Abstract
Background
We would evaluate the epidemiology, clinical aspects, and prognostic factors of patients of all ages admitted with human corona virus (HCoV).
Methods
This study was retrospectively performed at five university teaching hospitals between 1st January 2018 and 31th March 2020. Routine molecular testing using for multiplex real-time reverse transcription-polymerase chain reaction (RT-PCR) methods was conducted on the respiratory viruses. We assessed the demographics, laboratory findings, and treatment of patients infected with coronavirus.
Results
There were 807 coronavirus-infected patients from 24,311 patients with respiratory virus PCR test admitted to five hospitals over 27 months. All-cause mortality rates of patients admitted for seasonal HCoV disease were 3.1% in all patients and 10.8% in patients aged ≥18 years. The Cox proportional hazard regression analysis was performed in patients aged ≥18 years. After adjusting for other clinical variables, general weakness symptoms [hazard ratio (HR), 2.651; 95% confidence interval (CI), 1.147–6.125, P=0.023], National Early Warning Score (NEWS) ≥2 (HR, 5.485; 95% CI, 1.261–23.858, P=0.023), and coronavirus subtype OC43 (HR, 2.500; 95% CI, 1.060–5.897, P=0.036) were significantly associated with death from coronavirus.
Conclusions
Coronavirus infection can reveal a higher mortality rate in patients of ≥18 than those of <18 years, thus, adult patients require more careful treatment. Furthermore, in adult patients, the factors associated with death from coronavirus include general weakness symptoms, NEWS higher than 2, and OC43 subtype.
Keywords: Coronavirus, viral disease, mortality
Introduction
The coronavirus disease 19 (COVID-19) is responsible for pandemic outbreak with high mortality. Worldwide, more than 100,000,000 COVID 19 patients have occurred, resulting in more than 2,000,000 deaths. In Korea, 79,311 COVID 19 patients have occurred, resulting in 1,441 deaths with a mortality rate of 1.82%. (2nd February, 2020) (1). Before COVID-19, severe respiratory diseases from common community human coronaviruses (HCoV) including severe acute respiratory syndrome coronavirus 2 (SARS) and Middle-East respiratory syndrome coronavirus (MERS-CoV) had always been around (2-5). Species 229E, OC43, NL63, and HKU1 are commonly known subtypes of HCoVs (5). These HCoVs are considered relatively benign respiratory pathogens in humans leading to upper respiratory tract diseases (5), occasionally, it has been reported lower respiratory tract infections in immune-compromised patients or severe illnesses required acute care (6-8). Although HCoVs are widespread globally, their frequency and course of varies significantly according to ages, region, or season (2,3,5,8-10). Despite these features of their epidemiology, recent clinical studies (8-12) about coronaviruses have been conducted in children and adolescents; there are limited number of studies investigating patients of all ages (2,8). In addition, few studies about epidemiology about HCoVs have been done since 2018. Therefore, an analysis of the clinical course or prognostic factors of these common HCoV diseases could provide important information about epidemiology of common HCoV and other seasonal common respiratory viruses, and a clue to overcome the current COVID-19 pandemic. We would expand the existing evidence base and provide new insights into HCoV disease by analyzing the epidemiology, clinical aspects, and prognostic factors of patients admitted with coronavirus.
We present the following article/case in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-3190).
Methods
Study design
The study was performed at five university teaching hospitals located in four administrative districts in Korea. Two hospitals were an 820-bed in Anyang-si and a 737-bed in Dongtan-si, Gyeonggi-do. The other two hospitals were 585-bed and 600-bed hospitals in Seoul. The other hospital was a 404-bed in Chuncheon-si, Gangwon-do. We retrospectively analyzed the medical records of patients who were admitted with a laboratory-confirmed coronavirus between 1 January 2018 and 31 March 2020. Routine molecular testing for coV-229E, coV-OC43 and coV-NL63 was conducted using a multiplex real-time reverse transcription-polymerase chain reaction method. We also tested for other respiratory viruses including influenza virus, respiratory syncytial virus (RSV), human adenovirus, human rhinovirus, human metapneumovirus, bocavirus, enterovirus and parainfluenza virus. Samples for bacterial culture obtained from sputum or trans-trachea aspiration in sterile methods, following safety protocols and procedure. The collected samples were aseptically inoculated to pre-prepared sterile MacConkey agar (Oxoid, UK) media, Blood agar, Chocolate agar (Becton-Dickinson, Sparks, MD, USA) and incubated at 37 °C for 24 hours. Matrix-assisted laser desorption ionization-time of flight mass spectrometry with Vitek-MS (BioMerieux, Marcy I'Etoile, France) was used for identification.
