Abstract
Thymic epithelial tumours (TETs) represent a rare disease, yet they are the most common tumours of the anterior mediastinum. Due to the rare occurrence of TETs, evidence on optimal treatment is limited. Surgery is the treatment of choice in the management of TETs, while the role of postoperative radiotherapy (PORT) remains unresolved. PORT remains debated for thymomas, especially in completely resected stage II tumours, for which PORT may be more likely to benefit in the presence of aggressive histology (WHO subtype B2, B3) or extensive transcapsular invasion (Masaoka-Koga stage IIB). For stage III thymoma, evidence suggests an overall survival (OS) benefit for PORT after complete resection. For incompletely resected thymomas stage II or higher PORT is recommended. Thymic carcinomas at any stage with positive resection margins should be offered PORT. Radiotherapy plays an important role in the management of unresectable locally advanced TETs. Induction therapy (chemotherapy or chemoradiation) followed by surgery may be useful for locally advanced thymic malignancies initially considered as unresectable. Chemotherapy only is offered in patients with unresectable, metastatic tumours in palliative intent, checkpoint inhibitors may be promising for refractory diseases. Due to the lack of high-level evidence and the importance of a multidisciplinary approach, TETs should be discussed within a multidisciplinary team and the final recommendation should reflect individual patient preferences.
Keywords: Thymoma, thymic cancer, radiotherapy, postoperative radiotherapy (PORT)
Introduction
Thymic epithelial tumours (TETs) originate from the thymus and are classified as thymoma, thymic carcinoma (TC) or thymic neuroendocrine tumour (TNET) (1).
The incidence of all TETs is about 1.3–3.2 cases per million worldwide and most commonly diagnosed between the age of 40–60 years (2).
Thymomas are the most common anterior mediastinal tumour, accounting for up to 50% of all anterior mediastinal masses (3). The most relevant differential diagnoses are lymphomas and germ-cell tumours.
Thymic carcinomas are even less frequent (4), TNETs are the rarest of the three accounting for approximately 0.4% of all carcinoid tumours (5).
One-third of patients with thymoma present with autoimmune disorders, mainly myasthenia gravis which is particularly common in type AB, B1 and B2 thymomas and almost always associated with anti-acetylcholine receptor antibodies (6). Myasthenia gravis is a consequence of cross-reactivity of anti-thymoma immune response with the neuromuscular junction. In patients suffering from paraneoplastic myasthenia gravis, commonly used drugs such as ciprofloxacin, beta blockers, calcium channel blockers, muscle relaxants and verapamil among others, should not be administered as they can worsen the symptoms of myasthenia gravis. Contrast-enhanced computed tomography (CT) is essential for the diagnosis and to define the best strategy of care (7). CT is equal or superior to magnetic resonance imaging (MRI) for the diagnosis, except in the setting of cystic lesions (8).
Histopathology of TETs is complex and different histological classifications have been described in the literature. The WHO histologic classification system gives a detailed overview of the different histologic subtypes. Based on the morphological appearance of TETs and the lymphocyte/thymic epithelial cell (TEC) ratio, thymoma are classified as types A, AB, B1, B2 and B3 (9,10). Depending on the histological subtype, the prognosis of TET can vary significantly (11). Thymoma types A, AB, and B1 have a good 10-year overall survival (OS) of 90–95%. While thymomas show less aggressive behaviour, TC and TNET are associated with worse outcomes (12) with 5-year overall survival rates of approximately 55% for TC and 28% to 75% for TNET (13-16).
As a consequence of their rarity, our knowledge about the management of patients with TETs is mainly based on retrospective series, uncontrolled non-randomised trials or expert opinions (17,18). Studies including large numbers of patients are rare, especially when investigating multimodal treatment options. With the creation of the International Thymic Malignancies Interest Group (ITMIG) and other thymic working groups (e.g., ESTS, RHYTMIC), substantial efforts have been made to optimise the care of thymic malignancies (18). The selection of optimal management of TETs remains challenging and the role of radiotherapy is controversial. The current review will highlight TET management with a focus on radiotherapy.
Staging
The Masaoka-Koga classification, which was developed in 1981 (19) and revised in 1994 (20), is the most widely adopted staging system due to its prognostic significance (16,21,22). It is a pathology based grading system only assessable after surgical resection of the tumour.
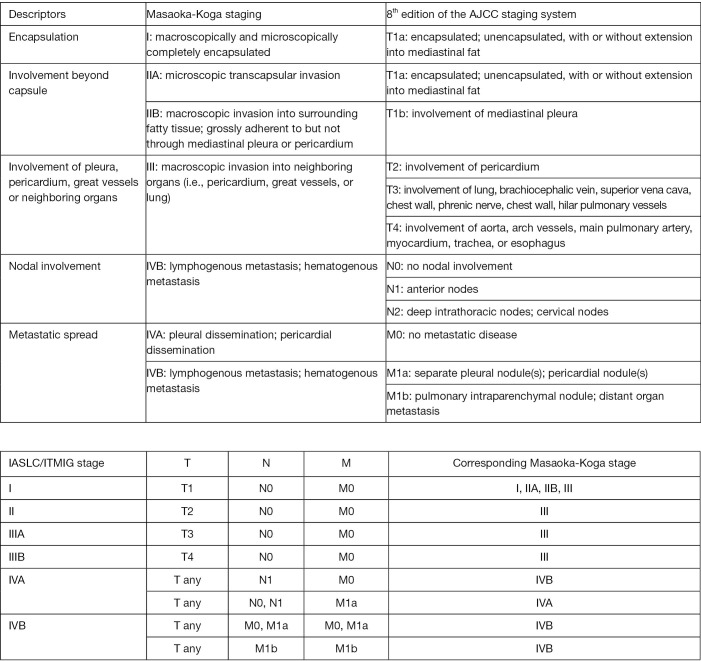
The ITMIG and the Thymic Domain of the Staging and Prognostic Factors Committee of the International Association for the Study of Lung Cancer (IASLC) has developed a TNM classification that has been incorporated into the 8th version of the AJCC and UICC staging systems (11) (Figure 1).
Figure 1.
Comparison between the Masaoka-Koga staging system and the TNM based (IASLC/ITMIG) staging system for TETs included in the AJCC 8th edition cancer staging system manual, adapted from Willmann et al. (17).
In the TNM staging system, Masaoka I, II and some stage III TETs correspond to stage I (T1N0M0) tumours. Major problem regarding PORT is to identify Masaoka-Koga stage II and III tumours within the TNM stage I group. The recommendation of the current ESMO guidelines is to keep decision-making based on the Masaoka-Koga system for PORT—until more data is available.
