Abstract
It is not known how specific the neural mechanisms underpinning empathy for different domains are. In the present study, we set out to test whether shared neural representations between first-hand pain and empathy for pain are pain-specific or extend to empathy for unpleasant affective touch as well. Using functional magnetic resonance imaging and psychopharmacological experiments, we investigated if placebo analgesia reduces first-hand and empathic experiences of affective touch, and compared them with the effects on pain. Placebo analgesia also affected the first-hand and empathic experience of unpleasant touch, implicating domain-general effects. However, and in contrast to pain and pain empathy, administering an opioid antagonist did not block these effects. Moreover, placebo analgesia reduced neural activity related to both modalities in the bilateral insular cortex, while it specifically modulated activity in the anterior midcingulate cortex for pain and pain empathy. These findings provide causal evidence that one of the major neurochemical systems for pain regulation is involved in pain empathy, and crucially substantiates the role of shared representations in empathy.
Keywords: empathy, fMRI, pain, placebo analgesia, touch
Introduction
There is an ongoing controversy about whether or not empathy relies on similar neuro-cognitive processes as the ones engaged when experiencing an emotion oneself (see review; Lamm et al. 2019). One specific discussion concerns whether overlapping neural activity observed during pain and empathy for pain indicates the same or different underlying processes (see review; Zaki et al. 2016). Several studies have consistently shown that experimentally induced analgesia not only reduces first-hand pain, but also reduces empathy for pain in a similar way and to a similar extent. This was associated with reduced amplitudes of pain event-related potentials (Rütgen, Seidel, Riecansky, et al. 2015a; Rütgen et al. 2018), and lower brain activation in areas associated with pain and pain empathy (Rütgen, Seidel, Silani, et al. 2015b). Furthermore, these effects are related to an opioidergic mechanism, as administration of the opioid antagonist naltrexone blocked the effects of placebo analgesia both for pain and pain empathy. Related findings have been reported for the painkiller acetaminophen, which reduces pain empathy as well (Mischkowski et al. 2016), and social learning about threats delivered to another person (Haaker et al. 2017). Together, such findings indicate that the brain processes pain and the witnessing of pain in another individual in a similar manner, thus lending support to the notion of shared representations between self and other (Zaki et al. 2016; Lamm et al. 2019).
From a conceptual and methodological point of view, the critical advance of these studies was the experimental manipulation of self-experienced pain, by means of cognitive and pharmacological manipulations. This would allow more specific and potentially causal conclusions on the neural and cognitive mechanisms underpinning the processing of other people’s pain, fostering evidence, and theory building that could potentially overcome the limitations of previous work that had predominantly been based on correlational methods (Lamm et al. 2011; Krishnan et al. 2016; Zaki et al. 2016). However, one key assumption that needs to be made in order to fully exploit the potential of this approach is that the experimentally induced placebo analgesia will act on pain specifically. However, if it (also) acts on domain-general processes, such as for example, the processing of unspecific negative affective states, then its effects on empathy might have resulted from a general blunting of affect. The findings would thus need to be interpreted differently, such as that pain empathy is grounded in the processing of negative affect, rather than in pain specifically. Testing the specificity for pain thus needs further causal manipulations and an assessment of their outcome on different domains or modalities. Related attempts have been successful in nonhuman animal research. Using a combination of electrophysiological recordings and transient lesions, a specific sharing of neural responses to self-experienced and vicariously experienced pain has recently been demonstrated in rats (Carrillo et al. 2019).
Assessing whether these findings translate to humans and their more complex models of affect sharing is thus a timely and imperative endeavor. The present rigorous investigation of the specificity of our own and related previous findings in humans is also motivated by ample indications that analgesia induced by either placebo or analgesics might have domain-general effects on negative and possibly even on positive affect, in addition to pain-specific effects. For instance, Koban and colleagues (2017) revealed that placebo analgesia also reduced the unpleasantness of romantic rejection, along with an increase of neural activation in the dorsolateral prefrontal cortex during both pain and rejection-related unpleasantness. In addition, a recent meta-analysis cast some doubts on the specificity of the effects of placebo analgesia on self-experienced pain (Zunhammer et al. 2018). By indicating that only moderate parts of reduced neural activation were related to early nociceptive processing, this analysis suggested that placebo analgesia may predominantly exert its effects by acting on other, possibly later aspects of the multidimensional experience of pain, such as affective and evaluative processes. These may include processes that are involved in different aspects of general emotional states and thus show limited specificity to the pain experience. Moreover, the opioidergic mechanism involved in placebo analgesia might partly operate via the reward system, and thus the modulation of positive affect (Scott et al. 2007). In line with this idea, it has been shown that remifentanil, a potent mu-opioid-receptor agonist, changed pleasantness ratings of affective pictures (Gospic et al. 2008).
In light of the doubts that these observations may cast on the previously assumed shared mechanism underlying analgesia and pain empathy, the present work tested the specificity of the effects of placebo analgesia on pain empathy (which were previously published; Rütgen, Seidel, Silani, et al. 2015b). Using functional magnetic resonance imaging (fMRI) and psychopharmacological administration experiments, we investigated whether placebo analgesia also reduces empathy for nonpainful negative and positive emotions induced by affective touch, as well as their first-hand experience. In brief, the results provide support for both domain-general and pain-specific mechanisms. Placebo analgesia was associated with reduced first-hand experience of unpleasant (but nonpainful) touch as well as empathy for such touch. Crucially, these effects were not modulated by the opioid system, as administration of the opioid antagonist naltrexone did not block them, while it clearly did for pain and pain empathy. Pain-specificity in placebo empathy analgesia was further corroborated by the fMRI findings, which showed no placebo effects on touch in the anterior midcingulate cortex (aMCC), while a modulation of activation in these areas was present for first-hand pain and empathy for pain. This suggests that the placebo effects on pain and unpleasant touch were driven by distinct neuro-cognitive and neuro-chemical processes, and highlights a specific role of the opioid system for sharing another person’s pain.
Materials and Methods
Participants
All participants were right-handed, had normal or corrected-to-normal vision and had no history of neurological or psychiatric disorders. Further exclusion criteria were past or present substance abuse, use of psychopharmaceuticals within the last 3 months, and pregnancy (all assessed by urine tests). Participants were recruited via advertisements posted at local universities. Written consent was obtained at the outset of the study, with the consent form including elements of deception regarding the experimental design, the placebo treatment, and the confederate. The study was approved by the local Institutional Review Board and was conducted according to the Declaration of Helsinki (World Medical Association 2013). All participants were reimbursed for their participation. The sample of the psychopharmacological experiment was independent of the sample of the fMRI experiment.
fMRI Experiment
A total of 120 healthy right-handed volunteers (Vienna university students) were randomly assigned to a control (n = 60; 38 females, 22 males) or a placebo group (n = 60, 43 females, 17 males). In total, 19 participants had to be excluded from the analysis, mainly because they were classified as nonresponders to the placebo manipulation (10 exclusions, see Supplement M1 for information on exclusion criteria), but also because of technical problems (such as partial malfunctioning of the fMRI scanner; 9 exclusions, 8 of them in the control group). All analyses reported in the present paper were carried out with the remaining 101 participants. The sample was identical to the one in Rütgen, Seidel, Silani, et al. (2015b), except for one female control subject, who did not complete fMRI scanning of the touch paradigm this paper is focusing on (final sample control group: n = 52, 33 females, 19 males; mean age ± SEM = 26.18 ± 0.61; placebo group: n = 49, 36 females, 13 males; mean age ± SEM = 24.59 ± 0.41).
Psychopharmacological Experiment
We used a double-blind, placebo-controlled between-subjects design. Fifty-seven healthy right-handed volunteers (Vienna university students; 28 female, 29 male, mean age ± SEM = 25.05 ± 0.41) were randomly assigned to a placebo-placebo (n = 29, 13 females, 16 males) or a placebo-naltrexone group (n = 28, 15 females, 13 males). Seven participants in total had to be excluded from the analysis, because of classification as nonresponders to the placebo manipulation (5 exclusions, 4 of them in the placebo-placebo group), or because of technical problems (2 exclusions in the placebo-naltrexone group). All analyses reported in the paper were carried out for the remaining 50 participants (identical sample as in Rütgen, Seidel, Silani, et al. (2015b); placebo-placebo group: n = 25, 12 females, 13 males, mean age ± SEM = 25.28 ± 0.75; placebo-naltrexone group: n = 25, 12 females, 13 males, mean age ± SEM = 25.00 ± 0.52.
Tasks
The following task descriptions apply to both the fMRI and the psychopharmacological experiment. To maximize comparability, all parameters (including timing and number of ratings) of the tasks were kept the same across both experiments.
