controlling vasculogenesis and arteriogenesis. This review examines the role of TRPV4 in vascular function, particularly in vascular dilation and constriction, vascular permeability, vascular remodeling, and vascular damage, along with possible mechanisms, and discusses the possibility of targeting TRPV4 for therapy.
Keywords: TRPV4, vasodilation, vasoconstriction, vascular permeability, vascular remodeling, vascular damage, therapeutic target
Introduction
Increasing evidence has shown that ion channels play numerous important roles in cell homeostasis, allowing the passage of specific ions (Hubner and Jentsch, 2002; Jentsch et al., 2004). Ion channels are critical for a variety of physiological and pharmacological functions, and their dysfunction can cause channelopathies associated with many kinds of diseases (Liu and Wang, 2019). Transient receptor potential (TRP) channels are non-selective cation channels originally found in Drosophila melanogaster, which form a large superfamily of cation channels involved in many sensory and signal transduction processes (Earley and Brayden, 2015). Activated TRPs depolarize the cellular membrane and activate voltage-dependent ion channels, leading to changes in intracellular Ca2+ concentrations ([Ca2+]i), playing an important regulatory role in cells (Michel, 2006).
The mammalian TRP channels can be subdivided into six subfamilies according to amino acid sequence homology: vanilloid (TRPV), classical or canonical (TRPC), polycystin (TRPP), melastatin (TRPM), ankyrin (TRPA), and mucolipin (TRPML) (Yin and Kuebler, 2010; Nilius and Owsianik, 2011). The TRP superfamily is implicated in a variety of external or internal stimuli-sensing and transmission functions including taste, smell, vision, pain, temperature, pH, osmotic pressure, mechanical stress, and many endogenous and exogenous ligands (Wong and Yao, 2011). Generally, TRPs reside in the plasma membrane, assembling homo- or hetero-oligomeric polymers into functional channels (Schaefer, 2005; He and Ma, 2016).
Transient receptor potential vanilloids contain six members (TRPV1–TRPV6) involved in many cellular functions (Randhawa and Jaggi, 2015a). TRPV4 is an osmo-mechanosensitive channel, which is permeable to non-selective cations, such as Ca2+, Na+, and Mg2+ ions (Randhawa and Jaggi, 2015b). Structurally, TRPV4 is an 871 amino acid protein with six transmembrane domains, an ion pore between the fifth and sixth transmembrane domains, an NH2-terminal ankyrin repeat domain, and a COOH-terminal cytoplasmic domain (Scheraga et al., 2017). The long amino terminus contains a proline-rich domain and a phosphoinositide-binding domain, and the carboxyl terminus contains a TRP box, a Ca2+/calmodulin (CaM)-binding region, and a PDZ domain (Goldenberg et al., 2015a). TRPV4 channels are expressed in most tissues, such as the vasculature, lungs, brain, heart, kidneys, salivary glands, liver, bladder, trachea, skin, bone, spleen, testes, dorsal root ganglions, and trigeminal ganglions (Goldenberg et al., 2015a; Randhawa and Jaggi, 2015b; Cui et al., 2020). Cell swelling activates TRPV4 by the phospholipase A2 (PLA2)-dependent generation of arachidonic acid (AA), and its subsequent metabolization to 5,6-epoxyeicosatrienoic acid (5,6-EET) by a cytochrome p450 (CYP) epoxygenase-dependent pathway (Vriens et al., 2004). Phorbol esters and heat activate TRPV4 depending on an aromatic residue at the N terminus of the third transmembrane domain (Vriens et al., 2004), suggesting that stimuli activating TRPV4 promotes channel-opening via distinct pathways. The main endogenous agonists include mechanical flow stimuli, lipid mediator AA (Cao et al., 2018), and its EET metabolites (Kotlikoff, 2005). The synthetic ligands 4α-phorbol 12,13-didecanoate (4α-PDD) (Vriens et al., 2007) and GSK1016790A (Thorneloe et al., 2017) can also activate TRPV4, as well as several natural products such as apigenin (Wei et al., 2017), eugenol (Peixoto-Neves et al., 2015), morin (Zhang et al., 2019), curcumin (Shao et al., 2019), hydroxysafflor yellow A (Yang et al., 2020), omega-3 fatty acid, (Zhu et al., 2020) and puerarin (Zhou et al., 2020). Additional antagonists of TRPV4 include RN1734 (Greenberg et al., 2017), HC067047 (Maqboul and Elsadek, 2018), GSK2193874 (Thorneloe et al., 2017), AB159908 (Arora et al., 2017), and GSK2798745 (Brooks et al., 2019).
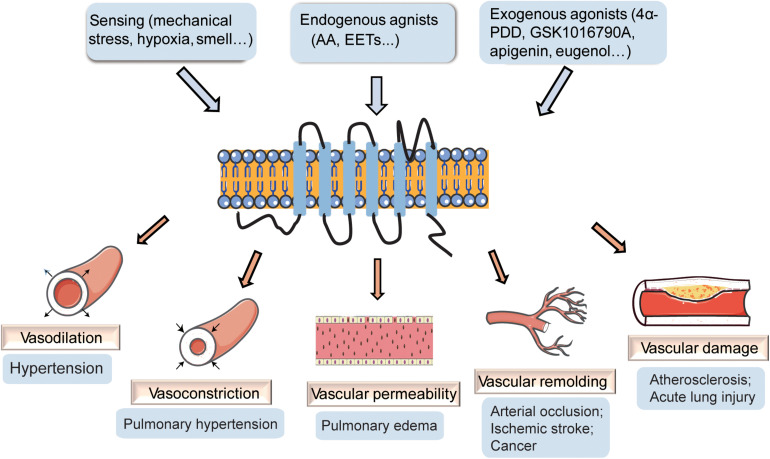
Transient receptor potential vanilloid 4 (TRPV4) channels have been reported to play vital roles in the regulation of cardiovascular homeostasis and are critically implicated in the regulation of cardiac remodeling, blood pressure, hypoxic preconditioning, pulmonary hypertension, and pulmonary edema (Randhawa and Jaggi, 2015b). TRPV4 channels are present in vascular smooth muscle cells (VSMCs), vascular endothelial cells, and perivascular nerves (Negri et al., 2019), and increasing studies have revealed the role of TRPV4 channels in the maintenance of vascular function. In the current review, we examine the reports on the involvement of TRPV4 in vascular function (Figure 1), particularly in vascular dilation and constriction, vascular permeability, vascular remodeling, and vascular damage, along with possible mechanisms and discuss its potential as a therapeutic target for vascular diseases.
FIGURE 1.
The effect of transient receptor potential vanilloid 4 (TRPV4) on vasculature. TRPV4 channels can be activated by stimuli-sensing, endogenous agonists, and exogenous agonists. The activation of TRPV4 participates in the regulation of vascular dilation and constriction, vascular permeability, vascular remolding, and vascular damage, playing important roles in vascular related diseases.
Transient Receptor Potential Vanilloid 4 and Vasodilation
Endothelium-Dependent Vasodilation
TRPV4 is expressed in rodent endothelial cells in both large-conductance and small resistance vessels (Filosa et al., 2013). The functional role of endothelial TRPV4 in vascular tone and reactivity has been largely investigated. The activation of TRPV4 contributes to the apparent relaxation of many kinds of vessels, such as mouse mesenteric arteries (Earley et al., 2009), mouse carotid artery (Loot et al., 2008), rat aortic artery (Willette et al., 2008), rat carotid artery, and rat arteria gracilis (Kohler et al., 2006). Recently, a study shows that shear forces enhance endothelial TRPV4 agonist sensitivity and link TRPV4 activation to acetylcholine (ACh)-mediated endothelium-dependent vasodilation in rat cremaster arterioles (Darby et al., 2018). In human coronary arterioles, flow- or AA-induced dilation is mediated by endothelial TRPV4 through Ca2+ entry and mitochondrial reactive oxygen species (ROS) signaling (Bubolz et al., 2012; Zheng et al., 2013), demonstrating that endothelial TRPV4 channels are involved in shear stress- and ACh-induced vasodilation (Hartmannsgruber et al., 2007; Zhang et al., 2009). TRPV4-induced vasodilation is almost completely abolished by the inhibition of nitric oxide synthase (NOS) in large vessels and by the inhibition of Ca2+-active K+ channels in small vessels (Filosa et al., 2013). Endothelial NOS (eNOS) inhibition impedes TRPV4 activator 4α-PDD- and shear stress-induced dilation in rat carotid arteries (Kohler et al., 2006), and the TRPV4 activator GSK1016790A fails to cause vasodilation in mouse aortic rings from eNOS–/– mice (Willette et al., 2008). These studies suggest that eNOS is very important for endothelial TRPV4-regulated vasodilation in large vessels. Both NOS and cyclooxygenase (COX) inhibition reduce 4α-PDD-induced dilation in rat carotid arteries, but NOS and COX inhibition is ineffective in the resistance artery (Filosa et al., 2013). In mouse mesenteric arteries, NOS inhibition and high K+ solution block shear stress-induced dilation, whereas COX inhibition has no effect (Baylie and Brayden, 2011), indicating that COX is not required to mesenteric arterial shear stress-induced dilation. Flow-mediated NO and endothelial-derived hyperpolarizing factor (EDHF) production are markedly reduced in the small mesenteric arteries of TRPV4–/– mice (Mendoza et al., 2010). Recently, it is reported that Piezo1 acts upstream of TRPV4 to induce pathological changes in endothelial cells due to shear stress (Swain and Liddle, 2020). ACh-TRPV4 regulates endothelium-dependent vasodilation mainly via EDHF through the induction of intermediate- and small-conductance Ca2+-sensitive K+ channels (IKCa and SKCa) in small mesenteric arteries (Sonkusare et al., 2012), suggesting that Ca2+-active K+ channels-mediated EDHF is the main pathway involved in TRPV4-regulated vasodilation in small vessels. Therefore, endothelial TRPV4-mediated vasodilation mainly via NO and EDHF production, and the relative contribution of each varies in different vessel types.