All clinical specimens were obtained from the upper respiratory tract using a nasopharynx or throat swab. The authors are accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the institutional review board of Hallym University Kangnam Sacred Heart Hospital (HKS 2020-04-027). Patient information was anonymized and de-identified before analysis; therefore, requirements for informed consent were waived. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Variables
We assessed the demographics of the patients with a coronavirus. Gender, age, body mass index (BMI), comorbidities and pre-hospital care were recorded on admission. We analyzed the clinical data on admission including the diagnosis, symptoms and duration of symptoms. Mortality was defined as the death of a patient during hospitalization. Chronic vascular disease is defined as old cerebral disease and ischemic heart disease. Chronic airway disease is defined as asthma and chronic obstructive pulmonary disease. Chronic kidney disease is defined as having a GFR of less than 50. Chronic liver disease is defined liver cirrhosis and chronic hepatitis B or C. The National Early Warning Score (NEWS) (13) on admission was used to evaluate the risk of coronavirus-infected patients. NEWS was assessed with measurement of seven items including respiratory rate, oxygen saturations, any supplemental oxygen, temperature, systolic blood pressure, mental status, and heart rate. The sum of NEWS is scored from 0 to 20 points. We used NEWS in patients over 18 years old. We also investigated the coronavirus infected patient's laboratory findings, serum mycoplasma antibody IgM and bacterial cultures grown from lower respiratory tract samples. Antibacterial agents and anti-viral agents were defined if a patient was prescribed during hospitalization. Mortality was calculated including death for any reason during hospitalization with coronavirus infection.
Statistics
Frequencies are expressed as numbers (%) and descriptive data are expressed as medians with the interquartile range (IQR). We compared the clinical features, viruses, and bacterial types according to age group. Elder group is patient older than 18 years and younger group is patient younger than 18 years because many more children were infected with a coronavirus. The Chi-square test or Fisher’s exact test were used for categorical variables, and continuous variables were compared using the Mann-Whitney U test. We analyzed the prognostic factors only in patients ≥18 years of age because mortality rate was significant in adults. There were patients who did not measure their weight or height at hospitalization, so the body mass indexes of 164 patients were missing, and the statistics were analyzed by processing the missing value. The univariate analysis of risk factors for mortality was performed by creating a categorized variable based on the median value of clinical and laboratory characteristics in adult patients infected with coronavirus. Factors significantly associated with survival were analyzed further in a Cox proportional hazard model to adjust for the potential confounding effects of each factor. Hazard ratios (HR) with 95% confidence intervals (CI) were used to report the results. The survival duration was defined as the date of follow-up after being discharged from hospital by analyzing medical records. The five variables were included in a Cox’s regression analysis because all were associated with P values <0.05 in a univariate analysis. However, we excluded corona subtype NL63 from the multivariate analysis, because of the influence of choices divided among the subtypes of coronavirus. Cumulative survival curves were derived using the Kaplan-Meier method with reference to corona subtype OC43, pneumonia, and general weakness. Statistical analysis was performed using SPSS software (version 18).
Results
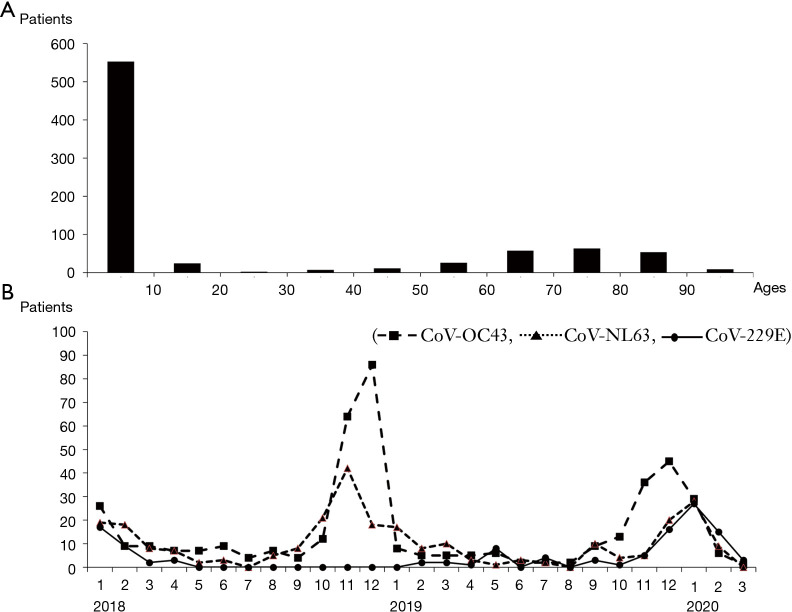
There were 807 coronavirus-infected patients (3.3%) from 24,311 patients with respiratory virus PCR test admitted to five hospitals over 27 months. According to the age group, 553 (69%) patients hospitalized for coronavirus infection were <10 years, followed by 63 (7.8%) patients in the 70s, and 58 (7.2%) patients in the 60s. The more patients were admitted in December 2019 as well as January and February 2020. The HCoVs OC43 and NL63 occurred more frequently during winter but decreased in summer, and 229E was rare in winter 2019 compared to 2018 and 2020 (Figure 1).
Figure 1.
Distribution of human coronavirus (HCoVs) case. (A) Age distributions of HCoVs; (B) monthly prevalence of HCoVs detected among admitted patients between 1 January 2018 and 31 March 2020.
