Abstract
Hyperinflammatory syndromes comprise a heterogeneous group of disorders characterized by severe inflammation, multiple organ dysfunction, and potentially death. In response to antigenic stimulus (e.g., SARS-CoV-2 infection), overactivated CD8+ T-cells and macrophages produce high levels of proinflammatory cytokines, such as IFN-γ, TNF-α, IL-6, and IL-12. Multiple inflammatory mediators implicated in hyperinflammatory syndromes utilize the Janus kinase–signal transducers and activators of transcription (JAK-STAT) cascade to propagate their biological function. Our findings demonstrate that oral ruxolitinib dosing designed to mimic clinically relevant JAK-STAT pathway inhibition significantly reduces the harmful consequences of immune overactivation in multiple hyperinflammatory models. In contrast to monoclonal antibody therapies targeting a single cytokine, ruxolitinib effectively downregulates the functional effect of multiple cytokines implicated in hyperinflammatory states, without broad immunosuppression.
Keywords: hyperinflammatory syndromes, cytokine release syndrome, cytokine storm syndrome, hemophagocytic lymphohistiocytosis, JAK inhibition
Introduction
The worldwide surge in morbidity and mortality due to hyperinflammatory syndromes brought by the COVID-19 pandemic has rekindled an interest in such pathophysiologies (Cao et al., 2020; Kale et al., 2020; Mangalmurti and Hunter, 2020). Hyperinflammatory syndromes, mainly cytokine storm syndrome (CSS) and cytokine release syndrome (CRS), are severe conditions characterized by the excessive activation of immune cells (T-cells and macrophages) and cytokine production leading to multiorgan failure and often death. While CSS and CRS share many clinical characteristics, there are important differences in the pathophysiology. CSS is observed after viral infections (Tisoncik et al., 2012), and acute respiratory distress syndrome (ARDS) constitutes the leading cause of mortality (Mehta et al., 2020b). CSS may also be associated with macrophage activation syndrome and hemophagocytic lymphohistiocytosis (HLH), with overlapping physiologies (Behrens and Koretzky, 2017). Patients with CSS are frequently refractory to the current standard of care (glucocorticoids and the chemotherapeutic agent etoposide), resulting in a clear clinical need to develop new therapeutic agents to limit inflammation, reduce cytokine levels, and ultimately prevent multiorgan failure and death (Meyer et al., 2020). In contrast, CRS is the result of an overactivation of immune cells often seen in the aftermath of immunotherapies such as chimeric antigen receptor T-cells (CAR-T) or bispecific antibodies (Lee et al., 2014; Huarte et al., 2020).
JAKs play an important role in signal transduction following cytokine and growth factor binding to their cognate cell surface receptors. Upon activation, JAKs phosphorylate transcription factor STATs, which results in dimerization and translocation of STATs to the nucleus to activate gene transcription and initiate an inflammatory response (Howell et al., 2019). Multiple cytokines implicated in hyperinflammatory syndromes (e.g., IL-2, IL-6, IL-7, IL-10, IFN-γ and G-CSF) rely on the JAK/STAT pathway. We and others have suggested the use of JAK inhibitors in several hyperinflammatory conditions (Meyer et al., 2020). ruxolitinib, an equipotent and selective JAK1 and JAK2 inhibitor (Quintás-Cardama et al., 2010), is FDA approved for the treatment of myelofibrosis, polycythemia vera, and acute steroid-refractory graft-versus-host-disease. Ongoing clinical trials are exploring the therapeutic potential of ruxolitinib in HLH (NCT04414098, NCT04355793 and NCT04551131) (Ahmed et al., 2019).
Here, we elucidate the effect of ruxolitinib at clinically achievable JAK/STAT pathway inhibition using several in vitro and in vivo models of hyperinflammation, including CRS and HLH, both primary [i.e., genetic] as well as secondary [i.e., non-genetic]. Our data show that ruxolitinib, but not anti-IL-1R nor anti-IL-6R monoclonal antibodies (mAb), significantly reduces cytokine expression by both T-cells and macrophages and ameliorates disease symptoms, thus supporting the hypothesis that generalized JAK/STAT pathway activation plays a critical role in hyperinflammatory syndromes and that its pharmacological inhibition may represent a viable therapeutic strategy.
Overall, ruxolitinib may represent a therapeutic intervention to address the need for more effective treatments for patients developing hyperinflammatory syndromes.
Methods
Animals
C57BL/6 and BALB/c mice were purchased from Taconic Biosciences (Rensselaer, NY, United States) and were approximately 8 weeks old. Ovalbumin (OVA) transgenic TCR mice (OT-1) and perforin deficient mice (Prf1 −/−) were purchased from the Jackson Laboratory (Bar Harbor, ME, United States). All mice were used in protocols approved by the Institutional Animal Care and Use Committees at the respective institutions.