Structurally, TRPV4 channels can interact with several proteins including other members of the TRP channel family or Ca2+-sensitive K+ channels to form functional complexes (Schaefer, 2005; He and Ma, 2016). The stimulation of Ca2+-sensing receptors activates heteromeric TRPV4-TRPC1 channels to induce endothelium-dependent vasorelaxation in rabbit and mouse mesenteric arteries (Greenberg et al., 2019). It is well known that most Ca2+-handling proteins from endothelial and SMCs are located in caveolae (Absi et al., 2007; Riddle et al., 2011), where Ca2+-relevant signal transduction starts (Isshiki and Anderson, 2003). Caveolar integrity is reported to be essential for PLA2-dependent EDHF signaling in porcine arteries (Graziani et al., 2004). The interaction between caveolin-1 (a structural caveolar protein) and TRPV4 is functionally important for 4α-PDD-induced Ca2+ increase and EDHF-mediated relaxation (Saliez et al., 2008). TRPV4-KCa2.3 complexes in caveolae play important roles in mouse mesenteric dilation (Goedicke-Fritz et al., 2015; He et al., 2017). These studies suggest the importance of caveolar microdomains in TRPV4-mediated vasodilation.
Additionally, endothelial TRPV4 channels are important for cerebral arteriole dilation (Zhang et al., 2013). The inhibition of TRPV4 and PLA2 partially reduce uridine triphosphate-induced Ca2+ influx in the endothelial cells of pressurized rat middle cerebral arteries (Marrelli et al., 2007). TRPV4−mediated Ca2+ influx in astrocytic endfeet and mouse neurons results in the recruitment of inositol 1,4,5-trisphosphate receptors (IP3Rs) and Ca2+−induced Ca2+ release (Dunn et al., 2013). Given that SKCa and IKCa channels are involved in modulating the basal tone in mouse parenchymal arterioles (Cipolla et al., 2009), TRPV4 channels probably influence ACh- and EDH-mediated dilation through IKCa and SKCa channel activation in the endothelial cells of cerebral arterioles (Diaz-Otero et al., 2018).
Taken together, the activation of endothelial TRPV4 induces the production of NO, prostaglandin (PGI2) and EDHF to regulate vascular dilation (Baylie and Brayden, 2011), and the effect seems to be dependent upon the vascular bed type. The reason probably due to that spatial organization of signaling elements in differential arteries determines selectively underlying mechanism for vasodilatation regulation (Ottolini et al., 2020).
Endothelium-Independent Vasodilation
TRPV4 channels are also widely expressed in the smooth muscle cells of various artery types from many species (Filosa et al., 2013). The functional coupling of smooth muscle TRPV4 with large-conductance Ca2+-activated K+ (KCa1.1) is implicated in vascular tone control. For example, in rat cerebral arteries, EETs activate TRPV4 in SMCs and induce Ca2+ entry-stimulated Ca2+ release from ryanodine receptors (RyR) in the sarcoplasmic reticulum (SR), contributing to Ca2+ sparks and subsequently activating KCa1.1 to stimulate smooth muscle hyperpolarization and vasodilation (Earley et al., 2005). Fawn hooded hypertensive rat cerebral arterial myocytes display a stretch-sensitive TRPV4-like single-channel current and its activation opened KCa1.1 single-channel current (Gebremedhin et al., 2017). In mouse mesenteric arteries, approximately 50% of the EET-induced vasodilation is reported to be endothelium-independent due to its direct action on the smooth muscle TRPV4-KCa1.1 axis (Earley et al., 2009). In rat retinal arterioles, GSK1016790A activates vasodilation in NO- and KCa1.1 channel-dependent manners in vivo (Mori et al., 2020). In the cerebral basal artery of cerebral ischemia-reperfusion injury rats, the vasorelaxation induced by the total flavonoids of rhododendron is linked to the TRPV4-KCa1.1 pathway in smooth muscle cells (Han et al., 2018). Since TRPC1 associates with the KCa1.1 channel to form a signal complex in vascular smooth muscles, controlling smooth muscle hyperpolarization and vascular tone (Kwan et al., 2009), the TRPV4-TRPC1-KCa1.1 complex is probably present in vascular smooth muscle. It has been reported that in isolated human left internal mammary arteries segments, 11,12-EET induces smooth muscle membrane hyperpolarization and vascular relaxation through the TRPV4-TRPC1-KCa1.1 complex (Ma et al., 2015), suggesting the involvement of the TRPV4-TRPC1-KCa1.1 complex in smooth muscle-mediated vascular dilation.
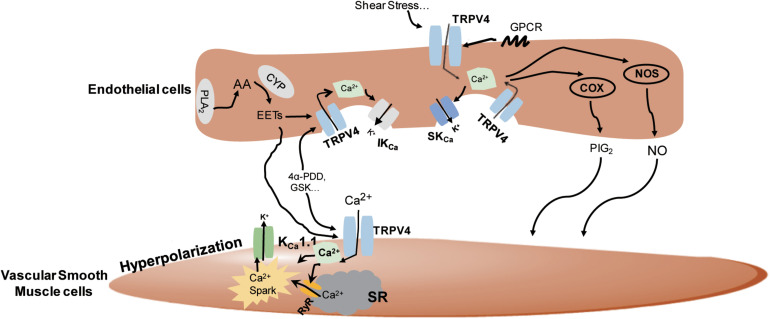
In summary, the TRPV4 channels can be activated by a wide range of stimuli and regulate vasorelaxation in both endothelium-dependent and endothelium-independent manners involving complicated distinct pathways (Figure 2).
FIGURE 2.
The underlying mechanisms of the regulation of TRPV4 in vasodilation. In the vascular endothelial cells, TRPV4 can be activated by stimuli-sensing, G protein-coupled receptors (GPCR) activation, epoxyeicosatrienoic acid (EETs), GSK1016790A (GSK), and 4α-PDD. Its activation leads to Ca2+ influx and the subsequent activation of nitric oxide synthase (NOS), cyclooxygenase (COX), and intermediate-conductance Ca2+-sensitive K+ channels/small conductance Ca2+-sensitive K+ channels (IKCa/SKCa) channels to induce nitric oxide (NO), prostacyclin (PGI2), and endothelial-derived hyperpolarizing factor (EDHF) production, respectively, and causes vasodilation. In smooth muscles, TRPV4 can be activated by endothelial-derived EETs or chemical agonists, leading to Ca2+ entry-induced Ca2+ release from ryanodine receptor (RyR) in the sarcoplasmic reticulum (SR), the stimulation of Ca2+ sparks, and the subsequent activation of KCa1.1 to stimulate smooth muscle hyperpolarization and vasodilation.
Transient Receptor Potential Vanilloid 4 and Vasoconstriction
In addition to the well-documented vasorelaxation, TRPV4 channel activation can evoke constriction in some vascular beds (Saifeddine et al., 2015; Dryn et al., 2016; Zhang et al., 2018). Physiologically, TRPV4 channels participate in the regulation of the adrenergic vascular contractile activity of pulmonary artery smooth muscle cells (PASMCs) (Dryn et al., 2016). TRPV4 is shown to participate in the PASMC contractile response to hypoxia as the inhibition of TRPV4 blocks hypoxia-induced Ca2+ influx and myosin light chain phosphorylation in human PASMCs (Goldenberg et al., 2015b). 4α-PDD induces extracellular Ca2+ entry and TRPV4 overexpression increases basal [Ca2+]i in hypoxic PASMCs, resulting in myogenic tone enhancement and the development of hypoxia-induced pulmonary hypertension (Yang et al., 2012). Moreover, TRPV4 contributes to 5-hydroxytryptamine (5-HT)-mediated pulmonary vasoconstriction in chronic hypoxic pulmonary hypertension (Xia et al., 2013). A recent study find that the inhibition of TRPV4 reverses steady-state myogenic tone and inhibits the pressure-evoked membrane depolarization, [Ca2+]i increase and constriction in neonatal pig pre-glomerular distal interlobular arteries (Soni et al., 2017). In addition, the blockade of TRPV4 channels attenuates angiotensin II (Ang II)-induced Ca2+ entry and constriction in the pre-glomerular microvessels of neonatal pigs (Soni et al., 2019).
