Abstract
Background:
Antibiotic exposure has been associated with worse outcomes with immune checkpoint inhibitors (ICIs) in cancer patients, likely due to disruption of the gut microbiome. Other commonly prescribed medications, such as proton pump inhibitors (PPIs) and histamine-2-receptor antagonists (H2RAs), are also known to disrupt the microbiome, but data on their association with ICI outcomes are conflicting.
Methods:
We conducted a retrospective, multicenter, international cohort study including 314 hepatocellular carcinoma (HCC) patients treated with ICIs from 2017 to 2019 to assess the association between PPI or H2RA exposure (up to 30 days before ICI) and overall survival. Secondary outcomes included overall response rate (ORR) and development of any treatment-related adverse events (AEs).
Results:
Baseline PPI/H2RA exposure was not associated with overall survival in univariable (HR 1.01, 95% CI 0.75–1.35) or multivariable analysis (HR 0.98, 95% CI 0.71–1.36). Baseline PPI/H2RA exposure was not associated with either ORR (OR 1.32, 95% CI 0.66–2.65) or AEs (OR 1.07, 95% CI 0.54–2.12) in multivariable analysis.
Conclusions:
Our results suggest that exposure to PPI/H2RA prior to ICIs does not adversely affect outcomes in HCC patients.
Keywords: antacid, hepatocellular carcinoma, immunotherapy, proton pump inhibitor
Introduction
There is growing interest in potential interactions between commonly prescribed medications, such as antibiotics, proton pump inhibitors (PPIs), steroids, and opioids, and cancer immunotherapy.1 Antibiotic exposure has been repeatedly linked to worse immunotherapy outcomes.2–8 Experimental evidence suggests that this association is mediated through the gut microbiome, which is disrupted by antibiotics.5,9–12 Several other commonly prescribed medication classes, including antacids such as PPIs and histamine-2-receptor antagonists (H2RAs), are also known to alter the gut microbiome,13–16 and recent reports have linked PPI usage to worse immunotherapy outcomes in lung and bladder cancer.7,17,18
Immune checkpoint inhibitors (ICIs) have become an important component of hepatocellular carcinoma (HCC) management. ICI agents were initially approved by the US Food and Drug Administration in 2017 for second-line treatment of advanced HCC on the basis of promising overall response rates (ORRs) of 20% in early phase studies.19,20 More recently, the combination of atezolizumab and bevacizumab became the first immunotherapy regimen to improve overall survival (OS) over sorafenib in advanced HCC in a phase III trial and has become standard of care.21 However, ORRs remain limited, underlining the need to identify markers and mediators of ICI resistance and response.
Given the increasing use of ICIs in HCC management and the lack of published data addressing the effect of antacids on outcomes in this setting, we conducted this observational study to test the associations of PPI/H2RA exposure before ICI treatment with survival and response.
Methods
Study population
The study population comprised HCC patients treated with immunotherapy between 2017 and 2019 at 11 tertiary referral centers in the United States, Europe, and Asia. An earlier version of the cohort with fewer sites was described previously.22 Included patients had a diagnosis of HCC in accordance with American Association for the study of Liver Disease23 and European Association for the Study of the Liver24 guidelines, received systemic ICI therapy (either monotherapy or in combination), and had measurable disease according to RECIST 1.125 criteria at ICI commencement. Baseline antacid data were not available from two US sites, both of which were excluded (N = 102). An additional eight patients were excluded due to missing baseline antacid data.
Clinical variables were obtained through manual review of electronic medical records by investigators at each site using a standardized data collection form, including specific fields for concomitant medications such as antacids. Antacids were defined as PPIs (omeprazole, lansoprazole, dexlansoprazole, esomeprazole, pantoprazole, rabeprazole) or H2RAs (famotidine, ranitidine, cimetidine, nizatidine). Data were censored on 20 February 2020. Disease staging was conducted by computerized tomography or magnetic resonance imaging prior to ICI initiation and at approximately 9-week intervals during treatment.
This study was conducted in accordance with the Declaration of Helsinki. The Institutional Review Board (IRB) at Imperial College London acted as the central IRB, whose review was accepted by all participating institutions’ IRBs (Ref. 17/WA/0161/R18009) (Supplemental Table 1). The central IRB determined that this research involved no greater than minimal risk and approved a waiver for informed consent.
Study design
This was a retrospective cohort study with a primary outcome of OS, measured from the date of initiation of ICI to the date of death from any cause or last follow-up. Secondary outcomes were ORR, defined as the proportion of patients with a best response of either complete response (CR) or partial response (PR) by RECIST v1.1 criteria; disease control rate (DCR), defined as CR, PR, or stable disease (SD) by RECIST v1.1 criteria; and the development of treatment-related adverse events (AEs) of any grade. AEs were identified from clinical notes in conjunction with laboratory and radiographic evidence. Events were deemed treatment-related based on known side-effect profiles of ICI drugs and the judgment of the treating physician, with study investigators validating the association during chart review. AEs were graded following the National Cancer Institute Common Terminology Criteria for Adverse Events versus 5.0. All outcome data were obtained from the electronic medical records of the individual institutions.
The primary predictor in the analysis was baseline antacid (H2RA or PPI) exposure within 30 days prior to ICI initiation. Exposure was defined as an active prescription in the medical record per clinical notes or prescription records. Data were also collected on antacid exposures concurrent with ICI treatment; that is, antacid exposures between the dates of ICI initiation and ICI cessation. Baseline H2RA and PPI exposure were also considered separately as secondary predictors. Additional clinically relevant covariates were age, sex, alpha-fetoprotein (AFP) >400 ng/ml, presence of cirrhosis (clinically or radiologically diagnosed), Barcelona Clinic Liver Cancer (BCLC) stage (A–B versus C–D), Child–Turcotte Pugh class, Eastern Cooperative Oncology Group (ECOG) performance status (0 versus ⩾1), and antibiotic exposure within 30 days prior to ICI.
Statistical analysis
Descriptive statistics were summarized using medians and interquartile ranges (IQRs) for continuous variables, and frequency and proportions for categorical variables. The Wilcoxon rank-sum test and Fisher’s exact test were used to compare the distribution of continuous and categorical variables between antacid exposures, respectively. AFP values were missing in 17 patients and were imputed as the median value. Median OS was determined using the Kaplan–Meier method, and Kaplan–Meier survival curves were compared using the log-rank test.
Univariable and multivariable Cox proportional hazards models were used to test the association of the predictors with OS. Some covariates for the multivariable model were selected a priori, based on the number of events in the dataset and existing literature. These covariates were: age, sex, geographic region, AFP > 400 ng/ml, BCLC (A–B versus C–D), and prior antibiotics, in addition to the primary predictor. Additional covariates significant in the univariable regression analysis were added to the multivariable model, provided they were not redundant with covariates already being included, for example, BCLC stage incorporates Child–Pugh score and performance status. The proportional hazards assumption was tested and the final model was stratified by geographic region; that is, separate baseline hazard functions were estimated for each geographic region. There was no evidence of multicollinearity with variance inflation factors of all predictors <5. A summary of each model was presented using hazard ratios (HRs) and 95% confidence intervals (CIs). Subgroup analyses were conducted using a model including the interaction term between antacid exposure and the grouping variable. The HR and 95% CI for antacid exposure in each subgroup was presented, along with the p value of the interaction term.
