Abstract
The aim of this review is to provide a systematic design guideline to users, particularly engineers interested in developing and deploying lung models, and biologists seeking to identify a suitable platform for conducting in vitro experiments involving pulmonary cells or tissues. We first discuss the state of the art on lung in vitro models, describing the most simplistic and traditional ones. Then, we analyze in further detail the more complex dynamic engineered systems that either provide mechanical cues, or allow for more predictive exposure studies, or in some cases even both. This is followed by a dedicated section on microchips of the lung. Lastly, we present a critical discussion of the different characteristics of each type of system and the criteria which may help researchers select the most appropriate technology according to their specific requirements. Readers are encouraged to refer to the tables accompanying the different sections where comprehensive and quantitative information on the operating parameters and performance of the different systems reported in the literature is provided.
Keywords: Lung models, in vitro models, aerosol exposure, fluidic systems, stretching systems
Introduction
Epithelial barriers regulate the passage from one domain to another, and are the body’s natural defense against external substances.1 Lung epithelium is one of the most permeable epithelial barriers of the human body2 and it is the object of different investigations regarding drug and nanoparticle (NP) delivery and toxicology. Recent developments in delivering drugs to the lung are driving the need for studies to evaluate the fate of inhaled medicines.3 In particular, inhalation of aerosolized drugs is a promising route for noninvasive targeted drug delivery to the lung.4 Additionally, researchers are focusing their attention on the adverse effects caused by inhaled nanoparticles and chemical compounds (which depend on their hazard), and on exposure.2 To understand what can and cannot cross the lung barrier and their effects on the human tissues, models have emerged to rigorously study and investigate these questions.
Both in vivo and in vitro models are used for lung pathology (such as infection, inflammation, cancer, small-airway pulmonary diseases), drug delivery, and toxicology studies. Indeed, animal models provide a means for testing hypotheses, such as the therapeutic efficacy of a drug candidate, in complex biological systems. In vivo models are important for the evaluation of drug deposition efficiency, or to study the effects of nanomaterials and inhaled chemicals on lungs and peripheral tissue.2,5–8 However, although they can recapitulate key pathological changes in some lung diseases, they are still limited in reiterating all features observed in humans due to fundamental differences in anatomy and physiology between humans and animals. The combination of differences in host immune responses to epithelial injury, pathology biomarkers, the extent of respiratory bronchioles, interdigitation of conducting airways, acinar size, and air-blood barrier thickness contribute to the varied sensitivity to inhaled toxicants between species.9 In addition to differences in lung physiology and responses to compounds, animal testing is also a sensitive topic from an ethical point of view and a transition to non-animal technologies is encouraged through national legislation.
In vitro models offer tightly controlled cellular environments that can be evaluated in real time, easily scaled and replicated, allowing the evaluation of the effects of drugs, chemical compounds, exhausts or NPs on lung tissues, and reducing the use of animal models and clinical studies. Leveraging these models could aid the discovery of novel therapeutic targets, may provide powerful, scalable screening platforms to test the effects of pharmaceuticals, and can act as an important preclinical step to bridge the gap between drug testing in animal models - which are expensive and have a high failure rate - and human clinical trials.10 In fact, several advanced in vitro systems have been recently used to model pathological conditions,11 revealing that, in some cases, they are able to perform a match comparison between the responses from normal cells and disease-exposed cells from the same patient, which is an important step toward personalized medical therapy.12
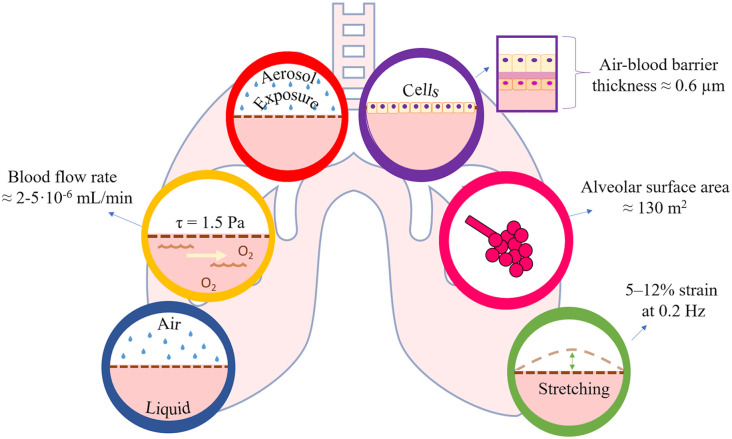
The term “physiologically relevant” is often used in the context of in vitro models and is referred to the likeness of the model with respect to the in vivo counterpart. Considering the microenvironment of the alveolus, which is the functional unit of the lung where gas exchange and particles absorption take place, the specifications for an ideal “physiologically relevant” engineered human in vitro model are:
Human-derived cells that compose the native alveolar barrier (thickness ≈ 0.6 µm,13 alveolar surface area ≈ 130 m2),14 consisting ideally of: an epithelial layer of simple squamous epithelium (i.e. pneumocytes and macrophages); a layer of endothelial cells of the capillary wall; and the basement membrane between the two. Lung cells must be cultured using defined protocols, without losing their phenotypic characteristics;
A fluidic system that reproduces the blood flow through the alveolar capillaries (mean velocity ≈ 1 mm/s, flow rate 2–5 mL/min in an adult)15,16 and provides adequate oxygenation and nutrients to the cell, as well as physiological shear stress to endothelial cells (around 1.5 Pa)17;
An air-liquid interface (ALI) that mimics in vivo microenvironment where the epithelial lung cells are in contact with humid air on one side (which may contain particulate matter in the form of droplets or aerosols) and blood on the other;
A substrate for growing cells with properties similar to native tissue in terms of chemical composition and biomechanical behavior. Moreover, to reproduce alveolar barrier motion during breathing, this substrate must be subjected to mechanical cyclic stretching (around 5%–12% strains at 0.2 Hz in physiological conditions, and up to 20% strain in some pathological conditions).18
To be physiologically relevant, an in vitro model should replicate as many as possible of the essential features of the tissue or organ it is intended to represent, and which are fundamental for the experimental endpoints that must be evaluated. Thus, alveolar models can be designed with some or all of these requirements (summarized in Figure 1) according to the type of study under consideration and the specific questions being addressed (e.g. a model used to study differentiation or inflammation will have different requirements for physiological relevance than a model used for toxicology or safety applications).
Figure 1.
Toward physiological relevance—main elements of the lung microenvironment that are desirable in an in vitro model.
The most important determinant of any in vitro model is the biological component, that is, the cells. Cells for in vitro models of the lung have been amply discussed in some excellent reviews and the reader is encouraged to refer to these for more in depth biological information and comparisons.19,20 Generally, they can be obtained from donors, primary cells, cell lines, or human pluripotent stem cells (hPSCs). Commonly employed human epithelial cell lines are the A549 and NCI-H441, for the assessment of the alveolar barrier, and the Calu-3, BEAS-2B, and 16HBE for the assessment of the bronchial barrier.2 A promising alternative to cell lines are the hPSCs, which open the possibility to develop patient-specific models.19 hPSCs could indeed win the debate over the ideal cell source, but currently the need for protocol optimization and standardization is still an obstacle. Additionally, lung epithelial cells can be co-cultured with vascular, neural, or immune cells such as macrophages, dendritic cells, and mast cells.1,2,19–23 The co-cultures enhance the reliability of the in vitro lung model, making them more similar to the complex in vivo microenvironment. Regardless of the type of cells, there seems to be an agreement among the scientific community: models in which cells are cultured at the air-liquid interface (ALI) better represent the physiological environment of the lung. Indeed, a wide variety of studies have been performed comparing the culture of lung cells in ALI and in submerged conditions, revealing that cells displayed phenotypic differences.20
Besides the cells, the choice of the most appropriate experimental setup is crucial for the design of an ad hoc in vitro model. A variety of models have been proposed using different engineering solutions that we will discuss in the next sections of this review. To facilitate the analysis of the devices, we have grouped them in:
Fluidic systems that provide adequate oxygenation and nutrients to the cells, as well as physiological shear stress.
Systems that combine ALI culture with direct and quantitative aerosol/smoke exposure, for toxicological studies and drug testing.
Devices that mimic mechanical stretch of lung tissues during breathing.
Lungs-on-chips, which mimic biological and/or biochemical processes at the micro-scale.
In each section we analyze the biological role of the mechanical stimuli and exposure on lung tissues and then critically assess the approaches that have been employed to recreate such dynamic conditions.
Dynamic lung models: Fluidic systems
Shear stress is the frictional force per unit surface area exerted at a fluid-solid interface when they are in relative motion. The vascular system of the lung is continuously exposed to shear stress from blood flow. Furthermore, shear stress is also generated on the gas side from the airflow exerted on the airways.2 It has been demonstrated that shear stress modulates different cellular phenomena such as morphology, proliferation, differentiation, metabolism, and communication.24
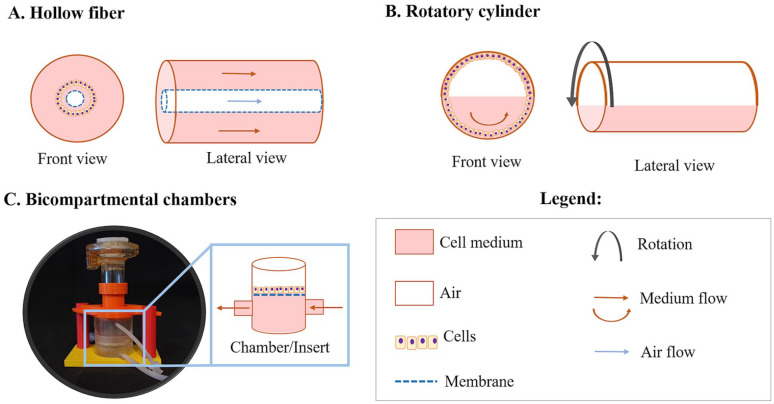
Several research groups have therefore developed dynamic systems that are able to provide shear stress while enhancing oxygen and nutrient diffusion at the same time. A variety of solutions have been adopted to generate cell cultures with medium flow using bioreactors with different configurations, as schematized in Figure 2.
Figure 2.
Different configurations of bioreactors designed to operate with millifluidics. Representations are not at scale. The photograph in panel C shows the MALI chamber with nebulizer.158
Hollow fiber bioreactors, for example, are common tools for performing dynamic cultures of many tissues25 and the lung is no exception.26 Here, air and cell culture media flow through the system through appropriate connectors providing the ability to modulate the environment both in the lumen of and surrounding the semipermeable fibers. An interesting feature of this type of system while comparing with other solutions, is that the cell culture can experience a nearly physiological air and fluid flow environment. Unlike cells grown in conventional 2D static culture systems, cells grown in these bioreactors show typical characteristics of differentiation.26 Another way of providing both ALI and adequate shear stress to the cells is by culturing them in rolling bioreactors.27 As the system rolls, the cells spend an equal amount of time in air and liquid, rendering this bioreactor a suitable tool to study the impact of ALI on the cell differentiation process. However, unlike hollow fiber-based systems, it does not faithfully represent the in vivo configuration. Finally, a prevalent choice for the provision of medium flow is through the use of bioreactors with a tubing system and pumps.28–34 In these models, well inserts are typically cultured with epithelial and endothelial cells, on the apical and basal sides, respectively. The endothelial compartment is connected to the tubing system and thus sustained by media flow, which can be controlled to regulate the level of shear stress on the cells. Exploring this approach, dynamic in vitro models of invasive pulmonary aspergillosis were set up to study the pharmacodynamics of voriconazole32 and isavuconazole.31 Toxicology studies have also been performed to evaluate the effects of NPs (e.g. gold NPs),34 or other airborne materials such as pollen.33 These examples illustrate that the use of flow systems is relevant for studies, where the role of shear stress on cell responses is assessed, and for absorption studies, where more complex kinetics and dynamics are considered. The characteristics of the systems employed to perform these studies are summarized in Table 1.
Table 1.
Specifications of fluidic systems developed for lung in vitro studies (n.m.: not mentioned). Values were compiled as published in the original sources, RWV: Synthecon Rotating Wall Vessel.
| Authors | Type of bioreactor | Medium volume | Cell type | Cell density | Flow rate | Shear stress | Study |
|---|---|---|---|---|---|---|---|
| Grek et al.26 | Hollow fiber | n.m. | MLE-15 | 8.6 × 104 cells/cm2 | Air flow: 1 × 10−2 mL/min; medium flow: n.m. | n.m. | Assessment of phenotypic characteristics |
| Ghaedi et al.27 | Rotating cylinder | 15 mL | iPSC-ATII and hATII | 2 × 105 cells/cm2 | n.m. | n.m. | Stem cell differentiation protocols |
| Jeans et al.32 | Inserts housed in bioreactors | 200 mL | Co-culture of HPAECs and A549s | 1 × 106 cells/cm2 and 5.5 × 105 cells/cm2 (respectively) | 0.17 mL/min | n.m. | Human-like voriconazole pharmacokinetics |
| Box et al.31 | Inserts housed in bioreactors | n.m. | Co-culture of HPAECs and A549s | 1 × 106 cells/cm2 and 5.5 × 105 cells/cm2 (respectively) | 0.17 mL/min | n.m. | Human-like isavuconazole pharmacokinetics |
| Blume et al.33 | Inserts housed in microfluidic circuit | n.m. | PBECs | n.m. | 5 × 10−4 mL/min | n.m. | Biological responses to pollen exposure |
| Breitner et al.34 | Multiwell plate connected to pump | n.m. | A549s | 8.4 × 104 cells/cm2 | 7.5 × 10−1 mL/min; | n.m. | Nanoparticle evaluation |
| Aufderheide et al.35 | Cultex LTC-C | 35 mL | NHBE048 | 1–1.5 × 105 cells/cm2 | Rotation speed: 3 rpm | n.m. | Cultivation of airway epithelial cells at ALI |
| Carterson et al.36 | RWV bioreactor | n.m. | A549s | 5 × 106 cells in microcarrier beads | n.m. | Note: The bioreactor delivers the same terminal velocity and consequently shear stress to similar-sized particles, independent of the rotation speed. | Interactions between Pseudomonas aeruginosa and lung epithelial cells |
| Cortiella et al.37 | RWV bioreactor | n.m. | mESCs | 2 × 106 cells/construct | 2 rpm | Note: The bioreactor delivers the same terminal velocity and consequently shear stress to similar-sized particles, independent of the rotation speed. | Acellular lung as a matrix to develop an engineered lung tissue |
| Crabbé et al.38 | RWV bioreactor | 50 mL | MSCs and C10 | 4 × 106 cells/scaffold (MSCs/C10) | 20 rpm | Note: The bioreactor delivers the same terminal velocity and consequently shear stress to similar-sized particles, independent of the rotation speed. | Enhance the cell repopulation of decellularized lungs |
| Wilkinson et al.39 | RWV bioreactor | n.m. | Lung organoids: FLFs, HUVECs, and SAECs; or iPSCs | 1 mL alginate beads + 4 × 106 FLFs/iPSC; 100 μL alginate beads + 1.5 × 105 SAECs | 4–30 rpm | Note: The bioreactor delivers the same terminal velocity and consequently shear stress to similar-sized particles, independent of the rotation speed. | Generation of self-assembled human lung tissue |
| Crabbé et al.40 | RWV bioreactor | n.m. | A549s | 5 × 106 cells in microcarrier beads | n.m. | Note: The bioreactor delivers the same terminal velocity and consequently shear stress to similar-sized particles, independent of the rotation speed. | Pseudomonas aeruginosa biofilm susceptibility on biotic surfaces |
Since the culture of cells in dynamic conditions became popular, optimized commercial solutions have been developed based on the operating principles represented in Figure 2. Among the commercial devices, lung models using Synthecon (Synthecon® Incorporated, Houston, Texas, USA) and Cultex LTC-C (Cultex® Technology, Hannover, Germany) have been widely reported. Synthecon systems are perfusion bioreactors that comprise a cylindrically shaped rotating vessel with a central gas transfer core, while the Cultex LTC-C system presents a hydraulic circuit with media flow facilitated by peristaltic pumps. The main application of the latter is the generation of comparable cultures for mechanistic and toxicological studies.41 On the other hand, Synthecon bioreactors are better tailored for cell/tissue engineering approaches. They have mainly been used to produce whole acellular lung as a matrix to support the development of engineered lung tissue,37,38 to evaluate antimicrobial efficacy against biofilm formation in 3D lung epithelial models,40 and for the generation of self-assembled human lung tissue (organoids)39 employed for disease modeling and drug discovery. The employment of more sophisticated fluidic devices is analyzed in the following section and they are an option for researchers looking for further complex systems compatible with exposure studies.