Ruxolitinib Modulation of Mouse Cytokine Release Syndrome Models
CRS was induced in BALB/c mice by ConA injection (20 mg/kg, IV). Animals were dosed with ruxolitinib (30 or 60 mg/kg, PO), anti-IL-1R mAb (25 mg/kg, IP) or anti-IL-6R mAb (25 mg/kg, IP) 60 min before (prophylactic) or 30 min after (therapeutic) CRS induction. Two hours after ConA injection, mice were sacrificed and serum was collected for cytokine measurement.
Hemophagocytic Lymphohistiocytosis Mouse Models
For the secondary HLH model, C57BL/6 mice were injected IP with 50 μg of CpG DNA on days 0, 2, 4, 6, and 8. Starting on day 5, mice were treated with vehicle (PO, BID), ruxolitinib (60 mg/kg, PO, BID), anti-IL-1R mAb (25 mg/kg, IP, QD), or anti-IL-6R mAb (25 mg/kg, IP, QD). In the primary (genetic) model of HLH, Prf1 −/− mice were IP infected with 2 × 105 PFU LCMV Armstrong and treated with control or ruxolitinib chow (2 g/kg) starting on day 4 post-infection (Meyer et al., 2020).
Transcriptomic Analysis
Splenic T-cells were isolated using a pan T-cell isolation kit and an autoMACS Pro Separator (Miltenyi Biotec, Bergisch Gladback, Germany). Approximately 2 × 106 cells were lysed in 500 µl Trizol (Invitrogen, Carlsbad, CA, United States) in gentleMACS M tubes. RNA was purified using a Trizol Plus RNA purification kit (Invitrogen). 100 ng of RNA was hybridized with the nCounter mouse immunology panel codeset (NanoString Technologies, Inc. Seattle, WA, United States) for 18 h. The cartridges were run on an nCounter SPRINT profiler (NanoString Technologies, Inc.). Data were analyzed using nSolver 4.0 Advanced Analysis software. p values were adjusted using the Benjamini-Hochberg method.
T-Cell Proliferation Assay
Splenocytes were incubated with CFSE to permit measurement of proliferation. T-cells were activated with Dynabeads (Thermo Fisher Scientific) at a 3:1 ratio, resuspended at a density of 0.5 × 106 cells/mL in 24-well plates, and treated with ruxolitinib at various concentrations. The plates were incubated for 7 days, and proliferation was determined by flow cytometry.
CD107a Degranulation Assay
Splenocytes from OT-1 or C57BL/6 were resuspended at 5 × 106 cells/mL in complete RPMI, 20 IU IL-2, anti-CD3 (5 μg/ml, plate bound) and anti-CD28 (1 μg/ml) antibodies, and increasing ruxolitinib concentrations. After 3–5 days, OT-1 cells were collected and stained overnight with an anti-CD107a antibody. The cultures were then incubated with OVA+ EG-7 tumor cells for 5 h at 37°C. Immediately following stimulation, cultures were washed once, surface stained with directly conjugated antibodies against CD3 and CD8, and analyzed by flow cytometry.
Activated Macrophage Models
Bone marrow was collected from C57BL/6 mice, and bone marrow cells were cultured in RPMI medium supplemented with 10% fetal bovine serum and 10 ng/mL M-CSF. On day 6, cells were treated with varying ruxolitinib concentrations and then incubated with 2.5 ng/ml lipopolysaccharide (LPS) on day 7. On day 8, cytokines were measured from supernatants. For the in vivo model, C57BL/6 mice were prophylactically dosed with vehicle, ruxolitinib (60 mg/kg, PO), anti-IL-1R mAb (25 mg/kg, IP), or anti-IL-6R mAb (25 mg/kg, IP). Mice were then challenged with LPS (5 µg per animal). Two hours after LPS injection, mice were euthanized, and a peritoneal lavage was performed.
Cytotoxicity Assay
Splenocytes from OT-1 mice were incubated in the presence of 2 µg/ml of the ovalbumin peptide, SIINFEKL, for 3 days. During this time, OVA-expressing EG-7 cells were transfected with a pGL3 luciferase plasmid (Promega, E1751) using lipofectamine 2000 (ThermoFisher, 11,668,030) according to manufacturer’s instructions. After 3 days, OT-1 cells were mixed with EG-7 target cells in a 5:1 ratio and incubated at 37° for 5 h. Following incubation, 50 µL of Bright-Glo luciferase reagent (Promega, E2610) was added to the cultures and fluorescence was measured by plate reader.
