Abstract
The term smooth uterine muscle of uncertain malignant potential (STUMP) indicates a group of uterine smooth muscle tumours that cannot be diagnosed unequivocally as malignant but does not fulfil the criteria for leiomyoma, or its variants. In this case, we present a woman treated for infertility who presented with an asymptomatic cervical mass, diagnosed as STUMP after three cycles of controlled ovarian stimulation. We reviewed the literature with particular emphasis on the effects of STUMP upon fertility, up-to-date guidance regarding the management of patients’ wishing fertility-sparing approaches and obstetric outcomes. To the best of our knowledge, this is the first case report of STUMP in a patient that has undergone multiple in vitro fertilization treatments as well as the first to provide a putative biological basis for the efficacy of gonadotropin-releasing hormone agonists, in this patient group.
Keywords: Obstetrics/gynaecology, oncology, genetics, bioinformatics
Introduction
The smooth muscle neoplasms are categorised as benign (leiomyomas), malignant (leiomyosarcomas) or smooth muscle tumours of uncertain malignant potential (STUMP), according to displayed characteristics including mitotic activity and proliferating capacity, cytological atypia and coagulative or tumour cell necrosis.1 The term STUMP indicates a group of uterine smooth muscle tumours that cannot be diagnosed unequivocally as malignant and raises concern that the lesion may behave in a malignant fashion.1 Nonetheless, the term ‘atypical leiomyoma’ has been incompatibly employed to include diverse entities, including STUMP, which does hold a risk of recurrence, as well as leiomyoma with bizarre nuclei, which does not pose such a risk when appropriately classified.1 Occasionally smooth muscle tumours may exhibit significant mitotic activity without cellular characteristics of malignancy.2
The clinical presentation and imaging modalities have not been proven adequate to distinguish STUMPs from other neoplastic entities. The symptoms of uterine STUMPs are similar to those of leiomyomas including vaginal bleeding, anaemia, abdominal pain or discomfort, dyspareunia, dysuria and other features of a pelvic mass.3,4 Accurate STUMP diagnosis can only be achieved by pathology evaluation of retrieved hysterectomy or myomectomy samples.1 The Stanford criteria of STUMP diagnosis include at least two of the following: diffuse moderate to severe atypia, a mitotic count of at least 10 mitotic figures or 10 high power fields and tumour cell necrosis.3,4
Consequently, diagnosis, surgical management, and follow-up of this neoplasm remains controversial, especially in pre-menopausal women who desire fertility. As the proliferation index of STUMP remains obscure due to the mixed cytological phenotypes, all tumours that have been categorised as STUMP should be considered as having some recurrence potential, even in cases where complete hysterectomy with an intact corpus has been performed.3,5 Consequently, while tumours without cytologic atypia and tumour cell necrosis but with a recorded high mitotic index carry a low risk of recurrence 8.7%, tumours without cytologic atypia and low mitotic index with recorded tumour cell necrosis carry a risk of recurrence as high as 26.7%.2 Regardless of mitotic index, a tumour with high cytological atypia and necrosis should be classified as malignant leiomyosarcoma, according to Stanford criteria.5 Here, we report a case a woman treated for infertility who presented with an asymptomatic cervical mass, diagnosed as STUMP after three cycles of controlled ovarian stimulation (COS).
