Abstract
Background
Ischemia-reperfusion injury following acute ST-segment elevation myocardial infarction (STEMI) is strongly related to inflammation. However, whether intracoronary (IC) tacrolimus, an immunosuppressant, can improve myocardial perfusion is uncertain.
Methods
A multicenter double-blind randomized controlled trial was conducted in Taiwan from 2014 to 2017. Among 316 STEMI patients with Killip class ≤ 3 undergoing primary percutaneous coronary intervention (PCI), 151 were assigned to the study group treated with IC tacrolimus 2.5 mg to the culprit vessel before first balloon inflation, and the remaining 165 were assigned to the placebo group receiving IC saline only. The primary endpoint was percentage of post-PCI TIMI-3 flow. The primary composite endpoints included achievement of TIMI-3 flow, TIMI- myocardial perfusion (TMP) grade, or 90-min ST-segment resolution (STR). The secondary endpoints were left ventricular ejection fraction (LVEF) and 1-month/1-year major adverse cardio-cerebral vascular events (MACCEs) (defined as death, myocardial infarction, stroke, target-vessel revascularization or re-hospitalization for heart failure).
Results
Although post-PCI TIMI-3 epicardial flow and MACCE rate at 1 month and 1 year did not differ between the two groups, TMP grade (2.54 vs. 2.23, p < 0.001) and 90-min STR (67% vs. 61%, p < 0.001) were significantly higher in the tacrolimus-treated group than in the placebo group. The STEMI patients treated with tacrolimus also had significantly higher 3D LVEF and less grade 2 or 3 LV diastolic dysfunction at 9 months compared to those without.
Conclusions
IC tacrolimus for STEMI improved coronary microcirculation and 9-month LV systolic and diastolic functions. However, the benefit of tacrolimus on clinical outcomes remains inconclusive due to insufficient patient enrollment.
Keywords: Left ventricular systolic and diastolic function, Microcirculation, Myocardial perfusion, ST-segment elevation myocardial infarction, Tacrolimus
INTRODUCTION
Early and rapid restoration of blood flow in infarct-related arteries (IRAs) and microvasculature via thrombolysis or primary percutaneous coronary intervention (PCI) is the gold standard treatment strategy for minimizing myocardial damage and preserving cardiac pumping function in the setting of ST-segment elevation myocardial infarction (STEMI).1,2 The grade of myocardial reperfusion, a strong index of preservation of heart function and improvement of prognostic outcome after primary PCI, not only depends upon the patency of the IRA but is also adversely influenced by the severity of microvascular dysfunction.3,4
Slow-flow or no-reflow phenomenon is known to contribute to unsuccessful myocardial reperfusion after primary PCI,5 and to be associated with the duration of myocardial ischemia,4,6 the burdens of thrombus formation, plaque content and ischemia-reperfusion (IR) injury.7,8 Additionally, IR injury is strongly related to inflammation that exacerbates myocardial damage even after successful reperfusion.7,9,10 Several experimental studies have also demonstrated that innate immune response11-13 followed by activated adaptive immune signaling11-13 play crucial roles in the regulation of inflammatory reactions after acute myocardial infarction (AMI). Taking into account the important role of inflammation in the reperfusion process,7,8,10,12 early suppression of inflammatory reactions and immune signaling may be important to attenuate the progression of AMI-induced myocardial damage following successful reperfusion therapy. To validate this hypothesis, we previously performed a series of experimental studies, the results of which demonstrated that the intra-coronary administration of cyclosporine or tacrolimus effectively attenuated inflammatory and immune reactions, limited infarct size, and preserved left ventricular (LV) function.14-16
Experimental studies have shown that tacrolimus is a more potent immunosuppressant14-16 and results in better long-term outcomes in renal transplantation compared with cyclosporine.17 In addition, a previous pilot study18 of the COAT-STEMI trial supported tacrolimus as being a safe and effective therapeutic agent for patients with STEMI undergoing primary PCI. Thus, the positive preliminary outcomes of our pilot study18 justified a phase III randomized-controlled clinical trial to investigate adjunctive tacrolimus therapy in patients with STEMI.
MATERIALS AND METHODS
Study design and patient population
The COAT-STEMI trial was a multicenter double-blind randomized-controlled trial conducted by three local hospitals and three tertiary medical centers in southern Taiwan from February 2014 to May 2017. Patients diagnosed with acute STEMI for < 12 hours and Killip classification ≤ 3 undergoing primary PCI were prospectively enrolled into the study. The study protocol was approved by the institutional review boards of each institute. Written informed consent was obtained from all study participants prior to enrollment.
Enrollment, inclusion and exclusion criteria
Patients with acute STEMI of Killip class ≤ 3 upon presentation at the emergency room (ER) aged ≥ 20 and ≤ 80 years undergoing primary PCI were prospectively consecutively enrolled. Primary PCI was performed for all eligible STEMI patients with onset of chest pain less than 12 hours.
The exclusion criteria were age < 20 or > 80 years, occurrence of recent myocardial infarction (MI) or stroke in the past three months, history of tacrolimus allergy or current use of immunosuppressant therapy, cardiogenic shock, infection and active inflammatory conditions, pregnancy or breastfeeding, liver cirrhosis, recipient of organ transplantation, hemodialysis, life expectancy estimated to be less than one year, cancer, patients still participating in other clinical trial, and those who refused to participate in the study.
Random allocation and blinding implementation
The study sequence was generated by a computerized random number generator. Due to concerns about the time required to explain detailed protocols in the urgent circumstance of acute STEMI, permuted block randomization was adopted to assign sample numbers equally to each group with a block size of ten. Blocking can ensure close balance of the numbers in each group at any time during atrial. Patients with STEMI Killip 1-3 were allocated into a tacrolimus-treated or placebo-controlled group in a 1:1 ratio according to an even or odd random number contained in a selected envelope. The envelopes were opened in consecutive order for enrollment just prior to the primary PCI by technicians who were blinded to case randomization. Additionally, the interventional cardiologists, echocardiographers, and senior technicians who performed the echocardiogram, the cardiologists who took care of the patients in the ward and outpatient department, and the clinical trial nurses were also completely blinded to the case randomization and treatment protocol until the end of the study period.