From Table 1, it is clear that the protocols are not universal for all the lung models cultured in dynamic conditions. Besides varying in their working principle, they also vary in scale: the “in-house” fluidic circuits go from micro to milli-scale and even commercial devices, such as the ones from Synthecon, can work with a wide range of volumes (from 1 to 50 mL for one of the available configurations, according to their website). Regarding the biological components, both cell density and cell medium flow rate vary greatly. For the models with cells cultured in 2D, the epithelial cell densities range from 8000 to 500,000 cells/cm2, but this range expands as the cellular complexity of the model increases (both in composition and in arrangement). When it comes to the flow rate, the data is not always available and in a number of cases it was derived from descriptions of the fluidic circuit set-ups. Remarkably, the flow rates are considerably higher than the pulmonary capillary flow rate of ≈2–5 10−6 mL/min in a human.15 Only the device developed by Blume et al.33 applied a medium flow (5 × 10−4 mL/min) that was relatively close to the physiological range. The shear stress provided by all these systems is often referred to as “low-shear stress” but the values are not estimated/presented. Clearly there is a need to harmonize reporting to enable more precise identification and implementation of the flow-related parameters in the studies. Only then can different approaches be compared, and meaningful correlations be identified between cellular response and a certain stimulus or type/magnitude of stimulus. Nonetheless, these bioreactor systems are a step forward in the design of advanced in vitro models, when compared with more traditional systems.
Knowing what we breathe: Combining ALI culture and aerosol/smoke exposure
Inhalation is an important route of exposure to particulates, both in the form of drugs if considering pharmaceutical therapies, and environmental particles.42 The goal of pharmaceutical therapies is to allow drug delivery into the lung with maximum efficiency, while the effect of environmental particles must be investigated to evaluate their potential toxicity on lung tissues. Therefore, several studies are focused on assessing the biopharmaceutics and toxicology following particulate exposure, and several models have been developed to investigate these aspects. Progress in this direction is represented by complex in vitro models that combine ALI culture and aerosol/smoke exposure making them suitable for studies on inhalation toxicology and pharmacology. The following subsections present both laboratory-made and commercial systems that belong to this category.
Laboratory-made systems for aerosol exposure
Over the years, several authors have focused on the design and characterization of innovative systems in terms of deposition efficiency and homogeneity, to maximize experimental reliability and throughput. Indeed, one of the essential requirements for exposure systems is the ability to present compounds or materials directly and reproducibly to cells in culture so as to allow dose-response analyses of airborne molecules or materials. They have been used principally to investigate the inflammatory and toxic effects of aerosolized compounds on lung tissue, combining ALI culture with direct exposure of gaseous contaminants (i.e. NO2 and O3),43,44 volatile organic compounds,45 brake powder,46 diesel exhaust particles,47–50 or micro/nanoparticles.51–53 The exposure is performed using different approaches: with gas generators,43,44 commercial microsprayers,51 flame spray synthesis,54 or specifically designed solutions. For example, Riediker et al. worked on a device that consisted in an exposure-box mounted around a car’s braking system to collect, purify, and nebulize brake powders;46 Cooney,47 Holder,48,49 and Oosting50 designed custom exposure systems and deposition chambers to evaluate the effect of diesel exhaust particles on lung cells cultured on Transwell inserts; another even simpler approach was proposed by Bakand et al., who placed cells cultured at ALI in a glass chamber at 37°C, together with filter paper soaked with volatile compounds (i.e. Toluene and Xylene).45
Other studies focused on the quantitative characterization of therapeutic aerosols in vitro, using modified pharmaceutical impactors and impingers, which operate on the principle of inertial impaction. These devices consist in a series of stages with a single or multiple nozzles or jets through which the aerosol flow is driven. If particles have sufficient inertia, they will impact on that particular stage collection plate; if not, they will remain entrained in the air stream and pass to the next stage where the process is repeated. This allows characterizing the particle size distribution of aerosols. The term “impactor” is generally used when the particles impact on a dry impaction plate or cup, while “impinger” refers to a liquid collection surface.55 For the aerodynamic assessment of fine particles, the European Pharmacopoeia recommends: the twin stage impinger (TSI), multi stage liquid impinger (MSLI), next generation impactor (NGI), and Andersen cascade impactor (ACI).55 Even though these tools are useful for evaluating the aerodynamic performance of aerosol formulations, they do not give information relating to drug dissolution and transport at the epithelia. Therefore, in order to study the deposition and transport of inhaled drugs across the epithelial barrier, several researchers modified these impactors inhouse incorporating in vitro cell based methods into classical impactors to provide a better understanding of the fate of microparticles after deposition in the respiratory tract.56–59
Another approach used to design predictive lung in vitro models consists in the introduction of media flow combined with exposure at ALI. This setup better reproduces the alveolar microenvironment, where the blood flow through the capillaries is reproduced by the media flowing through the “liquid side” of the ALI interface. The media flow also enhances oxygen and nutrient diffusion and provides shear stress to the cell surface. In this context, Tippe et al. modified the commercially available perfusion Minucell device (MINUCELL, D-93077 Bad Abbach, Germany) to evaluate the quantitative dosimetry of fine and ultrafine aerosol particles during in vitro exposure and permit an aerosol exposure by stagnation point flow.60 Successively, other authors used this approach to allow a dose-controlled exposure of ultrafine- and nano-particles.61,62
Finally, several studies show that applying an electric field during exposure leads to better control of particle precipitation, enhancing the deposition efficiency, reproducibility, and uniformity of particles on the cell culture surface. Therefore, electrode-assisted systems were used to evaluate the deposition and electrical discharge on cell layers during aerosolization, analyzing the toxicity of nebulized micro-63 and nano-particles,42,52,53,64 diesel exhaust,63,65,66 or air pollutants.67
Commercial aerosol and smoke exposure devices
The most commonly used commercial systems for the direct and quantitative exposure to aerosols are the Cultex® RFS system, the Vitrocell® exposure chambers, and the PreciseInhale. In these systems the aerosol generator is connected to an exposure chamber in which the well inserts (or Petri dishes) are placed, and the cell culture media is supplied individually to each well compartment, ensuring the ALI. Although Vitrocell systems are designed to perform ALI culture, they have been used to test both submerged and ALI experimental conditions, investigating the effects of each exposure scenario.68–71
The successful application of these commercial systems is demonstrated by the high number of toxicological testing studies to which they are applied. They can also be used to evaluate the therapeutic potential of new formulations. For instance, Lenz et al. investigated the effect of a commercial FDA-approved proteasome inhibitor (Bortezomib).72 Another example is Schmid et als.’ study of the biokinetic behavior of the immunosuppressive drug Cyclosporin A encapsulated in liposomes at the lung epithelial barrier.4 Still in the field of drug testing, Gerde et al. and Malmlöf et al. used the PreciseInhale to evaluate dissolution and adsorption in the lungs of drugs such as Fluticasone propionate,73,74 Budesonide,73,74 and Salmetrol.74 Tables 2 and 3 summarize the classes of substances and testing conditions using these systems, splitting them respectively into chemical/biological compounds and nanomaterials.
Table 2.
Chemical compounds aerosolized using the Cultex and Vitrocell exposure chambers.
| Tested substances | Authors | System used | Cell type | Cell density | Compound concentration | Aerosolization mode | |
|---|---|---|---|---|---|---|---|
| Biological compounds | Aspergillus fumigatus | Persoz et al.75 | Vitrocell | A549 | 1.8·104/cm2 | 7·108 spores/m3 | Texp: 30 min; Q: 5 ± 0.1 mL/min |
| Organic compounds | Benzene | Pariselli et al.76 | Cultex | A549 | 1.5·104/cm2 | 0.28 ± 0.03 ppmv | Texp: 1 h; Q: 2 mL/min |
| Dicarbonyls | Anderson et al.77 | Vitrocell | A549 | 53 533/cm2 | 15–65 ppm | Texp: 2/4 h; Flow rate: 3 mL/min | |
| Formaldehyde | Persoz et al.75 | Vitrocell | A549 | 1.8·104/cm2 | 50 µg/m3 | Texp: 30 min; Q: 5 ± 0.1 mL/min | |
| Bardet et al.78 | Vitrocell | hAECN | 1·105/cm2 | 200 µg/m3 |
Texp: 1 h for 1, 2, 3 times at 24-h intervals Q: 2 ± 0.1 mL/min |
||
| Toluene | Pariselli et al.76 | Cultex | A549 | 1.5·104/cm2 | 0.25 ± 0.06 ppmv | Texp: 1 h; Q: 2 mL/min | |
| Al Zallouha et al.79 | Vitrocell | A549 | 1.1·105/cm2 | 100 and 1000 ppm |
Texp: 1 h Q: 100 mL/min |
||
| Méausoone et al.80 | Vitrocell | BEAS-2B | 1500/cm2 | 100 and 1000 ppm | Texp: 1 h over 5 days; Q: 100 mL/min | ||
| Dihydroxyacetone (DHP) | Wang et al.81 | Vitrocell | NHTBE | Suspension of 4·105 cells/mL 100 µL added to a 24-well Transwell insert | DHP dissolved in DPBS to 0.2, 0.4, and 1 M | Texp: 30 s; Q: n.m. | |
| Phthalic anhydride | Chary et al.82 | Vitrocell | A549+THP-1+EA.hy 926 | EA.hy 926: 2.4·104/cm2
A549: 6·104/cm2 THP-1: 2.4·104/cm2 |
Stock solutions diluted in 50% (v/v) sterile water in PBS 1X | Texp: 15 min; Q: n.m. | |
| Trimellitic anhydride | |||||||
| Methyl salicylate | |||||||
| Acrolein | |||||||
| Limonene | Anderson et al.83 | Vitrocell | A549 | 2.8·104–1.1·105/cm2 | 20 ppm | Texp: 1–4 h; Q: 3 mL/min | |
| MucilAir | n.m. | 500 ppb |
Texp: 1 h per day, 5 days per week/4 weeks Q: 2 mL/min |
||||
| Inorganic compounds | Ozone | Anderson et al.83 | Vitrocell | A549 | 2.8·104–1.1·105/cm2 | 4 ppm | Texp: 1–4 h; Q: 3 mL/min |
| MucilAir | n.m. | 100 ppb |
Texp: 1 h per day, 5 days per week/4 weeks Q: 2 mL/min |
||||
| Phosgene | Olivera et al.84 | Vitrocell | 16HBE | 5.8·105/cm2 | 1, 2, 4, 8, 16, 32, and 64 ppm | Texp: 8 min; Q: 8.3 mL/min | |
| Copper(II) oxide micro | Aufderheide et al.85 | Cultex | A549 | 1·105/cm2 | n.m. | Texp: 15/30/60 min; Q: 30 mL/min | |
| Complex mixtures | Cigarette smoke | Okuwa et al.86 | Cultex | Chinese hamster lung cells | 1.1·105/cm2 | n.m. | According to ISO 3308 (35 mL puff volume, 2 s duration, 1 puff/min) Texp: 4 h; Q: 5 mL/min |
| Aufderheide et al.87 | Cultex | 16HBE14o- | n.m. | ||||
| Nara et al.88 | Cultex | CHO-K1 | 4.4–5.6·104/cm2 | ||||
| Rach et al.89 | Cultex | 16HBE14o- | 2.5·105/cm2 | ||||
| Aufderheide et al.40 | Cultex | NHBE | 1–1.5·105/cm2 | ||||
| Scheffler et al.90 | Cultex | NHBE+A549+CL 1548 | NHBE: 2.1·105/cm2
A549: 2.5·105/cm2 CL 1548: 2.5·105/cm2 |
||||
| Scheffler et al.91 | Cultex | NHBE | 2.1·105/cm2 | ||||
| E-liquid aerosol | Scheffler et al.90 | Cultex | NHBE+A549+CL 1548 | NHBE: 2.1·105/cm2
A549: 2.5·105/cm2 CL 1548: 2.5·105/cm2 |
|||
| Scheffler et al.91 | Cultex | NHBE | 2.1·105/cm2 | ||||
| Exhaust fumes (HFO: Heavy Fuel Oil; DF: Diesel Fuel, DE: Diesel Exhaust, DEPM: Diesel Exhaust Particulate Matter) | Oeder et al.92 | Vitrocell | A549/BEAS-2B | 8.9·104/cm2 | DF: 28 ± 1.5 µg/cm3
HFO: 56 ± 0.7 µg/cm3 |
According to ISO 8178-4 E2 Texp: 4 h |
|
| Sapcariu et al.93 | Vitrocell | RAW 264.7 | 2.1·105/cm2 | DF: 340 µg/cm3
HFO: 760 µg/cm3 |
|||
| Klein et al.94 | Vitrocell | A549 + THP-1 + EA.hy 926 + HMC1 | EA.hy 926: 2.4·105/cm2
A549: 1.2·105/cm2 THP-1: 2.4·105/cm2 HMC1: 1.2·105/cm2 |
Dose of DEPM: 40, 80 and 240 ng/cm2 |
Texp: 1 min 8 s (40 ng/cm2) 2 min 17 s (80 ng/cm2) 6 min 52 s (240 ng/cm2) Q: 5 ± 0.1 mL/min |
||
| Kooter et al.95 | Vitrocell | A549 | ~0.1·105/cm2 | n.m. | DE exposure according to the European Commission directive 2005/78/EC Texp: 1.5 h; |
||
| Tsukue et al.96 | Cultex | A549 | 1.67·106/cm2 | DEPM: 0.07–0.85 mg/m3 Gaseous components: 0.2–45.8 ppm |
Texp: 1 h; Q: 8.3 cc/min/insert | ||
| Ji et al.97 | PreciseInhale | PBEQ + MQ | PBEQ: 1·105/cm2
MQ: 5.6·105/cm2 |
Dose of DEPM: 1.7 µg/cm2 | Texp: 3 min; Q: 10 mL/min | ||
| Emission from laser printers | Tang et al.98 | Vitrocell | A549 | n.m. | n.m. | Texp: 1 h; Q: 5 mL/min | |
| Smoke particles emitted by a household log wood stove | Mülhopt et al.99 | Vitrocell | A549 | 8.6·104/cm2 | Particle density in wood exhaust: 2.7 g/cm3 | Stove fired according to DIN EN ISO 17225-5 Texp: 4 h; Q: 100 mL/min |
|
| Fly ash collected from a municipal waste incinerator | Diabaté et al.100 | Cultex | BEAS-2B THP-1 |
BEAS-2B: 1.1·105/cm2 | ~3.6·104 particles/cm3 | Texp: 1 h; Q: 300 mL/min | |
| Indoor gaseous pollutants | Bardet et al.78 | Vitrocell | hAECN | 1·105/cm2 | n.m. |
Texp: 1 h for 1, 2, 3 times at 24-h intervals Q: 2 ± 0.1 mL/min |
|
m.: not mentioned; Q: flow rate; Texp: exposure time.
Table 3.