Case report
A 37-year-old nulliparous Caucasian women was admitted to the IVF centre due to infertility. Her initial assessment including clinical examination, transvaginal ultrasound and biochemical profile revealed poor ovarian reserve (anti-Mullerian hormone (AMH) 0.8 ng/mL, follicle-stimulating hormone (FSH) 12.2 mIU/mL, estradiol (E2) 42 pg/mL, antral follicle count (AFC) 4 on Day 3 of menstrual cycle). The sperm count of her spouse was of normal range and good motility. She reported regular cycles and no other co-morbidities were recorded or known to the patient. Within the first year of her presentation, she underwent three cycles of COS, using a fixed gonadotropin-releasing hormone (GnRH) antagonist stimulation protocol with 300 IU of gonadotropins. Briefly, ovarian stimulation was initiated on Day 3 with 300 IU of human Menopausal Gonadotropins (hMG; Menopur; Ferring Pharmaceutical Hellas AE). She was re-evaluated on Day 5 of stimulation (Day 8 of the cycle), when a transvaginal sonogram was performed to evaluate follicular growth and serum E2 levels were re-measured. On Day 6 of stimulation or when a leading follicle reached a diameter of 14 mm, a commercially available GnRH antagonist, Cetrorelix acetate 0.25 mg/day, was initiated (Cetrotide; Merck Serono Hellas AE). Patient had serial evaluations as required per protocol. When at least two follicles reached an average diameter of 18–20 mm, final oocyte maturation was triggered with 10,000 IU of human chorionic gonadotropin (hCG; Pregnyl; Organon Greece Inc). Oocyte Pick Up (OPU) was carried out 34–36 h later. The patient underwent routine intracytoplasmic sperm injection (ICSI) based on the limited number of available oocytes. Three embryo transfers were carried out with no success.
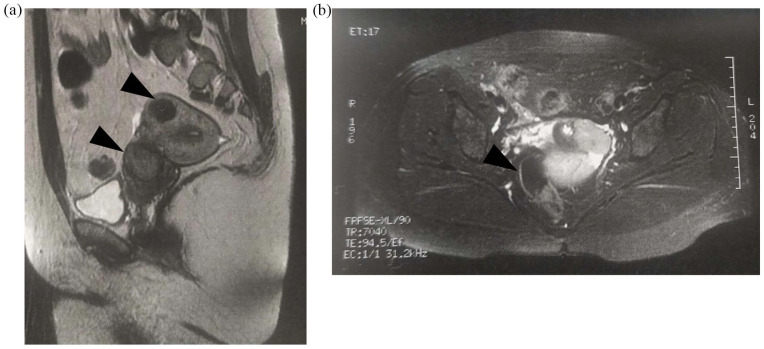
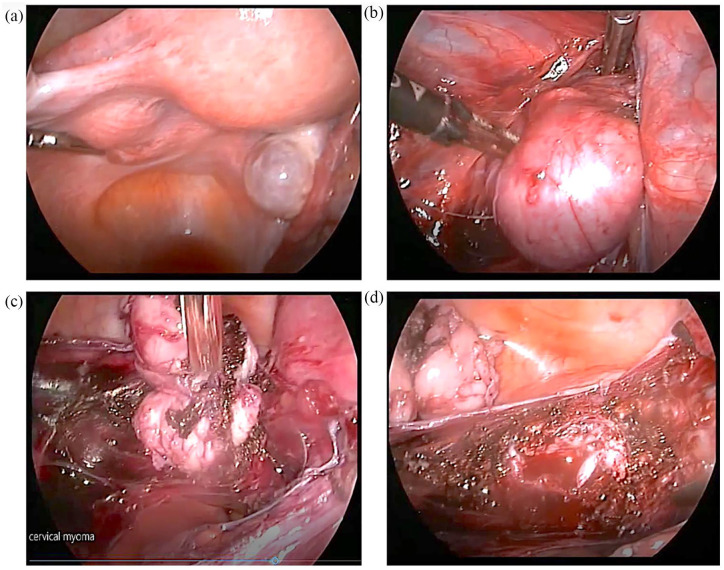
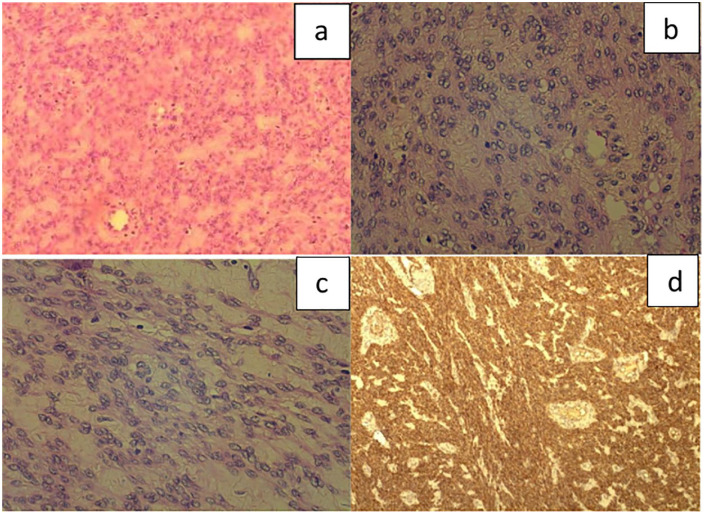
Six months after her last embryo transfer, the patient presented for evaluation prior to her fourth attempt. A transvaginal ultrasound on Day 3 was performed as usual and revealed a 5 cm cervical asymptomatic mass with possible diagnosis of leiomyoma, which was not previously recorded. Magnetic resonance imaging (MRI) images confirmed the finding with extension above the cardinal ligament, projecting in the peritoneal cavity (Figure 1). The patient underwent laparoscopic evaluation which confirmed the presence of a smooth, well-defined mass attached to the left side of the cervix which was removed. Considering the young of patient’s age, the lesion size and the MRI report, the lesion was removed by power morcellation (Figure 2). Frozen sections were negative for malignancy (Figure 