Univariable and multivariable logistic regression models were used to test the association of predictors with the secondary outcomes ORR, DCR, and AE. A similar set of covariates were selected for the multivariable models a priori, with additional covariates added based on significance in univariable analysis, absence of collinearity, and available degrees of freedom. A summary of each model was presented using odds ratios (OR) and 95% CIs.
All analyses were conducted in R version 4.0.0 (Vienna, Austria).
Results
Baseline characteristics
The cohort included 314 patients with HCC treated with ICIs between 2017 and 2019 at centers in Asia (N = 99, 31.5%), the United States (N = 154, 49%), and Europe (N = 61, 19.4%). Clinical characteristics of the cohort are reported in Table 1.
Table 1.
Baseline characteristics, by baseline antacid exposure.
| Variable | No antacid exposure (N = 204) | Antacid exposure (N = 110) | p value | All patients (N = 314) |
|---|---|---|---|---|
| Age (years) | 65.6 (59.1–72.6) | 67.9 (58.2–70.7) | 0.77 | 66 (58.7–71.6) |
| Male | 161 (78.9%) | 87 (79.1%) | 1 | 248 (79%) |
| USA | 119 (58.3%) | 35 (31.8%) | <0.001 | 154 (49%) |
| Europe | 23 (11.3%) | 38 (34.5%) | <0.001 | 61 (19.4%) |
| Asia | 62 (30.4%) | 37 (33.6%) | 0.634 | 99 (31.5%) |
| PD-1 monotherapy | 186 (91.2%) | 85 (77.3%) | <0.001 | 271 (86.3%) |
| PD-1/CTLA-4 Combination | 8 (3.9%) | 13 (11.8%) | 0.016 | 21 (6.7%) |
| PD-1/TKI Combination | 10 (4.9%) | 12 (10.9%) | 0.063 | 22 (7%) |
| First-line ICI | 93 (45.6%) | 44 (40%) | 0.404 | 137 (43.6%) |
| Second-line ICI | 102 (50%) | 56 (50.9%) | 0.906 | 158 (50.3%) |
| Third-line or later | 9 (4.4%) | 10 (9.1%) | 0.135 | 19 (6.1%) |
| Prior sorafenib | 110 (53.9%) | 65 (59.1%) | 0.406 | 175 (55.7%) |
| Prior local therapy | 182 (89.2%) | 95 (86.4%) | 0.467 | 277 (88.2%) |
| Cirrhosis | 147 (72.1%) | 78 (70.9%) | 0.896 | 225 (71.7%) |
| Chronic hepatitis B | 57 (27.9%) | 31 (28.2%) | 1 | 88 (28%) |
| Chronic hepatitis C | 76 (37.3%) | 42 (38.2%) | 0.903 | 118 (37.6%) |
| HBV/HCV co-infection | 6 (2.9%) | 2 (1.8%) | 0.718 | 8 (2.5%) |
| Non-viral liver disease | 65 (31.9%) | 35 (31.8%) | 1 | 100 (31.8%) |
| Alcoholic liver disease | 35 (17.2%) | 23 (20.9%) | 0.447 | 58 (18.5%) |
| Non-alcoholic steatohepatitis | 21 (10.3%) | 9 (8.2%) | 0.688 | 30 (9.6%) |
| Other liver disease | 7 (3.4%) | 4 (3.6%) | 1 | 11 (3.5%) |
| ECOG ⩾ 1 | 88 (43.1%) | 57 (51.8%) | 0.155 | 145 (46.2%) |
| BCLC A | 5 (2.5%) | 0 (0%) | 0.167 | 5 (1.6%) |
| BCLC B | 51 (25%) | 30 (27.3%) | 0.686 | 81 (25.8%) |
| BCLC C | 146 (71.6%) | 77 (70%) | 0.795 | 223 (71%) |
| BCLC D | 2 (1%) | 3 (2.7%) | 0.348 | 5 (1.6%) |
| Child–Pugh A | 137 (67.2%) | 78 (70.9%) | 0.441 | 215 (68.5%) |
| Child–Pugh B | 58 (28.4%) | 27 (24.5%) | 0.507 | 85 (27.1%) |
| Child–Pugh C | 7 (3.4%) | 3 (2.7%) | 1 | 10 (3.2%) |
| Portal venous thrombosis | 68 (33.3%) | 40 (36.4%) | 0.535 | 108 (34.4%) |
| Extrahepatic metastasis | 103 (50.5%) | 59 (53.6%) | 0.637 | 162 (51.6%) |
| No measurable intrahepatic disease | 26 (12.7%) | 9 (8.2%) | 0.261 | 35 (11.1%) |
| Multifocal (⩾3) intrahepatic nodules | 115 (56.4%) | 59 (53.6%) | 0.632 | 174 (55.4%) |
| Maximum diameter of largest lesion (cm) | 6.15 (3–12.5) | 6 (3.6–10.1) | 0.663 | 6 (3.2–11.2) |
| Alpha-fetoprotein (ng/ml) | 183.3 (10.9–2563.5) | 223.65 (14.25–4615.5) | 0.408 | 183.3 (12.4–2917.7) |
| Baseline PPI only† | NA | 85 (78%) | NA | 85 (27.1%) |
| Baseline H2RA only | NA | 17 (15.6%) | NA | 17 (5.4%) |
| Baseline PPI and H2RA exposure | NA | 7 (6.4%) | NA | 7 (2.2%) |
| No concurrent antacid‡ | 147 (72.4%) | 10 (9.3%) | <0.001 | 157 (50.5%) |
| Concurrent PPI only | 34 (16.7%) | 73 (67.6%) | <0.001 | 107 (34.4%) |
| Concurrent H2RA only | 9 (4.4%) | 19 (17.6%) | <0.001 | 28 (9%) |
| Concurrent PPI and H2RA exposure | 13 (6.4%) | 6 (5.6%) | 1 | 19 (6.1%) |
| Baseline antibiotic exposure | 14 (6.9%) | 26 (23.6%) | <0.001 | 40 (12.7%) |
| Baseline steroid exposure | 4 (2%) | 10 (9.1%) | 0.006 | 14 (4.5%) |
Data are presented as medians and interquartile ranges or counts and proportions.
One patient had baseline antacid exposure, but the type of antacid was not documented.
Two patients with baseline antacid exposure had missing data on concurrent antacid exposure; one patient without baseline antacid exposure had missing data on concurrent antacid exposure.
BCLC, Barcelona Clinic Liver Cancer; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ECOG, Eastern Cooperative Oncology Group; H2RA, histamine-2-receptor antagonist; HBV, hepatitis B virus; HCV, hepatitis C virus; ICI, immune checkpoint inhibitor; PD-1, programmed cell death protein 1; PPI, proton pump inhibitor; TKI, tyrosine kinase inhibitor.
