Abstract
Transthyretin cardiac amyloidosis (ATTR-CM) is an increasingly recognized cause of heart failure with preserved ejection fraction. Favorable prognosis depends on early diagnosis and correct treatment strategy. Among patients for whom there is a high clinical suspicion of cardiac amyloidosis, 99mTc-labeled bone avid scintigraphy including 99mTc-pyrophosphate (PYP) scintigraphy may be of diagnostic and prognostic importance. Various international guidelines support the non-biopsy diagnosis of ATTR-CM using 99mTc-PYP scintigraphy, yet emphasize the gap in standardization of acquisition and imaging analysis protocols, as well as the appropriateness of its clinical use. Therefore, a joint expert consensus has been reached by the Taiwan Society of Cardiology and the Society of Nuclear Medicine of the Republic of China, to advocate for the application of 99mTc-PYP scintigraphy in the diagnosis of ATTR-CM. This article aims to highlight the recommendations on image acquisition, qualitative and quantitative assessments of cardiac 99mTc-PYP uptake, and diagnostic algorithms. We hope the implementation of these recommendations in Taiwan will facilitate the process and enhance the diagnostic rate of ATTR-CM.
Keywords: Cardiac amyloidosis, Cardiomyopathy, Heart failure, Scintigraphy, 99mTc-pyrophosphate (PYP), Transthyretin
INTRODUCTION
Heart failure (HF) is the leading cause of cumulative hospitalization days for cardiovascular disease in Taiwan, and rates of re-hospitalization and mortality are high.1,2 Due to different etiologies and disease severity, there is a large gap in treatment response and prognosis.3-5
The incidence and prevalence of HF with preserved ejection fraction (HFpEF) have increased in recent years.6 Among the different causes of HFpEF, cardiac amyloidosis (CA), where amyloid deposition occurs in the heart, still poses challenges to physicians in early diagnosis and treatment.7,8 The majority (> 95%) of patients with cardiac amyloidosis are affected either by a misfolded immunoglobulin light chain (AL), or by transthyretin (TTR) deposition in the heart.8 Cardiomyopathy is a clinical feature of transthyretin amyloidosis (ATTR), which is an under-recognized systemic disease whereby the transthyretin protein misfolds to form fibrils that deposit in various tissues and organs. Transthyretin cardiac amyloidosis (ATTR-CM) can be divided into two major forms — wild-type ATTR (ATTRwt) and ATTR from a genetic mutation (ATTRm).7
The primary manifestations of ATTRwt are cardiac amyloidosis and carpal tunnel syndrome. The prevalence of ATTRwt in HFpEF patients with left ventricular hypertrophy (LVH) (wall thickness > 12 mm) is about 13%, and the prevalence of ATTRwt in patients with aortic stenosis after aortic valve replacement is 6-16%.9-11 The age of onset of ATTRm is variable, ranging from 30 to 80 years old. The symptoms of ATTRm correspond to different gene mutations. This hereditary form of ATTR manifests predominantly as polyneuropathy, cardiomyopathy, or mixed phenotype.12 The Transthyretin Amyloidosis Outcomes Survey (THAOS) study found that among symptomatic patients with TTR mutations, approximately half have neurological manifestations as their primary symptom, followed by cardiac abnormalities and mixed phenotype, each constituting approximately a quarter, respectively.12 ATTR genotypes and phenotypes are highly heterogeneous. Because there are no large-scale screening and registration studies, the actual prevalence of ATTR-CM in Taiwan remains unclear. The Ala97Ser mutation is the most common cause of ATTRm in Taiwan.13,14 It is considered both a form of cardiomyopathy and a polyneuropathic syndrome, and approximately 80% of them have LVH.14 Therefore, raising early awareness that ATTR-CM in Taiwan can manifest as a dominant cardiac phenotype mimicking hypertrophic cardiomyopathy (HCM) at presentation will facilitate the clinical recognition of this under-diagnosed disease.
ATTR is debilitating and associated with poor life expectancy, especially in those with cardiac dysfunction. Delayed or lack of treatment after diagnosis will result in poor prognosis, with a median survival period of only 2 to 3 years. Recently, a variety of new and promising treatment options have become available,7 including pharmacological therapy that slows or halts ATTR-CM progression and favorably affects clinical outcomes. Therefore, early recognition is essential to afford the best treatment efficacy.
Several clinical signs can raise suspicion for CA. These red flags include abnormalities on electrocardiography (ECG), echocardiography, cardiac magnetic resonance imaging (CMR), or cardiac biomarkers.15-18 Noninvasive methods including ECG, echocardiography, CMR, and nuclear scintigraphy can assist in diagnosis.19 LVH is a common indicator used to detect CA. Red flags of CA on ECG include a low QRS voltage in combination with increased left ventricle thickness, a pseudo-infarction pattern, conduction abnormality, and arrhythmia.19-21 Red flags on echocardiography indicating CA include thickening of the atrioventricular valves and interatrial septum, a speckled and granular appearance of the myocardium, and a base-to-apex strain gradient with relative apical sparing of longitudinal strain (the so-called "cherry-on-top pattern").19,22,23 CMR might show global diffuse myocardial late gadolinium enhancement in the subendocardial layers, elevated native T1 and extracellular volume fraction in T1 mapping.18,19,24 Endocardial biopsy is considered as the gold standard for the diagnosis of ATTR.19 Once CA is suspected, further confirmatory diagnosis could be reached through either non-invasive methods such as nuclear scintigraphy and the free light chain (FLC) test,25-27 or invasive methods such as biopsy and amyloid typing.