The median BMI of elder group with a coronavirus was 22.1 kg/m2, which was higher than that of younger group. Elder group was diagnosed with pneumonia when hospitalized, and young group was hospitalized for viral infection, bronchitis, or laryngitis. Elder group more often stayed in other hospitals or nursing homes before hospitalization compared with younger group. The main symptom of coronavirus-infected patients was fever in more than 90% of younger group, while 44.4% of elder group had a fever. Symptoms of general weakness and dyspnea were frequently reported in elder group, and coryza was reported in younger group all patients who died were elder group. All-cause mortality rates of patients admitted for seasonal HCoV disease were 3.1% in all patients, and 10.8% in patients aged 18 years or older (Table 1).
Table 1. Clinical characteristics of coronavirus-infected patients.
| Characteristics | All patients (n=807) | Elder group (n=232) | Younger group (n=575) | P value |
|---|---|---|---|---|
| Age, year | 2 (1–55) | 72 (61–81) | 1 (0–3) | – |
| Male | 467 (57.9%) | 130 (56.0%) | 337 (58.6%) | 0.503 |
| Body mass index, kg/m2 | 17.3 (16.0–20.1) | 22.1 (19.7–25.4) | 16.7 (15.7–18.1) | <0.001 |
| Smoking history | ||||
| Never smoked | 768 (95.2%) | 194 (83.6%) | 574 (99.8%) | <0.001 |
| Former smoked | 23 (2.9%) | 22 (9.5%) | 1 (0.2%) | <0.001 |
| Current smoked | 16 (2.1%) | 16 (6.9%) | 0 | <0.001 |
| Diagnosis | ||||
| Viral infection | 109 (13.5%) | 4 (1.7%) | 105 (18.3%) | <0.001 |
| Pneumonia | 315 (39.0%) | 141(60.8%) | 174 (30.3%) | <0.001 |
| Bronchitis | 112 (13.9%) | 5 (2.2%) | 107 (18.6%) | <0.001 |
| Laryngitis or pharyngitis | 78 (9.7%) | 2 (0.9%) | 76 (13.2%) | <0.001 |
| Febrile convulsion | 48 (5.9%) | 1 (0.4%) | 47 (8.2%) | <0.001 |
| Influenza | 25 (3.1%) | 10 (4.3%) | 15 (2.6%) | 0.196 |
| Acute exacerbation of COPD or asthma | 10 (1.2%) | 5 (2.2%) | 5 (0.9%) | 0.13 |
| Kawasaki disease | 8 (1.0%) | 0 | 8 (1.4%) | 0.073 |
| Other | 102 (12.6%) | 63 (27.2%) | 39 (6.8%) | <0.001 |
| Comorbidity | ||||
| Hypertension | 115 (14.3%) | 115 (49.6%) | 0 | <0.001 |
| Diabetes | 57 (7.1%) | 57 (24.6%) | 0 | <0.001 |
| Chronic airway disease | 48 (5.9%) | 44 (19.0%) | 4 (0.7%) | <0.001 |
| Cancer | 46 (5.7%) | 45 (19.4%) | 1 (0.2%) | <0.001 |
| Chronic vascular disease | 42 (5.2%) | 42 (18.1%) | 0 | <0.001 |
| Chronic kidney disease | 31 (3.8%) | 31 (13.4%) | 0 | <0.001 |
| Chronic liver disease | 8 (1%) | 8 (3.4%) | 0 | <0.001 |
| Residence type | ||||
| Home | 760 (94.2%) | 187 (80.7%) | 573 (99.6%) | <0.001 |
| Hospital | 38 (4.7%) | 37 (15.9%) | 1 (0.2%) | <0.001 |
| Health care center | 9 (1.1%) | 8 (3.4%) | 1 (0.2%) | <0.001 |
| Duration of symptoms, days | 3 (1–5) | 3 (1–7) | 2 (1–4) | 0.003 |
| Symptoms | ||||
| Fever | 622 (77.1%) | 103 (44.4%) | 519 (90.3%) | <0.001 |
| General weakness | 47 (5.8%) | 40 (17.2%) | 7 (1.2%) | <0.001 |
| Myalgia | 16 (2.0%) | 13 (5.6%) | 3 (0.5%) | <0.001 |
| Headache | 14 (1.7%) | 9 (3.9%) | 5 (0.9%) | 0.003 |
| Sore throat | 23 (2.9%) | 7 (3.0%) | 16 (2.8%) | 0.856 |
| Coryza | 290 (35.9%) | 13 (5.6%) | 277 (48.2%) | <0.001 |
| Cough | 509 (63.1%) | 101 (43.5%) | 408 (71.0%) | <0.001 |
| Sputum | 297 (37%) | 80 (37.7%) | 217 (37.7%) | 0.385 |
| Dyspnea | 116 (14.4%) | 73 (31.5%) | 43 (7.5%) | <0.001 |
| Hemoptysis | 11 (1.4%) | 11 (4.7%) | 0 | <0.001 |
| Vomiting | 92 (11.4%) | 15 (6.5%) | 77 (13.4%) | 0.005 |
| Diarrhea | 48 (5.9%) | 5 (2.2%) | 43 (7.5%) | 0.004 |
| Seizure | 60 (7.4%) | 4 (1.7%) | 56 (9.7%) | <0.001 |
| Lymph enlargement | 12 (1.5%) | 0 | 12 (2.1%) | 0.027 |
| NEWS | – | 2 (1–4) | – | – |
| Duration of admission, days | 4 (3–6) | 8 (4–15) | 3 (2–4) | <0.001 |
| Antiviral agent | 45 (5.6%) | 24 (10.4%) | 21 (3.6%) | <0.001 |
| Peramivir | 15 (1.9%) | 10 (4.3%) | 5 (0.9%) | |
| Tamiflu | 25 (3.1%) | 11 (4.8%) | 14 (2.4%) | |
| Other | 5 (2.2%) | 3 (1.3%) | 2 (0.3%) | |
| Antibacterial agent | 532 (65.9%) | 207 (89.2%) | 325 (56.5%) | <0.001 |
| ICU | 71 (8.7%) | 71 (30.2%) | 0 | <0.001 |
| Death | 25 (3.1%) | 25 (10.8%) | 0 | <0.001 |
Data are presented as the median value (interquartile range) or number. Elder group is patient ≥18 years and younger group is patient <18 years. COPD, chronic obstructive pulmonary disease; NEWS, National Early Warning Score; NEWS is calculated only in elder group.