Pathologically, the action of TRPV4 contributes to vascular contraction under some specific conditions. For example, in mouse aorta, the G protein-coupled receptor (GPCR) potentiation of TRPV4 action leads to vasoconstriction through COX-dependent prostanoid production and Tx receptor activation (Saifeddine et al., 2015). Additionally, in mouse aortas from hypertensive mice, GSK1016790A-activated TRPV4 channels increase [Ca2+]i, resulting in endothelium-dependent contraction through the activation of cytosolic phospholipase A2 (cPLA2) and COX2 (Zhang et al., 2018). These studies suggest that TRPV4 channel activation can increase [Ca2+]i and generate vasoconstricting prostanoids to constrict vessels under some specific pathological conditions.
Transient Receptor Potential Vanilloid 4 and Vascular Permeability
The current knowledge suggests that TRPV4 participates in the control of vascular permeability, particularly in the lung capillary endothelial barrier. The activation of TRPV4 channels is shown to increase lung epithelial-endothelial permeability and disrupt alveolar walls (Alvarez et al., 2006). TRPV4 activation causes Ca2+-dependent vascular hyperpermeability in lung, leading to the disruption of the alveolar septal barrier and acute lung injury following high-pressure ventilation (Hamanaka et al., 2007). It has been demonstrated that in human and mouse lung microvascular endothelial cells, exogenous H2O2 increases [Ca2+]i and barrier permeability via activation of TRPV4 through the Src kinase Fyn (Suresh et al., 2015). TRPV4 agonist 4α-PDD causes endothelial blebs and/or breaks in rat and mouse lungs, and increases lung endothelial permeability in a Ca2+-entry dependent manner (Alvarez et al., 2006). Mechanically, 4α-PDD induces pulmonary arterial endothelial cell barrier disruption through the mitochondrial redistribution of uncoupled eNOS secondary to a protein kinase C-dependent phosphorylation of eNOS at Threonine 495 (T495) (Lu et al., 2021). TRPV4 inhibition or deletion ameliorates endothelial permeability and fluid-induced lung injury, involving the release of Ang II and P-selectin (Bihari et al., 2017). The TRPV4 channels are also involved in the Pannexin 1-regulated lung vein permeability (Maier-Begandt and Comstra, 2021) and the vascular hyperpermeability induced by GPCR protease-activated receptor 1 (PAR1) in the airways and upper gastrointestinal tract of mice (Peng et al., 2020). In addition, deletion of the TRPV4 gene reduces colonic vascular endothelial permeability during dextran sulfate sodium-induced murine colonic inflammation, whereas the up-regulation of TRPV4 results in the progression of colonic inflammation by increasing vascular permeability (Matsumoto et al., 2018). These data indicates the importance of TRPV4 for vascular permeability, providing targetable pathways to regulate vascular permeability and barrier function, and to prevent inflammation-induced harmful effects of vascular leak.
Transient Receptor Potential Vanilloid 4 and Vascular Remodeling
TRPV4 participates in vascular remodeling regulation mainly by controlling vasculogenesis and arteriogenesis (Negri et al., 2019). TRPV4 expression is significantly increased in fluid shear stress-stimulated cerebral collateral circulation and 4α-PDD-induced TRPV4 activation enhances cerebral arteriogenesis (Schierling et al., 2011), while chronic hypoxia-induced vascular remodeling and right ventricle hypertrophy are blocked in a TRPV4-deficient mouse model (Yang et al., 2012), confirming the vital role of TRPV4 in vascular remodeling regulation. Numerous Ca2+-dependent transcription factors, such as nuclear factor of activated T cells, myocyte enhancer factor 2C, and calcineurin-dependent 1 are involved in the regulation of TRPV4 in arteriogenesis (Troidl et al., 2010). TRPV4 plays a key role in cytoskeletal reorganization and cell adhesion, which regulates endothelial cell proliferation and motility through mechanotransduction (Reddy et al., 2015; Adapala et al., 2016; Thoppil et al., 2016). The cyclical stretching-evoked stimulation of TRPV4 mediates cytoskeletal remodeling and cell reorientation through integrin-to-integrin signaling (Thodeti et al., 2009). TRPV4 participates in the calcium oscillations and VSMC migration induced by platelet-derived microvesicles (PMVs) (Li et al., 2021), implying that TRPV4 promotes VSMC migration under pathological conditions resulting in vascular remodeling regulation. Besides VSMCs, TRPV4 has also been known to regulate neovascularization by inducing endothelial cell proliferation and migration (Moccia, 2018). It is found that 4α-PDD-activated TRPV4 induces Ca2+ entry and proliferation in human brain capillary endothelial cells (Fiorio Pla et al., 2012) and such proliferation is partially inhibited by TRPV4-siRNA (Hatano et al., 2013), suggesting that endothelial TRPV4 partially mediates cell proliferation. A recent study in isolated lung microvascular endothelial cells from rats subjected to SU5416 [an inhibitor of vascular endothelial growth factor receptor-2 (VEGFR2)] plus hypoxia shows that endogenous ROS increases the basal levels of [Ca2+]i via TRPV4 activation and promotes aberrant migratory and proliferative capacity (Suresh et al., 2018). Evidence also indicates that 4α-PDD causes endothelial cell proliferation, triggering collateral vessel growth (Troidl et al., 2009). In addition, 4α-PDD decreases the infarct volume, improves the recovery of neurological function, and enhances vasculogenesis and neurogenesis in ischemic stroke rats (Chen et al., 2018). These results suggest that both VSMCs and endothelial cells play important roles in TRPV4-regulated vascular remodeling.
It is reported that TRPV4 negatively regulates tumor angiogenesis and tumor vessel maturation (Adapala et al., 2016). For instance, in tumor endothelial cells, the deletion of the TRPV4 gene induces cell proliferation, migration, sprouting angiogenesis, and abnormal tube formation in vitro accompanied by an increase in basal Rho activity (Thoppil et al., 2016). In contrast, the activation or overexpression of TRPV4 restores aberrant migration and angiogenesis by inhibiting the exacerbated Rho activity (Adapala et al., 2016). Additionally, a study on TRPV4 knockout tumors shows that silencing of the TRPV4 gene stimulates VEGF-regulated migration in vitro and increases the expression of VEGFR2 in vivo in the vasculature compared to control tumors (Kanugula et al., 2019). A recent study with endothelial-specific TRPV4 knockout mice demonstrates that the specific deletion of endothelial TRPV4 promotes tumor angiogenesis, growth, and metastasis compared to control mice (Kanugula et al., 2021), indicating that endothelial TRPV4 is a critical modulator of vascular integrity and tumor angiogenesis. The discrepant function of TRPV4 in vascular remodeling probably is dependent on the cell type and animal model used in different studies.
Transient Receptor Potential Vanilloid 4 and Vascular Damage
Reactive oxygen species production has been implicated in the pathogenesis of atherosclerosis, ischemia/reperfusion injury, obstructive sleep apnea, and other diseases, playing a critical role in vascular physiology and pathophysiology (Droge, 2002). GSK1016790A increases phosphorylation of eNOS and adenosine 3′,5′-monophosphate-activated protein kinase in the aorta and decreases leukocyte adhesion to tumor necrosis factor α-inflamed endothelium, and oral administration of GSK1016790A reduces atherosclerotic plaque formation in ApoE deficient mice with a western-type diet, suggesting that pharmacological activation of TRPV4 may serve as a potential therapeutic approach to treat atherosclerosis (Xu et al., 2016). However, recent study shows that genetic deletion or chemical antagonism of TRPV4 channels blocks lipopolysaccharide-triggered exacerbation of oxidized low-density lipoprotein (oxLDL)-mediated foam cell formation (Gupta et al., 2019), and TRPV4 deficiency prevents pathophysiological range matrix stiffness or scratch-induced exacerbation of oxLDL-induced foam cell formation (Goswami et al., 2017). Therefore, it still needs to deeply clarify the role of TRPV4 in atherogenesis development.
In addition, TRPV4 is a critical mediator of pressure-induced damage related to ventilator-induced lung injury, infarction, and heart failure (Balakrishna et al., 2014). GSK1016790A decreases right ventricular and systemic pressure, resulting in fatal circulatory collapse, accompanied by increased protein permeability, lung hemorrhage and fluid extravasation (Wandall-Frostholm et al., 2015). TRPV4 inhibition prevents edema and inflammation, and improves oxygen saturation and pulmonary function in HCl- and Cl2-induced chemical lung injury, suggesting TRPV4 inhibitors as a potential treatment for acute lung injury (Balakrishna et al., 2014). Furthermore, the inhibition of TRPV4 channels increases the viability of astrocytes against oxidative stress induced by mercaptosuccinate or buthionine sulfoximine (Bai and Lipski, 2010). It is suggested that after traumatic brain injury, excessive mitochondria-derived H2O2 activates BKCa channels via a TRPV4-dependent pathway in the VSMCs, impairing pressure-induced constriction of cerebral arteries (Szarka et al., 2018). The up-regulation or activation of TRPV4 contributes to endothelial damage leading to tissue damage, vascular destabilization, and blood-spinal cord barrier breakdown after spinal cord injury, whereas the knockdown or inhibition of TRPV4 prevents such effects (Kumar et al., 2020), indicating that the regulation of TRPV4 signaling might lead to new therapeutic strategies to protect endothelial cells and enhance repair after spinal cord injury.
