Abstract
Background
CHA2DS2-VASc score is a useful score to evaluate the risk of stroke in patients with atrial fibrillation (AF), and it has been shown to outperform CHADS2 score. Our recent cross-sectional study showed that CHA2DS2-VASc score was associated with an ankle-brachial index < 0.9. The aim of the current study was to evaluate whether CHA2DS2-VASc score is a useful predictor of new-onset peripheral artery occlusive disease (PAOD) and whether it can outperform CHADS2 and R2CHADS2 scores.
Methods
We used the National Health Insurance Research Database to survey 723750 patients from January 1, 2000 to December 31, 2001. CHADS2, R2CHADS2, and CHA2DS2-VASc scores were calculated for every patient. Finally, 280176 (score 0), 307209 (score 1), 61093 (score 2), 35594 (score 3), 18956 (score 4), 11032 (score 5), 6006 (score 6), 2696 (score 7), 843 (score 8), and 145 (score 9) patients were studied and followed to evaluate new-onset PAOD. We further divided the study patients into six groups: group 1 (score 0), group 2 (score 1-2), group 3 (score 3-4), group 4 (score 5-6), group 5 (score 7-8), and group 6 (score 9).
Results
Overall, 24775 (3.4%) patients experienced new-onset PAOD during 9.8 years of follow-up. The occurrence rate of PAOD increased from 1.3% (group 1) to 23.4% (group 6). Subgroup analysis by gender also showed an association between CHA2DS2-VASc score and the occurrence rate of PAOD. After multivariate analysis, groups 2-6 were significantly associated with new-onset PAOD. CHA2DS2-VASc score also outperformed CHADS2 and R2CHADS2 scores for predicting new-onset PAOD.
Conclusions
CHA2DS2-VASc score was a more powerful predictor of new-onset PAOD than CHADS2 and R2CHADS2 scores in patients without AF.
Keywords: CHADS2 score, CHA2DS2-VASc score, Peripheral arterial occlusive disease, R2CHADS2 score
INTRODUCTION
Peripheral arterial occlusive disease (PAOD) is one of the most common atherosclerotic vascular diseases, and shares similar risk factors to coronary artery disease and cerebrovascular disease.1,2 Major risk factors for PAOD include advanced age, dyslipidemia, hypertension, diabetes mellitus, and cigarette smoking.2,3 Furthermore, congestive heart failure, chronic kidney disease, stroke, race, elevated inflammatory markers, and obesity are also associated with the PAOD process.1-8
CHADS2 score is a useful scoring system to assess the risk of stroke in patients with atrial fibrillation (AF).9 A significant relationship between CHADS2 score and the annual risk of stroke has been reported in AF patients.10 In addition, CHADS2 score has also been reported to predict future cardiovascular outcomes in non-AF populations.11,12 R2CHADS2 score is another scoring system which combines CHADS2 score and impaired renal function.13 Because renal dysfunction is also a predictor of stroke and systemic embolization in patients with nonvalvular AF, Piccini et al. verified R2CHADS2 score in the ROCKET AF study and ATRIA study cohorts, and showed that R2CHADS2 score exhibited excellent predictive value for future stroke and systemic embolization in AF patients.13
Because older age, hypertension, diabetes, congestive heart failure, chronic kidney disease, and stroke are all risk factors for PAOD, our previous study showed that CHADS2 score and R2CHADS2 score were significantly associated with an ankle-brachial index (ABI) < 0.9 in non-AF patients.14,15 In addition, we also performed a nationwide cohort study using data from the National Health Insurance Research Database (NHIRD) and further confirmed the relationship between CHADS2 score and PAOD.16
CHA2DS2-VASc score has been shown to be more useful than CHADS2 score for predicting future stroke and systemic embolization in AF patients.17-19 In our recent study, we also found that CHA2DS2-VASc score was significantly associated with an ABI < 0.9 in non-AF patients.20 Hence, the aim of this study was to investigate whether CHA2DS2-VASc score is also a powerful predictor of new-onset PAOD in non-AF patients. In addition, we also wanted to further confirm whether CHA2DS2-VASc score can outperform CHADS2 score and R2CHADS2 score.