Surgery
The optimal therapy for TETs should be determined at a multidisciplinary setting (23-26). Treatment strategy for TET is primarily based on whether the tumour is resected upfront. The evaluation of resectability is based on tumour size and the surgeon’s expertise. TNM staging may even assist in estimating resectability: stage T1-3 refers to an extension amenable to surgical resection, while stage T4 is mostly considered unresectable (25).
Complete tumour resection represents the most effective therapy and most significant favourable prognostic factor, both on DFS and OS (22,27-32).
Generally, complete thymectomy, including the tumour, residual thymus and perithymic fat is preferred for stage I-II disease. Recently, the concept of thymectomy in stage I leaving residual thymic tissue and peri-thymic fat behind has emerged as an option in non-myasthenic TET patients (33). In stage III TET infiltrating surrounding structures, en bloc removal of all affected structures should be performed.
Surgical clips should be placed at the time of resection to areas of close margins, residual disease or tumour adhesion to unresected normal structures to help define target volumes for potential PORT.
The ITMIG/IASLC working groups proposed lymph node staging, which was the basis for the 8th version of the TNM classification. Routine removal of anterior mediastinal nodes is recommended, and systematic sampling of intrathoracic sites is encouraged in stage III/IV tumours. An even more extensive systematic nodal dissection is recommended for TC (high rate of lymphatic spread of 20%) (22,25,34).
For potentially resectable TETs (Masaoka-Koga stage III/IVa), a biopsy should be performed and induction chemotherapy with or without RT can be considered to improve the R0 resection rate as a curative-intent strategy (25,26,35-41). Two to four cycles of cisplatin-based combination regimens should be administered followed by radiological restaging to reassess resectability. About 10% of these patients will not be eligible for surgery after induction chemotherapy. For patients with unresectable disease, RT with (or without) chemotherapy is recommended (26).
Surgery for Masaoka-Koga stage IVa (especially in the case of pleural involvement) is not well defined and should be discussed in a multidisciplinary team (MDT). Tumour debulking, extrapleural pneumonectomy (EPP) and intracavitary pleural treatment are additional options for this stage (40,42).
Radiotherapy
Radiotherapy is used in various settings for the treatment of TETs: after surgical resection as postoperative radiotherapy (PORT), as definite treatment in unresectable TETs and in the neoadjuvant setting. Palliative radiotherapy for metastatic TETs is a feasible option either with typical conventional fractionation or stereotactic body radiation therapy (SBRT) (26). Thoracic RT for TETs is generally well tolerated.
Postoperative radiotherapy
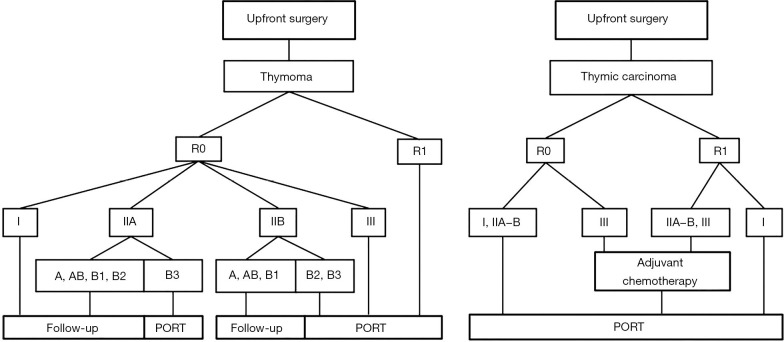
Some retrospective studies reported that PORT after incomplete resection could prevent or postpone postoperative recurrence, and improve the survival of patients in locally advanced stages (Masaoka-Koga I–III). Given the lack of prospective evidence, several investigators have utilised the SEER database and other national data sets to investigate the role of PORT for TETs (13,30,43-53). Basse et al. were the first to report on a large cohort of 828 consecutive patients with resected TETs, for whom a guidelines-based decision for PORT was prospectively made at an expert MDT. Data of this study indicate that the ESMO guidelines algorithms for PORT are relevant in routine clinical practice (23) (Figure 2).
Figure 2.
Treatment algorithm for resectable TET (Masaoka-Koga stage I-III) adapted from on the ESMO and RHYTHMIC network guidelines (25). R0, microscopically margin-negative resection, R1, removal of all macroscopic disease, but microscopic margins are positive for tumor; I-III, Masaoka-Koga stage; A, AB, B1−3, WHO subtype; PORT, postoperative radiotherapy.
Resectable stage I-II thymoma
In stage I thymoma, excellent local control rates after surgery were achieved in several studies, and there is no indication for PORT in this setting (17,45,47). In a randomised trial investigating PORT in 26 patients with stage I TETs did not demonstrate a survival benefit (54). This finding is consistent with one of the largest database analysis with 1244 patients in, which no significant impact on survival was shown with the use of PORT (49).
PORT is more controversial in stage II TETs. With R0 resection, many series report recurrence rates of <10% with or without PORT at 10 years. In a Japanese cohort of 257 stage II thymomas receiving PORT or surgery only, no difference was found with regards to recurrence rate (22). In contrast, PORT resulted in a trend towards a survival benefit of 19 months (134 versus 115 months, P=0.09) in 1,334 stage IIB thymoma patients of a SEER database analysis. After excluding patients surviving less than 4 months in this stage (4-month mortality 11.6% for the surgery only group and 0.6% for the PORT group), the difference in survival was not significant (P=0.45) (47).
In a subset analysis of the study of Jackson et al., including 633 patients, the impact of PORT for stage IIA did not reach significance but indicated longer OS in association with PORT for patients with stage IIB thymoma (P=0.004) (49). These findings align well with a study of the ITMIG database, which might provide the strongest evidence for PORT to date, supporting the use of PORT in stage II thymoma, even when completely resected; the greatest benefit was observed for the histologic subtypes WHO B1−3 (52). In the only prospective study of Basse et al. PORT was most commonly offered in B3 subtype TETs (23). While the most recent ITMIG study suggests that WHO types B1−3 benefit most from PORT (52), interestingly two NCDB studies demonstrate a greater benefit for the less aggressive WHO type A and AB (49,55). However, the ESMO/RYTHMIC guidelines recommend PORT only for stage IIA and B3 or stage IIB and B2−3 (23,25).
A survey among centres of ESTS members showed that in stage II thymoma, 61% of ESTS members consider PORT, while 39% of the centres state that they do not use PORT. However, most of the centres that offer PORT state the indication on an individual basis, depending on worse histological subtypes, incomplete resection and subdivision of stage II according to the Masaoka-Koga staging system into stage IIA and IIB (24).
