Abstract
Frisson is characterised by tingling and tickling sensations with positive or negative feelings. However, it is still unknown what factors affect the intensity of frisson. We conducted experiments on the stimulus characteristics and individual’s mood states and personality traits. Participants filled out self-reported questionnaires, including the Profile of Mood States, Beck Depression Inventory, and Big Five Inventory. They continuously indicated the subjective intensity of frisson throughout a 17-min experiment while listening to binaural brushing and tapping sounds through headphones. In the interviews after the experiments, participants reported that tingling and tickling sensations mainly originated on their ears, neck, shoulders, and back. Cross-correlation results showed that the intensity of frisson was closely linked to the acoustic features of auditory stimuli, including their amplitude, spectral centroid, and spectral bandwidth. This suggests that proximal sounds with dark and compact timbre trigger frisson. The peak of correlation between frisson and the acoustic feature was observed 2 s after the acoustic feature changed, suggesting that bottom-up auditory inputs modulate skin-related modalities. We also found that participants with anxiety were sensitive to frisson. Our results provide important clues to understanding the mechanisms of auditory–somatosensory interactions.
Keywords: Frisson, hearing, somatosensory, interaural level difference, proximal space, autonomous sensory meridian response (ASMR)
Introduction
Tactile sensation is considered to originate at the surface of the body. However, the modern view suggests that some skin-related modalities, such as affective touch, rely on interoception rather than on extraception (A. D. Craig, 2015; Tihanyi et al., 2018). In addition, skin sensation is affected by cognitive processes such as attention, expectations, and verbal suggestions (Bartels et al., 2014; Jensen et al., 2012). The rubber hand illusion can be caused by watching a rubber hand being stroked synchronously with one’s own hand; participants fail to localise the perceived position of their hand (Botvinick & Cohen, 1998). Thus, the integration of vision, touch, and proprioception plays a critical role in shaping the body schema (de Vignemont et al., 2005; Haggard et al., 2007; Maravita et al., 2003).
Regarding hearing, chills are known as a phenomenon in which auditory inputs cause bodily sensations such as goosebumps and shivers (Harrison & Loui, 2014; Huron & Margulis, 2011; Maruskin et al., 2012). However, there is a lack of operative consensus. An early study found that a chill, shudder, tingling, or tickling sensation defined as a thrill was elicited by happy music (Goldstein, 1980). Another study demonstrated that the same phenomenon defined as a chill was often induced by sad music (Panksepp, 1995). Previous findings on how personality traits affect chills have been controversial. Several researchers argued that Openness to experience, one of the Big Five personality traits, was most predictive of chills (Maruskin et al., 2012; McCrae, 2007; Nusbaum & Silvia, 2010). Others argued that chills correlate with Agreeableness (Panksepp, 1995) and Neuroticism (Maruskin et al., 2012). A concern is that most studies have focused on chills in the aesthetics domain, whereas we sometimes experience frisson in daily life. For example, the buzz of a mosquito, perceived to be moving around the head, can induce frisson (Honda et al., 2020). We should investigate determinant factors of frisson and chills from a different perspective.
We examined the degree to which frisson is affected by acoustic features of auditory stimuli as well as mood states and personality traits of individuals. We treat frisson as a broad concept of tingling and tickling sensations that are associated with sounds and accompanied with positive or negative emotions. Frisson is probably the most usable term because it integrates emotional intensity with tactile sensations and does not include potential meanings of cold chills (Harrison & Loui, 2014). From the perspective of cross-modal interactions, frisson is an intriguing phenomenon because sounds stimulate skin-related modalities. Participants sometimes report a tickling sensation when listening to recorded sounds of stroking an ear of a dummy head with a paintbrush (Kitagawa & Igarashi, 2005). The effects of auditory distractors on tactile discrimination are larger when the distractors are presented close to the head (20 cm) than when they are presented far from the head (70 cm) (Kitagawa et al., 2005). Self-produced action sounds can bias the body schema and its spatial boundaries (Tajadura-Jimenez et al., 2012). In addition, auditory feedback can modify the tactile perception of participants when they rub their hands together. When high-frequency components of the auditory feedback increase, the perceived roughness/moisture of the palmar skin decreases and its smoothness/dryness increases (Guest et al., 2002; Jousmäki & Hari, 1998). However, it is not clear what factors transform auditory inputs into frisson.
We used an auditory stimulus related to the autonomous sensory meridian response (ASMR), a phenomenon often characterised by a tingling electrostatic-like sensation across the scalp and back of the neck (Barratt & Davis, 2015). We considered the ASMR as a component of frisson. Participants, all of whom had never experienced ASMR triggers, were instructed to listen to binaural brushing and tapping sounds from headphones and continuously indicate the intensity of frisson. In Experiment 1, we used a test–retest paradigm to confirm the consistency of frisson within an individual. In Experiment 2, we checked participant’s mood states and personality traits. Participants were instructed to continuously indicate the intensity of frisson while listening to the ASMR stimuli throughout a 17-min trial. At the end of this experiment, participants were asked to report where their frisson sensation originated. We assumed no confounding factor between acoustic features and individual’s states/traits because these parameters are essentially independent of each other. We examined the degree to which bottom-up sensory evidence and individual’s states/traits are involved in the frisson experience.
Materials and methods
Participants
Ten Japanese college students (3 males and 7 females; mean age = 20.6 years, range = 20–21 years) participated in Experiment 1 for the test–retest reliability. Another 30 students (13 males and 17 females; mean age = 20.6 years, range = 18–23 years) participated in Experiment 2. Specifically, according to a priori power analysis with a power of 0.8 (α = .05), we required at least 26 participants for this experiment (r = .5, two-tailed t test). Participants were right-handed with normal hearing. None had ever viewed any video for the ASMR experience. This study was approved by the Ethics Committee of Chukyo University (approval no. RS18-023) and carried out in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects. All participants gave written informed consent after the procedures had been fully explained to them.
Stimuli and task procedures
We used the same auditory stimuli for Experiments 1 and 2. The auditory stimuli were made from an ASMR video with Creative Commons licences that was uploaded to the video-sharing site YouTube (https://www.youtube.com/watch?v=7MZtaAgqoTY). The total duration of the auditory stimuli was 1,020 s. Visual information was removed from the stimuli to focus on sound-induced frisson and exclude confounding effects of facial expressions on participant’s responses. Participants were instructed to dichotically listen to the stimuli through headphones (Sennheiser HD 599) and continuously indicate their sensation using a 3-point Likert-type scale: no frisson, weak frisson, or strong frisson. We told participants that frisson means a “zoku-zoku” sensation because this Japanese onomatopoeia includes the meanings of to be thrilled by joy and tremble from cold chills (Liao et al., 2017; Osaka et al., 2003, 2004). Their responses were collected via three keys on a computer keyboard with the sampling rate of 1 kHz. A key press indicating a response was held until a subsequent key press. The stimulus presentation and data collection were controlled using a PC with Presentation software (Neurobehavioral Systems, Berkeley, CA, USA).
The test–retest paradigm was used in Experiment 1. For the same participants, a retest session was conducted 2 weeks after the initial session. In Experiment 2, participants filled out three types of questionnaires, performed one trial of the frisson task, and participated in an interview. We confirmed that participants responded to the ASMR stimuli as we had intended. Participants were instructed to indicate where the frisson originated and whether the sensation moved to other areas of their body. They also reported the degree of their tingling and tickling sensations throughout a trial using a visual analogue scale (range = 0–100). They also reported whether their experience was pleasant or unpleasant. The scale ranged from negative to positive emotions (neutral = 50).