Empathy for Pain Paradigm
In this task, short-lasting (500 ms) and individually calibrated painful or nonpainful electrical stimulation was delivered to participants, or to another person (a confederate of the experimenters). Trials were structured as follows: First, an arrow (2000 ms) indicated the target (self vs. other) of the upcoming stimulus. The intensity of the upcoming stimulus was indicated by the color of this arrow (red: painful vs. green: nonpainful). After a jittered blank screen (3500 ± 1500 ms), the electrical stimulus (500 ms) was delivered during simultaneous presentation of another visual delivery stimulus (1000 ms). The latter consisted of a picture of the confederate’s face, shown with either a painful or a neutral expression, or, in case of self-directed stimulation, scrambled versions of these pictures were shown to control for visual stimulation. Depending on the stimulus category, these pictures were accompanied by either a red (painful) or green (nonpainful) flash in the lower right corner of the picture. The delivery cue was followed by a fixation cross (5000 ± 2500 ms), and an optional rating (self-directed: one rating question; other-directed: two rating questions; 6000-ms answering time per each question). After self-directed stimulation, participants rated their own pain (self-directed pain ratings), using the question “How painful was this stimulus for you?” on a seven-point rating scale ranging from “not at all” to “extremely painful.” After other-directed stimulation, participants rated the other person’s pain (other-directed pain ratings; “How painful was this stimulus for the other person?” answered using the same seven-point rating scale as for the self-directed pain ratings), as well as their own unpleasantness during other-directed stimulation (unpleasantness ratings; “How unpleasant did it feel when the other person was stimulated?”; seven-point scale, from “not at all” to “extremely unpleasant”). Ratings were collected in about one third of the trials in a pseudorandomized fashion. Between trials, a fixation cross (2000 ms) was presented. In sum, 15 trials per condition (i.e., self-directed pain/no pain; other-directed pain/no pain) were presented. Participants were instructed to empathize with the other person.
For more details, see Rütgen, Seidel, Silani, et al. (2015b).
Touch Paradigm
Following the empathy for pain paradigm, we applied a touch paradigm (Silani et al. 2013; Lamm et al. 2015) including 15 pleasant, 15 unpleasant and 15 neutral stimuli in pseudo-randomized order (see also Fig. 1). This paradigm consisted of two separate runs: In the first run (self-directed affective touch), the participant was stimulated to measure behavioral responses and brain activation related to the first-hand experience of affective touch. In the second run (empathy for affective touch) a confederate acting as a second participant was supposedly undergoing affective touch, and participants were instructed to empathize with her feelings. In every single self-directed trial, visual presentation of an object was accompanied by simultaneous stroking of the left palm at 1 Hz for 2 s in proximal-to-distal direction with a material whose touch resembled the touch of the object depicted on the screen. For example, touching the participant’s hand with down feathers was accompanied by the picture of a chick to elicit a pleasant affective touch experience. The stimuli had been selected in extensive pretesting based on maximum agreement among participants in terms of congruency between visual and somatosensory stimulus and emotional responses (see Supplemental Results R1.1 for paradigm validation test). In one third of the trials (5 per condition), participants were asked to rate the stimulation in that trial on a 9-point scale ranging from very unpleasant (left extreme of the scale) to very pleasant (right extreme) for either themselves or, supposedly, for the other participant (i.e., the confederate). Each single trial consisted of a jittered fixation cross (5000 + −2000 ms), followed by visuo-tactile stimulation (2000 ms) and a jittered blank screen (1500 + −1000 ms). In trials with ratings, the rating was presented after the jittered blank screen for 5000 ms and was followed by another jittered blank screen (1500 + −1000 ms). Other-directed trials were identical apart from the absence of tactile stimulation of the participant, and the instruction that participants should empathize with their feelings.
Figure 1.
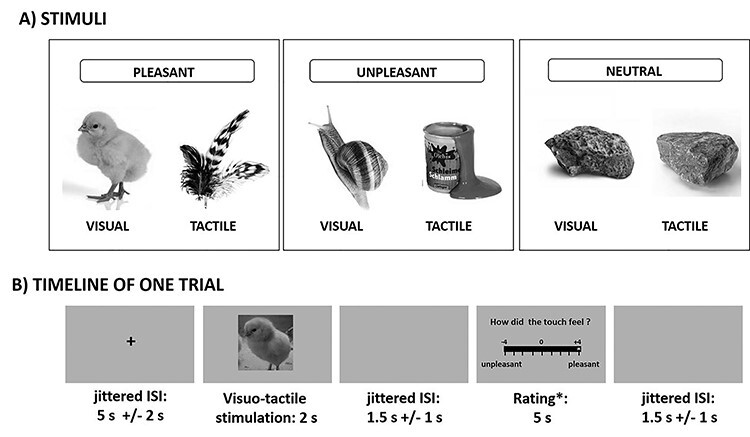
Stimulus examples and timeline. (A) Examples of stimuli of each valence (pleasant, unpleasant, neutral), depicting images and the actual object used for concurrent tactile stimulation. (B) Timeline of a single trial including a rating.
Procedures
After an initial screening procedure, participants who fulfilled inclusion criteria were invited to a single fMRI session (or behavioral session, in case of the psychopharmacological experiment). After being introduced to another participant, who was a confederate of the experimenters, they underwent a calibration procedure (for obtaining individually adjusted pain intensities for every single subject). Then, participants were instructed about the different parts of the experimental session, which were presented as two entirely independent experiments within the same study “to use scanning time efficiently and to reduce recruitment efforts.” Participants of the placebo group underwent a placebo analgesia induction procedure (see next section for details), and knew beforehand that they would receive pain medication. During the induction procedure, special care was taken not to suggest (explicitly or implicitly) any general effects on the processing of unpleasant affect, as our findings would be of limited conclusiveness otherwise. To avoid eliciting expectations of either positive or negative effects on empathic pain and on touch, we emphasized the independence of the experimental tasks and the potency of the medication as a painkiller without side effects. Also, during the instruction, we intentionally avoided addressing general effects of the medication beyond pain reduction, such as emotional blunting or reduced negative affect. Thus, the participants were expected to develop specific beliefs and expectations regarding their own pain processing, but not on somatosensory or affect processing, when directed neither to themselves nor to the other.
Then, both the participant and the confederate went into the scanner room, where the confederate was seated at a wooden table next to the scanner, with a computer screen replica placed on it. After the participant had been positioned in the scanner, the confederate left the scanner room without the participant being able to notice it. Instructions that were given over the loudspeakers were always addressed to both the participant and the confederate, to enhance the participant’s belief into the confederate’s ongoing presence in the scanner room. The participants went through the experimental tasks in the following order. First, the pain and empathy for pain paradigm (duration: 15 min) was completed. Then, the self-directed affective touch run (duration: 10 min) was conducted, followed by the empathy for affective touch run (duration: 10 min). The order of the touch runs was fixed in this way as prior experience with the touch items was necessary for participants to accurately empathize with the affective touch of the other person. A fixed order between the pain and touch tasks was chosen to maximize the homogeneity of placebo analgesia responses in the pain task. After an anatomical scan and resting state scan, the participant left the scanner, filled in post-experimental questionnaires, and was debriefed.
Placebo Induction in the fMRI Experiment
Full details on the placebo induction procedure can be found in Rütgen, Seidel, Silani, et al. (2015b). In short, participants in the placebo group were introduced to a medical doctor who administered a placebo pill presented as a highly effective as well as expensive (Waber et al. 2008) pain killer without side effects. They were told that this medication would considerably reduce their pain and that the aim of the study was to study its effects on brain activation. After 15 min waiting time, allegedly for the medication to take effect, the placebo analgesic effect was amplified by a procedure with several conditioning trials. Such combinations of verbal instructions and conditioning trials have been shown to be very effective in inducing robust placebo effects (see review; Wager and Atlas 2015), which last up to several hours or even days after successful conditioning (e.g., Colloca and Benedetti 2006; Colloca et al. 2010). Importantly, it was communicated explicitly to participants that they were the only ones receiving the analgesic, while the second participant (i.e., the confederate) would not receive any medication.
Placebo Induction in the Psychopharmacological Experiment
This experiment was largely identical to the fMRI experiment, but it involved additional administration of a pharmacological compound to half of the participants, in a double-blinded (between-subjects) fashion. The placebo analgesia induction procedure differed from the one in the fMRI experiment in one respect, which was that after the initial administration of a placebo pill and the conditioning procedure, participants received another pill (supposedly to strengthen the analgesic effects). This pill was the one that included either the opioid antagonist naltrexone, or starch (placebo). The rationale of this procedure was directly motivated by previous placebo analgesia research (see, e.g., Eippert, Bingel, et al. 2009a), and served to investigate opioidergically mediated placebo analgesia effects once induced by the administration of the inert pill and the conditioning procedure. Following the peak level at about 50 min after administration, naltrexone plasma levels have been shown to be stable for at least 2 h (Wall et al. 1981). In the present study, the touch paradigm immediately followed the empathy for pain paradigm, with a maximum starting point of 70 min after naltrexone administration. Thus, naltrexone medication effects were expected to persist over the whole course of the experimental tasks (which lasted 35 min in total), including the part when the affective touch task was performed.
fMRI Acquisition and Statistical Analysis
Data were acquired on a 3 T Siemens Tim Trio MRI System (Siemens Medical), using a multiband accelerated echoplanar imaging sequence (TE/TR = 33/1800 ms; voxel size 1.5 × 1.5 × 2 mm) (see details; Rütgen, Seidel, Silani, et al. 2015b). Data preprocessing was carried out in SPM12 (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm) using default settings unless specified differently. Preprocessing of data from the pain and the touch tasks were carried out in the exact same way. This included slice timing correction (reference = first slice), motion correction, spatial normalization to Montreal Neurological Institute (MNI) stereotactic space using an in-house scanner-specific EPI template, and spatial smoothing (6 mm Gaussian kernel). The scanner-specific template was generated from EPI data sets of 339 subjects all acquired on our own scanner using the iterative algorithm in the Advanced Normalization Tools (ANTs) toolbox (http://stnava.github.io/ANTs/). For this study, all individual data sets were normalized to this template and then transformed to MNI space using ANTs. Thresholds for excessive head movement were applied in accordance with acquired voxel size. Data analysis was performed based on a general linear model approach. The first-level design matrix of each subject contained 7 regressors: self-directed unpleasant touch, other-directed unpleasant touch, self-directed pleasant touch, other-directed pleasant touch, self-directed neutral touch, other-directed neutral touch, rating. For each condition, we modeled the 2 s time period of the affective touch and convolved them with SPM12’s standard canonical hemodynamic response function. Six realignment parameters for translation and rotation were included as additional nuisance regressors. Group statistics were calculated using second-level random effects analyses in SPM12.