Method of tacrolimus administration and procedure of primary PCI
The rationale of the tacrolimus dosage was described in our pilot study.18 To attain a higher concentration and direct therapeutic effect of tacrolimus in damaged myocardium, we adopted an intracoronary (IC) rather than intravenous route of administration. The patients in the tacrolimus-treated group were treated by the IC administration of 2.5 mg (0.5 mL) tacrolimus [Tacrolimus (5.0 mg/mL/amp), Astellas Taiwan, Fujisawa Ireland Co., Ltd. Killorglin Co. Kerry, Ireland] mixed with 200 mcg of nitroglycerin (2.0 mL) and 7.5 mL normal saline in a total of 10 mL prior to first balloon inflation or direct stent deployment. Those in the placebo-controlled group received IC 10 mL of saline infusion plus 200 mcg of nitroglycerin adjunctive to the standard PCI procedure. Operators were blinded to the study agent preparations. The procedure and protocol of the primary PCI have been described in our previous reports.2,4,5 After wiring across the obstructive lesion into the distal part of the IRA, a thrombus aspiration catheter was then tracked along the guide wire. Tacrolimus or normal saline was then slowly administered through the aspiration catheter to the IRA. The purposes of using thrombosuction catheters in both groups were to reduce thrombus burden with mechanical thrombectomy, to facilitate study or placebo agent infusion into the culprit vessel, and then to exactly administer the agent into the infarcted myocardium. Balloon dilatation and stenting were then performed 3 min after IC injection of these therapeutic agents. The primary PCI procedure was finished as routine course. All study patients in each group were admitted to a coronary care unit after the primary PCI to closely monitor their clinical condition and hemodynamic status for at least one day. Every study patient received guideline-directed treatment and management.19
Medications for STEMI
A heparin dose of 70-90 IU/kg body weight was administered to all patients before and during PCI. The patients received a loading dose of oral aspirin 300 mg and P2Y12 inhibitor (ticagrelor 180 mg or clopidogrel 300 mg orally) in the emergency room, followed by a maintenance dose of the anti-platelet agents for at least 12 months after the primary PCI. Other common medications for STEMI including renin-angiotensin-aldosterone system blockers, statins, beta-blockers, vasodilators, or diuretics were prescribed in accordance with guideline recommendations with the dosage adjusted appropriately.
Primary and secondary endpoints
The primary endpoint was to evaluate whether the IC administration of tacrolimus during primary PCI resulted in better final epicardial coronary flow as reflected in thrombolysis in myocardial infarction (TIMI)-3 flow. Additionally, because epicardial coronary flow and thrombus burden can influence downstream coronary microcirculatory and post-PCI myocardial perfusion status, we further defined the primary composite endpoints as achievement of epicardial TIMI-3 flow, TIMI-myocardial perfusion (TMP) grade or 90-min ST-segment resolution (STR). The purpose was to assess the overall impact of IC tacrolimus on the results of macro-/micro-coronary flow and myocardial perfusion by using a combination of angiographic and electrocardiographic findings. Regarding clinical outcomes, the secondary endpoints were defined as preservation of LV systolic and diastolic functions, as well as one-month and one-year major adverse cardio-cerebral vascular events (MACCEs), including death, MI, stroke, target-vessel revascularization and repeated hospitalization for heart failure.
Calculation of sample size for the specific objective
In this phase III clinical trial, for the primary endpoint of the study (i.e., the achievement of final TIMI-3 flow in the IRA), an estimated sample size of 600 subjects in each group were enrolled based on the estimation of effective size with an alpha = 0.05 and power = 90%, and an anticipation of final TIMI-3 flow of 91.0% in the placebo controls vs. 96% in the study group receiving tacrolimus therapy. Considering that the results of TMP grade (microcirculation) and 90-min STR (myocardial perfusion) were mainly dependent on post-PCI TIMI-3 epicardial flow status, we did not use the percentage of primary composite endpoints for the sample size evaluation.
Definitions of TIMI flow, TMP grade and 90-min ST-segment resolution
Angiographic assessments for the grade of TIMI flow and TMP were based on previous reports20,21 for the purposes of evaluating epicardial coronary, microcirculatory, and myocardial perfusion status. In addition, the measurement of 90-min STR using complete electrocardiograms (ECGs) at 90 min after primary PCI was based on previously reported criteria.22,23 Briefly, no, partial and complete STR were defined as < 30%, 30% to 70%, and > 70% reductions in amplitude of ST segments in infarct-associated leads on 90-min follow-up ECG, respectively, compared with the baseline. The results of TIMI flow, TMP grade and 90-min STR were assessed by two expert and experienced interventional cardiologists blinded to the study allocation.
Echocardiographic measurements
Conventional and advanced echocardiography was performed with standard 2-dimenional (2D) and 3D views, M-mode, tissue and color Doppler assessment, and speckle tracking technique. Digital images were collected and data were analyzed according to the standardized protocol released by the American Society of Echocardiography.24 Two independent echocardiographic specialists blinded to patient allocation individually measured and evaluated LV systolic and diastolic functions, LV global longitudinal strain (GLS), LV lateral and right ventricular (RV) systolic functions within 48 hours after PCI, and then 3 and 9 months after discharge using 2D or 3D echocardiography with off-line analysis.
Clinical follow-up
The STEMI patients in both groups completed at least 1 year of follow-up and received regular follow-up at cardiovascular (CV) outpatient clinics every 3 months or as needed. A research nurse or assistant at every participating institute recorded all clinical information including the presence or absence of MACCEs, adverse events or serious adverse events by using a case report form and regular telephone interviews. The clinical outcomes and adverse events were adjudicated by the patient’s attending physician in charge and study principal investigator. Data were filed in a case report form and entered into a computer after each clinical visit and on readmission for any reported event.
Statistical analysis
Values were expressed as mean ± standard deviation (SD), percentage, or number where appropriate. The clinical and laboratory data in the study and control groups were analyzed and compared using the independent t-test for continuous variables and chi-square test (or Fisher’s exact test) for categorical variables. We used intention-to-treat analysis and Kaplan-Meier analysis to estimate cumulative incidence rates of 1-year MACCEs and CV mortality at the end of the follow-up period, and the log-rank test to examine differences between two groups. To identify potential predictors of microcirculatory improvement, multivariate logistic regression analysis was performed to calculate the adjusted hazard ratio of individual variable for target outcomes. The model was adjusted for all statistically significant variables in univariate analysis, including tacrolimus treatment and other potential confounders that may have influenced outcomes. Subgroup analysis was performed to identify the subjects who potentially benefitted from IC tacrolimus therapy. Statistical analysis was performed using SPSS statistical software for Windows version 22 (SPSS for Windows, version 22; SPSS Inc., Chicago, IL, U.S.A.). A p-value < 0.05 was considered to be statistically significant.
RESULTS
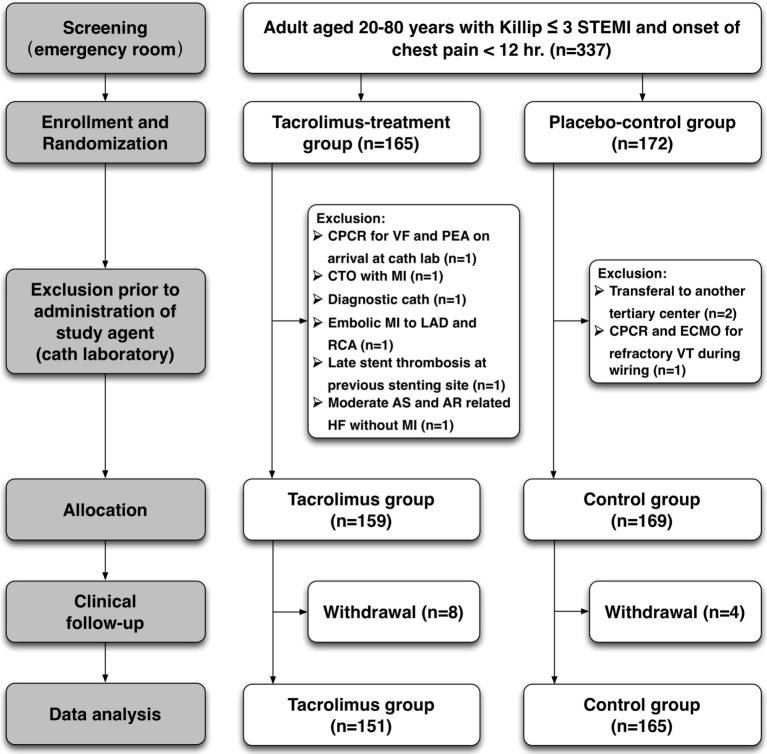
This clinical study intended to enroll 600 patients in the study group and 600 patients in the control group, but was prematurely terminated owing to difficulty in case enrollment. Between February 2014 and May 2017, a total of 337 consecutive subjects who met the criteria were prospectively enrolled into the study, including 165 patients assigned into the tacrolimus-treated group and 172 patients in the placebo-controlled group (Figure 1). In the tacrolimus group, 6 patients were excluded at the catheterization laboratory because of unexpected resuscitation events prior to PCI, unsuitable lesions, or being misdiagnosed as having STEMI, and 8 patients withdrew during the follow-up period. In addition, three cases were excluded and another four withdrew from the control group. Finally, a total of 151 and 165 patients in the study and control groups, respectively, were completely followed up for at least 1 year and objectively assessed for laboratory and clinical outcomes.