Data and Statistical Analysis
Data are reported as mean + SEM in the relevant figures. Differences between groups were analyzed by nonparametric Mann-Whitney test. Statistical analysis for multiple groups was performed by Kruskal-Wallis with Dunn’s post hoc test for nonparametric data sets or analysis of variance with Holm-Sidak’s test for parametric results. All tests were performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA, United States).
Results and Discussion
Ruxolitinib Reduces Exaggerated Cytokine Levels in Murine Models of Acute Hyper-Inflammation
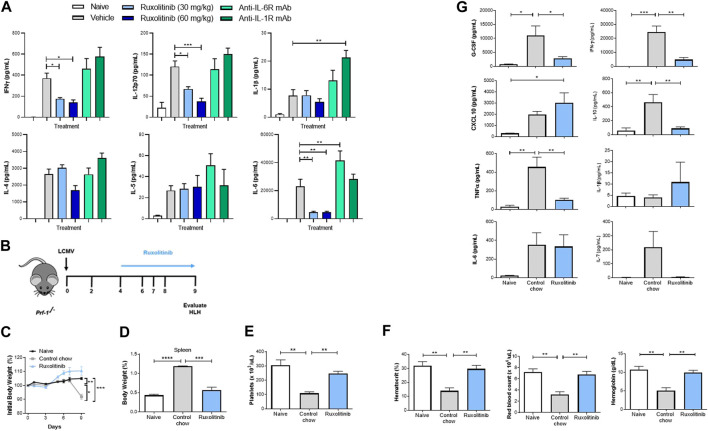
Cytokine production is the hallmark of CSS, and the majority of the implicated cytokines signal through the JAK/STAT pathway (Albeituni et al., 2019). We therefore examined whether JAK1/2 inhibition with ruxolitinib at doses that mimic clinically achievable human JAK/STAT target inhibition could be used as a therapeutic modality to dampen hyperinflammation without inducing broad immune suppression. First, using the potent T-cell mitogen ConA, we studied an in vivo model characterized by broad inflammatory cytokine release and lymphocyte proliferation (Gantner et al., 1995). Similar to individuals experiencing hyperinflammatory syndromes, mice receiving ConA have elevated serum levels of multiple inflammatory cytokines, as well as physiological changes, such as fever, malaise, hypotension, hypoxia, capillary leak, multiorgan toxicity, and potentially death. To study the effect of ruxolitinib in this model, animals were therapeutically dosed with 30 or 60 mg/kg ruxolitinib 30 min post ConA challenge. Two hours after ConA injection animals were sacrificed and serum was collected for cytokine analysis. When compared with vehicle-treated animals, ruxolitinib significantly reduced systemic levels of many CSS-implicated cytokines that signal through the JAK/STAT pathway (e.g., IL-6, IL-12, and IFN-γ) in a dose-dependent manner (Figure 1A). Importantly, other JAK-dependent cytokines did not show a significant reduction (e.g., IL-4 and IL-5), indicating that ruxolitinib treatment downregulated the exaggerated cytokine milieu, but did not induce broad immunosuppression. Ruxolitinib did not have a significant effect on cytokines independent of the JAK signaling pathway (e.g., IL-1β, Figure 1A).
FIGURE 1.
Ruxolitinib lowers cytokine levels in murine models of acute inflammation (A) BALB/c mice were challenged with Con-A and dosed 30 min later with vehicle control, 30 or 60 mg/kg of ruxolitinib, anti-IL-1 mAb (25 mg/kg), or anti-IL-6R mAb (25 mg/kg). Animals were sacrificed 120 min later, and serum was collected. Multiplex cytokine analysis was performed to quantify proinflammatory cytokine concentrations (B) Prf1−/− mice were infected with LCMV and treated with either control chow or ruxolitinib chow (ruxolitinib) starting on day 4 post-infection. Mice were euthanized on day 9 post-infection (C) Kinetics of body weight percentage in treatment-naive, control chow-treated, and ruxolitinib-treated mice (D) Spleen percentage of body weight in treatment-naive, control chow-treated, and ruxolitinib-treated mice (E,F) Blood analysis showing number of PLTs, HCT, levels of HB, and RBC counts (G) Levels of serum cytokines. N = 5 animals per group. Data are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Beyond inhibiting the JAK/STAT pathway, the use of monoclonal antibodies (mAb) such as those targeting IL-1 or IL-6 receptors has been explored as a treatment strategy against CSS (Mehta et al., 2020a). To understand these mechanisms further, some animals were dosed therapeutically with blocking mAb against IL-1R or IL-6R 30 min after ConA challenge. Interestingly, neither anti-IL-1R nor anti-IL-6R treatment was able to significantly reduce cytokine levels in our model (Figure 1A), indicating that inhibiting the JAK/STAT pathway effectively targets multiple inflammatory cytokines and clearly differentiates from strategies that block individual mediators (Meletiadis et al., 2020). While higher plasma levels of IL-6 and/or IL-1 can be attributed to the antibodies blocking the receptor, it is unclear why anti-IL-1R and anti-IL-6R failed to reduce other cytokines levels (Nishimoto et al., 2008).