3). The final histological examination revealed a smooth muscle neoplasm with epithelioid morphology (Figure 3(a)) with low to moderate nuclear atypia (Figure 3(b) and (c)) without necrotic areas or mitotic activity (p53 (–) Caldesmon (+) p16 (–) compatible with STUMP (Figure 3(d)). The case was reviewed by the oncology board of our institution which deemed that no further surgical treatment was required. One year after surgery the patient remained recurrence-free of disease.
Figure 1.
Patient MRI findings: (a) sagittal and (b) transverse sections.
Patient identifiable information has been removed from the images. Black arrows indicate areas of interest.
Figure 2.
Laparoscopic images depicting the stages of STUMP morcellation: (a) the presence of a smooth well-defined 5 cm mass attached to the left side of the cervix, (b) STUMP after opening the broad ligament, (c) solid adhesions of STUMP to adjacent tissues and (d) the bed of STUMP after its removal.
Figure 3.
Histological examination of section. Smooth muscle neoplasm identified with epithelioid morphology (a) (H–E ×100) and diffuse low to moderate nuclear atypia throughout the tumour (b) and (c), (H–E ×400). The mitotic count was low with less than five mitoses or 10 HPF. Areas of necrosis were not observed. Immunohistochemically, the neoplastic cells stained positive for calponin (d) (×40) and were negative for p16 and p53.
Discussion
As previously mentioned, diagnosis, surgical management, and follow-up of STUMP remains controversial, especially in pre-menopausal women who desire fertility, while the optimal management of the infertility in STUMP patients remains obscure. For such patients, a multidisciplinary approach should be pursued to assess tumour characteristics and the patient’s wishes for future pregnancy. While fertility preservation remains a significant factor of consideration in patients of child-bearing age, standardised protocols and guidelines of STUMP management in this patient group, have not been developed. Nonetheless, a number of studies have demonstrated that fertility-sparing approaches such as myomectomy or initial treatment with GnRH agonists remain feasible within this population.6,7 Current management strategies involve mainly surgical interventions including myomectomy by hysteroscopy, myomectomy by laparotomy or laparoscopy. Of note, warning have been raised regarding the use of electromechanical power morcellation.6 It is important to mention that the technique of power morcellation in a bag is suggested to minimise the risk of inadvertent tissue spread and should be performed especially in cases where the fast-growing mass is presented, even in young patients.