Aerosolized nanoparticles using the Cultex and Vitrocell exposure chambers.
| Tested NP/NT | Authors | System used | Primary NP/NT diameter | NP/NT concentration | Aerosolization mode | Cell type | Cell density |
|---|---|---|---|---|---|---|---|
| Zinc oxide | Xie et al.68 | Vitrocell | 25 nm | NPs suspended in sterile water (5 mg/mL) and diluted 1-, 2-, 10-, 50-, and 100-fold |
Texp: 10–20 min Q: 10 mL/min |
C10 | n.m. |
| Stoehr et al.71 | Vitrocell | 35 nm | 0.5 and 4.25 mg/mL in MilliQ dH2O |
Texp: 15 min Q: n.m. |
A549 | 2.4·104/cm2 | |
| Mihai et al.101 | Vitrocell | 25 nm | NP solution diluted at 0, 0.05, 0.20, 0.30, 0.50, 1.00, and 1.50 mg/mL |
Texp: 10 min Q: 10 mL/min |
C10 | 2.4·104/cm2 | |
| Silica | Klein et al.102 | Vitrocell | 50 nm | 1 g/L in PBS |
Texp: 30 min Q: 5 mL/min |
A549 THP-1 EA.hy 926 HMC-1 |
A549: 1.2·105/cm2
THP-1: 2.4·105/cm2 EA.hy 926: 2.4·105/cm2 HMC-1: 1.2·105/cm2 |
| Panas et al.103 | Vitrocell | 50 nm | 1, 3.25 and 7 mg/mL in dH2O |
Texp: 5 or 7 h Q: 100 mL/min |
A549 | 8.5·104/cm2 | |
| Pristine and carboxylated copper oxide NPs | Kooter et al.104 | Vitrocell | 10–20 nm | 6.15·105 and 1.65·106 particles/cm3 |
Texp: 1 h Q: 1.5 mL/min |
MucilAir | n.m. |
| Hybrid lipid-polymer | D’Angelo et al.105 | Vitrocell | ~150 nm | 0.5 and 0.9 mg/mL |
Texp: 10 and 30 min Q: 15 L/min |
16HBE14o-
MDM MDDC |
16HBE14o- : 0.55·106/cm2
MDM: 2.5·104/mL MDDC: 83·104/mL |
| Cerium oxide | Steinritz et al.106 | Cultex | 15–30 nm | Concentration of 25 µg/cm2 deposited mass within 15 min exposure |
Texp: 15, 30 and 60 min Q: 1.5 L/min |
A549 | 1·105/cm2 |
| Rach et al.89 | Cultex | 50–80 nm | Concentration of 25 µg/cm2 deposited mass within 15-min exposure |
Texp: 15–60 min Q: 5 mL/min |
A549 | 1·105/cm2 | |
| Kooter et al.107 | Vitrocell | 13.8 and 750 nm | 50 mg/m3 |
Texp: 1 h Q: 1.5 and 5 mL/min |
A549 BEAS-2B MucilAir |
A459 and BEAS-2B: 9524/cm2
MucilAir: NA |
|
| Loret et al.69 | Vitrocell | 29 nm | 7.9–105.7 mg/m3 |
Texp: 3 h Q: 5 mL/min |
A549 THP-1 |
A549: 17,130/cm2
THP-1: 1713/cm2 |
|
| Cappellini et al.108 | PreciseInhale | ~30 nm | 2.56 mg/mL in BSA |
Texp: 20 min Q: 5 mL/min |
A549 THP-1 |
A549: 30,000/cm2
THP-1: 46,666/cm2 |
|
| Titanium dioxide | Loret et al.69 | Vitrocell | 8, 21 and 100 nm | 10.6–113.5 mg/m3 |
Texp: 3 h Q: 5 mL/min |
A549 THP-1 |
A549: 17,130/cm2
THP-1: 1713/cm2 |
| Rach et al.89 | Cultex | 25 nm | Concentration of 25 µg/cm2 deposited mass within 15-min exposure |
Texp: 15–60 min Q: 5 mL/min |
A549 | 1·105/cm2 | |
| Steinritz et al.106 | Cultex | 25 nm | Concentration of 25 µg/cm2 deposited mass within 15-min exposure |
Texp: 15, 30 and 60 min Q: 1.5 L/min |
A549 | 1·105/cm2 | |
| Carbon black | Steinritz et al.106 | Cultex | 14 nm | ||||
| Magnesium oxide | Steinritz et al.106 | Cultex | n.m. | ||||
| Barium sulfate | Steinritz et al.106 | Cultex | n.m. | ||||
| Copper(II) oxide | Steinritz et al.106 | Cultex | 40 nm | ||||
| Aufderheide et al.85 | Cultex | 40–80 nm | n.m. |
Texp: 15 and 60 min Deposition rate: 25 µg/cm2/15 min |
A549 | 1·105/cm2 | |
| Copper | Kim et al.109 | Vitrocell | 25 nm | 1 mg/mL |
Texp: NA Q: 5 mL/min |
A549 | 1.7·105/cm2 |
| Elihn et al.110 | Cultex | 180 ± 1.5 nm | 105 particles/mL |
Texp: 4 h (constant and pulsed aerosol flow) Q: 20 mL/min |
A549 | 0.43·105/cm2 | |
| Gold | Bachler et al.111 | Vitrocell | 2, 7, 18, 46 and 80 nm | 170, 200, 300, 200, and 220 µg/mL |
Texp: 15 min Q: 5 mL/min |
A549 16HBE14o- MLE-12 |
0.56·106/cm2 |
| Durantie et al.112 | Vitrocell | ~ 32 nm | 0.05, 0.1, 0.25, and 0.5 mg/mL |
Texp: 10 min Q: n.m. |
A549 MDM MDDC |
A549: 120·104/cm2
MDM: 5.56·104/cm2 MDDC: 1436·104/cm2 |
|
| Chortarea et al.113 | Vitrocell | ~58 nm | 120 µg/mL |
Texp: n.m. Q: 5 L/min |
A549 MDM MDDC |
A549: 9714/mm2
MDM: 411/mm2 MDDC: 231/mm2 |
|
| Multi-walled carbon NTs | Chortarea et al.114 | Vitrocell | n.m. | 25, 125, and 250 µg/mL in Pluronic F127 |
Texp: n.m. Q: 5 L/min |
A549 MDM MDDC |
A549: 9714/mm2
MDM: 411/mm2 MDDC: 231/mm2 |
| Chortarea et al.115 | Vitrocell | n.m. | 250 µg/mL in Pluronic F127 |
Texp: n.m. Q: 5 L/min |
MucilAir | n.m. | |
| Beyeler et al.116 | Vitrocell | Length: 2–16 µm Inner diameter: 2–13 nm Outer diameter: 6–34 nm |
25 µg/mL in Pluronic F127 |
Texp: n.m. Q: 5 L/min |
Primary bronchial epithelial cells | n.m. | |
| Palladium | Ji et al.117 | PreciseInhale | 6–10 nm | n.m. |
Texp: 20 s, 45 s and 3 min Q: 10 mL/min |
PBEC MRC-5 |
PBEC: 1·105/cm2
MRC-5: 1·104/cm2 |
m.: not mentioned; Q: flow rate.; Texp: exposure time.
In addition to the systems for direct aerosol exposure, several commercial smoking machines combined with exposure chambers have been designed particularly for smoke inhalation simulation: examples are the Vitrocell VC smoking machine (Vitrocell® systems, Waldkirch, Germany) and the Borgwaldt systems (Borgwaldt KC, Hamburg, Germany), the latter usually paired with the British American Tobacco (BAT) exposure chamber.118
Several studies illustrate the value of these systems, which can be found in the papers by Thorne who provided a comprehensive review of the major tobacco smoke exposure systems available to 2013,118 and a comparison of in vitro data across multiple smoke exposure studies using reference cigarettes and considering three different smoking machines.119 In the latter review, Thorne demonstrated that in vitro dosimetry techniques can align data between contrasting setups and experimental protocols, resulting in a link between in vitro, in vivo, and human dosimetry studies.
Although smoking machines have been used principally to investigate the effects of cigarette smoke on lung tissues,120–129 in the last few years, several research groups have focused their efforts on studying the effects of next generation tobacco and nicotine products, namely e-cigarette aerosols and heated tobacco products.130–140
Table 4 shows all the commercial technologies mentioned here, which can be considered a good choice for advanced in vitro models if exposure conditions related with inhalation are a crucial aspect of the study.
Table 4.
Commercial systems for aerosol and smoke exposure, highlighting their similarities and differences.
| Aerosol Exposure | Smoke exposure | |||||||
|---|---|---|---|---|---|---|---|---|
| System | Cultex RFS | Vitrocell | PreciseInhale | Vitrocell VC | Borgwaldt systems | |||
| Exposure chambers | Powder chamber | Cloud system | ||||||
| Configuration | Stand alone | Combined with aerosols generators or gas supply systems | Stand alone | Stand alone | Combined with XposeALI cell exposure unit | Combined with DissolvIt module | Combined with the Vitrocell exposure chambers | Combined with the BAT chamber |
| Aerosolized substances | Airborne substances (gases, NPs, complex mixtures, fibers) | Airborne substances (gases, NPs, complex mixtures, fibers) | Specific for dry powders | Specific for liquid aerosols | Airborne particles | Specific for dry powders | Smoke generation | Smoke generation |
| Media flow | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| QCM | No | Yes | Yes | Yes | No | No | No | No |
To summarize, all the technologies mentioned above have somehow increased the complexity of lung models compared to traditional static culture. In these studies, the goal was to investigate the effect of aerosol/smoke deposition on lung tissues; fundamental to this scope is culturing cells at the ALI, to reproduce the in vivo deposition of the inhaled particulate. Some researchers devised very simple but functional solutions for their purposes (i.e. Bakand et al.),45 while others increased the model complexity by introducing culture medium flow, or performing electrical deposition. Finally, some authors modified pharmaceutical impactors, already used to characterize the particle size distribution of pharmaceutical aerosols, to obtain ad hoc in vitro models and study the deposition and transport of inhaled drugs. In addition to these laboratory-made devices, a large number of investigations are conducted using commercial systems, which at least allows some comparisons to be made between data from different studies. However, we should underline that although there are many reports on exposure systems, very little attention is paid to precise measures of dosimetry, which greatly reduces the strength of comparative analyses. (Source PubMed—from 2010 to 2020: 55 documents on inhalation exposure and dosimetry in vitro and 1076 on inhalation exposure in vitro). In general, commercial systems are always the best choice for obtaining comparable results within the scientific community. This is the reason why Cultex and Vitrocell systems have become so diffuse in aerosol deposition studies over the last years. On the other hand, for the quantitative characterization of therapeutic aerosols in vitro, modified pharmaceutical impactors and impingers can be a good alternative. Indeed, these devices recommended by the European Pharmacopoeia are specifically designed for evaluating the aerodynamic performance of aerosol formulations, and, when modified incorporating in vitro cell based methods, also give information relating to drug dissolution and transport at the epithelia. However, there are studies in which commercial systems or modified commercial systems are not appropriate, since they do not allow to reproduce/evaluate some elements. For example, in order to control particle precipitation and enhance the deposition efficiency, electrode-assisted systems are the best option. However, to our knowledge there are no commercial in vitro lung models able to apply an electric field during exposure, and for this reason several authors designed ad hoc devices to be used only with charged or chargeable particles. Finally, it should be noted that none of these devices (commercial or not) are able to reproduce the effect of the deformation of lung tissue which occurs during breathing, which may be a crucial modulator of the interaction between the alveoli and inhaled materials as discussed in the following section.
There is more to breathing than air exchange: Mimicking mechanical stretching
Over the last two decades it has become clear that mechanical stress and deformation influence the biological function and signaling of alveolar epithelial cells.141,142 For example, mechanical stretch of cultured alveolar type II cells leads to changes in surfactant secretion,143–145 cell injury or death,143,146–148 permeability,149–152 and cell migration.153 However, there are still many unanswered questions regarding the micromechanics of the alveoli and the way in which it affects the mechanisms involved in lung physiology.141,154 To better understand the effects of mechanical stimuli on lung epithelia by reproducing breathing motions, several research groups have developed systems able to apply cyclic stretch to cell culture supports.
Most of the currently available in vitro cell-stretching devices are covered in an excellent and systematic review by Doryab et al. that elucidates the relevance of cyclic mechanical forces in lung biology.155
In this section, we first discuss and analyze the motion and resulting strains in the alveolus and lung. We then describe milli and micrometer-sized stretching devices, outlining the combined effect of device dimensions, deformation mechanism and stretching directions, and their physiological relevance.
A brief description of the motion
During spontaneous breathing or mechanical ventilation, pulmonary tissues are permanently subjected to cyclic stretch with varying breathing frequency and volume amplitude in order to pair up with the metabolic state of the subject. In the resting state, the lungs expand and recoil with a frequency of about 0.2 Hz (12 cycles/min) and a tidal volume of around 10% of total lung capacity.18
At the macroscale, breathing movements are mainly related to the transpulmonary pressure (i.e. the difference between air pressure in the airways and the pressure at the pleural surface), elastic recoil (related to the high elastin content), and to muscular movements caused by the diaphragm and intercostal muscles. These forces are transmitted at the microscale thanks to the extracellular matrix network, causing linear strain (defined as the variation in alveolar radius with respect to the initial radius) between 4% and 12%.18 However, strain levels can increase or decrease in an injured or damaged lung, due to changes in the structure and mechanical properties.
Ex-vivo and computational studies have shown that different deformation mechanisms occur as a function of pressure-volume variations including: (i) recruitment/derecruitment of alveolar units; (ii) folding/unfolding of alveolar walls; (iii) change in alveolar shape (dodecahedral/spherical); (iv) isotropic stretching/destretching.154,156
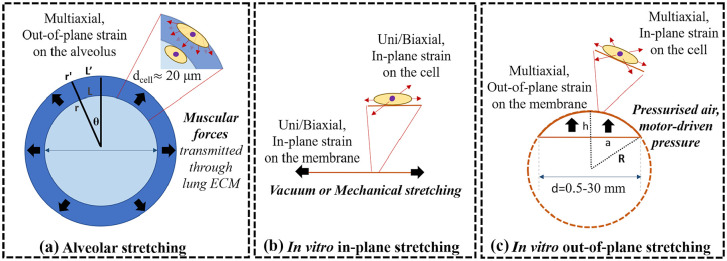
Ideally (i.e. without considering tissue anisotropy), the alveolus can be considered spherically symmetric, thus each plane passing from its center can be considered a plane of symmetry. Representing the alveolus as an isotropic thin walled sphere (Figure 3(a)), in conditions of small deformation the tangential strain (εtangential) can be defined as:
Figure 3.
Schematic representation of the: (a) alveolar, (b) in vitro in-plane, and (c) in vitro out-of-plane stretching. Black arrows represent the deformation directions, while red arrows the corresponding strain on the cells.
where L′ and L are the lengths of the arc under the angle θ in stretched and resting conditions respectively. Analogously, r′ and r are the radii of the alveolus in stretch and resting conditions. Thus, in conditions of isotropic stretching and relaxation, typical of resting state tidal breathing in vivo,154,156 the measured linear strain εlinear can be considered the same as that experienced by the epithelial barrier.
Similarly, in vitro systems with 2D circular or semi-spherical shapes as in Figure 3(b) and (c) are symmetric with respect to any plane perpendicular to the membrane rest plane and passing through the center.
The types of deformation applied to the substrate varies greatly but in general can be classified either as out-of-plane or in-plane. The first consists of a deformation characterized by the bulging in/upwards of the substrate (Figure 3(a) and (c)), whereas the second-one is the result of a lateral expansion of the substrate maintaining its original flat position/plane (Figure 3(b)). Furthermore, according to the direction of stress applied and the substrate’s relative displacement due to applied constraints the strains can be uniaxial, biaxial, multiaxial, or radial. This concept is often generalized, or even represented erroneously in the literature, so we provide a basic definition here. In Cartesian coordinates, uniaxial, biaxial, and multiaxial strains respectively occur in the case of a stretching along one, two, or more axial directions, while, in circular configurations, uniform stretching in all the directions along the radius results in a strain which is defined as radial. Tangential or circumferential strains have been also defined in spherical or semispherical out-of-plane deformations, referring to the uniform strains along the surface, perpendicular to the radius. Note that the alveolar barrier undergoes an out-of-plane deformation although linear and tangential strains are equal.
In most devices, the membrane radius ranges from 0.5 up to 30 mm,157 while the radius of curvature (R) of the membrane can be calculated as follows:
where a is the membrane radius and h is membrane displacement (Figure 3(c)). For example, for a 12-mm radius membrane, a 5% linear strain is equivalent to vertical displacement of 3.8 mm158 and R is equal to 20.7 mm. On the other hand, the dimensions of the cell and the radius of curvature of the alveolus are comparable (respectively ≈10 and ≈50 to 100 μm). Therefore, as shown in Figure 3, despite the fact that in vitro models are able to apply linear strains which recapitulate those observed in an alveolus, at the cell scale they generally fail in reproducing out-of-plane deformations. In fact, as demonstrated by recent studies, cells are able to respond to curvatures up to 1000 μm, a phenomenon defined as “curvotaxis,” which may result in cell re-orientation and different gene expression.159–162 On typical cell culture systems subject to out-of-plane deformation the effective radius of curvature of the membrane is out of the cell “curvature” sensing range and, consequently, they are likely to “feel” an in-plane deformation. Reproducing the in vivo curvature is important to mimic the cell native environment and the out-of-plane deformations at the cell scale. As the technology hardware for in vitro models improves, it should be possible to investigate this aspect with due attention to assess its importance in modulating cell responses to inhaled substances and therefore include it in the design criteria for physiological relevance.