For incompletely resected stage I−II thymoma tumours it is widespread practice to offer adjuvant RT to prevent local recurrence and improve survival (49).
Resectable stage III-IV thymoma
PORT is recommended after complete resection of stage III/IVA thymoma in an effort to prolong recurrence-free survival and OS (25). In R0 stage III, the benefit of RT is established (49,52). The large population-based study by Weksler et al. with 476 patients demonstrated an improved disease-specific survival (P=0.049) without significant improvement of OS for resected stage III thymoma with the addition of PORT compared to surgical resection alone (56). In the ITMIG study, completely resected stage III thymoma investigating PORT lead to 10-year OS rates of 79% with PORT versus 64% without (P=0.0005) (52). In contrast to these results, a pooled analysis of 13 retrospective studies compared PORT to no adjuvant treatment in thymoma stage II−III patients, no significant difference was observed (P=0.63) (57). Importantly, a potential selection bias with poorer prognosis patients receiving PORT cannot be excluded in the analysis of Korst et al.
If tolerable, aggressive multimodality treatment including induction chemotherapy, surgery and adjuvant RT may be considered in patients with localised stage IVA thymoma to improve their survival, although the benefit seems to be less pronounced than in stage III. Univariate analysis of the study from Modh et al. showed that patients with stage IVA disease or a poorer performance status had worse freedom from recurrence (17,58).
In cases of incomplete resection, PORT is usually proposed to reduce the risk of mediastinal progression (34,59). Patients also might benefit from an OS advantage with PORT after R+ resection (49).
Resectable thymic carcinoma/thymic neuroendocrine tumours
Whereas some studies suggest that adjuvant therapy may not be necessary for early-stage TCs (60), the ITMIG and ESTS database analysis shows an improved OS for patients receiving PORT compared with those who do not receive RT (4). An analysis of the Japanese Association for Research on the Thymus (JART) database that only included TC cases stage II and III, aimed to assess the impact of PORT. PORT only improved recurrence-free survival but not OS of stages II and III TC patients (50). For R+ resected TCs and TNETs PORT is recommended (4,16,29,43,50,61).
For TNETs there are even less clinical trials and thus less evidence on treatment effectiveness. A complete resection should always be attempted. TCs and TNETs are tumours with a high risk of recurrence, with recurrence rates from 20% to 30% in stage I and up to 80% in advanced disease. PORT is optional for R0 resected stage I tumours, should be considered for stage II tumours and is recommended for stage III/IVA tumours (4,16,25,50).
Neoadjuvant and/or adjuvant treatment is performed as in TCs (62).
The ESMO guidelines recommend that PORT should be started within three months after the surgical procedure on the basis of expert consensus (25).
Outlook
The magnitude of the effect from PORT in different stages of disease has yet to be quantified. As no randomised or even prospective study has been conducted to assess the effect of PORT on recurrence rates or survival for advanced stage TETs, available guidelines are based on low levels of evidence (25,63). Due to a lack of high evidence and the potential for a multidisciplinary approach, TETs should be discussed in a MDT and patient preferences should be incorporated in the decision-making. Phase III clinical trials assessing PORT for TET are currently being conducted in the People’s Republic of China and enrolling patients with stage II or III completely and incompletely resected thymomas and thymic cancers (Table 1). In the future, hopefully the results of these trials will provide good data on outcomes improving our clinical decision making.
Table 1. Brief overview of ongoing trials.
| Disease | Intervention | Comparator arm | Overall statue | Country | |
|---|---|---|---|---|---|
| NCT02633553 | Thymoma Masaoka-Koga II/III | Adjuvant RT (50 Gy) | No RT | Recruiting | China |
| NCT02014805 | Thymoma Masaoka-Koga II−IIIB | Adjuvant RT | No RT | Recruiting | China |
| NCT02633514 | Thymoma/TC R1/R2 | Adjuvant CRT with cisplatin/etoposide (60 Gy) | Adjuvant RT (60 Gy) | Recruiting | China |
Definitive radiotherapy
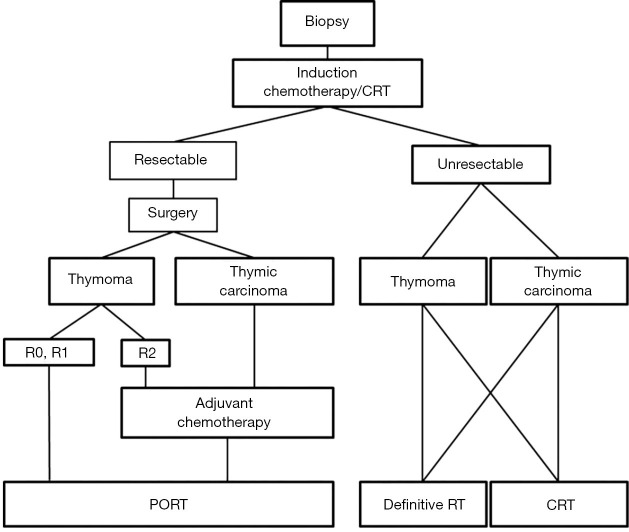
Definitive RT can be performed in unresectable TETs, or when surgery cannot be performed due to poor performance status or co-existing medical conditions. Definitive RT also refers to RT delivered when a macroscopically incomplete (R2) resection was previously performed (25) (Figure 3).
Figure 3.
Treatment algorithm for primary unresectable TET (Masaoka-Koga stage III-IVA) based on the ESMO and RHYTHMIC network guidelines (25).
Despite the lack of high-level evidence, definitive RT or chemoradiation (CRT) is generally performed for unresectable TETs (64). Most studies are retrospective, except for two prospective phase II studies. The landmark prospective trial of Loehrer et al. enrolled 23 patients who received induction chemotherapy with the cyclophosphamide, doxorubicin and cisplatin regimen and subsequent definitive RT to a total dose of 54 Gy. This strategy resulted in an overall response rate of 70% and a 5-year survival of 53%, comparing favorably with the results of incomplete resection (65). The largest prospective phase II trial is from Fan et al. with 56 patients evaluating the use of CRT with etoposide/cisplatin (EP) as the first-line treatment for advanced TETs (85.7% stage IV). The study demonstrated that intensity modulated radiation therapy with 54 Gy and EP is safe and effective for unresectable TETs. The objective response rate was 85.7%. The 5-year PFS of 29.5% and OS rate of 56.2% were promising (66). The results from these studies and other reports (64,67-70) indicate that a multimodality approach such as definitive CRT is an effective strategy, also for selected patients with advanced stage IVB thymomas or thymic carcinomas, even if the recommended first-line treatment in case of Masaoka-Koga stage IVB TETs is definitive chemotherapy followed by surgery or definitive RT depending on response and goal of palliation (25).
