Abstract
Purpose
Dichoptic training is becoming a popular tool in amblyopia treatment. Here we investigated the effects of dichoptic demasking training in children with amblyopia who never received patching treatment (NPT group) or were no longer responsive to patching (PT group).
Methods
Fourteen NPT and thirteen PT amblyopes (6–16.5 years; 24 anisometropic, two strabismus, and one mixed) received dichoptic demasking training for 17 to 22 sessions. They used the amblyopic eye (AE) to practice contrast discrimination between a pair of Gabors that were dichoptically masked by a band-filtered noise pattern simultaneously presented in the fellow eye (FE). Dichoptic learning was quantified by the increase of maximal tolerable noise contrast (TNC) for AE contrast discrimination. Computerized visual acuities and contrast sensitivity functions for both eyes and the Randot stereoacuity were measured before and after training.
Results
Training improved maximal TNC by six to eight times in both groups, along with a boost of AE acuities by 0.15 logMAR (P < 0.001) in the NPT group and 0.06 logMAR (P < 0.001) in the PT group. This visual acuity improvement was significantly dependent on the pretraining acuity. Stereoacuity was significantly improved by 41.6% (P = 0.002) in the NPT group and 64.2% (P < 0.001) in the PT group. The stereoacuity gain was correlated to the pretraining interocular acuity difference (r = −0.49, P = 0.010), but not to the interocular acuity difference change (r = −0.28, P = 0.15). Training improved AE contrast sensitivity in the NPT group (P = 0.009) but not the PT group (P = 0.76). Moreover, the learning effects in 12 retested observers were retained for 10 to 24 months.
Conclusions
Dichoptic training can improve, and sometimes even restore, the stereoacuity of amblyopic children, especially those with mild amblyopia (amblyopic VA ≦0.28 logMAR). The dissociation of stereoacuity gain and the interocular acuity difference change suggests that the stereoacuity gain may not result from a reduced interocular suppression in most amblyopes. Rather, the amblyopes may have learned to attend to, or readout, the stimulus information to improve stereopsis.
Keywords: dichoptic learning, amblyopia, patching history, children, stereopsis
Amblyopia is a developmental disorder of the visual cortex that arises from abnormal visual experience (e.g., strabismus or anisometropia) in early childhood.1,2 During normal binocular viewing, information from the amblyopic eye is suppressed, whereas the stronger eye dominates perception.2–7 A weakened ability of the amblyopic eye to modulate cortical response gain was created by an imbalance of interocular suppression that favors the dominant eye.4 In addition to decreased visual acuity, amblyopia is accompanied by binocular dysfunctions such as impaired stereoacuity.8,9 Therefore it has been argued that amblyopia is intrinsically a binocular problem, rather than a monocular one. This may explain why the conventional patching treatment, which forces the use of amblyopic eye (AE) with the fellow eye (FE) patch-covered, improves AE visual acuity more than stereoacuity.10–14
In the past decades, studies have shown that perceptual learning can improve visual functions in patients with amblyopia (see Levi et al.15 for a comprehensive review). Earlier perceptual learning studies mostly performed monocular training in AE with FE patched.16–20 For example, we reported that monocular training of a grating acuity task (cutoff spatial frequency) improved visual acuity in amblyopic children (ages similar to those in the current study) by 0.08 to 0.13 logMAR.16 However, monocular training does not directly address interocular suppression. More recent studies used dichoptic training, targeting binocular discordance directly via reducing interocular suppression, strengthening binocular fusion, or promoting binocular vision. Many dichoptic training studies use signal integration training paradigms,21–27 which require observers to integrate dichoptically presented task elements for successful task completion. To manipulate interocular suppression directly, previously we adopted a different dichoptic demasking training paradigm (detailed provided in Methods and Results), in which the observers were trained to discriminate the contrast or orientation of a Gabor stimulus presented to the amblyopic eyes while resisting dichoptic noise masking from the fellow eyes.28,29 The amblyopic observers were significantly more capable of discounting dichoptic noise masking after training. Moreover, dichoptic training further improved stereoacuity, but not AE visual acuity, in monocularly well-trained adults with amblyopia.28 These results support Levi et al.15 on the potential extra advantages of dichoptic training.
Binocular approaches for amblyopic children, such as dichoptic games, that rebalance contrast between two eyes to overcome suppression have been reported to induce visual acuity gains.30–39 However, their effects on stereoacuity are unclear. Some studies report that binocular treatments improved stereoacuity in some amblyopic children.35,40,41 For example, Kelly et al.40 reported that 20% of 41 amblyopic children (age 4–10 years) experienced stereoacuity improvements after nine to ten hours of binocular treatments (dichoptic game or movie). But other studies have shown no improvement in binocular functions.31–33 For example, Li et al.31 found that passive viewing of dichoptic movies for two weeks failed to improve stereoacuity in eight amblyopic children (age 4–10 years). The diverse outcomes could result from differences in treatment type, treatment duration, and sample inhomogeneity.15 Therefore it remains to be determined whether binocularity in children benefits from binocular treatments and what factors are associated with the outcomes.
Here we investigated the effects of dichoptic de-masking training on visual functions, especially stereoacuity, in children with amblyopia, and related the training effects to the history of patching treatment and the severity of amblyopia. These amblyopic children learned to use AE to perform contrast discrimination while resisting dichoptic noise masking simultaneously presented in FE. Learning was quantified by the maximal tolerable noise contrast (TNC) for AE contrast discrimination. To assess the improvements of visual functions, monocular visual acuity and contrast sensitivity, as well as binocular stereoacuity, were measured before and after training.
Methods
Observers
Twenty-seven amblyopic observers aged 6 to 16.5 years took part in this study. They were trained in the Tengzhou Central People's Hospital, Tengzhou City, Shandong province of China. Thirteen observers (seven boys and six girls, mean ± SD = 10.9 ± 2.8 years; Table 1) had been patch treated for more than 1.5 years, starting at the age of 6.6 ± 3.1 years, by the three ophthalmologist authors (LXY, FG, FC). The visual acuity of these observers had improved by 0.43 ± 0.18 logMAR on the tumbling E chart (missing SA2 data). There had been no acuity improvement in the previous six months before the current training. These observers formed the patch-treated (PT) group. The fourteen other observers (ten boys and four girls, mean ± SD = 10.4 ± 2.0 years; Table 2) had never received patching treatment. They formed the never patch-treated (NPT) group. Among them, four amblyopes (SB5, SB8, SB11, SB13) had worn their corrective lenses for six months, and they received no other therapy beyond glasses before training. The other 10 observers had untreated amblyopia before training. They were either newly diagnosed amblyopes (SB1, SB4, SB7, SB10, SB12, SB14) or amblyopes who were diagnosed younger (SB2, SB3, SB6, SB9) but did not take any treatment because of poor compliance. They were prescribed new glasses and wore them for at least two weeks (mean ± SD = 4.1 ± 2.4 weeks) before data collection. All observers had undergone part-time occlusion therapy during training. The prescribed dose during training was about 2.5 h/d on average. Besides, we obtained the pre-patching and post-patching visual acuity data of 15 age-matched amblyopic observers from the medical archives at the Beijing Tongren Hospital. These amblyopes received 2965 ± 362 hours of patching treatment starting at similar ages (10.2 ± 0.6 years).
Table 1.