The white blood cell, lymphocyte counts were higher in younger group, and C-reactive protein and procalcitonin levels were higher in elder group. The coronavirus subtypes comprised of 51.3% OC43, 33.1% NL63, and 15.4% 229E. Other respiratory virus co-infected cases were 57.4% in patients under the age of 18, more than 19.0% in elder group Influenza was the most common co-infected virus in elder group and respiratory syncytial virus A was the most frequent in younger group. The most common concomitantly infected bacteria were Klebsiella pneumonia and Pseudomonas aeruginosa in elder group and Mycoplasma in younger group (Table 2). The analysis of 25 mortality cases is shown in Table 3.
Table 2. Laboratory, viral, and bacterial findings of coronavirus infected patients.
| Characteristics | All patients (n=807) | Elder group (n=232) | Younger group (n=575) | P value |
|---|---|---|---|---|
| Laboratory finding | ||||
| White cell count, 103/μL | 10.3 (7.4–13.6) | 9.41 (6.74–13.2) | 10.8 (7.80–14.0) | 0.001 |
| Lymphocyte count, 103/μL | 2.99 (1.43–5.72) | 1.44 (0.88–3.37) | 3.50 (2.03–5.93) | <0.001 |
| Platelet count, 103/μL | 296 (229–377) | 245 (169–307) | 314 (246–395) | <0.001 |
| Hemoglobin, g/dL | 12.2 (11.3–13.0) | 12.0 (10.4–13.6) | 12.2 (11.6–12.8) | 0.207 |
| C-reactive protein, mg/dL | 16.4 (4.10–52.3) | 74.1 (20.9–149.2) | 10.5 (3.0–26.5) | <0.001 |
| Coronavirus subtype | ||||
| OC43 | 416 (51.5%) | 92 (39.7%) | 324 (56.3%) | <0.001 |
| NL63 | 267 (33.1%) | 61 (26.3%) | 206 (35.8%) | 0.009 |
| 229E | 124 (15.4%) | 79 (34.1%) | 45 (7.8%) | <0.001 |
| Co-infecting viruses | 374 (46.3%) | 44 (19.0%) | 330 (57.4%) | <0.001 |
| Influenza | 51 (6.3%) | 26 (11.2%) | 25 (4.3%) | |
| RSV A | 102 (12.6%) | 5 (2.2%) | 97 (16.8%) | |
| Rhinovirus | 100 (12.4%) | 6 (2.6%) | 94 (16.3%) | |
| Adeno virus | 86 (10.7%) | 8 (3.5%) | 78 (13.6%) | |
| RSV B | 39 (4.8%) | 4 (1.7%) | 35 (6.1%) | |
| Boca virus | 28 (3.5%) | 1 (0.4%) | 27 (4.7%) | |
| Enterovirus | 23 (2.9%) | 0 | 23 (4.0%) | |
| Parainfluenza virus | 23 (2.9%) | 0 | 23 (4.0%) | |
| Metapneumovirus | 14 (1.7%) | 3 (1.3%) | 11 (1.9%) | |
| Others | 4 (0.5%) | 0 | 4 (0.7%) | |
| Co-infecting bacteria | 65 (8.1%) | 40 (17.2%) | 25 (4.3%) | <0.001 |
| Mycoplasma | 21 (2.6%) | 1 (0.04%) | 20 (3.5%) | |
| Klebsiella pneumonia | 15 (1.9%) | 10 (4.3%) | 5 (0.9%) | |
| Pseudomonas aeruginosa | 9 (1.1%) | 9 (3.8%) | 0 | |
| Haemophilus influenza | 6 (0.74%) | 6 (2.6%) | 0 | |
| Staphylococcus aureus | 4 (0.5%) | 4 (1.7%) | 0 | |
| Others | 10 (1.2%) | 10 (4.3%) | 0 |
Data are presented as the median value (interquartile range) or number. Elder group is patient ≥18 years and younger group is patient <18 years. RSV, respiratory syncytial virus.