Figure 1.
Algorithm for patients’ enrollment and allocation. The supplemental figure shows how we screened and recruited study subjects at emergency room and catheterization laboratory, assigned two groups randomly, excluded those who were ineligible to enrollment, and did follow-up during study period. AR, aortic regurgitation; AS, aortic stenosis; CPCR, cardiopulmonary and cerebral resuscitation; ECMO, extracorporeal membrane oxygenation; LAD, left anterior descending artery; MI, myocardial infarction; PCI, percutaneous coronary intervention; PEA, pulseless electrical activity; RCA, right coronary artery; STEMI, ST-segment elevation myocardial infarction; VF, ventricular fibrillation; VT, ventricular tachycardia.
Baseline characteristics of the study and control groups (Table 1)
Table 1. Baseline, clinical and laboratory characteristics and medication between study and control groups.
| Variables | Tacrolimus (n = 151) | Control (n = 165) | p value |
| Baseline characteristics | |||
| Age, mean ± SD | 59.40 ± 11.85 | 58.55 ± 10.53 | 0.324 |
| Male gender, % (n) | 88.7% (134) | 85.5% (141) | 0.385 |
| Body height (cm) | 166.64 ± 7.44 | 165.60 ± 7.41 | 0.302 |
| Body weight (kg) | 72.72 ± 13.64 | 69.94 ± 11.87 | 0.094 |
| Body mass index | 26.14 ± 4.33 | 25.42 ± 3.59 | 0.194 |
| Smoker, % (n) | 51.7% (78) | 50.9% (84) | 0.853 |
| Hypertension, % (n) | 57.6% (87) | 57.6% (95) | 0.994 |
| Diabetes mellitus, % (n) | 31.8% (48) | 34.5% (57) | 0.603 |
| Hyperlipidemia, % (n) | 33.1% (50) | 33.9% (56) | 0.876 |
| Old myocardial infarction, % (n) | 8.6% (13) | 4.8% (8) | 0.180 |
| Old stroke, % (n) | 4.6% (7) | 4.9% (8) | 0.920 |
| History of CABG surgery, % (n) | 0.7% (1) | 0.0% (0) | 0.478 |
| History of PCI, % (n) | 6.0% (9) | 1.8% (3) | 0.054 |
| Laboratory data | |||
| WBC count (1000/μL), mean ± SD | 11.42 ± 3.40 | 10.67 ± 3.29 | 0.098 |
| Hemoglobin (g/dL) | 14.52 ± 2.01 | 14.39 ± 2.11 | 0.830 |
| Creatinine (mg/dL) | 1.13 ± 0.68 | 1.23 ± 1.10 | 0.783 |
| Serum potassium (mEq/L) | 3.87 ± 0.52 | 3.84 ± 0.50 | 0.534 |
| cTn-I (ng/mL) | 23.32 ± 96.40 | 27.52 ± 116.14 | 0.068 |
| CK-MB (ng/mL) | 50.68 ± 85.35 | 57.51 ± 108.16 | 0.580 |
| Peak cTn-I (ng/mL) | 66.32 ± 105.24 | 99.3 ± 522.76 | 0.519 |
| Peak CK-MB (ng/mL) | 194.60 ± 157.29 | 185.36 ± 183.96 | 0.252 |
| Blood sugar (mg/dL) | 170.96 ± 71.55 | 181.91 ± 80.91 | 0.418 |
| HbA1c (%) | 6.95 ± 1.98 | 6.94 ± 1.89 | 0.954 |
| Total cholesterol (mg/dL) | 183.56 ± 44.97 | 185.99 ± 48.79 | 0.787 |
| LDL-C (mg/dL) | 112.60 ± 34.19 | 113.81 ± 41.33 | 0.898 |
| HDL-C (mg/dL) | 40.49 ± 9.01 | 40.81 ± 12.90 | 0.543 |
| Triglyceride (mg/dL) | 143.53 ± 142.05 | 150.72 ± 117.14 | 0.619 |
| Clinical presentation at ER | |||
| Location of MI, % (n) | 0.963 | ||
| Anterior | 47.3% (71) | 46.3% (76) | |
| Inferior | 48.0% (72) | 49.4% (81) | |
| Lateral | 4.7% (7) | 4.3% (7) | |
| Killip classification, mean ± SD | 1.38 ± 0.65 | 1.55 ± 0.81 | 0.090 |
| Ventricular arrhythmia, % (n) | 8.6% (13) | 6.7% (11) | 0.525 |
| Complete heart block, % (n) | 5.3% (8) | 4.8% (8) | 0.845 |
| Atrial fibrillation, % (n) | 5.3% (8) | 4.2% (7) | 0.659 |
| Medications within 48 hours, % (n) | |||
| Aspirin | 93.3% (140) | 95.8% (158) | 0.342 |
| P2Y12 inhibitors | 98.0% (147) | 97.6% (161) | 1.000 |
| Oral anticoagulant | 3.3% (5) | 2.4% (4) | 0.741 |
| Statin | 79.3% (119) | 83.6% (138) | 0.325 |
| Beta blocker | 80.7% (121) | 80% (132) | 0.882 |
| Calcium channel blocker | 11.3% (17) | 8.5% (14) | 0.397 |
| ARB/ACEI | 82.7% (124) | 81.8% (135) | 0.844 |
| Diuretic | 11.3% (17) | 13.3% (22) | 0.590 |
| Isosorbide di-/mono-nitrate | 32.6% (30) | 18.6% (19) | 0.025 |
Data are expressed as mean ± standard deviation (SD) or % (n).
ARB/ACEI, angiotensin II type I receptor blocker/angiotensin converting enzyme inhibitor; CABG, coronary artery bypass graft; CK-MB, creatinine kinase MB isoenzyme; cTn-I, cardiac troponin-I; ER, emergency room; HbA1c, glycohemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; PCI, percutaneous coronary intervention; P2Y12 inhibitors, only clopidogrel and ticagrelor are available in Taiwan; WBC, white blood cells.