Bone-avid radiotracers including technetium-99m (99mTc)-pyrophosphate (PYP) have been reported to be useful in detecting CA.8,15-19,22-27 The exact mechanism is not fully understood. It may be related to amyloid microcalcification. Historically, 99mTc-PYP has been well-known for its role in diagnosing acute/recent myocardial infarction or rhabdomyolysis, thus also referred to as infarct or muscle scintigraphy. Semi-quantitative analyses based on the radiotracer uptake in the heart and surrounding ribs, expressed on a 0-3 point grading scale, help to distinguish different types of CA.25-27 Most patients with AL amyloidosis are of lower grades (grades 0-2), while patients with ATTR-CM are of higher grades (grades 2-3).25-27 In a study which enrolled 1,217 patients after excluding AL with suspected CA and using PYP scan grades 2-3 as criteria, the specificity and positive predictive values were both as high as 100%.28 PYP scan has thus been considered a suitable alternative to EMB in some patients suspect of ATTR-CM.29,30 In contrast to echocardiography, EMB can truly demonstrate amyloid fibrils in clinically suspected patients mimicking hypertensive heart disease or HCM. It strengthens the importance of pathological confirmation of CA from other HCM, albeit diagnostic yield of extracardiac biopsies with the presence of amyloid protein in high-risk patients with conduction disturbances and advanced age could be relatively low. A feasible and less invasive tool such as PYP scan will make diagnosis of ATTR-CM more applicable for both clinicians and patients.27
Last but not least, with respect to promising therapeutic outcome from recent clinical trials, imaging techniques and reporting format standardization are of increasing importance. The Taiwan Society of Cardiology (TSOC) and the Society of Nuclear Medicine of the Republic of China (SNMROC) apprehend the acute necessity of establishing advocacy statements on when and how to use nuclear imaging procedures, adjust relevant acquisition parameters, standardize reporting format, and facilitate earlier therapeutic intervention and clinical research.
99mTc-PYROPHOSPHATE SCINTIGRAPHY
Image acquisition procedures
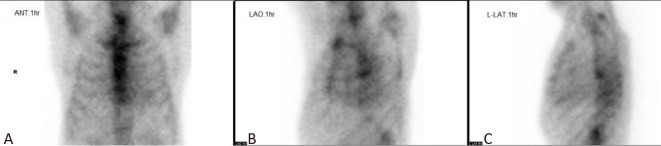
Recommendations for 99mTc-PYP scintigraphy acquisition parameters are listed in Table 1. Standard planar images for anterior, left anterior oblique and lateral views are shown in Figure 1. Bilateral shoulders should be visible in the image to evaluate for musculoskeletal manifestations of amyloid deposition, such as the shoulder pad sign or joint swelling.31 Symmetric renal uptake should be minimized in planar images to avoid interference with the myocardial counts.
Table 1. Recommendations for 99mTc-pyrophosphate scintigraphy acquisition parameters.
| Parameters | |
| Imaging procedure | |
| Specific preparation | None; fasting is not required |
| Scan | Rest scan |
| Dose of 99mTc-PYP | • Recommended: 20 mCi for 3-hour acquisition |
| • Adjust according to different models of instrument | |
| Time interval between injection and acquisition | • Recommended: 3-hour planar and SPECT |
| • Optional: 1-hour planar | |
| Imaging parameters | |
| Field of view | Cardiac or chest |
| Image type | Cardiac or chest planar and SPECT (or SPECT/CT) |
| Position | Supine |
| Energy window | 140 keV, 15-20% |
| Collimators | Low energy, high resolution |
| Matrix | 128 × 128 recommended (64 × 64 minimum required) for SPECT, 256 × 256 for planar |
| Pixel size | 2.3-6.5 mm (may change with different matrix) |
| Planar imaging specific parameters | |
| Number of views | Anterior, lateral, and left anterior oblique (LAO) |
| Detector configuration | 180 degrees |
| Image duration | 750,000 counts |
| Magnification | 1.45-1.5 |
| SPECT imaging specific parameters | |
| Angular range | 360 degrees |
| Detector configuration | 180 degrees |
| ECG gating | Off; non-gated imaging |
| Number of views/detectors | 32-64 |
| Time per stop | 20 seconds |
ECG, electrocardiography; keV, kilo-electronvolts; mCi, millicurie; PYP, pyrophosphate; SPECT, single-photon emission computed tomography; SPECT/CT, SPECT/computed tomography.
Figure 1.
Standard planar images for (A) ANT (anterior), (B) LAO (left anterior oblique) and (C) LAT (lateral) views, acquired at 1 hour (hr) and/or 3 hrs, respectively. Optimal field of view (FOV) should cover the whole chest region, including bilateral shoulders while avoiding highly radioactive sites, such as injection sites or areas with marked renal activity.
One-hour imaging results have higher detection sensitivity, while 3-hour imaging results offer higher detection specificity. This is because delayed clearance of blood pool activity after tracer injection is not uncommon in patients with poor renal function or low cardiac output. In our recommendation, 3-hour planar images are recommended for visual scoring and semi-quantitation and 1-hour images are optional. Single-photon emission computed tomography (SPECT) is necessary to distinguish blood pool activity from myocardial activity. In addition, SPECT/computed tomography (SPECT/CT) is preferred for attenuation correction and anatomical localization. It accentuates differences in myocardial uptake patterns, allowing for more accurate classifications — absent, focal, diffuse, or focal on diffuse. Therefore, an injection dose of 20 millicurie (mCi) of 99mTc PYP is recommended for 3-hour planar and SPECT imaging. Although delayed-time-point imaging is expected to improve qualitative and semi-quantitative results, SPECT or SPECT/CT at 1 hour with low blood activity is also acceptable. If SPECT or SPECT/CT is performed, planar images of left anterior oblique and lateral views are optional to shorten the image acquisition time.