Ruxolitinib Reduces Splenomegaly and Hyperinflammation in a Model of Primary Hemophagocytic Lymphohistiocytosis
HLH, the prototypical CSS (Meyer et al., 2020), is a rare and often fatal immune disorder characterized by overwhelming activation of T-cell and macrophage compartments and an autocrine loop of proinflammatory cytokines, resulting in unremitting fever, tissue damage and multiorgan failure. To explore the efficacy of oral ruxolitinib at doses which mimic achievable human JAK/STAT target inhibition in primary (i.e., genetic) HLH, we utilized the well-established LCMV infection model of Prf1 −/− mice (Albeituni et al., 2019; Das et al., 2016). To define the therapeutic efficacy of JAK inhibition, Prf1−/− mice were treated with phosphate-buffered saline or infected with 2 × 105 PFU LCMV Armstrong (Figure 1B). Beginning on day 4 post-infection, animals were fed with either control or ruxolitinib chow (2 g/kg) to achieve a JAK target inhibition comparable to 60 mg/kg PO, BID ruxolitinib (Incyte Corporation, Wilmington, DE, United States). On day 9 post LCMV infection, coinciding with the peak of T-cell antiviral immune response, animals were euthanized and organs harvested. Consistent with the published literature (Das et al., 2016), LCMV-infected animals developed anemia, splenomegaly and thrombocytopenia (Figures 1C–F and data not shown); however, following treatment with ruxolitinib, these parameters completely normalized (Figures 1C–F). Importantly, ruxolitinib reduced circulating levels of cytokines implicated in the hyperimmune response, such as G-CSF, IFN-γ, IL-10 and TNF-α (Figure 1G). Furthermore, we also detected a significant reduction in both the percentage and total numbers of IFNγ+TNFα− and IFNγ+TNFα+ CD44+CD62L−CD8+ effector memory T-cells in mice treated with ruxolitinib chow (Supplementary Material Figure S1).
Ruxolitinib Abrogates Splenomegaly and Hyperinflammation in a Mouse Model of Secondary Hemophagocytic Lymphohistiocytosis
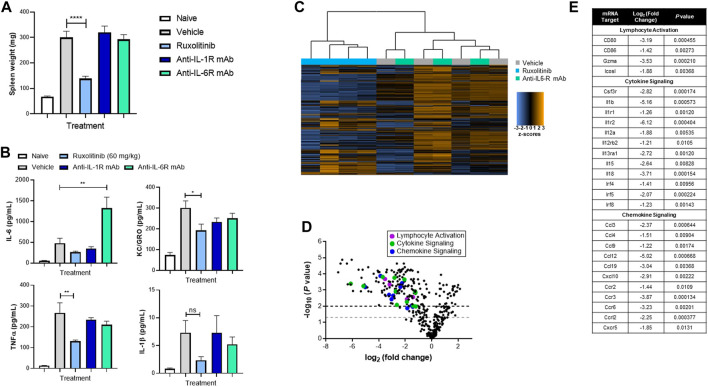
We previously showed that ruxolitinib treatment reduced symptoms in a mouse model of secondary HLH (Das et al., 2016; Albeituni et al., 2019), where hyperinflammation is triggered by immune events as opposed to genetic conditions. Next, we queried whether orally administered doses of ruxolitinib that mimic clinically achievable human JAK/STAT target inhibition would effectively reduce hyperinflammation. As shown in Figure 2A, when compared with vehicle-treated animals, mice receiving 60 mg/kg ruxolitinib had significantly reduced splenomegaly, which coincided with lower levels of pro-inflammatory cytokines KC/GRO (IL-8 functional analogue in rodents) and TNF-α in plasma (Figure 2B) and spleen (data not shown).
FIGURE 2.
Ruxolitinib abrogates hyperinflammation in a murine model of secondary HLH. C57BL/6 animals were injected with 50 μg of CpG DNA on days 0, 2, 4, 6, and 8. Starting on day 5, corresponding mice were treated with vehicle, ruxolitinib, anti-IL-1R mAb, or anti-IL-6R mAb. On day 9, animals were sacrificed, and spleen size (A) and cytokines (B) were measured. Data are representative of five independent experiments. *p < 0.05, ***p < 0.001, ****p < 0.0001 (C) Heatmap of splenic T-cell mRNA expression profiling panel, with blue indicating downregulation and orange indicating upregulation. Genes below threshold count (<20) were removed (D) Volcano plot of splenic T-cell differential gene expression between ruxolitinib and vehicle (baseline) treatment. Points above the gray and black dashed lines indicate adjusted p < 0.05 and < 0.01, respectively (E) Table highlighting individual differentially expressed proinflammatory genes.