We should also point out that in vivo and in vitro deformation mechanisms are generally different: in the alveolus, forces generated by the muscles are transmitted through the ECM; while, in vitro, membranes are stretched via pressurized air, vacuum or motor-driven systems, as described further in this section. The stress and strain distributions experienced by the cells may be affected by the deformation mechanism as stress concentrations may appear in correspondence of mechanical constraints, indenters, and membrane fixation points. Additionally, the stress on the alveolar is known to be related to the alveolar pressure level, which varies between −1 and +1 mmHg (101.3 ± 0.1 kPa) during normal breathing. On the contrary, pneumatic pressure devices typically apply pressures up to 7 kPa with respect to atmospheric pressure.158 Since it is known that high pressures may damage lung tissues in vivo (e.g. during mechanical ventilation), non-physiological pressure and stress levels are likely to alter cell behavior in vitro.163,164
Pneumatic and motor-driven devices
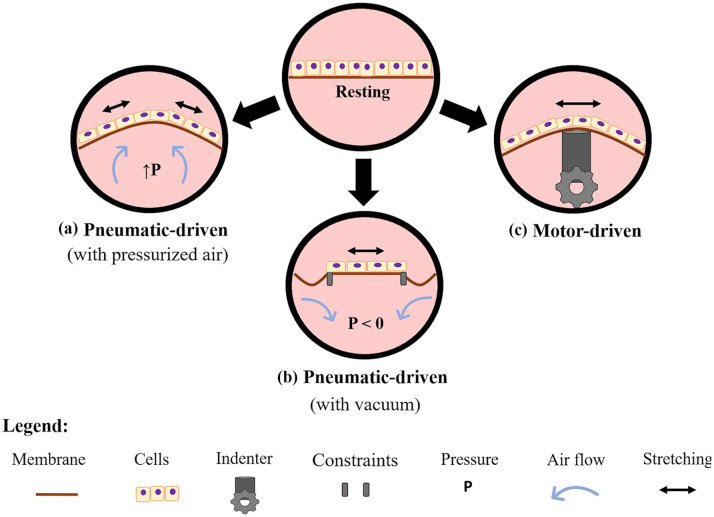
Figure 4 schematizes the most common methods for driving stretching motion in in vitro models of the lung. They are based either on the use of pneumatic actuation through the application of air over- or under-pressure or mechanical actuation with indenters.
Figure 4.
Scheme of the most common principles of actuation for stretching elastic cell culture supports in lung in vitro models. In pneumatic actuation, the support can be deformed either by inflowing air at controlled over pressure (a), or by applying a negative pressure (b), and (c) motor-driven convex surfaces or indenters cyclically deform the support.
As shown in Figure 4(a), pneumatic-driven devices deform the culture support using controlled air inflow (i.e. overpressure) or vacuum (i.e. underpressure). The over-pressurization of a chamber upon or underneath a flexible cell culture support is generally achieved thanks to pressure regulators (i.e. electro-valves), which allow the control of air pressure and of the stretching level. These devices appeared from 1989 and were based on precision-cut lung slices165 or non-permeable membranes, enabling the application of overpressure under the cells.166–168 A more complex device was developed to model bronchiole stretching.169 It was able to provide cyclic mechanical strain in combination with ALI. The cylindrical-shaped bronchioles constructed from human lung primary cells were vertically supported by a thin-walled silicone rubber tubing. The device applied mechanical stimulation by pulsing air through the silicone tubing, exerting dilatory forces on the engineered bronchiole. Finally, Cei et al.158,170 were the first to combine media flow, ALI, aerosol exposure, and cyclic mechanical strain in a single device to study drug and nanoparticle deposition and passage. Their system, known as MALI (Moving Air Liquid Interface bioreactor), consists of a two-compartment bioreactor with a moving membrane placed between an air-liquid interface, and a nebulizer for quantitative aerosol exposure experiments. In the MALI, an external electro-pneumatic regulator induces an increase of pressure in the apical chamber, while culture medium flows through the basal one; the difference between air pressure and hydrodynamic pressure results in an out-of-plane deformation of the membrane. Notably, MALI and its successor DALI are the first in vitro lung devices to be available as open source technologies.171
The second pneumatic-driven approach consists in deforming the cell substrate by applying vacuum underneath a non-porous elastic support (Figure 4(b)), thus they are neither able to model the air-liquid interface, nor to modulate the stretching level. Trepat et al. were among the first to design a cell-stretching system based on this working principle.172 Their device consisted in a well with a flexible-bottom a cylindrical loading post located underneath. When a negative pressure was applied under the annular outer region of the substrate, the central area was uniformly stretched, resulting in an in-plane deformation, only in two axes. This type of stretching does not recapitulate the multidirectional out-of-plane deformation that occurs in the alveolar wall. Nevertheless, the stimuli provided allowed the authors to understand how it could modify viscoelastic properties, structural integrity, and micromechanics of human alveolar epithelial cells.172,173 Peñuelas et al. used this device to evaluate the antioxidant role of human adult adipose tissue-derived stromal cells when human alveolar epithelial cells were subjected to injurious cyclic overstretching.174 Finally, a commercial pneumatic-driven device, is the Flexcell Tension System (Flexcell® International Corporation, Burlington, NC, USA). It allows cell culture on the top of a silicone membrane that is stretched in-plane thanks to a vacuum driven mechanism. Models of lung injury, lung inflammation, or lung tissue repair, as well as changes in cell sensitivity and permeability to compounds and cytokine have been studied with the Flexcell device.164,175
As schematized in Figure 4(c), motor-driven systems have been used to deform the cell supports by means of convex surfaces or indenters,148,176–183 leaving only one compartment for cell culture. In such systems, stretching level can be tuned by controlling motor displacement. For example, Tschumperlin and Margulies,148 Tsuda et al.,176 and Cavanaugh and Margulies177 used the cyclic movement of a motor-driven annular indenter to deform an elastomeric support (silicone membrane), evaluating the effects of the stretching on alveolar epithelial cells. In detail, the annular indenter contacted the bottom of the silicone membrane near the periphery of the cell culture surface, leading to the sliding of the membrane over the indenter. As result, the membrane stretches transversally with respect to the direction of the indenter motion,148 resulting in an in-plane deformation. Tschumperlin and Margulies used this device to study cell vulnerability to different stretching ranges;148 Cavanaugh and Margulies showed that applying cyclic stretch with higher amplitudes than the physiological ones led to a decrease of intracellular alveolar epithelial tight junction protein content and to an increase of the permeability.177 Finally, Tsuda et al.176 showed that the physical stress exerted on the alveolar epithelium by deposited fibrous particulate was greatly enhanced by the tidal cyclic motion of the epithelial cells. A commercial motor-driven device, the Strex cell stretching system (Strex Inc., San Diego, USA), was also developed. Here, cells are cultured within ad hoc designed chambers that are clamped both to a fixed frame and to a movable frame, which moves by connection to a stepping motor, leading to a uniaxial in-plane deformation of the seeding support. This system was used by Ito et al.184,185 to investigate the effects of mechanical stretch in pulmonary endothelial cells or airway smooth muscle cells.
Finally, Choe et al.186 designed a bioreactor system able to apply a mechanical cyclic stretch combined with ALI to characterize the effects of dynamic compression in ECM remodeling in a physiologically relevant 3D environment. The stretching device presented individual wells with movable inner walls designed to introduce lateral compressive strain, leading to an in-plane deformation of the substrate where cells were seeded. Cyclic compressive strain was imposed via a motor-driven mechanical arm. This device was also used by Tomei et al. to evaluate the effects of dynamic compression on lentiviral transduction in an in vitro airway wall model.187
Some alternatives to pneumatic- and motor-driven approaches have been also used for lung cell stretching.144,188–194 For instance, Skinner et al.144,192–194 used a solenoid unit to stretch a substrate seeded with cells. The substrate was fixed to a dish at one end and to a moving iron bar at the other end; the alternating electromagnetic field generated caused the iron bar to move back and forth, deforming the support on which cells were cultured.
In summary, the integration of membrane actuation systems in bioreactors has enabled cyclic movements reminiscent of breathing in vitro. Although the majority of these systems are capable of mimicking physiologically relevant (linear and tangential) strain levels, they fail in reproducing the actuation mechanism and the cyclic change in curvature at cell scales.
Lung-on-chips: Breathing at the microscale
The dimensions of engineered systems described in the previous section were comparable to those of traditional culture plates (multiwells, transwells, etc.), facilitating the transfer of cell culture protocols. But a big step in downscaling has taken place in recent years, since the design of complex microscaled fluidic devices, known as organ-on-chips, took off.195 Polydimethylsiloxane (PDMS),196 a well-known transparent, biocompatible, and easily moldable silicone, is commonly used to fabricate these devices by soft-lithography. These small chips possess microfabricated microchannels that can be continuously perfused and lined with living cells.197,198 Lung-on-chips have been thus proposed for drug testing, toxicology studies, and disease modeling.
Nalayanda et al.199 were the first to report a lung-on-chip platform in 2009 in the form of a miniaturized ALI set up. Media flow on the basal side guaranteed the nourishment of cells, while an open system on the apical side exposed the cells to air. Using this chip, they assessed the integrity and functionality of A549 monolayers. Some years later, Long et al.200 presented a similar device: the authors designed a two-chamber system to accommodate commercially available cell culture membrane supports. In this case, computational simulations were run to optimize the chip design and maximize gas transport on the liquid side of the alveolus.
Aiming at integrating more than one cell type in a chip, in 2015 Benam et al. described a microscaled system that hosted the co-culture of differentiated, mucociliary bronchiolar epithelium on the air side, and an underlying microvascular endothelium exposed to fluid flow.201 It was used to model complex and dynamic inflammatory responses of healthy and diseased lungs in vitro. A similar model was proposed by Jain et al.,202 with the difference that cells of the endothelial side experienced whole blood flow instead of cell culture media flow, allowing a quantitative analysis of inflammation-induced thrombosis. Still co-culturing epithelial with endothelial cells, this time together with pulmonary fibroblasts, Barkal et al.203 developed a microscale organotypic model of the human bronchiole for studying aspergillosis.
Showing that chips can also be used for exposure studies in the field of inhalation toxicology, Benam and co-workers reported a system that integrated a lung-on-chip microfluidic with a smoke generator and a micro-respirator that recapitulates human smoking behavior.204 This chip permitted the analysis of the effects of whole smoke, from both conventional tobacco and electronic cigarettes, delivered under physiologically relevant flow conditions.
With a completely different application, Li et al. used a lung-on-chip for studying long-term electrotaxis,205 evaluating cancer cell re-orientation and migration directionally under a physiological electric field. Their device did not present an ALI, but a simple cell culture channel divided into three segments of different widths, in order to allow the investigation of electrotactic migration.
Stretching lung-on-chips
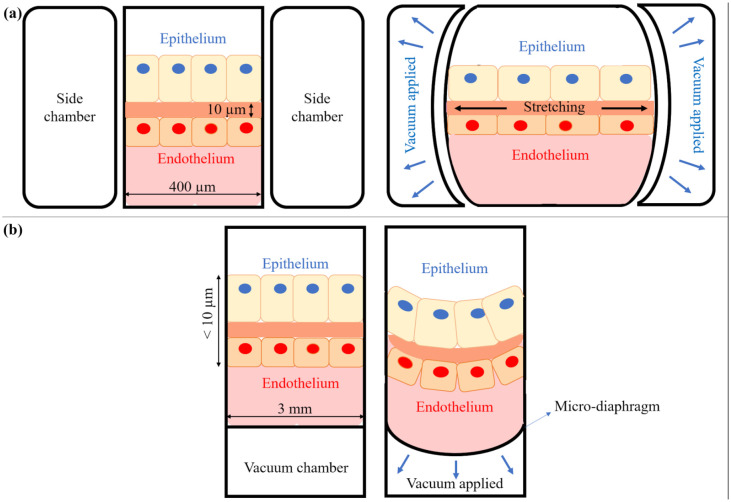
Despite being fairly complete and versatile devices, none of the chips mentioned above reported a mechanical stimulation of the cells able to reproduce breathing movements. Such functionality first appeared in 2008, in a device described by Kamotani et al.,206 and took a turning point in 2010 with the widely publicized chip developed by Huh et al.207 Kamotani et al’s. device was an array of miniature cell stretching chambers that enabled the study of the effects of mechanical strain in a parallel manner amenable to higher throughput screening.208 The system used microwells with flexible bottom membranes that were placed over piezoelectrically actuated pins that pushed against a membrane seeded with cells, applying an out-of-plane deformation of the seeded support. Huh et al. developed a lung-on-chip device able to apply a cyclic mechanical stretch to the cells.196,207–210 This microfabricated two-channel system employs the vacuum actuation method used in the FlexCell (see section on pneumatic devices above), but with a different configuration: the membrane is attached to a flexible frame placed between two chambers, the upper one for the airflow, and the bottom one for the media flow. The vacuum channels are located on the side and when vacuum is applied the frame moves leading to an in-plane uniaxial deformation of the membrane (Figure 5(a)). The device has been used for a number of applications: as an alveolar-capillary mimic to simulate bacteria and inflammatory cytokine responses207; in the nanotoxicology field to evaluate how cyclic mechanical stretching affects toxic and inflammatory response to silica nanoparticles207; in disease modeling and therapeutic substances studies, predicting the activity of a drug for pulmonary edema210 and recapitulating lung cancer growth, tumor dormancy, and responses to tyrosine kinase inhibitor therapy.211
Figure 5.
Schematic representation of two different working principles of breathing chips: (a) Huh et al.207 working principle: the microfabricated device uses compartmentalized PDMS microchannels to mimic the lung breathing sequence, and (b) Stucki et al.213,214 working principle: a micro-diaphragm actuated by an electro-pneumatic set-up leads to the cyclic motion of the cells.
Some microchips have been designed to couple different compartments that represent different organs, to study how they communicate. For instance, Liu et al. studied brain metastasis,212 by connecting an upstream “lung” with a downstream “brain,” characterized by a functional blood–brain barrier structure. The lung part of this microdevice was actuated like Huh’s chip.209 The concept of stretching lung-on-chips has been taken up by several teams, exploring other ways of actuating flexible substrates. An interesting device known as the breathing lung-on-chip device with a new design was fabricated by Stucki et al. in 2015.213,214 This chip was able to reproduce the cyclic out-of-plane motions that occur during breathing thanks to a micro-diaphragm that was actuated by an electro-pneumatic set-up. The fluidic part of the chip consisted of cell culture wells with porous and flexible membranes, while the micro-diaphragms were integrated into the pneumatic part and connected to pneumatic microchannels. Applying vacuum underneath the diaphragm led to its displacement and membrane motion, as shown in Figure 5(b). Given the dimensions of the device, it is the only system which reproduces a relevant out-of-plane deformation at cell scales. This chip was used to evaluate permeability properties of epithelial cell layers and to demonstrate that cell strain influences the metabolic activity and the cytokine secretion of primary human pulmonary alveolar epithelial cells obtained from patients. Felder et al. used a chip with the same diaphragm-like actuation to examine the influence of mechanical strain on alveolar epithelial wound healing in idiopathic pulmonary fibrosis.215 Another chip design for an out-of-plane deformation of the cell substrate by means of pneumatic actuations was that of Campillo et al.216 One of the main differences of this system, compared with Stucki’s chip, is that the flexible membrane deflects upwards by cyclically increasing gas pressure beneath it. Interestingly, Campillo’s device was used for a novel application: to study the effects of intermittent hypoxia, a hallmark of obstructive sleep apnea.
To sum up, the chips designed by Kamotani and Campillo are able to apply a cyclic deformation to the substrate, even though they do not present ALI and media flow. These features are instead present in both Huh’s and Stucki’s chips.207,213 Nevertheless, none of the mentioned devices presents a system to directly expose cells to compounds or NPs for allowing quantitative aerosol exposure experiments.