The Characteristics of the Amblyopic and Fellow Eyes in the PT Group
| Visual Acuity | Stereoacuity (Arcsec) | Patch Treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observer | Age (y) | Gender | Type | Strabismus | Eye | Correction | Pre | Post | Pre | Post | Starting Age/Length (y) | Starting Acuity | Training Sessions |
| SA1 | 9.5 | Male | A | None | AE (L) | +5.00 | 0.48 | 0.35 | F | 200 | 7.5/2 | 0.92 | 22 |
| FE (R) | Plano | −0.09 | −0.11 | 0 | |||||||||
| SA2 | 16.5 | Female | A | None | AE (L) | +3.50/−2.00 × 15 | 0.29 | 0.21 | F | 70 | 3/9 | Unknown | 22 |
| FE (R) | −3.50 | −0.03 | −0.05 | Unknown | |||||||||
| SA3 | 7.2 | Male | A | None | AE (R) | +3.50 | 0.13 | 0.11 | 200 | 40 | 5/2 | 0.92 | 22 |
| FE (L) | +3.75 | 0.05 | 0.01 | 0.40 | |||||||||
| SA4 | 10.4 | Male | A | None | AE (L) | +2.50/+0.75 × 115 | 0.59 | 0.51 | F | 200 | 6/4.5 | 1 | 21 |
| FE (R) | Plano | 0.08 | 0.05 | 0 | |||||||||
| SA5 | 6.0 | Female | A | None | AE (L) | +3.00 | 0.24 | 0.18 | F | 50 | 4/2 | 0.82 | 22 |
| FE (R) | +0.75 | 0.15 | 0.11 | 0.10 | |||||||||
| SA6 | 10.2 | Male | A | None | AE (L) | +6.00/+2.00 × 80 | 0.43 | 0.37 | F | 200 | 5/2 | 0.82 | 22 |
| FE (R) | +2.50/−2.00 × 85 | 0.11 | −0.05 | 0.52 | |||||||||
| SA7 | 10.2 | Female | A | None | AE (R) | +6.00 | 0.24 | 0.23 | 400 | 140 | 5/2 | 0.52 | 22 |
| FE (L) | +5.50 | 0.09 | 0.05 | 0.10 | |||||||||
| SA8 | 11.8 | Male | A | None | AE (L) | +0.75/+2.75 × 90 | 0.27 | 0.24 | 400 | 20 | 10/1.5 | 0.30 | 22 |
| FE (R) | +1.25/+0.75 × 80 | 0.06 | 0.01 | 0 | |||||||||
| SA9 | 14.0 | Male | A | None | AE (L) | +2.50 | 0.23 | 0.19 | 50 | 20 | 12/2 | 0.60 | 17 |
| FE (R) | Plano | −0.10 | −0.12 | −0.18 | |||||||||
| SA10 | 14.0 | Male | A | None | AE (R) | +6.00/+0.50 × 120 | 0.32 | 0.22 | 140 | 20 | 12/1.5 | 0.70 | 18 |
| FE (L) | +0.25/+0.50 × 60 | −0.15 | −0.14 | 0 | |||||||||
| SA11 | 10.0 | Female | A | None | AE (R) | +4.25 × 95 | 0.13 | 0.12 | 30 | 20 | 8/2 | 0.22 | 17 |
| FE (L) | +3.50 × 75 | 0 | −0.01 | 0 | |||||||||
| SA12 | 11.5 | Female | A | None | AE (R) | +3.50 | 0.13 | 0.11 | 50 | 20 | 4/2 | 0.40 | 18 |
| FE (L) | +4.00/+0.75 × 130 | 0.08 | 0.07 | 0.10 | |||||||||
| SA13 | 10.7 | Female | S | R 15∆ EsoT | AE (R) | −0.75 | 0.22 | 0.10 | F | F | 4/2 | 1 | 22 |
| FE (L) | −0.75/−0.50 × 10 | 0 | −0.10 | 0.30 | |||||||||
Pretraining and posttraining visual acuities were measured with a computerized crowded-E acuity test (except SA13 whose E chart acuity was used instead). The starting acuity was tested with a Tumbling E chart. The stereoacuity was evaluated with the Randot Stereo Test. Strabismus diagnosed by a cover test at a distance of 33 cm.
A, anisometropic; S, strabismic; AE, amblyopic eye; FE, fellow eye; L: left; R: right; EsoT, esotropia; ExoT, exotropia; F, failed the Randot Stereo Test.
Table 2.
The Characteristics of the Amblyopic and Fellow Eyes in the NPT Group
| Visual Acuity | Stereoacuity (Arcsec) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observer | Age (y) | Gender | Type | Strabismus | Eye | Correction | Pre | Post | Pre | Post | Treatment History | Training Sessions |
| SB1 | 12 | Female | A | None | AE (R) | +3.00 | 0.61 | 0.44 | F | 200 | No treatment | 21 |
| FE (L) | Plano | 0 | −0.14 | |||||||||
| SB2 | 8.8 | Male | A | None | AE (L) | +6.25/+0.75 × 95 | 0.68 | 0.43 | F | F | No treatment | 20 |
| FE (R) | +1.75/+0.75 × 90 | −0.02 | −0.08 | |||||||||
| SB3 | 9.3 | Male | A & S | L 15∆ EsoT | AE (L) | +3.00/+1.25 × 90 | 0.96 | 0.69 | F | F | No treatment | 21 |
| FE (R) | +1.50 | −0.04 | −0.02 | |||||||||
| SB4 | 7.5 | Male | A | None | AE (L) | +3.50/−3.50 × 115 | 0.33 | 0.16 | 400 | 40 | No treatment | 22 |
| FE (R) | +4.00/−2.00 × 175 | 0.15 | 0.12 | |||||||||
| SB5 | 11.8 | Male | A | None | AE (L) | +6.50/+0.75 × 100 | 0.39 | 0.36 | F | 200 | Glasses for 0.5 y, no patching | 22 |
| FE (R) | +6.75/+1.00 × 80 | 0.16 | 0.16 | |||||||||
| SB6 | 11.7 | Male | S | L 30∆ EsoT | AE (L) | −0.50/−1.25 × 170 | 0.57 | 0.35 | F | F | No treatment | 22 |
| FE (R) | −1.50 × 175 | 0.24 | 0.22 | |||||||||
| SB7 | 10.7 | Female | A | None | AE (R) | +1.00/+1.00 × 50 | 0.18 | 0.15 | 140 | 40 | No treatment | 20 |
| FE (L) | Plano | −0.03 | −0.02 | |||||||||
| SB8 | 7.2 | Female | A | None | AE (R) | +2.25/+1.50 × 60 | 0.45 | 0.32 | F | 30 | Glasses for 0.5 y, no patching | 22 |
| FE (L) | +1.25/+1.50 × 95 | 0.22 | 0.18 | |||||||||
| SB9 | 11.7 | Male | A | None | AE (R) | +4.00 | 0.51 | 0.39 | F | F | No treatment | 22 |
| FE (L) | Plano | 0.03 | 0.01 | |||||||||
| SB10 | 12 | Male | A | None | AE (R) | +0.50 | 0.21 | 0.02 | 200 | 30 | No treatment | 17 |
| FE (L) | +4.00 | −0.12 | −0.12 | |||||||||
| SB11 | 9.5 | Male | A | None | AE (R) | +4.50/−5.50 × 5 | 0.14 | 0.14 | 70 | 30 | Glasses for 0.5 y, no patching | 22 |
| FE (L) | +4.50/-6.00 × 175 | 0.14 | 0.14 | |||||||||
| SB12 | 9.0 | Female | A | None | AE (R) | +2.50/+0.75 × 95 | 0.44 | 0.26 | 200 | 70 | No treatment | 21 |
| FE (L) | Plano | 0.04 | 0.06 | |||||||||
| SB13 | 14.5 | Male | A | None | AE (R) | +6.00 | 0.60 | 0.54 | 400 | 400 | Glasses for 0.5 y, no patching | 20 |
| FE (L) | Plano | −0.17 | −0.10 | |||||||||
| SB14 | 9.5 | Male | A | None | AE (R) | +3.00/+1.00 × 70 | 0.77 | 0.54 | F | F | None | 18 |
| FE (L) | +1.00 × 105 | 0.15 | 0.09 | |||||||||
A, anisometropic; S, strabismic; AE, amblyopic eye; FE, fellow eye; L: left; R: right; EsoT, esotropia; ExoT, exotropia; F, failed the Randot Stereo Test.
Each observer's vision was best-corrected before training with a tumbling E acuity chart at the designated viewing distance of 5 m. Testing and training were performed with the observer wearing the best optical correction, and the visual acuity values reported throughout the article are for best-corrected acuity. The study adhered to the tenets of the Declaration of Helsinki and was approved by the ethics committees of Tengzhou Central People's Hospital. Informed consent was obtained from each observer's parent or guardian after an explanation of the nature and possible consequences of the study.
Apparatus
The setup was identical to those described in Liu and Zhang.28,29 Briefly, the stimuli were generated with Psychtoolbox-342 and presented on a 21-inch Sony G520 CRT monitor (2048 pixel × 1536 pixel, 0.19 mm × 0.19 mm pixel size, 75 Hz frame rate; Sony, Tokyo, Japan). The head of the observer was stabilized by a chin-and-head rest. Experiments were run in a dimly lit room. For cutoff frequency (grating acuity) and contrast sensitivity measurements, a 14-bit look-up table achieved with a video attenuator was used to linearize the luminance of the monitor (mean luminance = 27 cd/m2). For other tasks, an 8-bit look-up table was used (mean luminance = 50 cd/m2).