The cohort was predominantly male (N = 248, 79%), with a median age of 66 years (IQR 59–72). At baseline, 225 (71.7%) patients had clinical or radiographic evidence of cirrhosis. The most common underlying causes of liver disease were hepatitis C virus (N = 118, 37.6%) and hepatitis B virus (N = 88, 28%), followed by alcoholic liver disease (N = 58, 18.5%) and non-alcoholic steatohepatitis (N = 30, 9.6%). Liver function was preserved (Child–Pugh A) in most patients (N = 215, 68.5%).
Most patients (N = 223, 71%) met criteria for BCLC stage C HCC at the time of ICI initiation. The majority of patients were treated with anti-PD-1 monotherapy (N = 271, 86.3%). Half of the patients (N = 158, 50.3%) had received one prior systemic treatment, whereas 43.6% (N = 137) were naïve to systemic treatment. Most patients (N = 277, 88.2%) had also had prior local therapy, most commonly surgical resection (N = 103, 32.8%).
Treatment outcomes
There were 190 deaths (60.5%) during a median follow-up of 9.2 months (IQR 4.0–16.1). Based on best radiographic response, there were 123 patients with SD (39.2%), 31 with PRs (9.9%), and 21 with CRs (6.7%), resulting in a DCR of 55.7% and an ORR of 16.6%. Median OS in the whole cohort was 12.3 months (95% CI 9.9–15.7). The median duration of ICI treatment was 3.7 months (IQR 1.9–8.6). The most common reason for treatment discontinuation was progressive disease (N = 178, 56.7%). Treatment-related AEs developed in 33.8% of patients (N = 106), with 15.6% of patients (N = 49) experiencing AEs of grade 2 or higher. The most common AEs involved the liver (N = 41, 13.1%) and skin (N = 33, N = 10.5%).
Antacid exposures
Baseline exposure to antacids, either a PPI or a H2RA, within 30 days prior to ICI treatment was present in 35% of patients (N = 110) (Table 1). Most baseline exposures were to PPIs (N = 85, 27.1%), but some were exposed to H2RAs (N = 17, 5.4%), or both (N = 7, 2.2%). Among those with baseline antacid exposure, 90.9% (N = 100) also had antacid exposure concurrent with their ICI treatment.
Additional details on duration and indications for antacid usage were available for 224 patients, 76 of whom had baseline antacid exposure and 117 of whom had concurrent antacid exposure (Supplemental Tables 2 and 3). Most baseline antacids were prescribed for more than 4 weeks (77.6%), with the most common indications being acid reflux or dyspepsia (43.4%) and procedures or other prophylactic reasons (48.7%). Most concurrent antacid exposures originated from prescriptions beginning before ICI treatment (63.6%) and the most common indications were also acid reflux or dyspepsia (37.9%) and procedures or other prophylaxis (37.1%).
There were baseline differences between the patients with or without antacid exposures (Table 1). Antacid-exposed patients were more commonly from European sites (34.5% of exposed versus 11.3% of unexposed, p < 0.001) and less commonly from US sites (31.8% of exposed versus 58.3% of unexposed, p < 0.001). Antacid-exposed patients were less likely to have been treated with anti-PD-1 monotherapy (77.3% of exposed versus 91.2% of unexposed, p < 0.001), but more likely to have been treated with a combination of anti-PD-1 and anti-CTLA-4 agents (11.8% of exposed, 3.9% of unexposed, p = 0.02). Antacid-exposed patients were also more likely to have been exposed to antibiotics (23.6% of exposed versus 6.9% of unexposed, p < 0.001) or steroids (9.1% of exposed versus 2.0% of unexposed, p = 0.006) in the 30 days prior to ICI initiation.
Association of baseline antacid use with immunotherapy outcomes
In univariable analysis, baseline antacid exposure was not associated with OS (HR 1.01, 95% CI 0.75–1.35), nor was baseline PPI exposure (HR 1.14, 95% CI 0.84–1.54) or H2RA exposure (0.58, 95% CI 0.31–1.10) (Table 2). Factors associated with OS were: ECOG PS > 0 (HR 1.55, 95% CI 1.15–2.09), BCLC C or D (HR 1.45, 95% CI 1.04–2.03), Child–Pugh class B or C (HR 1.94, 95% CI 1.43–2.62), portal venous thrombosis (HR 1.73, 95% CI 1.29–2.32), multifocal (⩾3) intrahepatic nodules (HR 1.69, 95% CI 1.26–2.26), and AFP > 400 ng/ml (HR 1.39, 95% CI 1.04–1.85).
Table 2.
Univariable and multivariable Cox proportional hazards models for overall survival.
| Variable | Univariable HR (95% CI) | p value | Multivariable HR* (95% CI) | p value |
|---|---|---|---|---|
| Age (years) | 0.997 (0.99–1.01) | 0.642 | 1.00 (0.99–1.01) | 0.958 |
| Male | 1.07 (0.75–1.54) | 0.701 | 1.12 (0.77–1.61) | 0.560 |
| Geographic region | ||||
| Europe versus USA | 0.97 (0.67–1.41) | 0.866 | − | − |
| Asia versus USA | 0.83 (0.60–1.17) | 0.292 | − | − |
| Immunotherapy treatment | ||||
| PD-1/CTLA-4 versus PD-1 | 0.78 (0.44–1.37) | 0.380 | − | − |
| PD-1/TKI versus PD-1 | 0.80 (0.45–1.41) | 0.443 | − | − |
| Second-line or later | 1.15 (0.86–1.54) | 0.339 | − | − |
| Cirrhosis | 1.06 (0.77–1.45) | 0.740 | − | − |
| Liver disease | ||||
| HCV versus HBV | 0.85 (0.60–1.22) | 0.388 | − | − |
| HBV/HCV versus HBV | 0.16 (0.02–1.19) | 0.074 | − | − |
| Non-viral versus HBV | 1.09 (0.76–1.56) | 0.650 | − | − |
| ECOG ⩾ 1 | 1.55 (1.15–2.09) | 0.004 | − | − |
| BCLC C/D | 1.45 (1.04–2.03) | 0.030 | 1.22 (0.85–1.75) | 0.276 |
| Child–Pugh B/C | 1.94 (1.43–2.62) | <0.001 | − | − |
| Portal venous thrombosis | 1.73 (1.29–2.32) | <0.001 | 1.51 (1.10–2.08) | 0.011 |
| Extrahepatic metastasis | 1.06 (0.80–1.42) | 0.673 | − | − |
| ⩾3 intrahepatic nodules | 1.69 (1.26–2.26) | <0.001 | 1.83 (1.34–2.50) | <0.001 |
| Maximum diameter of largest lesion (cm) | 0.998 (0.99–1.00) | 0.503 | − | − |
| Alpha-fetoprotein >400 ng/ml | 1.39 (1.04–1.85) | 0.025 | 1.31 (0.96–1.79) | 0.084 |
| Baseline antacid exposure | 1.01 (0.75–1.35) | 0.971 | 0.98 (0.71–1.36) | 0.909 |
| Baseline PPI exposure | 1.14 (0.84–1.54) | 0.409 | − | − |
| Baseline H2RA exposure | 0.58 (0.31–1.10) | 0.095 | − | − |
| Baseline antibiotic exposure | 1.38 (0.92–2.06) | 0.122 | 1.23 (0.78–1.94) | 0.370 |
Stratified by geographic region.
BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ECOG, Eastern Cooperative Oncology Group; H2RA, histamine-2-receptor antagonist; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; PD-1, programmed cell death protein 1; PPI, proton pump inhibitor; TKI, tyrosine kinase inhibitor.
In multivariable analysis, baseline antacid exposure remained unassociated with OS (HR 0.98, 95% CI 0.71–1.36) (Table 2). Significant independent predictors of survival in this model were multifocal intrahepatic disease (HR 1.83, 95% CI 1.34–2.50) and portal venous thrombosis (HR 1.51, 95% CI 1.10–2.08).
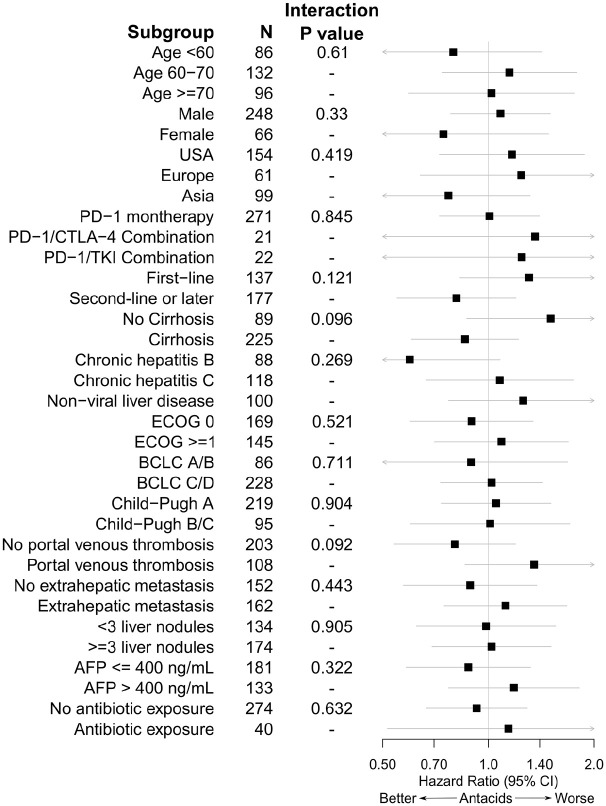
In subgroup analyses, there were trends towards heterogeneous effects of antacid exposure based on cirrhosis status (interaction p = 0.096) and the presence or absence of portal venous thrombosis (interaction p = 0.092) (Figure 1).
Figure 1.
Cox proportional hazards models for overall survival taking into account interactions between baseline antacid exposure and subgroup membership. Hazard ratios and 95% confidence intervals for antacid exposure are presented along with p values for the interaction term in each mod.
To control more strictly for possible confounding by baseline antibiotic exposure, we repeated the OS analysis while excluding the 40 patients who had received baseline antibiotics. Baseline antacid use was not associated with OS in univariable analysis (HR 0.92, 95% CI 0.66–1.29) nor multivariable analysis (HR 0.92, 95% CI 0.63–1.34) after excluding antibiotic-exposed patients.
Baseline antacid exposure was not associated with either ORR (univariable OR 1.06, 95% CI 0.57–1.98; multivariable OR 1.32, 95% CI 0.66–2.65) or DCR (univariable OR 1.56, 95% CI 0.95–2.57; multivariable OR 1.34, 95% CI 0.75–2.39). There were no significant associations with ORR in either univariable or multivariable analysis (Table 3). DCR was associated with AFP > 400 ng/ml (OR 0.57, 95% CI 0.36–0.9), European sites (OR 3.97, 95% CI 1.91–8.24), and chronic hepatitis C (OR 2.04, 95% CI 1.14–3.66) in univariable analysis. AFP > 400 ng/ml (OR 0.55, 95% CI 0.33–0.92) and European sites (OR 3.94, 95% CI 1.73–9.03) remained independently associated with DCR in multivariable analysis (Table 4).
Table 3.
Univariable and multivariable logistic regression for overall response.
| Variable | Univariable OR (95% CI) | p value | Multivariable OR (95% CI) | p value |
|---|---|---|---|---|
| Age (years) | 0.99 (0.97–1.02) | 0.459 | 0.99 (0.97–1.02) | 0.534 |
| Male | 0.95 (0.45–1.97) | 0.883 | 0.88 (0.41–1.85) | 0.730 |
| Geographic region | ||||
| Europe versus USA | 0.63 (0.26–1.53) | 0.306 | 0.53 (0.20–1.44) | 0.213 |
| Asia versus USA | 1.28 (0.66–2.48) | 0.462 | 1.16 (0.58–2.35) | 0.676 |
| Immunotherapy treatment | ||||
| PD-1/CTLA-4 versus PD-1 | 1.90 (0.70–5.17) | 0.209 | − | − |
| PD-1/TKI versus PD-1 | 0.53 (0.12–2.36) | 0.403 | − | − |
| Second line or later | 0.83 (0.45–1.51) | 0.537 | − | − |
| Cirrhosis | 1.74 (0.83–3.67) | 0.142 | − | − |
| Liver disease | ||||
| HCV versus HBV | 0.94 (0.46–1.9) | 0.852 | − | − |
| HBV/HCV versus HBV | 0.77 (0.08–6.99) | 0.812 | − | − |
| Non-viral versus HBV | 0.56 (0.25–1.25) | 0.159 | − | − |
| ECOG ⩾ 1 | 1.08 (0.59–1.97) | 0.799 | − | − |
| BCLC C/D | 0.83 (0.43–1.59) | 0.567 | − | − |
| Child–Pugh B/C | 1.33 (0.70–2.51) | 0.383 | − | − |
| Portal venous thrombosis | 1.04 (0.55–1.95) | 0.905 | − | − |
| Extrahepatic metastasis | 0.85 (0.47–1.55) | 0.602 | − | − |
| ⩾3 intrahepatic nodules | 0.66 (0.36–1.22) | 0.185 | − | − |
| Maximum diameter of largest lesion (cm) | 1.00 (0.99–1.01) | 0.994 | − | − |
| Alpha-fetoprotein >400 ng/ml | 0.95 (0.51–1.74) | 0.858 | − | − |
| Baseline antacid exposure | 1.06 (0.57–1.98) | 0.847 | 1.32 (0.66–2.65) | 0.429 |
| Baseline PPI exposure | 1.15 (0.60–2.18) | 0.676 | − | − |
| Baseline H2RA exposure | 1.10 (0.36–3.42) | 0.865 | − | − |
| Baseline antibiotic exposure | 0.67 (0.25–1.82) | 0.435 | 0.60 (0.20–1.74) | 0.343 |
BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ECOG, Eastern Cooperative Oncology Group; H2RA, histamine-2-receptor antagonist; HBV, hepatitis B virus; HCV, hepatitis C virus; OR, odds ratio; PD-1, programmed cell death protein 1; PPI, proton pump inhibitor; TKI, tyrosine kinase inhibitor.
