Abstract
Background
Microvascular invasion (MVI) is an independent risk factor associated with tumor recurrence and poor survival in patients with intrahepatic cholangiocarcinoma (ICC) after partial hepatectomy (PH). The potential impact of adjuvant TACE on the prognosis of patients with ICC involving MVI (ICC-MVI) remains uncertain. Our aim was to investigate the effectiveness of postoperative adjuvant transarterial chemoembolization (TACE) on ICC involving MVI.
Methods
Multicentric data consisted of 223 patients who underwent curative-intent PH for ICC-MVI from 2002–2015 were retrospectively analyzed. The impact of adjuvant TACE was evaluated using inverse probability of treatment weighting (IPTW) and propensity-score matched (PSM) analyses.
Results
No association between the TACE and the overall survival (OS) and recurrence rates was observed among the overall ICC-MVI patients. However, subgroup analyses revealed that adjuvant TACE favored OS (HR, 0.62; 95% CI, 0.39–0.99; P=0.047) and time to recurrence (TTR) (HR, 0.59; 95% CI, 0.36–0.97; P=0.037) among patients with elevated CA19-9 and those without lymphadenectomy (HR, 0.53; 95% CI, 0.30–0.93; P=0.027 for OS, and HR, 0.49; 95% CI, 0.28–0.87; P=0.015 for TTR, respectively). In the CA19-9 ≥39 U/L subgroup and Nx subgroup, adjuvant TACE was associated with higher 1-, 3-, and 5-year OS rates (P=0.033 and P=0.034, respectively) and lower corresponding recurrence rates (P=0.024 and P=0.023, respectively).
Conclusions
Among the ICC-MVI patients undergoing curative-intent PH, only those have elevated CA19-9 or who did not undergo lymphadenectomy might be suitable for adjuvant TACE.
Keywords: Intrahepatic cholangiocarcinoma (ICC), microvascular invasion (MVI), adjuvant therapy, TACE, partial hepatectomy (PH)
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common liver cancer after hepatocellular carcinoma (HCC) with an increasing incidence worldwide (1). Partial hepatectomy (PH) is currently the only well-established treatment aiming at cure for ICC. However, the long-term prognosis after PH for ICC is dismal due to high incidence of tumor recurrence (2). Efforts to investigate effective postoperative adjuvant therapy may improve the long-term survival outcomes in patients with ICC.
Adjuvant transarterial chemoembolization (TACE) has been demonstrated to be effective among patients undergoing curative-intent PH for HCC with aggressive histopathological features observed in previous studies (3-7). However, no universally accepted adjuvant treatment exists for surgically resected ICC. Although TACE is frequently used in patients with unresectable ICC (8-10), studies on adjuvant TACE among ICC patients undergoing PH are nevertheless limited. Previous studies reported that adjuvant TACE could prolong the overall survival (OS) of ICC patients with early recurrence (11) or advanced ICC (12). Recently, Jeong et al. showed that adjuvant TACE effectively improves prognosis of ICC related to hepatitis B virus (HBV)- with arterial phase enhancement in CT scans (13). Another study suggested that adjuvant TACE may be indicated for ICC patients with elevated serum gamma-glutamyl transferase (GGT) levels (14). These studies indicate that adjuvant TACE may improve the long-term outcomes in certain ICC patients.
Microvascular invasion (MVI) is a well-defined risk factor associated with tumor recurrence and poor OS in patients with ICC after PH (15-18). It is currently unclear whether adjuvant TACE improves the long-term prognosis in patients with ICC involving MVI (ICC-MVI) after PH is unclear. Our previous study revealed that adjuvant TACE following PH might be suitable for ICC patients with high ICC nomogram scores (≥77) (19). In this study, we used a database from a multicentric cohort of patients with ICC-MVI to evaluate the causal effects of adjuvant TACE on ICC-MVI after PH, using propensity score analyses to emulate a randomized controlled trial. We present the following article in accordance with the STROBE reporting checklist (Available at http://dx.doi.org/10.21037/jgo-20-443).
Methods
Study population
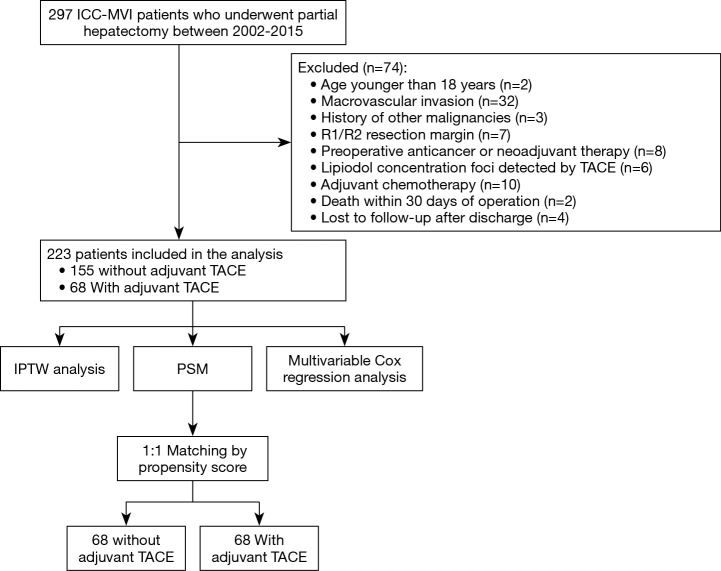
All patients with ICC-MVI undergoing curative-intent PH at the Eastern Hepatobiliary Surgery Hospital (Second Military Medical University, Shanghai), the Zhongda Hospital (Southeast University, Nanjing), and the Qin Huai Medical District of Eastern Theater General Hospital (Bayi Hospital, Nanjing) between December 2002 and November 2015 were considered to be enrolled in this retrospectively study. All of the enrolled patients were confirmed by histopathological findings. The inclusion criteria included patients with: (I) curative-intent PH for ICC; and (II) age older than 18 years. The exclusion criteria included patients with: (I) macrovascular invasion; (II) a history of other malignancies; (III) R1 and R2 resections; (IV) preoperative anticancer or neoadjuvant therapy; (V) lipiodol concentration foci detected by TACE; (VI) other postoperative adjuvant therapy (adjuvant chemotherapy, etc.); (VII) death within 30 days of operation and (VIII) loss to follow-up. The study flow chart is shown in Figure 1.