In our previous work (Rütgen, Seidel, Silani, et al. 2015b), we had shown that placebo analgesia reduces activation in areas that had previously been related to empathy for pain. The aim of the present study was to test pain-specificity of these previous results and to identify general and modality-specific placebo effects for pain and touch in self-experience and empathy. Our general analysis approach was, thus, as follows: First, we analyzed whether placebo effects on the processing of unpleasant and pleasant touch stimuli could be observed at all (which was the case for unpleasant touch). Second, we tested whether the placebo effects related to affective touch engaged similar brain areas as the placebo effects related to pain. As for the first step, analyses of group differences were confined to a priori selected and independently determined areas of interest (AOIs) based on previous work. Selection of the AOIs was guided by our previous work (Lamm et al. 2015), which had revealed the insular and orbitofrontal cortices (OFC) as the main areas associated with processing self- and other-directed unpleasant and pleasant touch, respectively. Due to differences to our previous work regarding relevant experimental parameters (shorter stimulation and higher number of trials in our current work), we decided to tailor the AOIs to our current sample. Group differences regarding unpleasant touch were thus analyzed in an AOI based on the conjunction self-unpleasant ∩ other-unpleasant (in all participants of the fMRI experiment), restricted to anatomical left and right insular cortex (IC). Regarding pleasant touch, the AOI was based on the conjunction self-pleasant ∩ other-pleasant (in all participants), restricted to anatomical OFC (anatomical areas as delineated in the automated anatomical labeling atlas; Tzourio-Mazoyer et al. 2002; created using the WFU Pick atlas version 2.3; Maldjian et al. 2003).
For the second step, that is, to test for the overlap of areas associated with pain and unpleasant touch-related placebo effects, we performed group comparisons (control group > placebo group) in the anatomically defined bilateral IC (Tzourio-Mazoyer et al. 2002) (as above, using the WFU Pick atlas) and aMCC (Vogt 2016). The latter was created with ITK-Snap (Yushkevich et al. 2016) by restricting the bilateral anatomical cingulate cortex (automated anatomical labeling atlas, as implemented in WFU Pick atlas) by the aMCC borders as described by Vogt (2016), and comprised the aMCC in both hemispheres. Both the insula and the aMCC were revealed as crucial during both unpleasantness and pain processing in past studies (Rütgen, Seidel, Riecansky, et al. 2015a; Xu et al. 2020). We decided against using regions of interest from our previous work on placebo analgesia and empathy (Rütgen, Seidel, Silani, et al. 2015b), where we had extracted mean activation from regions of interest derived from a pain empathy meta-analysis (specifically, the conjunction self-pain ∩ other-pain; Lamm et al. 2011), to avoid biasing the analysis towards specific subregions associated with pain and pain empathy only. This analysis thus allowed us to draw conclusions about shared as well as distinct placebo effects in the two modalities, pain and touch. Based on previous work on placebo analgesia (Wager et al. 2004; Eippert, Bingel, et al. 2009a; Geuter et al. 2013), multiple comparison correction was based on a family-wise error rate (P < 0.05) using small volume correction (SVC, as implemented in SPM12), within the regions of interest (for SVC, we combined all three ROIs into one ROI and reported the resulting peaks).
On top of the analyses focusing on areas selected a priori, we also performed a complementary exploratory whole-brain analysis of group differences in the touch conditions. These analyses were corrected using FWE-correction with P < 0.05 on the cluster-level across the whole brain.
Behavioral Measures Analysis
Statistical analyses of behavioral measures were performed using SPSS 18.0 (Statistical Packages for the Social Sciences, Version 18.0, SPSS Inc.) and the level of significance was set to P < 0.05. Our analysis approach consisted of the following steps: First, we ran a repeated-measure ANOVA including the factor valence (pleasant vs. unpleasant) on the self-report ratings in the control group as a validity check of the paradigm. This check was successful and is reported in Supplement R1.1. Second, we focused on a priori planned comparisons to test our main hypothesis, which was that the placebo manipulation resulted not only in a reduction of the first-hand experience of touch, but also of empathy for affective touch. These planned comparisons consisted of two-tailed t-tests for independent samples, which first tested whether ratings delivered after (pleasant or unpleasant) affective touch of oneself differed between the placebo and the control group, and then whether this was also the case for ratings of empathy for affective touch. Finally, a third independent-samples t-test examined whether the predicted reduction of empathy was of similar size as the reduction of the self-directed experience (i.e., a t-test comparing the group difference placebo—control for self-directed affective touch, with the same difference for empathy for affective touch). We also performed a complimentary 2 (target) × 2 (valence) × 2 (group) mixed-model ANOVA (“group”: placebo vs. control, or placebo-placebo vs. placebo-naltrexone; “valence”: pleasant vs. unpleasant; “target”: self vs. other). This aimed at assessing whether the experimental conditions produced significant variation in the data, which was tested by follow-up pair-wise comparisons in case of significant main effects or interactions. Results of this ANOVA are reported in Supplement R1.2. Except for the first ANOVA (paradigm validation), the joint analysis of ratings related to unpleasant versus pleasant touch required a scale-transformation so that all ratings were on the scale with positive values. This was achieved by reversing the sign of ratings delivered during unpleasant stimulation (see also Silani et al. 2013; Rütgen, Seidel, Riecansky, et al. 2015a). Bayesian statistics were used to further qualify our findings: These allow testing of null hypotheses by assessing strength of evidence in favor of either H0 or H1 (Rouder et al. 2009; Keysers et al. 2020) and enabled us to estimate the odds in favor of having obtained true null results. In Bayesian statistics, probabilities are indicated by the so-called Bayes Factor BF10 = P(Data|H1)/P(Data|H0); BF01 = P(Data|H0)/P(Data|H1). Thus, BF01 > 1 means that the H0 is more likely than the H1. BF01 < 1/3 is commonly interpreted as evidence for the H1, while BF01 > 3 is usually interpreted as substantial evidence for the H0, though such absolute thresholds should be used with some caution (Lee and Wagenmakers 2014; Rouder et al. 2018). BF01,H(0,x) refers to a BF used to test the alternative hypothesis that there is a difference between groups, represented as a half-normal distribution with a standard deviation (SD) of x, against H0, the hypothesis of no difference (Dienes 2014). BF+0 and BF−0 indicate one-tailed tests. BFs can be interpreted continuously, with a BF of 4 providing twice as much evidence as compared with a BF of 2.
Bayesian t-tests were also used to test the similarity of placebo effects on pain and touch (both self and other, see “Comparing placebo effects on pain and touch”) and to follow up nonsignificant effects of the psychopharmacological manipulation on affective touch (H0: Naltrexone-effect on pain, but not on unpleasant touch vs. H1: Naltrexone having an effect on both modalities, pain and touch). For the latter, we used an evidence-based prior (between-groups pain effect size from the psychopharmacological experiment) for testing these hypotheses in both self-directed (x = 0.61) and other-directed (x = 0.69) unpleasant touch data. On top, we performed three additional analyses per target (self/other), using different SDs, depending on prior effect sizes (results of these additional tests are reported in Supplement R1.5): in a similar fashion as for the first evidence-based prior, we used the between-groups pain effect size priors from the fMRI experiment (self-directed: x = 0.72; other-directed: x = 0.53) to estimate the probability of the data under the two hypotheses. Second, in order to compare the strength of the between-group effects for self-directed unpleasant touch in the fMRI versus the psychopharmacological experiment, we used the between-groups (=placebo effect) unpleasant touch effect size prior in the fMRI experiment (self-directed: x = 0.58; other-directed: x = 0.53). Third, we used an objective prior of x = 0.707. Bayes factors (BFs) were computed with JASP (JASP-Team 2020). A repeated measures ANOVA with the within-subjects factors “paradigm” (pain vs. touch) and “target” (self vs. other), and the between-subjects factor “group” (placebo-naltrexone vs. placebo-placebo) was conducted as a complementary frequentist analysis.