The single-institution analysis of Hao et al. demonstrates that patients treated with SBRT had an excellent local control with low rate of acute toxicities, which suggests that SBRT is feasible for the patients with TETs who are unable to undergo either surgery or conventionally fractionated radiation therapy or as a palliative therapy for metastases of TETs (71).
Treatment planning in TETs
Radiation oncologists should consult the surgeon to assess the operative findings to help determine the target volume at risk. The clinical target volume (CTV) for PORT should encompass the entire thymus (for partial resection cases), surgical clips, and any potential sites with residual disease. The CTV should be reviewed with the thoracic surgeon (26).
When the tumour has been completely resected, minimum fields for PORT should encompass the pre-operative extent of disease, as indicated by pre-operative imaging and regions of risk identified intra-operatively. The field may then encompass involved nodes and the site of a possible pleural implant.
Although TETs have been deemed radiosensitive for years, the benefit of dose escalation on local control has not been clearly been established. Arriagada et al. (72) reported similar local control rates with total doses inferior to 48 Gy or superior to 60 Gy, while in more recent series, no improvement regarding local control was observed with doses of 50 or >60 Gy (73).
Radiation doses for the adjuvant setting are typically between 45 to 55 Gy in the adjuvant setting [45 to 50 Gy for clear or close margins (73-75), 54 Gy for microscopically positive (R1) resection margins (76-78) and 60-66 Gy in the definitive treatment setting or after incomplete (R2) resection (25,26,63,76,79,80)], using a standard fractionation of 1.8−2 Gy per day. For definitive concomitant CRT a radiation dose of 54 Gy seems to be most commonly recommended (64).
Novel techniques, such as IMRT or proton beam RT, may reduce long-term radiation toxicity and thereby favourably shift the risk-benefit ratio of RT. Because these patients are younger and often long-term survivors, the mean dose to the heart should be as low as reasonably achievable (26,81). The first prospective study has recently shown that proton beam therapy in thymoma and TC patients is safe and feasible (82). A retrospective single-institution study found that adjuvant IMRT might achieve better outcomes than conventional RT as an adjuvant treatment of completely resected stage III thymoma patients (83).
The impact of IMRT, proton beam therapy, optimal dosing and radiation field design have yet to be accounted for in future prospective studies.
Neoadjuvant treatment
Induction therapy followed by surgery may be useful for locally advanced thymic malignancies initially considered unresectable (26,35-41). Patients with advanced disease (Masaoka-Koga stage III/IVA) are candidates for induction chemotherapy with or without RT. Cisplatin-based combination regimens should be administered (84,85). Usually, two to four cycles are administered before reassessing resectability of the tumour. A cohort of 22 patients with locally advanced thymoma and TC (stage III/IV) has been analysed prospectively to assess the efficacy of neoadjuvant CRT. After neoadjuvant CRT, R0 resection could be achieved in a majority (77%) of patients. The OS benefit was higher for patients that could be downstaged. Induction CRT might therefore be a feasible option to achieve complete resection in patients with locally advanced and highly aggressive tumours and thereby potentially improve their survival (86). This treatment option is also mentioned in the ESMO/RYTHMIC guideline (Figure 2).
Systemic treatment
Chemotherapy may be used in unresectable, advanced, metastatic or recurrent tumours, either as induction chemotherapy before intended radical local treatment, in combination with radiotherapy as potentially curative radio-chemotherapy, or as palliative systemic treatment.
Few studies have focused on the effectiveness of chemotherapy for thymoma. Postoperative chemotherapy is not recommended after R0–R1 resection of thymomas (25,29,33,63).
For patients with advanced TC stage II/III/IV, adjuvant chemotherapy is recommended, if not already administered in the neoadjuvant setting (33,63).
Chemotherapy only is offered in patients with unresectable, metastatic tumours in palliative intent. Cisplatin-based multi-agent combinations are considered standard of care. The preferred regimen for thymoma is cisplatin/doxorubicin/cyclophosphamide (CAP) (87-90). For TC carboplatin/paclitaxel is the preferred regime for first-line therapy (91-100).
Checkpoint inhibitors may be promising for refractory thymic neoplasms. Pembrolizumab has been studied in TC in a phase II trial and disease control was achieved in 75% of patients with a median OS of 24.9 months (101). Grade 3 to 4 myocarditis has been reported in 5% to 9% of patients with TCs receiving pembrolizumab (101,102). Nivolumab is currently studied (NIVOTHYM trial) by the European Organization for Research and Treatment of Cancer (EORTC) in patients with TC or type B3 thymoma as a second-line treatment (ClinicalTrials.gov Identifier: NCT03134118).
Recurrences
Recurrences of TETs occur frequently (~10–15% of all-stage resected tumours) and should be treated similar to primary disease if possible. The question of complete resection of these lesions should be considered and if not deemed possible, re-administration of a previously effective treatment must be discussed especially (103). Previous irradiation to the same area may pose a significant limitation to radiotherapy.
Follow-up
No prospective data are available to build recommendations on post-treatment oncological follow-up of patients. After primary treatment for resectable thymomas, NCCN guidelines recommend that surveillance for recurrence should include chest CT every 6 months for 2 years, then annually for 10 years for thymoma (104). MRI may be used for surveillance if patients cannot tolerate IV contrast and to decrease radiation if patients are young and are expected to be screened for many years. Given the risk of late recurrence for thymoma, surveillance should continue for at least 10 years. ESMO guidelines recommend for completely resected stage I/II thymomas CT scan every year for 5 years, then every 2 years. For stage III/IV thymomas and TCs or after R1−2 resection CT scans every 6 months for 2 years, and thereafter annually should be performed (26).
Patients with thymoma also have an increased risk for secondary malignancies, although no particular screening is recommended to detect these (3,105,106).
Conclusion
Radiotherapy plays an important role in the management of unresectable locally advanced thymic epithelial tumours. In the adjuvant setting RT remains debated, especially in completely resected stage II tumours, for which PORT may be more likely to benefit in the presence of aggressive histology (WHO subtype B2, B3) or extensive transcapsular invasion (Masaoka-Koga stage IIB). For stage III thymoma, evidence suggests an OS benefit for PORT after complete resection. For incompletely resected thymomas stage II or higher PORT is recommended. Thymic carcinomas at any stage with positive resection margins should be offered PORT.