There were no differences between the tacrolimus-treated and placebo-controlled groups in terms of age, gender, coronary artery disease (CAD) risk factors and relevant medical and procedural history. Laboratory studies showed that there were also no differences in complete blood count and serum creatinine between the two groups. Additionally, the initial and peak levels of myocardial injury biomarkers, blood sugar upon presentation, glycated hemoglobin (HbA1c) level and lipid profile were similar between the two groups.
There were also no significant differences in the incidence of infarct location, arrhythmia, and Killip class upon presentation between the two groups. The standard medications used by the patients were also similar, except for more isosorbide di-/mono-nitrate in the tacrolimus-treated than in the control group.
Time intervals from chest pain onset, presentation to ER and to PCI procedure, angiographic results and echocardiographic findings (Table 2)
Table 2. Procedural and echocardiographic information at baseline.
| Variables | Tacrolimus (n = 151) | Control (n = 165) | p value |
| Time of door to PCI | |||
| Onset of chest pain to door time (min), mean ± SD | 213.55 ± 236.38 | 154.72 ± 164.59 | 0.074 |
| Door to balloon time (min) | 63.09 ± 55.78 | 70.75 ± 81.91 | 0.333 |
| Procedure time (min) | 42.26 ± 19.43 | 39.42 ± 17.64 | 0.087 |
| Pre-PCI diameter stenosis (%) | 95.70 ± 11.19 | 95.43 ± 12.84 | 0.680 |
| Pre-PCI TIMI flow grade | 0.64 ± 0.93 | 0.58 ± 0.90 | 0.606 |
| Pre-PCI TIMI thrombus grades ≥ 4# | 82.1% (124) | 86.1% (142) | 0.338 |
| Post-PCI diameter stenosis (%) | 11.87 ± 9.04 | 10.74 ± 5.52 | 0.282 |
| Post-PCI TIMI flow grade | 2.89 ± 0.44 | 2.87 ± 0.39 | 0.223 |
| Type of implanted stents, % (n) | 95.7% (88) | 96.1% (98) | 0.881 |
| BMS | 23.9% (22) | 24.5% (25) | |
| DES | 71.7% (66) | 71.6% (73) | |
| Mechanical thrombectomy | 100% (151) | 100% (165) | 1.000 |
| Distal protection | 26.9% (25) | 28.4% (29) | 0.809 |
| GP IIb/IIIa inhibitor | 6.5% (6) | 8.8% (9) | 0.535 |
| IABP support | 9.3% (14) | 6.7% (11) | 0.382 |
| Left main disease, % (n) | 4.0% (6) | 3.6% (6) | 0.876 |
| No. of diseased vessel | 0.125 | ||
| 1-CAD, % (n) | 31.1% (47) | 38.8% (64) | |
| 2-CAD, % (n) | 35.8% (54) | 23.6% (39) | |
| 3-CAD, % (n) | 31.8% (48) | 35.8% (59) | |
| Echocardiographic parameters* | |||
| 2D LVEF (%), mean ± SD | 56.01 ± 10.56 | 55.79 ± 11.09 | 0.861 |
| LVEDD (mm) | 49.10 ± 10.09 | 48.55 ± 10.56 | 0.335 |
| LVESD (mm) | 32.95 ± 8.96 | 33.11 ± 11.44 | 0.746 |
| 3D LVEF (%), mean ± SD | 38.86 ± 25.69 | 37.43 ± 26.81 | 0.691 |
| LVEDV (mm3) | 89.13 ± 30.23 | 88.02 ± 31.15 | 0.978 |
| LVESV (mm3) | 41.26 ± 19.89 | 40.30 ± 21.51 | 0.726 |
| GLS by 2D-STE (%) | -13.17 ± 4.20 | -14.23 ± 4.14 | 0.088 |
| RLS by 2D-STE (%) | -10.30 ± 5.57 | -10.55 ± 5.96 | 0.774 |
| E/A ratio | 0.93 ± 0.54 | 1.08 ± 0.64 | 0.009 |
| Grade of LVDD, mean ± SD | 1.47 ± 0.57 | 1.70 ± 0.60 | 0.011 |
| % of grade 2 or 3 LVDD, % (n) | 48.3% (42) | 64.9% (63) | 0.023 |
| TAPSE (mm) | 20.77 ± 5.02 | 21.34 ± 5.37 | 0.494 |
Data are expressed as mean ± standard deviation (SD) or % (n).
BMS, bare-metal stent; CAD, coronary artery disease; DES, drug-eluting stent; E/A ratio, the ratio of the early (E) to late (A) ventricular filling velocities; GLS, global longitudinal strain; GP, glycoprotein; IABP, intra-aortic balloon pumping; LVDD, LV diastolic dysfunction; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume; PCI, percutaneous coronary intervention; RLS, regional longitudinal strain; TAPSE, tricuspid annular plane systolic excursion; TIMI, Thrombolysis In Myocardial Infarction; 2D or 3D, 2- or 3-dimentional echocardiography; 2D-STE, 2D speckle tracking echocardiography.
* Indicated transthoracic echocardiography was done within 48 h after primary PCI.
# TIMI thrombus grade ≥ 4 was recognized as high thrombus burden and defined as thrombus length longer than or equal to two vessel diameter (grade 4) or recently total occlusion (grade 5).
The time interval from chest pain onset to ER and duration of PCI procedure did not differ between the two groups. Additionally, the degree of pre-PCI/post-PCI vessel stenosis, the mean TIMI-flow grade and proportion of TIMI thrombus grade ≥ 4, as well as the number of diseased vessels were similar. The frequency of the types of stents showed no difference between the two groups. Moreover, there was no notable difference in the rates of utilization of intra-aortic balloons, GP IIb/IIIa inhibitors and distal protective devices. Although there was no difference in the baseline echocardiographic parameters between the two groups, the frequency of grade 2 or 3 LV diastolic dysfunction (LVDD) was higher in the placebo-controlled group than that in the tacrolimus-treated group.
Angiographic and electrocardiographic results, and time courses of echocardiographic evaluations (Table 3)
Table 3. Angiographic and electrocardiographic outcomes and serial echocardiographic findings.