Figure 1.
Flow chart of the study.
Clinicopathological factors and definitions
The patients’ clinicopathological data of the included patients were retrospectively collected. An R0 resection was defined as complete removal of nodules with a negative surgical margin confirmed by microscopic histological examination (20). The definition of MVI was microscopic tumor invasion in a portal vein, hepatic vein or large capsular vessel of the surrounding hepatic tissue contiguous to the tumor (21). All of the patients were restaged according to the AJCC TNM staging manual 8th edition (22). Tumor recurrence was defined as imaging findings or biopsy-proven tumor, and OS was defined as the interval between the date of PH and the date of patient death or the last follow-up. Time to recurrence (TTR) was the interval between the date of PH and the date of diagnosis of recurrence.
Postoperative adjuvant TACE
Adjuvant TACE was carried out within 2 months after PH using the Seldinger technique. Briefly, microcatheter was inserted into an appropriate hepatic artery through femoral artery, and iodized oil emulsion (3–5 mL), 5-fluorouracil (500 mg), hydroxycamptothecin (10 mg), and epirubicin (20 mg) were injected into the liver through the catheter.
Follow-up
Patients were followed-up once every 2 months in the first 2 years after surgery and then once every 3–6 months thereafter. In each follow-up visit, serum tumor markers that included carbohydrate antigen (CA) 19-9, carcinoembryonic antigen (CEA), and alpha-fetal protein (AFP); liver function; chest radiography; and abdominal ultrasound were routinely conducted. CT/MRI was performed every 6 months or earlier if clinically indicated. Once tumor recurrence was diagnosed, patients were treated with repeat resection, percutaneous ablation, TACE, radiotherapy, or supportive care.
Statistics analysis
Continuous variables expressed as median (range) or means ± standard deviations (SDs) were compared using Mann-Whitney or t test between the adjuvant TACE and non-TACE groups, whereas categorical variables were reported as numbers and percentages and compared using chi-square or Fisher test. The OS and recurrence rates were estimated using Kaplan-Meier and compared by log-rank test. Univariate and multivariate analyses of the entire data were performed using the Cox proportional hazards regression model for recurrence and OS. Propensity scores were calculated by a multivariable logistic regression model, with adjuvant TACE status regressed on age (continuous variable), sex (female/male), HBsAg (negative/positive), cirrhosis (no/yes), CEA level (<5/≥5 µg/L), CA 19-9 level (<39/≥39 U/mL), tumor size (≤5/>5 cm), tumor number (single/multiple), differentiation (well-moderate/poor), lymph node status (N0/Nx/N1), visceral peritoneum invasion (no/yes), and direct invasion of adjacent organs (no/yes). Nearest neighbor with a caliper of default width of the SD of the logit of the propensity score was used (23). Standardized mean difference (SMD) was used to compare balance among covariates, with a value of ≤0.10 indicating good balance (24). Inverse probability of treatment weighting (IPTW) based on the propensity score was used to construct a weighted cohort of patients who had similar baseline characteristics. To reduce the variability in the IPTW models, we used stabilized weights (25). A 1:1 propensity-score matched (PSM) analysis was also conducted. To assess the robustness of our results, a robust sandwich estimator was also used. In the subgroup analysis, Cox regression model was utilized to calculate the hazard ratios (HR) and 95% CI.
Statistical analyses were conducted using R software (version 3.5.1, R Foundation) and SAS (version 9.4; SAS Institute); PSM analyses were conducted using the MatchIt package (version 3.0.2). A two-tailed P value of less than 0.05 was considered statistically significant.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committees of each of the involved hospitals (NO. EHBHKY2020-K-027, 2020ZDSYLL010-P01, and 81YY-KYLL-19-05). Informed consent was routinely obtained before surgery.
Results
Baseline characteristics before and after IPTW
A total of 223 patients with ICC-MVI was enrolled in the study after 74 were excluded, among them 68 (30.5%) patients received adjuvant TACE (Figure 1). Table 1 shows the SMD of the baseline covariates before and after IPTW. Before IPTW, 60.0% (93/155) of patients in the non-TACE group and 44.1% (30/68) of patients in the adjuvant TACE group had elevated preoperative serum CA19-9 level (P=0.040); direct invasion of adjacent organ was more frequent in the non-TACE group (9.7% vs. 1.5%, P=0.044). Moreover, 33.5% (52/155) of patients in the non-TACE group and 52.9% (36/68) patients in the adjuvant TACE group did not undergo lymphadenectomy. Among the patients who underwent lymphadenectomy, lymph node metastasis rates were 48.5% (50/103) in the adjuvant TACE group and 31.2 (10/32) in the non-TACE group (P=0.007). In addition, 9.7% (15/155) patients in the non-TACE group and 1.5% (1/68) of patients in the adjuvant TACE group were at T4 stage (P=0.035).
