Abstract
Purpose
Diabetic retinopathy (DR) is a common cause of vision loss in working age adults and presents changes in retinal vessel oxygenation and morphology. The purpose of this study was to test the hypothesis that there is an association of retinal vessel oxygen saturation with vessel density (VD) and tortuosity in DR.
Methods
Ninety-five subjects were classified in the following groups: nondiabetic control (N = 25), no DR (N = 28), mild nonproliferative DR (NPDR; N = 21), moderate to severe NPDR (N = 14), or treated proliferative DR (PDR; N = 7). Retinal oximetry was performed to measure arterial and venous oxygen saturation (SO2A and SO2V) and calculate oxygen extraction fraction (OEF). Optical coherence tomography angiography (OCTA) was performed for measurements of VD and vessel tortuosity index (VTI).
Results
There were statistically significant differences in SO2A and SO2V among groups (P < 0.004). SO2A and SO2V were higher in the PDR group compared to the control group and SO2V was also higher in the moderate to severe NPDR group. VD differed significantly among groups (P = 0.003), whereas VTI was not significantly different (P = 0.22). Compared to the control group, VD was lower in moderate to severe NPDR and PDR groups. VD was also lower in the PDR group than that in the no DR group (P = 0.03). There was a significant correlation of VTI with SO2V (r = 0.32, P = 0.002) and OEF (r = −0.35, P = 0.001).
Conclusions
Retinal vessel morphology, oxygenation, and tissue oxygen extraction were associated with each other in a cohort of subjects with and without DR.
Translational Relevance
The findings of this study have the potential to improve clinical management of DR by providing better understanding of human disease pathophysiology and propelling future studies to identify multiple image-based biomarkers for improved disease diagnosis and monitoring.
Keywords: diabetic retinopathy (DR), imaging, retinal vasculature
Introduction
Among diabetes complications, diabetic retinopathy (DR) has a significant impact on vision and can eventually lead to blindness. In fact, DR is one of the most common causes of visual loss in working age adults.1–4 An underlying cause of vision-threatening DR pathologies is thought to be retinal hypoxia5 due to nonperfusion of the retinal capillaries during the progression of the disease.5–9 Currently, there are no clinical methods available to measure retinal tissue oxygen content. However, previous studies have shown increased retinal arterial and venous oxygen saturation (SO2A and SO2V) at stages of DR.10–15 Other studies have reported decreased difference between SO2A and SO2V or oxygen extraction fraction (OEF) in subjects with non-proliferative DR (NPDR), mild or no DR.11–13,15–17 Some studies have attributed the decrease in oxygen extraction to changes in the retinal vessel endothelium and regulatory function of pericytes.18–21
Morphological changes in retinal vessels have been shown to be present in DR. Retinal vessel density (VD) was reported to be decreased with progression of DR, primarily due to capillary nonperfusion.22–24 Additionally, tortuosity of retinal vessels was found to increase with progression of DR23,25,26 and eventually decrease in the severe proliferative stage.23 Although the mechanism of increased tortuosity is not entirely clear, studies suggested that it may be caused by damage to endothelial and autoregulatory cells, limiting the ability of the vessel walls to adjust to changes in blood flow,18,23,26 under normal physiology or hypoxic conditions.27
Alterations in retinal VD and vessel wall elasticity may impair the delivery to and uptake of oxygen by the tissue, thereby affecting vascular oxygen content and tissue oxygen extraction. The purpose of the current study was to test the hypothesis that there is an association between retinal vessel oxygen saturation and tortuosity in DR.
Methods
Subjects
This study was approved by an institutional review board of the University of Southern California (USC). Subjects were recruited from the USC retina clinic. Before subjects were enrolled, the research study was explained, and informed consents were obtained according to the tenets of the Declaration of Helsinki. A total of 95 subjects participated in the study. Based on retina examination by experienced retina specialists, subjects were classified28 as diabetic with no DR (N = 28), mild (N = 21), or moderate to severe (N = 14) with or without macular edema, proliferative DR (PDR; N = 7), or healthy nondiabetic control (N = 25). Subjects with PDR had received no treatment (N = 1), antivascular endothelium growth factor treatment (N = 1), panretinal photocoagulation treatment (N = 3), or both treatments (N = 2). Nondiabetic subjects with a history of any eye disease and subjects with diabetes with a history of eye diseases, other than DR, were excluded from this study.