Pragmatism and patient involvement are the key in this setting of a rare tumour and limited evidence.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jacek Jassem and Rafal Dziadziuszko) for the focused issue “Radiotherapy in thoracic malignancies” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-458
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-458). The focused issue “Radiotherapy in thoracic malignancies” was commissioned by the editorial office without any funding or sponsorship. Paul Martin Putora reports research support and educational grants to the department: AstraZeneca, Celgene, Takeda. The authors have no other conflicts of interest to declare.
References
- 1.Marx A, Chan JKC, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol 2015;10:1383-95. 10.1097/JTO.0000000000000654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jong WK, Blaauwgeers JLG, Schaapveld M, et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 2008;44:123-30. 10.1016/j.ejca.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 3.Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260-5. 10.1097/JTO.0b013e3181f1f62d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg 2015;149:95-101.e1012. 10.1016/j.jtcvs.2014.09.124 [DOI] [PubMed] [Google Scholar]
- 5.Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 6.Evoli A, Lancaster E. Paraneoplastic disorders in thymoma patients. J Thorac Oncol 2014;9:S143-S7. 10.1097/JTO.0000000000000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato Y, Yanagawa M, Hata A, et al. Volumetric analysis of the thymic epithelial tumors: correlation of tumor volume with the WHO classification and Masaoka staging. J Thorac Dis 2018;10:5822-32. 10.21037/jtd.2018.09.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seki S, Koyama H, Ohno Y, et al. Diffusion-weighted MR imaging vs. multi-detector row CT: Direct comparison of capability for assessment of management needs for anterior mediastinal solitary tumors. Eur J Radiol 2014;83:835-42. 10.1016/j.ejrad.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 9.Kondo K, Yoshizawa K, Tsuyuguchi M, et al. WHO histologic classification is a prognostic indicator in thymoma. Ann Thorac Surg 2004;77:1183-8. 10.1016/j.athoracsur.2003.07.042 [DOI] [PubMed] [Google Scholar]
- 10.Marx A, Ströbel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol 2014;9:596-611. 10.1097/JTO.0000000000000154 [DOI] [PubMed] [Google Scholar]
- 11.Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-S72. 10.1097/JTO.0000000000000290 [DOI] [PubMed] [Google Scholar]
- 12.Suster S, Rosai J. Thymic carcinoma. A clinicopathologic study of 60 cases. Cancer 1991;67:1025-32. [DOI] [PubMed] [Google Scholar]
- 13.Weksler B, Dhupar R, Parikh V, et al. Thymic carcinoma: a multivariate analysis of factors predictive of survival in 290 patients. Ann Thorac Surg 2013;95:299-303. 10.1016/j.athoracsur.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 14.Gal AA, Kornstein MJ, Cohen C, et al. Neuroendocrine tumors of the thymus: a clinicopathological and prognostic study. Ann Thorac Surg 2001;72:1179-82. 10.1016/S0003-4975(01)03032-6 [DOI] [PubMed] [Google Scholar]
- 15.Hsu C-H, Chan JK, Yin C-H, et al. Trends in the incidence of thymoma, thymic carcinoma, and thymic neuroendocrine tumor in the United States. PLoS One 2019;14:e0227197. 10.1371/journal.pone.0227197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruffini E, Detterbeck F, Van Raemdonck D, et al. Thymic carcinoma: a cohort study of patients from the European society of thoracic surgeons database. J Thorac Oncol 2014;9:541-8. 10.1097/JTO.0000000000000128 [DOI] [PubMed] [Google Scholar]
- 17.Willmann J, Rimner A. The expanding role of radiation therapy for thymic malignancies. J Thorac Dis 2018;10:S2555-64. 10.21037/jtd.2018.01.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drevet G, Collaud S, Tronc F, et al. Optimal management of thymic malignancies: current perspectives. Cancer Manag Res 2019;11:6803-14. 10.2147/CMAR.S171683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [DOI] [PubMed] [Google Scholar]
- 20.Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359-67. 10.1111/j.1440-1827.1994.tb02936.x [DOI] [PubMed] [Google Scholar]
- 21.Masaoka A. Staging system of thymoma. J Thorac Oncol 2010;5:S304-S12. 10.1097/JTO.0b013e3181f20c05 [DOI] [PubMed] [Google Scholar]
- 22.Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. 10.1016/S0003-4975(03)00555-1 [DOI] [PubMed] [Google Scholar]
- 23.Basse C, Thureau S, Bota S, et al. Multidisciplinary Tumor Board Decision Making for Postoperative Radiotherapy in Thymic Epithelial Tumors: Insights from the RYTHMIC Prospective Cohort. J Thorac Oncol 2017;12:1715-22. 10.1016/j.jtho.2017.07.023 [DOI] [PubMed] [Google Scholar]
- 24.Ruffini E, Van Raemdonck D, Detterbeck F, et al. Management of thymic tumors: a survey of current practice among members of the European Society of Thoracic Surgeons. J Thorac Oncol 2011;6:614-23. 10.1097/JTO.0b013e318207cd74 [DOI] [PubMed] [Google Scholar]
- 25.Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v40-v55. 10.1093/annonc/mdv277 [DOI] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network 2020. Thymomas and Thymic Carcinomas (version 1.2020, November 27, 2019). Available online: https://wwwnccnorg/professionals/physician_gls/pdf/thymicpdf.