Table 1. Clinicopathological characteristics of ICC patients with MVI before and after IPTW analysis.
| Variable | Unweighted population | IPTW weighted population | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=223) | Non-TACE (n=155) | Adjuvant TACE (n=68) | P value | SMD | Non-TACE (n=155) | Adjuvant TACE (n=68) | P value | SMD | ||
| Age in years | 51.4±10.6 | 51.7±10.6 | 50.7±10.7 | 0.506 | 0.097 | 51.3±10.5 | 52.2±12.3 | 0.698 | 0.077 | |
| Gender, n (%) | 0.092 | 0.282 | 0.892 | 0.024 | ||||||
| Female | 54 (24.2) | 43 (27.7) | 11 (16.2) | 37.6 (24.3) | 15.9 (23.2) | |||||
| Male | 169 (75.8) | 112 (72.3) | 57 (83.8) | 117.4 (75.7) | 52.4 (76.8) | |||||
| HBsAg, n (%) | 0.120 | 0.250 | 0.885 | 0.025 | ||||||
| Negative | 111 (49.8) | 83 (53.5) | 28 (41.2) | 78.1 (50.4) | 35.2 (51.6) | |||||
| Positive | 112 (50.2) | 72 (46.5) | 40 (58.8) | 76.9 (49.6) | 33.0 (48.4) | |||||
| Anti-HCV, n (%) | 0.092 | 0.246 | 0.054 | 0.266 | ||||||
| Negative | 221 (99.1) | 155 (100.0) | 66 (97.1) | 155.0 (100.0) | 65.9 (96.6) | |||||
| Positive | 2 (0.9) | 0 (0.0) | 2 (2.9) | 0.0 (0.0) | 2.3 (3.4) | |||||
| Cirrhosis, n (%) | 0.136 | 0.237 | 0.612 | 0.076 | ||||||
| No | 161 (72.2) | 117 (75.5) | 44 (64.7) | 112.8 (72.8) | 51.9 (76.1) | |||||
| Yes | 62 (27.8) | 38 (24.5) | 24 (35.3) | 42.2 (27.2) | 16.3 (23.9) | |||||
| Child-Pugh class, n (%) | 0.237 | 0.215 | 0.939 | 0.018 | ||||||
| A | 209 (93.7) | 143 (92.3) | 66 (97.1) | 144.9 (93.5) | 64.1 (93.9) | |||||
| B | 14 (6.3) | 12 (7.7) | 2 (2.9) | 10.1 (6.5) | 4.1 (6.1) | |||||
| CEA (ug/L), n (%) | 0.996 | 0.024 | 0.726 | 0.058 | ||||||
| <5 | 159 (71.3) | 110 (71.0) | 49 (72.1) | 111.4 (71.9) | 50.8 (74.5) | |||||
| ≥5 | 64 (28.7) | 45 (29.0) | 19 (27.9) | 43.5 (28.1) | 17.4 (25.5) | |||||
| CA 19-9 (U/mL), n (%) | 0.040 | 0.322 | 0.91 | 0.019 | ||||||
| <39 | 100 (44.8) | 62 (40.0) | 38 (55.9) | 70.3 (45.3) | 31.6 (46.3) | |||||
| ≥39 | 123 (55.2) | 93 (60.0) | 30 (44.1) | 84.7 (54.7) | 36.6 (53.7) | |||||
| Gross type of tumor, n (%) | 0.106 | 0.255 | 0.811 | 0.043 | ||||||
| MF | 180 (80.7) | 130 (83.9) | 50 (73.5) | 124.9 (80.6) | 53.8 (78.9) | |||||
| PI | 43 (19.3) | 25 (16.1) | 18 (26.5) | 30.1 (19.4) | 14.4 (21.1) | |||||
| Tumor diameter (cm), n (%) | 0.412 | 0.143 | 0.895 | 0.024 | ||||||
| ≤5 | 76 (34.1) | 56 (36.1) | 20 (29.4) | 52.9 (34.1) | 24.1 (35.3) | |||||
| >5 | 147 (65.9) | 99 (63.9) | 48 (70.6) | 102.1 (65.9) | 44.2 (64.7) | |||||
| Tumor number, n (%) | 0.416 | 0.141 | 0.662 | 0.078 | ||||||
| Solitary | 167 (74.9) | 119 (76.8) | 48 (70.6) | 116.4 (75.1) | 48.9 (71.7) | |||||
| Multiple | 56 (25.1) | 36 (23.2) | 20 (29.4) | 38.6 (24.9) | 19.3 (28.3) | |||||
| Differentiation, n (%) | 0.477 | 0.134 | 0.926 | 0.015 | ||||||
| Well/Moderate | 200 (89.7) | 141 (91.0) | 59 (86.8) | 136.6 (88.1) | 60.4 (88.6) | |||||
| Poor | 23 (10.3) | 14 (9.0) | 9 (13.2) | 18.4 (11.9) | 7.8 (11.4) | |||||
| Visceral peritoneum invasion, n (%) | 0.438 | 0.100 | 0.823 | 0.032 | ||||||
| No | 216 (96.9) | 151 (97.4) | 65 (95.6) | 149.8 (96.6) | 66.3 (97.2) | |||||
| Yes | 7 (3.1) | 4 (2.6) | 3 (4.4) | 5.2 (3.4) | 1.9 (2.8) | |||||
| Direct invasion, n (%) | 0.044 | 0.364 | 0.833 | 0.055 | ||||||
| No | 207 (92.8) | 140 (90.3) | 67 (98.5) | 143.8 (92.8) | 64.2 (94.1) | |||||