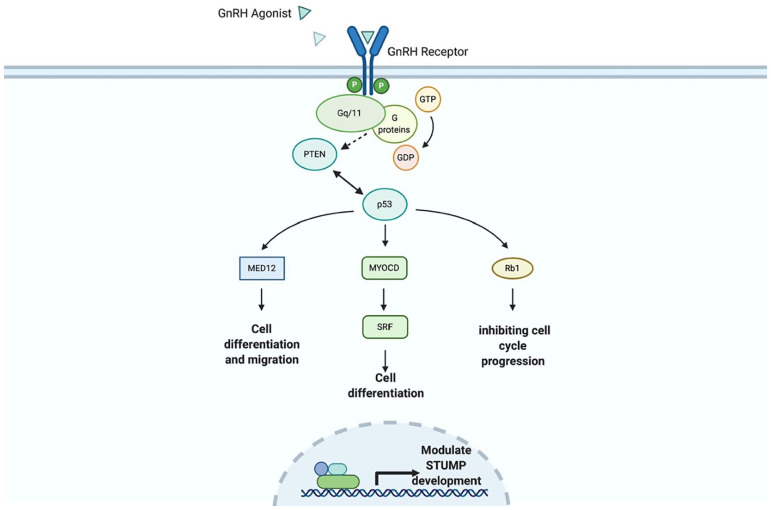
The GnRH agonists have been used to decrease the size of fibroids, especially in symptomatic women to resolve anaemia.7 Goserelin (GnRH agonist) has been shown to quantifiably decrease the size of STUMP prior surgical removal and consequently decrease the extend of the necessary myomectomy, albeit this approach has been used in a small number of studies.6,7 GnRH agonists may function through activation of Pten, Tp53, Myocd and Rb1, proteins which have been previously identified as deregulated in STUMP development (Figure 4).8 Nonetheless, GnRH agonists cannot be used for long time because of their side effects and the risk of osteoporosis. However, it is well known that the removal of myomas after GnRH treatment is difficult, taking into account that the diagnosis of STUMP cannot be suspected prior the surgical removal. The preoperative use of GnRH agonists seems to be beneficial in patients with only submucous fibroids.7 Benefits also include a resolution of preoperative anaemia.
Figure 4.
Putative mechanism of STUMP size modulation through GnRH agonists.
Putative pathway: GnRH agonist activates GnRH receptor which through currently unidentified, Gq/11 G-protein family members, activating Pten phosphatase which in turn activates Med12, Myocd and Rb1 leading to cell development and cell-cycle regulation potentially regulating and decreasing STUMP growth.9 Protein associations were mined and generated through the STRING V. 11 (https://string-db.org/), Cytoscape freeware platform and Agilent Literature plugin (V. 3.8) and the National Library of Medicine (US). Bethesda (MD): The Library; (15 July 2020). Available from: https://ghr.nlm.nih.gov/. Pathway was reconstructed through BioRender online design platform.
By inducing a state of hyperestrogenism during ovarian stimulation for IVF, COS theoretically may increase the size of leiomyomas. Surprisingly, it seems that COS does not modify the dimension of subserosal and intramural leiomyomas. Moreover, previous literature does not confirm any relationship between COS and the magnitude of the ovarian responsiveness. In 2003, a big follow-up study of 8714 women showed that IVF treatment did not affect the occurrence of uterine leiomyomas neither influenced the leiomyoma’s size.10 According to these data, we can consider that even, in our case, the treatment with gonadotropins for infertility had not affected the occurrence of STUMP. A recent retrospective study by Şahin et al.,11 which explored fertility and oncological outcomes in 57 patients with a STUMP diagnosis, identified similar recurrence rates (14%) for patients undergoing either hysterectomy or myomectomy for STUMP management, in agreement with a previous retrospective study by Guntupalli et al.9 A 37% (N 10 out of 27) of patients that underwent myomectomy, achieved pregnancy within the study follow-up period [57 (Min: 16 to Max: 125) months].11
In our case, we presented the infertile women with poor ovarian reserve. Her infertility treatment after surgery remains questionable as data in the literature regarding STUMP and the ovarian stimulation remain unclear. STUMP recurrence should be monitored and evaluated; ideally in a multidisciplinary approach, prior attempts for pregnancy have taken place.1,5,12 Even after conception, obstetric outcomes and prognosis remain stratified among patients with STUMP consequently great care should be given in monitoring these patients’ prior conception and throughout gestation to ensure the best possible outcomes. Annual surveillance with pelvic imaging (transvaginal ultrasound and MRI with gadolinium) potentially every 6 months depending on STUMP histologic features may be necessary in fertility-preserving patients to ensure timely management of potential recurrence.
Conclusion
There is no data in the literature regarding the influence of COS with gonadotropins on the development of STUMP in infertile women. Consequently, the risk uncertainty of malignancy and the lack of standardised management still complicate the decision process, especially for women who may require additional fertility treatments.