METHODS
Data source
We analyzed data from the NHIRD, which is published by the National Health Research Institute in Taiwan. The database contains 1000000 random subjects. The National Health Insurance (NHI) program offers a comprehensive and universal health insurance program to all citizens who have established a registered domicile for at least 4 months in Taiwan. The coverage includes inpatient and outpatient services, physical therapy, preventive health care, and home care. In 2004, 99% of the population of Taiwan were covered by the NHI program. The NHIRD is one of the most complete and also the largest population-based dataset in Taiwan. The patients’ privacy is protected because the original identification numbers of the patients are encrypted in the NHIRD. The extremely large sample size in the NHIRD provided a good opportunity to evaluate CHA2DS2-VASc score in predicting new-onset PAOD.
Study population
In total, 723750 patients who were aged ≥ 18 years with no past history of PAOD, rheumatic heart disease, or AF were identified in the NHIRD from January 1, 2000 to December 31, 2001.
Calculation of CHADS2 score, R2CHADS2 score, and CHA2DS2-VASc score
The CHADS2 score was calculated for each patient based on a scoring system in which 2 points were assigned for a history of stroke or transient ischemic attack and 1 point was assigned for age ≥ 75 years, the presence of hypertension, diabetes mellitus, and congestive heart failure.9,10 The R2CHADS2 score was calculated for each patient based on a scoring system in which 2 points were assigned for chronic kidney disease and a history of stroke, and 1 point was assigned for age ≥ 75 years, the presence of hypertension, diabetes mellitus, and congestive heart failure.13 The modified CHA2DS2-VASc score was calculated for each patient based on a point system in which 2 points were assigned for age ≥ 75 years and a history of stroke, and 1 point was assigned for congestive heart failure, hypertension, age between 65 and 74 years, diabetes mellitus, female sex, and vascular disease (coronary artery disease including acute coronary syndrome).
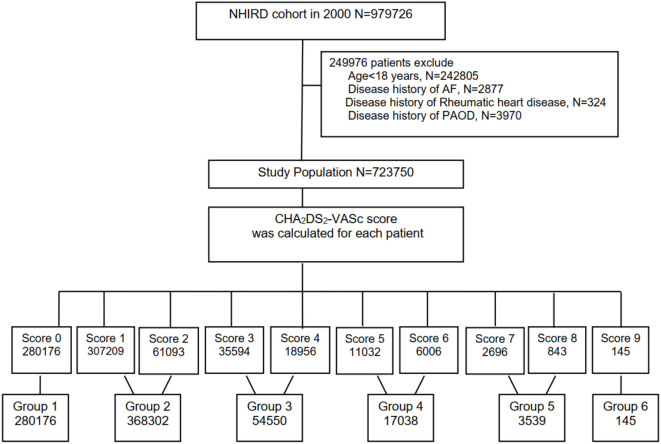
We used the following international classification of disease (ICD) codes: congestive heart failure (ICD-9-CM: 428), hypertension (ICD-9-CM: 401-405), diabetes mellitus (ICD-9-CM: 250), stroke (ICD-9-CM: 430-438), chronic kidney disease (ICD-9-CM: 250.4, 274.1, 283.11, 403, 404, 440.1, 442.1, 447.3, 572.4, 580-588, 642.1, 646.2), coronary artery disease including acute coronary syndrome (ICD-9-CM: 410-414), and PAOD (ICD-9-CM: 250.7, 443, 443.81, 443.9, 785.4, and 444.2). Furthermore, comorbidities including dyslipidemia and chronic obstructive pulmonary disease were also evaluated. The flowchart of the study patients is shown in Figure 1. In this study, 280176 (score 0), 307209 (score 1), 61093 (score 2), 35594 (score 3), 18956 (score 4), 11032 (score 5), 6006 (score 6), 2696 (score 7), 843 (score 8), and 145 (score 9) patients were enrolled. The study patients were further divided into 6 groups: group 1 (score 0), group 2 (score 1-2), group 3 (score 3-4), group 4 (score 5-6), group 5 (score 7-8), and group 6 (score 9). Medications of the patients including aspirin, β-blockers, calcium channel blockers (CCBs), angiotensin converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), diuretics, and statins were also obtained from the database.