Questionnaires of mood states and personality traits
Before an experimental trial, we gave participants questionnaires to avoid the effects of the ASMR stimuli on their emotions. We obtained self-reported measures of participant’s mood states and personality traits using the Profile of Mood States (POMS), Beck Depression Inventory (BDI-II), and Big Five Inventory (BFI). The POMS measures transient distinct mood states for the past 1 week and consists of six subscales: Tension-Anxiety (9 items), Depression (15 items), Anger-Hostility (12 items), Vigour (8 items), Fatigue (7 items), and Confusion (7 items) (McNair et al., 1971). Participants rated their mood with 65 adjectives (e.g., “Furious,” “Hopeless,” and “Carefree”) on a 5-point Likert-type scale from not at all to extremely. The BDI is widely used as an indicator of the severity of depression in adolescents and adults (Beck et al., 1996). Participants responded to 21 items based on their mental states over the past 2 weeks. The BFI is used to measure individual’s basic personality traits: Extraversion (8 items), Agreeableness (9 items), Conscientiousness (9 items), Neuroticism (8 items), and Openness (10 items) (John & Srivastava, 2001; Kondo et al., 2017). Participants rated these 44 items on a 5-point Likert-type scale ranging from strongly disagree to strongly agree. One participant was excluded from the subsequent analysis on mood states and personality traits because his or her data on the questionnaires were missing.
Data analyses
Behavioural data analysis
The time-series data of the frisson estimate were converted to numerical values of 0, 1, and 2. Based on the interviews with participants, the frisson sensation was visualised on areas of the body. We conducted a correlation analysis to check the relationship between averaged frisson estimate, mood states, and personality traits and calculated a significance level. Statistical analyses were carried out with Python, R (version 3.1.2), and IBM SPSS Statistics (version 23).
Acoustic features of auditory inputs
The cochlear spectrogram was calculated from the auditory stimuli presented to each ear. The sounds were recorded with an artificial ear (TYPE2015, ACO, Tokyo) with the sampling rate of 44.1 kHz. The recorded waveforms were decomposed into frequency components by a gammatone filter bank (Patterson et al., 1992). The centre frequencies of the filters were spaced one equivalent rectangular bandwidth (ERB) apart (Glasberg & Moore, 1990), and the lowest centre frequency was set to 20 Hz, resulting in 42 filters with the highest centre frequency of 20.562 kHz. To simulate nonlinear compression in the auditory periphery, amplitude envelopes of the frequency-decomposed signals were calculated with Hilbert transformation, powered by 0.3 (McDermott & Simoncelli, 2011) and down-sampled to 2 kHz.
The auditory features obtained for both the left and right channels were amplitude, spectral centroid, spectral bandwidth, and instantaneous roughness. The amplitude is the mean of the cochlear spectrogram over frequencies:
| (1) |
where f, t, and c denote a frequency bin in the ERB scale, time, and a channel indicating left or right, respectively. The term S(f, t, c) represents a cochlear spectrogram. The spectral centroid indicates the gravity centre in the frequency domain (Koumura & Furukawa, 2017):
| (2) |
The spectral bandwidth is the width of the mass distribution in the frequency domain:
| (3) |
Roughness is often calculated from a sound segment with a certain duration. To evaluate temporal variation of the stimuli, we calculated roughness at every time point, that is, an instantaneous roughness. For this roughness, the cochlear spectrogram at each centre frequency was filtered with a bandpass filter with a frequency between 30 and 150 Hz (Arnal et al., 2015). Instantaneous roughness was defined as
| (4) |
where Sbp denotes the bandpass-filtered spectrogram.
The four features described above were down-sampled to 10 Hz. We computed the average and absolute difference of the features in the left and right ears.
| (5) |
| (6) |
where F indicates amplitude, centroid, bandwidth, or roughness. This resulted in a total of 8 features: 4 (amplitude, spectral centroid, spectral bandwidth, and instantaneous roughness) × 2 (average and absolute difference). We refer to them as Ave- and Diff-amplitude, Ave- and Diff-centroid, Ave- and Diff-bandwidth, and Ave- and Diff-roughness.
Relationship between frisson estimate and acoustic features
We calculated cross-correlations to examine the similarity between the time course of the frisson estimate and acoustic features. The frisson estimate was z-scored for each participant and averaged over participants. The correlations were assessed using Spearman’s rank-order correlations (rs). Statistical significance of the correlation coefficient at the peak of the cross-correlogram was tested by comparing the peak correlation coefficient with 16,000 cross-correlograms calculated from randomly shifted time courses. We also calculated a cross-correlogram for each participant. The cross-correlations did not reach statistical significance for one participant on amplitude; for two participants on spectral centroid; and for five participants on spectral bandwidth. Consequently, we excluded the data of six participants from a repeated-measures analysis of variance (ANOVA) on the time lags of cross-correlations when comparing between the acoustic features (N = 24).
Relationship between frisson estimate and individual’s states/traits
We conducted a correlation analysis and factor analysis to specify the relationship between the intensity of frisson and participant’s mood states and personality traits. We assessed the frisson estimate (raw data) and the scores from the POMS, BDI-II, and BFI. Cronbach’s alpha of the measures reached a satisfactory level (range = .65–.93), suggesting that these measures have a high level of internal consistency. For the factor analysis, however, the BFI Agreeableness score was excluded because it was closely related to the BFI Conscientiousness score (r = .51, p = .005) and its reliability was relatively low (.65). Twelve variables were entered into the subsequent analyses. We checked the Kaiser–Meyer–Olkin (KMO) statistic using zero-order correlations and partial correlations to test whether the variables in our dataset were adequate to correlate. The KMO statistic (.67) indicated that a common factor underlays our dataset, because KMO statistic was higher than .50 for proceeding with a satisfactory factor analysis. Bartlett’s test of sphericity showed that correlations between the variables were greater than those expected by chance: χ2 = 255.04, p < .001. The factors were extracted by least squares estimation and then subjected to an oblique promax rotation.
Results
Frisson experience
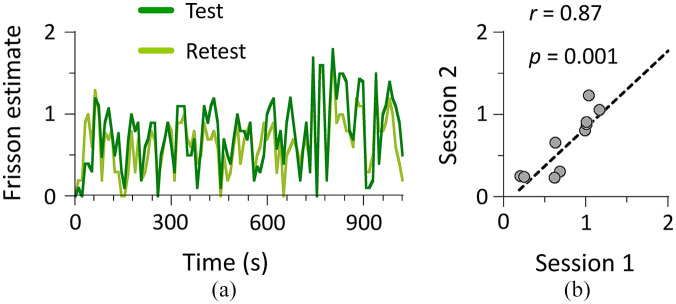
Figure 1 shows the results of the test–retest paradigm used in Experiment 1. The frisson estimate (mean ± SD) throughout a trial did not differ between the first (0.76 ± 0.33) and second (0.66 ± 0.38) sessions: t = 1.78, p = .101, Cohen’s d = 0.29. Importantly, the frisson estimate highly correlated between the two sessions (r = .87, p = .001). These results indicate that the frisson experience observed in this study had high reliability within each participant, whereas the intensity of frisson largely varied across participants.