Retinal findings were documented using our previously established retinal oximetry system16,17 and commercially available optical coherence tomography angiography (OCTA) instrument (Optovue Inc., Fremont, CA). Hematocrit (HCT) and hemoglobin A1C (HbA1c) were measured from blood samples obtained from a finger-prick and using a micro-hematocrit centrifuge (Unico, Dayton, NJ) and HbA1C analyzer (Siemens DCA Vantage Analyzer, Frimley, Camberley, UK). HCT and HbA1c data were not available in one subject in the no DR group and two subjects in the control group, respectively. Blood pressure (BP) was recorded using a wrist cuff to obtain the mean arterial pressure (MAP) = 1/3 (systolic BP) + 2/3 (diastolic BP). Three MAP measurements were averaged.
Oxygen Saturation and Extraction Fraction
Using our previously established retinal oximetry system,16,17 9 retinal reflectance images were acquired at oxygen-sensitive and oxygen-insensitive imaging wavelengths of 606 and 570 nm, within 3 seconds. Images acquired at each wavelength were registered using the stack-reg plug-in of ImageJ (National Institutes of Health, Bethesda, MD) to compensate for eye motion movements and then averaged. Retinal vessels within a circumpapillary region of interest (Fig. A) were automatically segmented and centerlines were generated by our previously published image analysis algorithm.17,29 From the mean image at each wavelength, optical densities (ODs) were calculated for each vessel as log (Ioutside/Iinside), where Iinside and Ioutside represent the average pixel intensity inside and outside the vessel, respectively. OD ratios (OD606/OD570), were adjusted and converted to SO2 values using a linear regression model, as previously described.17 Measurements in individual arteries and veins were averaged separately to calculate SO2A and SO2V per eye. OEF was calculated as: OEF = (SO2A – SO2V) / SO2A. OEF is the fraction of the total oxygen supplied by the retinal circulation that is extracted by the tissue. It is also defined by the ratio of oxygen metabolism to delivery, and thus independent of blood flow.
Figure.
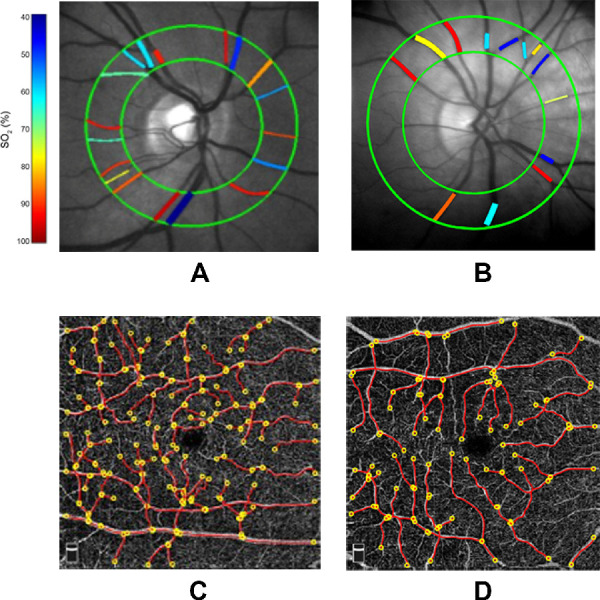
Examples of retinal images obtained in a (A) control subject and (B) moderate to severe nonproliferative diabetic retinopathy subject. Retinal vascular oxygen saturation (SO2) measurements in major arteries and veins are depicted in pseudo-color within a circumpapillary region bounded by green circles. Examples of optical coherence tomography angiography images acquired in the same (C) control subject and (D) moderate to severe nonproliferative diabetic retinopathy subject. Vessel centerlines (red lines) are overlaid on the vessels and vessel end points are indicated by yellow circles. Vessel tortuosity index (VTI) was determined by averaging measurements from all vessels.