Figure 1.
The flowchart of the study population. AF, atrial fibrillation; NHIRD, National Health Insurance Research Database; PAOD, peripheral arterial occlusive disease.
Statistical analysis
Data are presented as the mean and standard deviation for normally distributed continuous variables and proportions for categorical variables. Differences in categorical variables between groups were compared using the chi-square test. Differences in continuous values between groups were compared using the unpaired t test for normally distributed continuous variables and Mann-Whitney rank-sum test for skewed variables. Covariates of risk factors and time to developing PAOD were modeled using Cox regression analysis. ROC curves were used to compare different scores to assess the predictive ability for the risk of PAOD. A higher area under the curve (AUC) was considered to be better. A p value less than 0.05 was considered to be statistically significant. All data processing and statistical analyses were performed with SAS 9.2 software.
RESULTS
Table 1 shows the baseline characteristics of the study population. The mean age of the study population was 42 ± 17 years old. Most of the patients had a CHA2DS2-VASc score of 1 (42.4%). During the period from January 2000 to December 2009 with a mean follow-up duration of 9.8 years, 24775 (3.4%) patients experienced new-onset PAOD. The occurrence rates of new-onset PAOD from score 0 to score 9 were 1.32%, 2.36%, 7.35%, 9.78%, 13.50%, 15.09%, 15.55%, 18.47%, 19.22%, and 23.45%, respectively (Figure 2A). In addition, the occurrence rates of new-onset PAOD from group 1 to group 6 were 1.32%, 3.19%, 11.10%, 15.30%, 18.60%, and 23.45%, respectively (p < 0.001) (Figure 2B). We further performed subgroup analysis by gender to evaluate CHA2DS2-VASc score and the occurrence rate of new-onset PAOD. In the male patients, the occurrence rates of new-onset PAOD from group 1 to group 5 were 1.32%, 6.72%, 11.86%, 15.17%, and 18.45%, respectively (p < 0.001) (Figure 3A). In the female patients, the occurrence rates of new-onset PAOD from group 2 to group 6 were 2.46%, 10.65%, 15.30%, 18.71%, and 23.45%, respectively (p < 0.001) (Figure 3B). Due to gender issues, none of the males did had a score of 9 (group 6), and none of the females had a score of 0 (group 1).
Table 1. The baseline characteristics of the study population.
| Variables | Study population |
| Age (years) | 41.7 ± 16.8 |
| Gender (female) | 354327 (49.0%) |
| Newly-onset PAOD | 24775 (3.4%) |
| Group 1 (score 0) | 3709 (15.0%) |
| Group 2 (score 1-2) | 11732 (47.4%) |
| Group 3 (score 3-4) | 6041 (24.4%) |
| Group 4 (score 5-6) | 2599 (10.5%) |
| Group 5 (score 7-8) | 660 (2.7%) |
| Group 6 (score 9) | 34 (0.1%) |
| Comorbidities | |
| Hypertension | 96382 (13.3%) |
| Diabetes mellitus | 48145 (6.7%) |
| Dyslipidemia | 18366 (2.5%) |
| Cerebrovascular disease | 26086 (3.6%) |
| Heart failure | 10349 (1.4%) |
| Coronary artery disease | 22258 (3.1%) |
| Chronic kidney disease | 14809 (2.1%) |
| COPD | 23964 (3.3%) |
| Medications | |
| Aspirin | 4614 (0.6%) |
| β-blocker | 26975 (3.7%) |
| CCB | 44103 (6.1%) |
| ACEI | 21460 (3.0%) |
| ARB | 7248 (1.0%) |
| Diuretic | 10208 (1.4%) |
| Statin | 6391 (0.9%) |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; PAOD, peripheral arterial occlusive disease.