| Yes | 16 (7.2) | 15 (9.7) | 1 (1.5) | 11.2 (7.2) | 4.0 (5.9) | |||||
| Regional nodal metastasis, n (%) | 0.007 | 0.482 | 0.791 | 0.113 | ||||||
| N0 | 75 (33.6) | 53 (34.2) | 22 (32.4) | 51.0 (32.9) | 19.9 (29.2) | |||||
| Nx | 88 (39.5) | 52 (33.5) | 36 (52.9) | 62.1 (40.1) | 26.6 (39.0) | |||||
| N1 | 60 (26.9) | 50 (32.3) | 10 (14.7) | 41.8 (27.0) | 21.7 (31.8) | |||||
| T stage 8th, n (%) | 0.035 | 0.386 | 0.939 | 0.055 | ||||||
| T2 | 201 (90.1) | 137 (88.4) | 64 (94.1) | 139.4 (89.9) | 62.3 (91.3) | |||||
| T3 | 6 (2.7) | 3 (1.9) | 3 (4.4) | 4.5 (2.9) | 1.9 (2.8) | |||||
| T4 | 16 (7.2) | 15 (9.7) | 1 (1.5) | 11.2 (7.2) | 4.0 (5.9) | |||||
| TNM stage 8th, n (%) | 0.002 | 0.564 | 0.837 | 0.175 | ||||||
| Not Available | 88 (39.5) | 52 (33.5) | 36 (52.9) | 62.1 (40.1) | 26.6 (39.0) | |||||
| II | 66 (29.6) | 44 (28.4) | 22 (32.4) | 43.7 (28.2) | 19.9 (29.2) | |||||
| IIIA | 2 (0.9) | 2 (1.3) | 0 (0.0) | 2.3 (1.5) | 0 (0.0) | |||||
| IIIB | 67 (30.0) | 57 (36.8) | 10 (14.7) | 46.9 (30.3) | 21.7 (31.8) | |||||
ICC, intrahepatic cholangiocarcinoma; MVI, microvascular invasion; IPTW, Inverse Probability of Treatment Weighting; TACE, transarterial chemoembolization; SMD, standardized mean differences; HBsAg, hepatitis B surface antigen; Anti-HCV, antibody to hepatitis virus C; CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen 19-9; MF, mass-forming; PI, periductal infiltrative; N0, no regional lymph node metastasis; Nx, regional lymph node metastasis cannot be assessed; N1, regional lymph node metastasis present; TNM, tumor, node, metastases.
After IPTW, the covariates were well-balanced in the propensity-weighted cohort as presented in the Table 1. In addition, after 1:1 PSM, 68 patients were matched in each group and the baseline characteristics were well-balanced between the two groups (Table S1).
Prognosis of patients treated with or without adjuvant TACE before and after IPTW
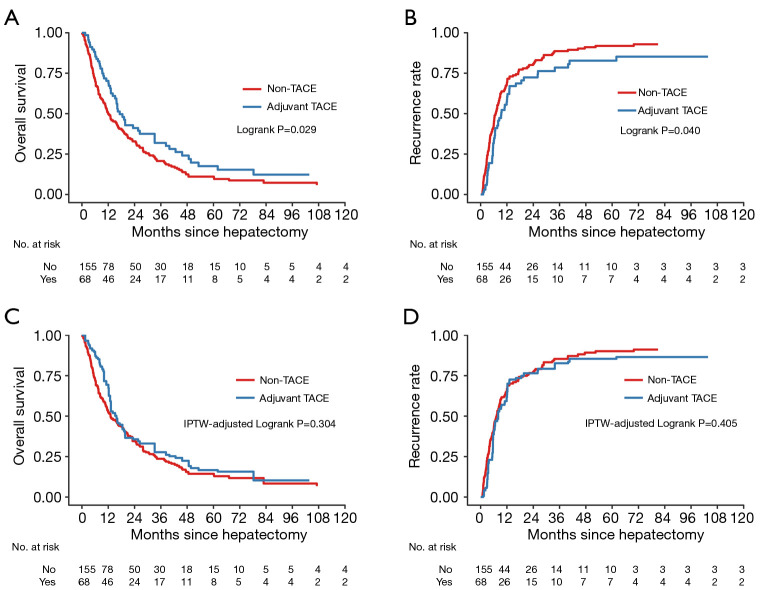
The study was censored on January 21, 2019. During a median follow-up of 80.9 months, tumor recurrence occurred in 42 patients (61.8%) in the adjuvant TACE group and 99 patients (63.9%) in the non-TACE group. Before IPTW, the median OS in the adjuvant TACE and non-TACE groups was 17.2 months and 12.0 months, respectively. The 1-, 3-, and 5-year OS rate in the adjuvant TACE group were 70.3%, 31.9%, and 17.5%, respectively, which were significantly higher than those in the non-TACE group (50.3%, 20.7%, and 11.0%, respectively; P=0.029; Figure 2A). The median TTR in the adjuvant TACE group and the non-TACE group was 9.4 months and 6.8 months, respectively. The 1-, 3-, and 5-year recurrence rates in the adjuvant TACE group were 57.2%, 78.5%, and 82.8%, respectively, which were significantly lower than those in the non-TACE adjuvant group (68.7%, 88.7%, and 91.9%, respectively; P=0.040; Figure 2B).
Figure 2.
Kaplan-Meier survival curves of OS and tumor recurrence before (A,B) and after (C,D) IPTW analysis among ICC-MVI patients who underwent curative-intent partial hepatectomy, according to adjuvant TACE status. The at-risk table shows the actual number of patients at risk. OS, overall survival; IPTW, inverse probability of treatment weighting; ICC, intrahepatic cholangiocarcinoma; MVI, microvascular invasion; TACE, transarterial chemoembolization.