Vessel Density and Tortuosity
Using a commercially available OCTA system (Avanti Optovue, Inc., Fremont, CA), images were acquired at a wavelength of 840 nm with an axial scan rate and axial scan depth resolution of 70 kHz and 5 µm, respectively. The instrument generated OCTA images in a 6 mm × 6 mm perifoveal region based on detection of red blood cell motion from repeated B-scans acquired in identical retinal locations. The instrument's software defined the superficial vascular complex within the nerve fiber and ganglion cell layers.
OCTA images with quality scores better than 3 out of 10 were selected for analysis. VD in the superficial vascular complex was determined using our previously published fractal analysis method.30 VD values ranged from zero to one, corresponding to the fraction of the imaged area occupied by vessels. OCTA images were also analyzed to determine vessel tortuosity along each vessel segment using our previously published method31 and calculate a mean vessel tortuosity index (VTI). The minimum value of VTI is zero, representing a straight line. VTI increases with higher variation in angles, number of critical points, and amplitude of curvature along the vessel centerline.
Statistical Analysis
Statistical Analysis System (SAS, version 9.4; SAS Institute Inc., Cary, NC) was used to analyze the data, and significance was accepted at P < 0.05. One eye of each subject was selected for the study. For subjects with data available in both eyes, one eye was selected at random by alternating the selection between the right and left eyes. Assumption of normality of data distribution was confirmed through the Shapiro-Wilk tests and graphical visualization of quantile-quantile plots. Demographics among groups (control, no DR, mild NPDR, moderate to severe NPDR, and PDR) were compared by ANOVA (age) and χ2 tests (eyes and sex). Race was compared using Fisher's exact test because the expected counts in more than 20% of the cells was less than 5. Comparison of all other continuous variables among groups was performed using ANOVA (HbA1c, VD, SO2A, SO2V, and OEF), or Kruskal-Wallis tests (MAP and VTI) for those that were not normally distributed. Homogeneity of variance of ANOVA analyses was confirmed by Levene's test. Post hoc analysis was performed using Tukey tests. Spearman rank correlation analysis was used to determine associations of VTI with SO2A, SO2V, and OEF. Partial correlations of VTI with SO2A, SO2V, and OEF were conducted while adjusting for HCT and HbA1c. The study had 90% power to detect a correlation coefficient of 0.32 or higher at the alpha level of 0.05 with a sample size of 95.
Results
Demographic characteristics of subjects stratified by group are shown in Table 1. There were no significant differences in sex (P = 0.64), eyes (P = 0.09), race (P = 0.52), or age (P = 0.43) among the control, no DR, mild NPDR, moderate to severe NPDR, and PDR groups.
Table 1.
Comparison of Demographics Among Groups
| Control | No DR | Mild NPDR | Moderate to | PDR | ||
|---|---|---|---|---|---|---|
| Variable | (N = 25) | (N = 28) | (N = 21) | Severe NPDR (N = 14) | (N = 7) | P Value |
| Sex | ||||||
| Male | 11 | 11 | 9 | 7 | 5 | 0.64 |
| Female | 14 | 17 | 12 | 7 | 2 | |
| Eye | ||||||
| Right | 11 | 12 | 16 | 6 | 5 | 0.09 |
| Left | 14 | 16 | 5 | 8 | 2 | |
| Race | ||||||
| Asian | 8 | 3 | 3 | 2 | 0 | 0.52 |
| African American | 2 | 3 | 1 | 0 | 0 | |
| White | 4 | 5 | 6 | 6 | 2 | |
| Hispanic | 11 | 17 | 11 | 6 | 5 | |
| Age (years) | ||||||
| 50 ± 14 | 55 ± 15 | 58 ± 15 | 56 ± 17 | 52 ± 12 | 0.43 |
Data presented in control, no diabetic retinopathy (no DR), mild nonproliferative diabetic retinopathy (mild NPDR), moderate to severe NPDR, and proliferative diabetic retinopathy (PDR) groups.