| Variables | Tacrolimus (n = 151) | Control (n = 165) | Odds ratio (95% CI) | p value |
| Post-PCI epicardial coronary flow | ||||
| TIMI flow grade, mean ± SD | 2.89 ± 0.44 | 2.87 ± 0.39 | 1.15 (0.67-1.99) | 0.606 |
| TIMI grade 2 or 3, % (n) | 96.7% (146) | 98.2% (162) | 0.54 (0.13-2.30) | 0.406 |
| TIMI grade 3, % (n) | 92.7% (139) | 88.5% (146) | 1.66 (0.76-3.61) | 0.200 |
| Post-PCI microcirculatory status | ||||
| TMP grade, mean ± SD | 2.54 ± 0.71 | 2.23 ± 0.77 | 1.78 (1.29-2.46) | < 0.001 |
| TMP grade 2 or 3, % (n) | 92.7% (140) | 84.1% (138) | 2.40 (1.14-5.02) | 0.021 |
| 90-min STR, mean ± SD | 0.67 ± 0.29 | 0.61 ± 0.33 | 4.94 (2.30-10.59) | < 0.001 |
| Persistent ST elevation, % (n) | 5.7% (9) | 11% (18) | 0.49 (0.21-1.18) | 0.112 |
| Composite endpoint of TIMI 3 flow, TMP grade 3, or complete 90-min STR | 90.7% (137) | 85.5% (141) | 1.94 (0.84-4.47) | 0.113 |
| 3-month echocardiography | ||||
| 2D LVEF (%), mean ± SD | 59.55 ± 11.67 | 61.03 ± 11.38 | 0.99 (0.98-1.02) | 0.929 |
| LVEDD (mm) | 49.57 ± 11.57 | 49.20 ± 10.63 | 1.00 (0.98-1.03) | 0.802 |
| LVESD (mm) | 33.63 ± 9.59 | 33.75 ± 8.54 | 1.01 (0.98-1.04) | 0.551 |
| 3D LVEF (%), mean ± SD | 57.66 ± 9.95 | 58.56 ± 10.36 | 0.99 (0.97-1.02) | 0.564 |
| LVEDV (mm3) | 89.28 ± 24.53 | 89.05 ± 25.37 | 1.00 (0.99-1.01) | 0.55 |
| LVESV (mm3) | 39.14 ± 18.53 | 37.90 ± 19.31 | 1.01 (0.99-1.02) | 0.472 |
| GLS by 2D-STE (%) | -15.92 ± 3.84 | -16.27 ± 4.20 | 0.97 (0.90-1.05) | 0.439 |
| RLS by 2D-STE (%) | -14.15 ± 4.95 | -14.18 ± 5.52 | 0.99 (0.94-1.05) | 0.778 |
| E/A ratio | 1.03 ± 0.56 | 1.42 ± 2.95 | 0.82 (0.54-1.24) | 0.348 |
| Grade of LVDD, mean ± SD | 1.68 ± 0.63 | 1.72 ± 0.61 | 0.83 (0.51-1.35) | 0.444 |
| % of grade 2 or 3 LVDD, % (n) | 65.4% (98) | 65.9% (108) | 0.98 (0.52-1.84) | 0.945 |
| TAPSE (mm) | 22.86 ± 5.32 | 22.81 ± 5.13 | 1.01 (0.96-1.07) | 0.694 |
| 9-month echocardiography | ||||
| 2D LVEF (%), mean ± SD | 61.27 ± 10.63 | 60.94 ± 11.04 | 1.00 (0.98-1.03) | 0.731 |
| LVEDD (mm) | 51.52 ± 6.54 | 50.59 ± 6.15 | 1.01 (0.98-1.03) | 0.550 |
| LVESD (mm) | 34.69 ± 7.34 | 33.95 ± 7.14 | 1.01 (0.98-1.04) | 0.659 |
| 3D LVEF (%), mean ± SD | 61.03 ± 8.64 | 57.96 ± 9.23 | 1.04 (1.003-1.08) | 0.031 |
| LVEDV (mm3) | 91.59 ± 26.03 | 92.64 ± 23.92 | 1.00 (0.99-1.01) | 0.769 |
| LVESV (mm3) | 36.73 ± 17.48 | 40.22 ± 18.94 | 0.99 (0.98-1.01) | 0.277 |
| GLS by 2D-STE (%) | -16.29 ± 3.36 | -16.12 ± 3.47 | 1.03 (0.94-1.12) | 0.564 |
| RLS by 2D-STE (%) | -14.78 ± 4.59 | -14.89 ± 4.13 | 1.00 (0.93-1.08) | 0.929 |
| E/A ratio | 0.95 ± 0.37 | 1.01 ± 0.49 | 0.71 (0.33-1.51) | 0.375 |
| Grade of LVDD, mean ± SD | 1.41 ± 0.65 | 1.69 ± 0.62 | 0.50 (0.30-0.84) | 0.009 |
| % of grade 2 or 3 LVDD, % (n) | 47.2% (71) | 63.1% (104) | 0.52 (0.28-0.99) | 0.048 |
| TAPSE (mm) | 23.25 ± 4.43 | 22.70 ± 5.28 | 1.02 (0.96-1.09) | 0.507 |
Data are expressed as mean ± standard deviation (SD) or % (n).
CI, confidence interval; E/A ratio, the ratio of the early (E) to late (A) ventricular filling velocities; GLS, global longitudinal strain; LVDD, LV diastolic dysfunction; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume; PCI, percutaneous coronary intervention; RLS, regional longitudinal strain; STR, ST-segment resolution; echo, echocardiography; TAPSE, tricuspid annular plane systolic excursion; TIMI, Thrombolysis In Myocardial Infarction; TMP, TIMI myocardial performance; 2D or 3D, 2- or 3-dimentional echocardiography; 2D-STE, 2D speckle tracking echocardiography.
The angiographic findings showed that the mean post-PCI TIMI flow grade and percentage of ≥ TIMI flow -2 or -3 did not differ between the two groups. However, mean post-PCI TMP grade and percentage of TMP grade 2 or 3 (i.e., an indicator for microvasculature) were significantly higher in the tacrolimus group than in the control group. Additionally, the 90-min ST-segment resolution (STR) exhibited a similar pattern to TMP grade inthe two groups. Furthermore, the primary composite endpoint, including post-PCI macro- and micro-circulatory status, also did not differ significantly between both groups. Supplemental Figure 1 shows that the effectiveness of tacrolimus therapy on myocardial perfusion and microcirculation was consistent in each subgroup regardless of age, gender, body mass index, underlying diseases, angiographic results, and thromboembolic prophylactic modalities.
Supplemental Figure 1.
Subgroup analysis for better improvement of coronary microcirculation. Forest plots demonstrates tacrolimus treatment was better than placebo to achieve post-PCI TMP grade 2/3 or 90-min STR > 50% among all subgroups. Data is displayed by % of event (number of patients). CAD, coronary artery disease; CI, confidence interval; GP, glycoprotein; MI, myocardial infarction; STR, ST-segment resolution; TIMI, Thrombolysis In Myocardial Infarction; TMP, TIMI myocardial performance.
Three-month LV systolic function was improved compared to baseline, but did not significantly differ between the study and control groups (Table 2). GLS and RV function were similar in both groups at baseline, and after 3 and 9 months of follow-up. Notably, the 9-month echocardiography showed significantly better LV systolic (3D LVEF) and diastolic functions in the tacrolimus-treated group than those in the placebo-controlled group. Interestingly, the further improvements in LV systolic and diastolic functions from 3 to 9 months were only observed in the tacrolimus-treated group (for 3D LVEF: 57.66% vs. 61.03%, p = 0.001; for grade of LVDD: 1.68 vs. 1.41, p = 0.013), but not in the placebo-controlled group (for 3D LVEF: 58.56% vs. 57.96%, p = 0.103; for grade of LVDD: 1.72 vs. 1.69, p = 0.672).