Results
Our analysis approach for identifying common versus distinct neural mechanisms underlying pain, touch, and their empathic experience, consisted of three steps: First, we tested for placebo effects on affective touch (“Placebo effects on affective touch”). Second, the psychopharmacological experiment aimed to provide causal evidence whether an opioidergic mechanism was also underpinning the placebo effects on affective touch and empathy, as it did for pain (“Opioidergic modulation of placebo effects”). Third, assessing the correspondence in brain activations related to placebo effects on pain and touch, and their empathic experience, allowed us to corroborate the findings from the opioidergic modulation experiment (“Domain-general and pain-specific placebo effects on brain activation”).
Behavioral Results—fMRI Experiment
Placebo Effects on Affective Touch
Planned comparisons for unpleasant touch revealed reduced unpleasant affect ratings in the placebo compared with the control group, in both self- and other-directed ratings (self-directed: t(99) = 2.42, P = 0.017, d = 0.48, BF10 = 2.75, mean ratings ± SEM: control group = −2.98 ± 0.14, placebo group = −2.40 ± 0.19; other-directed: t(99) = 2.67, P = 0.009, d = 0.53, BF10 = 4.75, mean ratings ± SEM: control group = −3.62 ± 0.14, placebo group = −3.04 ± 0.17; see Fig. 2A). The magnitude of these effects did not differ (t(99) = 0.013, P = 0.989, BF01 = 4.76), indicating that the placebo manipulation reduced the unpleasantness of first-hand touch and its empathic experience to a similar extent. Placebo analgesia had no effect on pleasant touch ratings, neither when touch was directed to the self (P = 0.87, BF01 = 4.71; mean ratings ± SEM: control group = 2.79 ± 0.16, placebo group = 2.83 ± 0.15) or to the other person (P = 0.28, BF01 = 2.85; mean ratings ± SEM: control group = 2.86 ± 0.14, placebo group = 3.05 ± 0.12). See Supplemental Results R1.2 for complete factorial analysis, and R1.1 for results of the successful paradigm validation check.
Figure 2.
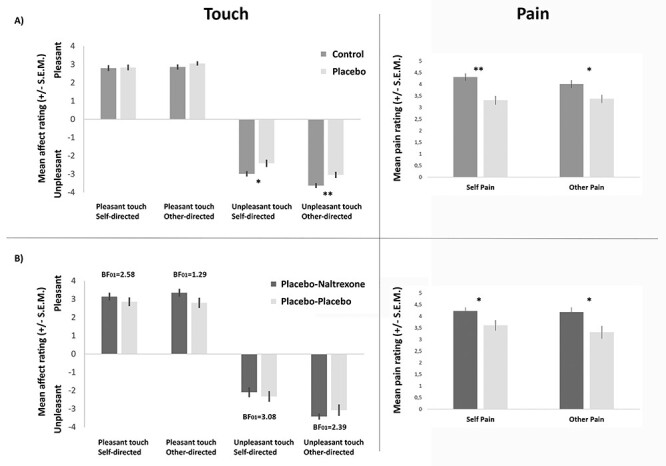
Behavioral results of the two experiments, showing that while placebo analgesia reduced unpleasantness of touch ratings, this was not underpinned by an opioidergic mechanism. Left panels: touch data. Right panels: pain data. (A) fMRI experiment (NControl/Placebo = 52/49): ratings of pleasant touch revealed no group differences, while the unpleasantness of both self- and other-directed unpleasant touch was significantly reduced in the placebo group, similar to pain and pain empathy. (B) Psychopharmacological experiment (N = 25/25): administration of the opioid antagonist naltrexone had no influence on ratings of affective touch in any of the four conditions (all P-values > 0.123), while it increased pain and empathy for pain. Asterisks (*P < 0.05, **P < 0.01) mark significant planned comparisons (independent samples t-tests, see text for detailed results). BF01 values are BFs in favor of the null hypothesis (i.e., no effect of naltrexone).
Comparing Placebo Effects on Pain and Touch
To test whether placebo effects on unpleasant touch were comparable with those on pain, we performed Bayesian t-tests on unpleasant touch, employing effect size priors from pain. These analyses revealed BF0+,H(0,1.02) = 0.22, for self-directed unpleasant touch, and BF0+,H(0,0.64) = 0.10 for other-directed unpleasant touch. This indicates placebo effects of similar extent in the two modalities, for both the self- and the other-directed conditions.
Behavioral Results—Psychopharmacological Experiment
Opioidergic Modulation of Placebo Effects
Planned comparisons testing whether naltrexone administration led to modulation of placebo effects on self- or other-directed affective touch revealed no significant effects in any of the conditions (unpleasant: self t(48) = −0.577, P = 0.566, BF01 = 3.08, mean ratings ± SEM: placebo-naltrexone group = −2.09 ± 0.26, placebo-placebo group = −2.31 ± 0.29; other t(36.84) = 0.974, P = 0.337, BF01 = 2.39, mean ratings ± SEM: placebo-naltrexone group = −3.41 ± 0.16, placebo-placebo group = −3.07 ± 0.31; pleasant: self t(48) = 0.876, P = 0.385, BF01 = 2.58, mean ratings ± SEM: placebo-naltrexone group = 3.14 ± 0.21, placebo-placebo group = 2.85 ± 0.23; other t(48) = 1.571, P = 0.123, BF01 = 1.29, mean ratings ± SEM: placebo-naltrexone group = 3.35 ± 0.21, placebo-placebo group = 2.79 ± 0.28; see Fig. 2B). See Supplemental Results R1.4 for complete factorial analysis. This was in stark contrast to the previously reported effects on pain and pain empathy, where substantial and significant effects had been found, in the same participants. To assess the likelihood of a true null finding for naltrexone effects on unpleasant touch (H0) over a possible effect on both pain and unpleasant touch (H1: similar effects of naltrexone on both modalities), we thus carried out Bayesian t-tests on the group differences for unpleasant touch in the psychopharmacological experiment, using evidence-based distributions as priors (as suggested by Gronau et al. 2017). Using effect sizes of naltrexone on placebo effects for pain as priors resulted in BF01,H(0, 0.61) = 2.76 for self-directed unpleasant touch (i.e., favoring H0 over H1 by 2.76 times), and BF01,H(0, 0.69) = 2.35 for other-directed unpleasant touch. See Supplement R1.5 for additional Bayesian analyses supporting these results when incorporating priors based on other evidence, as well as neutral priors. These analyses suggest that naltrexone had no modulatory effects on first-hand or empathic experiences of unpleasant affective touch, in comparison with pain, where it clearly did. Also, see Supplement R1.6 for an alternative frequentist analysis incorporating both pain and touch data, which yielded similar results.
Comparison of Placebo Effects in fMRI and Psychopharmacological Experiments
Since the fMRI and the psychopharmacological experiments had been performed in different samples, the lack of naltrexone effects in the psychopharmacological experiment could in principle be related to failure in inducing robust placebo analgesia effects in the latter. To assess this possible confound, we compared the placebo-placebo group of the psychopharmacological experiment to the placebo and the control groups of the fMRI experiment. There was no difference between the two groups that had undergone a more or less identical placebo induction procedure: ratings in the placebo–placebo group from the psychopharmacological experiment and from the placebo group in the fMRI experiment did not differ, regarding neither unpleasant nor pleasant touch (unpleasant self: t(72) = 0.085, P = 0.806, BF01 = 3.86; other: t(72) = 0.093, P = 0.926, BF01 = 3.95; pleasant self: t(72) = 0.246, P = 0.932, BF01 = 3.95, other: t(72) = 0.994, P = 0.323, BF01 = 2.60). However, the placebo-placebo group from the psychopharmacological experiment significantly differed from the control group of the fMRI experiment regarding unpleasantness ratings (self: t(75) = 2.323, P = 0.023, d = 0.56, BF10 = 2.40; other: t(33.36) = 1.618, P = 0.115, d = 0.42, BF10 = 1.13), but not regarding pleasant touch ratings (self: BF01 = 3.93; other: BF01 = 3.91). We also compared the placebo effects within and across experiments with a Bayesian approach. Using effect size priors of placebo effects in the fMRI experiment, we calculated BF0+ in a Bayesian one-sided t-test on the group difference between fMRI-control group and placebo-placebo group (psychopharmacological), which revealed BF0+, H(0, 0.47) = 0.19 for self-directed unpleasant touch, and BF0+, H(0, 0.53) = 0.41 for other-directed unpleasant touch, which indicates similarly strong placebo effects in the psychopharmacological experiment. Taken together, these results suggest that the placebo induction in the psychopharmacological experiment had similarly strong effects on touch as in the fMRI experiment, ruling out an alternative explanation for the lack of naltrexone effects on unpleasant touch. Neutral touch ratings were neither significantly influenced by placebo analgesia (all P-values > 0.662) nor by naltrexone (all P-values > 0.225).
Imaging Results
Placebo Effects on Affective Touch
The first set of fMRI analyses, which aimed at revealing the neural correlates of placebo analgesia effects on affective touch (tested in a priori selected and independently determined AOI based on previous work (Lamm et al. 2015); see methods for details), revealed lower activation during self-directed unpleasant touch in the placebo group in several parts of the bilateral anterior insula (contrast [self-unpleasant: control group > placebo group], P < 0.05, small-volume family-wise error corrected [SVC-FWE]). For other-directed unpleasant touch, a similar group difference was indicated in the right anterior insula (contrast [other-unpleasant: control group > placebo group], P < 0.05, SVC). See Table 1 for further details. Analyses of group differences for pleasant touch revealed no significant effects, which is in line with the absence of behavioral effects ([self-pleasant: control group > placebo group] and [other-pleasant: control group > placebo group]). None of the reverse contrasts (testing for higher activation in the placebo group) showed significant results either.