In IPTW analyses, no significant differences were found in the 1-, 3-, and 5-year OS rates (69.4%,27.7%, and 16.7% vs. 53.0%, 23.6%, and 14.3%, P=0.304; Figure 2C) and the corresponding recurrence rates (61.6%, 82.6%, and 85.4% vs. 66.1%, 85.3%, and 90.1%, P=0.405; Figure 2D) between the 2 weighted cohorts.
Prognosis of patients treated with or without adjuvant TACE before and after PSM
The 1:1 PSM analysis generated 68 pairs of patients, and the baseline characteristics of the 2 groups are shown in Table S1. Consistent with the findings by the IPTW analyses, similar results were obtained after the PSM analyses. Adjuvant TACE was not significantly associated with better long-term outcomes in the ICC patients with MVI (Figure S1).
Independent risk factors for prognosis of patients in the entire cohort
After univariate analysis (Table S2), multivariate analysis of all study patients showed that elevated serum CA19-9 level (HR, 1.38; 95% CI, 1.02–1.87; P=0.037) and lymph node involvement (N1 vs. N0: HR, 1.54; 95% CI, 1.05–2.26; P=0.026) were independent risk factors for tumor recurrence. With respect to OS, elevated serum CA19-9 level (HR, 1.53; 95% CI, 1.13–2.05; P=0.005), tumor diameter ≥5 cm (HR, 1.46; 95% CI, 1.06–2.00; P=0.019), multiple tumor (HR, 1.68; 95% CI, 1.21–2.33; P=0.002), direct invasion (HR, 1.81; 95% CI, 1.06–3.10; P=0.030), and lymph node involvement (N1 vs. N0: HR, 1.63; 95% CI, 1.13–2.36; P=0.009) were significant poor prognostic factors for OS in patients with ICC-MVI. Notably, the adjuvant TACE was not and independent factor for OS and recurrence in the whole cohort (Table 2).
Table 2. Multivariable cox regression analysis in the whole ICC-MVI patients (n=223).
| Variable | Tumor recurrence | OS | |||||
|---|---|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | HR | 95% CI | ||
| CA 19-9, U/mL, ≥39 vs. <39 | 0.037 | 1.38 | 1.02–1.87 | 0.005 | 1.53 | 1.13–2.05 | |
| Tumor diameter (cm), >5 vs. ≤5 | 0.248 | 1.21 | 0.88–1.66 | 0.019 | 1.46 | 1.06–2.00 | |
| Tumor number, Multiple vs. Solitary | 0.209 | 1.25 | 0.88–1.75 | 0.002 | 1.68 | 1.21–2.33 | |
| Direct invasion, Yes vs. No | 0.386 | 1.29 | 0.73–2.27 | 0.030 | 1.81 | 1.06–3.10 | |
| Regional nodal metastasis | |||||||
| Nx vs. N0 | 0.399 | 0.86 | 0.60–1.23 | 0.472 | 0.88 | 0.62–1.25 | |
| N1 vs. N0 | 0.026 | 1.54 | 1.05–2.26 | 0.009 | 1.63 | 1.13–2.36 | |
| Adjuvant TACE, Yes vs. No | 0.177 | 0.79 | 0.56–1.11 | 0.208 | 0.81 | 0.58–1.12 | |
ICC-MVI, intrahepatic cholangiocarcinoma involving microvascular invasion; OS, overall survival; HR, hazard ratio; CA19-9, carbohydrate antigen 19-9; N0, no regional lymph node metastasis; Nx, regional lymph node metastasis cannot be assessed; N1, regional lymph node metastasis present; TACE, transarterial chemoembolization.
Subgroup analyses for the effect of adjuvant TACE
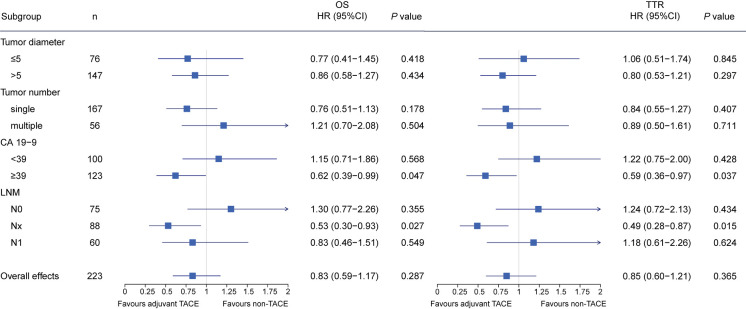
Next, we performed subgroup analyses by the independent risk factors identified by the multivariable cox regression analysis. As direct invasion was presented in only one patient in the TACE group, its subgroup was not analyzed. In the weighted cohort, Cox regression model revealed that adjuvant TACE favored for OS (HR, 0.62; 95% CI, 0.39–0.99; P=0.047) and TTR (HR, 0.59; 95% CI, 0.36–0.97; P=0.037) among patients with elevated CA19-9, as well as those without lymphadenectomy (HR, 0.53; 95% CI, 0.30–0.93; P=0.027 for OS, and HR, 0.49; 95% CI, 0.28–0.87; P=0.015 for TTR, respectively) No significant difference of prognosis between the TACE and non-TACE was observed among the patients in other subgroups (Figure 3). Similarly, results obtained from the PSM analyses are in accordance with the findings revealed by the IPTW analyses (Figure S2).
Figure 3.
Forest plot of impacts of adjuvant TACE on OS and tumor recurrence in subgroup patients after IPTW analysis. Squares represent point estimates for the hazard ratio as compared with non-TACE, and horizontal lines indicate the associated 95% CIs. Hazard ratios and P values were estimated with the use of propensity-weighted Cox proportional-hazards models. OS, overall survival; TTR, time to recurrence; IPTW, inverse probability of treatment weighting; TACE, transarterial chemoembolization.