Study Design
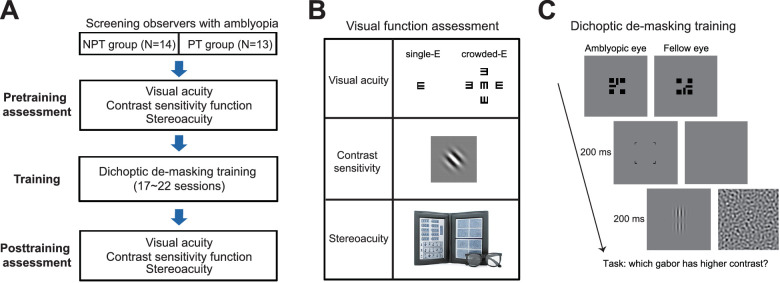
The experiment consisted of pretraining assessment, dichoptic demasking training, and posttraining assessment (Fig. 1A). Pretraining and posttraining assessments measured visual acuities and contrast sensitivity functions for AE and FE, respectively, and stereoacuity (Fig. 1B). Dichoptic demasking training took 21 sessions on average (mean ± SD = 20.7 ± 1.6 for the NPT group and 20.6 ± 1.8 for the PT group). Each training session consisted of 14 to 21 staircases and lasted for approximately 1 to 1.5 hours. The training frequency ranged from two to five daily sessions per week, which was more frequent during summer and winter breaks and varied among observers. The experiment lasted 3 months on average (mean ± SD = 85 ± 23 days). Three NPT observers (SB10, SB11, SB12) did not complete the pretraining contrast sensitivity assessment. One PT observer (SA13) did not complete the pretraining computerized-E acuity assessment. His/her Tumbling E chart acuity was used as VA (visual acuity) in data analysis.
Figure 1.
(A) A flowchart of the study design. Two groups of observers (NPT and PT) were recruited according to their treatment history. The experiment consisted of pretraining assessment, dichoptic demasking training, and posttraining assessment. (B) Visual function assessment. Top: Single-E and crowded-E acuities test; Middle: A Gabor patch used for contrast sensitivity measurement. Bottom: The Randot Stereo Test. (C) The dichoptic training paradigm of a contrast discrimination task. From top-left to bottom-right: binocular fusion was first achieved with the assistance of two half-crosses. Then a cue was presented for 200 ms to prime AE. A pair of collinear Gabors were then presented to AE for 200 ms, whereas a bandpass noise masker was presented to FE simultaneously. Observers judged which Gabor had higher contrast. AE, amblyopic eye; FE, fellow eye; NPT, never patch-treated; PT, patch-treated.
Dichoptic Training
The dichoptic stimuli (Fig. 1C) consisted of a pair of collinear vertical Gabors (Gaussian windowed sinusoidal gratings) presented in AE and a band-pass filtered white noise masker in FE. The two Gabors had the same spatial frequency at 30% of AE's pretraining cutoff frequency, standard deviation at one wavelength (the reciprocal of spatial frequency), orientation at 90°, phase at 90°, and a center-to-center distance of 4 wavelengths. The cutoff frequency of AE (mean ± SE = 15.9 ± 0.9 cycle per degree, cpd) was obtained via a cutoff frequency test before training. In contrast discrimination trials, one Gabor's contrast was set at 80%, and the other Gabor's contrast was 80% − 1.414 times of contrast discrimination threshold (with no masker presented in FE). The contrast discrimination threshold was premeasured for each observer with the same Gabor stimulus at a reference contrast of 80% (contrast discrimination threshold: mean ± SE = 15.7% ± 1.1%). The band-pass filtered noise masker was 512 × 512 pixels (4.4° × 4.4°) in size. To create the noise masker, a 512 × 512 pixels zero-mean white noise field was first generated, with each element being 2 × 2 pixels. The white noise field was then filtered in the frequency domain by a one-octave band-pass filter centered at the same frequency of the Gabors. A new noise masker was generated for every trial. The viewing distance was 0.8 m.
In the dichoptic training task, a trial began with binocular-fusion of two half-crosses (contrast 100%), each with four assisting squares, to align the two eyes in a four-mirror stereoscope (Fig. 1C). A whole cross was perceived when correct vergence on the target was achieved. For observers whose visual acuity difference between the two eyes exceeded 0.4 logMAR (six NPT observers and four PT observers), the contrast of the half cross and four assisting squares in FE was reduced to 60% to facilitate binocular fusion. For other observers, the contrast of these fixation aids was 100%. The observers pressed the space bar to initiate the trial as soon as the whole cross appeared stable. Immediately after the keypress, a 1.5° × 1.5° black empty square (edge width = 2 arcmin) was presented for 200 ms to AE to prime attention. This was followed by a dichoptic presentation of the Gabor stimuli and the noise masker for 200 ms.
During training, the observers judged which Gabor had higher contrast. The root mean square contrast of the noise masker was varied by a staircase following a three-up/one-down rule to achieve a 79.4% convergence rate. The step size of the staircase was 0.05 log units. Each staircase consisted of eight reversals (approximately 40–50 trials). The geometric mean of the last six reversals was taken as the maximal TNC for successful contrast discrimination. To ensure that the observers did not close the FE when seeing the stimuli, a white digit (“1” or “2”, 1.1° × 1.7° in size) centered in the noise masker in the FE was presented in 20% of the trials while a blank screen was presented in the AE. The observers needed to report the digit by a keypress (the mean correct rate = 96.0% ± 1.6%). Auditory feedback was given on incorrect responses in all trials.
Visual Function Assessment
Visual Acuity
In amblyopia, vision is often substantially worse when the target letter is presented with flanked letters than when it is presented alone, a phenomenon known as crowding.43 Therefore both single-letter acuity and crowded-letter acuity were measured to offer a comprehensive assessment of visual acuity.
Single-E and crowded-E acuities were tested with a custom computerized program at a viewing distance of 4 m. Single-E acuity was tested with a tumbling letter E (a minimal luminance black letter on a full-luminance white monitor screen). Crowded-E acuity was tested with a tumbling E target surrounded by four same-sized tumbling E letters at an edge-to-edge gap of one letter size. The crowded-E acuity was functionally similar to the conventional E acuity chart because both were influenced by visual crowding. The stroke and opening width of the E letters were one-fifth of the letter height.
The E acuities were all measured with a single-interval staircase procedure. The stimulus stayed on until a keypress by the observer. The task was to judge the orientation of the tumbling E (left, right, up, or down). All thresholds were estimated following a three-down/one-up staircase rule. Each staircase consisted of two preliminary reversals and four experimental reversals. The step size of the staircase was 0.05 log units. The geometric mean of the experimental reversals was taken as the threshold for each staircase run. Three staircases were run to determine single-E or crowded-E acuities. The computerized E-acuities test (the step size of the staircase was 0.05 log units) might be more reliable than the clinical E-chart test (size of optotypes changed by 0.1 log unit from line to line); therefore we only use the computerized acuity tests to evaluate VA.
Stereoacuity
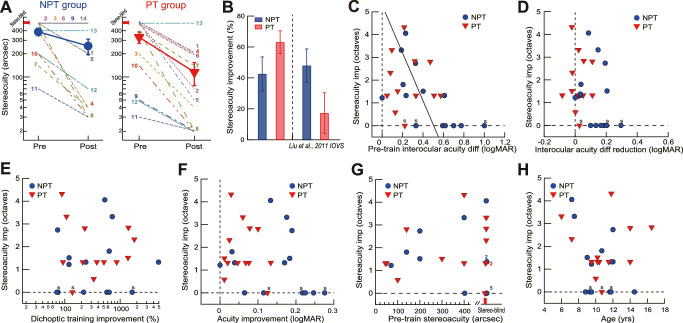
Stereoacuity is the smallest detectable depth difference that can be seen in binocular vision. The Randot Stereo Test (Stereo Optical Co, Inc, Chicago, IL, USA) was used to test stereoacuity under normal room lighting. Contoured circles at 10 levels of disparity ranging from 400 to 20 arcsec provide a graded sequence for testing. Observers wore polarizing glasses and looked at the test material at a viewing distance of 40 cm. Note that in Figure 4A, and for the convenience of data analysis, the stereoacuity for those who failed the Randot Stereo Test was set at 500 arcsec, a value below the lowest measurable score.
Figure 4.