Table 1.
Significant activation when testing for the neural correlates of placebo effects on unpleasant touch (contrasts: self-unpleasant: control > placebo group, and other-unpleasant: control > placebo group) and pleasant touch (contrasts: self-pleasant: control > placebo group, and other-pleasant: control > placebo group), small-volume-corrected P < 0.05 in bilateral anterior IC = insular cortex (unpleasant touch)/orbitofrontal cortex (pleasant touch), k = cluster size, MNI = Montreal Neurological Institute stereotactic space
| P(SVC-FWE) | k | t-value | x,y,z (MNI) | Anatomical region |
|---|---|---|---|---|
| Self-unpleasant: control > placebo group | ||||
| 0.02 | 83 | 3.61 | −36 12 0 | Left anterior IC |
| 0.044 | 28 | 3.35 | −26 28 8 | Left anterior IC |
| 0.012 | 71 | 3.72 | 32 28 0 | Right anterior IC |
| Other-unpleasant: control > placebo group | ||||
| 0.001 | 90 | 4.48 | 36 28 6 | Right anterior IC |
| Self-pleasant: control > placebo group | ||||
| No voxels surviving threshold. | ||||
| Other-pleasant: control > placebo group | ||||
| No voxels surviving threshold. | ||||
Complementary Exploratory Whole-Brain Analyses
The contrast (self-unpleasant: control group > placebo group) revealed activation in the fusiform gyrus, left primary and bilateral secondary somatosensory cortex, anterior insula and posterior insula, and the contrast (other-unpleasant: control group > placebo group) in the left fusiform gyrus and right secondary somatosensory cortex (for details refer to Supplement, Supplementary Tables S1 and S2). The corresponding contrasts for pleasant touch indicated activation differences in visual areas (fusiform gyrus, middle occipital gyrus; see Supplement, Supplementary Tables S3 and S4). The reverse contrasts (all conditions: placebo group > control group) did not reveal any significant differences.
Domain-General and Pain-Specific Placebo Effects on Brain Activation
To assess whether the effects of placebo analgesia on unpleasant touch were underpinned by similar or distinct areas as those modulated by placebo analgesia during pain processing, we tested for joint activations in pain and touch in the anatomically defined bilateral insula and aMCC. In the bilateral insula, this revealed reduced activation in the placebo group during pain and touch, both when self- and other-directed; these effects partially overlapped for self-directed pain and touch, but not at all for other-directed pain and touch. In the aMCC, however, no placebo effects on touch were found, while largely overlapping clusters were found for self- and other-directed pain. Figure 3 visualizes the domain-general along with modality-specific placebo effects resulting from these analyses (for full details on cluster size, location, and statistical estimates, see Table 2).
Figure 3.
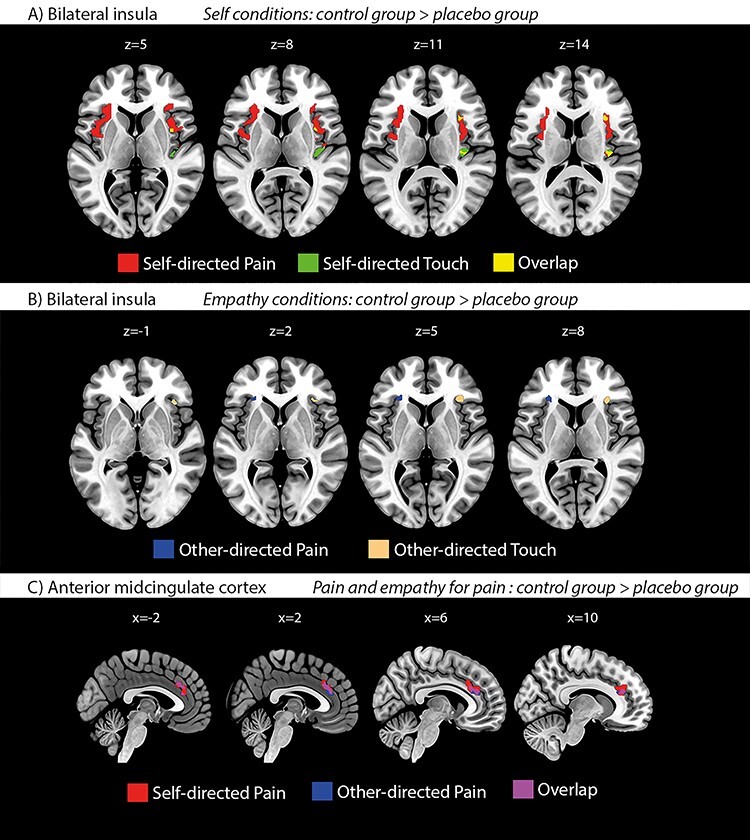
(A + B) Mass-univariate fMRI results of placebo effects on pain and unpleasant touch in the bilateral insula, demonstrating both domain-general and modality-specific placebo effects. Activation maps illustrate both overlapping (pain and touch; yellow) and nonoverlapping effects of placebo analgesia on self- (A; maps in upper row; red = pain; green = touch) and other-directed (B; maps in lower row; blue = pain; beige = touch) pain and touch, respectively (thresholded at P < 0.05, small-volume-corrected in bilateral insula). (C) Mass-univariate fMRI results of placebo effects on self- and other-directed pain in the aMCC, showing largely overlapping placebo effects for self- and other-directed pain. Importantly, this contrasts with the absence of such effects on unpleasant touch in this region (no activations surviving threshold). Activation maps illustrate effects of placebo analgesia on self-directed (red) and other-directed (blue) pain (overlap in purple). Thresholded at P < 0.05, small-volume-corrected in aMCC.
Table 2.
Significant activation when testing for placebo analgesia effects in unpleasant touch and pain in bilateral IC and aMCC
| PFWE-corr. | k | t-value | x,y,z (MNI) | Anatomical region |
|---|---|---|---|---|
| (A) Unpleasant touch placebo effects: | ||||
| Self-unpleasant touch: controls > placebo | ||||
| <0.001 | 80 | 5.29 | 38 –20 10 | Right posterior IC |
| Other-unpleasant touch: controls > placebo | ||||
| 0.008 | 56 | 4.48 | 36 28 6 | Right anterior IC |
| (B) Pain placebo effects: | ||||
| Self-pain: controls > placebo | ||||
| <0.001 | 590 | 5.25 | −8 16 30 | aMCC |
| <0.001 | 463 | 6.82 | −26 26 8 | Left anterior IC |
| <0.001 | 307 | 6.44 | 40 14 4 | Right anterior IC |
| <0.001 | 43 | 5.89 | 30 28 8 | Right anterior IC |
| 0.006 | 28 | 4.57 | 32 –18 16 | Right posterior IC |
| 0.007 | 51 | 4.55 | 38 –14 0 | Right posterior IC |
| 0.010 | 35 | 4.47 | 32 18 –12 | Right anterior IC |
| Other-pain: controls > placebo | ||||
| 0.023 | 232 | 4.23 | 16 36 18 | aMCC |
| 0.048 | 52 | 4.02 | −8 18 26 | aMCC |
| 0.001 | 41 | 4.75 | −26 30 2 | Left anterior IC |
Notes: (A) Significant clusters resulting from the contrasts (self-unpleasant: control group > placebo group) and (other-unpleasant: control group > placebo group). (B) Significant clusters resulting from the contrasts (self-pain: control group > placebo group) and (other-pain: control group > placebo group) (small-volume-corrected P < 0.05; k = cluster size, MNI = Montreal Neurological Institute stereotactic space).
In summary, the fMRI analyses revealed, first, the neural correlate of the selective placebo effects on unpleasant, but not on pleasant touch; second, modality-specific placebo modulation of pain and its empathic experience in the aMCC, where, in contrast, no placebo modulation of unpleasant touch was found; third, partially similar placebo modulation of self-directed pain and touch in the bilateral IC, but lateralized effects for other-directed pain (left AI) and touch (right AI).
Discussion
A series of previous studies using a variety of behavioral and neuroscience methods had consistently indicated that placebo analgesia also reduces empathy for pain (Rütgen, Seidel, Riecansky, et al. 2015a; Rütgen, Seidel, Silani, et al. 2015b; Rütgen et al. 2018). Based on these results, it had been suggested that empathy for pain is grounded in self pain, that is, it recruits neural functions that also underpin first-hand pain processing (see review; Lamm et al. 2019). The present work set out to test the specificity of these findings, as previous research, including our own, would not allow to rule out the interpretation that reduced empathy resulted from a domain-general (i.e., applying to negative affective states in general vs. being specific to pain) blunting of (negative) affect by placebo analgesia. The findings reported here suggest both domain-general and modality-specific mechanisms. For one thing, the behavioral and neural data of the fMRI experiment suggest domain-general effects, by revealing that induction of placebo analgesia not only acted on pain and pain empathy, but that it also reduces negative affect resulting from unpleasant touch, and empathy for such touch. However, two observations speak for additional modality-specific mechanisms and crucially complement these findings. First, the placebo effects on unpleasant touch were not modulated by causally manipulating opioidergic activity, by means of administration of the opioid antagonist naltrexone. Since previous results in the same sample of participants had shown such a modulation for pain and pain empathy, this suggests specificity of placebo effects on pain with regard to the underlying neurochemical mechanisms. Second, while placebo effects for both pain and touch were found in the insula, activity in the aMCC was specifically modulated by placebo for pain and empathy for pain, but not for touch, pointing towards a pain-specific mechanism and corroborating the psychopharmacological results by providing insights into the brain areas underpinning the distinct neurochemical mechanism.