Table 2 shows measurements of MAP, HbA1c, and HCT stratified by group. There was no significant difference in MAP among groups (P = 0.80). HbA1c was significantly different among groups (P < 0.0001). HbA1c levels were higher in the no DR, mild NPDR, moderate to severe NPDR, and PDR groups compared to the control group (P < 0.0001) and higher in the PDR group compared to the no DR group (P = 0.03). There was a statistically significant difference in HCT among groups (P = 0.001). HCT was lower in the mild NPDR group compared to the control group (P = 0.0001).
Table 2.
Comparison of Mean Arterial Pressure (MAP), Hemoglobin A1c (HbA1c), and Hematocrit (HCT) Among Groups
| Control | No DR | Mild NPDR | Moderate to | |||
|---|---|---|---|---|---|---|
| Metric | (N = 25) | (N = 28) | (N = 21) | Severe NPDR (N = 14) | PDR (N = 7) | P Value |
| MAP (mm Hg) | 103 ± 14 | 102 ± 16 | 100 ± 11 | 103 ± 17 | 108 ± 15 | 0.80 |
| HbA1c (%) | 5.4 ± 0.47a | 7.0 ± 1.1 | 7.7 ± 1.4 | 8.0 ± 1.4 | 8.4 ± 1.6 | <0.0001 |
| HCT (%) | 45 ± 4 | 43 ± 3b | 40 ± 5 | 43 ± 4 | 41 ± 5 | 0.001 |
Data presented in control, no diabetic retinopathy (no DR), mild nonproliferative diabetic retinopathy (mild NPDR), moderate to severe NPDR, and proliferative diabetic retinopathy (PDR) groups.
Bolded values indicate P < 0.05. Superscripts represent groups that had missing HbA1c or HCT data and thus a lower N value of subjects in that group,
N = 23.
N = 27.
Measurements of SO2A, SO2V, and OEF stratified by group are displayed in Table 3. SO2A was significantly different among groups (P = 0.002). SO2A was higher in the PDR group than the control group (P = 0.001). SO2v was significantly different among groups (P = 0.004). SO2v was higher in the moderate to severe NPDR (P = 0.01) and PDR (P = 0.04) groups compared to the control group. There was a marginal difference in OEF among groups (P = 0.07).
Table 3.
Comparison of Arterial and Venous Oxygen Saturation (SO2A and SO2V), Oxygen Extraction Fraction (OEF), Vessel Density (VD), and Vessel Tortuosity Index (VTI) Among Groups
| Control | No DR | Mild NPDR | Moderate to Severe | PDR | ||
|---|---|---|---|---|---|---|
| Metric | (N = 25) | (N = 28) | (N = 21) | NPDR (N = 14) | (N = 7) | P Value |
| SO2A (%) | 89 ± 7 | 94 ± 8 | 94± 10 | 95 ± 8 | 104 ± 12 | 0.002 |
| SO2V (%) | 53 ± 8 | 58 ± 9 | 56 ± 10 | 64 ± 13 | 64 ± 9 | 0.004 |
| OEF | 0.41 ± 0.08 | 0.39 ± 0.08 | 0.40 ± 0.10 | 0.33 ± 0.12 | 0.38 ± 0.08 | 0.07 |
| VD | 0.49 ± 0.04 | 0.48 ± 0.03 | 0.47 ± 0.05 | 0.45 ± 0.04 | 0.43 ± 0.05 | 0.003 |
| VTI | 0.50 ± 0.15 | 0.53 ± 0.14 | 0.51 ± 0.13 | 0.62 ± 0.18 | 0.55 ± 0.18 | 0.22 |
Data presented in control, no diabetic retinopathy (no DR), mild nonproliferative diabetic retinopathy (mild NPDR), moderate to severe NPDR, and proliferative diabetic retinopathy (PDR) groups.
Bolded values indicate P < 0.05.