Figure 1.
Results of Experiment 1 (N = 10). (a) Time-series data of averaged frisson estimate. Participants attended two test sessions (total duration of 1,020 s for each) that were conducted 2 weeks apart. They continuously indicated intensity of frisson using 3-point Likert-type scale while dichotically listening to auditory stimuli through headphones. (b) Circles in correlation plot represent mean frisson estimate over entire stimulus presentation.
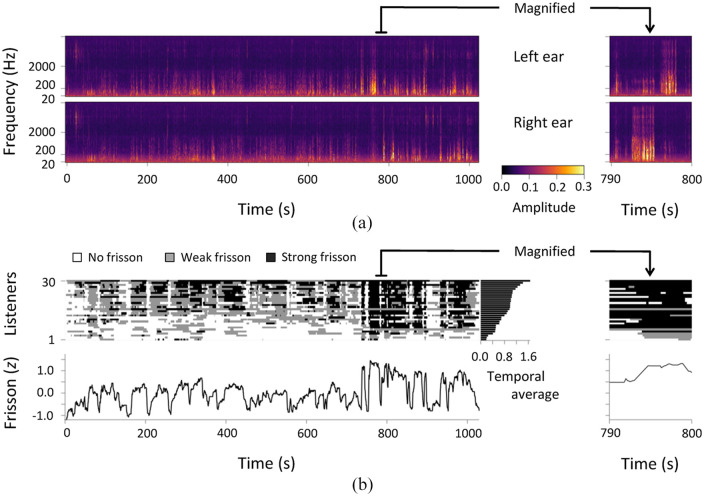
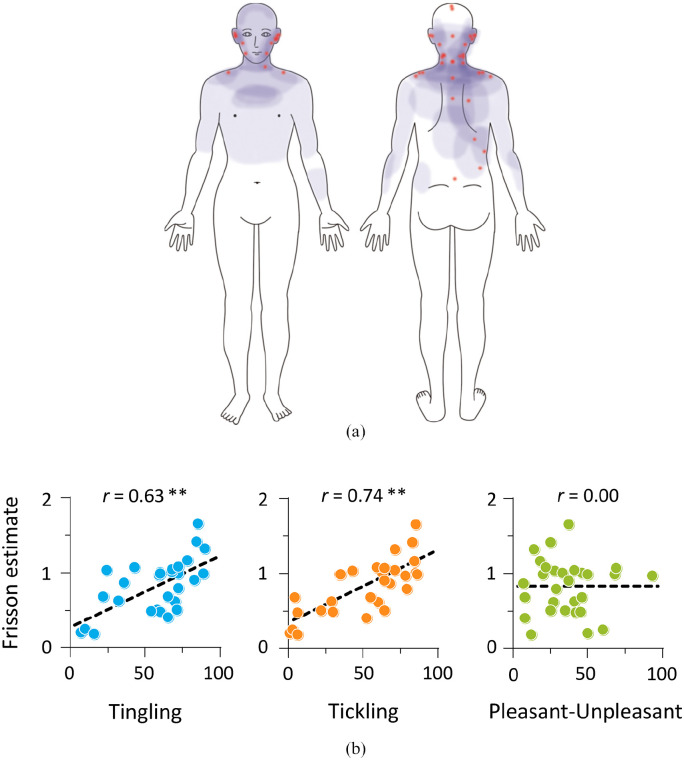
Figure 2 shows time-series data of auditory stimuli and frisson estimate in Experiment 2. The results indicate not only interindividual variations of the frisson experience but also similar temporal changes across participants. Ninety percent of the participants reported experiencing frisson on different parts of their bodies (Figure 3a). Of those parts, it mainly originated on the ears and vicinity (59%), neck (44%), shoulders (44%), and spine and back (30%). Using a visual analogue scale, participants indicated their subjective ratings of their tingling and tickling sensations and pleasant–unpleasant feelings throughout a trial (Table 1). Using the Shapiro–Wilk test, we identified that the frisson estimate and pleasant–unpleasant rating followed a normal distribution, but the subjective ratings of tingling and tickling did not (p < .05). The rating (mean ± SD) was 57.7 ± 24.0 for tingling and 51.2 ± 28.3 for tickling. More participants found the stimuli unpleasant (35.7 ± 20.2) than those who found them pleasant. We examined the degree to which these measures were associated with the frisson experience (Figure 3b). The frisson estimate (0.83 ± 0.36) positively correlated with the ratings of tingling and tickling sensations: r = .63, p < .001, and r = .74, p < .001. However, we did not find any significant correlation between frisson and pleasant–unpleasant feeling (r = .00, p = .98). Our results indicate that the participants had experienced frisson accompanied with tingling and tickling sensations, but not with pleasant–unpleasant feeling.
Figure 2.
Auditory stimuli and frisson estimate. (a) Cochlea spectrograms of stimuli recorded by artificial ear. (b) Results of frisson estimate (z-score) in Experiment 2 (N = 30). Top and bottom panels represent time-series data for each participant and averaged z-score across participants, respectively.
Figure 3.
Frisson, tingling, tickling, and pleasant–unpleasant sensations. (a) Illustration of frisson experience. Red dots indicate origin of “zoku-zoku” sensation, whereas pale-blue areas represent sensation spreading to other parts of body. (b) Scatter plots for relationship between frisson estimate and subjective ratings of tingling, tickling, and pleasant–unpleasant sensations. Dashed line represents linear regression.
**p < .01.
Table 1.
Descriptive statistics for frisson estimate and subjective sensations.
| Measure | M | SD | Min. | Max. | Skewness | Kurtosis | W | p |
|---|---|---|---|---|---|---|---|---|
| Frisson estimate | 0.83 | 0.36 | 0.19 | 1.66 | 0.06 | –0.23 | 0.967 | .477 |
| Visual analogue scale | ||||||||
| Tingling | 57.7 | 24.0 | 7 | 90 | –0.78 | –0.38 | 0.908 | .015 |
| Tickling | 51.2 | 28.3 | 1 | 86 | –0.55 | –1.01 | 0.899 | .009 |
| Pleasant–unpleasant | 35.7 | 20.2 | 7 | 93 | 0.82 | 0.98 | 0.949 | .174 |
W: Shapiro–Wilk normality test.