We will now discuss these findings in some more detail. Behavioral data of both experiments indicate that unpleasant touch was experienced as less unpleasant in participants who had undergone placebo analgesia than in participants from the control group. Interestingly, empathy for such touch was also reduced, extending previous findings linking reduced first-hand affect processing to a reduced sharing of another person’s affect to the domain of unpleasant touch. The placebo manipulation also showed similarly strong effects in both modalities (pain and touch) and for both conditions (self and empathy). In addition, the absence of effects related to pleasant touch contradicts the hypothesis of a generalized blunting of affect by placebo analgesia.
Since our previous work had consistently indicated an opioidergic mechanism for the effects of placebo analgesia on both pain and pain empathy (Rütgen, Seidel, Silani, et al. 2015b; Rütgen et al. 2018), the psychopharmacological experiment was crucial in terms of testing whether the domain-general effects on unpleasant affect were indeed underpinned by similar neural mechanisms. This experiment, however, showed that opioidergic blockade had no impact on how placebo affects the processing of unpleasant touch, contrasting the findings in the domain of pain. Importantly, the Bayesian analyses (as well as the complementary frequentist analysis) across modalities were specifically tailored to avoid unjustified conclusions solely based on significant differences in one, but not in the other condition (Nieuwenhuis et al. 2011). These analyses further supported the interpretation of an absence of effects of naltrexone on unpleasant touch, rather than a lack of sensitivity to pick them up. This was shown in both a within-subject comparison and a between-subject comparison with the larger fMRI sample. Directly comparing the between-groups effects on unpleasant touch found in the fMRI (effects induced by placebo analgesia) and the psychopharmacological experiment (effects induced by naltrexone) also revealed a higher probability for a null-effect in the latter. Complementary frequentist analyses confirmed these results. Note though that some of the BFs were in a range (<3) that is considered only anecdotal evidence for the H0, which calls for replication in larger samples. Moreover, the two groups that had undergone placebo analgesia were comparable. Taken together, this indicates that the absence of effects by the opioid antagonist cannot be explained by insufficient analgesia induction, or that the two samples are not comparable to start with.
The fMRI data revealed that placebo effects on the processing of unpleasant affect were associated with activation differences in the insula, in a subdivision previously related to the processing of unpleasant touch (Lamm et al. 2015). Comparing pain- and unpleasant touch-related placebo effects revealed both overlapping and distinct subdivisions in the IC, but pain-specific effects in the aMCC. The overlapping activations could be interpreted as a neural correlate of the domain-general effects, while the modality-specific effects suggest that (1) the insula contains parts that are specifically and distinctly related to the representation of pain and touch, and that (2) the aMCC is exclusively (in the sense of not including touch) involved in the placebo modulation of pain. This is in line with recent fMRI findings showing that specific parts of the anterior insula seem to specifically code for pain expectations, and not for the domain-general processing of aversive affect (Fazeli and Büchel 2018). In consistence with that, we found pain-specific involvement of the left AI. The modality-specific placebo modulation of pain in the aMCC is an especially noteworthy observation, considering its high density of mu-opioid receptors (Baumgärtner et al. 2006; Kantonen et al. 2020), along with recent reports of pain-specific multivariate representations in the aMCC (Kragel et al. 2018), which were clearly separable from negative emotion representations (that were represented in the ventromedial prefrontal cortex). Hence, these findings may pinpoint aMCC as the specific area mediating the modulation of opioidergic activity, seen in the psychopharmacological experiment. However, such a conclusion would require direct testing in a combined psychopharmacological and fMRI experiment. Also, absence of evidence for unpleasant touch modulation in the present study does not fully exclude a potential role of the aMCC in placebo effects on unpleasant touch.
From these findings, we thus infer moderate but consistently stronger evidence for an opioidergic mechanism in the domain of pain compared with the domain of unpleasant touch. Note that these findings are based on rather large sample sizes for both fMRI and psychopharmacological research, and a within-subject design, thus excluding the potential confound that between-sample variation might have caused the different effect patterns for pain and touch.
We will now put our findings in a broader perspective, with respect to the phenomena of empathy and placebo analgesia. Theoretical accounts of shared neural representations between first-hand and empathic affective experiences have recently gained momentum through evidence from animal research and multivariate analysis approaches. A study by Corradi-dell’Acqua and colleagues revealed a significant role of the anterior insula and the midcingulate cortex in processing both pain and disgust experiences related to self as well as others (Corradi-Dell’Acqua et al. 2016). Crucially, in the same study, the authors also showed modality and target specific neural responses, indicating (similar to the present study) both domain-generality and modality-specificity. In their study, they used a multivariate analysis approach, targeting fine-grained neural patterns. In contrast, Krishnan and colleagues employed a similar technique and found no shared neural patterns for first-hand and vicarious pain experience (Krishnan et al. 2016). This divergence could be caused by the nature of the employed tasks. While the former study employed closely matched cue-based tasks for self- and other-related experiences, Krishnan and colleagues compared neural responses to thermal pain (cue-based self-related pain task) with neural responses to imagining oneself being in a painful situation depicted on the screen. These tasks obviously differ in their complexity and the involved cognitive demands, and may therefore have biased the analysis towards the emergence of differences, rather than similarity. Moreover, the resolution of fMRI might be too low to identify shared activations even with the refined multivariate approach. This is why Carrillo and colleagues employed single-cell recordings and pharmacological “lesions” in a rat model of empathy (Carrillo et al. 2019). They elegantly demonstrated that a multivariate decoder can successfully predict self-experienced pain after being trained on observations of a conspecific in pain. This was specifically related to single neuron activities in the cingulate cortex, as shown by transient lesions of this region. These lesions further affected pain only, and not negative affect (fear) in general, which parallels our finding of pain-specific placebo modulation of the aMCC.
Placebo treatments—that is, the creation of expectations about effects in the absence of any physical or pharmacological treatment—are powerful tools for both basic research and clinical application (see reviews Benedetti and Amanzio 2013; Wager and Atlas 2015; Schwarz et al. 2016). They have been especially well-studied within the subfield of placebo analgesia (or, more precisely, hypoalgesia; Büchel et al. 2014). Also, placebo treatments have major effects on general emotional processes such as unpleasantness induced by threatening pictures (Petrovic et al. 2005). A recent meta-analysis suggested that placebo analgesia exerts only a small effect on early sensory components of pain processing that are closely associated with the nociceptive signal. Instead, there seems to be a larger placebo effect on domain-general phenomena such as stress, subjective affect and cognitive evaluation (Zunhammer et al. 2018). Our neuroimaging data indicate that the cross-modal placebo effect relies on this rather domain-general unpleasant affect mechanism associated with activation of the IC. Some findings on the whole-brain level (reported in Supplementary Tables S1 and S2) are though interesting to note: placebo analgesia led to a downregulation of the fusiform gyrus during both self-experienced and empathic unpleasant touch. Decreased activity in the fusiform gyrus in response to unpleasant pictures has previously been found in placebo analgesia studies and has been related to treatment expectancies (Petrovic et al. 2005). Decreased activity in somatosensory areas during self-experienced unpleasant touch is in line with the previously demonstrated role of somatosensory areas in attributing emotional value to self-directed affective touch (Gazzola et al. 2012). Another interesting finding on the whole-brain level is the downregulation of the inferior frontal gyrus during empathic unpleasant touch. The inferior frontal gyrus has been shown to be relevant for evaluating social signals (e.g., interpreting smiles, see Paracampo et al. (2017)). Predictive coding processes have been suggested to play a fundamental role in placebo responses (Petrovic et al. 2010; Büchel et al. 2014; Grahl et al. 2018). Predictive coding suggests that models of the world and the self (priors) are compared with input (e.g., nociceptive signals) and generate error signals in case of mismatch. The error signals may change the priors but also how the input is further processed. The subjective experience is then influenced both by the priors and the input signal. Expectations induced in a placebo treatment may be viewed as priors (Petrovic et al. 2010; Büchel et al. 2014). In the present dataset, predictive coding may explain why there is no effect of the placebo analgesia induction on pleasant touch (as it is not part of the prediction) but some effect on unpleasant touch (as there are overlapping components in the underlying information processing with pain processing). However, although a prediction may be relevant for both pain and unpleasant touch, this does not equal that the same modulating system (e.g., endogenous opioid system) is used to influence the underlying processing to be more in accord with the predictions.