The differences in 99mTc-PYP scintigraphy imaging acquisition parameters between our recommendations and those of the 2019 American Society of Nuclear Cardiology (ASNC) practice points26 are listed in Table 2.
Table 2. Differences in 99mTc-pyrophosphate scintigraphy acquisition parameters between ASNC* and TSOC/SNMROC consensus recommendations.
| TSOC and SNMROC recommendations | 2019 ASNC practice points recommendations* | |
| Imaging procedure | ||
| Dose of 99mTc-PYP | • Recommended: 20 mCi for 3-hour acquisition | • 10-20 mCi |
| • Adjust according to different models of instrument | ||
| Time interval between injection and acquisition | • Recommended: 3-hour planar and SPECT | • Recommended: 1-hour planar and SPECT |
| • Optional: 1-hour planar | • Optional: 3-hour SPECT or planar | |
| Imaging parameters | ||
| Matrix | • Recommended: 128 × 128 for SPECT | 64 × 64 minimum |
| • Required: 64 × 64 minimum for SPECT | ||
| • 256 × 256 for planar | ||
| Planar imaging specific parameters | ||
| Detector configuration | 180 degrees | 90 degrees |
| Magnification | 1.45-1.5 | 1.46 |
| SPECT imaging specific parameters | ||
| Number of views/detector | 32-64 | 40 |
ASNC, American Society of Nuclear Cardiology; ECG, electrocardiography; mCi, millicurie; PYP, pyrophosphate; SNMROC, Society of Nuclear Medicine of the Republic of China; SPECT, single-photon emission computed tomography; TSOC, Taiwan Society of Cardiology.
* Source: Dorbala S, et al. 2019 ASNC Practice Points.26
SEMI-QUANTITATION
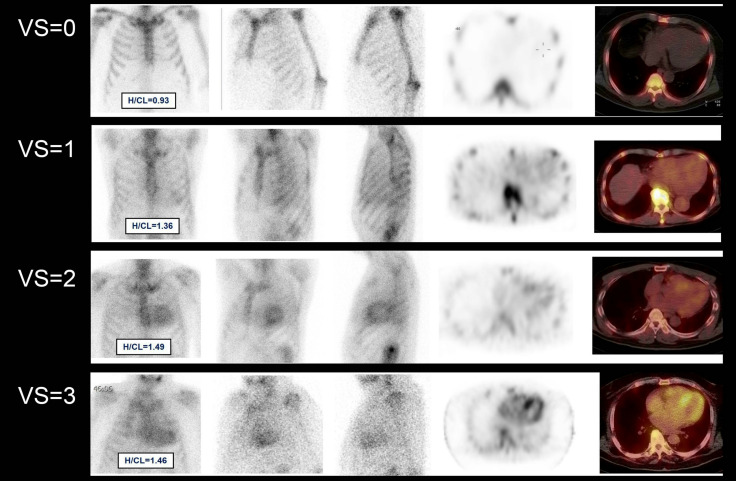
A visual grading scale is used to interpret the imaging results (Table 3). A visual grade of 0 is not suggestive of ATTR-CM. Grade 1 is equivocal. If clinically highly suspicious for CA, a specialist should perform further assessment. Grade 2 or 3 is diagnostic of ATTR-CM.
Table 3. Recommendations for standardized interpretation of 99mTc-pyrophosphate scintigraphy.
| Visual interpretation |
| • Evaluate for cardiac radiotracer uptake on planar and myocardial uptake on SPECT or SPECT/CT images. |
| • If SPECT images show radiotracer uptake in the myocardium, proceed with visual grading. In the absence of any myocardial tracer uptake, a visual grade of 0 is given. |
| Visual grading |
| The relative tracer uptake in the myocardium to ribs seen on planar and SPECT images is graded on the following scale: |
| • Grade 0: No myocardial uptake and normal bone uptake |
| • Grade 1: Myocardial uptake less than rib uptake |
| • Grade 2: Myocardial uptake equal to rib uptake |
| • Grade 3: Myocardial uptake greater than rib uptake with mild/absent rib uptake |
| H/CL ratio interpretation |
| If tracer uptake is not evident on SPECT images, obtaining the H/CL ratio is not recommended. H/CL ratios can help identify ATTR-CM if systemic AL amyloidosis is excluded by the following values: |
| • 1-hour images: H/CL ratio > 1.5 |
| • 3-hour images: H/CL ratio ≥ 1.3 |
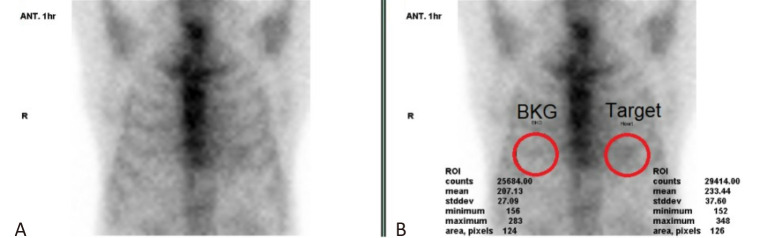
A heart to contralateral lung ratio (H/CL) on the planar anterior view is used for semi-quantitation as shown in Figure 2. A circular or elliptical region of interest (ROI) is drawn to include the left ventricle maximally while cautiously avoiding the sternal region and the adjacent lung. The ROI is mirrored to the contralateral chest, being careful to exclude any right heart or mediastinal activity and avoiding the subdiaphragmatic organs. Total and absolute mean counts are measured in each ROI. A H/CL ratio is calculated as the fraction of heart ROI mean counts to contralateral chest ROI mean counts. Alternatively, geometric means of the average counts of ROIs calculated from both anterior and posterior planar views can be used. Although different H/CL ratios and cut-off values for one hour or three hours have been reported,25-27,32 our consensus uphold the criteria of the 2019 ASNC recommendations25,26 and the 2020 JCS guideline.27 For one-hour images, an H/CL ratio > 1.5 is used to identify ATTR-CM. For 3-hour images, an H/CL ratio ≥ 1.3 is used to identify ATTR-CM.