Considering that both IL-1 and IL-6 are critical cytokines in CSS, tocilizumab and anakinra have been explored as potential treatments for patients with severe HLH (Dufranc et al., 2020; Mehta et al., 2020a). In our experimental model, treatment with either anti-IL-1R or anti-IL-6R mAb failed to significantly reduce splenomegaly or cytokine levels and indeed dramatically increased plasma levels of IL-6 (Figures 2A,B). These data suggest that blockade of the JAK/STAT pathway may differentiate in efficacy compared with targeting individual cytokines such as IL-1 or IL-6.
Ruxolitinib Downregulates the T-Cell Inflammatory Transcriptome in a Secondary Hemophagocytic Lymphohistiocytosis Model
To characterize the immunological pathway changes that underpin ruxolitinib-mediated efficacy in the murine secondary HLH model, transcriptome profiling using RNA isolated from splenic T-cells was performed. Principle component analysis revealed clustering of the expression profiles of T-cells from vehicle and anti-IL-6R−treated mice, while ruxolitinib-treated animals clustered separately (Figure 2C). This analysis confirmed that ruxolitinib, but not anti-IL-6R, treatment induced discrete changes in gene expression in HLH mice. Differential expression analysis further revealed that ruxolitinib downregulated many proinflammatory genes, including those involved in lymphocyte activation, cytokine signaling and chemokine signaling (Figure 2D). This analysis resulted in a list of 27 genes that we then analyzed for putative upstream regulators of gene expression (Figure 2E). Consistent with the known physiological mechanism of action of ruxolitinib (Spoerl et al., 2014; Verstovsek et al., 2017; Gozzetti et al., 2020; Zeiser et al., 2020), we confirmed that genes implicated in the response to IL-1 and IFN, as well as several T-cell trafficking chemokines, were the top gene regulators inhibited in ruxolitinib-treated mice.
Ruxolitinib Lowers Cytokine Production and Proliferation Without Impairing Degranulation
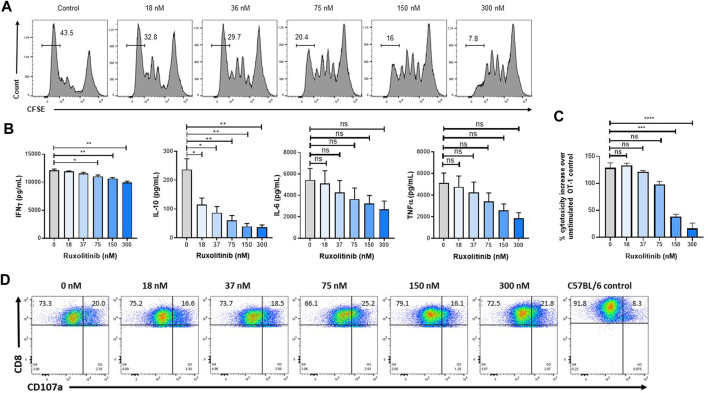
Because activated T-cells play an important antitumor role, the effect of ruxolitinib on T-cell expansion and effector function was studied. As a model for antigen-specific proliferation and cytolytic activity, we used the well-established OT-1 system, where lymphocytes express a transgenic TCR VαVβ5 specific for the OVA257–264 peptide (SIINFEKL) and restricted to H-2Kb. Antigen-specific T-cells were obtained from OT-1 spleens and expanded in vitro with anti-CD3/CD28 antibodies. As seen in Figure 3A, only concentrations of ruxolitinib higher than the in vitro IC50 (in the single-digit nanomolar range (Quintás-Cardama et al., 2010)) result in a reduction in proliferation, as measured with CFSE staining by flow cytometry (Quah et al., 2007). Importantly, ruxolitinib induced a significant and concentration-dependent reduction on the levels of several inflammatory cytokines implicated in CSS hyperactivity (e.g., IFN-γ and IL-10, with non-significant trends in IL-6 and TNF-α) (Figure 3B).
FIGURE 3.
Ruxolitinib reduces proliferation without affecting effector function of T-cells. To measure OT-1 CD8+ T-cell expansion, CFSE-labeled splenocytes were stimulated with SIINFEKL peptide and increasing ruxolitinib concentrations (A) Four days later, the number of divisions was calculated by flow cytometry (B) Supernatants were collected from the cultures and cytokines were quantified (C) To measure degranulation, OT-1 cultures were stimulated overnight with OVA-expressing EG-7 cells, and CD107a levels were measured by flow cytometry (D) OT-1 cultures were activated with OVA peptide and incubated with OVA-expressing EG-7 cells transfected with a luciferase plasmid, with luciferase activity being measured to quantify cytotoxicity. Data are representative of two independent experiments. *p < 0.05, **p < 0.01. **p < 0.001. ns indicates not significant.