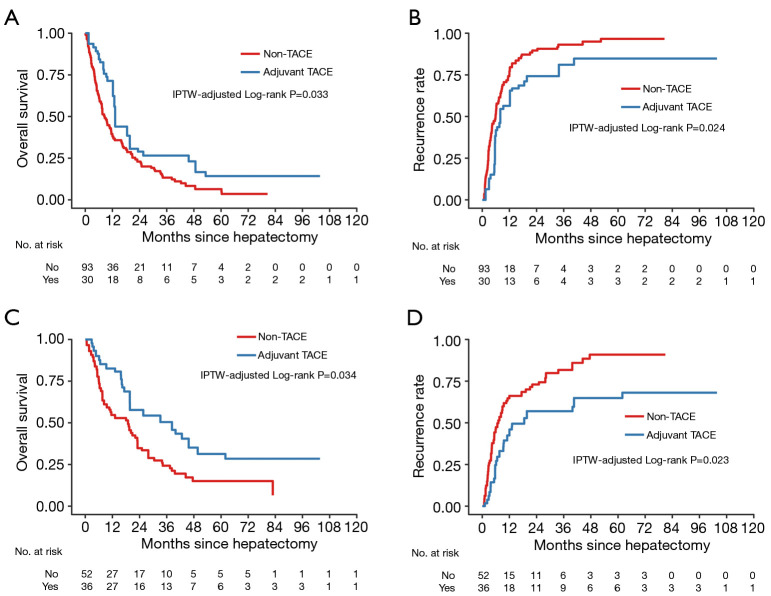
In the CA19-9 ≥39 U/L subgroup, the 1-, 3-, and 5-year OS rates were 71.4%, 26.6%, and 14.3% for TACE patients with a median OS of 13.2 months; and 39.5%, 13.3%, and 6.4% for non-TACE patients with a median OS of 8.6 months, respectively (P=0.033; Figure 4A). The median TTR was 8.0 months for TACE patients and 5.9 months for non-TACE patients. In addition, the 1-, 3-, and 5-year recurrence rates were 56.4%, 81.0%, and 84.8% in TACE patients, and 75.4%, 93.1%, and 96.6% in non-TACE patients, respectively (P=0.024; Figure 4B).
Figure 4.
Kaplan-Meier survival curves of OS and tumor recurrence among CA19-9 ≥39 U/L subgroup patients (A,B) and Nx subgroup patients (C,D) after IPTW. The at-risk table shows the actual number of patients at risk. OS, overall survival; IPTW, inverse probability of treatment weighting.
In the Nx subgroup, the median OS and TTR were 38.3 months and 18.9 months for TACE patients and 18.6 and 7.1 months for non-TACE patients. Adjuvant TACE was associated with higher 1-, 3-, and 5-year OS rates (82.6%, 50.4%, and 31.3% vs. 54.7%, 24.3%, and 15.0%, respectively; P=0.034; Figure 4C) and lower corresponding recurrence rates (42.4%, 57.0%, and 64.9%, vs. 66.1%, 81.7%, and 90.9%, respectively; P=0.023; Figure 4D).
Discussion
Our current study investigated the impact of adjuvant TACE on patients with ICC involving MVI after curative-intent PH. Our findings suggest that adjuvant TACE was only associated with lower recurrence and better OS in the ICC-MVI patients with elevated CA19-9 or without lymphadenectomy. These finding were consistent and robust across IPTW and PSM analyses.
Previously, many studies indicated that adjuvant TACE might provide survival benefits for patients with HCC when MVI presented (3,4,6,7,26). However, the potential impact of adjuvant TACE on ICC-MVI patients remains uncertain. In this study, our preliminary results before IPTW and PSM showed that adjuvant TACE was associate with better postoperative long-term outcomes in the ICC patients with MVI. However, when compared to the baseline clinicopathological characteristics, some selected biases were observed, which might account for the differences in prognosis between these 2 groups. Patients in the non-TACE group had higher incidences of elevated serum CA19-9 level, direct invasion of adjacent organ, and lymph node involvement than those in the adjuvant TACE group. Patients in these two groups differed significantly by these factors which have been widely proven as independent risk factors for postoperative prognosis of ICC patients by various studies (27-30). After the baseline characteristics of the 2 groups were balanced by IPTW and PSM, the results showed that adjuvant TACE was not associated with improved prognosis among the overall ICC-MVI patients.
It is interesting to note that in subgroup analyses significant TTR and OS differences between the adjuvant TACE and non-TACE groups were observed in the CA19-9 ≥39 U/L and Nx subgroups. The reasons for the TACE’s positive impacts on these populations are not clear from our work. In this study, lymphadenectomy was performed in 135 patients (60.5%), 60 (44.4%) of whom had LN metastasis. This result indicated that ICC involving MVI has a relative higher rate of LN metastasis when compared to that reported in previous studies (31). Moreover, various studies have demonstrated that elevated preoperative serum CA19-9 was associated with LN metastasis (32-36). Concerning 80% or more of hepatic lymph drains through the periportal lymphatic system to the hilar LNs (37), we hypothesized that TACE may result in necrosis or inhibition of micrometastatic foci lay in the deep lymphatic system of the liver when elevated CA19-9 or nonlymphadenectomy was presented. Further studies are needed to better understand the underlying mechanisms.