Table 3. Characteristics of 25 mortality cases.
| Age | Sex | Diagnosis | Other hospital or healthcare center | Symptom | Co-morbidity | NEWS | HCoV subtype | Combined pathogen | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| 51 | Female | Pneumonia | Yes | Fever | 3 | OC43 | Pseudomonas aeruginosa | Pneumonia | |
| 53 | Male | Hepatic failure | No | Fever, general weakness | Diabetes, pancreatic cancer | 2 | OC43 | Pancreatic cancer | |
| 55 | Female | Cerebral infarction | No | Decreased mentality | 2 | 229E | Cerebral infarction | ||
| 60 | Male | Pneumonia | Yes | Fever, dyspnea | Hypertension | 2 | OC43 | Acinetobacter baumannii | Pneumonia |
| 65 | Male | Pneumonia | No | Cough, sputum, dyspnea | COPD, diabetes | 13 | OC43 | Streptococcus agalactiae | Pneumonia |
| 68 | Male | Pharyngitis | No | General weakness | Lung cancer | 8 | OC43 | Pneumonia | |
| 69 | Male | Pneumonia | No | General weakness, cough, sputum, dyspnea | Diabetes, cancer | 4 | OC43 | Rhinovirus; E. coli | Pneumonia |
| 69 | Male | Pneumonia | No | No | Lung cancer | 10 | OC43 | Lung cancer | |
| 73 | Female | Pneumonia | No | Dyspnea | Hypertension | 4 | 229E | Pneumonia | |
| 75 | Female | Pneumonia | No | Vomiting | 1 | 229E | Pneumonia | ||
| 77 | Female | Pneumonia | Yes | Fever, general weakness | Old CI | 2 | OC43 | Pneumonia | |
| 77 | Male | Acute respiratory lung disease | No | Dyspnea | 3 | OC43 | Pneumonia | ||
| 77 | Male | Pneumonia | No | General weakness | Diabetes, hypertension, old CI, chronic kidney disease | 7 | OC43 | Brain hemorrhage | |
| 78 | Male | Pneumonia | No | General weakness | Diabetes, hypertension | 1 | 229E | Pneumonia | |
| 79 | Female | Pneumonia | No | Fever, vomiting | Hypertension, lymphoma | 2 | OC43 | Pneumonia | |
| 79 | Female | Pneumonia | Yes | Fever, myalgia | Hypertension, old CI | 6 | OC43 | Influenza, Klebsiella pneumoniae | Pneumonia |
| 79 | Male | Pneumonia | Yes | Fever, general weakness | Old CI | 8 | NL63 | Adeno virus | Pneumonia |
| 80 | Female | Pneumonia | No | General weakness | Hypertension, chronic kidney disease | 16 | OC43 | Influenza | Pneumonia |
| 81 | Female | Pneumonia | No | Fever, cough | 8 | 229E | Pneumonia | ||
| 82 | Male | Pneumonia | No | Coryza, cough, sputum, dyspnea | Hypertension, heart failure | 2 | OC43 | Respiratory syncytial virus A | Pneumonia |
| 82 | Female | Heart failure | No | Dyspnea | 4 | OC43 | Pneumonia | ||
| 83 | Female | Pneumonia | Yes | Fever | Multiple myeloma | 11 | OC43 | Influenza | Pneumonia |
| 86 | Female | Pneumonia | No | Cough, sputum, dyspnea | Diabetes, hypertension | 3 | 229E | Pneumonia | |
| 91 | Male | Pneumonia | Yes | General weakness, cough, dyspnea | Asthma, diabetes, hypertension | 2 | 229E | Pneumonia | |
| 91 | Female | Pneumonia | No | General weakness | 2 | Influenza | Pneumonia |
CI, cerebral infarction.
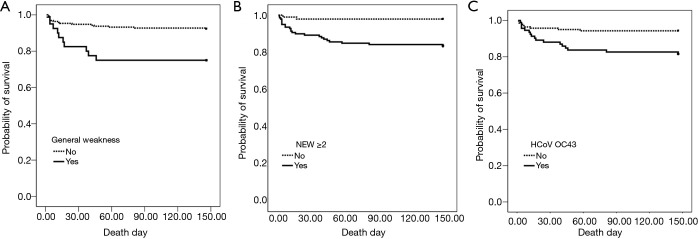
In the univariate analysis of the risk of mortality for patients admitted for coronavirus, it was higher in those with the pneumonia, general weakness, the 2 points or more on the NEWS, CRP ≥74 mg/dL, or the OC43 corona subtype. After adjusting for other clinical variables, general weakness symptoms [hazard ratio (HR), 2.651; 95% confidence interval (CI), 1.147–6.125, P=0.023], NEWS ≥2 (HR, 5.485; 95% CI, 1.261–23.858, P=0.023), and coronavirus subtype OC43 (HR, 2.500; 95% CI, 1.060–5.897, P=0.036) (Table 4). Figure 2 shows the survival curve.
