Abstract
Herein, we describe three patients affected by metastatic colorectal cancer (mCRC) experiencing infection by severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) and reduction of disease burden during coronavirus disease 2019 (COVID-19) course. Insights into tumor-associated angiotensin-converting enzyme (ACE)-2 expression and lymphocyte function suggest a correlation between host/SARS-Cov-2 infection and tumor burden reduction. This may shed new light into (a) the infection mechanism of SARS-CoV-2 virus and (b) the multiple aspects of a composite antiviral immune response with potential paradoxical and unexpected applications.
Keywords: chemotherapy, colorectal cancer, COVID-19, NK cells, SARS-CoV-2
Introduction
Colorectal cancer (CRC) is the third cause of cancer-related deaths worldwide. The therapeutic panorama has been enriched by biologic therapies [anti-angiogenic anti-EGFR (epidermal growth factor receptor) drugs] in association with standard chemotherapies (fluorouracil, irinotecan and oxaliplatin). However, the survival of metastatic disease rarely lasts 24 months.1
In the last year, since SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) has spread from Wuhan (China) to worldwide, many oncological patients undergoing COVID-19 (coronavirus disease 2019) experienced, among other effects, treatment delay. In fact, according to several institutional policies, patients positive to molecular pharyngo-nasal swabs cannot undergo restored, or commence, chemotherapy until after three consecutive negative molecular pharyngo-nasal swabs.
In this scenario, three patients affected by metastatic CRC (mCRC) displayed radiologic reduction of disease burden during their COVID-19 experience. The patients’ consent for anonymized publication was obtained and archived in accordance with regulatory authorities.
Case descriptions
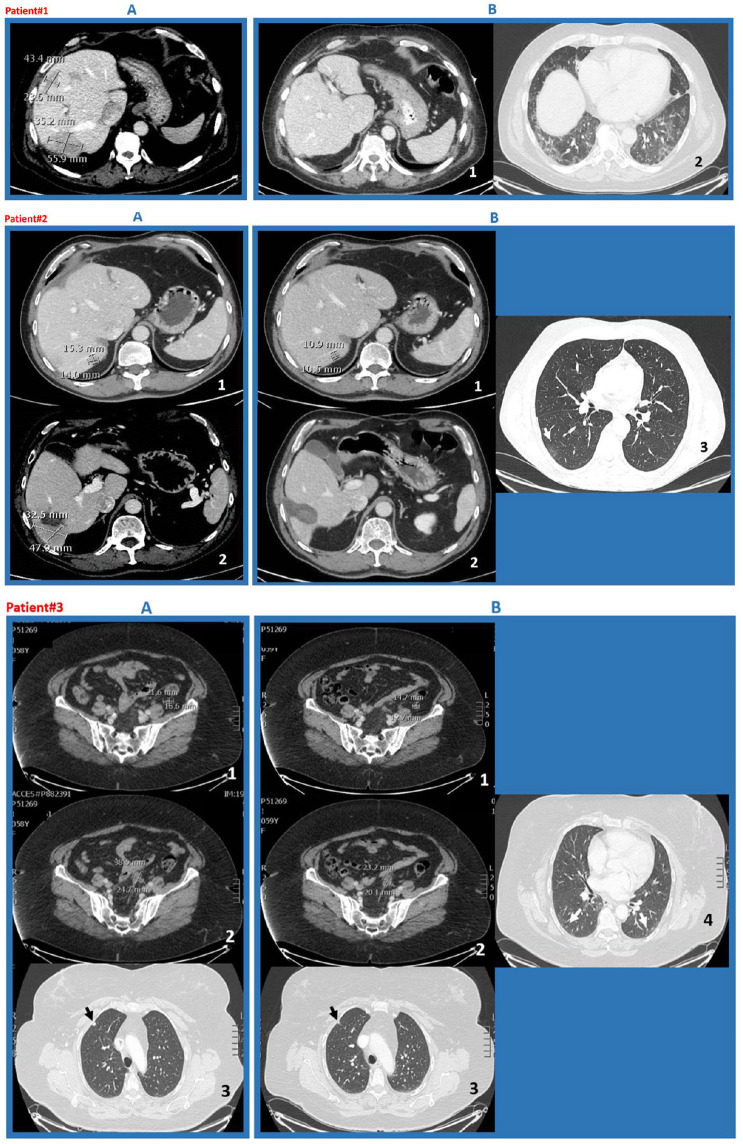
Patient 1 is a 65-year-old man who received a right hemicolectomy in October 2019. Histologic and genetic examination revealed a pT4apN1b RAS and BRAF wild-type, MSS (microsatellite stable) adenocarcinoma and he presented with liver metastases. Disease stabilization (with no change in tumor size) was obtained after 7 months of first-line chemotherapy FOLFOX/BEVA [oxaliplatin on day 1 at the dose of 85 mg/m2 as a 2 h infusion, concurrently with leucovorin 400 mg/m2, followed by a bolus of 5-fluorouracil 400 mg/m2 (FU), and a 48 h infusion of 5-fluorouracil 2400 mg/m2 (FA) and bevacizumab 5 mg/kg (BEVA) on day 1, every 2 weeks] as documented by CT (computed tomography) in August 2020. Following therapy was FU/FA/BEVA every 2 weeks as maintenance/depotentiated treatment, with the last cycle performed on 2 October 2020, for a total of three cycles. Thereafter, he developed severe COVID-19, from which he recovered in November 2020 when the CT scan showed complete response with regression of hepatic lesions (Figure 1). The immunoglobulin G (IgG) anti-SARS-CoV-2 titer was 1455 U/ml.