In our series, all of the ICC involved MVI, and the long-term outcomes in these patients after PH were relatively poor with high recurrence rate and low OS rates. These results revealed that MVI is an adverse factor for recurrence and death of patients with ICC. Various studies have documented that MVI may lead to intra- and extrahepatic disseminations through the portal vein or hepatic vein (38), thus presenting an aggressive tumor biological behavior associated with advanced tumor characteristics and a higher risk of tumor recurrence (17,39). Furthermore, unlike macrovascular invasion, MVI cannot be diagnosed before surgery, it only can be determined by microscopic histological examination after surgery (38). As such, postoperative adjuvant therapy to improve long-term prognosis should be considered for these patients (40). In this study, the association between the adjuvant TACE and better survival was only observed among ICC-MVI patients with elevated CA19-9 and Nx. Therefore, the role of other adjuvant therapeutic strategies in the remaining ICC-MVI patients needs to be investigated.
In addition, we guess that as a locoregional therapy, TACE might offer an advantage in the adjuvant setting in patients with high risk for early intrahepatic recurrence. While, adjuvant chemotherapy may inhibit the invasion and metastasis of tumor cells systemically, adjuvant TACE could potentially improve outcomes in resected ICC-MVI patients that will receive adjuvant modern chemotherapy as well.
The current study has several limitations. First, patient selection for adjuvant TACE was not randomized, so potential selection bias may exist. We have attempted to directly address this basic limitation using the IPTW and PSM analyses. Another limitation of this study is that half of the enrolled patients were diagnosed with HBV-related ICC; whether our findings can be confirmed in other study populations is still uncertain. Third, the drug, dosage, and frequency of adjuvant TACE may affect the outcomes of patients with ICC. Further well-designed randomized clinical trials with an ideal regimen of adjuvant TACE will thus be necessary in the future. Last but not least, although we have collected the largest number of ICC patients with MVI so far, the relatively small sample size limits our power to come to any formal conclusions.
In conclusion, among the ICC-MVI patients who underwent curative-intent PH, only those have elevated CA19-9 or who did not undergo lymphadenectomy might be suitable for adjuvant TACE. Further studies are needed to confirm the results of this study.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China [81871988 to C.Z.J.], the Jiangsu Province Key Research and Development Program [BE2019747 to C.Z.J.], the Six Talent Peaks Project of Jiangsu Province [SWYY-007 to C.Z.J.], and the State Key Project on Infectious Diseases of China [2017ZX10203204 to F.S.].
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committees of each of the involved hospitals (NO. EHBHKY2020-K-027, 2020ZDSYLL010-P01, and 81YY-KYLL-19-05). Informed consent was routinely obtained before surgery.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jgo-20-443
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jgo-20-443
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at http://dx.doi.org/10.21037/jgo-20-443). The authors declare no conflict of interest.
References
- 1.Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017;24:1073274817729245. 10.1177/1073274817729245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. 10.1016/j.jhep.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Ren Z, Chen Y, et al. Adjuvant Transarterial Chemoembolization for HBV-Related Hepatocellular Carcinoma After Resection: A Randomized Controlled Study. Clin Cancer Res 2018;24:2074-81. 10.1158/1078-0432.CCR-17-2899 [DOI] [PubMed] [Google Scholar]
- 4.Qi YP, Zhong JH, Liang ZY, et al. Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma involving microvascular invasion. Am J Surg 2019;217:739-44. 10.1016/j.amjsurg.2018.07.054 [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Li H, Guo L, et al. Tumor Size Affects Efficacy of Adjuvant Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma and Microvascular Invasion. Oncologist 2019;24:513-20. 10.1634/theoncologist.2018-0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YY, Wang LJ, Xu D, et al. Postoperative adjuvant transcatheter arterial chemoembolization should be considered selectively in patients who have hepatocellular carcinoma with microvascular invasion. HPB (Oxford) 2019;21:425-33. 10.1016/j.hpb.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 7.Wei W, Jian PE, Li SH, et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety. Cancer Commun (Lond) 2018;38:61. 10.1186/s40880-018-0331-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehm LM, Jayakrishnan TT, Miura JT, et al. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol 2015;111:213-20. 10.1002/jso.23781 [DOI] [PubMed] [Google Scholar]
- 9.Gusani NJ, Balaa FK, Steel JL, et al. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): a single-institution experience. J Gastrointest Surg 2008;12:129-37. 10.1007/s11605-007-0312-y [DOI] [PubMed] [Google Scholar]
- 10.Savic LJ, Chapiro J, Geschwind JH. Intra-arterial embolotherapy for intrahepatic cholangiocarcinoma: update and future prospects. Hepatobiliary Surg Nutr 2017;6:7-21. 10.21037/hbsn.2016.11.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen WF, Zhong W, Liu Q, et al. Adjuvant transcatheter arterial chemoembolization for intrahepatic cholangiocarcinoma after curative surgery: retrospective control study. World J Surg 2011;35:2083-91. 10.1007/s00268-011-1171-y [DOI] [PubMed] [Google Scholar]
- 12.Li T, Qin LX, Zhou J, et al. Staging, prognostic factors and adjuvant therapy of intrahepatic cholangiocarcinoma after curative resection. Liver Int 2014;34:953-60. 10.1111/liv.12364 [DOI] [PubMed] [Google Scholar]