Table 4. Prognostic factors for coronavirus infected patients in elder group.
| Characteristics | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | CI | P value | HR | CI | P value | ||
| Age ≥72 years | 2.26 | 0.974–5.244 | 0.058 | ||||
| Male | 1.579 | 0.719–3.464 | 0.255 | ||||
| Body mass index ≤22.1 kg/m2 | 1.289 | 0.569–2.920 | 0.543 | ||||
| Smoker | 1.075 | 0.369–3.134 | 0.894 | ||||
| Pneumonia | 2.936 | 1.101–7.832 | 0.031 | 1.881 | 0.683–5.179 | 0.221 | |
| Comorbidity | |||||||
| Diabetes | 1.139 | 0.476–2.727 | 0.77 | ||||
| Chronic airway disease | 2.959 | 0.697–12.559 | 0.141 | ||||
| Cancer | 1.787 | 0.771–4.141 | 0.176 | ||||
| Chronic kidney disease | 2 | 0.471–8.490 | 0.347 | ||||
| Hospital and health care center | 2.86 | 1.225–6.676 | 0.015 | 1.858 | 0.769–4.490 | 0.169 | |
| Symptoms | |||||||
| Fever | 1.027 | 0.466–2.261 | 0.948 | ||||
| General weakness | 3.586 | 1.610–7.990 | 0.002 | 2.651 | 1.147–6.125 | 0.023 | |
| Cough | 2.472 | 0.987–6.191 | 0.053 | ||||
| Sputum | 2.83 | 0.971–8.244 | 0.057 | ||||
| Dyspnea | 1.76 | 0.799–3.877 | 0.161 | ||||
| NEWS ≥2 | 8.741 | 2.060–37.086 | 0.003 | 5.485 | 1.261–23.858 | 0.023 | |
| Antiviral agent | 2.127 | 0.798–5.667 | 0.131 | ||||
| Antibacterial agent | 2.834 | 0.383–20.951 | 0.308 | ||||
| Laboratory finding | |||||||
| White cell count ≥9.4, 103/μL | 1.881 | 0.831–4.258 | 0.129 | ||||
| Lymphocyte count ≥1.44, 103/μL | 1.345 | 0.611–2.865 | 0.462 | ||||
| Platelet count ≤245, 103/μL | 2.055 | 0.887–4.762 | 0.093 | ||||
| Hemoglobin ≤12.0, g/dL | 2.11 | 0.910–4.893 | 0.082 | ||||
| C-reactive protein ≥74, mg/dL | 3.929 | 1.566–9.857 | 0.004 | 1.918 | 0.717–5.135 | 0.195 | |
| Coronavirus subtype | |||||||
| OC43 | 3.452 | 1.488–8.005 | 0.004 | 2.500 | 1.060–5.897 | 0.036 | |
| NL63 | 0.108 | 0.015–0.797 | 0.029 | ||||
| 229E | 0.755 | 0.315–1.808 | 0.528 | ||||
| Co-infecting influenza | 0.046 | 0.000–197.997 | 0.261 | ||||
| Co-infecting bacteria | |||||||
| Klebsiella pneumonia | 1.013 | 0.137–7.490 | 0.99 | ||||
| Pseudomonas aeruginosa | 0.961 | 0.130–7.103 | 0.969 | ||||
HR, hazard ratio; CI, confidence interval.
Figure 2.
Kaplan-Meier survival curves showing the effects of (A) general weakness, (B) NEWS ≥2, (C) HCoV OC43 of 232 hospitalized patients with coronavirus infection.
Discussion
All-cause mortality rates of patients admitted for seasonal HCoV disease were 3.1% in all patients, and 10.8% in adults. This high mortality rate contrasts with the fact that coronavirus is typically a benign infection that causes a mild upper respiratory infection (5). The mortality rate in this study was lower than the global mortality rate of 4.1% and higher than the mortality rate of South Korea (2.1%) due to COVID-19 as of February 2021. The reason for the higher mortality in our study might be that we analyzed patients requiring hospitalization, while the COVID-19 mortality rate was calculated including asymptomatic patients. In the study about HCoV respiratory tract infections in adults admitted to acute care (8), all-cause mortality was high at 7%, which was similar to adult mortality rate in our study. The one-year mortality rate was 25.8% in patents ≥60 years who were hospitalized for RSV infection (14). The mortality was 13.3% in patients over 65 years of age and 26.8% in intensive care unit in our study. In our study, the mortality rate was high in the elderly. The reasons for the high mortality rate in older patients with viral infection can be thought of many comorbid diseases, poor immunity, or a high probability of pneumonia (15). The world’s population is aging due to declines in both mortality and birth rates (16,17). Therefore, such an increase in the number of elderly patients will lead to a higher mortality rate from respiratory viral diseases in the future.
In our study, the majority of patients (71%) with coronavirus were under 18 years old. There were 45% of coronavirus cases under 18 years old in US (2) and 80% under 15 years old in Zambia (18). It can be seen that coronavirus infection occurs frequently at younger age.