Figure 1.
CT scans of patients 1–3.
Patient 1 (a) CT scan shows multiple metastatic liver lesions with relative measures (August 2020); (b) CT scan shows complete regression of metastases (November 2020); (c) persistent diffuse interstitial thickening, coarse reticular patterns, and parenchymal bands that were SARS-CoV-2 associated (November 2020). Patient 2 (a)(i) and (ii) CT scan show metastatic liver lesions at VI and VII segments with the relative measures (August 2020); (b)(i) and (ii) CT scan shows reduction of lesion at VII segment and disappearance of that at VI segment (December 2020); (iii) some persistent interstitial thickening that was SARS-CoV-2 associated (December 2020). Patient 3 (a)(i) and (ii) CT scan evidencing multiple measurable nodules on the peritoneum surface; and (iii) a subpleural small nodule in right upper lobe (September 2020); (b)(i) and (ii) reduction of peritoneal and (iii) lung disease; (iv) some persistent interstitial thickening that was SARS-CoV-2 associated (October 2020).
CT, computed tomography; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Patient 2 is a 58-year-old man who underwent a left hemicolectomy in February 2019. The histologic and genetic assessment showed a pT3pN0 RAS mutated, BRAF wild-type, MSS adenocarcinoma. At diagnosis, he presented with three unresectable liver metastatic lesions treated with pre-operative FOLFOX/BEVA (6 months) followed by multiple metastasectomies in November 2019. Thereafter, the patient refused post-operative chemotherapy. In August 2020, he progressed at liver segments VI and VII as documented with CT scan; concomitantly, he developed mild symptomatic COVID-19. CT scan in December 2020 reported reduction of the liver metastatic lesion at segment VII (15.3 × 14.0 mm versus 10.9 × 10.5 mm) and disappearance of the lesion at segment VI (Figure 1). The IgG anti-SARS-CoV-2 titer was 188.40 U/ml.
Patient 3 is a 60-year-old woman who received a left hemicolectomy in March 2019 for a pT3pN2a RAS and BRAF wild-type, MSS adenocarcinoma followed by 6 months adjuvant FOLFOX therapy (oxaliplatin on day 1 at the dose of 85 mg/m2 as a 2 h infusion, concurrently with leucovorin 400 mg/m2, followed by a bolus of FU and a 48 h infusion of FA). In June 2020, the disease progressed on positron emission tomography and CT total-body scan, with multiple measurable nodules on the peritoneum surface (standard uptake value >3) and a small nodule in the right lung. The patient started first-line chemotherapy with FOLFIRI/PANI (irinotecan 180 mg/m2 on day 1 plus leucovorin 400 mg/m2, followed by an FA bolus and an FU infusion, and panitumumab 6 mg/kg on day 1, every 2 weeks). After six cycles, CT scan in September 2020 displayed disease stabilization (with no change in tumor size) and, concomitantly mild COVID-19. She recovered from COVID-19 at the end of October. Unexpected reduction of peritoneal and lung disease was reported at the CT scan following COVID-19 (Figure 1). IgG anti-SARS-CoV-2 titer by Electro-Chemi-Luminescence Immuno Assay (ECLIA) was 1216 U/ml.