The impact of dichoptic training on stereoacuity. (A) Pretraining and posttraining stereoacuity for NPT and PT groups. The large symbols indicate group means. The digits indicate individual observers (see Tables 1 and 2). The arrows on the y-axis indicate amblyopic observers who failed the Randot Stereo Test (stereoblind). Their stereoacuity was set at 500 arcsec, the lowest score, for data analysis. (B) Mean improvement of stereoacuity. Left: NPT and PT groups in the current study. Right: Replotted NPT and PT data after monocular training in a previous study.16 Error bars = 1 SEM. (C) The improvement of stereoacuity as a function of the pre-training interocular acuity difference. The line shows a Deming regression fitting. Strabismic observers are indicated by the letter “s.” (D–H) The improvement of stereoacuity against the reduction of interocular acuity difference (D), the improvement of dichoptic demasking training (E), the improvement of visual acuity (F), the pretraining stereoacuity (G), and children age (H). NPT, never patch-treated; PT, patch-treated.
Contrast Sensitivity
Acuity measures only the smallest resolvable details, but not the ability to see larger ones. The contrast sensitivity function (CSF) provides a more comprehensive evaluation of spatial vision. CSF describes an observer's sensitivity (i.e., 1/contrast threshold) to sinusoidal gratings of various spatial frequencies. Therefore CSF is an additional tool to document changes in visual functions during the treatment of amblyopia.44
Contrast sensitivity was measured with a Gabor stimulus (σ = 0.9°, orientation = ± 45° from vertical). The spatial frequencies of the Gabor were 3/4, 1/2, 1/4, and 1/16 times the cutoff spatial frequency determined with a cutoff frequency measurement before training. For the cutoff frequency measurement task, the stimulus was a 0.29° × 0.29° sharp-edged full-contrast square-wave grating tilted ± 45° from vertical.
The contrast sensitivity and cutoff frequency measurement were all measured with a single-interval staircase procedure at a viewing distance of 4 m. The stimulus stayed on until a keypress by the observer. The task was to judge the orientation of the grating (tilted to the left or right from vertical). Each staircase consisted of two preliminary reversals and six experimental reversals. The step size of the staircase was 0.05 log units for contrast sensitivity measurements and 0.03 log units for cutoff frequency measurements. Three staircases were run to determine cutoff frequency and the contrast sensitivity to each spatial frequency. The order of all staircases for all spatial frequencies followed a randomly permuted table. Each observer's AE and FE had different tables. Staircases were run consecutively for one eye before being switched to the other eye.
The mean CSFs were fitted with a difference of Gaussians function: . Here y stood for the contrast sensitivity, x for the spatial frequency, A1 and A2 for the amplitudes, and σ1 and σ2 for the standard deviations.
Results
Perceptual Learning Improves Dichoptic Demasking
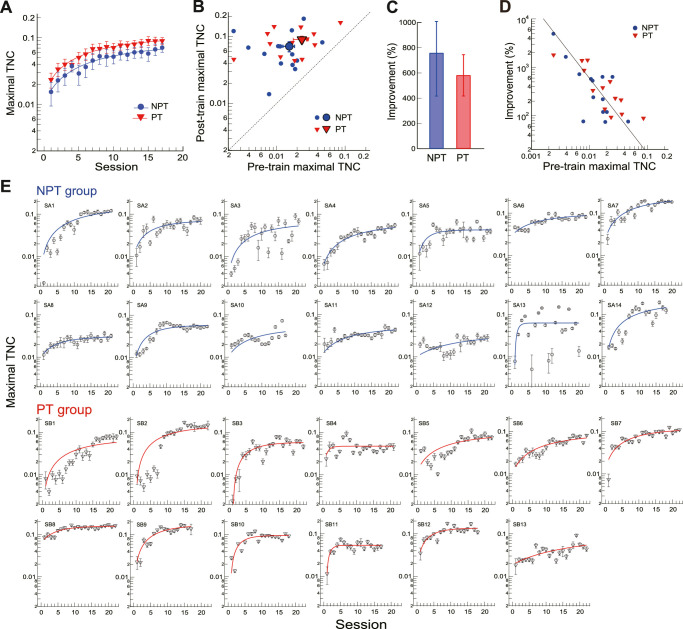
During the dichoptic training, the AE performed contrast discrimination under dichoptic noise masking from the FE (Fig. 1C). Significant learning was evident as the maximal TNC increased during the course of dichoptic training (Figs. 2A and 2B). We used the percent improvement (PI = (threshold_post/threshold_pre - 1)*100) to quantify the amount of learning. Training improved the maximal TNC of the NPT group by 747% ± 342% (t13 = 2.22, P = 0.045, Cohen's d = 0.59; two-tailed paired t-test here and later unless specified), from a root mean square contrast of 0.015 ± 0.003 to 0.070 ± 0.012 (Figs. 2A–2C). Likewise, training improved the maximal TNC of the PT group by 580% ± 164% (t12 = 3.55, P = 0.004, Cohen's d = 0.99), from a root mean square contrast of 0.023 ± 0.005 to 0.090 ± 0.011 (Figs. 2A–2C). A mixed-design ANOVA suggested a significant main factor of training (F1,25 = 63.38, P < 0.001, η2 = 0.72), a nonsignificant main factor of group (F1,25 = 2.01, P = 0.17, η2 = 0.07), and a nonsignificant interaction between training and group (F1,25 = 0.60, P = 0.45, η2 = 0.023). Moreover, the amount of dichoptic demasking learning appeared to depend on the pretraining maximal TNC, as shown by the Deming regression fit on the log-log plot (slope = −1.53, R2 = 0.57, P < 0.001) (Fig. 2D), suggesting that those with poorer pretraining maximal TNC tended to have more room for dichoptic learning. This correlation was consistent with previous studies45,46 showing that the learning speed and amount were strongly coupled to pretraining performance levels.
Figure 2.
The effects of dichoptic de-masking training on maximal TNC for AE contrast discrimination task. (A) Session by session changes of the mean maximal TNC for AE contrast discrimination in NPT and PT groups. The lines are the best-fitting exponential functions. (B) Comparisons of individual and mean posttraining and pretraining maximal TNCs. The large symbols represent the group means. (C) Mean percent improvement of performance in NPT and PT groups. (D) Dichoptic demasking learning as a function of pretraining maximal TNC. Data are plotted on a logarithmic scale, and a Deming regression line is plotted. (E) Maximal TNC as a function of training sessions for all observers. In each panel, the line is the exponential fit of the data. Error bars = 1 SEM. AE, amblyopic eye; NPT, never patch-treated; PT, patch-treated; TNC, tolerable noise contrast.
To quantify the learning rate, we used an exponential function: Maximal TNC = y0 + a (1-e−x/τ) to fit the training-induced change of maximal TNC (smooth curves in Figs. 2A and 2E), where x was the training session, y0 the maximal TNC at x = 0, a the asymptotic maximal TNC with sufficient training, and τ the time constant corresponding to the training time needed to reach 63% of asymptotic performance.47,48 The time constants were 11.36 ± 3.73 and 9.26 ± 1.60 sessions for NPT and PT groups, respectively (Fig. 2A), which were not significantly different between each other (independent-samples t-test, P = 0.28). Besides, the other two parameters y0 and a were not significantly different between NPT and PT groups (independent-samples t-test, y0: P = 0.85; a: P = 0.46). There were large individual variabilities, as indicated by the different improvements of maximal TNC or the time constants of learning across observers. However, no significant correlation was evident between these two indexes (r = 0.18, P = 0.36). Although learning is variable in different observers (17–22 sessions), there is no correlation of training frequency to the improvement of Maximal TNC (r = 0.14, P = 0.49) and to the time constant (r = 0.38, P = 0.052).
Visual Acuity Changes After Dichoptic Demasking Training
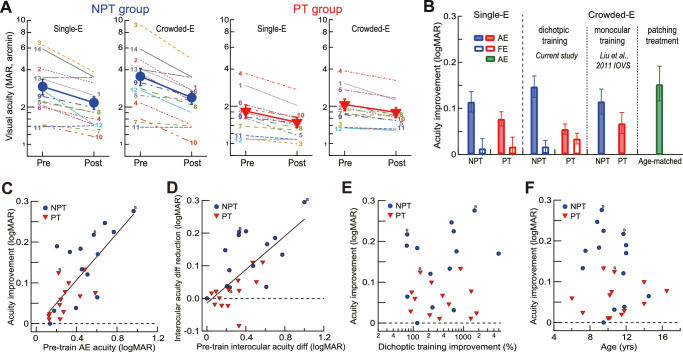
Figure 3A shows the AE visual acuities of the NPT and PT groups before and after training. A repeated-measures ANOVA suggested a significant main effect of training (F1,24 = 17.02, P < 0.001, η2 = 0.42), indicating significantly improved AE visual acuities of both groups and a significant main effect of acuity test type (F1,24 = 19.83, p < 0.001, η2 = 0.45) because crowded-E acuities tended to be lower than single-E acuities across the groups as a result of visual crowding.