We then measured the ability of OT-1 antigen-specific lymphocytes to kill target cells after being activated in the presence of ruxolitinib. OT-1 splenocytes activated in the presence of increasing doses of ruxolitinib only saw a reduction in lytic activity at the highest concentrations of ruxolitinib (Figure 3C). Next, we measured the capacity of OT-1 antigen-specific lymphocytes to degranulate in response to OVA-expressing EG-7 tumor cells. As seen in Figure 3D, even T-cells expanded at the higher ruxolitinib concentrations (300 nM) were able to upregulate CD107a in response to the OVA antigen (Betts et al., 2003), indicating that ruxolitinib does not have an adverse effect on the capacity of antigen-specific T-cells to degranulate upon tumor cell encountering. As a negative control, C57BL/6 splenocytes did not degranulate when cocultured with OVA-expressing EG-7 tumor cells (Figure 3D). Therefore, while ruxolitinib treatment reduced proliferation rates and cytokine production of lymphocytes, it did not affect the lytic activity.
Ruxolitinib Significantly Decreases IL-6 Production by Macrophages
Along with T-cells, macrophages are the main cellular subset implicated in hyperinflammatory conditions (Giavridis et al., 2018; Norelli et al., 2018; Crayne et al., 2019). We therefore investigated the effect of JAK1/2 inhibition on cytokine production by macrophages. First, murine bone marrow–derived macrophages were expanded in vitro with M-CSF, and ruxolitinib was added to the cultures on day 6. Treatment with ruxolitinib reduced IL-6 production following stimulation with LPS in a concentration-dependent manner, indicating that the pharmacologic activity of ruxolitinib in reducing production of inflammatory cytokines is not exclusive to T-cells (Figure 4A). We also observed a non-significant trend toward reduction in IL-12p70 and TNF-α (Figure 4A).
FIGURE 4.
Ruxolitinib lowers cytokine production by murine macrophages and human PBMCs (A) Bone marrow–derived macrophages were expanded in vitro with granulocyte-colony stimulating factor, and ruxolitinib was added to the cultures 24 h in advance of being activated with 5 ng/ml LPS. Culture supernatants were collected 24 h post-LPS stimulation, and multiplex cytokine analysis was performed (B) C57BL/6 mice were prophylactically dosed with vehicle, ruxolitinib, anti-IL-1R, or anti-IL-6R for 3 days before intraperitoneal LPS injection. Cytokines were measured from peritoneal lavages 2 h after injection. Data are representative of two independent experiments. *p < 0.05, **p < 0.01 (C) PBMCs from healthy volunteers were stimulated in vitro with anti-CD3 and anti-CD28 antibodies and increasing concentrations of ruxolitinib. Seven days later, supernatants were collected, and cytokines were measured by multiplex cytokine analysis. Data are normalized to individual donors.*p < 0.05.
We then queried whether ruxolitinib, anti-IL-1R, or anti-IL-6R mAbs would effectively reduce in vivo cytokine production by peritoneal macrophages. Mice were prophylactically treated with vehicle, ruxolitinib (PO), anti-IL-1R (IP), or anti-IL-6R (IP) for 3 days before receiving an intraperitoneal LPS injection. Two hours after LPS injection, cytokines were measured from peritoneal lavage fluid. Only ruxolitinib-treated animals showed a significant reduction in IL-6 levels (Figure 4B), as well as a trend toward IL-12 reduction (data not shown). The inability of anti-IL-6R to reduce IL-6 levels is consistent with clinical studies that have noted an increase in serum IL-6 levels in patients with Castleman disease (Nishimoto et al., 2008), CAR-T cells (Quintás-Cardama et al., 2010) or COVID-19 (Luo et al., 2020) treated with tocilizumab.
Ruxolitinib Reduces Cytokine Production by Human PBMCs
To explore the translational validity of the mouse studies, T-cells were obtained from freshly isolated human PBMCs of healthy adults. Following activation with anti-CD3/CD28−coated beads (Richman et al., 2018) and treatment with ruxolitinib, concentrations of several inflammatory cytokines were measured after 7 days of culture. As seen in Figure 4C, when compared with DMSO control, concentrations of ruxolitinib associated with the cellular IC50 significantly reduced levels of key CSS cytokines (IL-6, IL-10, IL-13, TNF-α and IFN−γ).