- 27.Detterbeck F, Youssef S, Ruffini E, et al. A review of prognostic factors in thymic malignancies. J Thorac Oncol 2011;6:S1698-S704. 10.1097/JTO.0b013e31821e7b12 [DOI] [PubMed] [Google Scholar]
- 28.Okumura M, Shiono H, Inoue M, et al. Outcome of surgical treatment for recurrent thymic epithelial tumors with reference to world health organization histologic classification system. J Surg Oncol 2007;95:40-4. 10.1002/jso.20671 [DOI] [PubMed] [Google Scholar]
- 29.Ruffini E, Detterbeck F, Van Raemdonck D, et al. Tumours of the thymus: a cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg 2014;46:361-8. 10.1093/ejcts/ezt649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel S, Macdonald OK, Nagda S, et al. Evaluation of the role of radiation therapy in the management of malignant thymoma. Int J Radiat Oncol Biol Phys 2012;82:1797-801. 10.1016/j.ijrobp.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 31.Ried M, Marx A, Götz A, et al. State of the art: diagnostic tools and innovative therapies for treatment of advanced thymoma and thymic carcinoma. Eur J Cardiothorac Surg 2016;49:1545-52. 10.1093/ejcts/ezv426 [DOI] [PubMed] [Google Scholar]
- 32.Ströbel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22:1501-9. 10.1200/JCO.2004.10.113 [DOI] [PubMed] [Google Scholar]
- 33.Falkson CB, Bezjak A, Darling G, et al. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol 2009;4:911-9. 10.1097/JTO.0b013e3181a4b8e0 [DOI] [PubMed] [Google Scholar]
- 34.Scorsetti M, Leo F, Trama A, et al. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol 2016;99:332-50. 10.1016/j.critrevonc.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 35.Okereke IC, Kesler KA, Freeman RK, et al. Thymic carcinoma: outcomes after surgical resection. Ann Thorac Surg 2012;93:1668-72; discussion 1672-3. 10.1016/j.athoracsur.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 36.Park S, Park IK, Kim YT, et al. Comparison of Neoadjuvant Chemotherapy Followed by Surgery to Upfront Surgery for Thymic Malignancy. Ann Thorac Surg 2019;107:355-62. 10.1016/j.athoracsur.2018.08.055 [DOI] [PubMed] [Google Scholar]
- 37.Ruffini E, Guerrera F, Brunelli A, et al. Report from the European Society of Thoracic Surgeons prospective thymic database 2017: a powerful resource for a collaborative global effort to manage thymic tumours. Eur J Cardiothorac Surg 2019;55:601-9. 10.1093/ejcts/ezy448 [DOI] [PubMed] [Google Scholar]
- 38.Kanzaki R, Kanou T, Ose N, et al. Long-term outcomes of advanced thymoma in patients undergoing preoperative chemotherapy or chemoradiotherapy followed by surgery: a 20-year experience. Interact Cardiovasc Thorac Surg 2019;28:360-7. 10.1093/icvts/ivy276 [DOI] [PubMed] [Google Scholar]
- 39.Riely GJ, Huang J. Induction therapy for locally advanced thymoma. J Thorac Oncol 2010;5:S323-S6. 10.1097/JTO.0b013e3181f20e90 [DOI] [PubMed] [Google Scholar]
- 40.Wright CD, Choi NC, Wain JC, et al. Induction chemoradiotherapy followed by resection for locally advanced Masaoka stage III and IVA thymic tumors. Ann Thorac Surg 2008;85:385-9. 10.1016/j.athoracsur.2007.08.051 [DOI] [PubMed] [Google Scholar]
- 41.Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer 2004;44:369-79. 10.1016/j.lungcan.2003.12.010 [DOI] [PubMed] [Google Scholar]
- 42.Yellin A, Simansky DA, Ben-Avi R, et al. Resection and heated pleural chemoperfusion in patients with thymic epithelial malignant disease and pleural spread: a single-institution experience. J Thorac Cardiovasc Surg 2013;145:83-7; discussion 87-9. 10.1016/j.jtcvs.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 43.Erratum: Omasa M, Date H, Sozu T, Sato T, Nagai K, Yokoi K, Okamoto T, Ikeda N, Tanaka F, and Maniwa Y, and the Japanese Association for Research on the Thymus. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: The Japanese Association for Research on the Thymus Database Study. Cancer. doi: 10.1002/cncr.29166. Cancer 2015;121:2102. [DOI] [PubMed]
- 44.Lim YJ, Kim HJ, Wu HG. Role of Postoperative Radiotherapy in Nonlocalized Thymoma: Propensity-Matched Analysis of Surveillance, Epidemiology, and End Results Database. J Thorac Oncol 2015;10:1357-63. 10.1097/JTO.0000000000000619 [DOI] [PubMed] [Google Scholar]
- 45.Forquer JA, Rong N, Fakiris AJ, et al. Postoperative radiotherapy after surgical resection of thymoma: differing roles in localized and regional disease. Int J Radiat Oncol Biol Phys 2010;76:440-5. 10.1016/j.ijrobp.2009.02.016 [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa K, Yokoi K, Nakajima J, et al. Is Thymomectomy Alone Appropriate for Stage I (T1N0M0) Thymoma? Results of a Propensity-Score Analysis. Ann Thorac Surg 2016;101:520-6. 10.1016/j.athoracsur.2015.07.084 [DOI] [PubMed] [Google Scholar]
- 47.Fernandes AT, Shinohara ET, Guo M, et al. The role of radiation therapy in malignant thymoma: a Surveillance, Epidemiology, and End Results database analysis. J Thorac Oncol 2010;5:1454-60. 10.1097/JTO.0b013e3181e8f345 [DOI] [PubMed] [Google Scholar]
- 48.Mariano C, Ionescu DN, Cheung WY, et al. Thymoma: a population-based study of the management and outcomes for the province of British Columbia. J Thorac Oncol 2013;8:109-17. 10.1097/JTO.0b013e318276241c [DOI] [PubMed] [Google Scholar]
- 49.Jackson MW, Palma DA, Camidge DR, et al. The Impact of Postoperative Radiotherapy for Thymoma and Thymic Carcinoma. J Thorac Oncol 2017;12:734-44. 10.1016/j.jtho.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 50.Omasa M, Date H, Sozu T, et al. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: the Japanese Association for Research on the Thymus Database Study. Cancer 2015;121:1008-16. 10.1002/cncr.29166 [DOI] [PubMed] [Google Scholar]