Transient Receptor Potential Vanilloid 4 as a Potential Therapeutic Target
TRPV4 channels are widely expressed in various tissues, playing important roles in many physiological and pathophysiological processes (Goldenberg et al., 2015a; Randhawa and Jaggi, 2015a). The activation of TRPV4 channels has been implicated in pulmonary hypertension (Suresh et al., 2018), hyponatremia (Carreno et al., 2009; Tian et al., 2009), neurodegenerative skeletal muscle dysplasia (Auer-Grumbach et al., 2010), and bone disorders (Mizoguchi et al., 2008) and its inhibition presents a potential therapeutic strategy for pain, gastrointestinal disorders, edema, and lung diseases (Grace et al., 2017). Therefore, TRPV4 channels become a potential therapeutic target in numerous diseases.
Increasing evidence has indicated that TRPV4 participates in the regulation of systemic vasculature reactivity and the maintenance of cardiovascular homeostasis (Randhawa and Jaggi, 2015b). TRPV4 plays an important role in blood pressure control. The activation of TRPV4 stimulates the relaxation of peripheral resistance arteries and regulates arterial pressure (Earley et al., 2009). TRPV4 activity is markedly impaired in Ang II-induced hypertensive mice (Sonkusare et al., 2014). TRPV4 channels probably function as a compensatory pathway to induce vasorelaxation under the condition of NO blockage (Feletou and Vanhoutte, 2006). Thus, TRPV4 might become a therapeutic target in hypertension treatment. Additionally, TRPV4 is involved in converting mechanical forces on the airways to molecular and transcriptional events, regulating the development of lung and stabilization of pulmonary vasculature (Morgan et al., 2018). Recent evidence suggests that activating pulmonary capillary endothelial TRPV4 channels enhance pulmonary venous pressure-induced edema and TRPV4 blockade prevents the increased vascular permeability and pulmonary edema (Thorneloe et al., 2012), highlighting a pharmacological therapeutic potential of TRPV4 inhibition for pulmonary edema induced by heart failure. The TRPV4 antagonist GSK2193874 mitigates the increases in lung weight after heart failure (Michel, 2006; Thorneloe et al., 2012) and TRPV4 inhibition or genetic deletion suppresses pulmonary inflammation and improved pulmonary function in the HCl-and Cl2- induced injury model (Balakrishna et al., 2014). These data indicate that TRPV4 might be a therapeutic target for pulmonary disease. It is showed that matrix stiffness promotes foam cell formation in a TRPV4-dependent manner by regulating the uptake of oxLDL in macrophages, suggesting the importance of TRPV4 channels for development of atherosclerotic lesions (Goswami et al., 2017). Therefore, targeting TRPV4 might be a potential therapeutic tactic to treat atherosclerosis.
Apart from cardiovascular diseases, TRPV4 has been found to affect diabetes-related complications. Recent studies have shown that TRPV4 is implicated in the replication of β-cells, and the production and secretion of insulin (Skrzypski et al., 2013). TRPV4 agonists exert insulinotropic effects on pancreatic β-cell lines, but extend activation resulted in cell dysfunction and death (Billert et al., 2017). TRPV4 agonists promote vasorelaxation and improve cardiovascular function in a rodent type 2 diabetes model, and TRPV4 antagonists reduce high-fat diet-induced obesity, insulin resistance, and diabetes-related complications (Hu et al., 2020), suggesting its potential as a therapeutic target for such diseases. Gastrointestinal inflammation increases TRPV4 expression and enhances channel activation (D’Aldebert et al., 2011; Cenac et al., 2015), and TRPV4 inhibitors demonstrate therapeutical potential for treating inflammation and abdominal pain (Holzer, 2011). Research has suggested that blocking TRPV4 activity might be a strategy for scleroderma treatment (Sharma et al., 2017). TRPV4 channels participate in the KCa3.1-regulated proliferation of human bronchial smooth muscle cells in the process of chronic asthma (Yu et al., 2017), indicating that it is a potential therapeutic target for chronic asthma treatment. TRPV4 has also been determined to have a pathological role in the activation and proliferation of hepatic stellate cells (Song et al., 2014; Zhan and Li, 2018), suggesting a potential therapeutic target for liver fibrosis treatment. Following cerebral hypoxia and ischemia, TRPV4 expression and activity are enhanced in hippocampal astrocytes, indicating the involvement of TRPV4 in the pathogenesis of astroglial reactivity after an ischemic insult (Butenko et al., 2012).
Some safety concerns about global TRPV4 agonism or antagonism have been raised as TRPV4 have both favorable and unfavorable effects on the same tissue. For example, TRPV4 is essential for endothelial barrier integrity, and TRPV4 inhibitors could be beneficial for pulmonary edema. However, the primary role of TRPV4 in hypoxic pulmonary vasoconstriction indicates the detriment of TRPV4 inhibition to patients with lung disease (Yang et al., 2012; Morty and Kuebler, 2014; Goldenberg et al., 2015a). Additionally, TRPV4 inhibition also represents a much-needed “two-pronged attack” against ARDS, for its barrier-stabilizing effect coupled with anti-inflammatory properties (Goldenberg et al., 2015a). The therapeutic target potential of TRPV4 is currently being studied in the clinical trial with a first-in-class, highly potent, selective TRPV4 channel blocker GSK2798745 from GlaxoSmithKline (Moran, 2018) (Goyal et al., 2019). A recent clinical trial study shows that GSK2798745 does not affect lipopolysaccharide-induced elevation of barrier permeability and inflammation (Mole et al., 2020). Because discrepant effect of TRPV4 on many tissues and systemic TRPV4 blockage may be detrimental, targeting the signaling pathways upstream of TRPV4 activation might be an alternative strategy.
Summary
The dysfunction of TRP channels is associated with endothelial dysfunction, which is reflected by NO bioavailability decrease, vascular smooth muscle tonicity dysregulation, endothelial barrier dysfunction, oxidative damage increase, and angiogenic disorder. The current review summarizes reports about the role of TRPV4 in vascular dilation and constriction, vascular permeability, vascular remodeling, and vascular damage, and discusses possible mechanisms. TRPV4 channels can be activated by a wide range of stimuli by means of distinct pathways and its impact on regulating vascular function is shown to vary between vessels and pathological conditions. The activation of TRPV4 channels contributes to vasodilation involving endothelial NO, PGI2, and EDHF pathways, or directly cause VSMC hyperpolarization and vasodilation. On the contrary, in some specific vascular beds or under specific pathological conditions, TRPV4 activation evokes constriction. TRPV4 also participates in the control of the lung capillary endothelial barrier, lung injury and vascular remodeling regulation. Since the complicated and discrepant function of TRPV4 in same tissues, more studies about cell-type specific role of TRPV4 are required to fully understand its pathobiological and beneficial potential under various pathophysiological conditions in the future. Additionally, the upstream of TRPV4 regulation is still need to be more investigated, as well as the precise function switching and underlying mechanism on the same tissues. Given that TRPV4 has equivocal effects on the same tissue, the safety concerns about global TRPV4 agonism or antagonism need to be studied. Therefore, whether agents targeting TRPV4 clinically have dual impacts on the regulation of vascular function in humans under different pathological conditions remains to be determined.
Author Contributions
LL, MG, XL, ZW, and TZ performed document searching and reviewing. LL, MG, JY, YL, and FY contributed to literature review. XW and LF provided a critical reading of the manuscript. TZ initiated the project and wrote the manuscript. All authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- ACh
acetylcholine
- Ang
angiotensin
- ARDS
acute respiratory distress syndrome
- [Ca2+]i
intracellular Ca2+ concentration
- COX
cyclooxygenase
- cPLA2
cytosolic phospholipase A2
- EDHF
endothelial-derived hyperpolarizing factor
- EET
epoxyeicosatrienoic acid
- GPCR
G protein-coupled receptors
- IKCa
intermediate-conductance Ca2+-sensitive K+ channels
- KCa1.1
large-conductance Ca2+-activated K+ channel
- NO
nitric oxide
- NOS
nitric oxide synthase
- PGI2
prostacyclin
- PAR1
protease activated receptor 1
- PASM
pulmonary artery smooth muscle
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
- SKCa
small conductance Ca2+-sensitive K+ channels
- TRP
transient receptor potential
- TRPV4
transient receptor potential vanilloid 4
- VEGFR
vascular endothelial growth factor receptor
- VSMC
vascular smooth muscle cell
- 4 α-PDD
4 α-phorbol 12,13-didecanoate
- 5-HT
5-hydroxytryptamine.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (Grant 81800430 to TZ), China Postdoctoral Science Foundation (Grant 2018M642172 to TZ), Postdoctoral Research funding program of Jiangsu Province (Grant 2020Z427 to XW), and the program of Wuxi Municipal Health Commission (to TZ).
