Abstract
The importance of fetal magnetic resonance imaging (MRI) in the prenatal diagnosis of central nervous system (CNS) anomalies is rapidly increasing. Fetal MRI represents a third level examination usually performed, as early as 18–20 weeks of gestational age, when a second level (expert) neuro-ultrasonography (US) evaluation raises the suspicion of a CNS anomaly or when a genetic disorder is known. Compared to the US, MRI has the advantage to allow a better visualization and characterization of brain structures so to detect anomalies not visible in the US, thus resulting in relevant implications for parent counselling and pregnancy management. Moreover, the improvement of MRI technologies permits to obtain ultrafast sequences, which minimize the drawback of movement artifacts, and to perform advanced studies. This review aims at providing a practical guide for trainees and fellows who are approaching fetal MRI. In the first part, we provide information about indications, safety and protocols based on the state-of-the-art sequences, with a mention on the innovations related to the use of a 3T scanner. The second part is focused on the normal development of the human fetal brain related to its MR appearance, whose knowledge is essential to detect possible abnormalities. The last section briefly describes the most frequent abnormalities in the fetal brain and spine as depicted by MRI.
Keywords: Fetal, magnetic resonance imaging (MRI), brain
Introduction
Fetal magnetic resonance imaging (MRI) or in utero MRI (iuMRI) is an important diagnostic tool in the field of prenatal diagnosis, and its use has widely spread during the last two decades thanks to advances in technology and new applications.
Although ultrasonography (US) remains the first imaging modality in the assessment of fetal central nervous system (CNS), some anomalies cannot be adequately characterized by US alone and, in such cases, MRI may play a crucial role thanks to its superior diagnostic accuracy which may impact on pregnancy management (1).
The aim of this review is to provide the basic knowledge requested for trainees and fellows before approaching brain iuMRI. We initially focus on indications, safety, and protocols. We therefore overview the main changes that the fetal brain undergoes during in utero development and briefly describe the most frequent fetal CNS pathologies and their MRI findings.
Indications
In utero MRI of the CNS is a third level examination that is indicated exclusively after a second level (expert) neuro-US is performed by a qualified ultrasonographer, both from an obstetric or radiological background (2,3).
It is indicated as early as 18–20 weeks of gestational age in case of (2):
❖ Abnormal findings at fetal US, such as ventricular dilatation, midline anomalies, suspected cortical malformations, posterior fossa abnormalities, abnormal fetal brain biometry.
❖ Pregnancy at risk for brain injuries: twin-twin transfusion syndrome (TTTS), intrauterine growth restriction (IUGR), thrombocytopenia, maternal hypoxia, and maternal TORCH infections (toxoplasmosis, other agents, rubella, cytomegalovirus, herpes simplex virus). In such cases, MRI can be indicated even with a normal ultrasound picture.
❖ Multiple extracerebral fetal malformations.
❖ Family history of genetic disorders involving the CNS.
In utero MRI is not routinely performed before the 18th week of gestational age due to the small size of the fetus and because movements usually do not allow to add any significant diagnostic information to US examination. Moreover, some structures such as corpus callosum or the cerebellar vermis are not fully developed before the 18 weeks and they are therefore not adequately evaluated (2). Despite MRI provides better information in late second and third trimesters, in several countries the deadline for pregnancy termination is within the 24th–25th weeks. Therefore, iuMRI is frequently performed before that age so to play a crucial role in terms of parent counselling and pregnancy management (4,5).
Safety
There are no demonstrated side effects or delayed sequelae when iuMRI is performed at 1.5 Tesla, without administration of contrast media (6). The safety of 3 Tesla scanning is still under debate with concerns about possible theoretical risks related to an increased amount of radiofrequency power deposition in fetal tissue, measured in the form of specific absorption rate (SAR), and to the elevated acoustic noise intensity as a result of rapid gradient field switching. Anyway, even at 3 Tesla, it is possible not to exceed the SAR limit of 2 W/kg for maternal whole-body exposure imposed by the Food and Drug Administration, and there is no evidence of acoustic damage to fetus, especially with the new noise reduction technologies acting on fast gradient switches (6-8). iuMRI is currently considered a safe and effective imaging modality with no demonstrated negative effects to the developing human fetus both at 1.5 and at 3 Tesla. Contraindications are the same as for any MRI examination, and they must be excluded before scanning (9).
Setting and MRI protocol
Informed consent must be obtained from the mother before the examination. Moreover, the radiologist should collect information about the gestational age, established by first-trimester ultrasound, clinical assessment, and ultrasound findings (5).