Measurements of VD and VTI stratified by group are shown in Table 3. VD differed significantly among groups (P = 0.003). VD was lower in the moderate to severe NPDR (P = 0.04) and PDR (P = 0.01) groups compared to the control group. VD was also lower in the PDR group than in the no DR group (P = 0.03). There was not a statistically significant difference in VTI among groups (P = 0.22).
There was a positive correlation between VTI and SO2V (rs = 0.32, P = 0.002, N = 95). The correlation between VTI and SO2A was not significant (rs = 0.05, P = 0.62, N = 95). VTI and OEF were negatively correlated (rs = −0.35, P = 0.001, N = 95). The significance of these relationships was unaffected by adjustment for HCT and HbA1c.
Discussion
Previous studies have shown increased retinal vascular oxygen saturation and tortuosity with progression of DR.11–14 Here, alterations in retinal vascular morphologic and oxygenation metrics at stages of DR were demonstrated and associations between these metrics were reported for the first time. The results confirmed the hypothesis that higher perifoveal vessel tortuosity was correlated with higher retinal venous oxygen saturation and lower fraction of extracted oxygen.
In the current study, an increase in retinal SO2A was shown in PDR compared to less advanced stages of DR. This finding is in agreement with previous studies that showed elevation of SO2A with increasing severity of DR.9,11,13,32–34 Previous studies in DR have reported increased SO2V14,15,33 and decreased retinal arteriovenous SO2 difference and OEF.12,13 In the current study, OEF was not found to be significantly decreased, although a trend in OEF reduction was observed. Maintained OEF suggests the relative rate of oxygen metabolism to delivery was not altered, despite potential changes in either parameter. Previous studies have shown reduced OEF in NPDR.15,17 Heterogeneity of DR, as well as separate grouping of stages of NPDR and limited sample size in the current study, likely contributed to an undetected statistically significant reduction in OEF. Several factors may account for the observed elevation retinal vascular SO2 in DR. First, increased glycosylated hemoglobin has been shown to exhibit higher affinity to oxygen.35–37 Therefore, oxygen may be more tightly bound to hemoglobin and diffuses less readily to the tissue, which can account for higher SO2 as oxygen remains in the lumen and attached to hemoglobin.37 Second, endothelial dysfunction and loss of regulatory cells due to DR19,20,38 may adversely affect the maintenance of homeostasis and the permeability to oxygen. Third, cellular dysfunction or loss due to hyperglycemia or vascular supply may result in a reduction in oxygen demand by the retinal tissue.
VD was reduced in moderate to severe NPDR and PDR, consistent with previous studies.22,23,39 The reduction in vessel density is due to capillary nonperfusion and the progressive loss of capillary cells.23 Previous studies have shown increased retinal vessel tortuosity during progression of DR.23,25,26 An increase in advanced glycation end products causes pericyte loss in DR18,40 and impairs the ability of cells to respond to certain biochemical signals, such as nitric oxide, which is important in the functioning of smooth muscle cell relaxation during vasodilation.38 Studies have shown reduced flow-mediated vasodilation in DR, suggesting endothelial dysfunction.19,21 Overall, increased vessel tortuosity in DR is likely due to impairment of the retinal vessel endothelial support and function,18 as well as due to tissue hypoxia.41
The results showed correlations of increased VTI with increased SO2V and decreased OEF, although a causal relation between these metrics is not indicated, requiring a different study design. Increased vessel tortuosity can occur in DR due to impairment of the retinal vessel endothelial support and function18,19,33 coupled with increased blood flow.42,43 Moreover, retinal vessels have been shown to be tortuous and elongated under DR, hypobaric hypoxia, and central retinal vein occlusion.41,44,45 On the other hand, decreased OEF (ratio of oxygen metabolism to oxygen delivery) is due to a decrease in oxygen metabolism and / or an increase in oxygen delivery. A decrease in OEF in DR can result with increased SO2V, which is suggestive of reduced oxygen metabolism coupled with increased blood flow, causing an increase in oxygen delivery.