- 51.Lim YJ, Kim E, Kim HJ, et al. Survival Impact of Adjuvant Radiation Therapy in Masaoka Stage II to IV Thymomas: A Systematic Review and Meta-analysis. Int J Radiat Oncol Biol Phys 2016;94:1129-36. 10.1016/j.ijrobp.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 52.Rimner A, Yao X, Huang J, et al. Postoperative Radiation Therapy Is Associated with Longer Overall Survival in Completely Resected Stage II and III Thymoma-An Analysis of the International Thymic Malignancies Interest Group Retrospective Database. J Thorac Oncol 2016;11:1785-92. 10.1016/j.jtho.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang JH, Kim HJ, Wu H-G, et al. Postoperative radiotherapy for completely resected stage II or III thymoma. J Thorac Oncol 2011;6:1282-6. 10.1097/JTO.0b013e31821f9662 [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Lu N, Wang M, et al. Postoperative radiotherapy for stage I thymoma: a prospective randomized trial in 29 cases. Chin Med J (Engl) 1999;112:136-8. [PubMed] [Google Scholar]
- 55.Boothe D, Orton A, Thorpe C, et al. Postoperative Radiotherapy in Locally Invasive Malignancies of the Thymus: Patterns of Care and Survival. J Thorac Oncol 2016;11:2218-26. 10.1016/j.jtho.2016.07.032 [DOI] [PubMed] [Google Scholar]
- 56.Weksler B, Shende M, Nason KS, et al. The role of adjuvant radiation therapy for resected stage III thymoma: a population-based study. Ann Thorac Surg 2012;93:1822-8; discussion 1828-9. 10.1016/j.athoracsur.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 57.Korst RJ, Kansler AL, Christos PJ, et al. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg 2009;87:1641-7. 10.1016/j.athoracsur.2008.11.022 [DOI] [PubMed] [Google Scholar]
- 58.Modh A, Rimner A, Allen PK, et al. Treatment Modalities and Outcomes in Patients With Advanced Invasive Thymoma or Thymic Carcinoma: A Retrospective Multicenter Study. Am J Clin Oncol 2016;39:120-5. 10.1097/COC.0000000000000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Detterbeck FC. Evaluation and treatment of stage I and II thymoma. J Thorac Oncol 2010;5:S318-22. 10.1097/JTO.0b013e3181f20dab [DOI] [PubMed] [Google Scholar]
- 60.Sakai M, Onuki T, Inagaki M, et al. Early-stage thymic carcinoma: is adjuvant therapy required? J Thorac Dis 2013;5:161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fu H, Gu ZT, Fang WT, et al. Long-Term Survival After Surgical Treatment of Thymic Carcinoma: A Retrospective Analysis from the Chinese Alliance for Research of Thymoma Database. Ann Surg Oncol 2016;23:619-25. 10.1245/s10434-015-4825-4 [DOI] [PubMed] [Google Scholar]
- 62.Filosso PL, Ruffini E, Solidoro P, et al. Neuroendocrine tumors of the thymus. J Thorac Dis 2017;9:S1484-S90. 10.21037/jtd.2017.10.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Girard N, Mornex F, Van Houtte P, et al. Thymoma: a focus on current therapeutic management. J Thorac Oncol 2009;4:119-26. 10.1097/JTO.0b013e31818e105c [DOI] [PubMed] [Google Scholar]
- 64.Komaki R, Gomez DR. Radiotherapy for thymic carcinoma: adjuvant, inductive, and definitive. Front Oncol 2014;3:330. 10.3389/fonc.2013.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loehrer PJ, Sr, Chen M, Kim K, et al. Cisplatin, doxorubicin, and cyclophosphamide plus thoracic radiation therapy for limited-stage unresectable thymoma: an intergroup trial. J Clin Oncol 1997;15:3093-9. 10.1200/JCO.1997.15.9.3093 [DOI] [PubMed] [Google Scholar]
- 66.Fan XW, Yang Y, Wang HB, et al. Intensity-modulated Radiotherapy plus Etoposide/Cisplatin for Patients with Limited Advanced Unresectable Thymic Epithelial Tumors: A Prospective Phase II Study. Int J Radiat Oncol Biol Phys 2020;107:98-105. 10.1016/j.ijrobp.2019.12.045 [DOI] [PubMed] [Google Scholar]
- 67.Venuta F, Rendina EA, Pescarmona EO, et al. Multimodality treatment of thymoma: a prospective study. Ann Thorac Surg 1997;64:1585-91; discussion 1591-2. 10.1016/S0003-4975(97)00629-2 [DOI] [PubMed] [Google Scholar]
- 68.Chen YY, Huang CH, Tang Y, et al. Concurrent chemoradiotherapy for unresectable thymic carcinoma. Chang Gung Med J 2004;27:515-22. [PubMed] [Google Scholar]
- 69.Hishida T, Nomura S, Yano M, et al. Long-term outcome and prognostic factors of surgically treated thymic carcinoma: results of 306 cases from a Japanese Nationwide Database Study. Eur J Cardiothorac Surg 2016;49:835-41. 10.1093/ejcts/ezv239 [DOI] [PubMed] [Google Scholar]
- 70.Han L, Zhang B, Wu S. Successful concurrent chemoradiotherapy with cisplatin plus etoposide after incomplete resection for advanced thymic carcinoma. Oxf Med Case Reports 2019;2019:omz070. 10.1093/omcr/omz070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hao XJ, Peng B, Zhou Z, et al. Prospective Study of Stereotactic Body Radiation Therapy for Thymoma and Thymic Carcinoma: Therapeutic Effect and Toxicity Assessment. Sci Rep 2017;7:13549. 10.1038/s41598-017-12909-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arriagada R, Bretel JJ, Caillaud JM, et al. Invasive carcinoma of the thymus. A multicenter retrospective review of 56 cases. Eur J Cancer Clin Oncol 1984;20:69-74. 10.1016/0277-5379(84)90036-1 [DOI] [PubMed] [Google Scholar]
- 73.Zhu G, He S, Fu X, et al. Radiotherapy and prognostic factors for thymoma: a retrospective study of 175 patients. Int J Radiat Oncol Biol Phys 2004;60:1113-9. 10.1016/j.ijrobp.2004.05.013 [DOI] [PubMed] [Google Scholar]
- 74.Chen YD, Feng QF, Lu HZ, et al. Role of adjuvant radiotherapy for stage II thymoma after complete tumor resection. Int J Radiat Oncol Biol Phys 2010;78:1400-6. 10.1016/j.ijrobp.2009.09.066 [DOI] [PubMed] [Google Scholar]
- 75.Harnath T, Marx A, Ströbel P, et al. Thymoma-a clinico-pathological long-term study with emphasis on histology and adjuvant radiotherapy dose. J Thorac Oncol 2012;7:1867-71. 10.1097/JTO.0b013e3182745f73 [DOI] [PubMed] [Google Scholar]
- 76.Gomez D, Komaki R, Yu J, et al. Radiation therapy definitions and reporting guidelines for thymic malignancies. J Thorac Oncol 2011;6:S1743-8. 10.1097/JTO.0b013e31821ea60c [DOI] [PubMed] [Google Scholar]
- 77.Gomez D, Komaki R. Technical advances of radiation therapy for thymic malignancies. J Thorac Oncol 2010;5:S336-43. 10.1097/JTO.0b013e3181f20ea2 [DOI] [PubMed] [Google Scholar]