Figure 2.
Quantitation of cardiac 99mTc-PYP uptake using the H/CL ratio. (A) Planar anterior view, (B) Region of interest (ROI) demonstration. Circular target ROI (red circles), which includes the heart, is drawn over the planar anterior image, and is mirrored over the contralateral lung. Total and mean counts are calculated, and the H/CL ratio is derived from mean counts of ROIs. BKG, background; H/CL, heart to contralateral lung; PYP, pyrophosphate; stddev, standard deviation.
99mTc-PYP quantitative SPECT is a feasible and objective tool for assessing the burden of CA in the diagnosis of ATTR-CM.33,34 Dual-isotope SPECT using 99mTc-PYP/ 201Tl might augment visual differentiation compared to single-isotope SPECT.35 However, the methodology is not standardized. As such, further investigation is warranted.
The recently introduced cadmium zinc telluride SPECT cameras have the potential to reduce radiation exposure to patients and shorten imaging time. The value of 99mTc PYP imaging utilizing the newer "cardiac only" SPECT cameras also needs further validation.36-40
INTERPRETATION AND REPORTING
Recommendations for image interpretation and reporting of 99mTc-PYP scintigraphy are listed in Tables 3 and 4.
Table 4. Recommendations for standardized reporting of 99mTc-pyrophosphate scintigraphy.
| Parameters | Components | |||
| Demographics | Patient name, age, sex, study date and reason, previous imaging procedures, and results of biopsy if available. | |||
| Heart failure severity (NYHA classification, LVEF %), recent myocardial infarction history (within 3 months), and renal function (eGFR or cCr within 3-6 months, or presence of ESRD) should be provided as supportive information (recommended) | ||||
| Methods | Image acquisition parameters and technique, dose and mode of radiotracer administration, time interval between injection and scan, and scan (planar and SPECT) performed (required) | |||
| Findings | Quality of the image | |||
| Visual grading interpretation in relation to rib uptake (required) | ||||
| Semi-quantitative H/CL ratio (required) | ||||
| Ancillary findings | Consider attenuation correction in CT interpretation if SPECT/CT scanners are used (recommended) | |||
| Conclusions | 1. Findings can be interpreted and categorized as follows: | |||
| Visual Score | H/CL ratio at 1-hour | H/CL ratio at 3-hour | ||
| Not suggestive | 0 | < 1 | < 1.3 (negative) | |
| Strongly suggestive | 2 or 3 | > 1.5 | ≥ 1.3 (positive) | |
| Equivocal | 1 | 1-1.5 | ||
| 2. Interpret the results in the context of prior cardiac evaluation, assessment for systemic AL amyloidosis using serum and urine immunofixation, and serum free light chain assay. | ||||
| 3. If echo/CMR are strongly positive and 99mTc-PYP scan is negative, consider further evaluation including endomyocardial biopsy. | ||||
| Notes | A negative or mildly positive 99mTc-PYP does NOT exclude AL | |||
| Equivocal results could represent either AL or early ATTR |
AL, immunoglobulin light chain amyloidosis; ATTR, transthyretin amyloidosis; cCr, creatinine clearance; CMR, cardiac magnetic resonance; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease; H/CL ratio, heart to contralateral lung ratio; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PYP, pyrophosphate; SPECT, single-photon emission computed tomography;SPECT/CT, SPECT/computed tomography. Adapted and modified from reference 25, 26.
Interpretation of 99mTc-PYP scintigraphy should include a visual evaluation of planar and SPECT images for any radiotracer uptake, visual grading, and the semi-quantitative H/CL ratio. In the absence of any tracer uptake in the myocardium on SPECT imaging, a visual grade of 0 is given. Visual scores ≥ 2 on planar imaging are regarded as ATTR-positive. An H/CL ratio > 1.5 on one-hour imaging or ≥ 1.3 on three-hour imaging is indicative of ATTR-CM if plasma cell dyscrasia has been excluded. If myocardial uptake patterns on SPECT are focal or focal on diffuse, use the maximal uptake for visual grading is recommended.
After excluding AL, 99mTc-labeled bone tracer scintigraphy is used to diagnose ATTR-CM with high sensitivity and specificity.37 False positives on 99mTc-PYP scans may arise from AL, blood pool uptake, rib fractures, myocardial infarction, quinine drug toxicity, or other rare forms of CA. False negatives may be due to minimal myocardial infiltration (early-stage disease) or scar tissue formation after myocardial infarction. Therefore, the severity of heart failure [i.e., New York Heart Association (NYHA) functional classification or left ventricular ejection fraction (LVEF) percentage], a recent history of myocardial infarction (within 3 months), and current renal function [estimated glomerular filtration rate (eGFR) or creatinine clearance (cCr) within 3-6 months, or presence of end-stage renal disease] are recommended supportive information to consider to avoid false positives. Visual grading and semi-quantitative H/CL ratios are required. SPECT/CT with attenuation correction is also recommended to distinguish blood pool or rib activity. If background or blood pool activity is still high, further delayed imaging may be considered. An overall interpretation of the findings separates patients into three categories — not suggestive, strongly suggestive, or equivocal — for ATTR-CM. Examples of 99mTc-PYP scintigraphy are shown in Figures 3, 4, 5, 6, 7.