References
- Absi M., Burnham M. P., Weston A. H., Harno E., Rogers M., Edwards G. (2007). Effects of methyl beta-cyclodextrin on EDHF responses in pig and rat arteries; association between SK(Ca) channels and caveolin-rich domains. Br. J. Pharmacol. 151 332–340. 10.1038/sj.bjp.0707222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adapala R. K., Thoppil R. J., Ghosh K., Cappelli H. C., Dudley A. C., Paruchuri S., et al. (2016). Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene 35 314–322. 10.1038/onc.2015.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez D. F., King J. A., Weber D., Addison E., Liedtke W., Townsley M. I. (2006). Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ. Res. 99 988–995. 10.1161/01.RES.0000247065.11756.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P. D., Di Gregorio M., He P., McCulloch C. A. (2017). TRPV4 mediates the Ca(2+) influx required for the interaction between flightless-1 and non-muscle myosin, and collagen remodeling. J. Cell Sci. 130 2196–2208. 10.1242/jcs.201665 [DOI] [PubMed] [Google Scholar]
- Auer-Grumbach M., Olschewski A., Papic L., Kremer H., McEntagart M. E., Uhrig S., et al. (2010). Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat. Genet. 42 160–164. 10.1038/ng.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J. Z., Lipski J. (2010). Differential expression of TRPM2 and TRPV4 channels and their potential role in oxidative stress-induced cell death in organotypic hippocampal culture. Neurotoxicology 31 204–214. 10.1016/j.neuro.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Balakrishna S., Song W., Achanta S., Doran S. F., Liu B., Kaelberer M. M., et al. (2014). TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 307 L158–L172. 10.1152/ajplung.00065.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylie R. L., Brayden J. E. (2011). TRPV channels and vascular function. Acta Physiol. (Oxf) 203 99–116. 10.1111/j.1748-1716.2010.02217.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihari S., Dixon D. L., Lawrence M. D., De Bellis D., Bonder C. S., Dimasi D. P., et al. (2017). Fluid-induced lung injury-role of TRPV4 channels. Pflugers Arch. 469 1121–1134. 10.1007/s00424-017-1983-1 [DOI] [PubMed] [Google Scholar]
- Billert M., Skrzypski M., Sassek M., Szczepankiewicz D., Wojciechowicz T., Mergler S., et al. (2017). TRPV4 regulates insulin mRNA expression and INS-1E cell death via ERK1/2 and NO-dependent mechanisms. Cell Signal 35 242–249. 10.1016/j.cellsig.2017.03.018 [DOI] [PubMed] [Google Scholar]
- Brooks C. A., Barton L. S., Behm D. J., Eidam H. S., Fox R. M., Hammond M., et al. (2019). Discovery of GSK2798745: a clinical candidate for inhibition of transient receptor potential vanilloid 4 (TRPV4). ACS Med. Chem. Lett. 10 1228–1233. 10.1021/acsmedchemlett.9b00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubolz A. H., Mendoza S. A., Zheng X., Zinkevich N. S., Li R., Gutterman D. D., et al. (2012). Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. Am. J. Physiol. Heart. Circ. Physiol. 302 H634–H642. 10.1152/ajpheart.00717.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenko O., Dzamba D., Benesova J., Honsa P., Benfenati V., Rusnakova V., et al. (2012). The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS One 7:e39959. 10.1371/journal.pone.0039959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S., Anishkin A., Zinkevich N. S., Nishijima Y., Korishettar A., Wang Z., et al. (2018). Transient receptor potential vanilloid 4 (TRPV4) activation by arachidonic acid requires protein kinase A-mediated phosphorylation. J. Biol. Chem. 293 5307–5322. 10.1074/jbc.M117.811075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno F. R., Ji L. L., Cunningham J. T. (2009). Altered central TRPV4 expression and lipid raft association related to inappropriate vasopressin secretion in cirrhotic rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296 R454–R466. 10.1152/ajpregu.90460.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenac N., Bautzova T., Le Faouder P., Veldhuis N. A., Poole D. P., Rolland C., et al. (2015). Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology 149 433.e437–444.e437. 10.1053/j.gastro.2015.04.011 [DOI] [PubMed] [Google Scholar]
- Chen C. K., Hsu P. Y., Wang T. M., Miao Z. F., Lin R. T., Juo S. H. (2018). TRPV4 activation contributes functional recovery from ischemic stroke via angiogenesis and neurogenesis. Mol. Neurobiol. 55 4127–4135. 10.1007/s12035-017-0625-0 [DOI] [PubMed] [Google Scholar]
- Cipolla M. J., Smith J., Kohlmeyer M. M., Godfrey J. A. (2009). SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke 40 1451–1457. 10.1161/STROKEAHA.108.535435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y. Y., Li M. Y., Li Y. T., Ning J. Y., Gou X. C., Shi J., et al. (2020). Expression and functional characterization of transient receptor potential vanilloid 4 in the dorsal root ganglion and spinal cord of diabetic rats with mechanical allodynia. Brain Res. Bull. 162 30–39. 10.1016/j.brainresbull.2020.05.010 [DOI] [PubMed] [Google Scholar]
- D’Aldebert E., Cenac N., Rousset P., Martin L., Rolland C., Chapman K., et al. (2011). Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology 140 275–285. 10.1053/j.gastro.2010.09.045 [DOI] [PubMed] [Google Scholar]
- Darby W. G., Potocnik S., Ramachandran R., Hollenberg M. D., Woodman O. L., McIntyre P. (2018). Shear stress sensitizes TRPV4 in endothelium-dependent vasodilatation. Pharmacol. Res. 133 152–159. 10.1016/j.phrs.2018.05.009 [DOI] [PubMed] [Google Scholar]
- Diaz-Otero J. M., Yen T. C., Fisher C., Bota D., Jackson W. F., Dorrance A. M. (2018). Mineralocorticoid receptor antagonism improves parenchymal arteriole dilation via a TRPV4-dependent mechanism and prevents cognitive dysfunction in hypertension. Am. J. Physiol. Heart Circ. Physiol. 315 H1304–H1315. 10.1152/ajpheart.00207.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W. (2002). Free radicals in the physiological control of cell function. Physiol. Rev. 82 47–95. 10.1152/physrev.00018.2001 [DOI] [PubMed] [Google Scholar]
- Dryn D., Melnyk M., Kizub I., Hu H., Soloviev A., Zholos A. (2016). The role Of Trpv4 cation channels in the regulation of phenylephrine-induced contraction of rat pulmonary arter. Fiziol. Zh. 62 79–86. 10.15407/fz62.02.079 [DOI] [PubMed] [Google Scholar]
- Dunn K. M., Hill-Eubanks D. C., Liedtke W. B., Nelson M. T. (2013). TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc. Natl. Acad. Sci. U.S.A. 110 6157–6162. 10.1073/pnas.1216514110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S., Brayden J. E. (2015). Transient receptor potential channels in the vasculature. Physiol. Rev. 95 645–690. 10.1152/physrev.00026.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S., Heppner T. J., Nelson M. T., Brayden J. E. (2005). TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ. Res. 97 1270–1279. 10.1161/01.RES.0000194321.60300.d6 [DOI] [PubMed] [Google Scholar]
- Earley S., Pauyo T., Drapp R., Tavares M. J., Liedtke W., Brayden J. E. (2009). TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am. J. Physiol. Heart Circ Physiol. 297 H1096–H1102. 10.1152/ajpheart.00241.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. (2006). Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb. Vasc. Biol. 26 1215–1225. 10.1161/01.ATV.0000217611.81085.c5 [DOI] [PubMed] [Google Scholar]
- Filosa J. A., Yao X., Rath G. (2013). TRPV4 and the regulation of vascular tone. J. Cardiovasc Pharmacol. 61 113–119. 10.1097/FJC.0b013e318279ba42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorio Pla A., Ong H. L., Cheng K. T., Brossa A., Bussolati B., Lockwich T., et al. (2012). TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene 31 200–212. 10.1038/onc.2011.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin D., Zhang D. X., Weihrauch D., Uche N. N., Harder D. R. (2017). Detection of TRPV4 channel current-like activity in Fawn Hooded hypertensive (FHH) rat cerebral arterial muscle cells. PLoS One 12:e0176796. 10.1371/journal.pone.0176796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedicke-Fritz S., Kaistha A., Kacik M., Markert S., Hofmeister A., Busch C., et al. (2015). Evidence for functional and dynamic microcompartmentation of Cav-1/TRPV4/K(Ca) in caveolae of endothelial cells. Eur. J. Cell Biol. 94 391–400. 10.1016/j.ejcb.2015.06.002 [DOI] [PubMed] [Google Scholar]