Opioids play a prominent role in pain regulation. One of their main roles seems to be to engage the descending pain modulation system, which enables regulation of pain by inhibitory influence on early (spinal) nociceptive signaling (Fairhurst et al. 2007; Eippert, Finsterbusch, et al. 2009b). In addition, opioids supposedly exert their analgesic effects by acting on cerebral structures and thus possibly on more downstream consequences of the nociceptive signals (Eippert, Bingel, et al. 2009a). Notably, anterior insula and aMCC are distinctly activated by opioids (Petrovic et al. 2002; Wise et al. 2002; Leppä et al. 2006) and have high opioid receptor concentration (Willoch et al. 2004)—making these regions not only key regions in processing pain and affective experience but also highly malleable to opioid regulation. Placebo analgesia acts via a number of cognitive and neural mechanisms that are not yet entirely understood. What seems clear though is that with respect to the neurochemical underpinnings, the opioid system plays a prominent role in placebo analgesia (see reviews; Benedetti and Amanzio 2013; Peciña and Zubieta 2015; Wager and Atlas 2015), but also other neurochemical mechanisms seem to be involved, such as the endocannabinoid system (Benedetti et al. 2011). For instance, studies using opioid receptor imaging measured by positron emission tomography (PET) have consistently indicated that placebo analgesia involves increased opioid activity in anterior insula and aMCC (Zubieta et al. 2005; Wager et al. 2007; Peciña and Zubieta 2015). Moreover, early evidence suggested that the blockade of opioidergic neurotransmission attenuated placebo analgesia (Levine et al. 1978; Amanzio and Benedetti 1999), complemented by more recent findings directly indicating that brainstem and spinal mechanisms indeed are engaged in this blockade (Eippert, Finsterbusch, et al. 2009b). The core finding of our study is that blockade of the opioid system only affects pain and pain empathy but not unpleasant touch. It suggests that one of the hallmark features of pain, that is, the involvement of the endogenous opioid system, seems irrelevant for the first-hand experience of unpleasant touch and empathy for such touch (or, to be more conservative, much less relevant than for pain). Thus, domain general effects on affect processing cannot solely explain our previously shown effects of placebo analgesia on empathy for pain. This would also be in line with recent correlational PET evidence indicating increased opioidergic activity during the observation of pain in others (Karjalainen et al. 2017), and a recent study showing that naltrexone interferes with vicarious learning of pain (Haaker et al. 2017). Taken together, our study thus provides another important piece of information that empathy for pain engages similar neuro-cognitive mechanisms as the first-hand experience of pain.
While the present study crucially expands the insights of previous work, the following limitations also require some attention and should be addressed in future studies. First, we did not counterbalance the order of the parts of the experiments involving pain and affective touch (see Methods). This arbitrary decision, which was motivated by our primary focus on the pain task and the cover-story (i.e., the study being about effects of a painkiller on brain activity during pain processing), could have been problematic if the effects either of the placebo analgesia or of the opioid blockade had been waning over time. However, it is unlikely that this was the case. Corollary analyses of pain and unpleasant touch ratings at the outset and the end of both tasks revealed similar degrees of placebo analgesia (see Supplemental Results R1.3). Naltrexone, on the other hand, shows receptor binding that by far outlasts the hour within which we performed both parts of the experiment (Lee et al. 1988).
Second, one might argue that the intensity of pain and unpleasant touch were insufficiently matched, so that possible differences between domains could be explained, for example, by differences in salience (Mouraux et al. 2011). However, this would only account for the similar effects found in the fMRI experiment, but not in the psychopharmacological experiment (which moreover showed similar overall ratings when comparing the groups that had undergone placebo analgesia). Moreover, the mean intensity of self-reported pain and touch were in a similar scale range (pain: 51% of maximum, unpleasant touch: 50.4%; pleasant touch: 57.4%). Finally, we only focused on opioidergic mechanisms and their role in placebo analgesia. Our study thus remains naïve with respect to other neuro-chemical mechanisms that might explain how placebo analgesia affects unpleasant touch, calling for future research. For the same reason, we are not making any claims regarding the general role of the opioid system in affective touch processing.
In conclusion, this study adds to an extensive research line and a growing body of evidence aiming at a more mechanistic understanding of empathy. It shows that while domain-general effects can explain some aspects of previous findings related to unpleasant affect, there is specificity with respect to the opioidergic mechanism underlying pain and its empathic experience. Notwithstanding independent replication and extension, this suggests that the opioid system and thus a hallmark feature of pain regulation plays a specific role in empathy for pain, and its regulation.
Supplementary Material
Contributor Information
Markus Rütgen, Social, Cognitive and Affective Neuroscience Unit, Department of Cognition, Emotion, and Methods in Psychology, Faculty of Psychology, University of Vienna, 1010 Vienna, Austria.
Eva-Maria Wirth, Social, Cognitive and Affective Neuroscience Unit, Department of Cognition, Emotion, and Methods in Psychology, Faculty of Psychology, University of Vienna, 1010 Vienna, Austria.
Igor Riečanský, Social, Cognitive and Affective Neuroscience Unit, Department of Cognition, Emotion, and Methods in Psychology, Faculty of Psychology, University of Vienna, 1010 Vienna, Austria; Department of Behavioural Neuroscience, Institute of Normal and Pathological Physiology, Centre of Experimental Medicine, Slovak Academy of Sciences, 813 71 Bratislava, Slovakia.
Allan Hummer, MR Center of Excellence, Medical University of Vienna, 1090 Vienna, Austria; Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, 1090 Vienna, Austria.
Christian Windischberger, MR Center of Excellence, Medical University of Vienna, 1090 Vienna, Austria; Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, 1090 Vienna, Austria.
Predrag Petrovic, Department of Clinical Neuroscience, Karolinska Institute, 171 76 Stockholm, Sweden.
Giorgia Silani, Department of Clinical and Health Psychology, Faculty of Psychology, University of Vienna, 1010 Vienna, Austria.
Claus Lamm, Social, Cognitive and Affective Neuroscience Unit, Department of Cognition, Emotion, and Methods in Psychology, Faculty of Psychology, University of Vienna, 1010 Vienna, Austria.
Notes
Each author declares to have no competing interests related to this study. Conflict of Interest. None declared.
Funding
The Viennese Science and Technology Fund (WWTF, CS11-016 to C.L.); the Austrian Science Fund (FWF, P32686 to C.L., M.R., and G.S.).
Author Contributions
M.R., E.-M.W., I.R., P.P., G.S., and C.L. designed research; M.R., E.-M.W., I.R., and A.H. performed research; M.R., E.-M.W., I.R., A.H., C.W., and C.L. analyzed data; M.R., E.-M.W., and C.L. wrote the first draft of the paper, and all authors edited and approved the final version.
Data Availability
Group-level statistical maps are stored at NeuroVault (https://identifiers.org/neurovault.collection:9206). Behavioral data are stored on the Open Science Framework website (https://osf.io/v93pz/).
References
- Amanzio M, Benedetti F. 1999. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 19:484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgärtner U, Buchholz H-G, Bellosevich A, Magerl W, Siessmeier T, Rolke R, Höhnemann S, Piel M, Rösch F, Wester H-J. 2006. High opiate receptor binding potential in the human lateral pain system. Neuroimage. 30:692–699. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M. 2013. Mechanisms of the placebo response. Pulm Pharmacol Ther. 26:520–523. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Rosato R, Blanchard C. 2011. Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nat Med. 17:1228–1230. [DOI] [PubMed] [Google Scholar]
- Büchel C, Geuter S, Sprenger C, Eippert F. 2014. Placebo analgesia: a predictive coding perspective. Neuron. 81:1223–1239. [DOI] [PubMed] [Google Scholar]
- Carrillo M, Han Y, Migliorati F, Liu M, Gazzola V, Keysers C. 2019. Emotional mirror neurons in the Rat’s anterior cingulate cortex. Curr Biol. 29:1301–1312e1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. 2006. How prior experience shapes placebo analgesia. Pain. 124:126–133. [DOI] [PubMed] [Google Scholar]
- Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. 2010. How the number of learning trials affects placebo and nocebo responses. Pain. 151:430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi-Dell’Acqua C, Tusche A, Vuilleumier P, Singer T. 2016. Cross-modal representations of first-hand and vicarious pain, disgust and fairness in insular and cingulate cortex. Nat Commun. 7:10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienes Z. 2014. Using Bayes to get the most out of non-significant results. Front Psychol. 5:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. 2009a. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 63:533–543. [DOI] [PubMed] [Google Scholar]
- Eippert F, Finsterbusch J, Bingel U, Büchel C. 2009b. Direct evidence for spinal cord involvement in placebo analgesia. Science. 326:404–404. [DOI] [PubMed] [Google Scholar]
- Fairhurst M, Wiech K, Dunckley P, Tracey I. 2007. Anticipatory brainstem activity predicts neural processing of pain in humans. Pain. 128:101–110. [DOI] [PubMed] [Google Scholar]
- Fazeli S, Büchel C. 2018. Pain related expectation and prediction error signals in the anterior insula are not related to aversiveness. J Neurosci. 38(29):6461–6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola V, Spezio ML, Etzel JA, Castelli F, Adolphs R, Keysers C. 2012. Primary somatosensory cortex discriminates affective significance in social touch. Proc Natl Acad Sci. 109:E1657–E1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter S, Eippert F, Hindi Attar C, Büchel C. 2013. Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage. 67:227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospic K, Gunnarsson T, Fransson P, Ingvar M, Lindefors N, Petrovic P. 2008. Emotional perception modulated by an opioid and a cholecystokinin agonist. Psychopharmacology (Berl). 197:295–307. [DOI] [PubMed] [Google Scholar]
- Grahl A, Onat S, Büchel C. 2018. The periaqueductal gray and Bayesian integration in placebo analgesia. Elife. 7:e32930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronau QF, Ly A, Wagenmakers E-J. 2019. Informed Bayesian t-tests. Am Stat. 74:137–143. [Google Scholar]
- Haaker J, Yi J, Petrovic P, Olsson A. 2017. Endogenous opioids regulate social threat learning in humans. Nat Commun. 8:15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JASP Team . 2020. JASP (Version 0.13.1) [Computer software].