Taken together, our preclinical studies using doses of ruxolitinib that mimic clinically achievable JAK/STAT target inhibition demonstrate profound downregulation of the proinflammatory cytokine milieu implicated in CRS and CSS pathophysiology. These experimental findings may have implications for the treatment of primary and secondary HLH (Jianguo et al., 2020), as well as other hyperinflammatory conditions (Capochiani et al., 2020; Del Valle et al., 2020; Karki et al., 2020; Yeleswaram et al., 2020).
Acknowledgments
This study was funded by Incyte Corporation. Editorial assistance was provided by Envision Pharma Group, Inc. (Philadelphia, PA, United States), and funded by Incyte Corporation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committees of Incyte and St. Jude Children’s Research Hospital.
Author Contributions
EH, KN, and PS conceived the experiments. MP, KV, BF, RB, and SA performed experiments and analyzed the results. EH wrote the initial draft and prepared figures with subsequent writing and figure preparation undertaken by MP, KN, and PS All authors reviewed the manuscript.
Funding
This study was funded by Incyte Corporation.
Conflict of Interest
The authors declare that this study received funding from Incyte Corporation. The funder had the following involvement with the study: EH, MP, BF and PS are employees and shareholders of Incyte Corporation, and they conceived, performed and analyzed experiments, and wrote the manuscript.
The handling editor declared a past co-authorship with one of the authors EH.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.650295/full#supplementary-material.
Abbreviations
mAb, monoclonal antibodies; PFU, plaque-forming unit; LCMV, lymphocytic choriomeningitis virus; CFSE, carboxyfluorescin succinimidyl ester; WT, wild type; M-CSF, macrophage colony-stimulating factor; IFN, interferon; G-CSF, granulocyte-colony stimulating factor; Con-A, Concanavalin-A; IP, intraperitoneal; IV, intravenous; BID, twice daily, QD, once daily; DMSO, dimethyl sulfoxide.
References
- Ahmed A., Merrill S. A., Alsawah F., Bockenstedt P., Campagnaro E., Devata S., et al. (2019). Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial. Lancet Haematol. 6, e630–e637. 10.1016/s2352-3026(19)30156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeituni S., Verbist K. C., Tedrick P. E., Tillman H., Picarsic J., Bassett R., et al. (2019). Mechanisms of action of ruxolitinib in murine models of hemophagocytic lymphohistiocytosis. Blood 134, 147–159. 10.1182/blood.2019000761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens E. M., Koretzky G. A. (2017). Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol. 69, 1135–1143. 10.1002/art.40071 [DOI] [PubMed] [Google Scholar]
- Betts M. R., Brenchley J. M., Price D. A., De Rosa S. C., Douek D. C., Roederer M., et al. (2003). Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunological Methods 281, 65–78. 10.1016/s0022-1759(03)00265-5 [DOI] [PubMed] [Google Scholar]
- Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., et al. (2020). Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 146 (1), 137–146.e3. 10.1016/j.jaci.2020.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capochiani E., Frediani B., Iervasi G., Paolicchi A, Sani S, Roncucci P., et al. (2020). Ruxolitinib rapidly reduces acute respiratory distress syndrome in COVID-19 disease. Analysis of data collection from RESPIRE protocol. Front. Med. (Lausanne) 7, 466. 10.3389/fmed.2020.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crayne C. B., Albeituni S., Nichols K. E., Cron RQ. (2019). The immunology of macrophage activation syndrome. Front. Immunol. 10, 119. 10.3389/fimmu.2019.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R., Guan P., Sprague L., Verbist K., Tedrick P., An Q. A., et al. (2016). Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood 127, 1666–1675. 10.1182/blood-2015-12-684399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle D. M., Kim-Schulze S., Huang H. H., Beckmann N. D., Nirenberg S., Wang B. et al. (2020). An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 26 (10), 1636–1643. 10.1038/s41591-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufranc E., Del Bello A., Belliere J., Kamar N., Fagur S.; TAIDI (Toulouse Acquired Immune Deficiency and Infection) study group (2020). IL6-R blocking with tocilizumab in critically ill patients with hemophagocytic syndrome. Crit. Care 24, 166. 10.1186/s13054-020-02878-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner F., Leist M., Lohse A. W., Germann P. G., Tiegs G. (1995). Concanavalin A-induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology 21, 190–198. 10.1002/hep.1840210131 [DOI] [PubMed] [Google Scholar]