- Goldenberg N. M., Ravindran K., Kuebler W. M. (2015a). TRPV4: physiological role and therapeutic potential in respiratory diseases. Naunyn Schmiedebergs Arch. Pharmacol. 388 421–436. 10.1007/s00210-014-1058-1 [DOI] [PubMed] [Google Scholar]
- Goldenberg N. M., Wang L., Ranke H., Liedtke W., Tabuchi A., Kuebler W. M. (2015b). TRPV4 is required for hypoxic pulmonary vasoconstriction. Anesthesiology 122 1338–1348. 10.1097/ALN.0000000000000647 [DOI] [PubMed] [Google Scholar]
- Goswami R., Merth M., Sharma S., Alharbi M. O., Aranda-Espinoza H., Zhu X., et al. (2017). TRPV4 calcium-permeable channel is a novel regulator of oxidized LDL-induced macrophage foam cell formation. Free Radic Biol. Med. 110 142–150. 10.1016/j.freeradbiomed.2017.06.004 [DOI] [PubMed] [Google Scholar]
- Goyal N., Skrdla P., Schroyer R., Kumar S., Fernando D., Oughton A., et al. (2019). Clinical pharmacokinetics, safety, and tolerability of a novel, first-in-class TRPV4 ion channel inhibitor, GSK2798745, in healthy and heart failure subjects. Am. J. Cardiovasc Drugs 19 335–342. 10.1007/s40256-018-00320-6 [DOI] [PubMed] [Google Scholar]
- Grace M. S., Bonvini S. J., Belvisi M. G., McIntyre P. (2017). Modulation of the TRPV4 ion channel as a therapeutic target for disease. Pharmacol. Ther. 177 9–22. 10.1016/j.pharmthera.2017.02.019 [DOI] [PubMed] [Google Scholar]
- Graziani A., Bricko V., Carmignani M., Graier W. F., Groschner K. (2004). Cholesterol- and caveolin-rich membrane domains are essential for phospholipase A2-dependent EDHF formation. Cardiovasc Res. 64 234–242. 10.1016/j.cardiores.2004.06.026 [DOI] [PubMed] [Google Scholar]
- Greenberg H. Z. E., Carlton-Carew S. R. E., Khan D. M., Zargaran A. K., Jahan K. S., Vanessa Ho W. S., et al. (2017). Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced nitric oxide production and vasorelaxation in rabbit mesenteric arteries. Vascul. Pharmacol. 96-98 53–62. 10.1016/j.vph.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. Z. E., Carlton-Carew S. R. E., Zargaran A. K., Jahan K. S., Birnbaumer L., Albert A. P. (2019). Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced relaxations and nitric oxide production in mesenteric arteries: comparative study using wild-type and TRPC1(-/-) mice. Channels (Austin) 13 410–423. 10.1080/19336950.2019.1673131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N., Goswami R., Alharbi M. O., Biswas D., Rahaman S. O. (2019). TRPV4 is a regulator in P. gingivalis lipopolysaccharide-induced exacerbation of macrophage foam cell formation. Physiol. Rep. 7:e14069. 10.14814/phy2.14069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka K., Jian M. Y., Weber D. S., Alvarez D. F., Townsley M. I., Al-Mehdi A. B., et al. (2007). TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am. J. Physiol. Lung Cell Mol. Physiol. 293 L923–L932. 10.1152/ajplung.00221.2007 [DOI] [PubMed] [Google Scholar]
- Han J., Xu H. H., Chen X. L., Hu H. R., Hu K. M., Chen Z. W., et al. (2018). Total flavone of rhododendron improves cerebral ischemia injury by activating vascular TRPV4 to induce endothelium-derived hyperpolarizing factor-mediated responses. Evid. Based Complement Alternat Med. 2018:8919867. 10.1155/2018/8919867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmannsgruber V., Heyken W. T., Kacik M., Kaistha A., Grgic I., Harteneck C., et al. (2007). Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One 2:e827. 10.1371/journal.pone.0000827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano N., Suzuki H., Itoh Y., Muraki K. (2013). TRPV4 partially participates in proliferation of human brain capillary endothelial cells. Life Sci. 92 317–324. 10.1016/j.lfs.2013.01.002 [DOI] [PubMed] [Google Scholar]
- He D., Pan Q., Chen Z., Sun C., Zhang P., Mao A., et al. (2017). Treatment of hypertension by increasing impaired endothelial TRPV4-KCa2.3 interaction. EMBO Mol. Med. 9:1491–1503. 10.15252/emmm.201707725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D. X., Ma X. (2016). Transient receptor potential channel C5 in cancer chemoresistance. Acta Pharmacol. Sin 37 19–24. 10.1038/aps.2015.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. (2011). Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol. Ther. 131 142–170. 10.1016/j.pharmthera.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Ding Y., Li Q., Shi R., He Y. (2020). Transient receptor potential vanilloid 4 channels as therapeutic targets in diabetes and diabetes-related complications. J. Diabetes Investig. 11 757–769. 10.1111/jdi.13244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner C. A., Jentsch T. J. (2002). Ion channel diseases. Hum. Mol. Genet. 11 2435–2445. 10.1093/hmg/11.20.2435 [DOI] [PubMed] [Google Scholar]
- Isshiki M., Anderson R. G. (2003). Function of caveolae in Ca2+ entry and Ca2+-dependent signal transduction. Traffic 4 717–723. 10.1034/j.1600-0854.2003.00130.x [DOI] [PubMed] [Google Scholar]
- Jentsch T. J., Hubner C. A., Fuhrmann J. C. (2004). Ion channels: function unravelled by dysfunction. Nat. Cell Biol. 6 1039–1047. 10.1038/ncb1104-1039 [DOI] [PubMed] [Google Scholar]
- Kanugula A. K., Adapala R. K., Jamaiyar A., Lenkey N., Guarino B. D., Liedtke W., et al. (2021). Endothelial TRPV4 channels prevent tumor growth and metastasis via modulation of tumor angiogenesis and vascular integrity. Angiogenesis [Online ahead of print] 10.1007/s10456-021-09775-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanugula A. K., Adapala R. K., Midha P., Cappelli H. C., Meszaros J. G., Paruchuri S., et al. (2019). Novel noncanonical regulation of soluble VEGF/VEGFR2 signaling by mechanosensitive ion channel TRPV4. FASEB J. 33 195–203. 10.1096/fj.201800509R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler R., Heyken W. T., Heinau P., Schubert R., Si H., Kacik M., et al. (2006). Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 26 1495–1502. 10.1161/01.ATV.0000225698.36212.6a [DOI] [PubMed] [Google Scholar]
- Kotlikoff M. I. (2005). EDHF redux: EETs, TRPV4, and Ca2+ sparks. Circ. Res. 97 1209–1210. 10.1161/01.RES.0000196741.99904.e4 [DOI] [PubMed] [Google Scholar]
- Kumar H., Lim C. S., Choi H., Joshi H. P., Kim K. T., Kim Y. H., et al. (2020). Elevated TRPV4 levels contribute to endothelial damage and scarring in experimental spinal cord injury. J. Neurosci. 40 1943–1955. 10.1523/JNEUROSCI.2035-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan H. Y., Shen B., Ma X., Kwok Y. C., Huang Y., Man Y. B., et al. (2009). TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ. Res. 104 670–678. 10.1161/CIRCRESAHA.108.188748 [DOI] [PubMed] [Google Scholar]
- Li S. S., Gao S., Chen Y., Bao H., Li Z. T., Yao Q. P., et al. (2021). Platelet-derived microvesicles induce calcium oscillations and promote VSMC migration via TRPV4. Theranostics 11 2410–2423. 10.7150/thno.47182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang K. (2019). Exploiting the diversity of ion channels: modulation of ion channels for therapeutic indications. Handb. Exp. Pharmacol. 260 187–205. 10.1007/164_2019_333 [DOI] [PubMed] [Google Scholar]
- Loot A. E., Popp R., Fisslthaler B., Vriens J., Nilius B., Fleming I. (2008). Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res. 80 445–452. 10.1093/cvr/cvn207 [DOI] [PubMed] [Google Scholar]
- Lu Q., Zemskov E. A., Sun X., Wang H., Yegambaram M., Wu X., et al. (2021). Activation of the mechanosensitive Ca(2+) channel TRPV4 induces endothelial barrier permeability via the disruption of mitochondrial bioenergetics. Redox Biol. 38:101785. 10.1016/j.redox.2020.101785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhang P., Li J., Lu J., Ge J., Zhao Z., et al. (2015). Epoxyeicosatrienoic acids act through TRPV4-TRPC1-KCa1.1 complex to induce smooth muscle membrane hyperpolarization and relaxation in human internal mammary arteries. Biochim. Biophys. Acta 1852 552–559. 10.1016/j.bbadis.2014.12.010 [DOI] [PubMed] [Google Scholar]