Figure 2.
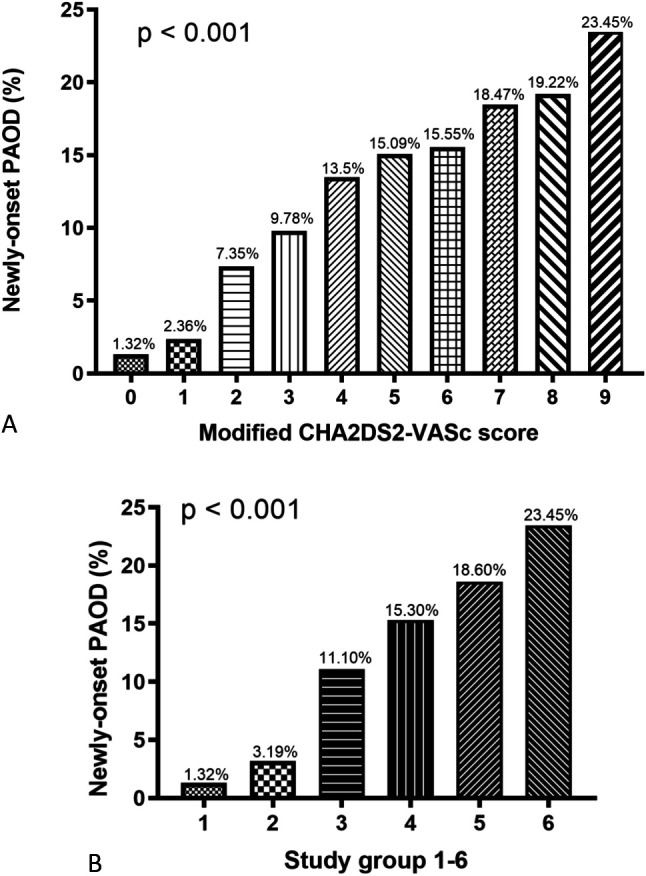
The occurrence rate of newly-onset peripheral arterial occlusive disease (PAOD) progressively increased from CHA2DS2-VASc score 0 to 9 (A) and from study group 1 to group 6 (B).
Figure 3.
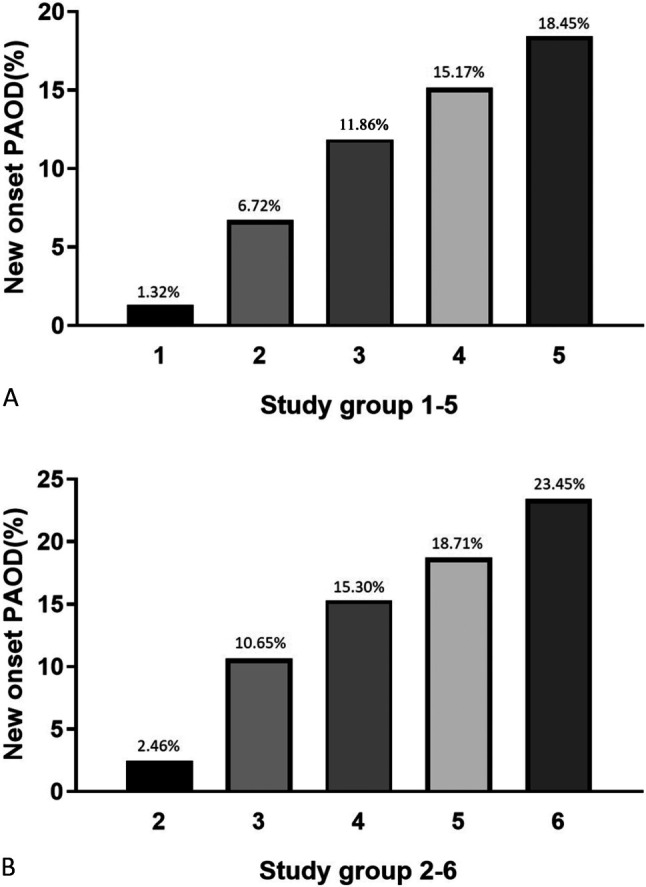
The occurrence rate of newly-onset peripheral arterial occlusive disease (PAOD) progressively increased from study group 1 to group 5 in male subgroup (A) and from study group 2 to group 6 in female subgroup (B).