- Giavridis T., van der Stegen S. J. C., Eyquem J., Hamieh M., Piersigilli A., Sadelain M. (2018). CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 24, 731–738. 10.1038/s41591-018-0041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzetti A., Capochiani E., Bocchia M. (2020). The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19. Leukemia 34 (1), 2815-2816. 10.1038/s41375-020-01038-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell M. D., Kuo F. I., Smith P. A. (2019). Targeting the Janus kinase family in autoimmune skin diseases. Front. Immunol. 10, 2342. 10.3389/fimmu.2019.02342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte E., O'Conner R. S., Peel M. T., Nunez-Cruz S., Leferovich J., Juvekar A., et al. (2020). Itacitinib (INCB039110), a JAK1 inhibitor, reduces cytokines associated with cytokine release syndrome induced by CAR T-cell therapy. Clin. Cancer Res. 26 (23), 6299–6309. 10.1158/1078-0432.CCR-20-1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jianguo L., Zhixuan Z., Rong L., et al. (2020). Ruxolitinib in alleviating the cytokine storm of hemophagocytic lymphohistiocytosis. Pediatrics 146 (2), e20191301. 10.1542/peds.2019-1301 [DOI] [PubMed] [Google Scholar]
- Kale S. D., Mehrkens B. N., Stegman M. M., Kastelberg B., Carnes H., McNeill R. J., et al. (2020). Small" intestinal immunopathology plays a "big" role in lethal cytokine release syndrome, and its modulation by interferon-gamma, IL-17a, and a Janus kinase inhibitor. Front. Immunol. 11, 1311. 10.3389/fimmu.2020.01311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R., Sharma B. R., Tuladhar S., Williams E. P., Zalduondo L., Samir P., et al. (2020). Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell 184 (1), 149–168.e17. 10.1016/j.cell.2020.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. W., Gardner R., Porter D. L., Louis C. U., Ahmed N., Jensen M., et al. (2014). Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195. 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. (2020). Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 92, 814–818. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalmurti N., Hunter C. A. (2020). Cytokine storms: understanding COVID-19. Immunity 53, 19–25. 10.1016/j.immuni.2020.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., Cron R. Q., Hartwell J., Manson J. J., Tattersall R. S. (2020a). Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2. 358–367. 10.1016/S2665-9913(20)30096-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D. F., Brown M., Sanchez E., Tattersall R. S., Manson J. J. (2020b). COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet 395, 1033–1034. 10.1016/s0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletiadis J., Tsiodras S., Tsirigotis P. (2020). Interleukin-6 blocking vs. JAK-STAT inhibition for prevention of lung injury in patients with COVID-19. Infect. Dis. Ther. 9, 707–713. 10.1007/s40121-020-00326-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer L. K., Verbist K. C., Albeituni S., Scull B. P., Bassett R. C., Stroh A. N., et al. (2020). JAK/STAT pathway inhibition sensitizes CD8 T cells to dexamethasone-induced apoptosis in hyperinflammation. Blood 136, 657–668. 10.1182/blood.2020006075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N., Terao K., Mima T., Nakahara H., Takagi N., Kakehi T. (2008). Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 112, 3959–3964. 10.1182/blood-2008-05-155846 [DOI] [PubMed] [Google Scholar]
- Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M., et al. (2018). Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748. 10.1038/s41591-018-0036-4 [DOI] [PubMed] [Google Scholar]
- Quah B. J. C., Warren H. S., Parish C. R. (2007). Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat. Protoc. 2, 2049–2056. 10.1038/nprot.2007.296 [DOI] [PubMed] [Google Scholar]
- Quintás-Cardama A., Vaddi K., Liu P., Manshouri T., Li J., Scherle P. A., et al. (2010). Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 115, 3109–3117. 10.1182/blood-2009-04-214957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman S. A., Nunez-Cruz S., Moghimi B., Li L. Z., Gershenson Z. T., Mourelatos Z., et al. (2018). High-affinity GD2-specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunol. Res. 6, 36–46. 10.1158/2326-6066.cir-17-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoerl S., Mathew N. R., Bscheider M., Schmitt-Graeff A., Chen S., Mueller T., et al. (2014). Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood 123, 3832–3842. 10.1182/blood-2013-12-543736 [DOI] [PubMed] [Google Scholar]
- Tisoncik J. R., Korth M. J., Simmons C. P., Farrar J., Martin T. R., Katze M. G. (2012). Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 76, 16–32. 10.1128/mmbr.05015-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S., Mesa R. A., Gotlib J., Gupta V., DiPersio J. F., Catalano J. V., et al. (2017). Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J. Hematol. Oncol. 10, 55. 10.1186/s13045-017-0417-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeleswaram S., Smith P., Burn T., Covington M., Juvekar A., Li Y., et al. (2020). Inhibition of cytokine signaling by ruxolitinib and implications for COVID-19 treatment. Clin. Immunol. 218, 108517. 10.1016/j.clim.2020.108517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiser R., von Bubnoff N., Butler J., Mohty M., Niederwieser D., Or R., et al. (2020). Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N. Engl. J. Med. 382 (19), 1800–1810. 10.1056/NEJMoa1917635 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.