Figure 3.
Visual scores. Examples of visual scores from 0 to 3 based on 99mTc-PYP planar (anterior, left anterior oblique, and lateral views), axial SPECT, and axial fused SPECT/CT images at 3 hours post-injection of radiotracer. H/CL ratio, heart to contralateral lung ratio; PYP, pyrophosphate; SPECT/CT, single-photon emission computed tomography/computed tomography.
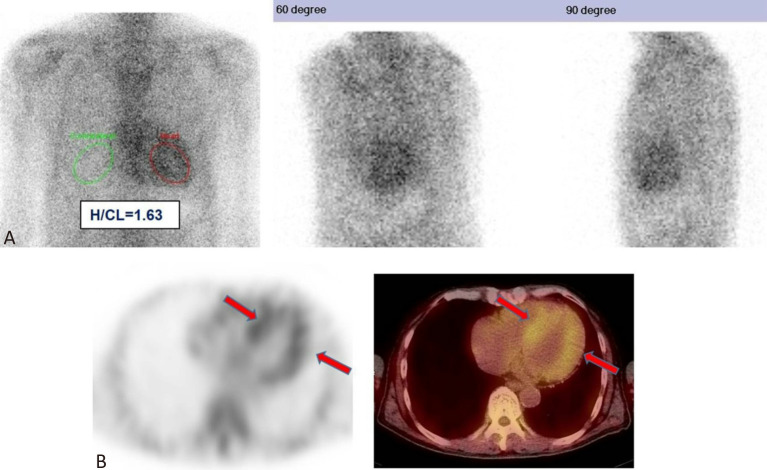
Figure 4.
ATTR amyloidosis, hereditary subtype. A 62-year-old man presented with a two-year history of progressive numbness and tingling in all four limbs. In the last six months, he developed exertional dyspnea. Coronary catheterization revealed patent coronary arteries and endomyocardial biopsy showed amyloidosis cardiomyopathy. 99mTc-PYP planar (A) and SPECT/CT (B) imaging demonstrated diffuse intense uptake in the LV myocardium at 3 hours post-injection, significantly greater than rib activity with mildly decreased rib uptake (red arrows, visual score of 3). The H/CL ratio at 3 hours post-injection was 1.63. Nerve conduction velocity exam revealed sensorimotor polyneuropathy with axonal degeneration, manifesting as bilateral median entrapment neuropathy. Urine, blood, and cerebral spinal fluid immunofixation electrophoresis showed no evidence of monoclonal gammopathy. Sural nerve biopsy also revealed amyloid neuropathy. Familial amyloid polyneuropathy stemming from a transthyretin (TTR) Ala97Ser mutation was further confirmed. ATTR, transthyretin amyloidosis; H/CL, heart to contralateral lung; LV, left ventricular; PYP, pyrophosphate; SPECT/CT, single-photon emission computed tomography/computed tomography.
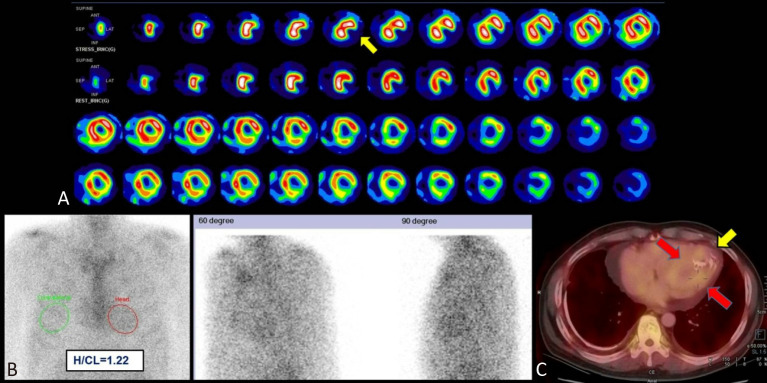
Figure 5.
AL amyloidosis. A 60-year-old man who presented with paroxysmal nocturnal dyspnea and orthopnea was diagnosed with heart failure with preserved ejection fraction of 58.2% (NYHA Functional Classification II). Standardized dipyridamole stress myocardial perfusion imaging showed scarring at the apical inferolateral wall (A, yellow arrow), but cardiac angiography revealed patent coronary arteries (figure not shown). Further 99mTc-PYP planar and SPECT/CT images demonstrated diffuse, mildly elevated tracer activity in the LV myocardium at 3 hours post-injection (B, red circle; and C, red arrows), equal to rib activity (visual score of 2). The H/CL ratio at 3 hours post-injection was 1.22. SPECT/CT images revealed the increased tracer uptake unseen at the LV apex, corresponding to an area of scarred myocardium (C, calcification seen at yellow arrow). Endomyocardial biopsy confirmed the patient has AL amyloidosis. Interstitial plasmacytosis (10-20%) was seen in the bone marrow biopsy, and a diagnosis of plasma cell myeloma was given. AL, immunoglobulin light chain amyloidosis; H/CL, heart to contralateral lung; LV, left ventricular; NYHA, New York Heart Association; PYP, pyrophosphate; SPECT/CT, single-photon emission computed tomography/computed tomography.