Correlations between frisson and acoustic features
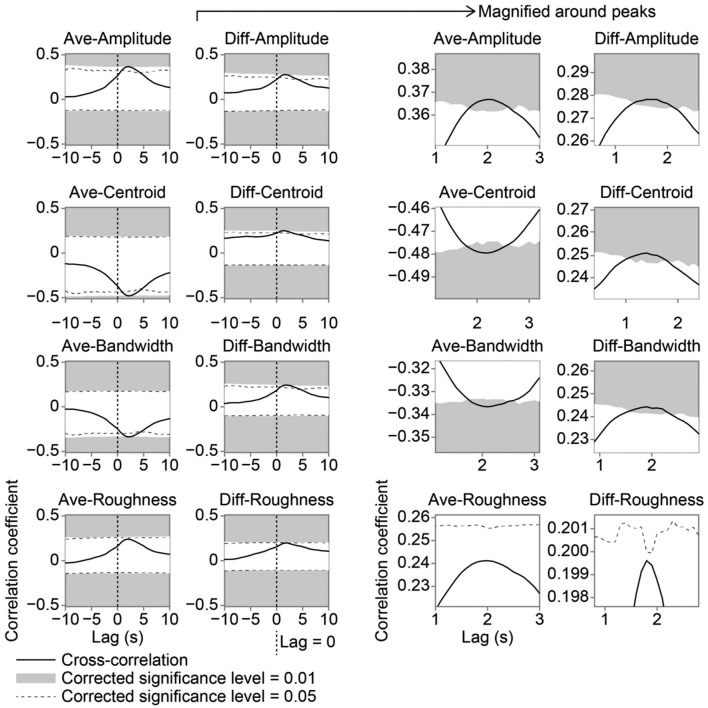
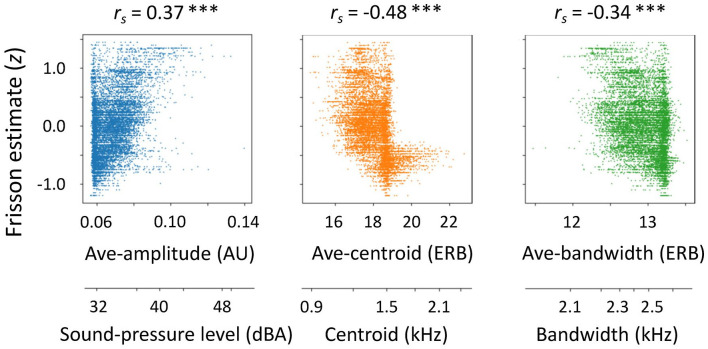
We conducted a cross-correlation analysis to examine whether and how each acoustic feature contributed to the frisson estimate as a function of time (Figure 4). The acoustic features we examined were amplitude, spectral centroid, spectral bandwidth, and instantaneous roughness. We computed an average (Ave) of the features presented to the left and right ears and the difference (Diff) in the features between the two ears. Regarding the Ave-features, the frisson estimate positively correlated with the Ave-amplitude feature (rs = .37, p < .001), indicating that auditory stimuli with larger sound-pressure levels were greater frisson estimates (Figure 4; also see Figure 5). The sound-pressure level (around 40 dBA) of the stimuli was generally low and comparable with the noise level of a quiet library. There were also silent periods between stimulus presentations. Together with these factors, we interpret the results as the following: soft sounds close to the ears strengthen the frisson experience. The frisson estimate negatively correlated with the Ave-centroid feature (rs = –.48, p < .001) and Ave-bandwidth feature (rs = –.34, p < .001) (Figure 4); the former indicates that sounds with dark timbre (a centroid frequency of less than 1.5 kHz) are related to frisson, whereas the latter indicates that compact sounds are associated with frisson (Figure 5). In contrast, the correlation between the frisson estimate and Ave-roughness feature did not reach statistical significance (rs = .24, p = .088).
Figure 4.
Cross-correlations between frisson estimate (z-score) and acoustic features. Areas shaded in grey and dashed lines represent statistical significance after Bonferroni correction over eight features. Right panels show magnified plots around peaks of cross-correlations. Positive time lag indicates that changes in acoustic features are followed by frisson.
Figure 5.
Correlations between frisson estimate (z-score) and acoustic features. Frisson estimate is shifted by peak lags of cross-correlations. Dots represent data for each time point (100 ms bin) throughout experimental trial. When Ave-amplitude feature was larger, frisson estimate was greater. Sound-pressure level (around 40 dBA) of stimuli was generally low. In contrast, when Ave-centroid and Ave-bandwidth features were smaller, frisson estimate was greater. Number lines below panels indicate physical dimensions transformed from acoustic feature values.
AU: arbitrary unit; ERB: equivalent rectangular bandwidth scale; rs: Spearman’s rank correlation coefficient.
***p < .001 (corrected for multiple comparisons).
We also found significant cross-correlations between the frisson estimate and all the Diff-features except for instantaneous roughness: rs = .28, p < .001, for the Diff-amplitude feature; rs = .25, p < .001, for the Diff-centroid feature; rs = .24, p < .001, for the Diff-bandwidth feature; and rs = .20, p = .053, for the Diff-roughness feature (Figure 4). This means that because the differences in the three acoustic features between left and right ears were larger, the frisson estimate became larger. Intriguingly, the peak of cross-correlation coefficients had a time lag of around 2 s. These results indicate that changes in the acoustic features are closely linked to changes in frisson.
We further examined the time lag of cross-correlations using the individual differences approach. The Ave- and Diff-roughness features were excluded from the subsequent analyses because their cross-correlations did not reach statistical significance. We conducted a 2 (Ave and Diff) × 3 (amplitude, centroid, and bandwidth) ANOVA on the time lags to the peak of cross-correlations (Figure 6). The time lag of the Diff-features (1.78 ± 0.17 s) was shorter than that of the Ave-features (2.22 ± 0.19 s): F(1, 23) = 25.38, p < .001, = 0.53. The main effect of the acoustic features did not reach statistical significance—1.93 ± 0.15 s for amplitude; 1.81 ± 0.14 s for centroid; 2.17 ± 0.26 s for bandwidth; F(2, 46) = 2.45, p = .097, = 0.10—and neither did the interaction: F(2, 46) = 2.51, p = .092, = 0.10. Therefore, our results suggest that effects of interaural differences in the acoustic features on frisson precede than those of averages of them.
Figure 6.
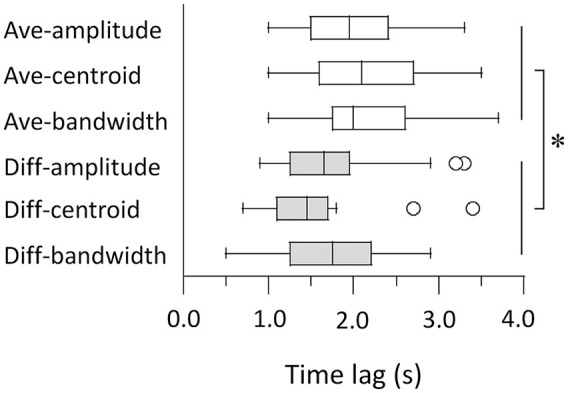
Time lags of peak cross-correlations for acoustic features. Box-and-whisker plots indicate median, 25th and 75th percentiles, and outliers for distribution across participants.
*p < .05.
Correlations between frisson and individual’s states/traits
We have thus far discussed the effects of acoustic features on frisson. We next investigated whether frisson is associated with the mood states and personality traits of the participants, which were assessed using the POMS, BDI-II, and BFI (Table 2). The frisson estimate significantly correlated with the POMS Tension-Anxiety score: r = .52, p = .004 (Table 3). The overall results indicate that individual’s mood states, relative to his or her personality traits, are involved in the frisson experience. We used a multiple regression analysis to specify the degree to which the 12 states/traits scores contribute to the frisson estimate. The results of a stepwise analysis showed that the POMS Tension-Anxiety score accounted for 27.2% of the variance of the frisson estimate: adjusted R2 = .245, F(1, 27) = 10.06, p = .004. However, the contribution of the other measures was not significant. Thus, it is likely that individuals with anxiety are sensitive to frisson.
Table 2.