Table 2 shows the multivariate Cox regression analysis for the prediction of new-onset PAOD. After adjusting for chronic kidney disease, dyslipidemia, chronic obstructive pulmonary disease, and medications (aspirin, ACEIs, ARBs, β-blockers, CCBs, diuretics, and statins), chronic kidney disease, dyslipidemia, chronic obstructive pulmonary disease, the use of ACEIs, beta-blockers, CCBs, diuretics and statins, and group 2 to 6 (p < 0.001) were significantly associated with new-onset PAOD.
Table 2. Multivariate Cox regression analysis for prediction of newly-onset PAOD.
| Variable | HR | Lower 95% CI | Upper 95% CI | p value |
| Chronic kidney disease | 1.53 | 1.30 | 1.79 | < 0.001 |
| Dyslipidema | 1.65 | 1.39 | 1.96 | < 0.001 |
| COPD | 1.32 | 1.15 | 1.51 | < 0.001 |
| Medication | ||||
| Aspirin | 1.21 | 0.88 | 1.66 | 0.247 |
| ACEI | 1.29 | 1.07 | 1.55 | 0.007 |
| ARB | 0.91 | 0.69 | 1.18 | 0.463 |
| β-blocker | 1.58 | 1.35 | 1.86 | < 0.001 |
| CCB | 1.68 | 1.44 | 1.96 | < 0.001 |
| Diuretic | 1.38 | 1.13 | 1.69 | 0.002 |
| Statin | 1.47 | 1.14 | 1.91 | 0.003 |
| Group of CHA2DS2-VASc score | ||||
| Group 1 (score = 0) | 1 | - | - | - |
| Group 2 (score 1-2) | 1.89 | 1.78 | 2.01 | < 0.001 |
| Group 3 (score 3-4) | 4.21 | 3.75 | 4.72 | < 0.001 |
| Group 4 (score 5-6) | 5.69 | 4.77 | 6.78 | < 0.001 |
| Group 5 (score 7-8) | 7.61 | 5.77 | 10.04 | < 0.001 |
| Group 6 (score 9) | 8.21 | 2.61 | 25.89 | < 0.001 |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; PAOD, peripheral arterial occlusive disease.
In addition, we further performed subgroup analysis by gender using Cox regression analysis for the prediction of new-onset PAOD, and the results are shown in Table 3. In the male patients, group 1 (score 0) was regarded as the baseline, and the hazard ratio (HR) showed a gradual increase from group 2 to group 5 (p < 0.001). In the female patients, group 2 (score 1-2) was regarded as the baseline, and the HR showed a gradual increase from group 3 to group 6 (p < 0.001). In both gender subgroups, CHA2DS2-VASc score was significantly associated with new-onset PAOD.
Table 3. Cox regression analysis for prediction of newly-onset PAOD in subgroup of male and female.
| Variable | HR | Lower 95% CI | Upper 95% CI | p value |
| Group of male | ||||
| Group 1 (score = 0) | 1 | - | - | - |
| Group 2 (score 1-2) | 5.23 | 5.00 | 5.46 | < 0.001 |
| Group 3 (score 3-4) | 9.55 | 9.06 | 10.06 | < 0.001 |
| Group 4 (score 5-6) | 12.64 | 11.77 | 13.57 | < 0.001 |
| Group 5 (score 7-8) | 16.01 | 13.60 | 18.85 | < 0.001 |
| Group 6 (score 9) | - | - | - | |
| Group of female | ||||
| Group 2 (score 1-2) | 1 | - | - | - |
| Group 3 (score 3-4) | 4.55 | 4.37 | 4.73 | < 0.001 |
| Group 4 (score 5-6) | 6.79 | 6.44 | 7.17 | < 0.001 |
| Group 5 (score 7-8) | 8.58 | 7.85 | 9.39 | < 0.001 |
| Group 6 (score 9) | 11.42 | 8.15 | 15.99 | < 0.001 |
CI, confidence interval; HR, hazard ratio; PAOD, peripheral arterial occlusive disease.