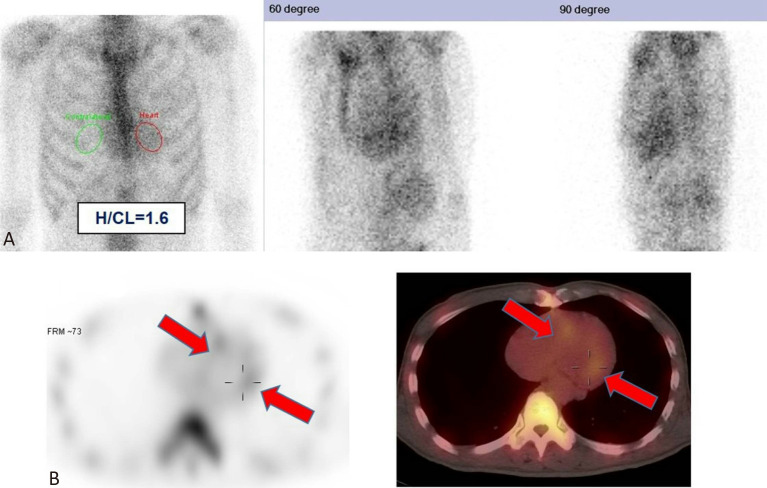
Figure 6.
ATTR amyloidosis, hereditary subtype. A 59-year-old man presented with a one-year history of progressive bilateral ascending numbness, as well as orthostatic hypotension, frequent diarrhea, and frequent episodes of palpitation. Many of his family members were diagnosed with familial amyloidotic polyneuropathy. A transthyretin gene study was performed with positive confirmation of the Ala97Ser mutation. 99mTc-PYP planar (A) and SPECT/CT (B) images demonstrated a visual score of 2, with radiotracer uptake localized to the LV myocardium (red arrows). The H/CL ratio at 3 hours post-injection was 1.6. ATTR, transthyretin amyloidosis; H/CL, heart to contralateral lung; LV, left ventricular; PYP, pyrophosphate; SPECT/CT, single-photon emission computed tomography/computed tomography.
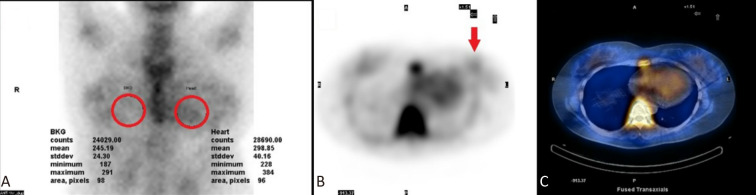
Figure 7.
Breast uptake overlapping the heart in planar and SPECT images. (A) Although the ROI (red circles) was well-delineated in planar view, marked breast physiological uptake was also noted. (B) An axial SPECT slice reveals marked breast uptake (red arrow) and blood pool activity. (C) SPECT/CT fusion images reveal potential causes for false positives of cardiac 99mTc-PYP uptake including blood pool activity, soft tissue uptake such as in the breast, osseous uptake, lung parenchymal lesions, overlying devices, or myocardial infarction. SPECT or SPECT/CT clearly demonstrates uptake in the myocardium or other areas. BKG, background; PYP, pyrophosphate; ROI, region of interest; SPECT/CT, single-photon emission computed tomography/computed tomography; stddev, standard deviation.
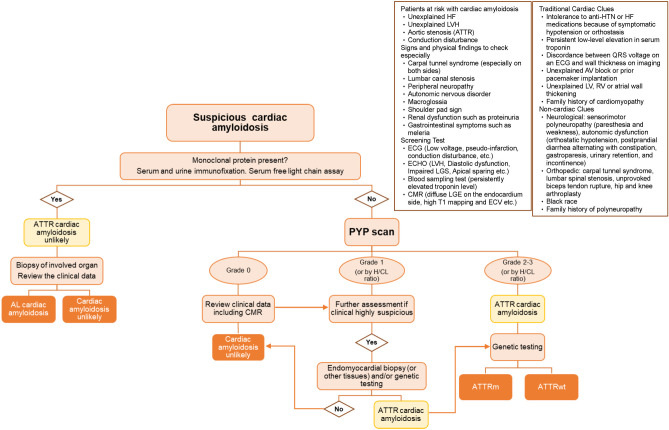
There is an increasing trend towards the use of noninvasive testing with 99mTc-PYP for the evaluation of ATTR-CM. In addition, monoclonal protein testing and SPECT imaging are critical components for ruling out AL and distinguishing myocardial retention from blood pooling.36,41 The diagnostic combination of the FLC test and 99mTc-PYP scintigraphy can reveal four possible outcomes. Firstly, if both tests are negative, it is unlikely to be AL or ATTR-CM. Secondly, if the FLC test is negative and 99mTc-PYP scintigraphy is positive with grade 2-3 radiotracer uptake, ATTR-CM is implicated. Thirdly, if the FLC test is positive and Tc-99mTc-PYP scintigraphy is negative, it points to AL, and histological confirmation is required. Lastly, if FLC test is positive and 99mTc-PYP scintigraphy shows visual grade of 1 to 3, further histological confirmation is necessary to differentiate between AL and ATTR-CM. To diagnosis ATTR-CM noninvasively (i.e. not requiring EMB), the patient should demonstrate both a positive 99mTC-PYP scan (visual grade 2 or greater) and a negative FLC test. Otherwise, a single negative or mildly positive 99mTc-PYP scan does not exclude AL. Equivocal results (visual grade 1) on 99mTc-PYP scintigraphy only could represent either AL or early ATTR-CM. The clinical information should be clearly reviewed and an EMB may be needed in the case of equivocal imaging findings or discordant data. Herein, we proposed a simplified clinical diagnostic algorithm of CA where the presence of monoclonal proteins in serum and urine should be analyzed before performing the 99mTc-PYP scintigraphy (Figure 8).