Figure 3.
The impact of dichoptic training on visual acuities. (A) Pretraining and posttraining single-E and crowded E-acuities for NPT AEs and PT AEs. The filled symbols indicate group means. The digits indicate individual observers (see Tables 1 and 2). Because one PT observer (SA13) did not complete the pretraining computerized-E acuity assessment, his/her data were not included here. The mean of the PT group was based on the other 12 observers. (B) Mean acuity improvement of NPT and PT AEs and FEs. We also replotted data from our previous study16 for comparison. Error bars = 1 SEM. (C) The correlation between AE acuity improvement and pretraining AE acuity. The solid line shows Deming regression for all observers. Three strabismic observers were indicated by the letter “s.” (D) The correlation between the interocular acuity difference reduction and the pretraining interocular acuity difference. The solid line shows Deming regression for all observers. (E) The visual acuity improvement versus dichoptic demasking learning. (F) The visual acuity improvement versus age. AE, amblyopic eye; FE, fellow eye; NPT, never patch-treated; PT, patch-treated.
On average, the single-E acuity was improved by 0.11 ± 0.02 logMAR in NPT AEs (Fig. 3B, P < 0.001, from 0.42 ± 0.06 to 0.31 ± 0.05 logMAR) and 0.08 ± 0.02 logMAR in PT AEs (P < 0.001, from 0.23 ± 0.05 to 0.15 ± 0.04 logMAR) and unchanged in NPT FEs (P = 0.60) and PT FEs (P = 0.43). The crowded-E acuity was improved by 0.15 ± 0.02 logMAR in NPT AEs (Fig. 3B, P < 0.001, from 0.50 ± 0.06 to 0.35 ± 0.05 logMAR) and 0.06 ± 0.01 logMAR in PT AEs (P < 0.001, from 0.29 ± 0.04 to 0.23 ± 0.03 logMAR), unchanged in the NPT FEs (0.02 ± 0.01 logMAR, P = 0.32), and improved in PT FEs (0.03 ± 0.01 logMAR, P = 0.014). For amblyopic eyes, the improvement of E chart acuity (NPT: 0.23 ± 0.04 logMAR; PT: 0.11 ± 0.01 logMAR) was correlated with the improvement of computerized crowded-E acuity (r = 0.69, P < 0.001), but not with that of single-E acuity (r = 0.38, P = 0.053), probably because E chart acuity and computerized crowded-E acuity were functionally similar (both influenced by visual crowding). Hence, for better comparison with other studies, we will use computerized crowded-E acuity as visual acuity in the rest of the article. In this manner, training reduced the interocular acuity difference in NPT children by 0.13 ± 0.02 logMAR (t13 = 5.72, P < 0.001, Cohen's d = 1.53), but not in PT children (t12 = 1.43, P = 0.18, Cohen's d = 0.40).
The crowded-E acuity improvements in each group (0.15 logMAR in the NPT group and 0.06 logMAR in the PT group, Fig. 3B) were comparable to those in our previous study (data replotted in Fig. 3B, P = 0.26 for NPT eyes and P = 0.88 for PT eyes) that performed monocular grating acuity training in similar-aged children in the same experimental setting (13 NPT observers, 11.6 ± 0.9 years; 10 PT observers, 11.8 ± 0.9 years),16 suggesting that monocular and dichoptic perceptual learning are both effective in improving AE visual acuity. Also, the crowded E acuity improvement in the NPT eyes was comparable to the E chart acuity improvement (0.16 ± 0.05 logMAR, t14 = 3.47, P = 0.004; Fig. 3B green bar) in the age-matched amblyopic control group after extended patching treatment (∼3000 hours; P = 0.85, two-tailed parametric t-test), suggesting that the visual acuity improvements in the NPT eyes could not be simply attributed to occlusion that accompanied the dichoptic training.
The improvement of AE visual acuity was significantly correlated to the pre-training visual acuity (r = 0.75, P < 0.001, Fig. 3C), which was evident in both NPT children (r = 0.69, P = 0.006) and PT children (r = 0.59, P = 0.036) (Fig. 3C). Moreover, the reduction of interocular acuity difference was significantly correlated to the pretraining interocular acuity difference (r = 0.65, P < 0.001, Fig. 3D), which was evident in both NPT children (r = 0.58, P = 0.029) and PT children (r = 0.60, P = 0.031) (Fig. 3D). However, the AE acuity gain was not correlated to dichoptic demasking learning in neither NPT children (r = 0.23, P = 0.44) nor PT children (r = −0.19, P = 0.54) (Fig. 3E). It was not correlated to the age of NPT children (r = −0.31, P = 0.29) and PT children (r = 0.15, P = 0.62) either (Fig. 3F).
Stereoacuity Changes After Dichoptic Demasking Training
For all observers, dichoptic training improved the stereoacuity from 358.5'' ± 34.6'' to 186.7'' ± 34.6'', or by 52.5% ± 6.7% (1.54 ± 0.25 octaves; t26 = 7.89, P < 0.001, Cohen's d = 1.52). Specifically, the stereoacuity in the NPT group was improved from 386.4'' ± 42.8'' to 256.9'' ± 57.7'', or by 41.6% ± 10.4% (1.24 ± 0.36 octaves; t13 = 3.99, P = 0.002, Cohen's d = 1.07; Figs. 4A and 4B); and the stereoacuity in the PT group was improved from 328.5'' ± 55.6'' to 115.4'' ± 38.2'', or by 64.2% ± 7.1% (1.87 ± 0.33 octaves; t12 = 8.99, P < 0.001, Cohen's d = 2.49; Figs. 4A and 4B). The NPT children showed comparable stereoacuity gains to those who performed monocular grating acuity training in our previous study16 (data replotted in Fig. 4B, P = 0.67), but the PT children showed more stereoacuity gains (P = 0.008, Fig. 4B) (this advantage over monocular training might exclude the possibility that the current stereoacuity gain was simply a result of the test-retest effect of the Randot Stereo Test).
The stereoacuity gain was negatively correlated to the pretraining interocular acuity difference (r = −0.49, P = 0.010, Fig. 4C), indicating that children with mild amblyopia (amblyopic VA ≤ 0.28 logMAR) would have more stereoacuity gain than those with more severe amblyopia. Therefore the treatment would be less effective for NPT children because more children had severe amblyopia in this group (Fig. 4C). However, the stereoacuity gain was not associated with a reduction of interocular acuity difference (r = −0.28, P = 0.15) (Fig. 4D), suggesting a dissociation of stereoacuity gain and the change of interocular acuity difference.
Individually, all PT children except strabismic SA13 showed stereoacuity improvement. Among them, five, including two who had no measurable pretraining stereoacuity, had their stereoacuity improved by 3.12 ± 0.34 octaves (87.3% ± 2.5%), reaching clinical significance (a stereoacuity gain of ≥2 octaves49,50). The other seven had their stereoacuity improved by 1.24 ± 0.1 octaves (56.9% ± 4.0%). Importantly, six PT observers regained normal stereoacuity (20–40 arcsec) after training. NPT children showed larger individual variations in stereoacuity gain: five with milder amblyopia (initial interocular difference ≤0.3 log units) regained normal stereoacuity (20–40 arcsec), two stereoblinds (initial interocular difference >0.3 log units) started to have measurable stereoacuity (200 arcsec), and five stereoblinds, including two strabismic, still had no measurable stereoacuity after training.
The stereoacuity improvement was not correlated to dichoptic demasking learning (r = −0.05, P = 0.86, Fig. 4E), to the AE acuity improvement (r = −0.29, P = 0.15, Fig. 4F), to the pretraining stereoacuity (r = −0.11, P = 0.57, Fig. 4G), to children age (r = −0.18, P = 0.38, Fig. 4H).