Discussion
In this paper we overview existing lung in vitro models starting from the simplest static models up to more elaborate engineered systems that better reproduce the mechanophysical stimuli present in vivo.
Although they have proven useful, the traditional static models whether cultured at ALI, and/or in co-culture, or even arranged in 3D, are not fully representative of the complexity of the dynamic lung environment. One side of this aspect has been addressed through the use of fluidic systems, such as bioreactors or microfluidic chips in combination with ALI culture (Table 5). Interestingly, most of the studies reported in Table 5 concern two main applications: inhalation toxicology and aerosol drug delivery testing. In this context, the ability to aerosolize particles is a must for investigating the interactions between cells and inhaled particles, as is the ability to accurately dose the amount of material coming in contact with the cells.217 As discussed in the section on exposure systems, there are commercial devices that meet such characteristics and are becoming standards for toxicology, including the testing of cigarette and tobacco products, since in these cases a high level of reproducibility is demanded. Moreover, the requirement for reproducibility reflects the favored use of commercial 3D cell models in several of the studies reported in the section on exposure systems and summarized in Table 4. Nevertheless, although there is a choice of commercial platforms to perform exposure studies, the effects of external aerosolized compounds are also mediated by factors that these systems cannot reproduce, such as the rhythmic contraction during the breathing. Hence the development of engineered systems that apply mechanical stimuli to cells is still a growing research field. Table 6 summarizes the systems that provide mechanical stretching. Pneumatic and motor-driven actuation are the main methods to stretch cell culture substrates, although microfluidic systems are also becoming widespread. Indeed, the first microchips posed a lurking “competition” to the milli-scaled devices present at the time, since these micro-platforms are a versatile solution for different applications, as shown in Tables 5 and 6. However, despite the advantage of requiring a low amount of material and space, which may allow the integration of multicompartmental models in a single chip, microscaled devices present several drawbacks. As reported by Mattei et al.218 these systems provide high wall shear stress, due to the high surface to volume ratio, and are subjected to edge-effects, since a large portion of cells lie at the periphery of the system and do not interact properly with other cells. They are also known to be tricky to handle, requiring a great deal of patience and expertise.219
Table 5.
Systems that apply a media flow (dynamic systems) or combine ALI with aerosol/smoke exposure.
| Scale | Type of product | Medium flow | ALI | Aerosolization | Applications | Examples | |
|---|---|---|---|---|---|---|---|
| Macro | Commercial | No exposure | Yes | Yes | No | Organoids for personalized disease modeling, tissue engineering, evaluation of biofilm formation | Synthecon bioreactor |
| Toxicology | Cultex LTC-C | ||||||
| ALI+exposure | Yes | Yes | Yes | Toxicology, evaluation of drugs and cancer mechanism | Cultex RFS | ||
| Vitrocell exposure chamber | |||||||
| PreciseInhale | |||||||
| Smoke exposure—toxicology | Vitrocell Smoking machine | ||||||
| Borgwaldt Smoking machine | |||||||
| Laboratory-made systems | No | Yes | Yes | Toxicology | Bakand et al.,45 Blank et al.,51 Riediker et al.,46 Switalla et al.,44 Cooney et al.,47 Holder et al.,48,49 Oosting et al.,50 Rothen-Rutishauser et al.,54 | ||
| Study the deposition and transport of inhaled drugs | Fiegel et al.,57 Cooney et al.,58 Haghi et al.,56 Grainger et al.,59 | ||||||
| Toxicology, electrostatic precipitation mechanisms | Savi et al.,52 Stevens et al.,53 Volckens et al.,67 De Bruijne et al.,63 Stoehr et al.,65 Holder et al.,64 Frijns et al.,42 Hawley et al.,66 | ||||||
| Yes | No | No | Cell-NP interaction | Breitner et al.34 | |||
| Yes | No | Patho-physiological stretching models, stem cell differentiation | Grek et al.,26 Jeans et al.,32 Ghaedi et al.,27 Blume et al.,33 Box et al.31 | ||||
| Yes | Toxicology | Tarkington et al.,43 Tippe et al.,60 Bitterle et al.,61, Lenz et al.62 | |||||
| Micro | Lungs-on-chips | Yes | Yes | No | Evaluation of chip efficiency, design optimization of liquid-phase flow patterns, long-term electrotaxis study, disease models | Nalayanda et al.,199 Long et al.,200 Benam et al.,201,204 Li et al.,205 Jain et al.202 | |
| Lung inflammation mechanisms | Barkal et al.203 | ||||||
| Yes | Analysis of the effects of whole smoke | Benam et al.204 | |||||
Table 6.
Systems that apply mechanical stretching.
| Scale | Actuation method | Flow | ALI | Co-culture | 3D | Authors/commercial system | Strain type | Strain range |
|---|---|---|---|---|---|---|---|---|
| Macro | Pneumatic | No | No | No | No | Winston et al.167, Gorfien et al.166, Pugin et al.168 | Multiaxial, out-of-plane | 0%–15% |
| Trepat et al.172,173 | Biaxial, in-plane | 0%–20% | ||||||
| Yes | Dassow et al.165 | Multiaxial, out-of-plane | 10%–25% | |||||
| Yes | No | Peñuelas et al.174 | Biaxial, in-plane | 15% | ||||
| Yes | No | Yes | Miller et al.169 | Multiaxial, out-of-plane | 2% | |||
| Yes | Yes | No | No | Cei et al.158 | Multiaxial, out-of-plane | 5%–17% | ||
| NA | NA | FlexCell (commercial)163 | Biaxial, in-plane | 8%–22% | ||||
| Motor-driven | No | No | No | No | Tschumperlin et al.148, Tsuda et al.176, Cavanaugh et al.177 | Multiaxial, in-plane | 0%–25% | |
| StrexCell (commercial) | Uniaxial, in-plane | 0%–30% | ||||||
| Yes | Yes | Yes | Choe et al.186, Tomei et al.187 | Uniaxial, in-plane | 0%–30% | |||
| Micro (lungs-on-chips) | Pneumatic | No | No | No | No | Campillo et al.216 | Multiaxial, out-of-plane | 0%–20% |
| Yes | Yes | No | No | Felder et al.215 | Multiaxial, out-of-plane | 0%–20% | ||
| Yes | No | Stucki et al.213,214 | Multiaxial, out-of-plane | 10% | ||||
| Huh et al.207,209,210, Hassel et al.211, Liu et al.212 | Uniaxial, in-plane | 10% | ||||||
| Motor-driven | No | No | No | No | Kamotani et al.206 | Multiaxial, out-of-plane | 0%–25% |
NA: not applicable.
Clearly, the literature from the past 20 years in engineering lung in vitro models describes remarkable progresses, but as yet these advanced systems fail in fully recapitulating the in vivo environment. To date, the main issues are related to mimicking: (i) the alveolar architecture (dimension, spherical structure, and interconnection with adjacent alveoli in the acinus and with the capillaries that surround the alveolus), which may affect aerosol deposition, transport, and cell stretching; (ii) mechanical properties (e.g. membrane elastic and viscoelastic properties), which may influence cell behavior; (iii) biochemical properties related to the presence of a surfactant layer that, besides avoiding alveolar collapse or hyperextension, is likely to interfere with the passage of substances (and pathogens) across the cell barrier.220
It is well known that micro-scale extracellular matrix properties strongly influence cellular growth, migration, and differentiation, as well as cellular response to mechanical and biochemical signals.221–226 This aspect is true for every organ type, but there are tissues whose function hinges on their intricate structures, and this is the case of the alveoli. Addressing the issue of alveolar architecture, several authors are focusing their efforts in building materials for generating complex 3D structures able to recreate these biophysically and biochemically entangled networks. In this direction, Grigoryan et al.227 used stereolithography to build soft hydrogels containing such biomimetic and multivascular architectures. They managed to print a bioinspired alveolar model with an ensheathing vasculature, which was also able to sustain a cyclic ventilation with humified oxygen gas, maintain the viability of mammalian cell lines, and support the normal function and differentiation of primary human stem cells. This work represents an important step forward in combining an alveolar-like architecture with the cyclic stretching movement that mimics breathing. However, work still needs to be performed in order to have a coherent approximation of scalable lung-specific design. With the goal of obtaining in vivo-like structures, also Erben et al.228 used stereolithography to print mm-sized high precision 3D scaffolds at micrometer resolution.
As far as mechanical properties are concerned, as demonstrated in the section describing alveolar motion, despite the fact that stretching devices are able to apply (patho)physiological strain levels, they are not able to fully replicate the three-dimensional nature and scales of alveolar stretching. Indeed, in most of the cases the systems provide an in-plane stretch and, also in the case of out-of-plane stretching, membrane fixation, constraints, and indenter contact points likely result in non-uniform (and hence difficult to characterize and control) strain distributions. However, it should be noted that the assumption of isotropic breathing may be an oversimplification of the in vivo dynamics, which is probably affected by intrinsic tissue anisotropy. Another crucial mechanical aspect which is often overlooked is the elastic modulus of the cell culture substrate compared with the lung, which is a highly stretchable soft tissue with an elastic modulus of the order of 3 kPa. Most of the materials used as flexing substrates, such as PDMS, are very stiff with elastic moduli of the order of megapascals. Thus, the forces or pressures required to deform the substrates are much higher than experienced in the alveoli.
As a result, devices with mechanical stimuli comply at different levels with the engineering requirements mentioned in the Introduction. They therefore dictate distinct applications (or restrictions on the application, if we may say). In fact, most of the stretching devices presented in Table 6 do not provide media flow, and even in the cases where technical solutions were adopted to culture cells at ALI, aerosol exposure was not contemplated. The concomitant presence of cell stretching, flow, and a reproducible aerosol exposure system definitely poses an engineering challenge. Up to date such achievement has been reached in only one device at the “milliscale.”158 The present challenge of engineered lung models is the design of the “all-in-one device,” which combines all the features existing in the lung (i.e. lung architecture, stretching movement, and aerosol exposure). This is the direction in which many research efforts are pointed, with the prospect of replacing, at least partially, animal models with in vitro models. However, not all studies need a holistic approach. When engineering an in vitro model, its application should set the requirements of the design. For example, when modeling lung tissues (e.g. bronchi) that differ from the alveolus—which is the functional unit that deforms during the breathing—it is not necessary to mimic cyclic mechanical stretch; while in applications that do not foresee toxicological studies or testing the impact of inhaled substances, an exposure chamber will not enhance the reliability of the model. Therefore, when developing a lung model, the key is to identify which relevant physiological parameters should be reproduced according to the research question being addressed and the context of the future experiments. Similarly, lung device-users need to define the application and then choose the device accordingly.
Another important point which needs to be addressed is the possibility to monitor or interrogate the cells during culture, performing the measurement necessary for the study. While media collection for different cell assays is usually enabled by the presence of valves in the fluidic circuits or in the culture chambers, other measurements can be a challenge. For example, evaluating barrier integrity is fundamental for passage studies. However, although the presence of an intact barrier can be visually monitored in a qualitative manner, not all the devices are optically transparent and compatible with microscopes. Quantitative information can be obtained using transepithelial electrical resistance/impedance (TEER/TEEI) measurements, but they are not easily integrated at ALI. Therefore, further efforts are also needed to develop efficient and non-invasive monitoring systems for the evermore sophisticated devices we engineer.28
After overcoming the technical challenges of developing advanced cell culture tissues, there are still other hurdles to face. Naturally, the acceptance of these devices for the day-to-day use in common laboratories might not be easy. To overcome a possible resistance two approaches are crucial: engineers should work in close collaboration with the final users (biologists, toxicologists, among others) of the device in its development phase; make the device compatible as much as possible with common lab instruments and assays. In this way, the validation of the system becomes a similar process to that of testing any other new practice/assay. Positive and negative controls, as well as multiple replicates, are essential for the verification of the results. In the context of testing a bioreactor, for instance, it would consist of first testing individually each of the dynamic cues (taken then as variables) the system can provide. On the same line of thought, it is advisable to start using the devices in simple context and for small experiments, rather than adding too many variables to the set-up. From a biological point of view, researchers might want to consider starting by culturing more robust and reliable cell sources, such as cell lines, to perform the proof of concept of the device. Once this is accomplished, other cell types and more complex cellular arrangements can be included in the protocol. Ideally, in a further step, different labs and research groups would have the opportunity to test their protocols on the new system.
On a final note, the future direction of lung in vitro models will depend greatly not only on the upcoming technologies in the engineering field, but also on the ever-changing motivations to use them. This aspect appears even more clear nowadays when the COVID-19 pandemic highlights the importance of having ad hoc reliable and predictive in vitro models for a systematic study of respiratory diseases. Interestingly, the pandemic has also brought home the impact of open-source technologies for rapid and efficacious solutions to biomedical emergencies.229 Although many of the in vitro devices described in this review are commercial systems, there is still plenty of scope for new developments based on open-source collaborative design which may help address some of the issues such as mimicking lung complexity in a simple to use system, handling, and non-destructive intermediate and end point analysis.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work leading to this paper has received funding from the European Union’s H2020 research and innovation programme under grant agreement no. 760813 (PATROLS).