Discussion
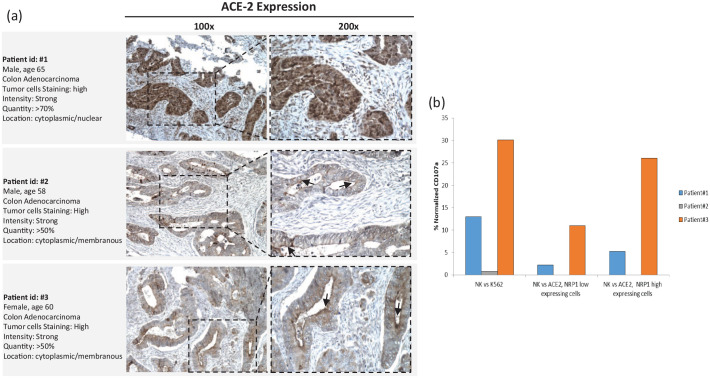
SARS-CoV-2 uses the viral spike (S) protein for host-cell attachment and entry through binding of ACE-2 (angiotensin-converting enzyme 2).2 The host protease furin cleaves the full-length precursor S glycoprotein into two associated polypeptides: S1 and S2. Cleavage of S generates a polybasic Arg-Arg-Ala-Arg carboxyl-terminal sequence on S1 that binds to cell-surface neuropilin-1 (NRP-1) and NRP-2 receptors. Blocking this interaction by ribonucleic acid interference or selective inhibitors reduces SARS-CoV-2 entry and infectivity in cell culture.3,4 Although wide distribution of ACE-2 across human tissues, including lung, liver, stomach, ileum, colon, and kidney was reported,4 alveolar pneumocytes type 2, the SARS-CoV-2 main target cell, actually expressed rather low levels of ACE-2.4–6 Hence, the SARS-CoV-2 may depend on co-receptor or other auxiliary membrane proteins to facilitate its infection. NRP-1 could represent such an ACE-2-potentiating factor by promoting the interaction of the virus with ACE-2. NRP-1 is expressed in various tissues of the body.7–9 Thus, we can hypothesize that the SARS-CoV-2 may infect ACE-2/NRP-1-expressing colon-cancer cells, evoking a direct immune response against the infected cells. Moreover, SARS-Cov-2 binding to ACE-2/NRP-1 in CRC cells might provoke cytokine release, which could enable attracting tumor microenvironment (TME) immune cells: this could be sufficient to explain an anti-tumor effect. Furthermore, cross-reactivity with viral antigens may also be involved in T (via T-cell receptor interactions) or NK (natural killer) lymphocytes [via ADCC (antibody-dependent cellular cytotoxicity)] activation against CRC cells. However, the natural immunity lymphocytes (including NK cells), which are non-major-histocompatibility-complex restricted, quickly react toward transformed or antibody-targeted cells (killing via ADCC). SARS-Cov-2 resolution is likely due to the presence of antibodies, which concomitantly eliminate infected cells (via ADCC) and prevent virus entry into new target cells. Further study of the SARS-Cov-2-infected TME (programmed cell-death protein-1/programmed cell-death ligand-1 pathway, tumor-infiltrating lymphocytes (TILs), tumor-associated macrophages, myeloid-derived suppressor cells, intratumoral cytokines patterns, etc.) will be crucial to understand (a) the consequences of eventual viral replication into tumor cells expressing high ACE-2, and (b) the involved lymphocyte sub-populations. In this regard, as a supporting hypothesis, Patients 1, 2, and 3 convincingly expressed ACE-2 [Figure 2, panel (a)] and patients 1 and 3 isolated NKs, displaying higher degranulation [Figure 2, panel (b)] toward ACE-2-/NRP-1-positive cells.
Figure 2.
Representative whole section IHC staining of ACE-2, and patients’ peripheral NKs display higher degranulation toward ACE-2/NRP-1-expressing cells.
(a) Representative whole section IHC (immunohistochemistry) staining of ACE-2. Staining was done with HPA000288 Sigma-Aldrich, 1:250 dilution, HIER pH9. Patient 1 showed strong, diffuse cytoplasmic/nuclear staining for ACE-2 in colonic cancer cells. Patient 2 showed strong, diffuse cytoplasmic/membranous ACE-2 expression, with apical linear staining pattern, to seal the apical intercellular spaces of glandular epithelia and interfacing to the paracellular macromolecules (arrow). Patient 3 showed strong and diffuse cytoplasmic/membranous ACE-2 expression with apical linear staining pattern (arrow). (b) Patients peripheral NKs display higher degranulation toward ACE-2-/NRP-1-expressing cells. HCT 116, human colon cancer cells, were low ACE-2 and NRP-1 expressing; IGROV-1, human ovarian cancer cells, were high ACE-2 and NRP-1 expressing (Tumor cell lines were puchased from American Tissue Cultutre Collection, www.lgcstandards-atcc.org). Purified NK cells (0.1 × 106) from patients’ peripheral blood were incubated with K562 cells (E:T ratio 10:1) and degranulation was evaluated through the lysosomal protein LAMP-1 (CD107a-PE, Clone H4A3, BD Pharmingen™). Patient 1 and patient 3 NKs displayed higher activity toward ACE-2-positive/NRP-1-positive-cells.