Descriptive statistics for individual’s states/traits.
| Measure | M | SD | Min. | Max. | Reliability |
|---|---|---|---|---|---|
| POMS | |||||
| Tension-Anxiety | 13.5 | 6.9 | 1 | 31 | .82 |
| Depression | 16.4 | 12.3 | 0 | 46 | .93 |
| Anger-Hostility | 11.3 | 9.6 | 0 | 34 | .93 |
| Vigour | 10.6 | 6.7 | 0 | 30 | .91 |
| Fatigue | 11.9 | 6.0 | 2 | 27 | .86 |
| Confusion | 11.4 | 6.1 | 0 | 25 | .84 |
| BDI-II | 12.7 | 8.1 | 2 | 31 | .81 |
| BFI | |||||
| Extraversion | 21.2 | 6.0 | 10 | 34 | .80 |
| Agreeableness | 28.0 | 6.2 | 10 | 38 | .65 |
| Conscientiousness | 26.2 | 5.9 | 13 | 36 | .76 |
| Neuroticism | 26.7 | 6.0 | 9 | 35 | .80 |
| Openness | 29.5 | 7.0 | 16 | 41 | .76 |
Reliability was calculated using Cronbach’s alpha. POMS: Profile of Mood States; BDI-II: Beck Depression Inventory; BFI: Big Five Inventory.
Table 3.
Correlations between frisson estimate and individual’s states/traits.
| Measure | Correlation | |
|---|---|---|
| r | p | |
| POMS | ||
| Tension-Anxiety | .521 | .004 |
| Depression | .420 | .023 |
| Anger-Hostility | .267 | .162 |
| Vigour | –.141 | .465 |
| Fatigue | .455 | .013 |
| Confusion | .416 | .025 |
| BDI-II | .281 | .140 |
| BFI | ||
| Extraversion | –.101 | .595 |
| Agreeableness | .093 | .623 |
| Conscientiousness | .135 | .476 |
| Neuroticism | .318 | .087 |
| Openness | –.266 | .155 |
Value in bold font is significant after false discovery rate (FDR) correction (n = 29). POMS: Profile of Mood States; BDI-II: Beck Depression Inventory; BFI: Big Five Inventory.
We conducted a factor analysis to further examine the relationships between the frisson estimate and states/traits scores. Two factors, with eigenvalues larger than 1, were extracted from the 12 subscales (Figure 7). The first factor, with an eigenvalue of 5.78 before the promax rotation, was heavily loaded on the frisson estimate, all the POMS subscores except the Vigour score, BDI score, and BFI Neuroticism score (factor loadings, 0.48–0.93). The second factor, with an eigenvalue of 1.68, was loaded on the BFI Extraversion and Openness scores and POMS Vigour score (0.49–0.97). The first and second factors were termed “anxiety” and “activity,” respectively. The contribution of the “anxiety” factor (0.51) to the frisson estimate was much greater than that of the “activity” factor (0.05).
Figure 7.
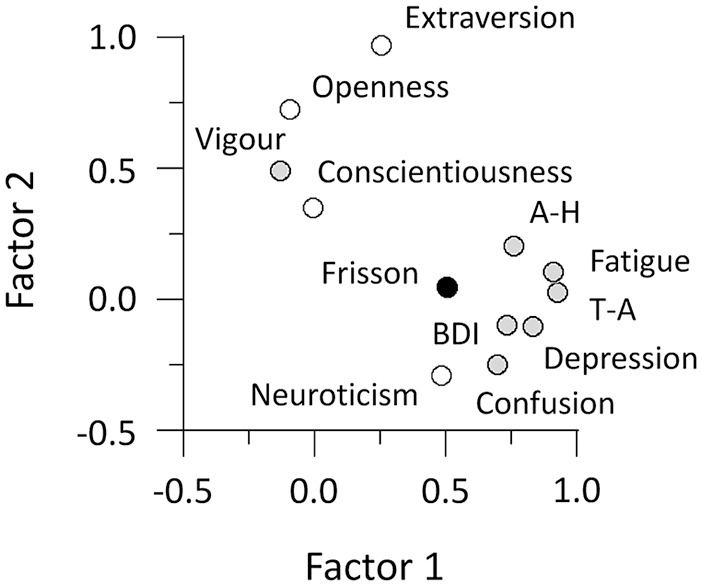
Results of factor analysis. Filled and open circles indicate subscales of questionnaires on individual’s mood states and personality traits, respectively. Factors 1 and 2 can be considered as “anxiety” and “activity” factors.
A-H: Anger-Hostility; T-A: Tension-Anxiety.
Discussion
The experimental results show that sound-induced frisson mainly originated on their ears, neck, and shoulders. Frisson was closely linked to tingling and tickling sensations but not to the pleasant–unpleasant dimension. The cross-correlation results indicate that the frisson experience was associated with particular acoustic features, including amplitude, spectral centroid, and spectral bandwidth, but not with instantaneous roughness. The peak of the correlation between frisson and the acoustic feature was observed 2 s after the acoustic feature changed. A factor analysis demonstrates that frisson was associated with participant’s mood states, except his or her Neuroticism personality. In what follows, we discuss the physiological and emotional aspects of frisson, effects of stimulus characteristics on frisson, and the relationship between the frisson experience and individual’s moods/traits.
We assumed that the ASMR is a component of sound-induced frisson. Although there is a view that the ASMR is related to musical chills (del Campo & Kehle, 2016), the two phenomena appear to be different in terms of physiological and emotional aspects. A previous study on chills revealed that most participants experienced goosebumps on their arms, whereas less than half felt something along their spines (D. G. Craig, 2005). In this study, however, small numbers of participants reported experiencing frisson on their arms (15%) and legs (0%). A profound emotional experience, such as weeping, sometimes arises in response to musical chills (Mori & Iwanaga, 2017; Panksepp, 1995; Sloboda, 1991). In contrast, many individuals expect to feel relaxed, a decrease in negative mood, and sleep better due to the ASMR (Barratt & Davis, 2015). Considering these findings, we can imagine that musical chills and ASMR are mainly modulated by the sympathetic and parasympathetic nervous systems, respectively. Sound-induced frisson, on the contrary, not only shares common tactile sensation that the ASMR induces but also includes unpleasant emotional response that the ASMR does not induce. It is thus surmised that sound-induced frisson reflects more complicated dynamic interactions between the sympathetic and parasympathetic nervous systems. Further studies are required to investigate the correspondence between subjective emotional and physiological responses such as pupillary response and skin conductance response, to understand the underlying neural mechanism.
Positive aspects, such as pleasant tingling, have been emphasised in the literature on the ASMR. However, participants in this study did not necessarily have pleasant feelings. A previous study showed that chills from music were associated with pleasant valence, whereas chills from human voices, animal calls, and environmental sounds were related to unpleasant valence (Grewe et al., 2011). Another study using a cluster analysis revealed that tingling sensation and goosebumps were pleasant, whereas coldness and shivers were unpleasant (Maruskin et al., 2012). Indeed, people may respond differently to various types of stimuli; frisson or chills have a wide range of affective states. It should be noted that the participants did not consume any ASMR media before our experiment. Thus, we speculate that positive emotions and pleasant feelings accompanied with the ASMR are acquired by an individual’s perceptual learning. This idea is consistent with the fact that ASMR stimuli are widely varied, for instance, whispering, grooming, repetitive movements, and natural sounds. Musical chills occur at the moment of surprising changes in the musical composition and increase with a listener’s familiarity with the piece (Panksepp, 1995). Therefore, we believe that an individual’s preference to ASMR stimuli is converged by accumulating the experience of various stimuli and sharpening the positive aspects of frisson.