- Maier-Begandt D., Comstra H. S. (2021). A venous-specific purinergic signaling cascade initiated by Pannexin 1 regulates TNFα-induced increases in endothelial permeability. Sci. Signal 14:eaba2940. 10.1126/scisignal.aba2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqboul A., Elsadek B. (2018). Expression profiles of TRPV1, TRPV4, TLR4 and ERK1/2 in the dorsal root ganglionic neurons of a cancer-induced neuropathy rat model. PeerJ 6:e4622. 10.7717/peerj.4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelli S. P., O’Neil R. G., Brown R. C., Bryan R. M., Jr. (2007). PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 292 H1390–H1397. 10.1152/ajpheart.01006.2006 [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Yamaba R., Inoue K., Utsumi D., Tsukahara T., Amagase K., et al. (2018). Transient receptor potential vanilloid 4 channel regulates vascular endothelial permeability during colonic inflammation in dextran sulphate sodium-induced murine colitis. Br. J. Pharmacol. 175 84–99. 10.1111/bph.14072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza S. A., Fang J., Gutterman D. D., Wilcox D. A., Bubolz A. H., Li R., et al. (2010). TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am. J. Physiol. Heart Circ. Physiol. 298 H466–H476. 10.1152/ajpheart.00854.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M. C. (2006). Transient receptor potential (TRP) channels as drug targets. Naunyn Schmiedebergs Arch. Pharmacol. 373 271–272. 10.1007/s00210-006-0071-4 [DOI] [PubMed] [Google Scholar]
- Mizoguchi F., Mizuno A., Hayata T., Nakashima K., Heller S., Ushida T., et al. (2008). Transient receptor potential vanilloid 4 deficiency suppresses unloading-induced bone loss. J. Cell Physiol. 216 47–53. 10.1002/jcp.21374 [DOI] [PubMed] [Google Scholar]
- Moccia F. (2018). Endothelial Ca(2+) signaling and the resistance to anticancer treatments: partners in crime. Int. J. Mol. Sci. 19:217. 10.3390/ijms19010217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole S., Harry A., Fowler A., Hotee S., Warburton J., Waite S., et al. (2020). Investigating the effect of TRPV4 inhibition on pulmonary-vascular barrier permeability following segmental endotoxin challenge. Pulm Pharmacol. Ther. 64:101977. 10.1016/j.pupt.2020.101977 [DOI] [PubMed] [Google Scholar]
- Moran M. M. (2018). TRP channels as potential drug targets. Annu. Rev. Pharmacol. Toxicol. 58 309–330. 10.1146/annurev-pharmtox-010617-052832 [DOI] [PubMed] [Google Scholar]
- Morgan J. T., Stewart W. G., McKee R. A., Gleghorn J. P. (2018). The mechanosensitive ion channel TRPV4 is a regulator of lung development and pulmonary vasculature stabilization. Cell Mol. Bioeng 11 309–320. 10.1007/s12195-018-0538-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori A., Takeda K., Sakamoto K., Nakahara T. (2020). Activation of transient receptor potential vanilloid 4 channels dilates rat retinal arterioles through nitric oxide- and BKCa channel-dependent mechanisms in vivo. Naunyn Schmiedebergs Arch. Pharmacol. 393 35–41. 10.1007/s00210-019-01707-1 [DOI] [PubMed] [Google Scholar]
- Morty R. E., Kuebler W. M. (2014). TRPV4: an exciting new target to promote alveolocapillary barrier function. Am. J. Physiol. Lung Cell Mol. Physiol. 307 L817–L821. 10.1152/ajplung.00254.2014 [DOI] [PubMed] [Google Scholar]
- Negri S., Faris P., Berra-Romani R., Guerra G., Moccia F. (2019). Endothelial transient receptor potential channels and vascular remodeling: extracellular Ca(2 +) entry for angiogenesis, arteriogenesis and vasculogenesis. Front. Physiol. 10:1618. 10.3389/fphys.2019.01618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Owsianik G. (2011). The transient receptor potential family of ion channels. Genome Biol. 12:218. 10.1186/gb-2011-12-3-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolini M., Daneva Z., Chen Y. L., Cope E. L., Kasetti R. B., Zode G. S., et al. (2020). Mechanisms underlying selective coupling of endothelial Ca(2+) signals with eNOS vs. IK/SK channels in systemic and pulmonary arteries. J. Physiol. 598 3577–3596. 10.1113/jp279570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto-Neves D., Wang Q., Leal-Cardoso J. H., Rossoni L. V., Jaggar J. H. (2015). Eugenol dilates mesenteric arteries and reduces systemic BP by activating endothelial cell TRPV4 channels. Br. J. Pharmacol. 172 3484–3494. 10.1111/bph.13156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S., Grace M. S., Gondin A. B., Retamal J. S., Dill L., Darby W., et al. (2020). The transient receptor potential vanilloid 4 (TRPV4) ion channel mediates protease activated receptor 1 (PAR1)-induced vascular hyperpermeability. Lab. Invest. 100 1057–1067. 10.1038/s41374-020-0430-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randhawa P. K., Jaggi A. S. (2015a). TRPV1 and TRPV4 channels: potential therapeutic targets for ischemic conditioning-induced cardioprotection. Eur. J. Pharmacol. 746 180–185. 10.1016/j.ejphar.2014.11.010 [DOI] [PubMed] [Google Scholar]
- Randhawa P. K., Jaggi A. S. (2015b). TRPV4 channels: physiological and pathological role in cardiovascular system. Basic Res. Cardiol. 110:54. 10.1007/s00395-015-0512-7 [DOI] [PubMed] [Google Scholar]
- Reddy K., Khaliq A., Henning R. J. (2015). Recent advances in the diagnosis and treatment of acute myocardial infarction. World J. Cardiol. 7 243–276. 10.4330/wjc.v7.i5.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle M. A., Hughes J. M., Walker B. R. (2011). Role of caveolin-1 in endothelial BKCa channel regulation of vasoreactivity. Am. J. Physiol. Cell Physiol. 301 C1404–C1414. 10.1152/ajpcell.00013.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifeddine M., El-Daly M., Mihara K., Bunnett N. W., McIntyre P., Altier C., et al. (2015). GPCR-mediated EGF receptor transactivation regulates TRPV4 action in the vasculature. Br. J. Pharmacol. 172 2493–2506. 10.1111/bph.13072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliez J., Bouzin C., Rath G., Ghisdal P., Desjardins F., Rezzani R., et al. (2008). Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117 1065–1074. 10.1161/CIRCULATIONAHA.107.731679 [DOI] [PubMed] [Google Scholar]
- Schaefer M. (2005). Homo- and heteromeric assembly of TRP channel subunits. Pflugers Arch. 451 35–42. 10.1007/s00424-005-1467-6 [DOI] [PubMed] [Google Scholar]
- Scheraga R. G., Southern B. D., Grove L. M., Olman M. A. (2017). The role of transient receptor potential vanilloid 4 in pulmonary inflammatory diseases. Front. Immunol. 8:503. 10.3389/fimmu.2017.00503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierling W., Troidl K., Apfelbeck H., Troidl C., Kasprzak P. M., Schaper W., et al. (2011). Cerebral arteriogenesis is enhanced by pharmacological as well as fluid-shear-stress activation of the Trpv4 calcium channel. Eur. J. Vasc Endovasc. Surg. 41 589–596. 10.1016/j.ejvs.2010.11.034 [DOI] [PubMed] [Google Scholar]
- Shao J., Han J., Zhu Y., Mao A., Wang Z., Zhang K., et al. (2019). Curcumin induces endothelium-dependent relaxation by activating endothelial TRPV4 channels. J. Cardiovasc Transl. Res. 12 600–607. 10.1007/s12265-019-09928-8 [DOI] [PubMed] [Google Scholar]
- Sharma S., Goswami R., Merth M., Cohen J., Lei K. Y., Zhang D. X., et al. (2017). TRPV4 ion channel is a novel regulator of dermal myofibroblast differentiation. Am. J. Physiol. Cell Physiol. 312 C562–C572. 10.1152/ajpcell.00187.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypski M., Kakkassery M., Mergler S., Grotzinger C., Khajavi N., Sassek M., et al. (2013). Activation of TRPV4 channel in pancreatic INS-1E beta cells enhances glucose-stimulated insulin secretion via calcium-dependent mechanisms. FEBS Lett. 587 3281–3287. 10.1016/j.febslet.2013.08.025 [DOI] [PubMed] [Google Scholar]