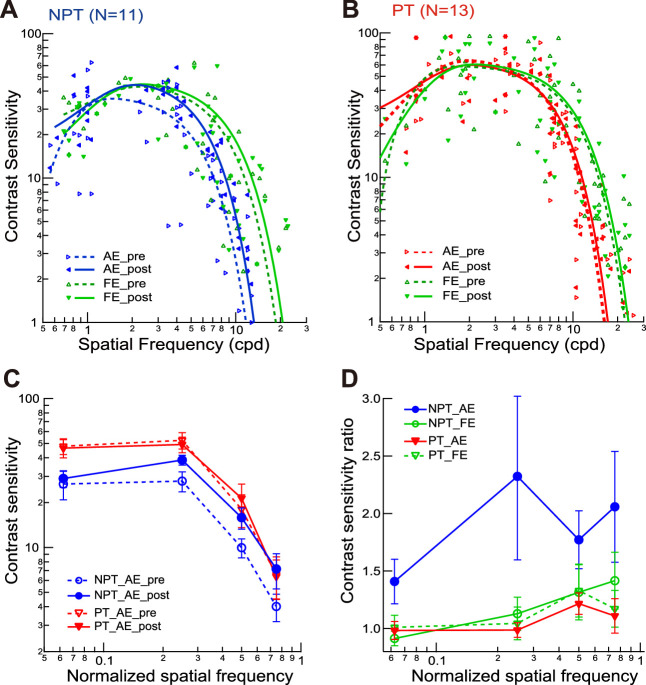
Contrast Sensitivity Changes After Dichoptic Demasking Training
The pretraining and posttraining CSFs were measured in AEs and FEs. The Gabor spatial frequencies were 3/4, 1/2, 1/4, and 1/16 times the pretraining cutoff spatial frequency. Individuals’ contrast sensitivity at each spatial frequency was plotted in Figures 5A and 5B (see Supplementary Fig. S1 for details). On the basis of these data points, we fitted the mean CSFs with a difference of Gaussian function (curves in Figs. 5A and 5B). The pretraining AE CSFs of both groups showed a loss of contrast sensitivity at high spatial frequencies. The mean pretraining cutoff spatial frequencies of the AEs were 14.8 ± 0.9 cpd in the NPT group and 18.1 ± 1.9 cpd in the PT group, lower than those of the FEs at 19.4 ± 1.6 cpd in the NPT group and 25.5 ± 1.5 cpd in the PT group (P = 0.002 and 0.004, respectively).
Figure 5.
The impact of dichoptic training on contrast sensitivity functions. (A, B) The mean contrast sensitivity functions of the AEs and FEs before and after training in NPT (A) and PT (B) groups, along with individual data points. Each curve is the best fitting of a difference-of-Gaussian function. (C) The mean contrast sensitivity functions for AEs and FEs before and after training, with stimulus spatial frequencies normalized by the corresponding cutoff spatial frequencies. (D) The ratios of the pretraining and posttraining normalized contrast sensitivity functions in AEs and FEs. Error bars = 1 SEM. AE, amblyopic eye; FE, fellow eye; NPT, never patch-treated; PT, patch-treated.
We replotted AE and FE CSFs with the spatial frequencies normalized by pretraining cutoff spatial frequencies (Fig. 5C) and the ratios of normalized CSFs pretraining and posttraining (Fig. 5D). A repeated-measures ANOVA compared the contrast sensitivities at four normalized spatial frequencies before and after training. For the NPT group, there was a significant main effect of training in AEs (F1,10 = 10.62, P = 0.009, η2 = 0.52) and spatial frequency (F3,30 = 21.57, P < 0.001, η2 = 0.68), but insignificant interaction between training and spatial frequency (F3,30 = 1.67, P = 0.19, η2 = 0.14). These effects can be appreciated in Figure 5D, in that AEs in the NPT group showed overall improvement of contrast sensitivities at all normalized spatial frequencies. There was no significant impact of training on FE CSF (F1,10 = 0.18, P = 0.68, η2 = 0.02). For the PT group, training had no significant impacts on the CSFs of AEs (F1,12 = 0.10, P = 0.76, η2 = 0.008) and FEs (F1,12 = 0.12, P = 0.73, η2 = 0.01).
Retention
To examine long-term retention of the treatment effects on visual acuity and stereoacuity, twelve observers who were able to complete the clinical follow-up were retested 10 to 24 months (mean ± SD = 18.1 ± 5.4) after they finished the dichoptic demasking training. Among them, six were PT observers (SA2, SA3, SA5, SA7, SA8, SA10), and six were NPT observers (SB2, SB3, SB4, SB6, SB8, SB9). The retention visual acuity and stereoacuity were not significantly different from those measured immediately after training (t11 = 0.54, P = 0.60 and t11 = 1.60, P = 0.14, respectively), suggesting that the treatment effects persisted for an extended period.
Discussion
In recent years, dichoptic training that aims to manipulate interocular suppression has shown promise in treating amblyopes. In this study, we used a dichoptic demasking training method to train children with amblyopia. Training significantly improved AE visual acuity and stereoacuity in NPT and PT groups. The stereoacuity gain was associated with pretraining interocular acuity difference, but not with the change of interocular acuity difference. Because a stronger interocular suppression was associated with a greater interocular difference in visual acuity,51 the latter dissociation might suggest that stereoacuity gain may not result from reduced interocular suppression. Instead, these children may have learned better to read out contrast signals from dichoptically presented noise to improve stereopsis.
Patching treatment is known to be effective for amblyopic children younger than six to seven years old.14,52,53 Approximately 120 hours of patching can improve visual acuity by one line (0.1 logMAR) in children three to eight years old.54 However, the effectiveness of patching treatment decreases steeply with age,53 and patching is not always successful, and there are also compliance and recurrence issues. Perceptual learning offers a new option for treating amblyopia. In the current study, the average AE improvements of single-E and crowded-E acuities in NPT AEs (0.13 logMAR) and PT AEs (0.07 logMAR) were comparable to the NPT eyes (0.13 logMAR) and PT eyes (0.08 logMAR) in our previous study that trained amblyopic children (ages similar to those in the current study) with a monocular grating acuity task for 40 to 60 sessions.16 It should be emphasized that PT children had all been compliant with the previous patching and reached a VA plateau under patching treatment. The VA gains by perceptual learning in the PT eyes (0.06 logMAR), although small, may bear clinical significance when added to previous VA gains after patching. The VA gains by perceptual learning in the NPT eyes, which might benefit from both refractive correction and part-time occlusion,55,56 were comparable to those (0.16 logMAR) in the age-matched control group after extended patching treatment (∼3000 hours), suggesting that perceptual learning combined with traditional treatment may speed up the time to recovery in children with amblyopia. Also, improved acuity was shown in some PT FEs after training. The FEs of many amblyopes are not as good as those in normally sighted people, because recent scientific evidence showed that ocular motor, visual, and visuomotor deficits were present with fellow eye monocular viewing and with binocular viewing.57–60 Training may have general effects on FE acuity. Nevertheless, our results suggest that monocular and dichoptic perceptual learning are both effective in improving AE visual acuity.
However, binocular training may have advantages over monocular training in stereoacuity improvements. Previous studies have shown that for amblyopic children who are no longer responsive to patching treatment, even if their AEs have achieved normal visual acuity, they rarely restored normal binocularity.11,12,61,62 In our study, all PT children except one in our study showed stereoacuity improvement. Five in our study had their stereoacuities improved by ≥2 octaves after dichoptic training, reaching clinical significance.49,50 Overall these children had greater stereoacuity gains than PT children after monocular grating acuity training in our previous study,16 suggesting that dichoptic training that targeted reducing interocular suppression may be more efficient in improving binocular function for children who are no longer responsive to patch treatment. These data are also consistent with recent findings of ours28 that dichoptic demasking training may produce extra gains of stereoacuity, but not visual acuity, in adults with amblyopia after prolonged monocular training. Our results thus support the argument of Levi et al.15 for the potential advantages of dichoptic training.