The AUCs for CHADS2 score, R2CHADS2 score, and CHA2DS2-VASc score for the prediction of new-onset PAOD were 0.709, 0.713, and 0.726, respectively. There were significant differences in the AUC between R2CHADS2 score and CHADS2 score, CHA2DS2-VASc score and R2CHADS2 score, and CHA2DS2-VASc score and CHADS2 score (all p < 0.001, Table 4).
Table 4. Comparison of AUC between CHADS2 score, R2CHADS2 score, and CHA2DS2-VASc score.
| Comparison of AUC | p value | |
| CHA2DS2-VASc score vs. CHADS2 score | 0.726 vs. 0.709 | < 0.001 |
| R2CHADS2 score vs. CHADS2 score | 0.713 vs. 0.709 | < 0.001 |
| CHA2DS2-VASc score vs. R2CHADS2 score | 0.726 vs. 0.713 | < 0.001 |
AUC, area under curve.
DISCUSSION
There were three major findings in this nationwide cohort study. First, the occurrence rate of new-onset PAOD progressively increased from the patients with a CHA2DS2-VASc score 0 to score 9 and from group 1 to group 6 (1.32% to 23.45%). Second, CHA2DS2-VASc score was a useful tool in predicting new-onset PAOD in non-AF patients not only in the whole population but also in subgroup analysis of males and females. Third, CHA2DS2-VASc score outperformed CHADS2 score and R2CHADS2 score for predicting the risk of new-onset PAOD.
CHADS2 score, R2CHADS2 score, CHA2DS2-VASc score and the risk of new-onset PAOD
PAOD is a systemic atherosclerotic process which is associated with high morbidity and mortality, especially in patients with acute limb ischemia or critical limb ischemia.21-24 Although new medications such as rivaroxaban and advances in endovascular therapy have improved the outcomes of PAOD,25-27 PAOD is still associated with extremely high rates of morbidity and mortality. Risk factors for PAOD include older age, hypertension, diabetes mellitus, dyslipidemia, cigarette smoking, chronic kidney disease, congestive heart failure, stroke, elevated inflammatory markers, and other vascular bed diseases.1-8 CHADS2 score is regarded as a useful score to assess the risk of stroke in patients with AF.9,10 However, some recent studies have extended the usage of CHADS2 score to patients without AF, such as those with coronary artery disease and acute coronary syndrome.11,12 Because PAOD is also a vascular disease which shares similar risk factors with coronary artery disease, CHADS2-related scores such as CHADS2, R2CHADS2 and CHA2DS2-VASc score might also be associated with the risk of PAOD. An ABI < 0.9 has been reported to be a reliable diagnostic tool for PAOD, and our previous studies found that these three scores were all associated with an ABI < 0.9.14,15,20
In the present study, we found that CHA2DS2-VASc score could not only predict the risk of new-onset PAOD in non-AF patients, it also outperformed CHADS2 score and R2CHADS2 score for the risk prediction of PAOD. In the modified CHA2DS2-VASc score in our study, vascular disease was defined as coronary artery disease including acute coronary syndrome. Because we aimed to predict the future risk of PAOD, we did not include PAOD in the component of vascular disease, which is similar to our previous cross-sectional study.20
Another important finding of our study was related to gender. Compared to the men, the women had higher rates of PAOD despite having fewer risk factors for cardiovascular disease. Hiramoto et al. reported that although men and women shared similar risk factors for an ABI ≤ 0.9, women were more likely to have an ABI ≤ 1.0 and ABI ≤ 0.9 compared to men (all p < 0.001).28 Kumakura et al. also showed sex-related differences in Japanese patients with PAOD. In their study, women more frequently had critical limb ischemia and diabetes mellitus, and below the knee lesions were more severe in women than in men.29 In Taiwan, Chang et al. also reported that young and middle-aged female patents with diabetic and those on a low income had a tendency to undergo amputation due to PAOD.30 Our previous nationwide cohort study evaluating CHADS2 score and the risk of new-onset PAOD also showed that female gender was significantly associated with the risk of new-onset PAOD after multivariable analysis.16 Furthermore, subgroup analysis by gender also showed that CHA2DS2-VASc score was significantly associated with new-onset PAOD in the current study.