Table 4.
Univariable and multivariable logistic regression for disease control rate.
| Variable | Univariable OR (95% CI) | p value | Multivariable OR (95% CI) | p value |
|---|---|---|---|---|
| Age (years) | 1.02 (1.00–1.05) | 0.028 | 1.01 (0.99–1.03) | 0.472 |
| Male | 0.98 (0.55–1.74) | 0.930 | 1.16 (0.62–2.18) | 0.648 |
| Geographic region | ||||
| Europe versus USA | 3.97 (1.91–8.24) | <0.001 | 3.94 (1.73–9.03) | 0.001 |
| Asia versus USA | 1.2 (0.70–2.04) | 0.509 | 1.40 (0.75–2.60) | 0.296 |
| Immunotherapy treatment | ||||
| PD-1/CTLA-4 versus PD-1 | 2.02 (0.76–5.37) | 0.160 | − | − |
| PD-1/TKI versus PD-1* | − | − | − | − |
| Second line or later | 1.08 (0.68–1.73) | 0.741 | − | − |
| Cirrhosis | 1.02 (0.60–1.71) | 0.955 | − | − |
| Liver disease | ||||
| HCV versus HBV | 2.04 (1.14–3.66) | 0.016 | 1.88 (0.95–3.71) | 0.069 |
| HBV/HCV versus HBV | 5.26 (0.59–46.9) | 0.138 | 8.25 (0.84–81.9) | 0.070 |
| Non-viral versus HBV | 1.55 (0.85–2.81) | 0.152 | 1.08 (0.54–2.16) | 0.836 |
| ECOG ⩾ 1 | 0.89 (0.56–1.43) | 0.637 | − | − |
| BCLC C/D | 0.88 (0.52–1.49) | 0.635 | 0.93 (0.52–1.63) | 0.790 |
| Child–Pugh B/C | 0.78 (0.47–1.29) | 0.325 | − | − |
| Portal venous thrombosis | 0.94 (0.57–1.54) | 0.802 | − | − |
| Extrahepatic metastasis | 1.07 (0.67–1.7) | 0.790 | − | − |
| ⩾3 Intrahepatic nodules | 0.66 (0.41–1.06) | 0.083 | − | − |
| Maximum diameter of largest lesion (cm) | 1.01 (0.997–1.02) | 0.198 | − | − |
| Alpha-fetoprotein >400 ng/ml | 0.57 (0.36–0.92) | 0.021 | 0.55 (0.33–0.92) | 0.024 |
| Baseline antacid exposure | 1.56 (0.95–2.57) | 0.079 | 1.34 (0.75–2.39) | 0.332 |
| Baseline PPI exposure | 1.31 (0.79–2.2) | 0.299 | − | − |
| Baseline H2RA exposure | 2.29 (0.82–6.44) | 0.115 | − | − |
| Baseline antibiotic exposure | 0.72 (0.37–1.44) | 0.355 | 0.63 (0.29–1.39) | 0.252 |
All 20 patients treated with PD-1/TKI attained disease control.
BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ECOG, Eastern Cooperative Oncology Group; H2RA, histamine-2-receptor antagonist; HBV, hepatitis B virus; HCV, hepatitis C virus; OR, odds ratio; PD-1, programmed cell death protein 1; PPI, proton pump inhibitor; TKI, tyrosine kinase inhibitor.
Baseline antacid exposure was associated with development of any AE (OR 1.85, 95% CI 1.14–3.05) in univariable analysis, but not multivariable analysis (OR 1.07, 95% CI 0.54–2.12) (Table 5). Predictors independently associated with development of any AE were European site (OR 8.33, 95% CI 2.64–26.4) and maximum diameter of the largest nodule in centimeters (OR 1.05, 95% CI 1.03–1.07). Compared with anti-PD-1 monotherapy, combination treatments were associated with AEs in univariable (PD-1/CTLA-4 OR 5.1, 95% CI 1.98–13.1; PD-1/TKI 8.17, 95% CI 2.89–23.1) but not multivariable analysis (PD-1/CTLA-4 OR 1.08, 95% CI 0.29–4.06; PD-1/TKI OR 1.68, 95% CI 0.41–6.89).
Table 5.
Univariable and multivariable logistic regression for any treatment-related adverse event.
| Variable | Univariable OR (95% CI) | p value | Multivariable OR (95% CI) | p value |
|---|---|---|---|---|
| Age (years) | 1.02 (0.995–1.04) | 0.131 | 0.999 (0.97–1.03) | 0.960 |
| Male | 0.66 (0.38–1.16) | 0.149 | 0.69 (0.33–1.47) | 0.341 |
| Geographic region | ||||
| Europe versus USA | 9.58 (4.84–18.9) | <0.001 | 8.33 (2.64–26.4) | <0.001 |
| Asia versus USA | 2.14 (1.21–3.81) | 0.009 | 0.61 (0.24–1.55) | 0.295 |
| Immunotherapy treatment | ||||
| PD-1/CTLA-4 versus PD-1 | 5.10 (1.98–13.1) | <0.001 | 1.08 (0.29–4.06) | 0.908 |
| PD-1/TKI versus PD-1 | 8.17 (2.89–23.1) | <0.001 | 1.68 (0.41–6.89) | 0.469 |
| Second line or later | 0.96 (0.60–1.53) | 0.848 | − | − |
| Cirrhosis | 0.75 (0.45–1.26) | 0.277 | − | − |
| Liver disease | ||||
| HCV versus HBV | 1.90 (1.02–3.51) | 0.042 | 1.69 (0.74–3.86) | 0.213 |
| HBV/HCV versus HBV* | − | − | − | − |
| Non-viral versus HBV | 2.30 (1.22–4.32) | 0.010 | 1.37 (0.58–3.25) | 0.475 |
| ECOG ⩾ 1 | 0.55 (0.34–0.89) | 0.014 | − | − |
| BCLC C/D | 0.88 (0.52–1.48) | 0.634 | 0.70 (0.37–1.35) | 0.288 |
| Child–Pugh B/C | 0.65 (0.38–1.09) | 0.104 | − | − |
| Portal venous thrombosis | 0.92 (0.56–1.51) | 0.732 | − | − |
| Extrahepatic metastasis | 1.23 (0.77–1.97) | 0.384 | − | − |
| ⩾3 intrahepatic nodules | 0.81 (0.51–1.30) | 0.386 | − | − |
| Maximum diameter of largest lesion (cm) | 1.03 (1.01–1.04) | <0.001 | 1.05 (1.03–1.07) | <0.001 |
| Alpha-fetoprotein >400 ng/ml | 1.40 (0.87–2.25) | 0.161 | 1.15 (0.62–2.13) | 0.650 |
| Baseline antacid exposure | 1.85 (1.14–3.01) | 0.013 | 1.07 (0.54–2.12) | 0.850 |
| Baseline PPI exposure | 1.84 (1.11–3.05) | 0.018 | − | − |
| Baseline H2RA exposure | 0.63 (0.24–1.63) | 0.338 | − | − |
| Baseline antibiotic exposure | 0.71 (0.34–1.48) | 0.356 | 1.17 (0.44–3.1) | 0.750 |
No patients with HBV/HCV co-infection (N = 8) developed treatment-related adverse events.
BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ECOG, Eastern Cooperative Oncology Group; H2RA, histamine-2-receptor antagonist; HBV, hepatitis B virus; HCV, hepatitis C virus; OR, odds ratio; PD-1, programmed cell death protein 1; PPI, proton pump inhibitor; TKI, tyrosine kinase inhibitor.
Discussion
In this multicenter retrospective cohort study of patients with advanced HCC and treated with ICI therapy, there was no association between OS and exposure to PPIs or H2RAs within 30 days prior to ICI initiation. Furthermore, there were no significant associations between antacid exposure and secondary outcomes such as OR, disease control, or AEs.
Previous observational studies of antacid exposure and immunotherapy outcomes have yielded mixed results. Several single-center retrospective studies focusing on patients with non-small cell lung cancer (NSCLC) or melanoma have failed to find an association between PPI use and immunotherapy outcome.26–29 Two of these studies were able to detect significant associations between immunotherapy outcome and antibiotic use, but not PPI exposure.27,29 Another study using a combination of retrospective and early phase clinical trial data found significant associations between outcomes and antibiotic exposure, but not PPI exposure.5
Conversely, post hoc analyses of phase II and III clinical trial data have found independent associations between PPI exposure and immunotherapy outcomes. Using data from the Checkmate 069 trial comparing ipilimumab versus ipilimumab with nivolumab in melanoma, Homicsko et al.30 found significantly reduced ORR, progression-free survival, and OS among PPI-exposed patients; these associations were maintained in multivariable analysis. Chalabi et al.7 and Hopkins et al.17 have also recently reported large analyses showing negative associations between PPI exposure and atezolizumab outcomes in lung and bladder cancer, respectively.
Chalabi et al.7 used data from the OAK and POPLAR trials of atezolizumab versus chemotherapy to examine the impact of antibiotic and PPI exposure in 1512 immunotherapy-treated patients with NSCLC. They found that both antibiotic and PPI exposure were independently associated with reduced OS among atezolizumab-treated patients, but not chemotherapy-treated patients. However, the interaction between PPIs and treatment was not significant.
Hopkins et al.17 examined PPI use as a predictor of outcomes among 1360 bladder cancer patients treated with atezolizumab or chemotherapy in the IMvigor210 and IMvigor211 trials. They similarly found that PPI exposure was an independent predictor of worse OS and PFS in atezolizumab-treated but not chemotherapy-treated patients; in this case, the interaction between PPI and treatment was statistically significant. Overall, these analyses from clinical trials are the most compelling evidence thus far that PPI usage can influence immunotherapy outcome in solid tumors.
Unlike prior studies, our study focuses on patients with HCC. The question of antacid exposure and immunotherapy is particularly relevant in HCC for both clinical and mechanistic reasons. Patients with chronic liver disease and HCC are often prescribed PPIs, whether they may be clinically indicated or not.31 Our results suggest that those with indications for PPIs may use them prior to immunotherapy without adversely affecting outcomes.
Mechanistically, the negative findings of our study raise questions of whether the immune microenvironment or microbiome in HCC differs from lung and bladder cancer to the extent that medication exposures have less influence on immunotherapy outcomes. The liver has been recognized as a site of myeloid-derived suppressor cell accumulation,32 and liver metastases have been associated with worse immunotherapy outcomes in lung cancer.33,34 Both cirrhosis and HCC are associated with altered gut microbiota and the microbiome has been linked with progression of cirrhosis to HCC.35–38
It is possible that the baseline dysbiosis of cirrhotic HCC patients limits the impact of medication-induced perturbations to the microbiome. Intriguingly, we found a suggestive but non-significant interaction (p = 0.096) between antacid exposure and cirrhosis status with regards to OS. One recent study of eight HCC patients reported microbial differences between immunotherapy-responders and non-responders but excluded patients with advanced liver disease.39 More microbiome data from immunotherapy-treated patients are needed to understand the interactions between medication exposures, the microbiome, and immunotherapy outcomes in HCC.
Another mechanistic link between PPIs and immunotherapy outcomes may be through the pH of the tumor microenvironment. Pre-clinical studies have linked the acidity of the tumor microenvironment with T-cell anergy and immune escape.40,41 Experiments in mice have demonstrated that systemic PPIs can increase intra-tumoral pH,42 which can in turn improve response to immunotherapy.41 The net effect of PPIs on immunotherapy outcomes may depend on the balance between detrimental impacts on the microbiome versus beneficial changes in the tumor microenvironment.
The external validity of these findings is bolstered by the size and geographic diversity of this cohort. Unlike clinical trials, which have been mostly limited to patients with preserved liver function, this observational cohort reflects a real-world clinical population. In-depth characterization of each patient’s tumor also allowed us to control for potential confounders such as performance status, liver function, disease stage, and antibiotic exposure.
This study has several limitations. Although our cohort size compares favorably with other retrospective studies examining this question,26–29 power may have been limited to detect significant effects. Our definition of antacid exposure did not incorporate duration or dose of antacid treatment, and medication adherence could not be assessed from the medical record alone. However, this definition and its limitations is consistent the definition of exposure used in prior studies, including the large studies of Chalabi et al.7 and Hopkins et al.17 Although we captured antibiotic and steroid use, we did not record use of other common medications which may also influence the microbiome or the immune system. Ultimately, this retrospective analysis was exploratory and the findings are hypothesis-generating. Untangling causal relationships between medications, the microbiome, and cancer immunotherapy will require larger retrospective clinical datasets, in-depth mechanistic studies, and randomized clinical trials, where feasible.