Comparison of clinical outcomes at different time points (Table 4)
Table 4. Comparison of in-hospital, 1-month, and 1-year clinical outcomes between two groups.
| Outcome | Tacrolimus (n = 151) | Control (n = 165) | Odds ratio (95% CI) | p value |
| In-hospital outcome | ||||
| NYHA functional class, mean ± SD | 1.29 ± 1.13 | 1.31 ± 1.10 | 0.98 (0.80-1.20) | 0.845 |
| Pulmonary edema, % (n) | 9.9% (15) | 12.7% (21) | 0.76 (0.38-1.53) | 0.436 |
| Sepsis, % (n) | 4.6% (7) | 4.2% (7) | 1.01 (0.38-3.20) | 0.865 |
| Stroke, % (n) | 0.0% (0) | 0.0% (0) | -- | -- |
| Recurrent MI, % (n) | 0.0% (0) | 0.0% (0) | -- | -- |
| Staged PCI, % (n) | 30.5% (46) | 23.6% (39) | 1.42 (0.86-2.33) | 0.172 |
| Length of hospital stay (days), mean ± SD | 6.33 ± 3.80 | 6.98 ± 6.30 | 0.98 (0.93-1.02) | 0.282 |
| 1-month outcomes | ||||
| MACCE, % (n) | 3.3% (5) | 4.8% (8) | 0.67 (0.22-2.10) | 0.494 |
| Death | 2.0% (3) | 2.4% (4) | 0.81 (0.18-3.68) | 0.786 |
| Recurrent acute MI | 0% (0) | 0% (0) | -- | -- |
| Stroke | 0.0% (0) | 0.0% (0) | -- | -- |
| Re-hospitalization for ADHF | 2.0% (3) | 3.0% (5) | 0.65 (0.15-2.75) | 0.552 |
| 1-year outcomes | ||||
| MACCE, % (n) | 11.3% (17) | 11.5% (19) | 0.98 (0.49-1.95) | 0.943 |
| Death | 2.6% (4) | 4.3% (7) | 0.61 (0.18-2.13) | 0.438 |
| Recurrent acute MI | 4.7% (7) | 2.5% (4) | 1.94 (0.56-6.75) | 0.300 |
| Stroke | 0.7% (1) | 0.6% (1) | 1.08 (0.07-17.45) | 0.956 |
| Re-hospitalization for ADHF | 4.1% (6) | 5.0% (8) | 0.80 (0.27-2.37) | 0.691 |
| Staged PCI, % (n) | 22.8% (34) | 17.4% (28) | 1.40 (0.80-2.46) | 0.234 |
| CABG surgery, % (n) | 0.7% (1) | 0.0% (0) | -- | 1.000 |
| Hospitalization for infection, % (n) | 2.2% (2) | 2.0% (2) | 1.11 (0.15-8.05) | 0.917 |
Data are expressed as mean ± standard deviation (SD) or % (n).
ADHF, acute decompensated heart failure; CABG, coronary artery bypass grafting; CI, confidence interval; MACCE, major adverse cardiovascular and cerebral vascular events; MI, myocardial infarction; NYHA Fc, New York Heart Association functional class; PCI, percutaneous coronary intervention.
During hospitalization, the incidence rates of CV/non-CV clinical events, stage PCI to non-IRAs, and duration of hospitalization did not differ between the tacrolimus-treated and placebo-controlled groups. By 1 month and 1 year after hospital discharge, the incidence of MACCEs was similar between the two groups. In addition, at 1 year, the incidence of stage PCI or coronary artery bypass surgery also did not differ between the two groups. Interestingly, the 1-year CV mortality rate was lower in the tacrolimus-treated than in the placebo-controlled group (2.6% vs. 4.3%), although it did not reach statistical significance (p = 0.440) (Figure 2). Of note, the incidence of infection during hospitalization was quite low in the study group, suggesting that tacrolimus did not suppress normal immune responses in the patients receiving tacrolimus therapy.
Figure 2.
Kaplan-Meier curves for cumulative incidences of MACCE and CV death during one-year follow-up period. Kaplan-Meier survival analysis with Log-rank test displays there were no significant differences in 1-year MACCE and CV mortality between two groups. CV, cardiovascular; MACCE, major adverse cardio-/cerebro-vascular events.
DISCUSSION
This prospective, multicenter, randomized placebo-control trial to investigate the therapeutic effects of IC tacrolimus during primary PCI yielded several relevant clinical implications. First, after myocardial reperfusion therapy, the tacrolimus-treated group had significantly higher TMP grade and 90-min STR than the placebo-controlled group. Second, the short-term and long-term LV systolic and diastolic functions were more preserved in the tacrolimus group than in the control group. However, although 1-year CV mortality tended to be lower in the tacrolimus group, this underpowered study did not show a significant additional benefit on survival with tacrolimus therapy.
Early and rapid restoration of the blood flow in the IRAs using primary PCI is a mainstay of therapy for acute STEMI,19,25 however this therapeutic strategy frequently causes IR injury, resulting in slow-flow and no-reflow phenomenon and further myocardial damage.5,7,10 Thus, restoration of blood flow in IRAs by primary PCI is recognized as being a "double-edge sword".7 Several studies have also demonstrated that IR injury can elicit inflammation, oxidative stress, platelet activation7,9,10 and innate and adaptive immune responses11-13 after primary PCI, and therefore the adverse responses from IR injury contribute to further death of cardiomyocytes.11,26,27 These issues7,9-11,26,27 are the rationale behind the strong sense of mission to perform this phase III clinical trial, aiming to find a way to resolve IR injury following primary PCI.
The essential finding of this study is that the IC administration of tacrolimus prior to first balloon inflammation achieved better TMP grade 2 or 3 and 90-min STR than placebo, highlighting that this adjunctive therapy to primary PCI could be a therapeutic choice to improve coronary microcirculation and attenuate myocardial injury. Clinically, 9-month LVEF and LV diastolic function were further improved in the tacrolimus group compared with those in the control group. Previous studies have shown that early achievement of TMP grade 2 to 3 and rapid electrocardiographic STR after primary PCI are independently predictive of recovery of LV contractility and favorable clinical outcomes.28-30 Thus, our findings are comparable to the findings of previous studies.28-30 The proposed mechanisms regarding how IC tacrolimus improves coronary microcirculatory and LV function through suppressing IR injury after myocardial reperfusion therapy are summarized in Supplemental Figure 2.
Supplemental Figure 2.
How IC tacrolimus improves coronary microcirculation and LV function through suppressing IR injury during primary PCI. Combined reperfusion strategies of primary PCI and IC tacrolimus restored epicardial coronary and facilitated microcirculatory blood flow, and then attained better improvement of LV systolic and diastolic functions. CAD, coronary artery disease; CK-MB, creatinine kinase MB isoenzyme; cTn-I, cardiac troponin-I; EKG, electrocardiography; IC, intracoronary; IR, ischemia-reperfusion; LV, left ventricular; PCI, percutaneous coronary intervention; ROS, reactive oxygen species; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis In Myocardial Infarction; TMP, TIMI myocardial performance.