- Kantonen T, Karjalainen T, Isojärvi J, Nuutila P, Tuisku J, Rinne J, Hietala J, Kaasinen V, Kalliokoski K, Scheinin H. 2020. Interindividual variability and lateralization of μ-opioid receptors in the human brain. Neuroimage. 217:116922. [DOI] [PubMed] [Google Scholar]
- Karjalainen T, Karlsson HK, Lahnakoski JM, Glerean E, Nuutila P, Jaaskelainen IP, Hari R, Sams M, Nummenmaa L. 2017. Dissociable roles of cerebral mu-opioid and type 2 dopamine receptors in vicarious pain: a combined PET-fMRI study. Cereb Cortex. 27:4257–4266. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V, Wagenmakers E-J. 2020. Using Bayes factor hypothesis testing in neuroscience to establish evidence of absence. Nat Neurosci. 23:788–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban L, Kross E, Woo C-W, Ruzic L, Wager TD. 2017. Frontal-brainstem pathways mediating placebo effects on social rejection. J Neurosci. 37:3621–3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel PA, Kano M, Van Oudenhove L, Ly HG, Dupont P, Rubio A, Delon-Martin C, Bonaz BL, Manuck SB, Gianaros PJ. 2018. Generalizable representations of pain, cognitive control, and negative emotion in medial frontal cortex. Nat Neurosci. 21:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Woo C-W, Chang LJ, Ruzic L, Gu X, Lopez-Sola M, Jackson PL, Pujol J, Fan J, Wager TD. 2016. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. Elife. 5:e15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. 2011. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 54:2492–2502. [DOI] [PubMed] [Google Scholar]
- Lamm C, Rütgen M, Wagner IC. 2019. Imaging empathy and prosocial emotions. Neurosci Lett. 693:49–53. [DOI] [PubMed] [Google Scholar]
- Lamm C, Silani G, Singer T. 2015. Distinct neural networks underlying empathy for pleasant and unpleasant touch. Cortex. 70:79–89. [DOI] [PubMed] [Google Scholar]
- Lee MC, Wagner HN, Tanada S, Frost JJ, Bice AN, Dannals RF. 1988. Duration of occupancy of opiate receptors by naltrexone. J Nucl Med. 29:1207–1211. [PubMed] [Google Scholar]
- Lee MD, Wagenmakers E-J. 2014. Bayesian cognitive modeling: a practical course. Cambridge: Cambridge University Press. [Google Scholar]
- Leppä M, Korvenoja A, Carlson S, Timonen P, Martinkauppi S, Ahonen J, Rosenberg PH, Aronen HJ, Kalso E. 2006. Acute opioid effects on human brain as revealed by functional magnetic resonance imaging. Neuroimage. 31:661–669. [DOI] [PubMed] [Google Scholar]
- Levine J, Gordon N, Fields H. 1978. The mechanism of placebo analgesia. Lancet. 312:654–657. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Mischkowski D, Crocker J, Way BM. 2016. From painkiller to empathy killer: acetaminophen (paracetamol) reduces empathy for pain. Soc Cogn Affect Neurosci. 11:1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. 2011. A multisensory investigation of the functional significance of the “pain matrix”. Neuroimage. 54:2237–2249. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers E-J. 2011. Erroneous analyses of interactions in neuroscience: a problem of significance. Nat Neurosci. 14:1105–1107. [DOI] [PubMed] [Google Scholar]
- Paracampo R, Tidoni E, Borgomaneri S, Di Pellegrino G, Avenanti A. 2017. Sensorimotor network crucial for inferring amusement from smiles. Cereb Cortex. 27:5116–5129. [DOI] [PubMed] [Google Scholar]
- Peciña M, Zubieta J-K. 2015. Molecular mechanisms of placebo responses in humans. Mol Psychiatry. 20:416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. 2005. Placebo in emotional processing—induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 46:957–969. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, Ingvar M. 2010. A prefrontal non-opioid mechanism in placebo analgesia. Pain. 150:59–65. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. 2002. Placebo and opioid analgesia—imaging a shared neuronal network. Science. 295:1737–1740. [DOI] [PubMed] [Google Scholar]
- Rouder JN, Haaf JM, Aust F. 2018. From theories to models to predictions: a Bayesian model comparison approach. Communication Monographs. 85:41–56. [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G. 2009. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev. 16:225–237. [DOI] [PubMed] [Google Scholar]
- Rütgen M, Seidel E-M, Pletti C, Riečanský I, Gartus A, Eisenegger C, Lamm C. 2018. Psychopharmacological modulation of event-related potentials suggests that first-hand pain and empathy for pain rely on similar opioidergic processes. Neuropsychologia. 116:5–14. [DOI] [PubMed] [Google Scholar]
- Rütgen M, Seidel E-M, Riecansky I, Lamm C. 2015a. Reduction of empathy for pain by placebo analgesia suggests functional equivalence of empathy and first-hand emotion experience. J Neurosci. 35:8938–8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rütgen M, Seidel E-M, Silani G, Riečanský I, Hummer A, Windischberger C, Petrovic P, Lamm C. 2015b. Placebo analgesia and its opioidergic regulation suggest that empathy for pain is grounded in self pain. Proc Natl Acad Sci. 112:E5638–E5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz KA, Pfister R, Büchel C. 2016. Rethinking explicit expectations: connecting placebos, social cognition, and contextual perception. Trends Cogn Sci. 20:469–480. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta J-K. 2007. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 55:325–336. [DOI] [PubMed] [Google Scholar]
- Silani G, Lamm C, Ruff CC, Singer T. 2013. Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. J Neurosci. 33:15466–15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. 2002. Automated anatomical Labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 15:273–289. [DOI] [PubMed] [Google Scholar]
- Vogt BA. 2016. Midcingulate cortex: structure, connections, homologies, functions and diseases. J Chem Neuroanat. 74:28–46. [DOI] [PubMed] [Google Scholar]
- Waber RL, Shiv B, Carmon Z, Ariely D. 2008. Commercial features of placebo and therapeutic. JAMA. 299:1016–1017. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY. 2015. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 16:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. 2004. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 303:1162–1167. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta J-K. 2007. Placebo effects on human μ-opioid activity during pain. Proc Natl Acad Sci. 104:11056–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall ME, Brine DR, Perez-Reyes M. 1981. Metabolism and disposition of naltrexone in man after oral and intravenous administration. Drug Metab Dispos. 9:369–375. [PubMed] [Google Scholar]
- Willoch F, Schindler F, Wester HJ, Empl M, Straube A, Schwaiger M, Conrad B, Tölle TR. 2004. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C] diprenorphine PET study. Pain. 108:213–220. [DOI] [PubMed] [Google Scholar]
- Wise RG, Rogers R, Painter D, Bantick S, Ploghaus A, Williams P, Rapeport G, Tracey I. 2002. Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. Neuroimage. 16:999–1014. [DOI] [PubMed] [Google Scholar]
- World Medical Association. 2013. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 310:2191–2194. [DOI] [PubMed] [Google Scholar]
- Xu A, Larsen B, Baller EB, Scott JC, Sharma V, Adebimpe A, Basbaum AI, Dworkin RH, Edwards RR, Woolf CJ. 2020. Convergent neural representations of experimentally-induced acute pain in healthy volunteers: a large-scale fMRI meta-analysis. Neurosci Biobehav Rev. 112:300–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Gao Y, Gerig G, editors. 2016. ITK-SNAP: an interactive tool for semi-automatic segmentation of multi-modality biomedical images. In: 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE, p. 3342–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Wager TD, Singer T, Keysers C, Gazzola V. 2016. The anatomy of suffering: understanding the relationship between nociceptive and empathic pain. Trends Cogn Sci. 20:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J-K, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. 2005. Placebo effects mediated by endogenous opioid activity on μ-opioid receptors. J Neurosci. 25:7754–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunhammer M, Bingel U, Wager TD, Consortium PI. 2018. Placebo effects on the neurologic pain signature a meta-analysis of individual participant functional magnetic resonance imaging data. JAMA Neurol. 75:1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Group-level statistical maps are stored at NeuroVault (https://identifiers.org/neurovault.collection:9206). Behavioral data are stored on the Open Science Framework website (https://osf.io/v93pz/).