- 78.Ruffini E, Venuta F. Management of thymic tumors: a European perspective. J Thorac Dis 2014;6 Suppl 2:S228-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mornex F, Resbeut M, Richaud P, et al. Radiotherapy and chemotherapy for invasive thymomas: a multicentric retrospective review of 90 cases. The FNCLCC trialists. Fédération Nationale des Centres de Lutte Contre le Cancer. Int J Radiat Oncol Biol Phys 1995;32:651-9. 10.1016/0360-3016(95)00079-E [DOI] [PubMed] [Google Scholar]
- 80.Myojin M, Choi NC, Wright CD, et al. Stage III thymoma: pattern of failure after surgery and postoperative radiotherapy and its implication for future study. Int J Radiat Oncol Biol Phys 2000;46:927-33. 10.1016/S0360-3016(99)00514-3 [DOI] [PubMed] [Google Scholar]
- 81.Vogel J, Lin L, Simone CB, 2nd, et al. Risk of major cardiac events following adjuvant proton versus photon radiation therapy for patients with thymic malignancies. Acta Oncol 2017;56:1060-4. 10.1080/0284186X.2017.1302097 [DOI] [PubMed] [Google Scholar]
- 82.Vogel J, Berman AT, Lin L, et al. Prospective study of proton beam radiation therapy for adjuvant and definitive treatment of thymoma and thymic carcinoma: Early response and toxicity assessment. Radiother Oncol 2016;118:504-9. 10.1016/j.radonc.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 83.Fan C, Feng Q, Chen Y, et al. Postoperative radiotherapy for completely resected Masaoka stage III thymoma: a retrospective study of 65 cases from a single institution. Radiat Oncol 2013;8:199. 10.1186/1748-717X-8-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Girard N, Lal R, Wakelee H, et al. Chemotherapy definitions and policies for thymic malignancies. J Thorac Oncol 2011;6:S1749-55. 10.1097/JTO.0b013e31821ea5f7 [DOI] [PubMed] [Google Scholar]
- 85.Girard N. Chemotherapy and targeted agents for thymic malignancies. Expert Rev Anticancer Ther 2012;12:685-95. 10.1586/era.12.29 [DOI] [PubMed] [Google Scholar]
- 86.Korst RJ, Bezjak A, Blackmon S, et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: a phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg 2014;147:36-46.e1. 10.1016/j.jtcvs.2013.08.061 [DOI] [PubMed] [Google Scholar]
- 87.Kondo K. Optimal therapy for thymoma. J Med Invest 2008;55:17-28. 10.2152/jmi.55.17 [DOI] [PubMed] [Google Scholar]
- 88.Okuma Y, Saito M, Hosomi Y, et al. Key components of chemotherapy for thymic malignancies: a systematic review and pooled analysis for anthracycline-, carboplatin- or cisplatin-based chemotherapy. J Cancer Res Clin Oncol 2015;141:323-31. 10.1007/s00432-014-1800-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rajan A, Giaccone G. Chemotherapy for thymic tumors: induction, consolidation, palliation. Thorac Surg Clin 2011;21:107-14, viii. 10.1016/j.thorsurg.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 90.Schmitt J, Loehrer PJ, Sr. The role of chemotherapy in advanced thymoma. J Thorac Oncol 2010;5:S357-60. 10.1097/JTO.0b013e3181f21129 [DOI] [PubMed] [Google Scholar]
- 91.Lemma GL, Lee JW, Aisner SC, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol 2011;29:2060-5. 10.1200/JCO.2010.32.9607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hirai F, Yamanaka T, Taguchi K, et al. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol 2015;26:363-8. 10.1093/annonc/mdu541 [DOI] [PubMed] [Google Scholar]
- 93.Furugen M, Sekine I, Tsuta K, et al. Combination chemotherapy with carboplatin and paclitaxel for advanced thymic cancer. Jpn J Clin Oncol 2011;41:1013-6. 10.1093/jjco/hyr089 [DOI] [PubMed] [Google Scholar]
- 94.Maruyama R, Suemitsu R, Okamoto T, et al. Persistent and aggressive treatment for thymic carcinoma. Results of a single-institute experience with 25 patients. Oncology 2006;70:325-9. 10.1159/000097944 [DOI] [PubMed] [Google Scholar]
- 95.Weide LG, Ulbright TM, Loehrer PJ, Sr, et al. Thymic carcinoma. A distinct clinical entity responsive to chemotherapy. Cancer 1993;71:1219-23. [DOI] [PubMed] [Google Scholar]
- 96.Lucchi M, Mussi A, Ambrogi M, et al. Thymic carcinoma: a report of 13 cases. Eur J Surg Oncol 2001;27:636-40. 10.1053/ejso.2001.1197 [DOI] [PubMed] [Google Scholar]
- 97.Yoh K, Goto K, Ishii G, et al. Weekly chemotherapy with cisplatin, vincristine, doxorubicin, and etoposide is an effective treatment for advanced thymic carcinoma. Cancer 2003;98:926-31. 10.1002/cncr.11606 [DOI] [PubMed] [Google Scholar]
- 98.Igawa S, Murakami H, Takahashi T, et al. Efficacy of chemotherapy with carboplatin and paclitaxel for unresectable thymic carcinoma. Lung Cancer 2010;67:194-7. 10.1016/j.lungcan.2009.03.031 [DOI] [PubMed] [Google Scholar]
- 99.Koizumi T, Takabayashi Y, Yamagishi S, et al. Chemotherapy for advanced thymic carcinoma: clinical response to cisplatin, doxorubicin, vincristine, and cyclophosphamide (ADOC chemotherapy). Am J Clin Oncol 2002;25:266-8. 10.1097/00000421-200206000-00012 [DOI] [PubMed] [Google Scholar]
- 100.Kanda S, Koizumi T, Komatsu Y, et al. Second-line chemotherapy of platinum compound plus CPT-11 following ADOC chemotherapy in advanced thymic carcinoma: analysis of seven cases. Anticancer Res 2007;27:3005-8. [PubMed] [Google Scholar]
- 101.Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018;19:347-55. 10.1016/S1470-2045(18)30062-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cho J, Kim HS, Ku BM, et al. Pembrolizumab for Patients With Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J Clin Oncol 2019;37:2162-70. 10.1200/JCO.2017.77.3184 [DOI] [PubMed] [Google Scholar]
- 103.Lara PN, Jr, Bonomi PD, Faber LP. Retreatment of recurrent invasive thymoma with platinum, doxorubicin, and cyclophosphamide. Chest 1996;110:1115-7. 10.1378/chest.110.4.1115 [DOI] [PubMed] [Google Scholar]
- 104.Marom EM. Imaging thymoma. J Thorac Oncol 2010;5:S296-S303. 10.1097/JTO.0b013e3181f209ca [DOI] [PubMed] [Google Scholar]
- 105.Kumar V, Garg M, Goyal A, et al. Changing pattern of secondary cancers among patients with malignant thymoma in the USA. Future Oncol 2018;14:1943-51. 10.2217/fon-2017-0626 [DOI] [PubMed] [Google Scholar]
- 106.Araki T, Nishino M, Gao W, et al. Anterior Mediastinal Masses in the Framingham Heart Study: Prevalence and CT Image Characteristics. Eur J Radiol Open 2015;2:26-31. 10.1016/j.ejro.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as