Another possibility is that emotional reactions to frisson depend on acoustic features of auditory stimuli. Unpleasant feelings are sometimes evoked by the roughness in sounds such as screams (Arnal et al., 2015). The perceptual attribute can be differentiated from other communication signals such as speech. In the present study, the cross-correlation between the frisson estimate and Ave-roughness feature did not reach statistical significance. This may be a reason that frisson was not necessarily a pleasant experience for several participants. Thus, it is worth considering how acoustic features of auditory stimuli affect the pleasant–unpleasant dimension of the ASMR experience.
We demonstrated a positive correlation between the Ave-amplitude feature and frisson estimate. The sound-pressure level of the stimuli was generally low. Thus, the results indicate that soft sounds close to the ears strengthen frisson. This is consistent with the finding that the intensity of frisson is greater for sounds moving around the head than for static sounds (Honda et al., 2020). In a previous study, participants reported a stronger tickling sensation when the sound of an ear being stroked was presented with headphones than when the sound was presented via a loudspeaker placed 80 cm from their ears (Kitagawa & Igarashi, 2005). Furthermore, we found that a larger Diff-amplitude feature resulted in a greater frisson estimate. This suggests that sound sources biased in either one ear or the other are associated with the frisson experience because the Diff-amplitude feature corresponds to an interaural level difference (ILD). Therefore, auditory–somatosensory interactions observed as frisson are probably related to sound-localisation ability in the space close to the head.
Most sound-localisation studies have focused on auditory events in the distal region (i.e., distances of more than 1 m from participants) (Brungart & Rabinowitz, 1999). This may be because the detection of distant events in an environment is believed to be necessary for the survival of humans and mammals (Heffner & Heffner, 1992; Kitagawa & Spence, 2006). In general, the ILD and interaural time difference (ITD) are respectively used as localisation cues when high- and low-frequency sounds reach the ears (Moore, 2013; Rayleigh, 1907). ILDs can be accurately obtained at high frequencies (more than 1.5 kHz) because diffraction due to the head hardly occurs, whereas ITDs can be easily detected at low frequencies because phase ambiguity hardly occurs. Intriguingly, a previous study demonstrated that ILDs, even at low frequencies, increase when a sound source is located less than 50 cm from the head (Brungart & Rabinowitz, 1999). Thus, we believe that sounds that appear closer are more likely to induce frisson.
The sounds in the proximal space produce tingling and tickling sensations on the skin of the ears, neck, shoulders, and spine. The auditory and somatosensory systems have similar temporal frequency channels (Occelli et al., 2011; Yau et al., 2009). Many neurophysiological studies have investigated somatosensory responses in the auditory cortex. Neuronal responses in the caudomedial area of the auditory cortex have been elicited through cutaneous stimulation on the neck and hands of monkeys (Fu et al., 2003; Schroeder et al., 2001). Tactile stimulation has been shown to enhance the activity of posterior auditory areas (Foxe et al., 2002; Kayser et al., 2005; Schürmann et al., 2006). In contrast, little is known about how neurons in the cerebral cortex encode information on auditory distance. A substantial study demonstrated that tactile-sensitive neurons in the ventral premotor area respond to auditory stimuli presented within 30 cm of the head and that neural responses decrease when they are presented at distances of 50 cm from the head (Graziano et al., 1999). The traditional view on sensory systems is that different sensory information is separately analysed in unisensory areas and subsequently projected into multisensory areas (Felleman & Van Essen, 1991). However, recent fMRI studies have revealed that auditory stimulation influences the somatosensory area (Liang et al., 2013; Pérez-Bellido et al., 2018). Thus, the present findings on frisson could contribute to clarifying neural mechanisms of auditory–somatosensory integration.
Our results indicate that the Ave-centroid and Ave-bandwidth features negatively correlated with the frisson estimate. First, the spectral centroid has been shown to correlate with the perceptual brightness of musical sounds with clear pitch (Almeida et al., 2017). Although our stimuli were non-musical sounds, a previous study revealed that both pitched and unpitched sounds can produce timbre in a common perceptual space, which includes the spectral centroid as one of its major dimensions (Lakatos, 2000). Thus, it is likely that sounds with a dark impression are closely linked to the frisson experience. Second, sounds with a narrowband of frequencies are generally perceived as originating from common sources (Cusack & Roberts, 1999). Thus, we can imagine that frisson is triggered by sounds with compact and sharp timbre than by diffuse noise-like sounds.
The POMS Tension-Anxiety score positively correlated with the frisson estimate, suggesting that participants with anxiety are sensitive to frisson. This result may be explained by increased self-monitoring of psychological and physiological states (Fredborg et al., 2017). A previous study reported that people in a depressive state had significantly gained from experiencing the ASMR (Barratt & Davis, 2015). In the present study, the correlation between the BDI score and frisson estimate were positive but did not reach statistical significance. The discrepancy in these results is possibly derived from the differences in analytic procedures: group comparison and individual-based correlation. The relationship between the ASMR and depressive state may be a nonlinear association. More importantly, a factor analysis revealed that “anxiety” and “activity” factors were extracted from 12 parameters of individual’s mood states and personality traits. The “anxiety” factor is composed of the frisson estimate, individual’s mood states, and BFI Neuroticism score. It has been found that personality traits, such as the BFI Neuroticism and Openness, contributed to musical chills (McCrae, 2007; Nusbaum & Silvia, 2010; Panksepp, 1995) and the ASMR (Fredborg et al., 2017). However, no single trait has emerged as a consistent predictor of the experience (Maruskin et al., 2012). Thus, we propose that sensitivity to the frisson experience largely depends on individual’s mood states, rather than on his or her personality traits.
Psychophysical studies have demonstrated that an interaction between auditory and somatosensory information occurs easily in the space behind the back when visual cues are not available (Kóbor et al., 2006; Zampini et al., 2007). However, they did not consider the effects of acoustic features on skin-related sensations and subjective feelings. An essential part of the frisson observed in this study is that auditory stimuli produce different forms of perception at the surface of the body or inside it. We demonstrated that different levels of frisson were associated with different levels of the acoustic features, namely, amplitude, spectral centroid, and spectral bandwidth. This suggests that dark, loud, and compact sounds induce frisson. We also found that the participants with anxiety tended to experience stronger frisson. A future study should investigate whether the findings can be generalised to other situations because all types of ASMR stimuli were not used in the present study. However, our findings can open a new avenue of cross-modal investigations to understand the mechanisms of auditory–somatosensory interactions.
Acknowledgments
We thank two anonymous reviewers for their thoughtful comments on an earlier version of this manuscript. We also thank the Institute for Advanced Collaborative Research at Chukyo University for its generous support.
Footnotes
Author Contributions: Conceptualisation: T.K., M.N., and H.M.K.; Data curation: T.K. and H.M.K.; Formal analysis: T.K. and H.M.K.; Funding acquisition: H.M.K.; Investigation: H.M.K.; Methodology: T.K. and H.M.K.; Project administration: H.M.K.; Resources: T.K., M.N., H.I.L., and H.M.K.; Supervision: H.M.K.; Writing—original draft: T.K., H.I.L., and H.M.K.; Writing—review and editing: T.K., M.N., H.I.L., and H.M.K.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by JSPS KAKENHI grants (Nos. 17K04494 and 20H01789 to H.M.K.).