The current study presented certain limitations. The relatively small sample size in some DR groups may have limited the statistical power to detect some differences among groups. Nevertheless, the sample size of all subjects had adequate power to establish correlations that were not influenced by DR stage. Additionally, the findings were based on data obtained in treated and untreated DR, hence heterogeneity of subjects may have had a variable effect on other aspects of retinal pathophysiology. Under normal physiology, the retinal and choroidal circulations predominately supply oxygen to the inner retinal neurons and photoreceptors, respectively. However, oxygen supply by the dual circulations may be altered under pathologic conditions. Using enhanced depth imaging OCT, several studies have investigated changes in the choroid due to DR.46 Overall, changes in the choroidal thickness were shown to be variable, whereas choroidal vascularity metrics were abnormal and decreased with worsening of DR. One limitation of the current study was that vascular oxygen saturation was only evaluated in the retinal circulation, thus the potential contribution of choroidal circulation to the findings was not considered. The effect of history of uncontrolled hypertension on the results was not considered, although at the time of imaging, subjects had similar BP. Because oxygenation and tortuosity measurements were obtained from different retinal regions, future studies are needed to assess the effect of retinal region and vessel size on these relationships. In addition, VTI measurements consisted of manual selection of vessel segments on a binary image based on an observer's subjective identification of locations of vessel branching. Therefore, in future studies, development of automatic segmentation for VTI measurements will improve the methodology. As technology advances, the current measurement methods may be improved, however, to our knowledge, the methods used in this study are among the best-established techniques in the field today.
In conclusion, the study showed a positive correlation of VTI with SO2V and a negative correlation with OEF, suggesting changes in these parameters develop under similar hyperglycemic situations and the pathological processes common in DR. The findings provide a better understanding of DR and may eventually lead to improved management of people with diabetes.
Acknowledgments
Supported by National Institutes of Health (NIH) grants EY030115, DK104393, and EY029220 and Research to Prevent Blindness.
Disclosure: S.L. Auvazian, None; J. Cano, None; S. Leahy, None; P. Karamian, None; A. Kashani, None; A. Moshfeghi, None; H. Ameri, None; N.P. Blair, None; M. Shahidi, None
References
- 1. Roy MS, Klein R, O'Colmain BJ, Klein BE, Moss SE, Kempen JH.. The prevalence of diabetic retinopathy among adult type 1 diabetic persons in the United States. Arch Ophthalmol. 2004; 122: 546–551. [DOI] [PubMed] [Google Scholar]
- 2. Kempen JH, O'Colmain BJ, Leske MC, et al.. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004; 122: 552–563. [DOI] [PubMed] [Google Scholar]
- 3. Zhang X, Saaddine JB, Chou CF, et al.. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010; 304: 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baker RS. Diabetic retinopathy in African Americans: vision impairment, prevalence, incidence, and risk factors. Int Ophthalmol Clin. 2003; 43: 105–122. [DOI] [PubMed] [Google Scholar]
- 5. AS Al-Kharashi. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J Ophthalmol. 2018; 32: 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW.. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002; 47(Suppl. 2): S253–S262. [DOI] [PubMed] [Google Scholar]
- 7. Silva PS, Dela Cruz AJ, Ledesma MG, et al.. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology. 2015; 122: 2465–2472. [DOI] [PubMed] [Google Scholar]
- 8. Niki T, Muraoka K, Shimizu K.. Distribution of capillary nonperfusion in early-stage diabetic retinopathy. Ophthalmology. 1984; 91: 1431–1439. [DOI] [PubMed] [Google Scholar]