Our results confirmed that CHADS2 score, R2CHADS2 score, and CHA2DS2-VASc score were significantly associated with new-onset PAOD. Furthermore, AUC analysis of the three scoring systems showed that CHA2DS2-VASc score had a better predictive value than CHADS2 score and R2CHADS2 score for the prediction of new-onset PAOD.
Study limitations
There are several limitations to this study. First, personal information such as cigarette smoking, body mass index, and physical activity was not available from the NHIRD, and these factors might be correlated with PAOD. Second, disease misclassification and inadequate diagnostic coding were possible in the NHIRD. The occurrence of PAOD was also based on the diagnostic code registered by the physicians responsible for the treatment of the patients.
CONCLUSION
Our study of patients without AF demonstrated that CHA2DS2-VASc score was independently associated with new-onset PAOD, and that CHA2DS2-VASc score outperformed CHADS2 score and R2CHADS2 score for predicting the risk of new-onset PAOD. Hence, CHA2DS2-VASc score is a more powerful tool in predicting the development of PAOD in non-AF patients.
Acknowledgments
This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes (Registered number 98178). The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes. The authors also thank the Statistical Analysis Laboratory, Department of Internal Medicine, Kaohsiung Medical University Hospital.
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
REFERENCES
- 1.Brevetti G, Giugliano G, Brevetti L, et al. Inflammation in peripheral artery disease. Circulation. 2010;122:1862–1875. doi: 10.1161/CIRCULATIONAHA.109.918417. [DOI] [PubMed] [Google Scholar]
- 2.Bartholomew JR, Olin JW. Pathophysiology of peripheral arterial disease and risk factors for its development. Cleve Clin J Med. 2006;73 Suppl 4:S8–S14. doi: 10.3949/ccjm.73.suppl_4.s8. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the united states: results from the national health and nutrition examination survey, 1999-2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee A, Fowkes FG, Rothwell PM. Associations between peripheral artery disease and ischemic stroke: implications for primary and secondary prevention. Stroke. 2010;41:2102–2107. doi: 10.1161/STROKEAHA.110.582627. [DOI] [PubMed] [Google Scholar]
- 5.Meves SH, Diehm C, Berger K, et al. Peripheral arterial disease as an independent predictor for excess stroke morbidity and mortality in primary-care patients: 5-year results of the getabi study. Cerebrovasc Dis. 2010;29:546–554. doi: 10.1159/000306640. [DOI] [PubMed] [Google Scholar]
- 6.Topakian R, Nanz S, Rohrbacher B, et al. High prevalence of peripheral arterial disease in patients with acute ischaemic stroke. Cerebrovasc Dis. 2010;29:248–254. doi: 10.1159/000267850. [DOI] [PubMed] [Google Scholar]
- 7.Gallego P, Roldan V, Marin F, et al. Ankle brachial index as an independent predictor of mortality in anticoagulated atrial fibrillation. Eur J Clin Invest. 2012;42:1302–1308. doi: 10.1111/eci.12004. [DOI] [PubMed] [Google Scholar]
- 8.Adesunloye BA, Valadri R, Mbaezue NM, et al. Impact of peripheral arterial disease on functional limitation in congestive heart failure: results from the national health and nutrition examination survey (1999-2004). Cardiol Res Pract. 2012;2012:306852. doi: 10.1155/2012/306852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gage BF, van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292. doi: 10.1161/01.CIR.0000145172.55640.93. [DOI] [PubMed] [Google Scholar]
- 10.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 11.Welles CC, Whooley MA, Na B, et al. The CHADS2 score predicts ischemic stroke in the absence of atrial fibrillation among subjects with coronary heart disease: data from the heart and soul study. Am Heart J. 2011;162:555–561. doi: 10.1016/j.ahj.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poci D, Hartford M, Karlsson T, et al. Role of the CHADS2 score in acute coronary syndromes: risk of subsequent death or stroke in patients with and without atrial fibrillation. Chest. 2012;141:1431–1440. doi: 10.1378/chest.11-0435. [DOI] [PubMed] [Google Scholar]