When possible, mother should have been fasting for at least 4 hours since low glucose levels help decreasing fetal motions and should be examined with an empty bladder to prevent urinary urge during imaging (2). The patient is scanned in supine position or, if not tolerated (polyhydramnios, multiple pregnancies, late gestational age, vena cava compression, back pain), in the left lateral decubitus. No sedatives are routinely used for mother or fetus. Contrast agent administration is contraindicated (10).
At present, fetal MRI is mostly performed using a 1.5 Tesla superconducting magnet with the abdominal or cardiac phase-array coil. 3 Tesla scanners are being more and more used because of the advantages of increasing the signal-to-noise ratio (SNR) and allowing the use of advanced techniques such as SWI, MRI spectroscopy (MRS), Diffusion tensor imaging (DTI) and resting state functional MRI (rsfMRI). On the other hand, 3 Tesla scanning is associated with more artefacts (i.e., standing wave, conductivity, and susceptibility artefacts) especially in case of polyhydramnios or maternal obesity; in these cases, 1.5 Tesla should be preferred (8).
A standard fetal brain MRI protocol at 1.5 T should include at least (Figure 1) (1,2,5):
Figure 1.
Main sequences used in current clinical fetal MRI. MRI, magnetic resonance imaging.
❖ A localizer (generally a T2-weighted single-shot fast spin-echo) using 6 mm slices in the maternal coronal plane to identify fetal position.
❖ T2-weighted fast or turbo spin-echo sequences [half-Fourier acquisition single-shot turbo spin-echo (HASTE)/single-shot fast spin echo (SS-FSE)/single-shot turbo spin echo (SS-TSE)] on axial, coronal, and sagittal planes relative to the fetal head, with thin slices (3–4 mm), no interslice gap and a field of view (FOV) ranging from 240 to 350 mm. This sequence represents the pillar of fetal MRI, since acquiring slice- per-slice has the great advantage of reducing fetal movement artifacts that will be limited only to those slices acquired during fetal motion. For sagittal plane scanning, is advisable to prescribe an odd number of slices, so the mid-sagittal one of the slabs would better encompass midline structures as, pituitary, chiasm, aqueduct, and vermis.
❖ Balanced steady-state gradient echo sequences [true fast imaging with steady-state precession (TRUE-FISP)/fast imaging employing steady-state acquisition (FIESTA)/balanced fast field echo (b-FFE)], may be acquired with reduced thickness (2–3 mm) and are useful for better characterization of smaller structures as cranial nerves, but also basal cistern anatomy, inner ear, cerebellar cortex or vermian lobules.
❖ T1-weighted images, at least on axial plane, typically acquired using two-dimensional gradient echo (2D GRE) sequence or a faster version of the GRE sequence called ultra-fast gradient echo sequences or turbo fast low-angle shot (FLASH). This sequence is usually acquired with a slice thickness between 4 and 6 mm, with or without an interslice gap of 0.2–0.4 mm. To limit motion artifacts, it is preferred performing during a maternal breath-hold. T1-weighted images may also be acquired with a version of FSE, although the number of slices is limited and maternal breath-hold is needed, such sequence provides better contrast resolution than GRE methods, although spatial resolution is not as good. T1-weighted sections are preferred to evaluate to detect haemorrhages, haemorrhagic necrosis foci, and focal or linear calcifications.
❖ Echo-planar T2*-weighted GRE section may also be acquired to better identify haemorrhagic foci, especially if associated with venous sinus thrombosis.
❖ Echo-planar diffusion-weighted imaging (DWI) (b_factor: 0–600 s/mm2, 4–6 mm thick) with apparent diffusion coefficient (ADC) maps reconstruction have provided valuable information in case of acute or hyperacute ischemic lesions in conditions as surviving monochorionic twin fetus or presence of vascular malformation.
❖ A single-shot or echo-planar version of the FLAIR sequence may be applied in order to help in detecting intraventricular anomalies (i.e., choroid plexus cysts) and it could be added to the protocol in case of ventriculomegaly or difficult visualization of ventricular content by US (11).
❖ At 3 Tesla, SWI sequence can be used to better depict calcifications and blood products (2) instead of the more traditional EPI-T2*-weighted mentioned above.
❖ Sequences used in research or advanced clinical settings (Figure 2): DTI and MRS are optional sequences, technically challenging in utero. With the spread the use of 3 Tesla these techniques may become more feasible, thanks to the reduction of the acquisition time, the higher SNR and, for MRS, the increased chemical shift that allows a higher metabolite resolution (8).