ACE-2, angiotensin-converting enzyme 2; E, effector; NK, natural killer cells; NRP, neuropilin; T, target.
In the period September–October 2020, 60 mCRC patients were observed. Interestingly, seven patients, not included in this report, developed COVID-19, and recovered from it in a median time of 34 days. In February 2021, four patients displayed undetectable humoral response (IgG <0.04 U/ml) and three showed IgG 0.8, 1.1, 3.6 U/ml. Although a detailed clinical–pathological description of this series is beyond the scope of this report, these patients did not show tumor burden reduction.
A characterization of TILs in metastatic tissue is lacking in our report. However, in CRC, there is a strong and repeatable association between microsatellite instability high (MSI-high) and abundant infiltration by CD3+ T cells. Nevertheless, the described patients were MSS. This may suggest that the observed phenomenon is more likely linked to effector cells from innate immunity.
Interestingly, a previous report linked SARS-CoV-2 infection with neoplastic course in Hodgkin lymphoma.10 Here, we described, for the first time, improvement in mCRC disease in three patients undergoing COVID-19. Our observation may contribute to generate hypotheses on the infection mechanism of the SARS-CoV-2 virus and the multiple aspects of a composite antiviral immune response in cancer patients.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Alessandro Ottaiano  https://orcid.org/0000-0002-2901-3855
https://orcid.org/0000-0002-2901-3855
Contributor Information
Alessandro Ottaiano, SSD-Innovative Therapies for Abdominal Metastases, Department of Abdominal Oncology, Istituto Nazionale Tumori di Napoli, IRCCS ‘G. Pascale,’ via M. Semmola, Naples 80131, Italy.
Stefania Scala, Microenvironment Molecular Targets, Istituto Nazionale Tumori di Napoli, IRCCS ‘G. Pascale,’ Naples, Italy.
Crescenzo D’Alterio, Microenvironment Molecular Targets, Istituto Nazionale Tumori di Napoli, IRCCS ‘G. Pascale,’ Naples, Italy.
Annamaria Trotta, Microenvironment Molecular Targets, Istituto Nazionale Tumori di Napoli, IRCCS ‘G. Pascale,’ Naples, Italy.
Annamaria Bello, Microenvironment Molecular Targets, Istituto Nazionale Tumori di Napoli, IRCCS ‘G. Pascale,’ Naples, Italy.
Giuseppina Rea, Microenvironment Molecular Targets, Istituto Nazionale Tumori di Napoli, IRCCS ‘G. Pascale,’ Naples, Italy.
Carmine Picone, Department of Radiology, Istituto Nazionale Tumori di Napoli, IRCCS ‘G. Pascale,’ Naples, Italy.
Mariachiara Santorsola, SSD-Innovative Therapies for Abdominal Metastases, Department of Abdominal Oncology, Istituto Nazionale Tumori di Napoli, IRCCS ‘G. Pascale,’ Naples, Italy.
Antonella Petrillo, Department of Radiology, Istituto Nazionale Tumori di Napoli, IRCCS ‘G. Pascale,’ Naples, Italy.
Guglielmo Nasti, SSD-Innovative Therapies for Abdominal Metastases, Department of Abdominal Oncology, Istituto Nazionale Tumori di Napoli, IRCCS ‘G. Pascale,’ Naples, Italy.
References
- 1. Nappi A, Berretta M, Romano C, et al. Metastatic colorectal cancer: role of target therapies and future perspectives. Curr Cancer Drug Targets 2018; 18: 421–429. [DOI] [PubMed] [Google Scholar]
- 2. Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A 2020; 117: 11727–11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daly JL, Simonetti B, Klein K, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020; 370: 861–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cantuti-Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020; 370: 856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li MY, Li L, Zhang Y, et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020; 9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol 2020; 251: 228–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kofler N, Simons M. The expanding role of neuropilin: regulation of transforming growth factor-β and platelet-derived growth factor signaling in the vasculature. Curr Opin Hematol 2016; 23: 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jubb AM, Strickland LA, Liu SD, et al. Neuropilin-1 expression in cancer and development. J Pathol 2012; 226: 50–60. [DOI] [PubMed] [Google Scholar]
- 10. Challenor S, Tucker D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br J Haematol 2021; 192: 415. [DOI] [PubMed] [Google Scholar]