The stereoacuity improvement varied in NPT children. Five had no measurable stereoacuity after training (initial interocular difference > 0.3 log unit), but five with milder amblyopia (initial interocular difference ≤0.3 log unit) regained normal stereoacuity. A recent study also reported that many stereoblind children remained stereoblind after two weeks of binocular training.41 In this study, most children had moderate to severe amblyopia (initial interocular difference ≥0.3 log unit). So were children in our NPT group. Therefore dichoptic training may be more effective for those with mild amblyopia, as suggested by Figure 4C, which is also consistent with a meta-analysis on the behavioral training effects for adult amblyopia.63
What is learned in dichoptic learning and how can it result in improving visual acuity and stereoacuity? Previously we investigated the mechanisms of amblyopic dichoptic demasking learning by testing two hypotheses.29 The low-level hypothesis assumed that dichoptic training reduces physiological interocular suppression in the amblyopic visual cortex, which restores at least part of the functionality of binocular vision. In contrast, the high-level hypothesis assumed that training might lead to better attention or reading out contrast or orientation signals from dichoptically presented noise to discount the effects of direct interocular suppression to improve visual function.63 The results supported the high-level hypothesis by demonstrating that AE dichoptic de-masking learning of contrast and orientation discrimination can transfer nearly completely to an orthogonal orientation with double training,64,65 so that new orientation or contrast signals can be read out from noise equally effectively. High-level brain areas may learn the rules of reweighting the noisy visual inputs from the amblyopic visual cortex for better readout.29 Our current results dissociated stereoacuity gain and the change of interocular suppression. The PT group exhibited greater stereoacuity gain even though the interocular suppression was unchanged, which is not expected if the amblyopic observers learn to discount interocular suppression directly through dichoptic training. It is likely the amblyopic observers may learn to be more capable of picking up the trained stimulus signals under the influence of dichoptic noise. The dissociation between stereoacuity gain and the change of interocular suppression suggests that training might lead to better attention to, or readout of, AE inputs, to counter the impacts of attentional bias to FEs or physiological interocular suppression.63 It certainly requires further research to decide whether the dichoptic training in amblyopic children is due to cognitive learning effects as the amblyopic adults.
Our current study has its limitations. First, our results are largely based on anisometropic amblyopes. Mechanisms underlying strabismic and anisometropic amblyopia are thought to be different.66,67 The training amount required for the recovery of stereo function may be different in strabismic and anisometropic amblyopia.15 For a more balanced evaluation of dichoptic training, it is necessary to collect data from all types of amblyopia in future studies. Second, our results may be specific to the particular dichoptic training paradigm used. We present a target in the amblyopic eye and a masker in the fellow eye. The observers are purposely trained to counter the masking effects from the fellow eye. In other dichoptic training paradigms, each eye is presented with a part of the stimuli, and the observer is required to integrate information from the two eyes for successful task completion. The training principles and the underlying mechanisms may be distinct between these paradigms. Further evaluation of the effectiveness and mechanisms between different training procedures is needed. Third, to appropriately differentiate the beneficial effects of perceptual learning and the standard treatment such as occlusion14 and refractive correction,68 we might need large-scale randomized clinical trials.
Conclusions
Dichoptic training can improve and even restore the stereoacuity of children with amblyopia, especially those with mild amblyopia. The dissociation between stereoacuity gain and the change of interocular acuity difference suggests that stereoacuity gain may not result from reduced interocular suppression. Rather children with amblyopia may have learned to better attend to, or readout, the stimulus information to improve stereopsis.
Supplementary Material
Acknowledgments
The authors thank Dennis Levi, Lei Liu, and Cong Yu for their insightful comments and discussions.
Supported by the Natural Science Foundation of China Grants 31470975 and 31970978.
Disclosure: X.-Y. Liu, None; Y.-W. Zhang, None; F. Gao, None; F. Chen, None; J.-Y. Zhang, None
References
- 1. Birch EE. Amblyopia and binocular vision. Progr Retinal Eye Res. 2013; 33: 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kiorpes L, Kiper DC, O'Keefe LP, Cavanaugh JR, Movshon JA. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. J Neurosci. 1998; 18: 6411–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harrad R, Sengpiel F, Blakemore C. Physiology of suppression in strabismic amblyopia. Br J Ophthalmol. 1996; 80: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shooner C, Hallum LE, Kumbhani RD, et al.. Asymmetric dichoptic masking in visual cortex of amblyopic macaque monkeys. J Neurosci. 2017; 37: 8734–8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sengpiel F, Blakemore C. The neural basis of suppression and amblyopia in strabismus. Eye (Lond). 1996; 10(Pt 2): 250–258. [DOI] [PubMed] [Google Scholar]
- 6. Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963; 26: 1003–1017. [DOI] [PubMed] [Google Scholar]
- 7. Hess RF, Thompson B, Baker DH. Binocular vision in amblyopia: structure, suppression and plasticity. Ophthalmic Physiol Opt. 2014; 34: 146–162. [DOI] [PubMed] [Google Scholar]
- 8. McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. J Vis. 2003; 3: 380–405. [DOI] [PubMed] [Google Scholar]
- 9. Giaschi D, Lo R, Narasimhan S, Lyons C, Wilcox LM. Sparing of coarse stereopsis in stereodeficient children with a history of amblyopia. J Vis. 2013; 13(10): 17. [DOI] [PubMed] [Google Scholar]
- 10. Scheiman MM, Hertle RW, Beck RW, et al.. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch Ophthalmol. 2005; 123: 437–447. [DOI] [PubMed] [Google Scholar]
- 11. Wallace DK, Lazar EL, Melia M, et al.. Stereoacuity in children with anisometropic amblyopia. J AAPOS. 2011; 15: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Birch EE, Wang J. Stereoacuity outcomes after treatment of infantile and accommodative esotropia. Optom Vis Sci. 2009; 86: 647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong AM New concepts concerning the neural mechanisms of amblyopia and their clinical implications. Can J Ophthalmol. 2012; 47: 399–409. [DOI] [PubMed] [Google Scholar]
- 14. Pediatric Eye Disease Investigator Group Writing, C, Rutstein RP, Quinn GE, Lazar EL, et al.. A randomized trial comparing Bangerter filters and patching for the treatment of moderate amblyopia in children. Ophthalmology. 2010; 117: 998–1004 e1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levi DM, Knill DC, Bavelier D. Stereopsis and amblyopia: a mini-review. Vision Res. 2015; 114: 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu XY, Zhang T, Jia YL, Wang NL, Yu C. The therapeutic impact of perceptual learning on juvenile amblyopia with or without previous patching treatment. Invest Ophthalmol Vis Sci. 2011; 52: 1531–1538. [DOI] [PubMed] [Google Scholar]
- 17. Li RW, Provost A, Levi DM. Extended perceptual learning results in substantial recovery of positional acuity and visual acuity in juvenile amblyopia. Invest Ophthalmol Vis Sci. 2007; 48: 5046–5051. [DOI] [PubMed] [Google Scholar]
- 18. Polat U, Ma-Naim T, Spierer A. Treatment of children with amblyopia by perceptual learning. Vision Res. 2009; 49: 2599–2603. [DOI] [PubMed] [Google Scholar]
- 19. Polat U, Ma-Naim T, Belkin M, Sagi D. Improving vision in adult amblyopia by perceptual learning. Proc Natl Acad Sci USA. 2004; 101: 6692–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levi DM, Polat U. Neural plasticity in adults with amblyopia. Proc Natl Acad Sci USA. 1996; 93: 6830–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ding J, Levi DM. Recovery of stereopsis through perceptual learning in human adults with abnormal binocular vision. Proc Natl Acad Sci USA. 2011; 108: E733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Astle AT, McGraw PV, Webb BS. Recovery of stereo acuity in adults with amblyopia. BMJ Case Rep. 2011; 2011: bcr0720103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xi J, Jia WL, Feng LX, Lu ZL, Huang CB. Perceptual learning improves stereoacuity in amblyopia. Invest Ophthalmol Vis Sci 2014, 55, 2384–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hess RF, Mansouri B, Thompson B. A new binocular approach to the treatment of amblyopia in adults well beyond the critical period of visual development. Restor Neurol Neurosci. 2010; 28: 793–802. [DOI] [PubMed] [Google Scholar]