- 13.Jeong S, Zheng B, Wang J, et al. Transarterial Chemoembolization: A Favorable Postoperative Management to Improve Prognosis of Hepatitis B Virus-associated Intrahepatic Cholangiocarcinoma after Surgical Resection. Int J Biol Sci 2017;13:1234-41. 10.7150/ijbs.21149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Z, Liu S, Yi Y, et al. Serum gamma-glutamyl transferase levels affect the prognosis of patients with intrahepatic cholangiocarcinoma who receive postoperative adjuvant transcatheter arterial chemoembolization: A propensity score matching study. Int J Surg 2017;37:24-8. 10.1016/j.ijsu.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Pang S, Si-Ma H, et al. Specific risk factors contributing to early and late recurrences of intrahepatic cholangiocarcinoma after curative resection. World J Surg Oncol 2019;17:2. 10.1186/s12957-018-1540-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng Z, Lei Z, Si A, et al. Modifications of the AJCC 8th edition staging system for intrahepatic cholangiocarcinoma and proposal for a new staging system by incorporating serum tumor markers. HPB (Oxford) 2019;21:1656-66. [DOI] [PubMed] [Google Scholar]
- 17.Hu LS, Weiss M, Popescu I, et al. Impact of microvascular invasion on clinical outcomes after curative-intent resection for intrahepatic cholangiocarcinoma. J Surg Oncol 2019;119:21-9. 10.1002/jso.25305 [DOI] [PubMed] [Google Scholar]
- 18.Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg 2014;149:432-8. 10.1001/jamasurg.2013.5168 [DOI] [PubMed] [Google Scholar]
- 19.Li J, Wang Q, Lei Z, et al. Adjuvant Transarterial Chemoembolization Following Liver Resection for Intrahepatic Cholangiocarcinoma Based on Survival Risk Stratification. Oncologist 2015;20:640-7. 10.1634/theoncologist.2014-0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Z, Yang P, Qu S, et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford) 2015;17:422-7. 10.1111/hpb.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Lei Z, Xia Y, et al. Association of Preoperative Antiviral Treatment With Incidences of Microvascular Invasion and Early Tumor Recurrence in Hepatitis B Virus-Related Hepatocellular Carcinoma. JAMA Surg 2018;153:e182721. 10.1001/jamasurg.2018.2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amin MB, Gress DM, Vega LRM, et al. editors. AJCC Cancer Staging Manual. eighth edition. New York: Springer International Publishing, 2017. [Google Scholar]
- 23.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150-61. 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol 2001;54:387-98. 10.1016/S0895-4356(00)00321-8 [DOI] [PubMed] [Google Scholar]
- 25.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014;33:1242-58. 10.1002/sim.5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun JJ, Wang K, Zhang CZ, et al. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who have Hepatocellular Carcinoma with Microvascular Invasion. Ann Surg Oncol 2016;23:1344-51. 10.1245/s10434-015-5008-z [DOI] [PubMed] [Google Scholar]
- 27.Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg 2014;149:565-74. 10.1001/jamasurg.2013.5137 [DOI] [PubMed] [Google Scholar]
- 28.Cho SY, Park SJ, Kim SH, et al. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol 2010;17:1823-30. 10.1245/s10434-010-0938-y [DOI] [PubMed] [Google Scholar]
- 29.Doussot A, Groot-Koerkamp B, Wiggers JK, et al. Outcomes after Resection of Intrahepatic Cholangiocarcinoma: External Validation and Comparison of Prognostic Models. J Am Coll Surg 2015;221:452-61. 10.1016/j.jamcollsurg.2015.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. 10.1200/JCO.2012.41.5984 [DOI] [PubMed] [Google Scholar]
- 31.de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5. 10.1200/JCO.2011.35.6519 [DOI] [PubMed] [Google Scholar]
- 32.Meng ZW, Lin XQ, Zhu JH, et al. A nomogram to predict lymph node metastasis before resection in intrahepatic cholangiocarcinoma. J Surg Res 2018;226:56-63. 10.1016/j.jss.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 33.Yoh T, Hatano E, Seo S, et al. Preoperative criterion identifying a low-risk group for lymph node metastasis in intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2018;25:299-307. 10.1002/jhbp.552 [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto Y, Sugiura T, Todaka A, et al. Surgical Indication for Advanced Intrahepatic Cholangiocarcinoma According to the Optimal Preoperative Carbohydrate Antigen 19-9 Cutoff Value. World J Surg 2018;42:3331-40. 10.1007/s00268-018-4605-y [DOI] [PubMed] [Google Scholar]
- 35.Yamada T, Nakanishi Y, Okamura K, et al. Impact of serum carbohydrate antigen 19-9 level on prognosis and prediction of lymph node metastasis in patients with intrahepatic cholangiocarcinoma. J Gastroenterol Hepatol 2018. [Epub ahead of print]. doi:. 10.1111/jgh.14124 [DOI] [PubMed] [Google Scholar]
- 36.Chang ME, Lei HJ, Chen MH, et al. Evaluation of prognostic factors and implication of lymph node dissection in intrahepatic cholangiocarcinoma: 10-year experience at a tertiary referral center. J Chin Med Assoc 2017;80:140-6. 10.1016/j.jcma.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 37.Morine Y, Shimada M. The value of systematic lymph node dissection for intrahepatic cholangiocarcinoma from the viewpoint of liver lymphatics. J Gastroenterol 2015;50:913-27. 10.1007/s00535-015-1071-2 [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez-Perálvarez M, Luong TV, Andreana L, et al. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol 2013;20:325-39. 10.1245/s10434-012-2513-1 [DOI] [PubMed] [Google Scholar]
- 39.Sasaki K, Margonis GA, Andreatos N, et al. Preoperative Risk Score and Prediction of Long-Term Outcomes after Hepatectomy for Intrahepatic Cholangiocarcinoma. J Am Coll Surg 2018;226:393-403. 10.1016/j.jamcollsurg.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 40.Cloyd JM, Pawlik TM. Adjuvant Therapy for Biliary Tract Cancers: New Evidence to Resolve Old Questions. J Oncol Pract 2018;14:723-4. 10.1200/JOP.18.00682 [DOI] [PubMed] [Google Scholar]