Figure 8.
The proposed diagnostic algorithm of suspected cardiac amyloidosis in Taiwan. AL, immunoglobulin light chain amyloidosis; ATTR, transthyretin amyloidosis; AV, atrioventricular; CMR, cardiac magnetic resonance; ECG, electrocardiography; ECV, extracellular volume fraction; Gr., grade; H/CL ratio, heart to contralateral lung ratio; HF, heart failure; HTN, hypertension; LGE, late gadolinium enhancement; LGS, longitudinal global strain; LV, left ventricle; LVH, left ventricular hypertrophy; PYP, pyrophosphate; RV, right ventricle. (Adapted and modified from references 25, 41).
CONCLUSIONS
Transthyretin cardiac amyloidosis is an increasingly recognized cause of HFpEF. Favorable prognosis depends on early diagnosis and correct treatment strategy. This article highlights the recommendations of 99mTc-PYP imaging among patients with clinical suspicion of cardiac amyloidosis. The diagnostic accuracy of ATTR-CM can be further improved and therapies applied earlier through the implementation of these recommendations in Taiwan.
Acknowledgments
This advocacy is a joint collaboration between the Taiwan Society of Cardiology and the Society of Nuclear Medicine of the Republic of China, and is partly supported by the Ministry of Science and Technology of Taiwan (MOST 107-2314-B-418-006-MY3). We thank the Rare Disease medical affairs team of Pfizer Taiwan Inc. for providing editorial support. Pfizer Taiwan has no conflict of interests throughout the preparation or publication of this manuscript.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Lin GM, Li YH, Yin WH, et al. The obesity-mortality paradox in patients with heart failure in Taiwan and a collaborative meta-analysis for east Asian patients. Am J Cardiol. 2016;118:1011–1018. doi: 10.1016/j.amjcard.2016.06.056. [DOI] [PubMed] [Google Scholar]
- 2.Wang CC, Chang HY, Yin WH, et al. TSOC-HFrEF registry: a registry of hospitalized patients with decompensated systolic heart failure: description of population and management. Acta Cardiol Sin. 2016;32:400–411. doi: 10.6515/ACS20160704A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang HY, Wang CC, Wei J, et al. Gap between guidelines and clinical practice in heart failure with reduced ejection fraction: results from TSOC-HFrEF registry. J Chin Med Assoc. 2017;80:750–757. doi: 10.1016/j.jcma.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Chang HY, Wang CC, Wu YW, et al. One-year outcomes of acute decompensated systolic heart failure in Taiwan: lessons from TSOC-HFrEF registry. Acta Cardiol Sin. 2017;33:127–138. doi: 10.6515/ACS20170202A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang CC, Wu CK, Tsai ML, et al. 2019 focused update of the guidelines of the Taiwan Society of Cardiology for the diagnosis and treatment of heart failure. Acta Cardiol Sin. 2019;35:244–283. doi: 10.6515/ACS.201905_35(3).20190422A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868–1877. doi: 10.1056/NEJMcp1511175. [DOI] [PubMed] [Google Scholar]
- 7.Griffin JM, Maurer MS. Transthyretin cardiac amyloidosis: a treatable form of heart failure with a preserved ejection fraction. Trends Cardiovasc Med. 2021;31:59–66. doi: 10.1016/j.tcm.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurer MS, Elliott P, Comenzo R, et al. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135:1357–1377. doi: 10.1161/CIRCULATIONAHA.116.024438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castano A, Narotsky DL, Hamid N, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38:2879–2887. doi: 10.1093/eurheartj/ehx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nitsche C, Scully PR, Patel KP, et al. Prevalence and outcomes of concomitant aortic stenosis and cardiac amyloidosis. J Am Coll Cardiol. 2021;77:128–139. doi: 10.1016/j.jacc.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–2594. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 12.Coelho T, Maurer MS, Suhr OB. THAOS - The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29:63–76. doi: 10.1185/03007995.2012.754348. [DOI] [PubMed] [Google Scholar]
- 13.Chao HC, Liao YC, Liu YT, et al. Clinical and genetic profiles of hereditary transthyretin amyloidosis in Taiwan. Ann Clin Transl Neurol. 2019;6:913–922. doi: 10.1002/acn3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai HJ, Huang KC, Liang YC, et al. Cardiac manifestations and prognostic implications of hereditary transthyretin amyloidosis associated with transthyretin Ala97Ser. J Formos Med Assoc. 2020;119:693–700. doi: 10.1016/j.jfma.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly JP, Hanna M. Cardiac amyloidosis: an update on diagnosis and treatment. Cleve Clin J Med. 2017;84:12–26. doi: 10.3949/ccjm.84.s3.02. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Lopez E, Lopez-Sainz A, Garcia-Pavia P. Diagnosis and treatment of transthyretin cardiac amyloidosis. Progress and hope. Rev Esp Cardiol (Engl Ed) 2017;70:991–1004. doi: 10.1016/j.rec.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Naharro A, Baksi AJ, Hawkins PN, Fontana M. Diagnostic imaging of cardiac amyloidosis. Nat Rev Cardiol. 2020;17:413–426. doi: 10.1038/s41569-020-0334-7. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqi OK, Ruberg FL. Cardiac amyloidosis: an update on pathophysiology, diagnosis, and treatment. Trends Cardiovasc Med. 2018;28:10–21. doi: 10.1016/j.tcm.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126:1286–1300. doi: 10.1161/CIRCULATIONAHA.111.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dungu JN, Anderson LJ, Whelan CJ, Hawkins PN. Cardiac transthyretin amyloidosis. Heart. 2012;98:1546–1554. doi: 10.1136/heartjnl-2012-301924. [DOI] [PubMed] [Google Scholar]