Acknowledgments
The authors thank Kashyap Anjali Hema, University of Aberdeen senior medical student, for reviewing the manuscript for English language editing.
Footnotes
Author contributions: T.O., K.S.L., and M.P. contributed to concept, analysis, format, revision, literature search, and manuscript drafting. T.O. and V.N. conducted the operation. K.S.L. and G.K. contributed to editing. T.O. and V.N. gave expert opinion.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors state that the manuscript is original; all the authors were active participants, and take full responsibility for this article, which they have read and approved, and that it is not simultaneously under consideration by other publication. The authors report no conflicts of interest. Contribution to the paper of each author is indicated.
Ethical approval: Case report was approved by the Leto Hospital IRB ethics committee and the patient with patient identifiers removed for data protection purposes (Protocol number: 125Δ796EZ/2020).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
IRB approval: Patient informed consent was obtained for image use for education and academic purposes. Case report was approved by the Leto Hospital IRB ethics committee and the patient with patient identifiers removed for data protection purposes.
ORCID iD: Kastora Stavroula Lila  https://orcid.org/0000-0003-4470-5267
https://orcid.org/0000-0003-4470-5267
References
- 1. Lu Z, Chen J. WHO classification of tumours of female reproductive organs. Zhonghua Bing Li Xue Za Zhi 2014; 43: 649–650. [PubMed] [Google Scholar]
- 2. Miettinen M. Smooth muscle tumors of soft tissue and non-uterine viscera: biology and prognosis. Mod Pathol 2014; 27 (Suppl. 1): S17–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ip PP, Tse KY, Tam KF. Uterine smooth muscle tumors other than the ordinary leiomyomas and leiomyosarcomas: a review of selected variants with emphasis on recent advances and unusual morphology that may cause concern for malignancy. Adv Anat Pathol 2010; 17(2): 91–112. [DOI] [PubMed] [Google Scholar]
- 4. Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms: a clinicopathologic study of 213 cases. Am J Surg Pathol 1994; 18(6): 535–558. [PubMed] [Google Scholar]
- 5. Huo L, Wang D, Wang W, et al. Oncologic and reproductive outcomes of uterine smooth muscle tumor of uncertain malignant potential: a single center retrospective study of 67 cases. Front Oncol 2020; 10: 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parker WH, Kaunitz AM, Pritts EA, et al. US Food and Drug Administration’s guidance regarding morcellation of leiomyomas: well-intentioned, but is it harmful for women? Obst Gynecol 2016; 127(1): 18–22. [DOI] [PubMed] [Google Scholar]
- 7. Gutmann JN, Corson SL. GnRH agonist therapy before myomectomy or hysterectomy. J Minimal Invas Gynecol 2005; 12(6): 529–537. [DOI] [PubMed] [Google Scholar]
- 8. Croce S, Ducoulombier A, Ribeiro A, et al. Genome profiling is an efficient tool to avoid the STUMP classification of uterine smooth muscle lesions: a comprehensive array-genomic hybridization analysis of 77 tumors. Mod Pathol 2018; 31(5): 816–828. [DOI] [PubMed] [Google Scholar]
- 9. Guntupalli SR, Ramirez PT, Anderson ML, et al. Uterine smooth muscle tumor of uncertain malignant potential: a retrospective analysis. Gynecol Oncol 2009; 113(3): 324–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klip H, van Leeuwen FE, Schats R, et al. Risk of benign gynaecological diseases and hormonal disorders according to responsiveness to ovarian stimulation in IVF: a follow-up study of 8714 women. Hum Reprod 2003; 18(9): 1951–1958. [DOI] [PubMed] [Google Scholar]
- 11. Şahin H, Karatas F, Coban G, et al. Uterine smooth muscle tumor of uncertain malignant potential: fertility and clinical outcomes. J Gynecol Oncol 2019; 30(4): e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ha HI, Choi MC, Heo JH, et al. A clinicopathologic review and obstetric outcome of uterine smooth muscle tumor of uncertain malignant potential (STUMP) in a single institution. Eur J Obstet Gynecol Reprod Biol 2018; 228: 1–5. [DOI] [PubMed] [Google Scholar]