ORCID iD: Roberta Nossa  https://orcid.org/0000-0003-3055-2327
https://orcid.org/0000-0003-3055-2327
References
- 1. Arumugasaamy N, Navarro J, Kent Leach J, et al. In vitro models for studying transport across epithelial tissue barriers. Ann Biomed Eng 2019; 47: 1–21. [DOI] [PubMed] [Google Scholar]
- 2. Fröhlich E, Salar-Behzadi S. Toxicological assessment of inhaled nanoparticles: Role of in vivo, ex vivo, in vitro, and in Silico Studies. Int J Mol Sci 2014; 15: 4795–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forbes B, Ehrhardt C. Human respiratory epithelial cell culture for drug delivery applications. Eur J Pharm Biopharm 2005; 60: 193–205. [DOI] [PubMed] [Google Scholar]
- 4. Schmid O, Jud C, Umehara Y, et al. Biokinetics of aerosolized liposomal ciclosporin a in human lung cells in vitro using an air-liquid cell interface exposure system. J Aerosol Med Pulm Drug Deliv 2017; 30: 411–424. [DOI] [PubMed] [Google Scholar]
- 5. Mathias NR, Yamashita F, Lee VHL. Respiratory epithelial cell culture models for evaluation of ion and drug transport. Adv Drug Delivery Rev. Epub ahead of print 1996. DOI: 10.1016/S0169-409X(96)00420-6. [DOI] [Google Scholar]
- 6. Semmler-Behnke M, Kreyling WG, Schulz H, et al. Nanoparticle delivery in infant lungs. Proc Natl Acad Sci USA. Epub ahead of print 2012. DOI: 10.1073/pnas.1119339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Warheit DB, Brock WJ, Lee KP, et al. Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: Impact of surface treatments on particle toxicity. Toxicol Sci. Epub ahead of print 2005. DOI: 10.1093/toxsci/kfi331. [DOI] [PubMed] [Google Scholar]
- 8. Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv Drug Deliv Rev 2006; 58: 1030–1060. [DOI] [PubMed] [Google Scholar]
- 9. Proudfoot AG, McAuley DF, Griffiths MJD, et al. Human models of acute lung injury. DMM Dis Model Mech 2011; 4: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robinson NB, Krieger K, Khan F, et al. The current state of animal models in research: a review. Int J Surg 2019; 72: 9–13. [DOI] [PubMed] [Google Scholar]
- 11. Gribaldo L and WM. Advanced non-animal models in biomedical research: respiratory tract diseases: executive summary. Luxembourg, 2020. Epub ahead of print 2020. DOI: 10.2760/725821. [DOI] [Google Scholar]
- 12. Hoeng J, Bovard D, Peitsch MC. Organ-on-a-chip: engineered microenvironments for safety and efficacy testing. Elsevier Science, 2019, pp. 1–523. [Google Scholar]
- 13. Irvin CG, Bates JHT. Measuring the lung function in the mouse: The challenge of size. Respir Res 2003; 4 : 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsia CCW, Hyde DM, Weibel ER. Lung structure and the intrinsic challenges of gas exchange. Compr Physiol 2016; 6: 827–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fung YC, Sobin SS. Pulmonary alveolar blood flow. Circ Res 1972; 30: 470–490. [DOI] [PubMed] [Google Scholar]
- 16. Ivanov KP, Kalinina MK, Levkovich YI. Blood flow velocity in capillaries of brain and muscles and its physiological significance. Microvasc Res 1981; 22: 143–155. [DOI] [PubMed] [Google Scholar]
- 17. Davies PF, Tripathi SC. Mechanical stress mechanisms and the cell: An endothelial paradigm. Circ Res 1993; 72: 239–245. [DOI] [PubMed] [Google Scholar]
- 18. Waters CM, Roan E, Navajas D. Mechanobiology in lung epithelial cells: Measurements, perturbations, and responses. Compr Physiol 2012; 2: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller AJ, Spence JR. In vitro models to study human lung development, disease and homeostasis. Physiology 2017; 32: 246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhowmick R, Gappa-Fahlenkamp H. Cells and culture systems used to model the small airway epithelium. Lung 2016; 194: 419–428. [DOI] [PubMed] [Google Scholar]
- 21. Nahar K, Gupta N, Gauvin R, et al. In vitro, in vivo and ex vivo models for studying particle deposition and drug absorption of inhaled pharmaceuticals. Eur J Pharm Sci 2013; 49: 805–818. [DOI] [PubMed] [Google Scholar]
- 22. Schilders KAA, Eenjes E, Riet S van, et al. Regeneration of the lung: lung stem cells and the development of lung mimicking devices. Respir Res 2016; 17: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nichols JE, Niles JA, Vega SP, et al. Modeling the lung: design and development of tissue engineered macro- and micro-physiologic lung models for research use. Exp Biol Med 2014; 239: 1135–1169. [DOI] [PubMed] [Google Scholar]
- 24. Chen H, Yu Z, Bai S, et al. Microfluidic models of physiological or pathological flow shear stress for cell biology, disease modeling and drug development. TrAC Trends Anal Chem 2019; 117: 186–199. [Google Scholar]
- 25. Costa J, Ahluwalia A. Advances and current challenges in intestinal in vitro model engineering: a digest. Front Bioeng Biotechnol 2019; 7: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grek CL, Newton DA, Qiu Y, et al. Characterization of alveolar epithelial cells cultured in semipermeable hollow fibers. Exp Lung Res 2009; 35: 155–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghaedi M, Mendez JJ, Bove PF, et al. Alveolar epithelial differentiation of human induced pluripotent stem cells in a rotating bioreactor. Biomaterials. 2014; 35: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cacopardo L, Costa J, Giusti S, et al. Real-time cellular impedance monitoring and imaging of biological barriers in a dual-flow membrane bioreactor. Biosens Bioelectron 2019; 140: 111340. [DOI] [PubMed] [Google Scholar]
- 29. Giusti S, Sbrana T, La Marca M, et al. A novel dual-flow bioreactor simulates increased fluorescein permeability in epithelial tissue barriers. Biotechnol J 2014; 9: 1175–1184. [DOI] [PubMed] [Google Scholar]
- 30. Mazzei D, Guzzardi MA, Giusti S, et al. A low shear stress modular bioreactor for connected cell culture under high flow rates. Biotechnol Bioeng 2010; 106: 127–137. [DOI] [PubMed] [Google Scholar]
- 31. Box H, Livermore J, Johnson A, et al. Pharmacodynamics of isavuconazole in a dynamic In Vitro model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2016; 60: 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jeans AR, Howard SJ, Al-Nakeeb Z, et al. Pharmacodynamics of voriconazole in a dynamic in vitro model of invasive pulmonary aspergillosis: implications for in vitro susceptibility breakpoints. J Infect Dis 2012; 206: 442–452. [DOI] [PubMed] [Google Scholar]
- 33. Blume C, Reale R, Held M, et al. Temporal monitoring of differentiated human airway epithelial cells using microfluidics. PLoS One 2015; 10: e0139872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Breitner EK, Hussain SM, Comfort KK. The role of biological fluid and dynamic flow in the behavior and cellular interactions of gold nanoparticles. J Nanobiotechnology 2015; 13: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aufderheide M, Scheffler S, Ito S, et al. Ciliatoxicity in human primary bronchiolar epithelial cells afterrepeated exposure at the air-liquid interface with native mainstreamsmoke of K3R4F cigarettes with and without charcoal filter. Exp Toxicol Pathol 2015; 67: 407–411. [DOI] [PubMed] [Google Scholar]
- 36. Carterson AJ, Höner Zu, Bentrup K, Ott CM, et al. A549 lung epithelial cells grown as three-dimensional aggregates: Alternative tissue culture model for Pseudomonas aeruginosa pathogenesis. Infect Immun 2005; 73: 1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cortiella J, Niles J, Cantu A, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A 2010; 16: 2565–2580. [DOI] [PubMed] [Google Scholar]
- 38. Crabbé A, Liu Y, Sarker SF, et al. Recellularization of decellularized lung scaffolds is enhanced by dynamic suspension culture. PLoS One 2015; 10: e0126846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilkinson DC, Alva-Ornelas JA, Sucre JMS, et al. Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Stem Cells Transl Med 2017; 6: 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crabbé A, Liu Y, Matthijs N, et al. Antimicrobial efficacy against Pseudomonas aeruginosa biofilm formation in a three-dimensional lung epithelial model and the influence of fetal bovine serum. Sci Rep 2017; 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aufderheide M, Förster C, Beschay M, et al. A new computer-controlled air-liquid interface cultivation system for the generation of differentiated cell cultures of the airway epithelium. Exp Toxicol Pathol 2016; 68: 77–87. [DOI] [PubMed] [Google Scholar]
- 42. Frijns E, Verstraelen S, Stoehr LC, et al. A novel exposure system termed NAVETTA for in vitro laminar flow electrodeposition of nanoaerosol and evaluation of immune effects in human lung reporter cells. Environ Sci Technol 2017; 51: 5259–5269. [DOI] [PubMed] [Google Scholar]
- 43. Tarkington BK, Reen Wu, Wei-Min Sun, et al. In vitro exposure of tracheobronchial epithelial cells and of tracheal explants to ozone. Toxicology 1994; 88: 51–68. [DOI] [PubMed] [Google Scholar]
- 44. Switalla S, Knebel J, Ritter D, et al. Effects of acute in vitro exposure of murine precision-cut lung slices to gaseous nitrogen dioxide and ozone in an air-liquid interface (ALI) culture. Toxicol Lett 2010; 196: 117–124. [DOI] [PubMed] [Google Scholar]
- 45. Bakand S, Winder C, Khalil C, et al. A novel in vitro exposure technique for toxicity testing of selected volatile organic compounds. J Environ Monit 2006; 8: 100–105. [DOI] [PubMed] [Google Scholar]
- 46. Riediker M, Gasser M, Perrenoud A, et al. A system to test the toxicity of brake wear particles. In: 12th Int ETH-conference combust gener nanoparticles, Zürich, 23–25 June 2008. [Google Scholar]
- 47. Cooney DJ, Hickey AJ. Cellular response to the deposition of diesel exhaust particle aerosols onto human lung cells grown at the air-liquid interface by inertial impaction. Toxicol Vitr 2011; 25: 1953–1965. [DOI] [PubMed] [Google Scholar]
- 48. Holder AL, Lucas D, Goth-Goldstein R, et al. Inflammatory response of lung cells exposed to whole, filtered, and hydrocarbon denuded diesel exhaust. Chemosphere 2007; 70: 13–19. [DOI] [PubMed] [Google Scholar]
- 49. Holder AL, Lucas D, Goth-goldstein R, et al. Cellular response to diesel exhaust particles strongly depends on the exposure method. Toxicol Sci 2008; 103: 108–115. [DOI] [PubMed] [Google Scholar]
- 50. Oostingh GJ, Papaioannou E, Chasapidis L, et al. Toxicology in Vitro development of an on-line exposure system to determine freshly produced diesel engine emission-induced cellular effects. Toxicol Vitr 2013; 27: 1746–1752. [DOI] [PubMed] [Google Scholar]
- 51. Blank F, Rothen-Rutishauser BM, Schurch S, et al. An optimized in vitro model of the respiratory tract wall to study particle cell interactions. J Aerosol Med 2006; 19: 392–405. [DOI] [PubMed] [Google Scholar]
- 52. Savi M, Kalberer M, Lang D, et al. A novel exposure system for the efficient and controlled deposition of aerosol particles onto cell cultures. Environ Sci Technol 2008; 42: 5667–5674. [DOI] [PubMed] [Google Scholar]
- 53. Stevens JP, Zahardis J, MacPherson M, et al. A new method for quantifiable and controlled dosage of particulate matter for in vitro studies: the electrostatic particulate dosage and exposure system (EPDExS). Toxicol Vitro 2008; 22: 1768–1774. [DOI] [PubMed] [Google Scholar]
- 54. Rothen-Rutishauser B, Grass RN, Blank F, et al. Direct combination of nanoparticle fabrication and exposure to lung cell cultures in a closed setup as a method to simulate accidental nanoparticle exposure of humans. Environ Sci Technol 2009; 43: 2634–2640. [DOI] [PubMed] [Google Scholar]
- 55. Coplay Scientific website, 2020, https://www.copleyscientific.com/.
- 56. Haghi M, Traini D, Young P. In vitro cell integrated impactor deposition methodology for the study of aerodynamically relevant size fractions from commercial pressurised metered dose inhalers. Pharm Res 2014; 31: 1779–1787. [DOI] [PubMed] [Google Scholar]
- 57. Fiegel J, Ehrhardt C, Schaefer UF, et al. Large porous particle impingement on lung epithelial cell monolayers–toward improved particle characterization in the lung. Pharm Res 2003; 20: 788–796. [DOI] [PubMed] [Google Scholar]
- 58. Cooney D, Kazantseva M, Hickey AJ. Development of a size-dependent aerosol deposition model utilising human airway epithelial cells for evaluating aerosol drug delivery. Altern Lab Anim 2004; 32: 581–590. [DOI] [PubMed] [Google Scholar]
- 59. Grainger CI, Greenwell LL, Martin GP, et al. The permeability of large molecular weight solutes following particle delivery to air-interfaced cells that model the respiratory mucosa. Eur J Pharm Biopharm 2009; 71: 318–324. [DOI] [PubMed] [Google Scholar]
- 60. Tippe A, Heinzmann U, Roth C. Deposition of fine and ultrafine aerosol particles during exposure at the air/cell interface. J Aerosol Sci 2002; 33: 207–218. [Google Scholar]
- 61. Bitterle E, Karg E, Schroeppel A, et al. Dose-controlled exposure of A549 epithelial cells at the air-liquid interface to airborne ultrafine carbonaceous particles. Chemosphere 2006; 65: 1784–1790. [DOI] [PubMed] [Google Scholar]
- 62. Lenz A-G, Karg E, Brendel E, et al. Inflammatory and oxidative stress responses of an alveolar epithelial cell line to airborne zinc oxide nanoparticles at the air-liquid interface: a comparison with conventional, submerged cell-culture conditions. Biomed Res Int 2013; 2013: 652632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. De Bruijne K, Ebersviller S, Sexton KG, et al. Design and testing of electrostatic aerosol in vitro exposure system (EAVES): an alternative exposure system for particles. Inhal Toxicol 2009; 21: 91–101. [DOI] [PubMed] [Google Scholar]
- 64. Holder AL, Marr LC. Toxicity of silver nanoparticles at the air-liquid interface. Biomed Res Int 2013; 2013: 328934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stoehr LC, Madl P, Boyles MSP, et al. Enhanced deposition by electrostatic field-assistance aggravating diesel exhaust aerosol toxicity for human lung cells. Environ Sci Technol 2015; 49: 8721–8730. [DOI] [PubMed] [Google Scholar]
- 66. Hawley B, McKenna D, Marchese A, et al. Time course of bronchial cell inflammation following exposure to diesel particulate matter using a modified EAVES. Toxicol Vitr 2014; 28: 829–837. [DOI] [PubMed] [Google Scholar]
- 67. Volckens J, Dailey L, Walters G, et al. Direct particle-to-cell deposition of coarse ambient particulate matter increases the production of inflammatory mediators from cultured human airway epithelial cells. Environ Sci Technol 2009; 43: 4595–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xie Y, Williams NG, Tolic A, et al. Aerosolized ZnO nanoparticles induce toxicity in alveolar type II epithelial cells at the air-liquid interface. Toxicol Sci 2012; 125: 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Loret T, Peyret E, Dubreuil M, et al. Air-liquid interface exposure to aerosols of poorly soluble nanomaterials induces different biological activation levels compared to exposure to suspensions. Part Fibre Toxicol 2016; 13: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tollstadius BF, Silva ACG da, Pedralli BCO, et al. Carbendazim induces death in alveolar epithelial cells: A comparison between submerged and at the air-liquid interface cell culture. Toxicol Vitr 2019; 58: 78–85. [DOI] [PubMed] [Google Scholar]
- 71. Stoehr LC, Endes C, Radauer-Preiml I, et al. Assessment of a panel of interleukin-8 reporter lung epithelial cell lines to monitor the pro-inflammatory response following zinc oxide nanoparticle exposure under different cell culture conditions. Part Fibre Toxicol 2015; 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lenz A-G, Stoeger T, Cei D, et al. Efficient bioactive delivery of aerosolized drugs to human pulmonary epithelial cells cultured in air-liquid interface conditions. Am J Respir Cell Mol Biol 2014; 51: 526–35. [DOI] [PubMed] [Google Scholar]
- 73. Gerde P, Malmlöf M, Havsborn L, et al. DissolvIt: an in vitro method for simulating the dissolution and absorption of inhaled dry powder drugs in the lungs. Assay Drug Dev Technol 2017; 15: 77–88. [DOI] [PubMed] [Google Scholar]
- 74. Malmlöf M, Nowenwik M, Meelich K, et al. Effect of particle deposition density of dry powders on the results produced by an in vitro test system simulating dissolution- and absorption rates in the lungs. Eur J Pharm Biopharm 2019; 139: 213–223. [DOI] [PubMed] [Google Scholar]
- 75. Persoz C, Leleu C, Achard S, et al. Sequential air-liquid exposure of human respiratory cells to chemical and biological pollutants. Toxicol Lett 2011; 207: 53–59. [DOI] [PubMed] [Google Scholar]
- 76. Pariselli F, Sacco MG, Ponti J, et al. Effects of toluene and benzene air mixtures on human lung cells (A549). Exp Toxicol Pathol 2009; 61: 381–386. [DOI] [PubMed] [Google Scholar]
- 77. Anderson SE, Jackson LG, Franko J, et al. Evaluation of dicarbonyls generated in a simulated indoor air environment using an in vitro exposure system. Toxicol Sci 2010; 115: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bardet G, Achard S, Loret T, et al. A model of human nasal epithelial cells adapted for direct and repeated exposure to airborne pollutants. Toxicol Lett 2014; 229: 144–149. [DOI] [PubMed] [Google Scholar]
- 79. Al Zallouha M, Landkocz Y, Brunet J, et al. Usefulness of toxicological validation of VOCs catalytic degradation by air-liquid interface exposure system. Environ Res 2017; 152: 328–335. [DOI] [PubMed] [Google Scholar]
- 80. Méausoone C, El Khawaja R, Tremolet G, et al. In vitro toxicological evaluation of emissions from catalytic oxidation removal of industrial VOCs by air/liquid interface (ALI) exposure system in repeated mode. Toxicol Vitr 2019; 58: 110–117. [DOI] [PubMed] [Google Scholar]
- 81. Wang Y, Wu Q, Muskhelishvili L, et al. Assessing the respiratory toxicity of dihydroxyacetone using an in vitro human airway epithelial tissue model. Toxicol Vitr 2019; 59: 78–86. [DOI] [PubMed] [Google Scholar]
- 82. Chary A, Serchi T, Moschini E, et al. An in vitro coculture system for the detection of sensitization following aerosol exposure. ALTEX 2019; 36: 403–418. [DOI] [PubMed] [Google Scholar]
- 83. Anderson SE, Khurshid SS, Meade BJ, et al. Toxicological analysis of limonene reaction products using an in vitro exposure system. Toxicol Vitr 2013; 27: 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Olivera DS, Hoard-Fruchey H, Sciuto AM. Evaluation of an in vitro screening model to assess phosgene inhalation injury. Toxicol Mech Methods 2017; 27: 45–51. [DOI] [PubMed] [Google Scholar]