ORCID iD: Hirohito M Kondo  https://orcid.org/0000-0002-7444-4996
https://orcid.org/0000-0002-7444-4996
References
- Almeida A., Schubert E., Smith J., Wolfe J. (2017). Brightness scaling of periodic tones. Attention, Perception, & Psychophysics, 79, 1892–1896. 10.3758/s13414-017-1394-6 [DOI] [PubMed] [Google Scholar]
- Arnal L. H., Flinker A., Kleinschmidt A., Giraud A. L., Poeppel D. (2015). Human screams occupy a privileged niche in the communication soundscape. Current Biology, 25, 2051–2056. 10.1016/j.cub.2015.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt E. L., Davis N. J. (2015). Autonomous Sensory Meridian Response (ASMR): A flow-like mental state. PeerJ, 3, e851. 10.7717/peerj.851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels D. J., van Laarhoven A. I., Haverkamp E. A., Wilder-Smith O. H., Donders A. R., van Middendorp H., . . . Evers A. W. (2014). Role of conditioning and verbal suggestion in placebo and nocebo effects on itch. PLOS ONE, 9, e91727. 10.1371/journal.pone.0091727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. T., Steer R. A., Brown G. (1996). Manual for the Beck Depression Inventory–II. Psychological Corporation. [Google Scholar]
- Botvinick M., Cohen J. (1998). Rubber hands “feel” touch that eyes see. Nature, 391, 756. 10.1038/35784 [DOI] [PubMed] [Google Scholar]
- Brungart D. S., Rabinowitz W. M. (1999). Auditory localization of nearby sources. Head-related transfer functions. Journal of the Acoustical Society of America, 106, 1465–1479. 10.1121/1.427180 [DOI] [PubMed] [Google Scholar]
- Craig A. D. (2015). How do you feel? An interoceptive moment with your neurobiological self. Princeton University Press. [Google Scholar]
- Craig D. G. (2005). An exploratory study of physiological changes during “chills” induced by music. Musicae Scientiae, 9, 273–287. 10.1177/102986490500900207 [DOI] [Google Scholar]
- Cusack R., Roberts B. (1999). Effects of similarity in bandwidth on the auditory sequential streaming of two-tone complexes. Perception, 28, 1281–1289. 10.1068/p2804 [DOI] [PubMed] [Google Scholar]
- del Campo M. A., Kehle T. J. (2016). Autonomous sensory meridian response (ASMR) and frisson: Mindfully induced sensory phenomena that promote happiness. International Journal of School, 4, 99–105. [Google Scholar]
- de Vignemont F., Ehrsson H. H., Haggard P. (2005). Bodily illusions modulate tactile perception. Current Biology, 15, 1286–1290. 10.1016/j.cub.2005.06.067 [DOI] [PubMed] [Google Scholar]
- Felleman D. J., Van Essen D. C. (1991). Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex, 1, 1–47. 10.1093/cercor/1.1.1 [DOI] [PubMed] [Google Scholar]
- Foxe J. J., Wylie G. R., Martinez A., Schroeder C. E., Javitt D. C., Guilfoyle D., . . . Murray M. M. (2002). Auditory-somatosensory multisensory processing in auditory association cortex: An fMRI study. Journal of Neurophysiology, 88, 540–543. 10.1152/jn.2002.88.1.540 [DOI] [PubMed] [Google Scholar]
- Fredborg B., Clark J., Smith S. D. (2017). An examination of personality traits associated with autonomous sensory meridian response (ASMR). Frontiers in Psychology, 8, Article 247. 10.3389/fpsyg.2017.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. M. G., Johnston T. A., Shah A. S., Arnold L., Smiley J., Hackett T. A., . . . Schroeder C. E. (2003). Auditory cortical neurons respond to somatosensory stimulation. Journal of Neuroscience, 23, 7510–7515. 10.1523/JNEUROSCI.23-20-07510.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasberg B. R., Moore B. C. J. (1990). Derivation of auditory filter shapes from notched-noise data. Hearing Research, 47, 103–138. 10.1016/0378-5955(90)90170-T [DOI] [PubMed] [Google Scholar]
- Goldstein A. (1980). Thrills in response to music and other stimuli. Physiological Psychology, 8, 126–129. 10.3758/BF03326460 [DOI] [Google Scholar]
- Graziano M. S., Reiss L. A., Gross C. G. (1999). A neuronal representation of the location of nearby sounds. Nature, 397, 428–430. 10.1038/17115 [DOI] [PubMed] [Google Scholar]
- Grewe O., Katzur B., Kopiez R., Altenmüller E. (2011). Chills in different sensory domains: Frisson elicited by acoustical, visual, tactile and gustatory stimuli. Psychology of Music, 39, 220–239. 10.1177/0305735610362950 [DOI] [Google Scholar]
- Guest S., Catmur C., Lloyd D., Spence C. (2002). Audiotactile interactions in roughness perception. Experimental Brain Research, 146, 161–171. 10.1007/s00221-002-1164-z [DOI] [PubMed] [Google Scholar]
- Haggard P., Christakou A., Serino A. (2007). Viewing the body modulates tactile receptive fields. Experimental Brain Research, 180, 187–193. 10.1007/s00221-007-0971-7 [DOI] [PubMed] [Google Scholar]
- Harrison L., Loui P. (2014). Thrills, chills, frissons, and skin orgasms: Toward an integrative model of transcendent psychophysiological experiences in music. Frontiers in Psychology, 5, Article 790. 10.3389/fpsyg.2014.00790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner R. S., Heffner H. E. (1992). Visual factors in sound localization in mammals. Journal of Comparative Neurology, 317, 219–232. 10.1002/cne.903170302 [DOI] [PubMed] [Google Scholar]
- Honda S., Ishikawa Y., Konno R., Imai E., Nomiyama N., Sakurada K., . . . Nakatani M. (2020). Proximal binaural sound can induce subjective frisson. Frontiers in Psychology, 11, Article 316. 10.3389/fpsyg.2020.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huron D., Margulis E. H. (2011). Music, expectancy, and thrills. In Juslin P. A., Sloboda J. A. (Eds.), Handbook of music and emotion: Theory, research, applications (pp. 575–604). Oxford University Press. [Google Scholar]
- Jensen K. B., Kaptchuk T. J., Kirsch I., Raicek J., Lindstrom K. M., Berna C., . . . Kong J. (2012). Nonconscious activation of placebo and nocebo pain responses. Proceedings of the National Academy of Sciences of the United States of America, 109, 15959–15964. 10.1073/pnas.1202056109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John O. P., Srivastava S. (2001). The big-five trait taxonomy: History, measurement, and theoretical perspectives. In Pervin L. A., John O. P. (Eds.), Handbook of personality: Theory and research (2nd ed., pp. 102–138). Guilford Press. [Google Scholar]
- Jousmäki V., Hari R. (1998). Parchment-skin illusion: Sound-biased touch. Current Biology, 8, R190. 10.1016/S0960-9822(98)70120-4 [DOI] [PubMed] [Google Scholar]
- Kayser C., Petkov C. I., Augath M., Logothetis N. K. (2005). Integration of touch and sound in auditory cortex. Neuron, 48, 373–384. 10.1016/j.neuron.2005.09.018 [DOI] [PubMed] [Google Scholar]
- Kitagawa N., Igarashi Y. (2005). Tickle sensation induced by hearing a sound. Japanese Journal of Psychonomic Science, 24, 121–122. [Google Scholar]