Figure 2.

Main fetal MR advanced techniques and research sequences.
Overall, the examination time should be in between 15 and 40 minutes, depending on fetal size and gestational age, mother comfort, active fetal motion requiring repeated sequence acquisition, the need to perform advanced-research sequences.
Normal brain development at fetal MRI
The knowledge of normal brain development is fundamental for the interpretation of CNS fetal anomalies (12). Here follows a synthetic resume of the main processes leading to the formation-organization of the fetal brain which can be monitored at fetal MRI spatial resolution scale: these processes are disrupted in case of fetal brain genetically-based maldevelopment or clastic lesions; their knowledge helps understanding the genesis of the anomalies studied by MRI.
Commissuration
Commissuration is the process of forming cerebral commissures through the growth of axonal fibers across the midline from one hemisphere to the other, connecting homologous parts of the hemispheres. It continues until roughly 18–19 weeks. Therefore, at gestational age of current clinical fetal MRI the corpus callosum should already fully appear with all its parts.
Cortical formation
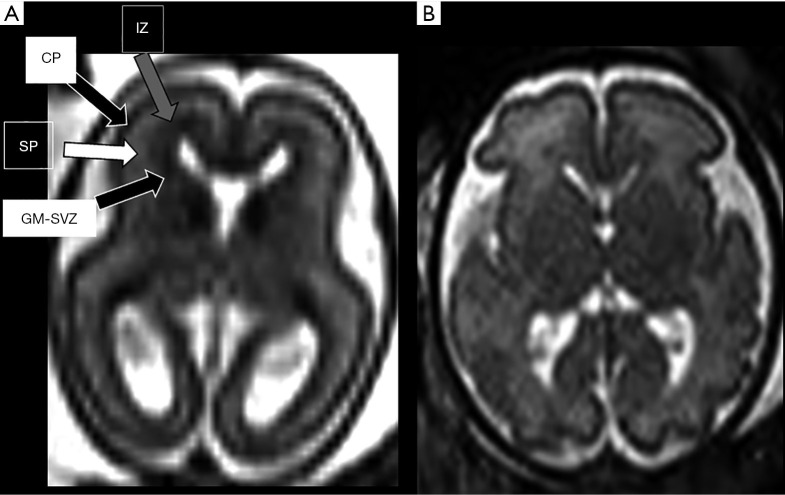
Corticogenesis starts around 8–10 weeks with the proliferation of neurons and glia in the germinal matrices. Neuronal cells then migrate through the fetal cerebral hemisphere using the radial glial cells as a scaffold and subsequently organize on the surface of the hemispheres to become the cerebral cortex (the whole process is therefore schematically described as composed of three largely overlapping processes: proliferation, migration and organisation). Because of the migration process, during the second trimester of pregnancy multiple transient layers appear evident in the cerebral mantle and are quite prominent in both histology and MRI imaging (Figure 3). They are well depicted between approximately the 19 and 26 weeks of gestational age: at MRI, the innermost layer is the germinal matrix-periventricular zone, appearing as a dark band on T2-weighted images and bright on T1-weighted images, lining the lateral ventricles. The most external layer is represented by the cortical plate that, as the germinal matrix-periventricular zone, appears markedly hypointense on T2. Between the germinal matrix-periventricular zone and the cortical plate there is the intermediate zone, the real future white matter, (iso-hypointense on T2), and the subplate (hyperintense on T2) (13). By the end of the second trimester, this layered pattern gradually disappears.
Figure 3.
Axial 3 mm thick T2-weighted ss-FSE sections at the level of basal ganglia in normal 22-week (A) and 30-week (B) gestation foetuses respectively. Note how the normal layering of the developing brain [different arrows pointing the different layers in (A)] tends to be less marked with gestation until it disappears. As a technical tip: TE value used at 22 weeks was above 120 ms and below that value at older gestational age, since in younger foetuses brain water content is higher and longer T2 values benefit from longer TE to allow for optimal contrast resolution. CP, cortical plate; GM-SVZ, germinal matrix-subventricular zone; IZ, intermediate zone; SP, subplate.