However, although the results of TMP grade and 90-min STR improved after tacrolimus therapy, the clinical outcome of 1-year MACCEs did not differ between the study and control groups. This discrepancy could be attributed to 1) the smaller sample size owing to early termination of the trial, 2) mild severity of STEMI in this trial, and 3) no mitochondrial protective effect provided by the immunosuppressant tacrolimus agent.31 In the pilot study of the COAT-STEMI trial18 for achievement of TIMI-3 flow 96% in the tacrolimus group vs. 91% in the control group (i.e. 5.0% difference), a total of 600 patients in each group were required to reach statistical significance. However, only 316 patients participated in the current trial. The achievement rate of TIMI-3 flow was 92.7% vs. 88.5% (i.e., 4.2% difference) in the tacrolimus vs. control group, respectively. Thus, an insufficient patient number could partially explain the statistical insignificance and may have led to the results being underpowered. Based on the achievement rate of TIMI-3 flow between the two groups, a case number of 320 or more in each group would have been necessary for a statistical survival significance. Additionally, most of the STEMI patients presented with Killip class 1or 2 in this trial, and therefore the therapeutic effect of tacrolimus in protecting the heart against IR injury and post-MI inflammation might not be so obvious. Also, several reasons, including exclusion of cardiogenic shock, early/comprehensive primary PCI, high quality of intensive care unit care and very low incidence of MACCEs among these relatively mild to moderate STEMI patients may explain why there was no difference in untoward clinical outcomes during 1 year of follow-up between the two groups.
Tacrolimus and cyclosporin are two well-known calcineurin-inhibitory immunosuppressants used to prevent organ rejection after kidney or liver transplantation.32 Compared with cyclosporin, tacrolimus has stronger cytotoxic immunosuppression14-16 and provides better long-term outcomes in renal transplantation.17 Similar to the current study, a previous study33 demonstrated that IV cyclosporine in patients with AMI resulted in microcirculatory improvement, but did not results in better clinical outcomes or prevent adverse LV remodeling at 1 year. When we took a closer look at these two studies, there were a few important issues to be clarified. First, the route of administration of these two immunosuppressant drugs was different between our and the previous studies. To further enhance the anti-inflammatory and immunosuppressant effects in IRA, we adopted IC rather than IV administration of tacrolimus to treat STEMI in the present study, guaranteeing a higher concentration of tacrolimus in the IRA for a maximum therapeutic effect on damaged myocardium compared with IV route of cyclosporine in the prior study.33 Regrettably, our strategy of IC tacrolimus, a more potent immunosuppressant plus more concentrated local effect, did not offer better clinical outcomes than IV cyclosporin for the STEMI patients undergoing primary PCI. This might be because tacrolimus does not have effects on preventing apoptosis-related mitochondrial dysfunction and opening of mitochondria permeability transition pores that are provided by cyclosporin.31,34,35 Therefore, unlike statins36 and colchicine37,38 that play an anti-inflammatory role in AMI, the findings of our and the previous studies suggest that immunosuppressants targeting calcineurin function in addition to myocardial reperfusion therapy failed to result in further improvements in clinical outcomes in the therapeutic aspect of STEMI.
This study has limitations. First, the sample size was too small to conclude the clinical benefit of IC tacrolimus therapy, and the clinical results might be underpowered. This could mainly be attributed to the fact that most patients were afraid of this novel study design with administering the study agent in an urgent situation. Therefore, due to concerns about the safety and side effects, quite a lot of the STEMI patients were unwilling to participate in our clinical trial. Moreover, the door to balloon time is always emphasized to be as fast as possible, so that regrettably would not allow the patients to have sufficient time to consider their willingness or discuss the study in detail. To clarity whether IC tacrolimus is beneficial for MI-associated clinical outcomes, a larger-scale randomized trial is encouraged. Second, this clinical trial was principal investigator-initiated, therefore, limited financial support was a big problem. Furthermore, owing to enrollment difficulty, an extension of the study period for recruitment of more STEMI patients was not approved by the institutional review board committees in some collaborated institutes. Accordingly, the study was terminated in May 2017. Finally, all study participants in both groups received mechanical thrombectomy first to facilitate IC administration of the study or placebo agent, and therefore some outcome benefits from thrombosuction should be considered.
CONCLUSIONS
IC tacrolimus-assisted primary PCI aimed to suppress myocardial IR injury provided STEMI patients with better microvascular perfusion status and greater improvement in 9-month LV systolic and diastolic functions. However, because this study is underpowered, the benefits of this adjunctive immunosuppressant therapy on STEMI remain inconclusive.
Acknowledgments
This study was supported by a program grant from Chang Gung Memorial Hospital, Chang Gung University (Grant numbers: CMRPG8C1111 and CMRPG8C1112). The authors are grateful to professors Cheuk-Man Yu for consultation of echocardiographic studies, John Y. Chiang for polishing the English, Dr. Chi-Hsiang Chu for trial data analysis and statistical work, and Dr. Hsin-Ju Chiang for illustration design. We appreciate Drs. Cheng-Hsu Yang, Chieh-Jen Chen, Sheng-Ying Chung, Shu-Kai Hsueh, Wei-Chieh Lee, and Hsin-Ju Chiang for case enrollment and data collection. We also thank the Clinical Trial Center, Kaohsiung Chang Gung Memorial Hospital for statistical consultation and support.
This study was performed in Kaohsiung Chang Gung Memorial Hospital, Kaohsiung Veterans General Hospital, National Cheng Kung University Hospital, Chiayi Chang Gung Memorial Hospital, Ditmanson Medical Foundation Chia-Yi Christian Hospital, and Pingtung Christian Hospital in southern Taiwan.
Clinical trials No.: ISRCTN38455499.
ETHIC APPROVAL AND CONSENT TO PARTICIPATE
The IRB numbers of the collaborative institutes are as follows: IRB No. of Kaohsiung and Chiayi Chang Gung Memorial Hospitals: 103-1417C; IRB No. of Kaohsiung Veterans General Hospital: VGHKS14-CT10-17; IRB No. of National Cheng Kung University Hospital: B-BR-103-031; IRB No. of Chia-Yi Christian Hospital: 103052; IRB No. of Pingtung Christian Hospital: IRB397A-1. Written informed consent was obtained from all study participants prior to enrollment.
CONFLICT OF INTEREST
All authors report no conflict of interest.
FINANCIAL SUPPORT
None.
CONSENT FOR PUBLICATION
Not applicable.