- 9. Hardarson SH, Stefansson E.. Retinal oxygen saturation is altered in diabetic retinopathy. Br J Ophthalmol. 2012; 96: 560–563. [DOI] [PubMed] [Google Scholar]
- 10. Stefansson E. Oxygen and diabetic eye disease. Graefes Arch Clin Exp Ophthalmol. 1990; 228: 120–123. [DOI] [PubMed] [Google Scholar]
- 11. Tayyari F, Khuu LA, Flanagan JG, Singer S, Brent MH, Hudson C.. Retinal blood flow and retinal blood oxygen saturation in mild to moderate diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015; 56: 6796–6800. [DOI] [PubMed] [Google Scholar]
- 12. Fondi K, Wozniak PA, Howorka K, et al.. Retinal oxygen extraction in individuals with type 1 diabetes with no or mild diabetic retinopathy. Diabetologia. 2017; 60: 1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jorgensen CM, Hardarson SH, Bek T.. The oxygen saturation in retinal vessels from diabetic patients depends on the severity and type of vision-threatening retinopathy. Acta Ophthalmol. 2014; 92: 34–39. [DOI] [PubMed] [Google Scholar]
- 14. Kashani AH, Lopez Jaime GR, Saati S, Martin G, Varma R, Humayun MS. Noninvasive assessment of retinal vascular oxygen content among normal and diabetic human subjects: a study using hyperspectral computed tomographic imaging spectroscopy. Retina. 2014; 34: 1854–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blair NP, Wanek J, Felder AE, et al.. Retinal oximetry and vessel diameter measurements with a commercially available scanning laser ophthalmoscope in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017; 58: 5556–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Felder AE, Wanek J, Blair NP, et al.. The effects of diabetic retinopathy stage and light flicker on inner retinal oxygen extraction fraction. Invest Ophthalmol Vis Sci. 2016; 57: 5586–5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Felder AE, Wanek J, Blair NP, Shahidi M.. Inner retinal oxygen extraction fraction in response to light flicker stimulation in humans. Invest Ophthalmol Vis Sci. 2015; 56: 6633–6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Curtis TM, Gardiner TA, Stitt AW.. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye (Lond). 2009; 23: 1496–1508. [DOI] [PubMed] [Google Scholar]
- 19. Yun JS, Ko SH, Kim JH, et al.. Diabetic retinopathy and endothelial dysfunction in patients with type 2 diabetes mellitus. Diabetes Metab J. 2013; 37: 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergers G, Song S.. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005; 7: 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malecki MT, Osmenda G, Walus-Miarka M, et al.. Retinopathy in type 2 diabetes mellitus is associated with increased intima-media thickness and endothelial dysfunction. Eur J Clin Invest. 2008; 38: 925–930. [DOI] [PubMed] [Google Scholar]
- 22. Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH.. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016; 57: OCT362–OCT370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee H, Lee M, Chung H, Kim HC.. Quantification of retinal vessel tortuosity in diabetic retinopathy using optical coherence tomography angiography. Retina. 2018; 38: 976–985. [DOI] [PubMed] [Google Scholar]
- 24. Lavia C, Couturier A, Erginay A, Dupas B, Tadayoni R, Gaudric A.. Reduced vessel density in the superficial and deep plexuses in diabetic retinopathy is associated with structural changes in corresponding retinal layers. PLoS One. 2019; 14: e0219164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klein R, Lee KE, Danforth L, et al.. The relationship of retinal vessel geometric characteristics to the incidence and progression of diabetic retinopathy. Ophthalmology. 2018; 125: 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sasongko MB, Wong TY, Nguyen TT, Cheung CY, Shaw JE, Wang JJ.. Retinal vascular tortuosity in persons with diabetes and diabetic retinopathy. Diabetologia. 2011; 54: 2409–2416. [DOI] [PubMed] [Google Scholar]