- 21.Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–1212. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 22.Dubrey SW, Hawkins PN, Falk RH. Amyloid diseases of the heart: assessment, diagnosis, and referral. Heart. 2011;97:75–84. doi: 10.1136/hrt.2009.190405. [DOI] [PubMed] [Google Scholar]
- 23.Fontana M, Corovic A, Scully P, Moon JC. Myocardial amyloidosis: the exemplar interstitial disease. JACC Cardiovasc Imaging. 2019;12:2345–2356. doi: 10.1016/j.jcmg.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 24.Arbustini E, Merlini G. Early identification of transthyretin-related hereditary cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7:511–514. doi: 10.1016/j.jcmg.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Dorbala S, Ando Y, Bokhari S, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2-evidence base and standardized methods of imaging. J Nucl Cardiol. 2019;26:2065–2123. doi: 10.1007/s12350-019-01760-6. [DOI] [PubMed] [Google Scholar]
- 26.Dorbala S BS, Miller E, Bullock-Palmer R, et al. 99mTechnetium-pyrophosphate imaging for transthyretin cardiac amyloidosis. 2019 ASNC Practice Points. Accessed on March 11, 2021.;Available at [https://www.asnc.org/Files/Amyloid/ASNC%20Practice%20Point-99mTechnetium-Pyrophosphate.2019.pdf] [Google Scholar]
- 27.Kitaoka H, Izumi C, Izumiya Y, et al. JCS 2020 guideline on diagnosis and treatment of cardiac amyloidosis. Circ J. 2020;84:1610–1671. doi: 10.1253/circj.CJ-20-0110. [DOI] [PubMed] [Google Scholar]
- 28.Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076–1084. doi: 10.1016/j.jacc.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 29.Bokhari S, Castano A, Pozniakoff T, et al. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6:195–201. doi: 10.1161/CIRCIMAGING.112.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanna M, Ruberg FL, Maurer MS, et al. Cardiac scintigraphy with technetium-99m-labeled bone-seeking tracers for suspected amyloidosis: JACC review topic of the week. J Am Coll Cardiol. 2020;75:2851–2862. doi: 10.1016/j.jacc.2020.04.022. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman JE, Dempsey NG, Sanchorawala V. Systemic amyloidosis caused by monoclonal immunoglobulins: soft tissue and vascular involvement. Hematol Oncol Clin North Am. 2020;34:1099–1113. doi: 10.1016/j.hoc.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Castano A, Haq M, Narotsky DL, et al. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol. 2016;1:880–889. doi: 10.1001/jamacardio.2016.2839. [DOI] [PubMed] [Google Scholar]
- 33.Dorbala S, Kijewski MF, Park MA. Quantitative bone-avid tracer SPECT/CT for cardiac amyloidosis: a crucial step forward. JACC Cardiovasc Imaging. 2020;13:1364–1367. doi: 10.1016/j.jcmg.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Ren C, Ren J, Tian Z, et al. Assessment of cardiac amyloidosis with (99m)Tc-pyrophosphate (PYP) quantitative SPECT. EJNMMI Phys. 2021;8:3. doi: 10.1186/s40658-020-00342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamarappoo B, Otaki Y, Manabe O, et al. Simultaneous Tc-99m PYP/Tl-201 dual-isotope SPECT myocardial imaging in patients with suspected cardiac amyloidosis. J Nucl Cardiol. 2020;27:28–37. doi: 10.1007/s12350-019-01753-5. [DOI] [PubMed] [Google Scholar]
- 36.Poterucha TJ, Elias P, Bokhari S, et al. Diagnosing transthyretin cardiac amyloidosis by technetium 99m pyrophosphate: a test in evolution. JACC Cardiovasc Imaging. 2020;S1936-878X(20)30816-0 doi: 10.1016/j.jcmg.2020.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Treglia G, Glaudemans A, Bertagna F, et al. Diagnostic accuracy of bone scintigraphy in the assessment of cardiac transthyretin-related amyloidosis: a bivariate meta-analysis. Eur J Nucl Med Mol Imaging. 2018;45:1945–1955. doi: 10.1007/s00259-018-4013-4. [DOI] [PubMed] [Google Scholar]
- 38.Flaherty KR, Morgenstern R, Pozniakoff T, et al. (99m)Technetium pyrophosphate scintigraphy with cadmium zinc telluride cameras is a highly sensitive and specific imaging modality to diagnose transthyretin cardiac amyloidosis. J Nucl Cardiol. 2020;27:371–380. doi: 10.1007/s12350-019-01831-8. [DOI] [PubMed] [Google Scholar]
- 39.Manrique A, Dudoignon D, Brun S, et al. Quantification of myocardial (99m)Tc-labeled bisphosphonate uptake with cadmium zinc telluride camera in patients with transthyretin-related cardiac amyloidosis. EJNMMI Res. 2019;9:117. doi: 10.1186/s13550-019-0584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorbala S, Park MA, Cuddy S, et al. Absolute quantitation of cardiac (99m)Tc-pyrophosphate using cadmium zinc telluride-based SPECT/CT. J Nucl Med. 2020 Sep 4;jnumed.120.247312 doi: 10.2967/jnumed.120.247312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kittleson MM, Maurer MS, Ambardekar AV, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020;142:e7–22. doi: 10.1161/CIR.0000000000000792. [DOI] [PubMed] [Google Scholar]