The outbreaks for each subtype of coronavirus (Figure 1B), generally started in October and were prevalent from late autumn to winter until January and January the following year. In a US study dealing with the outbreak of coronavirus from 2014 to 2017, the outbreak was around January every year, and in the Zambia study, which saw an outbreak from December 2018 to December 2019, the outbreak was from September to November 2018 (2,18). It can be seen that coronavirus occurs a lot from late autumn to early spring.
The patterns of corona infection in adults and children are very different. More than 60% of adult coronavirus patients were hospitalized with pneumonia, but about 30% of patients <18 years had pneumonia. These data show that coronavirus infection more often progresses to a lower respiratory tract infection in adults. Fever occurs in 90% of cases in younger age, but in only 44% of adult. In adults, particularly older adults, basal temperature is low and the inability to control temperature makes the febrile reaction weak, so there is often no fever from the infection (15). In addition, symptoms of upper respiratory infection including coryza, sore throat were more common in coronavirus patients in younger age, whereas general weakness or shortness of breath often occurred in adults. This is related to the observation that low respiratory infection or systemic disease such as pneumonia was more common in adults.
Like other studies of coronavirus-infected patients (2,19), the OC43 type was the most prevalent among the coronavirus subtypes, and 229E was less frequent, but adult coronavirus patients were more infected with 229E in our study. Other studies (5,10) have reported that coronaviruses are often co-infected with other respiratory viruses. The presence of co-infection, particularly RSV, is associated with an increased likelihood of low respiratory tract infection in multivariate models (20). However, in our study, several respiratory viruses were co-infected in younger patients with coronavirus, whereas co-infection with respiratory viruses in adult except influenza was very low. One study from South Africa detected a co-infecting respiratory virus in 49% of RSV cases and 32% of influenza cases (21). Another study of respiratory pathogen diversity and co-infections in rural Zambia (18) showed that respiratory viral co-infection was less in adults than in children, but it was not related to the difference with clinical severity.
In our study, coronavirus-infected patients with general weakness, higher NEWS, and HCoV OC43 had a poorer prognosis associated with mortality. Patients with severe COVID19 disease had more prominent laboratory abnormalities including lymphocytopenia and leukopenia than those with less severe disease (22). However, sex, age, and lymphocytopenia were not associated with mortality of coronavirus-infected patients in our study. Recent studies have shown that frailty and complaints general weakness, exhaustion, and low physical activity in elderly patients are related to the onset and prognosis of critical illness (23-25). NEWS has been reported to predict prognosis well in COVID 19 patients (26). Patients with OC43 had two-fold odds of requiring O2 or intubation than those with other strains, while no difference in mortality or ICU admission was observed in a study of coronavirus-infected patients receiving acute care (8). In our study, mortality was higher in patients infected with HCoV OC43 compared to the other subtypes. The reason for HCoV OC43 has more virulent prognosis is still unclear and requires further study.
Our study had several limitations. We only included patients who were admitted to the hospital, thus representing a subset of the more critically ill patients with HCoV infection in the community. However, a study of hospitalized patients provides important information for prognosis and treatment, including the death of patients from severe disease. Another limitation of the study is that viral diseases have different local distributions and characteristics, which limits the generalization of our findings to other institutions. Data were collected and analyzed from five hospitals in administratively different regions. Another limitation of the study is that the subjective symptoms may have been underestimated because children under the age of 1. However, since mortality analysis was only conducted over the age of 18, symptoms could be analyzed for prognostic factor.
Our study analyzed the clinical features and prognosis of patients admitted with coronavirus. In our study, the mortality rate of all admitted patients with coronavirus was 3.1% and 10.8% in adults. In an aging society, mortalities from various respiratory viruses including coronavirus could be inevitably increased. Therefore, it will be further needed studies on the clinical features, prognosis, and risk factors of respiratory viruses, which were known to cause upper respiratory tract infections with benign course in the past. Additionally, patients admitted by coronavirus should be treated carefully if they have general weakness symptoms, NEWS higher than 2, or the OC43 subtype.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of Hallym University Kangnam Sacred Heart Hospital (HKS 2020-04-027). Patient information was anonymized and de-identified before analysis; therefore, requirements for informed consent were waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-3190
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-3190
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3190). KSJ serves as an unpaid editorial board member of Journal of Thoracic Disease. The other authors have no conflicts of interest to declare.