- 85. Aufderheide M, Halter B, Möhle N, et al. The CULTEX RFS: a comprehensive technical approach for the in vitro exposure of airway epithelial cells to the particulate matter at the air-liquid interface. Biomed Res Int 2013; 2013: 734137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Okuwa K, Tanaka M, Fukano Y, et al. In vitro micronucleus assay for cigarette smoke using a whole smoke exposure system: a comparison of smoking regimens. Exp Toxicol Pathol 2010; 62: 433–440. [DOI] [PubMed] [Google Scholar]
- 87. Aufderheide M, Scheffler S, Möhle N, et al. Analytical in vitro approach for studying cyto- and genotoxic effects of particulate airborne material. Anal Bioanal Chem 2011; 401: 3213–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nara H, Fukano Y, Nishino T, et al. Detection of the cytotoxicity of water-insoluble fraction of cigarette smoke by direct exposure to cultured cells at an air-liquid interface. Exp Toxicol Pathol 2013; 65: 683–688. [DOI] [PubMed] [Google Scholar]
- 89. Rach J, Budde J, Möhle N, et al. Direct exposure at the air-liquid interface: Evaluation of an in vitro approach for simulating inhalation of airborne substances. J Appl Toxicol 2014; 34: 506–515. [DOI] [PubMed] [Google Scholar]
- 90. Scheffler S, Dieken H, Krischenowski O, et al. Cytotoxic evaluation of e-liquid aerosol using different lung-derived cell models. Int J Environ Res Public Health 2015; 12: 12466–12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Scheffler S, Dieken H, Krischenowski O, et al. Evaluation of e-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int J Environ Res Public Health 2015; 12: 3915–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Oeder S, Kanashova T, Sippula O, et al. Particulate matter from both heavy fuel oil and diesel fuel shipping emissions show strong biological effects on human lung cells at realistic and comparable in vitro exposure conditions. PLoS One 2015; 10: e126536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sapcariu SC, Kanashova T, Dilger M, et al. Metabolic profiling as well as stable isotope assisted metabolic and proteomic analysis of RAW 264.7 macrophages exposed to ship engine aerosol emissions: Different effects of heavy fuel oil and refined diesel fuel. PLoS One 2016; 11: e0157964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Klein SG, Cambier S, Hennen J, et al. Endothelial responses of the alveolar barrier in vitro in a dose-controlled exposure to diesel exhaust particulate matter. Part Fibre Toxicol 2017; 14: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kooter IM, Alblas MJ, Jedynska AD, et al. Alveolar epithelial cells (A549) exposed at the air-liquid interface to diesel exhaust: First study in TNO’s powertrain test center. Toxicol Vitr 2013; 27: 2342–2349. [DOI] [PubMed] [Google Scholar]
- 96. Tsukue N, Okumura H, Ito T, et al. Toxicological evaluation of diesel emissions on A549 cells. Toxicol Vitr 2010; 24: 363–369. [DOI] [PubMed] [Google Scholar]
- 97. Ji J, Upadhyay S, Xiong X, et al. Multi-cellular human bronchial models exposed to diesel exhaust particles: assessment of inflammation, oxidative stress and macrophage polarization. Part Fibre Toxicol 2018; 15: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tang T, Gminski R, Könczöl M, et al. Investigations on cytotoxic and genotoxic effects of laser printer emissions in human epithelial A549 lung cells using an air/liquid exposure system. Environ Mol Mutagen 2012; 53: 125–135. [DOI] [PubMed] [Google Scholar]
- 99. Mülhopt S, Dilger M, Diabaté S, et al. Toxicity testing of combustion aerosols at the air-liquid interface with a self-contained and easy-to-use exposure system. J Aerosol Sci 2016; 96: 38–55. [Google Scholar]
- 100. Diabaté S, Mülhopt S, Paur HR, et al. The response of a co-culture lung model to fine and ultrafine particles of incinerator fly ash at the air-liquid interface. ATLA Altern to Lab Anim 2008; 36: 285–298. [DOI] [PubMed] [Google Scholar]
- 101. Mihai C, Chrisler WB, Xie Y, et al. Intracellular accumulation dynamics and fate of zinc ions in alveolar epithelial cells exposed to airborne ZnO nanoparticles at the air-liquid interface. Nanotoxicology 2015; 9: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Klein SG, Serchi T, Hoffmann L, et al. An improved 3D tetraculture system mimicking the cellular organisation at the alveolar barrier to study the potential toxic effects of particles on the lung. Part Fibre Toxicol 2013; 10: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Panas A, Comouth A, Saathoff H, et al. Silica nanoparticles are less toxic to human lung cells when deposited at the air-liquid interface compared to conventional submerged exposure. Beilstein J Nanotechnol 2014; 5: 1590–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kooter I, Ilves M, Gröllers-Mulderij M, et al. Molecular signature of asthma-enhanced sensitivity to CuO nanoparticle aerosols from 3D cell model. ACS Nano 2019; 13: 6932–6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. D’Angelo I, Costabile G, Durantie E, et al. Hybrid lipid/polymer nanoparticles for pulmonary delivery of siRNA: development and fate upon in vitro deposition on the human epithelial airway barrier. J Aerosol Med Pulm Drug Deliv 2018; 31: 170–181. [DOI] [PubMed] [Google Scholar]
- 106. Steinritz D, Möhle N, Pohl C, et al. Use of the cultex radial flow system as an in vitro exposure method to assess acute pulmonary toxicity of fine dusts and nanoparticles with special focus on the intra- and inter-laboratory reproducibility. Chem Biol Interact 2013; 206: 479–490. [DOI] [PubMed] [Google Scholar]
- 107. Kooter IM, Gröllers-Mulderij M, Steenhof M, et al. Cellular effects in an in vitro human 3D cellular airway model and A549/BEAS-2B in vitro cell cultures following air exposure to cerium oxide particles at an air–liquid interface. Appl Vitr Toxicol 2016; 2: 56–66. [Google Scholar]
- 108. Cappellini F, Di Bucchianico S, Karri V, et al. Dry generation of CeO2 nanoparticles and deposition onto a co-culture of A549 and THP-1 cells in air-liquid interface—dosimetry considerations and comparison to submerged exposure. Nanomaterials 2020; 10: 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kim JS, Peters TM, O’Shaughnessy PT, et al. Validation of an in vitro exposure system for toxicity assessment of air-delivered nanomaterials. Toxicol Vitr 2013; 27: 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Elihn K, Cronholm P, Karlsson HL, et al. Cellular dose of partly soluble Cu particle aerosols at the air-liquid interface using an in vitro lung cell exposure system. J Aerosol Med Pulm Drug Deliv 2013; 26: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bachler G, Losert S, Umehara Y, et al. Translocation of gold nanoparticles across the lung epithelial tissue barrier: Combining in vitro and in silico methods to substitute in vivo experiments. Part Fibre Toxicol 2015; 12: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Durantie E, Vanhecke D, Rodriguez-Lorenzo L, et al. Biodistribution of single and aggregated gold nanoparticles exposed to the human lung epithelial tissue barrier at the air-liquid interface. Part Fibre Toxicol 2017; 14: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chortarea S, Fytianos K, Rodriguez-Lorenzo L, et al. Distribution of polymer-coated gold nanoparticles in a 3D lung model and indication of apoptosis after repeated exposure. Nanomedicine 2018; 13: 1169–1185. [DOI] [PubMed] [Google Scholar]
- 114. Chortarea S, Clift MJD, Vanhecke D, et al. Repeated exposure to carbon nanotube-based aerosols does not affect the functional properties of a 3D human epithelial airway model. Nanotoxicology 2015; 9: 983–993. [DOI] [PubMed] [Google Scholar]
- 115. Chortarea S, Barosova H, Clift MJD, et al. Human asthmatic bronchial cells are more susceptible to subchronic repeated exposures of aerosolized carbon nanotubes at occupationally relevant doses than healthy cells. ACS Nano 2017; 11: 7615–7625. [DOI] [PubMed] [Google Scholar]
- 116. Beyeler S, Chortarea S, Rothen-Rutishauser B, et al. Acute effects of multi-walled carbon nanotubes on primary bronchial epithelial cells from COPD patients. Nanotoxicology 2018; 12: 699–711. [DOI] [PubMed] [Google Scholar]
- 117. Ji J, Hedelin A, Malmlöf M, et al. Development of combining of human bronchial mucosa models with XposeALI® for exposure of air pollution nanoparticles. PLoS One 2017; 12: e0170428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Thorne D, Adamson J. A review of in vitro cigarette smoke exposure systems. Exp Toxicol Pathol 2013; 65: 1183–1193. [DOI] [PubMed] [Google Scholar]
- 119. Thorne D, Bishop E, Haswell L, et al. A case study for the comparison of in vitro data across multiple aerosol exposure studies with extrapolation to human dose. Appl Vitr Toxicol 2018; 4: 167–179. [Google Scholar]
- 120. Garcia-Canton C, Errington G, Anadon A, et al. Characterisation of an aerosol exposure system to evaluate the genotoxicity of whole mainstream cigarette smoke using the in vitro γH2AX assay by high content screening. BMC Pharmacol Toxicol 2014; 15: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Majeed S, Frentzel S, Wagner S, et al. Characterization of the Vitrocell® 24/48 in vitroaerosol exposure system using mainstream cigarette smoke. Chem Cent J 2014; 8: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Azzopardi D, Haswell LE, Foss-Smith G, et al. Evaluation of an air-liquid interface cell culture model for studies on the inflammatory and cytotoxic responses to tobacco smoke aerosols. Toxicol Vitr 2015; 29: 1720–1728. [DOI] [PubMed] [Google Scholar]
- 123. Zhang S, Li X, Xie F, et al. Evaluation of whole cigarette smoke induced oxidative stress in A549 and BEAS–2B cells. Environ Toxicol Pharmacol 2017; 54: 40–47. [DOI] [PubMed] [Google Scholar]
- 124. Geraghty P, Baumlin N, Salathe MA, et al. Glutathione peroxidase-1 suppresses the unfolded protein response upon cigarette smoke exposure. Mediators Inflamm 2016; 2016: 9461289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Unwalla HJ, Ivonnet P, Dennis JS, et al. Transforming growth factor-β1 and cigarette smoke inhibit the ability of β2-agonists to enhance epithelial permeability. Am J Respir Cell Mol Biol 2015; 52: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sailland J, Grosche A, Baumlin N, et al. Role of Smad3 and p38 signalling in cigarette smoke-induced CFTR and BK dysfunction in primary human bronchial airway epithelial cells. Sci Rep 2017; 7: 10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mathis C, Poussin C, Weisensee D, et al. Human bronchial epithelial cells exposed in vitro to cigarette smoke at the air-liquid interface resemble bronchial epithelium from human smokers. Am J Physiol - Lung Cell Mol Physiol 2013; 304: 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Talikka M, Kostadinova R, Xiang Y, et al. The response of human nasal and bronchial organotypic tissue cultures to repeated whole cigarette smoke exposure. Int J Toxicol 2014; 33: 506–517. [DOI] [PubMed] [Google Scholar]
- 129. Schmid A, Baumlin N, Ivonnet P, et al. Roflumilast partially reverses smoke-induced mucociliary dysfunction. Respir Res 2015; 16: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Neilson L, Mankus C, Thorne D, et al. Development of an in vitro cytotoxicity model for aerosol exposure using 3D reconstructed human airway tissue; application for assessment of e-cigarette aerosol. Toxicol Vitr 2015; 29: 1952–1962. [DOI] [PubMed] [Google Scholar]
- 131. Fields W, Maione A, Keyser B, et al. Characterization and application of the VITROCELL VC1 smoke exposure system and 3D EpiAirway models for toxicological and e-cigarette evaluations. Appl Vitr Toxicol 2017; 3: 68–83. [Google Scholar]
- 132. Adamson J, Jaunky T, Thorne D, et al. Characterisation of the borgwaldt LM4E system for in vitro exposures to undiluted aerosols from next generation tobacco and nicotine products (NGPs). Food Chem Toxicol 2018; 113: 337–344. [DOI] [PubMed] [Google Scholar]
- 133. Thorne D, Larard S, Baxter A, et al. The comparative in vitro assessment of e-cigarette and cigarette smoke aerosols using the γH2AX assay and applied dose measurements. Toxicol Lett 2017; 265: 170–178. [DOI] [PubMed] [Google Scholar]
- 134. Behrsing H, Aragon M, Adamson J, et al. Characterization of a vitrocell VC1 using nicotine dosimetry: an essential component toward standardized in vitro aerosol exposure of tobacco and next generation nicotine delivery products. Appl Vitr Toxicol 2018; 4: 159–166. [Google Scholar]
- 135. Iskandar AR, Zanetti F, Marescotti D, et al. Application of a multi-layer systems toxicology framework for in vitro assessment of the biological effects of Classic Tobacco e-liquid and its corresponding aerosol using an e-cigarette device with MESHTM technology. Arch Toxicol 2019; 93: 3229–3247. [DOI] [PubMed] [Google Scholar]
- 136. Czekala L, Simms L, Stevenson M, et al. Toxicological comparison of cigarette smoke and e-cigarette aerosol using a 3D in vitro human respiratory model. Regul Toxicol Pharmacol 2019; 103: 314–324. [DOI] [PubMed] [Google Scholar]
- 137. Bishop E, Haswell L, Adamson J, et al. An approach to testing undiluted e-cigarette aerosol in vitro using 3D reconstituted human airway epithelium. Toxicol Vitr 2019; 54: 391–401. [DOI] [PubMed] [Google Scholar]
- 138. Ishikawa S, Matsumura K, Kitamura N, et al. Application of a direct aerosol exposure system for the assessment of biological effects of cigarette smoke and novel tobacco product vapor on human bronchial epithelial cultures. Regul Toxicol Pharmacol 2018; 96: 85–93. [DOI] [PubMed] [Google Scholar]
- 139. Iskandar AR, Martin F, Leroy P, et al. Comparative biological impacts of an aerosol from carbon-heated tobacco and smoke from cigarettes on human respiratory epithelial cultures: A systems toxicology assessment. Food Chem Toxicol 2018; 115: 109–126. [DOI] [PubMed] [Google Scholar]
- 140. Jaunky T, Adamson J, Santopietro S, et al. Assessment of tobacco heating product THP1.0. Part 5: In vitro dosimetric and cytotoxic assessment. Regul Toxicol Pharmacol 2018; 93: 52–61. [DOI] [PubMed] [Google Scholar]
- 141. Ren Y, Zhan Q, Hu Q, et al. Static stretch induces active morphological remodeling and functional impairment of alveolar epithelial cells. Respiration 2009; 78: 301–311. [DOI] [PubMed] [Google Scholar]
- 142. Heise RL, Stober V, Cheluvaraju C, et al. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J Biol Chem 2011; 286: 17435–17444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Edwards YS. Stretch stimulation: Its effects on alveolar type II cell function in the lung. Comp Biochem Physiol A Mol Integr Physiol 2001; 129: 245–260. [DOI] [PubMed] [Google Scholar]
- 144. Liu M, Tanswell AK, Post M. Mechanical force-induced signal transduction in lung cells. Am J Physiol 1999; 277: L667–L683. [DOI] [PubMed] [Google Scholar]
- 145. Wirtz HR, Dobbs LG. The effects of mechanical forces on lung functions. Respir Physiol 2000; 119: 1–17. [DOI] [PubMed] [Google Scholar]
- 146. Arold SP, Bartolák-Suki E, Suki B. Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Am J Physiol Lung Cell Mol Physiol 2009; 296: L574–L581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hammerschmidt S, Kuhn H, Gessner C, et al. Stretch-induced alveolar type II cell apoptosis: Role of endogenous bradykinin and PI3K-Akt signaling. Am J Respir Cell Mol Biol 2007; 37: 699–705. [DOI] [PubMed] [Google Scholar]
- 148. Tschumperlin DJ, Margulies SS. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am J Physiol 1998; 275: L1173–L1183. [DOI] [PubMed] [Google Scholar]
- 149. Cavanaugh KJJ, Oswari J, Margulies SS, et al. Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol 2001; 25: 584–591. [DOI] [PubMed] [Google Scholar]
- 150. Cohen TS, Cavanaugh KJ, Margulies SS. Frequency and peak stretch magnitude affect alveolar epithelial permeability. Eur Respir J 2008; 32: 854–861. [DOI] [PubMed] [Google Scholar]
- 151. DeFronzo RA, Ferrannini E, Sato Y, et al. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest 1981; 68: 1468–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Schmekel B, Borgstrom L, Wollmer P. Exercise increases the rate of pulmonary absorption of inhaled terbutaline. Chest 1992; 101: 742–745. [DOI] [PubMed] [Google Scholar]
- 153. Desai LP, Chapman KE, Waters CM. Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1. Am J Physiol Lung Cell Mol Physiol 2008; 295: L958–L965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Roan E, Waters CM. What do we know about mechanical strain in lung alveoli? Am J Physiol Cell Mol Physiol 2011; 301: L625–L635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Doryab A, Tas S, Taskin MB, et al. Evolution of bioengineered lung models: recent advances and challenges in tissue mimicry for studying the role of mechanical forces in cell biology. Adv Funct Mater 2019; 29: 1903114. [Google Scholar]
- 156. Knudsen L, Ochs M. The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochem Cell Biol 2018; 150: 661–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Guenat OT, Berthiaume F. Incorporating mechanical strain in organs-on-a-chip: lung and skin. Biomicrofluidics 2018; 12: 042207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Cei D, Ali D, Lenz A-G, et al. Development of a dynamic in vitro stretch model of the alveolar interface with aerosol delivery. Biotechnol Bioeng 2021; 118: 690–702. [DOI] [PubMed] [Google Scholar]
- 159. Callens SJP, Uyttendaele RJC, Fratila-Apachitei LE, et al. Substrate curvature as a cue to guide spatiotemporal cell and tissue organization. Biomaterials 2020; 232: 119739. [DOI] [PubMed] [Google Scholar]
- 160. Assoian RK, Bade ND, Cameron CV, et al. Cellular sensing of micron-scale curvature: a frontier in understanding the microenvironment. Open Biol 2019; 9: 190155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Werner M, Petersen A, Kurniawan NA, et al. Cell-perceived substrate curvature dynamically coordinates the direction, speed, and persistence of stromal cell migration. Adv Biosyst 2019; 3: e1900080. [DOI] [PubMed] [Google Scholar]
- 162. Yamashita T, Nishina T, Matsushita I, et al. Air-pressure-driven separable microdevice to control the anisotropic curvature of cell culture surface. Anal Sci 2020; 36: 1015–1019. [DOI] [PubMed] [Google Scholar]
- 163. FLEXCELL®. FLEXCELL® website, 2020, https://www.flexcellint.com/category/tension.