- 27. Cheng RW, Yusof F, Tsui E, et al.. Relationship between retinal blood flow and arterial oxygen. J Physiol. 2016; 594: 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilkinson CP, Ferris FL 3rd, Klein RE, et al.. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003; 110: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 29. Moss HE, Treadwell G, Wanek J, DeLeon S, Shahidi M.. Retinal vessel diameter assessment in papilledema by semi-automated analysis of SLO images: feasibility and reliability. Invest Ophthalmol Vis Sci. 2014; 55: 2049–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cano J, Farzad S, Khansari MM, et al.. Relating retinal blood flow and vessel morphology in sickle cell retinopathy. Eye (Lond). 2020; 34: 886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khansari MM, O'Neill W, Lim J, Shahidi M.. Method for quantitative assessment of retinal vessel tortuosity in optical coherence tomography angiography applied to sickle cell retinopathy. Biomed Opt Express. 2017; 8: 3796–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dong Y, Lin L, Yan H, et al.. Shifts in retinal vessel diameter and oxygen saturation in Chinese type 2 diabetes mellitus patients. BMC Ophthalmol. 2016; 16: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hammer M, Vilser W, Riemer T, et al.. Diabetic patients with retinopathy show increased retinal venous oxygen saturation. Graefes Arch Clin Exp Ophthalmol. 2009; 247: 1025–1030. [DOI] [PubMed] [Google Scholar]
- 34. Khoobehi B, Firn K, Thompson H, Reinoso M, Beach J.. Retinal arterial and venous oxygen saturation is altered in diabetic patients. Invest Ophthalmol Vis Sci. 2013; 54: 7103–7106. [DOI] [PubMed] [Google Scholar]
- 35. Bunn HF, Gabbay KH, Gallop PM.. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978; 200: 21–27. [DOI] [PubMed] [Google Scholar]
- 36. Ditzel J. Affinity hypoxia as a pathogenetic factor of microangiopathy with particular reference to diabetic retinopathy. Acta Endocrinol Suppl (Copenh). 1980; 238: 39–55. [PubMed] [Google Scholar]
- 37. Castilho EM, Glass ML, Manco JC.. The effects of 2,3-diphosphoglycerate, adenosine triphosphate, and glycosylated hemoglobin on the hemoglobin-oxygen affinity of diabetic patients. Braz J Med Biol Res. 2003; 36: 731–737. [DOI] [PubMed] [Google Scholar]
- 38. Avogaro A, Fadini GP, Gallo A, Pagnin E, de Kreutzenberg S.. Endothelial dysfunction in type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2006; 16(Suppl. 1): S39–S45. [DOI] [PubMed] [Google Scholar]
- 39. Kaizu Y, Nakao S, Sekiryu H, et al.. Retinal flow density by optical coherence tomography angiography is useful for detection of nonperfused areas in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2018; 256: 2275–2282. [DOI] [PubMed] [Google Scholar]
- 40. Stitt AW, Curtis TM.. Advanced glycation and retinal pathology during diabetes. Pharmacol Rep. 2005; 57(Suppl.): 156–168. [PubMed] [Google Scholar]
- 41. Benitez-Aguirre P, Craig ME, Sasongko MB, et al.. Retinal vascular geometry predicts incident retinopathy in young people with type 1 diabetes: a prospective cohort study from adolescence. Diabetes Care. 2011; 34: 1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cuypers MH, Kasanardjo JS, Polak BC.. Retinal blood flow changes in diabetic retinopathy measured with the Heidelberg scanning laser Doppler flowmeter. Graefes Arch Clin Exp Ophthalmol. 2000; 238: 935–941. [DOI] [PubMed] [Google Scholar]
- 43. Schmetterer L, Wolzt M.. Ocular blood flow and associated functional deviations in diabetic retinopathy. Diabetologia. 1999; 42: 387–405. [DOI] [PubMed] [Google Scholar]
- 44. MacCormick IJ, Somner J, Morris DS, et al.. Retinal vessel tortuosity in response to hypobaric hypoxia. High Alt Med Biol. 2012; 13: 263–268. [DOI] [PubMed] [Google Scholar]
- 45. Muraoka Y, Tsujikawa A, Kumagai K, et al.. Retinal vessel tortuosity associated with central retinal vein occlusion: an optical coherence tomography study. Invest Ophthalmol Vis Sci. 2014; 55: 134–141. [DOI] [PubMed] [Google Scholar]
- 46. Hamadneh T, Aftab S, Sherali N, Vetrivel Suresh R, Tsouklidis N, An M. Choroidal changes in diabetic patients with different stages of diabetic retinopathy. Cureus. 2020; 12: e10871. [DOI] [PMC free article] [PubMed] [Google Scholar]