References
- 1.Hedquaters CDM. Coronavirus Disease-19, Republic of Korea. Available online: http://ncov.mohw.go.kr/
- 2.Killerby ME, Biggs HM, Haynes A, et al. Human coronavirus circulation in the United States 2014-2017. J Clin Virol 2018;101:52-6. 10.1016/j.jcv.2018.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 2018;23:130-7. 10.1111/resp.13196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Costa VG, Moreli ML, Saivish MV. The emergence of SARS, MERS and novel SARS-2 coronaviruses in the 21st century. Arch Virol 2020;165:1517-26. 10.1007/s00705-020-04628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogimi C, Kim YJ, Martin ET, et al. What's New With the Old Coronaviruses? J Pediatric Infect Dis Soc 2020;9:210-7. 10.1093/jpids/piaa037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogimi C, Greninger AL, Waghmare AA, et al. Prolonged Shedding of Human Coronavirus in Hematopoietic Cell Transplant Recipients: Risk Factors and Viral Genome Evolution. J Infect Dis 2017;216:203-9. 10.1093/infdis/jix264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogimi C, Waghmare AA, Kuypers JM, et al. Clinical Significance of Human Coronavirus in Bronchoalveolar Lavage Samples From Hematopoietic Cell Transplant Recipients and Patients With Hematologic Malignancies. Clin Infect Dis 2017;64:1532-9. 10.1093/cid/cix160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozak R, Prost K, Yip L, et al. Severity of coronavirus respiratory tract infections in adults admitted to acute care in Toronto, Ontario. J Clin Virol 2020;126:104338. 10.1016/j.jcv.2020.104338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varghese L, Zachariah P, Vargas C, et al. Epidemiology and Clinical Features of Human Coronaviruses in the Pediatric Population. J Pediatric Infect Dis Soc 2018;7:151-8. 10.1093/jpids/pix027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng ZQ, Chen DH, Tan WP, et al. Epidemiology and clinical characteristics of human coronaviruses OC43, 229E, NL63, and HKU1: a study of hospitalized children with acute respiratory tract infection in Guangzhou, China. Eur J Clin Microbiol Infect Dis 2018;37:363-9. 10.1007/s10096-017-3144-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talbot HK, Shepherd BE, Crowe JE, Jr, et al. The pediatric burden of human coronaviruses evaluated for twenty years. Pediatr Infect Dis J 2009;28:682-7. 10.1097/INF.0b013e31819d0d27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jean A, Quach C, Yung A, et al. Severity and outcome associated with human coronavirus OC43 infections among children. Pediatr Infect Dis J 2013;32:325-9. 10.1097/INF.0b013e3182812787 [DOI] [PubMed] [Google Scholar]
- 13.Eckart A, Hauser SI, Kutz A, et al. Combination of the National Early Warning Score (NEWS) and inflammatory biomarkers for early risk stratification in emergency department patients: results of a multinational, observational study. BMJ Open 2019;9:e024636. 10.1136/bmjopen-2018-024636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ackerson B, Tseng HF, Sy LS, et al. Severe Morbidity and Mortality Associated With Respiratory Syncytial Virus Versus Influenza Infection in Hospitalized Older Adults. Clin Infect Dis 2019;69:197-203. 10.1093/cid/ciy991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fung HB, Monteagudo-Chu MO. Community-acquired pneumonia in the elderly. Am J Geriatr Pharmacother 2010;8:47-62. 10.1016/j.amjopharm.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 16.Bagshaw SM, Webb SA, Delaney A, et al. Very old patients admitted to intensive care in Australia and New Zealand: a multi-centre cohort analysis. Crit Care 2009;13:R45. 10.1186/cc7768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler RA, Adhikari NK, Bhagwanjee S. Clinical review: critical care in the global context--disparities in burden of illness, access, and economics. Crit Care 2008;12:225. 10.1186/cc6984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loevinsohn G, Hardick J, Sinywimaanzi P, et al. Respiratory pathogen diversity and co-infections in rural Zambia. Int J Infect Dis 2021;102:291-8. 10.1016/j.ijid.2020.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuypers J, Martin ET, Heugel J, et al. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics 2007;119:e70-6. 10.1542/peds.2006-1406 [DOI] [PubMed] [Google Scholar]
- 20.Gerna G, Campanini G, Rovida F, et al. Genetic variability of human coronavirus OC43-, 229E-, and NL63-like strains and their association with lower respiratory tract infections of hospitalized infants and immunocompromised patients. J Med Virol 2006;78:938-49. 10.1002/jmv.20645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pretorius MA, Madhi SA, Cohen C, et al. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness--South Africa, 2009-2010. J Infect Dis 2012;206 Suppl 1:S159-65. 10.1093/infdis/jis538 [DOI] [PubMed] [Google Scholar]
- 22.Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708-20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brummel NE, Bell SP, Girard TD, et al. Frailty and Subsequent Disability and Mortality among Patients with Critical Illness. Am J Respir Crit Care Med 2017;196:64-72. 10.1164/rccm.201605-0939OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chong E, Ho E, Baldevarona-Llego J, et al. Frailty and Risk of Adverse Outcomes in Hospitalized Older Adults: A Comparison of Different Frailty Measures. J Am Med Dir Assoc 2017;18:638 e7-e11. [DOI] [PubMed]
- 25.Mahalingam M, Moore JX, Donnelly JP, et al. Frailty Syndrome and Risk of Sepsis in the REasons for Geographic And Racial Differences in Stroke (REGARDS) Cohort. J Intensive Care Med 2019;34:292-300. 10.1177/0885066617715251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu H, Yao N, Qiu Y. Predictive Value of 5 Early Warning Scores for Critical COVID-19 Patients. Disaster Med Public Health Prep 2020. [Epub ahead of print]. doi: . 10.1017/dmp.2020.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as