- Kitagawa N., Spence C. (2006). Audiotactile multisensory interactions in human information processing. Japanese Psychological Research, 48, 158–173. https://doi.org/10.1111./j.1468-5884.2006.00317.x [Google Scholar]
- Kitagawa N., Zampini M., Spence C. (2005). Audiotactile interactions in near and far space. Experimental Brain Research, 166, 528–537. 10.1007/s00221-005-2393-8 [DOI] [PubMed] [Google Scholar]
- Kóbor I., Füredi L., Kovács G., Spence C., Vidnyánszky Z. (2006). Back-to-front: Improved tactile discrimination performance in the space you cannot see. Neuroscience Letters, 400, 163–167. 10.1016/j.neulet.2006.02.037 [DOI] [PubMed] [Google Scholar]
- Kondo H. M., Farkas D., Denham S. L., Asai T., Winkler I. (2017). Auditory multistability and neurotransmitter concentrations in the human brain. Philosophical Transactions of the Royal Society B, 372, 20160110. 10.1098/rstb.2016.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumura T., Furukawa S. (2017). Context-dependent effect of reverberation on material perception from impact sound. Scientific Reports, 7, Article 16455. 10.1038/s41598-017-16651-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos S. (2000). A common perceptual space for harmonic and percussive timbres. Perception and Psychophysics, 62, 1426–1439. 10.3758/bf03212144 [DOI] [PubMed] [Google Scholar]
- Liang M., Mouraux A., Hu L., Iannetti G. D. (2013). Primary sensory cortices contain distinguishable spatial patterns of activity for each sense. Nature Communications, 4, 1979. 10.1038/ncomms2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H.-I., Nakatani M., Miyazaki H., Furukawa S. (2017, June). Sensing frisson by material sounds [Paper presentation]. The 21st annual meeting of the association for the scientific study of consciousness. [Google Scholar]
- Maravita A., Spence C., Driver J. (2003). Multisensory integration and the body schema: Close to hand and within reach. Current Biology, 13, R531–R539. 10.1016/s0960-9822(03)00449-4 [DOI] [PubMed] [Google Scholar]
- Maruskin L. A., Thrash T. M., Elliot A. J. (2012). The chills as a psychological construct: Content universe, factor structure, affective composition, elicitors, trait antecedents, and consequences. Journal of Personality and Social Psychology, 103, 135–157. 10.1037/a0028117 [DOI] [PubMed] [Google Scholar]
- McCrae R. R. (2007). Aesthetic chills as a universal marker of openness to experience. Motivation and Emotion, 31, 5–11. 10.1007/s11031-007-9053-1 [DOI] [Google Scholar]
- McDermott J. H., Simoncelli E. P. (2011). Sound texture perception via statistics of the auditory periphery: Evidence from sound synthesis. Neuron, 71, 926–940. 10.1016/j.neuron.2011.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D. M., Lorr M., Droppleman L. (1971). Manual for the Profile of Mood States. Educational and Industrial Testing Service. [Google Scholar]
- Moore B. C. J. (2013). An introduction to the psychology of hearing (6th ed.). Brill. [Google Scholar]
- Mori K., Iwanaga M. (2017). Two types of peak emotional responses to music: The psychophysiology of chills and tears. Scientific Reports, 7, Article 46063. 10.1038/srep46063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum E. C., Silvia P. J. (2010). Shivers and timbres: Personality and the experience of chills from music. Social Psychological and Personality Science, 2, 199–204. 10.1177/1948550610386810 [DOI] [Google Scholar]
- Occelli V., Spence C., Zampini M. (2011). Audiotactile interactions in temporal perception. Psychonomic Bulletin & Review, 18, 429–454. 10.3758/s13423-011-0070-4 [DOI] [PubMed] [Google Scholar]
- Osaka N., Osaka M., Kondo H., Morishita M., Fukuyama H., Shibasaki H. (2003). An emotion-based facial expression word activates laughter module in the human brain: A functional magnetic resonance imaging study. Neuroscience Letters, 340, 127–130. 10.1016/S0304-3940(03)00093-4 [DOI] [PubMed] [Google Scholar]
- Osaka N., Osaka M., Morishita M., Kondo H., Fukuyama H. (2004). A word expressing affective pain activates the anterior cingulate cortex in the human brain: An fMRI study. Behavioural Brain Research, 153, 123–127. 10.1016/j.bbr.2003.11.013 [DOI] [PubMed] [Google Scholar]
- Panksepp J. (1995). The emotional sources of “chills” induced by music. Music Perception, 13, 171–207. 10.2307/40285693 [DOI] [Google Scholar]
- Patterson R. D., Robinson K., Holdsworth J., McKeown D., Zhang C., Allerhand M. (1992). Complex sounds and auditory images. In Cazals Y., Horner K., Demany L. (Eds.), Auditory physiology and perception: Proceedings of the 9th international symposium on hearing (pp. 429–446). Pergamon. [Google Scholar]
- Pérez-Bellido A., Anne Barnes K., Crommett L. E., Yau J. M. (2018). Auditory frequency representations in human somatosensory cortex. Cerebral Cortex, 28, 3908–3921. 10.1093/cercor/bhx255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayleigh O. M. (1907). On our perception of sound direction. Philosophical Magazine, 13, 214–232. 10.1080/14786440709463595 [DOI] [Google Scholar]
- Schroeder C. E., Lindsley R. W., Specht C., Marcovici A., Smiley J. F., Javitt D. C. (2001). Somatosensory input to auditory association cortex in the macaque monkey. Journal of Neurophysiology, 85, 1322–1327. 10.1152/jn.2001.85.3.1322 [DOI] [PubMed] [Google Scholar]
- Schürmann M., Caetano G., Hlushchuk Y., Jousmaki V., Hari R. (2006). Touch activates human auditory cortex. Neuroimage, 30, 1325–1331. 10.1016/j.neuroimage.2005.11.020 [DOI] [PubMed] [Google Scholar]
- Sloboda J. A. (1991). Music structure and emotional response: Some empirical findings. Psychology of Music, 19, 110–120. 10.1177/0305735691192002 [DOI] [Google Scholar]
- Tajadura-Jimenez A., Valjamae A., Toshima I., Kimura T., Tsakiris M., Kitagawa N. (2012). Action sounds recalibrate perceived tactile distance. Current Biology, 22, R516–R517. 10.1016/j.cub.2012.04.028 [DOI] [PubMed] [Google Scholar]
- Tihanyi B. T., Ferentzi E., Beissner F., Köteles F. (2018). The neuropsychophysiology of tingling. Consciousness and Cognition, 58, 97–110. 10.1016/j.concog.2017.10.015 [DOI] [PubMed] [Google Scholar]
- Yau J. M., Olenczak J. B., Dammann J. F., Bensmaia S. J. (2009). Temporal frequency channels are linked across audition and touch. Current Biology, 19, 561–566. 10.1016/j.cub.2009.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampini M., Torresan D., Spence C., Murray M. M. (2007). Auditory-somatosensory multisensory interactions in front and rear space. Neuropsychologia, 45, 1869–1877. 10.1016/j.neuropsychologia.2006.12.004 [DOI] [PubMed] [Google Scholar]