In conclusion, these observational data suggest that PPIs and H2RAs do not adversely impact ICI therapy outcomes in patients with advanced HCC. These findings require validation in prospective cohorts and provide motivation for mechanistic studies to dissect the interactions of medication exposures and the microbiome in HCC as compared to other solid tumors.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211010937 for Antacid exposure and immunotherapy outcomes among patients with advanced hepatocellular carcinoma by Tomi Jun, Umut Ozbek, Sirish Dharmapuri, Camille Hardy-Abeloos, Huili Zhu, Jung-Yi Lin, Nicola Personeni, Tiziana Pressiani, Naoshi Nishida, Pei-Chang Lee, Chieh-Ju Lee, Hannah Hildebrand, Neil Nimkar, Sonal Paul, Petros Fessas, Muntaha Naeem, Dominik Bettinger, Uqba Khan, Anwaar Saeed, Yi-Hsiang Huang, Masatoshi Kudo, Lorenza Rimassa, Thomas U. Marron, David J. Pinato and Celina Ang in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359211010937 for Antacid exposure and immunotherapy outcomes among patients with advanced hepatocellular carcinoma by Tomi Jun, Umut Ozbek, Sirish Dharmapuri, Camille Hardy-Abeloos, Huili Zhu, Jung-Yi Lin, Nicola Personeni, Tiziana Pressiani, Naoshi Nishida, Pei-Chang Lee, Chieh-Ju Lee, Hannah Hildebrand, Neil Nimkar, Sonal Paul, Petros Fessas, Muntaha Naeem, Dominik Bettinger, Uqba Khan, Anwaar Saeed, Yi-Hsiang Huang, Masatoshi Kudo, Lorenza Rimassa, Thomas U. Marron, David J. Pinato and Celina Ang in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-3-tam-10.1177_17588359211010937 for Antacid exposure and immunotherapy outcomes among patients with advanced hepatocellular carcinoma by Tomi Jun, Umut Ozbek, Sirish Dharmapuri, Camille Hardy-Abeloos, Huili Zhu, Jung-Yi Lin, Nicola Personeni, Tiziana Pressiani, Naoshi Nishida, Pei-Chang Lee, Chieh-Ju Lee, Hannah Hildebrand, Neil Nimkar, Sonal Paul, Petros Fessas, Muntaha Naeem, Dominik Bettinger, Uqba Khan, Anwaar Saeed, Yi-Hsiang Huang, Masatoshi Kudo, Lorenza Rimassa, Thomas U. Marron, David J. Pinato and Celina Ang in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors acknowledge the infrastructure support provided by the Tisch Cancer Institute and the Icahn School of Medicine at Mount Sinai. DJP acknowledges infrastructural support by the Cancer Research UK Imperial Centre and the Imperial Experimental Cancer Medicine Centre and the Imperial College Tissue Bank.
Footnotes
Author contributions: TJ and CA conceived and designed the study. SD, NP, TP, NN, PCL, CJL, HH, NN, SP, PF, MN, UK, AS, YHH, MK, LR, TM, and DJP made substantial contributions to the acquisition of data. TJ, UO, and JYL analyzed the data. TJ and CA interpreted the data. TJ drafted the manuscript. All authors reviewed the work for important intellectual content.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: DJP is supported by grant funding from the Wellcome Trust Strategic Fund (grant number PS3416). Research reported in this publication was supported in part by the National Cancer Institute Cancer Center (Support Grant P30CA196521-01 awarded to the Tisch Cancer Institute of the Icahn School of Medicine at Mount Sinai) and used the Biostatistics Shared Resource Facility. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest statement: DJP received lecture fees from ViiV Healthcare and Bayer Healthcare and travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, and Astra Zeneca; received research funding (to institution) from MSD and BMS.
LR reports receiving consulting fees from Amgen, ArQule, AstraZeneca, Basilea, Bayer, Celgene, Eisai, Exelixis, Hengrui, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Sanofi; lectures fees from AbbVie, Amgen, Eisai, Gilead, Incyte, Ipsen, Lilly, Roche, Sanofi; travel fees from Ipsen; and institutional research funding from Agios, ARMO BioSciences, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Roche. NP reports receiving consulting fees from Amgen, Merck Serono, Servier; lectures fees from AbbVie, Gilead, Lilly; travel fees from Amgen, ArQule; and institutional research funding from Basilea, Merck Serono, Servier. TP reports receiving institutional research funding from Lilly.
Ethics approval and consent to participate: This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board in each participating institution.
ORCID iDs: Tomi Jun  https://orcid.org/0000-0002-2120-1704
https://orcid.org/0000-0002-2120-1704
Lorenza Rimassa  https://orcid.org/0000-0001-9957-3615
https://orcid.org/0000-0001-9957-3615
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Tomi Jun, Division of Hematology and Medical Oncology, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Umut Ozbek, Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Sirish Dharmapuri, Division of Hematology and Medical Oncology, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Camille Hardy-Abeloos, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Huili Zhu, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Jung-Yi Lin, Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Nicola Personeni, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; Medical Oncology and Hematology Unit, Humanitas Cancer Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy.
Tiziana Pressiani, Medical Oncology and Hematology Unit, Humanitas Cancer Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy.
Naoshi Nishida, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Pei-Chang Lee, Division of Gastroenterology and Hepatology, Department of Medicine, Taipei; Veterans General Hospital, Institute of Clinical Medicine, School of Medicine, National Yang-Ming University, Taipei.
Chieh-Ju Lee, Division of Gastroenterology and Hepatology, Department of Medicine, Taipei; Veterans General Hospital, Institute of Clinical Medicine, School of Medicine, National Yang-Ming University, Taipei.
Hannah Hildebrand, Division of Medical Oncology, Department of Medicine, Kansas University Cancer Center, Westwood, KS, USA.
Neil Nimkar, New York Presbyterian Brooklyn Methodist Hospital, Brooklyn, NY, USA.
Sonal Paul, New York Presbyterian Brooklyn Methodist Hospital, Brooklyn, NY, USA.
Petros Fessas, Department of Surgery and Cancer, Imperial College London, Hammersmith Hospital, London, UK.
Muntaha Naeem, Department of Surgery and Cancer, Imperial College London, Hammersmith Hospital, London, UK.
Dominik Bettinger, Department of Medicine II, Faculty of Medicine, Medical Center University of Freiburg, University of Freiburg, Freiburg, Germany.
Uqba Khan, Division of Hematology and Oncology, Weill Cornell Medicine/New York Presbyterian Hospital, New York, NY, USA.
Anwaar Saeed, Division of Medical Oncology, Department of Medicine, Kansas University Cancer Center, Westwood, KS, USA.
Yi-Hsiang Huang, Division of Gastroenterology and Hepatology, Department of Medicine, Taipei; Veterans General Hospital, Institute of Clinical Medicine, School of Medicine, National Yang-Ming University, Taipei.
Masatoshi Kudo, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Lorenza Rimassa, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; Medical Oncology and Hematology Unit, Humanitas Cancer Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy.
Thomas U. Marron, Division of Hematology and Medical Oncology, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
David J. Pinato, Imperial Centre for Translational and Experimental Medicine (ICTEM), 72 Du Cane Road, White City, London, W12 0NN, UK Division of Oncology, Department of Translational Medicine, Piemonte Orientale University, Novara, Italy.
Celina Ang, Division of Hematology and Medical Oncology, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, 1 Gustave Levy Place, Box 1079, New York, NY 10029, USA.