- 164. Cacopardo L, Mattei G, Ahluwalia A. A new load-controlled testing method for viscoelastic characterisation through stress-rate measurements. Materialia 2020; 9: 100552. [Google Scholar]
- 165. Dassow C, Wiechert L, Martin C, et al. Biaxial distension of precision-cut lung slices. J Appl Physiol 2010; 108: 713–721. [DOI] [PubMed] [Google Scholar]
- 166. Gorfien SF, Winston FK, Thibault LE, et al. Effects of biaxial deformation on pulmonary artery endothelial cells. J Cell Physiol 1989; 139: 492–500. [DOI] [PubMed] [Google Scholar]
- 167. Winston FK, Macarak EJ, Gorfien SF, et al. A system to reproduce and quantify the biomechanical environment of the cell. J Appl Physiol 1989; 67: 397–405. [DOI] [PubMed] [Google Scholar]
- 168. Pugin J, Dunn I, Jolliet P, et al. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol 1998; 275: 1040–1050. [DOI] [PubMed] [Google Scholar]
- 169. Miller C, George S, Niklason L. Developing a tissue-engineered model of the human bronchiole. J Tissue Eng Regen Med 2010; 4: 619–627. [DOI] [PubMed] [Google Scholar]
- 170. Cei D. Development of a dynamic model of the alveolar interface for the study of aerosol deposition. In: XIX International conference on mechanics in medicine and biology, Bologna, Italy, 2014. [Google Scholar]
- 171. UBORA: Open biomedical engineering e-platform for innovation through education, 2020, https://platform.ubora-biomedical.org/projects/9f0e14d2-9757-4510-a07a-4ade1dde1311.
- 172. Trepat X, Grabulosa M, Puig F, et al. Viscoelasticity of human alveolar epithelial cells subjected to stretch. Am J Physiol Lung Cell Mol Physiol 2004; 287: L1205–L1034. [DOI] [PubMed] [Google Scholar]
- 173. Trepat X, Puig F, Gavara N, et al. Effect of stretch on structural integrity and micromechanics of human alveolar epithelial cell monolayers exposed to thrombin. Am J Physiol Lung Cell Mol Physiol 2006; 290: 1104–1110. [DOI] [PubMed] [Google Scholar]
- 174. Peñuelas O, Melo E, Sánchez C, et al. Antioxidant effect of human adult adipose-derived stromal stem cells in alveolar epithelial cells undergoing stretch. Respir Physiol Neurobiol 2013; 188: 1–8. [DOI] [PubMed] [Google Scholar]
- 175. FLEXCELL®. FLEXCELL ® web site - publications, 2020, https://www.flexcellint.com/publication/lung.
- 176. Tsuda A, Stringer BK, Mijailovich SM, et al. Alveolar cell stretching in the presence of fibrous particles induces interleukin-8 responses. Am J Respir Cell Mol Biol 1999; 21: 455–462. [DOI] [PubMed] [Google Scholar]
- 177. Cavanaugh KJ, Margulies SS. Measurement of stretch-induced loss of alveolar epithelial barrier integrity with a novel in vitro method. Am J Physiol Cell Physiol 2002; 283: C1801-C1808. [DOI] [PubMed] [Google Scholar]
- 178. Hung CT, Williams JL. A method for inducing equi-biaxial and uniform strains in elastomeric membranes used as cell substrates. J Biomech 1994; 27: 227–232. [DOI] [PubMed] [Google Scholar]
- 179. Schaffer JL, Rizen M, L’Italien GJ, et al. Device for the application of a dynamic biaxially uniform and isotropic strain to a flexible cell culture membrane. J Orthop Res 1994; 12: 709–719. [DOI] [PubMed] [Google Scholar]
- 180. Lee AA, Delhaas T, Waldman LK, et al. An equibiaxial strain system for cultured cells. Am J Physiol Cell Physiol 1996; 27: C1400–C1408. [DOI] [PubMed] [Google Scholar]
- 181. Andersen KL, Norton LA. A device for the application of known simulated orthodontic forces to human cells in vitro. J Biomech 1991; 24: 649–654. [DOI] [PubMed] [Google Scholar]
- 182. Vandenburgh HH. A computerized mechanical cell stimulator for tissue culture : effects on skeletal muscle organogenesis. In Vitro Cell Dev Biol 1988; 24: 609–619. [DOI] [PubMed] [Google Scholar]
- 183. Hasegawa S, Sato S, Saito S, et al. Mechanical stretching increases the number of cultured bone cells synthesizing DNA and alters their pattern of protein synthesis. Calcif Tissue Int 1985; 37: 431–436. [DOI] [PubMed] [Google Scholar]
- 184. Ito S, Kume H, Naruse K, et al. A novel Ca2+ influx pathway activated by mechanical stretch in human airway smooth muscle cells. Am J Respir Cell Mol Biol 2008; 38: 407–413. [DOI] [PubMed] [Google Scholar]
- 185. Ito S, Suki B, Kume H, et al. Actin cytoskeleton regulates stretch-activated Ca2+ influx in human pulmonary microvascular endothelial cells. Am J Respir Cell Mol Biol 2010; 43: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Choe MM, Sporn PHS, Swartz MA. Extracellular matrix remodeling by dynamic strain in a three-dimensional tissue-engineered human airway wall model. Am J Respir Cell Mol Biol 2006; 35: 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Tomei AA, Choe MM, Swartz MA. Effects of dynamic compression on lentiviral transduction in an in vitro airway wall model. Am J Physiol - Lung Cell Mol Physiol 2008; 294: 79–86. [DOI] [PubMed] [Google Scholar]
- 188. Akbari S, Shea HR. Microfabrication and characterization of an array of dielectric elastomer actuators generating uniaxial strain to stretch individual cells. J Micromech Microeng 22. Epub ahead of print 2012. DOI: 10.1088/0960-1317/22/4/045020. [DOI] [Google Scholar]
- 189. Cei D, Costa J, Gori G, et al. A bioreactor with an electro-responsive elastomeric membrane for mimicking intestinal peristalsis. Bioinspir Biomim 2016; 12: 016001. [DOI] [PubMed] [Google Scholar]
- 190. Poulin A, Imboden M, Sorba F, et al. An ultra-fast mechanically active cell culture substrate. Sci Rep 2018; 8: 9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Costa J, Ghilardi M, Mamone V, et al. Bioreactor with electrically deformable curved membranes for mechanical stimulation of cell cultures. Front Bioeng Biotechnol 2020; 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Skinner SJM, Somervell CE, Olson DM. The effects of mechanical stretching on fetal rat lung cell prostacyclin production. Prostaglandins 1992; 43: 413–433. [DOI] [PubMed] [Google Scholar]
- 193. Liu M, Skinner SJM, Xu J, et al. Stimulation of fetal rat lung cell proliferation in vitro by mechanical stretch. Am J Physiol 1992; 263: 376–383. [DOI] [PubMed] [Google Scholar]
- 194. Liu M, Xu J, Souza P, et al. The effect of mechanical strain on fetal rat lung cell proliferation: omparision of two- and three-dimensional culture systems. Vitr Cell Dev Biol 1995; 858–866. [DOI] [PubMed] [Google Scholar]
- 195. Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends in Cell Biology 2011; 21: 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Pedraza E, Brady AC, Fraker CA, et al. Synthesis of macroporous poly(dimethylsiloxane) scaffolds for tissue engineering applications. J Biomater Sci Polym Ed. 2013; 24: 1041–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Niemeyer BF, Zhao P, Tuder RM, et al. Advanced microengineered lung models for translational drug discovery. SLAS Discov 2018; 23: 777–789. [DOI] [PubMed] [Google Scholar]
- 198. Mittal R, Woo FW, Castro CS, et al. Organ-on-chip models: implications in drug discovery and clinical applications. J Cell Physiol 2019; 234: 8352–8380. [DOI] [PubMed] [Google Scholar]
- 199. Nalayanda DD, Puleo C, Fulton WB, et al. An open-access microfluidic model for lung-specific functional studies at an air-liquid interface. Biomed Microdevices 2009; 11: 1081–1089. [DOI] [PubMed] [Google Scholar]
- 200. Long C, Finch C, Esch M, et al. Design optimization of liquid-phase flow patterns for microfabricated lung on a chip. Ann Biomed Eng 2012; 40: 1255–1267. [DOI] [PubMed] [Google Scholar]
- 201. Benam KH, Villenave R, Lucchesi C, et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods 2015; 13: 151–157. [DOI] [PubMed] [Google Scholar]
- 202. Jain A, Barrile R, van der Meer AD, et al. Primary human lung alveolus-on-a-chip model of intravascular thrombosis for assessment of therapeutics. Clin Pharmacol Ther 2018; 103: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Barkal LJ, Procknow CL, Álvarez-Garciá YR, et al. Microbial volatile communication in human organotypic lung models. Nat Commun 2017; 8: 1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Benam KH, Novak R, Nawroth J, et al. Matched-comparative modeling of normal and diseased human airway responses using a microengineered breathing lung chip. Cell Syst 2016; 3: 456–466.e4. [DOI] [PubMed] [Google Scholar]
- 205. Li Y, Xu T, Zou H, et al. Cell migration microfluidics for electrotaxis-based heterogeneity study of lung cancer cells. Biosens Bioelectron 2017; 89: 837–845. [DOI] [PubMed] [Google Scholar]
- 206. Kamotani Y, Bersano-Begey T, Kato N, et al. Individually programmable cell stretching microwell arrays actuated by a Braille display. Biomaterials 2008; 29: 2646–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Huh D, Matthews BD, Mammoto A, et al. Reconstituting organ-level lung functions on a chip. Science 2010; 328: 1662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Huh D, Kim HJ, Fraser JP, et al. Microfabrication of human organs-on-chips. Nat Protoc 2013; 8: 2135–2157. [DOI] [PubMed] [Google Scholar]
- 209. Huh D. A human breathing lung-on-a-chip. Ann Am Thorac Soc 2015; 12: S42–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Huh D, Leslie DC, Matthews BD, et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med 2012; 4: 159ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Hassell BA, Goyal G, Lee E, et al. Human organ chip models recapitulate orthotopic lung cancer growth, therapeutic responses, and tumor dormancy in vitro. Cell Rep 2017; 21: 508–516. [DOI] [PubMed] [Google Scholar]
- 212. Liu W, Song J, Du X, et al. AKR1B10 (Aldo-keto reductase family 1 B10) promotes brain metastasis of lung cancer cells in a multi-organ microfluidic chip model. Acta Biomater 2019; 91: 195–208. [DOI] [PubMed] [Google Scholar]
- 213. Stucki AO, Stucki JD, Hall SRR, et al. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip 2015; 15: 1302–1310. [DOI] [PubMed] [Google Scholar]
- 214. Stucki JD, Hobi N, Galimov A, et al. Medium throughput breathing human primary cell alveolus-on-chip model. Sci Rep 2018; 8: 14359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Felder M, Trueeb B, Stucki AO, et al. Impaired wound healing of alveolar lung epithelial cells in a breathing lung-on-a-chip. Front Bioeng Biotechnol 2019; 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Campillo N, Jorba I, Schaedel L, et al. A novel chip for cyclic stretch and intermittent hypoxia cell exposures mimicking obstructive sleep apnea. Front Physiol 2016; 7: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Poli D, Mattei G, Ucciferri N, et al. An integrated in vitro–in silico approach for silver nanoparticle dosimetry in cell cultures. Ann Biomed Eng 2020; 48: 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Mattei G, Giusti S, Ahluwalia A. Design criteria for generating physiologically relevant in vitro models in bioreactors. Processes. Epub ahead of print 2014. DOI: 10.3390/pr2030548. [DOI] [Google Scholar]
- 219. Halldorsson S, Lucumi E, Gómez-Sjöberg R, et al. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron 2015; 63: 218–231. [DOI] [PubMed] [Google Scholar]
- 220. Bracco L. Journal of medical - clinical research & reviews. J Med Clin Res Rev 2020; 4: 1–3. [Google Scholar]
- 221. Burgstaller G, Oehrle B, Gerckens M, et al. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J 2017; 50: 1601805. [DOI] [PubMed] [Google Scholar]
- 222. Torgersen J, Qin X-H, Li Z, et al. Hydrogels for two-photon polymerization: a toolbox for mimicking the extracellular matrix. Adv Funct Mater 2013; 23: 4542–4554. [Google Scholar]
- 223. Mandal K, Wang I, Vitiello E, et al. Cell dipole behaviour revealed by ECM sub-cellular geometry. Nat Commun 2014; 5: 5749. [DOI] [PubMed] [Google Scholar]
- 224. Engelhardt S, Hoch E, Borchers K, et al. Fabrication of 2D protein microstructures and 3D polymer-protein hybrid microstructures by two-photon polymerization. Biofabrication 2011; 3: 25003. [DOI] [PubMed] [Google Scholar]
- 225. Guilak F, Cohen DM, Estes BT, et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009; 5: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Zhang F, King MW. Biodegradable polymers as the pivotal player in the design of tissue engineering scaffolds. Adv Healthc Mater 2020; 9: 1901358. [DOI] [PubMed] [Google Scholar]
- 227. Grigoryan B, Paulsen SJ, Corbett DC, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019; 364: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Erben A, Hörning M, Hartmann B, et al. Precision 3D-printed cell scaffolds mimicking native tissue composition and mechanics. Adv Healthc Mater 2020; 9: e2000918. [DOI] [PubMed] [Google Scholar]
- 229. De Maria C, Di Pietro L, Ravizza A, et al. Chapter 2 - Open-source medical devices: Healthcare solutions for low-, middle-, and high-resource settings. In: EBT-CEH Iadanza. (ed.) Clinical engineering handbook. Academic Press, 2020, pp. 7–14. [Google Scholar]