REFERENCES
- 1.Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 2.Yip HK, Wu CJ, Chang HW, et al. Comparison of impact of primary percutaneous transluminal coronary angioplasty and primary stenting on short-term mortality in patients with cardiogenic shock and evaluation of prognostic determinants. Am J Cardiol. 2001;87:1184–1188; a1184. doi: 10.1016/s0002-9149(01)01491-6. [DOI] [PubMed] [Google Scholar]
- 3.Michaels AD, Gibson CM, Barron HV. Microvascular dysfunction in acute myocardial infarction: focus on the roles of platelet and inflammatory mediators in the no-reflow phenomenon. Am J Cardiol. 2000;85:50b–60b. doi: 10.1016/s0002-9149(00)00811-0. [DOI] [PubMed] [Google Scholar]
- 4.Yip HK, Wu CJ, Chang HW, et al. Effect of the PercuSurge GuardWire device on the integrity of microvasculature and clinical outcomes during primary transradial coronary intervention in acute myocardial infarction. Am J Cardiol. 2003;92:1331–1335. doi: 10.1016/j.amjcard.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Yip HK, Chen MC, Chang HW, et al. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: predictors of slow-flow and no-reflow phenomenon. Chest. 2002;122:1322–1332. doi: 10.1378/chest.122.4.1322. [DOI] [PubMed] [Google Scholar]
- 6.Denktas AE, Anderson HV, McCarthy J, Smalling RW. Total ischemic time: the correct focus of attention for optimal ST-segment elevation myocardial infarction care. JACC Cardiovasc Interv. 2011;4:599–604. doi: 10.1016/j.jcin.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito H, Maruyama A, Iwakura K, et al. Clinical implications of the ‘no reflow’ phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223–228. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- 9.Moens AL, Claeys MJ, Timmermans JP, Vrints CJ. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100:179–190. doi: 10.1016/j.ijcard.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 11.Varda-Bloom N, Leor J, Ohad DG, et al. Cytotoxic T lymphocytes are activated following myocardial infarction and can recognize and kill healthy myocytes in vitro. J Mol Cell Cardiol. 2000;32:2141–2149. doi: 10.1006/jmcc.2000.1261. [DOI] [PubMed] [Google Scholar]
- 12.Sheu JJ, Chang LT, Chiang CH, et al. Prognostic value of activated toll-like receptor-4 in monocytes following acute myocardial infarction. Int Heart J. 2008;49:1–11. doi: 10.1536/ihj.49.1. [DOI] [PubMed] [Google Scholar]
- 13.Yip HK, Wang PW, Chang LT, et al. Cytotoxic T lymphocyte antigen 4 gene polymorphism associated with ST-segment elevation acute myocardial infarction. Circ J. 2007;71:1213–1218. doi: 10.1253/circj.71.1213. [DOI] [PubMed] [Google Scholar]
- 14.Chua S, Leu S, Sheu JJ, et al. Intra-coronary administration of tacrolimus markedly attenuates infarct size and preserves heart function in porcine myocardial infarction. J Inflamm (Lond) 2012;9:21. doi: 10.1186/1476-9255-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang CH, Sheu JJ, Tsai TH, et al. Effect of tacrolimus on myocardial infarction is associated with inflammation, ROS, MAP kinase and Akt pathways in mini-pigs. J Atheroscler Thromb. 2013;20:9–22. doi: 10.5551/jat.14316. [DOI] [PubMed] [Google Scholar]
- 16.Sheu JJ, Sung PH, Leu S, et al. Innate immune response after acute myocardial infarction and pharmacomodulatory action of tacrolimus in reducing infarct size and preserving myocardial integrity. J Biomed Sci. 2013;20:82. doi: 10.1186/1423-0127-20-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jurewicz WA. Tacrolimus versus cyclosporin immunosuppression: long-term outcome in renal transplantation. Nephrol Dial Transplant. 2003;18 Suppl 1:i7–i11. doi: 10.1093/ndt/gfg1028. [DOI] [PubMed] [Google Scholar]
- 18.Sung P, Chen Y, Chen H, et al. Intra-coronary administration of tacrolimus prior to first-balloon inflation attenuates infarct size and improves left ventricular function in patients with ST-segment elevation myocardial infarction (COAT-STEMI) undergoing primary coronary intervention. J Clin Trials. 2014;4:2167-0870.1000179. [Google Scholar]
- 19.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 20.The thrombolysis in myocardial infarction (TIMI) trial. Phase I findings. N Engl J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 21.van't Hof AW, Liem A, Suryapranata H, et al. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. 1998;97:2302–2306. doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 22.Wong CK, de la Barra SL, Herbison P. Does ST resolution achieved via different reperfusion strategies (fibrinolysis vs percutaneous coronary intervention) have different prognostic meaning in ST-elevation myocardial infarction? A systematic review. Am Heart J. 2010;160:842–848. doi: 10.1016/j.ahj.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 23.White HD, Wong CK, Gao W, et al. New ST-depression: an under-recognized high-risk category of ‘complete’ ST-resolution after reperfusion therapy. Eur Heart J Acute Cardiovasc Care. 2012;1:210–221. doi: 10.1177/2048872612454841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galderisi M, Henein MY, D'Hooge J, et al. Recommendations of the European Association of Echocardiography: how to use echo-Doppler in clinical trials: different modalities for different purposes. Eur J Echocardiogr. 2011;12:339–353. doi: 10.1093/ejechocard/jer051. [DOI] [PubMed] [Google Scholar]
- 25.Gerczuk PZ, Kloner RA. An update on cardioprotection: a review of the latest adjunctive therapies to limit myocardial infarction size in clinical trials. J Am Coll Cardiol. 2012;59:969–978. doi: 10.1016/j.jacc.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 26.Sheu JJ, Chua S, Sun CK, et al. Intra-coronary administration of cyclosporine limits infarct size, attenuates remodeling and preserves left ventricular function in porcine acute anterior infarction. Int J Cardiol. 2011;147:79–87. doi: 10.1016/j.ijcard.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 28.Schroder R, Dissmann R, Bruggemann T, et al. Extent of early ST segment elevation resolution: a simple but strong predictor of outcome in patients with acute myocardial infarction. J Am Coll Cardiol. 1994;24:384–391. doi: 10.1016/0735-1097(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 29.Angeja BG, Gunda M, Murphy SA, et al. TIMI myocardial perfusion grade and ST segment resolution: association with infarct size as assessed by single photon emission computed tomography imaging. Circulation. 2002;105:282–285. doi: 10.1161/hc0302.103588. [DOI] [PubMed] [Google Scholar]
- 30.Brener SJ, Dizon JM, Mehran R, et al. Complementary prognostic utility of myocardial blush grade and ST-segment resolution after primary percutaneous coronary intervention: analysis from the HORIZONS-AMI trial. Am Heart J. 2013;166:676–683. doi: 10.1016/j.ahj.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Norman KG, Canter JA, Shi M, et al. Cyclosporine A suppresses keratinocyte cell death through MPTP inhibition in a model for skin cancer in organ transplant recipients. Mitochondrion. 2010;10:94–101. doi: 10.1016/j.mito.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9–19. doi: 10.1111/j.1749-6632.1993.tb17137.x. [DOI] [PubMed] [Google Scholar]
- 33.Cung TT, Morel O, Cayla G, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 34.Oka N, Wang L, Mi W, et al. Cyclosporine A prevents apoptosis-related mitochondrial dysfunction after neonatal cardioplegic arrest. J Thorac Cardiovasc Surg. 2008;135:123–130, 130.e121-122. doi: 10.1016/j.jtcvs.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Sharov VG, Todor A, Khanal S, et al. Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. J Mol Cell Cardiol. 2007;42:150–158. doi: 10.1016/j.yjmcc.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 37.Deftereos S, Giannopoulos G, Angelidis C, et al. Anti-inflammatory treatment with colchicine in acute myocardial infarction: a pilot study. Circulation. 2015;132:1395–1403. doi: 10.1161/CIRCULATIONAHA.115.017611. [DOI] [PubMed] [Google Scholar]
- 38.Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]