- Song Y., Zhan L., Yu M., Huang C., Meng X., Ma T., et al. (2014). TRPV4 channel inhibits TGF-beta1-induced proliferation of hepatic stellate cells. PLoS One 9:e101179. 10.1371/journal.pone.0101179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni H., Peixoto-Neves D., Matthews A. T., Adebiyi A. (2017). TRPV4 channels contribute to renal myogenic autoregulation in neonatal pigs. Am. J. Physiol. Renal Physiol. 313 F1136–F1148. 10.1152/ajprenal.00300.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni H., Peixoto-Neves D., Olushoga M. A., Adebiyi A. (2019). Pharmacological inhibition of TRPV4 channels protects against ischemia-reperfusion-induced renal insufficiency in neonatal pigs. Clin. Sci. (Lond) 133:CS20180815. 10.1042/CS20180815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare S. K., Bonev A. D., Ledoux J., Liedtke W., Kotlikoff M. I., Heppner T. J., et al. (2012). Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336 597–601. 10.1126/science.1216283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare S. K., Dalsgaard T., Bonev A. D., Hill-Eubanks D. C., Kotlikoff M. I., Scott J. D., et al. (2014). AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci. Signal 7:ra66. 10.1126/scisignal.2005052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh K., Servinsky L., Jiang H., Bigham Z., Yun X., Kliment C., et al. (2018). Reactive oxygen species induced Ca(2+) influx via TRPV4 and microvascular endothelial dysfunction in the SU5416/hypoxia model of pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 314 L893–L907. 10.1152/ajplung.00430.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh K., Servinsky L., Reyes J., Baksh S., Undem C., Caterina M., et al. (2015). Hydrogen peroxide-induced calcium influx in lung microvascular endothelial cells involves TRPV4. Am. J. Physiol. Lung Cell Mol. Physiol. 309 L1467–L1477. 10.1152/ajplung.00275.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. M., Liddle R. A. (2020). Piezo1 acts upstream of TRPV4 to induce pathological changes in endothelial cells due to shear stress. J. Biol. Chem. 296:100171. 10.1074/jbc.RA120.015059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarka N., Pabbidi M. R., Amrein K., Czeiter E., Berta G., Pohoczky K., et al. (2018). Traumatic brain injury impairs myogenic constriction of cerebral arteries: role of mitochondria-derived H(2)O(2) and TRPV4-Dependent activation of BK(ca) channels. J. Neurotrauma 35 930–939. 10.1089/neu.2017.5056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thodeti C. K., Matthews B., Ravi A., Mammoto A., Ghosh K., Bracha A. L., et al. (2009). TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ. Res. 104 1123–1130. 10.1161/CIRCRESAHA.108.192930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoppil R. J., Cappelli H. C., Adapala R. K., Kanugula A. K., Paruchuri S., Thodeti C. K. (2016). TRPV4 channels regulate tumor angiogenesis via modulation of Rho/Rho kinase pathway. Oncotarget 7 25849–25861. 10.18632/oncotarget.8405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe K. S., Cheung M., Bao W., Alsaid H., Lenhard S., Jian M. Y., et al. (2012). An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci. Transl. Med. 4:159ra148. 10.1126/scitranslmed.3004276 [DOI] [PubMed] [Google Scholar]
- Thorneloe K. S., Cheung M., Holt D. A., Willette R. N. (2017). Properties of the trpv4 agonist GSK1016790A and the TRPV4 antagonist GSK2193874. Physiol. Rev. 97 1231–1232. 10.1152/physrev.00019.2017 [DOI] [PubMed] [Google Scholar]
- Tian W., Fu Y., Garcia-Elias A., Fernandez-Fernandez J. M., Vicente R., Kramer P. L., et al. (2009). A loss-of-function nonsynonymous polymorphism in the osmoregulatory TRPV4 gene is associated with human hyponatremia. Proc. Natl. Acad. Sci. U.S.A. 106 14034–14039. 10.1073/pnas.0904084106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troidl C., Nef H., Voss S., Schilp A., Kostin S., Troidl K., et al. (2010). Calcium-dependent signalling is essential during collateral growth in the pig hind limb-ischemia model. J. Mol. Cell Cardiol. 49 142–151. 10.1016/j.yjmcc.2010.03.021 [DOI] [PubMed] [Google Scholar]
- Troidl C., Troidl K., Schierling W., Cai W. J., Nef H., Mollmann H., et al. (2009). Trpv4 induces collateral vessel growth during regeneration of the arterial circulation. J. Cell Mol. Med. 13 2613–2621. 10.1111/j.1582-4934.2008.00579.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J., Owsianik G., Janssens A., Voets T., Nilius B. (2007). Determinants of 4 alpha-phorbol sensitivity in transmembrane domains 3 and 4 of the cation channel TRPV4. J. Biol. Chem. 282 12796–12803. 10.1074/jbc.M610485200 [DOI] [PubMed] [Google Scholar]
- Vriens J., Watanabe H., Janssens A., Droogmans G., Voets T., Nilius B. (2004). Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. U.S.A. 101 396–401. 10.1073/pnas.0303329101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandall-Frostholm C., Dalsgaard T., Bajoriunas V., Olivan-Viguera A., Sadda V., Beck L., et al. (2015). Genetic deficit of KCa 3.1 channels protects against pulmonary circulatory collapse induced by TRPV4 channel activation. Br. J. Pharmacol. 172 4493–4505. 10.1111/bph.13234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Gao P., Pu Y., Li Q., Yang T., Zhang H., et al. (2017). Activation of TRPV4 by dietary apigenin antagonizes renal fibrosis in deoxycorticosterone acetate (DOCA)-salt-induced hypertension. Clin. Sci. (Lond) 131 567–581. 10.1042/cs20160780 [DOI] [PubMed] [Google Scholar]
- Willette R. N., Bao W., Nerurkar S., Yue T. L., Doe C. P., Stankus G., et al. (2008). Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: part 2. J. Pharmacol. Exp. Ther. 326 443–452. 10.1124/jpet.107.134551 [DOI] [PubMed] [Google Scholar]
- Wong C. O., Yao X. (2011). TRP channels in vascular endothelial cells. Adv. Exp. Med. Biol. 704 759–780. 10.1007/978-94-007-0265-3_40 [DOI] [PubMed] [Google Scholar]
- Xia Y., Fu Z., Hu J., Huang C., Paudel O., Cai S., et al. (2013). TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am. J. Physiol. Cell Physiol. 305 C704–C715. 10.1152/ajpcell.00099.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Liu B., Yin M., Koroleva M., Mastrangelo M., Ture S., et al. (2016). A novel TRPV4-specific agonist inhibits monocyte adhesion and atherosclerosis. Oncotarget 7 37622–37635. 10.18632/oncotarget.9376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Wang R., Cheng X., Qu H., Qi J., Li D., et al. (2020). The vascular dilatation induced by Hydroxysafflor yellow A (HSYA) on rat mesenteric artery through TRPV4-dependent calcium influx in endothelial cells. J. Ethnopharmacol. 256:112790. 10.1016/j.jep.2020.112790 [DOI] [PubMed] [Google Scholar]
- Yang X. R., Lin A. H., Hughes J. M., Flavahan N. A., Cao Y. N., Liedtke W., et al. (2012). Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 302 L555–L568. 10.1152/ajplung.00005.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Kuebler W. M. (2010). Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem. Biophys. 56 1–18. 10.1007/s12013-009-9067-2 [DOI] [PubMed] [Google Scholar]
- Yu Z., Wang Y., Qin L., Chen H. (2017). Functional cooperation between KCa3.1 and TRPV4 channels in bronchial smooth muscle cell proliferation associated with Chronic Asthma. Front. Pharmacol. 8:559. 10.3389/fphar.2017.00559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L., Li J. (2018). The role of TRPV4 in fibrosis. Gene 642 1–8. 10.1016/j.gene.2017.10.067 [DOI] [PubMed] [Google Scholar]
- Zhang D. X., Mendoza S. A., Bubolz A. H., Mizuno A., Ge Z. D., Li R., et al. (2009). Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension 53 532–538. 10.1161/HYPERTENSIONAHA.108.127100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Papadopoulos P., Hamel E. (2013). Endothelial TRPV4 channels mediate dilation of cerebral arteries: impairment and recovery in cerebrovascular pathologies related to Alzheimer’s disease. Br. J. Pharmacol. 170 661–670. 10.1111/bph.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Sun C., Li H., Tang C., Kan H., Yang Z., et al. (2018). TRPV4 (Transient Receptor Potential Vanilloid 4) mediates endothelium-dependent contractions in the aortas of hypertensive mice. Hypertension 71 134–142. 10.1161/HYPERTENSIONAHA.117.09767 [DOI] [PubMed] [Google Scholar]
- Zhang X., Mao A., Xiao W., Zhang P., Han X., Zhou T., et al. (2019). Morin induces endothelium-dependent relaxation by activating TRPV4 channels in rat mesenteric arteries. Eur. J. Pharmacol. 859:172561. 10.1016/j.ejphar.2019.172561 [DOI] [PubMed] [Google Scholar]
- Zheng X., Zinkevich N. S., Gebremedhin D., Gauthier K. M., Nishijima Y., Fang J., et al. (2013). Arachidonic acid-induced dilation in human coronary arterioles: convergence of signaling mechanisms on endothelial TRPV4-mediated Ca2+ entry. J. Am. Heart Assoc. 2:e000080. 10.1161/JAHA.113.000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Wang Z., Guo M., Zhang K., Geng L., Mao A., et al. (2020). Puerarin induces mouse mesenteric vasodilation and ameliorates hypertension involving endothelial TRPV4 channels. Food Funct. 11 10137–10148. 10.1039/d0fo02356f [DOI] [PubMed] [Google Scholar]
- Zhu Y., Wen L., Wang S., Zhang K., Cui Y., Zhang C., et al. (2020). Omega-3 fatty acids improve flow-induced vasodilation by enhancing TRPV4 in arteries from diet-induced obese mice. Cardiovasc Res. [Online ahead of print] 10.1093/cvr/cvaa296 [DOI] [PubMed] [Google Scholar]