- 25. Hess RF, Mansouri B, Thompson B. A binocular approach to treating amblyopia: antisuppression therapy. Optom Vis Sci. 2010; 87: 697–704. [DOI] [PubMed] [Google Scholar]
- 26. Li J, Thompson B, Deng D, Chan LY, Yu M, Hess RF. Dichoptic training enables the adult amblyopic brain to learn. Curr Biol. 2013; 23: R308–309. [DOI] [PubMed] [Google Scholar]
- 27. Vedamurthy I, Nahum M, Huang SJ, et al.. A dichoptic custom-made action video game as a treatment for adult amblyopia. Vis Res. 2015; 114: 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu XY, Zhang JY. Dichoptic training in adults with amblyopia: Additional stereoacuity gains over monocular training. Vis Res. 2018; 152: 84–90. [DOI] [PubMed] [Google Scholar]
- 29. Liu XY, Zhang JY. Dichoptic de-masking learning in adults with amblyopia and its mechanisms. Invest Ophthalmol Vis Sci 2019; 60: 2968–2977. [DOI] [PubMed] [Google Scholar]
- 30. Kelly KR, Jost RM, Dao L, Beauchamp CL, Leffler JN, Birch EE. Binocular iPad game vs patching for treatment of amblyopia in children: a randomized clinical trial. JAMA Ophthalmol. 2016; 134: 1402–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li SL, Reynaud A, Hess RF, et al.. Dichoptic movie viewing treats childhood amblyopia. J AAPOS. 2015; 19: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li SL, Jost RM, Morale SE, et al.. A binocular iPad treatment for amblyopic children. Eye (Lond). 2014; 28: 1246–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Birch EE, Li SL, Jost RM, et al.. Binocular iPad treatment for amblyopia in preschool children. J AAPOS. 2015; 19: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holmes JM, Manh VM,, Lazar EL, et al.. Effect of a binocular iPad game vs part-time patching in children aged 5 to 12 years with amblyopia: a randomized clinical trial. JAMA Ophthalmol. 2016; 134: 1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Webber AL, Wood JM, Thompson B. Fine motor skills of children with amblyopia improve following binocular treatment. Invest Ophthalmol Vis Sci. 2016; 57: 4713–4720. [DOI] [PubMed] [Google Scholar]
- 36. Gambacorta C, Nahum M, Vedamurthy I, et al.. An action video game for the treatment of amblyopia in children: a feasibility study. Vis Res. 2018; 148: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gao TY, Guo CX, Babu RJ, et al.. Effectiveness of a binocular video game vs placebo video game for improving visual functions in older children, teenagers, and adults with amblyopia: a randomized clinical trial. JAMA Ophthalmol. 2018; 136: 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Manh VM, Holmes JM, Lazar EL, et al.. A randomized trial of a binocular iPad game versus part-time patching in children aged 13 to 16 years with amblyopia. Am J Ophthalmol. 2018; 186: 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bossi M, Tailor VK, Anderson EJ, et al.. Binocular therapy for childhood amblyopia improves vision without breaking interocular suppression. Invest Ophthalmol Vis Sci. 2017; 58: 3031–3043. [DOI] [PubMed] [Google Scholar]
- 40. Kelly KR, Jost RM, Wang YZ, et al.. Improved binocular outcomes following binocular treatment for childhood amblyopia. Invest Ophthalmol Vis Sci 2018; 59: 1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Knox PJ, Simmers AJ, Gray LS, Cleary M. An exploratory study: prolonged periods of binocular stimulation can provide an effective treatment for childhood amblyopia. Invest Ophthalmol Vis Sci 2012; 53: 817–824. [DOI] [PubMed] [Google Scholar]
- 42. Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997; 10: 437–442. [PubMed] [Google Scholar]
- 43. Levi DM. Crowding—an essential bottleneck for object recognition: a mini-review. Vis Res 2008; 48: 635–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sjöstrand J. Contrast sensitivity in children with strabismic and anisometropic amblyopia. A study of the effect of treatment. Acta Ophthalmol (Copenh). 1981; 59: 25–34. [DOI] [PubMed] [Google Scholar]
- 45. Fahle M, Henke-Fahle S. Interobserver variance in perceptual performance and learning. Invest Ophthalmol Vis Sci. 1996; 37: 869–877. [PubMed] [Google Scholar]
- 46. Astle AT, Li RW, Webb BS, Levi DM, McGraw PV. A Weber-like law for perceptual learning. Sci Rep. 2013; 3: 1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Levi DM, Li RW, Silver MA, Chung ST. Sequential perceptual learning of letter identification and “uncrowding” in normal peripheral vision: Effects of task, training order, and cholinergic enhancement. J Vis. 2020; 4: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li RW, Ngo C, Nguyen J, Levi DM. Video-game play induces plasticity in the visual system of adults with amblyopia. PLoS Biol. 2011, 9, e1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adams WE, Leske DA, Hatt SR, Holmes JM. Defining real change in measures of stereoacuity. Ophthalmology. 2009; 116: 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fawcett SL, Birch EE. Validity of the Titmus and Randot circles tasks in children with known binocular vision disorders. J AAPOS. 2003; 7: 333–338. [DOI] [PubMed] [Google Scholar]
- 51. Li J, Thompson B, Lam CS, et al.. The role of suppression in amblyopia. Invest Ophthalmol Vis Sci. 2011; 52: 4169–4176. [DOI] [PubMed] [Google Scholar]
- 52. Stewart CE, Moseley MJ, Stephens DA, Fielder AR. Treatment dose-response in amblyopia therapy: the Monitored Occlusion Treatment of Amblyopia Study (MOTAS). Invest Ophthalmol Vis Sci. 2004; 45: 3048–3054. [DOI] [PubMed] [Google Scholar]
- 53. Fronius M, Cirina L, Ackermann H, Kohnen T, Diehl CM. Efficiency of electronically monitored amblyopia treatment between 5 and 16 years of age: new insight into declining susceptibility of the visual system. Vision Res. 2014; 103: 11–19. [DOI] [PubMed] [Google Scholar]
- 54. Stewart CE, Stephens DA, Fielder AR, Moseley MJ, Cooperative R. Objectively monitored patching regimens for treatment of amblyopia: randomised trial. BMJ 2007; 335: 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cotter SA, Pediatric Eye Disease Investigator Group, Edwards AR, et al.. Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology. 2006; 113: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stewart CE, Moseley MJ, Fielder AR, Stephens DA, Cooperative M. Refractive adaptation in amblyopia: quantification of effect and implications for practice. Br J Ophthalmol. 2004; 88: 1552–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Simmers AJ, Ledgeway T, Hess RF, McGraw PV. Deficits to global motion processing in human amblyopia. Vis Res. 2003; 43;729–738. [DOI] [PubMed] [Google Scholar]
- 58. Knox PJ, Ledgeway T, Simmers AJ. The effects of spatial offset, temporal offset and image speed on sensitivity to global motion in human amblyopia. Vis Res. 2013; 86: 59–65. [DOI] [PubMed] [Google Scholar]
- 59. Stifter E, Burggasser G, Hirmann E, Thaler A, Radner W. Monocular and binocular reading performance in children with microstrabismic amblyopia. Br J Ophthalmol. 2005; 89: 1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Birch EE, Kelly KR, Giaschi DE. Fellow eye deficits in amblyopia. J Binocul Vis Ocul Motil. 2019; 69: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stewart CE, Wallace MP, Stephens DA, Fielder AR, Moseley MJ, Cooperative M. The effect of amblyopia treatment on stereoacuity. J AAPOS. 2013; 17: 166–173. [DOI] [PubMed] [Google Scholar]
- 62. Saxena R, Puranik S, Singh D, Menon V, Sharma P, Phuljhele S. Factors predicting recurrence in successfully treated cases of anisometropic amblyopia. Ind J Ophthalmol. 2013; 61: 630–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tsirlin I; Colpa L; Goltz HC; Wong AM. Behavioral training as new treatment for adult amblyopia: a meta-analysis and systematic review. Invest Ophthalmol Vis Sci. 2015; 56: 4061–4075. [DOI] [PubMed] [Google Scholar]
- 64. Xiao LQ, Zhang JY, Wang R, Klein SA, Levi DM, Yu C. Complete transfer of perceptual learning across retinal locations enabled by double training. Curr Biol. 2008; 18;1922–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang JY, Zhang GL, Xiao LQ, Klein SA, Levi DM, Yu C. Rule-based learning explains visual perceptual learning and its specificity and transfer. J Neurosci. 2010; 30: 12323–12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hess RF, Pointer JS. Differences in the neural basis of human amblyopia: the distribution of the anomaly across the visual field. Vis Res. 1985; 25: 1577–1594. [DOI] [PubMed] [Google Scholar]
- 67. Levi DM, Klein S. Differences in Vernier discrimination for grating between strabismic and anisometropic amblyopes. Invest Ophthalmol Vis Sci. 1982; 23: 398–407. [PubMed] [Google Scholar]
- 68. Moseley MJ, Fielder AR, Stewart CE. The optical treatment of amblyopia. Optom Vis Sci. 2009; 86: 629–633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.