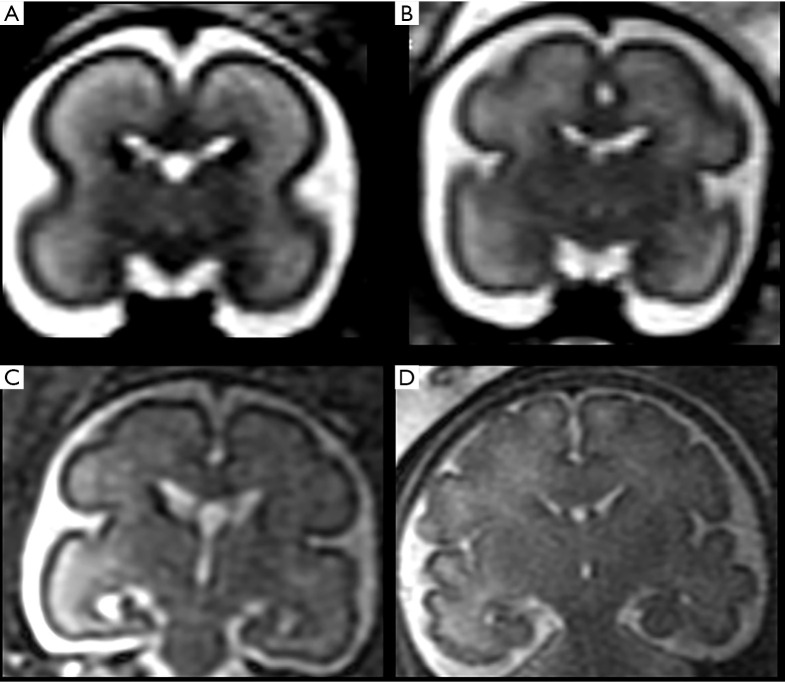
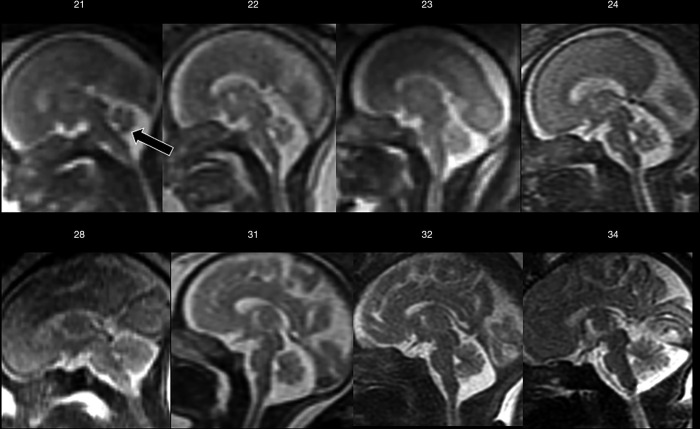
Later, cortical organization and a series of intimately related processes such as axonogenesis, dendritogenesis and synapse formation occur and consequently the newly formed cerebral cortex starts folding progressively, forming gyri and sulci. These processes (gyration and sulcation) follow a specific pattern that can be well depicted by MRI (Figures 4 and 5). As an example, before the 20th week of gestation the brain appears almost completely smooth, with only the Sylvian fissures recognizable at 18th weeks. Between the 22nd–23rd week a progressive sulcation starts, and it continues accelerating until the 34–35 weeks when the gyration appears almost complete. It must be stressed that sulcation process may not evolve precisely symmetric and it may be delayed in twin gestations (13).
Figure 4.
Coronal 3 mm thick T2-weighted ss-FSE sections at the level of Monro foramina in normal 22 (A), 26 (B), 28 (C) and 33 (D) weeks gestation foetuses, respectively. Note the increased progressive brain surface sulcation with gestational age. ss-FSE, single-shot fast spin echo.
Figure 5.
Mid-sagittal T2-weighted ss-FSE sections from increasing gestational age (week indicated) showing progressive sulcation and the relationship between cerebral hemispheres and cerebellar vermis through gestation. Note how already at 21-week gestation the vermis completely covers the fourth ventricle (arrow). ss-FSE, single-shot fast spin echo.