References
- 1. Hussain N, Naeem M, Pinato DJ. Concomitant medications and immune checkpoint inhibitor therapy for cancer: causation or association? Hum Vaccin Immunother. Epub ahead of print 23 June 2020. DOI: 10.1080/21645515.2020.1769398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tinsley N, Zhou C, Tan G, et al. Cumulative antibiotic use significantly decreases efficacy of checkpoint inhibitors in patients with advanced cancer. Oncologist. Epub ahead of print 10 July 2019. DOI: 10.1634/theoncologist.2019-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pinato DJ, Howlett S, Ottaviani D, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol 2019; 5: 1774–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greally M, Chou JF, Chatila WK, et al. Clinical and molecular predictors of response to immune checkpoint inhibitors in patients with advanced esophagogastric cancer. Clin Cancer Res 2019; 25: 6160–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359: 91–97. [DOI] [PubMed] [Google Scholar]
- 6. Hopkins AM, Kichenadasse G, Karapetis CS, et al. Concomitant antibiotic use and survival in urothelial carcinoma treated with atezolizumab. Eur Urol 2020; 78: 540–543. [DOI] [PubMed] [Google Scholar]
- 7. Chalabi M, Cardona A, Nagarkar DR, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol 2020; 31: 525–531. [DOI] [PubMed] [Google Scholar]
- 8. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 2018; 29: 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015; 350: 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018; 359: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018; 359: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 2015; 350: 1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jackson MA, Goodrich JK, Maxan M-E, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016; 65: 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut 2016; 65: 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dial S, Delaney JAC, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005; 294: 2989–2995. [DOI] [PubMed] [Google Scholar]
- 16. Gupta RW, Tran L, Norori J, et al. Histamine-2 receptor blockers alter the fecal microbiota in premature infants. J Pediatr Gastroenterol Nutr 2013; 56: 397–400. [DOI] [PubMed] [Google Scholar]
- 17. Hopkins AM, Kichenadasse G, Karapetis CS, et al. Concomitant proton pump inhibitor use and survival in urothelial carcinoma treated with atezolizumab. Clin Cancer Res 2020; 26: 5487–5493. [DOI] [PubMed] [Google Scholar]
- 18. Buti S, Bersanelli M, Perrone F, et al. Effect of concomitant medications with immune-modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: development and validation of a novel prognostic index. Eur J Cancer 2021; 142: 18–28. [DOI] [PubMed] [Google Scholar]
- 19. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19: 940–952. [DOI] [PubMed] [Google Scholar]
- 20. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020; 382: 1894–1905. [DOI] [PubMed] [Google Scholar]
- 22. Pinato DJ, Kaneko T, Saeed A, et al. Immunotherapy in hepatocellular cancer patients with mild to severe liver dysfunction: adjunctive role of the ALBI Grade. Cancers (Basel) 2020; 12: 1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018; 67: 358–380. [DOI] [PubMed] [Google Scholar]
- 24. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 25. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 26. Mukherjee S, Ibrahimi S, Khalid B, et al. Do proton pump inhibitors modulate the efficacy of anti-PD-1/PD-L1 therapy? A retrospective study. J Oncol Pharm Pract 2019; 25: 762–764. [DOI] [PubMed] [Google Scholar]
- 27. Hakozaki T, Okuma Y, Omori M, et al. Impact of prior antibiotic use on the efficacy of nivolumab for non-small cell lung cancer. Oncol Lett 2019; 17: 2946–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trabolsi A, Winter M, Rodriguez E. Proton pump inhibitors and response to immune check-point inhibitors: single center study. JCO 2019; 37 (Suppl. 15): e14092. [Google Scholar]
- 29. Zhao S, Gao G, Li W, et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer 2019; 130: 10–17. [DOI] [PubMed] [Google Scholar]
- 30. Homicsko K, Richtig G, Tuchmann F, et al. LBA2 Proton pump inhibitors negatively impact survival of PD-1 inhibitor based therapies in metastatic melanoma patients. Ann Oncol 2018; 29(Suppl. 10): 1. [Google Scholar]
- 31. Cole HL, Pennycook S, Hayes PC. The impact of proton pump inhibitor therapy on patients with liver disease. Aliment Pharmacol Ther 2016; 44: 1213–1223. [DOI] [PubMed] [Google Scholar]
- 32. Ilkovitch D, Lopez DM. The liver is a site for tumor induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res 2009; 69: 5514–5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cortellini A, Tiseo M, Banna GL, et al. Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ⩾50%. Cancer Immunol Immunother 2020; 69: 2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Botticelli A, Cirillo A, Scagnoli S, et al. The agnostic role of site of metastasis in predicting outcomes in cancer patients treated with immunotherapy. Vaccines (Basel) 2020; 8: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bajaj JS, Heuman DM, Hylemon PB, et al. The cirrhosis dysbiosis ratio defines changes in the gut microbiome associated with cirrhosis and its complications. J Hepatol 2014; 60: 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen Y, Yang F, Lu H, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011; 54: 562–572. [DOI] [PubMed] [Google Scholar]
- 37. Ponziani FR, Bhoori S, Castelli C, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology 2019; 69: 107–120. [DOI] [PubMed] [Google Scholar]
- 38. Fox JG, Feng Y, Theve EJ, et al. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut 2010; 59: 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zheng Y, Wang T, Tu X, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer 2019; 7: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bellone M, Calcinotto A, Filipazzi P, et al. The acidity of the tumor microenvironment is a mechanism of immune escape that can be overcome by proton pump inhibitors. Oncoimmunology 2013; 2: e22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, et al. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res 2016; 76: 1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Calcinotto A, Filipazzi P, Grioni M, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res 2012; 72: 2746–2756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211010937 for Antacid exposure and immunotherapy outcomes among patients with advanced hepatocellular carcinoma by Tomi Jun, Umut Ozbek, Sirish Dharmapuri, Camille Hardy-Abeloos, Huili Zhu, Jung-Yi Lin, Nicola Personeni, Tiziana Pressiani, Naoshi Nishida, Pei-Chang Lee, Chieh-Ju Lee, Hannah Hildebrand, Neil Nimkar, Sonal Paul, Petros Fessas, Muntaha Naeem, Dominik Bettinger, Uqba Khan, Anwaar Saeed, Yi-Hsiang Huang, Masatoshi Kudo, Lorenza Rimassa, Thomas U. Marron, David J. Pinato and Celina Ang in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359211010937 for Antacid exposure and immunotherapy outcomes among patients with advanced hepatocellular carcinoma by Tomi Jun, Umut Ozbek, Sirish Dharmapuri, Camille Hardy-Abeloos, Huili Zhu, Jung-Yi Lin, Nicola Personeni, Tiziana Pressiani, Naoshi Nishida, Pei-Chang Lee, Chieh-Ju Lee, Hannah Hildebrand, Neil Nimkar, Sonal Paul, Petros Fessas, Muntaha Naeem, Dominik Bettinger, Uqba Khan, Anwaar Saeed, Yi-Hsiang Huang, Masatoshi Kudo, Lorenza Rimassa, Thomas U. Marron, David J. Pinato and Celina Ang in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-3-tam-10.1177_17588359211010937 for Antacid exposure and immunotherapy outcomes among patients with advanced hepatocellular carcinoma by Tomi Jun, Umut Ozbek, Sirish Dharmapuri, Camille Hardy-Abeloos, Huili Zhu, Jung-Yi Lin, Nicola Personeni, Tiziana Pressiani, Naoshi Nishida, Pei-Chang Lee, Chieh-Ju Lee, Hannah Hildebrand, Neil Nimkar, Sonal Paul, Petros Fessas, Muntaha Naeem, Dominik Bettinger, Uqba Khan, Anwaar Saeed, Yi-Hsiang Huang, Masatoshi Kudo, Lorenza Rimassa, Thomas U. Marron, David J. Pinato and Celina Ang in Therapeutic Advances in Medical Oncology