- 13.Piccini JP, Stevens SR, Chang Y, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the rocket af (rivaroxaban once-daily, oral, direct factor xa inhibition compared with vitamin k antagonism for prevention of stroke and embolism trial in atrial fibrillation) and atria (anticoagulation and risk factors in atrial fibrillation) study cohorts. Circulation. 2013;127:224–232. doi: 10.1161/CIRCULATIONAHA.112.107128. [DOI] [PubMed] [Google Scholar]
- 14.Hsu PC, Lin TH, Lee WH, et al. Association between the CHADS2 score and an ankle-brachial index of 0.9 in patients without atrial fibrillation. J Atheroscler Thromb. 2014;21:322–328. doi: 10.5551/jat.21212. [DOI] [PubMed] [Google Scholar]
- 15.Hsu PC, Lee WH, Chiu CA, et al. R2CHADS2 score is significantly associated with ankle-brachial index < 0.9 in patients without atrial fibrillation. Atherosclerosis. 2014;236:307–311. doi: 10.1016/j.atherosclerosis.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Hsu PC, Chiu CA, Chu CY, et al. CHADS2 score and risk of new-onset peripheral arterial occlusive disease in patients without atrial fibrillation: a nationwide cohort study in Taiwan. J Atheroscler Thromb. 2015;22:490–498. doi: 10.5551/jat.27284. [DOI] [PubMed] [Google Scholar]
- 17.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 18.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 19.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with eacts. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 20.Hsu PC, Lee WH, Lee HC, et al. Association between modified CHA2DS2-VASc score with ankle-brachial index < 0.9. Sci Rep. 2018;8:1175. doi: 10.1038/s41598-018-19243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (tasc ii). J Vasc Surg. 2007;45 Suppl S:S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 22.Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence, risk factors, outcome, and prognosis of ischemic peripheral arterial events: implications for prevention. Circulation. 2015;132:1805–1815. doi: 10.1161/CIRCULATIONAHA.115.016424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). Tasc working group. Transatlantic inter-society consensus (tasc). J Vasc Surg. 2000;31:S1–S296. [PubMed] [Google Scholar]
- 24.Mustapha JA, Katzen BT, Neville RF, et al. Determinants of long-term outcomes and costs in the management of critical limb ischemia: a population-based cohort study. J Am Heart Assoc. 2018;7:e009724. doi: 10.1161/JAHA.118.009724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thukkani AK, Kinlay S. Endovascular intervention for peripheral artery disease. Circ Res. 2015;116:1599–1613. doi: 10.1161/CIRCRESAHA.116.303503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 27.Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:219–229. doi: 10.1016/S0140-6736(17)32409-1. [DOI] [PubMed] [Google Scholar]
- 28.Hiramoto JS, Katz R, Weisman S, et al. Gender-specific risk factors for peripheral artery disease in a voluntary screening population. J Am Heart Assoc. 2014;3:e000651. doi: 10.1161/JAHA.113.000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumakura H, Kanai H, Araki Y, et al. Sex-related differences in Japanese patients with peripheral arterial disease. Atherosclerosis. 2011;219:846–850. doi: 10.1016/j.atherosclerosis.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 30.Chang NT, Chan CL, Lu YT, et al. Invasively-treated incidence of lower extremity peripheral arterial disease and associated factors in Taiwan: 2000-2011 nationwide hospitalized data analysis. BMC Public Health. 2013;13:1107. doi: 10.1186/1471-2458-13-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]