Myelination
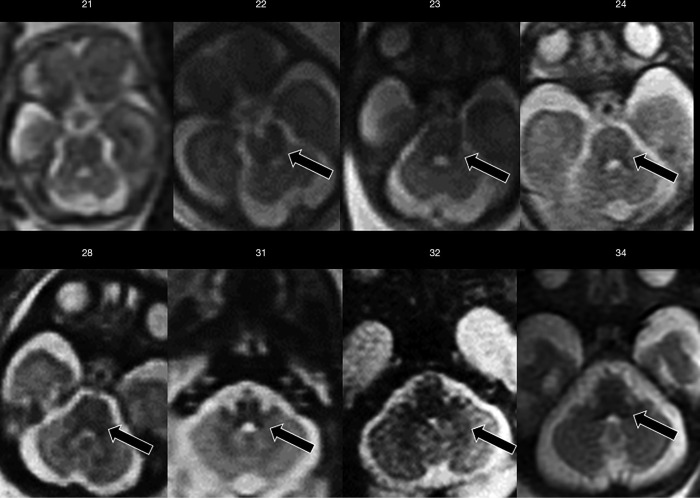
The myelination process starts in utero and continues after birth up to about 3 years of age. This process follows a caudo-cranial and dorsal-ventral direction. As an example, at 20 weeks of gestation, it is possible to see myelin deposition as a progressively increasing T1- and decreasing T2-signal in the posterior brainstem (Figure 6), at 33 weeks in the posterior limb of the internal capsule, at 35 weeks in the optic tracts and subcortical peri Rolandic white matter (14). Diffusion-weighted imaging (DWI) is a helpful sequence for the evaluation of the myelination process, being more sensitive than T1 and T2-weighted sequences to detect myelin-associated signal changes (14).
Figure 6.
Axial T2-weighted ss-FSE sections from increasing gestational age (week indicated) showing initial myelination process, as reduced T2-weighted signal, at the level of the tegmental brainstem and deep cerebellar white matter (arrows). ss-FSE, single-shot fast spin echo.
Biometry
Between the 20 and 40 weeks of gestational age the human brain undergoes a phase of accelerated growth manifesting in increase in volume, with changes in brain biometry. Therefore, evaluation of biometry is a fundamental step in prenatal brain imaging. The main brain and skull diameters must be compared to the normal reference values for gestational age available in literature (14-16). Free available centile calculators are also available on-line (https://www.developingbrain.co.uk/fetalcentiles/).
Main fetal CNS anomalies
Ventriculomegaly
Ventriculomegaly is defined as an atrial width >10 mm, measured in the axial plane, and it constitutes the most common indication for fetal MRI (2,17,18). The morphology of lateral ventricles changes throughout pregnancy: occipital horns appear prominent during the first gestational weeks and gradually decrease in size after the 23rd week; nevertheless, atrial width remains stable (18). In utero MRI allows better depiction of brain abnormalities possibly associated with ventriculomegaly, which can as well give insight into both its aetiology and the neurodevelopmental outcome. Therefore, in prognostic terms, the role of fetal MRI in this setting is to define the possible aetiology and to look for associated anomalies.
Ventriculomegaly is considered mild if atrial size is between 10–12 mm, moderate between 12.1–15 mm and severe if greater than 15 mm (17). Associated abnormalities can be identified in up to 70% of cases, including CNS and/or non-CNS malformations, such as agenesis of the corpus callosum, cortical malformations, periventricular heterotopia, cerebellar malformations, hemimegaloencephaly, periventricular white matter injury, porencephaly, multicystic encephalomalacia, intraventricular haemorrhage, and germinal matrix haemorrhage. If no additional structural abnormalities or chromosomal aberrance are noted, ventriculomegaly is defined as “isolated” and the prognosis primarily depends on its grade. Progression of the dilatation at follow-up, asymmetrical (>2 mm difference) and bilateral ventriculomegaly are other factors that have been linked to a worse prognosis (19). Isolated mild ventriculomegaly represents a challenge in terms of parent counselling and outcome. In fact, the majority (94–98%) of children with isolated mild ventriculomegaly detected by ultrasound are healthy at birth, and the role of fetal MRI in this group is still debated. Despite this, the diagnostic work-up must be meticulous, including serological evaluation for infections, chromosomal investigation, and serial ultrasounds to exclude progression (17,20).
Corpus callosum dysgenesis
The corpus callosum is the main and by far the largest commissural pathway in the brain. Its development starts at 8 weeks of gestation, acquiring its final shape by 20 weeks (although maturation and growth go on until postnatal life) (3). Suspected corpus callosum agenesis (ACC) is a frequent indication for fetal MRI since ACC is the commonest developmental abnormality. However, the term ACC implies a complete agenesis of the corpus callosum, while partial agenesis or hypoplasia of the corpus callosum also exist. In addition, in the spectrum of possible callosal dysgenesis, too thick or too short CCs may also be encountered. With this respect, US is relatively poor in diagnosing anomalies associated with ACC or milder callosal anomalies compared to MRI, and a fetal MRI should be performed in any case of suspicion on antenatal US (3).
While US diagnosis of ACC relies on indirect signs such as absence of the cavum septi pellucidi, dilatation of ventricular atria and occipital horns, and inferior orientation of the medial hemispheric sulci, fetal MRI can directly visualize the corpus callosum in the sagittal plane, appearing as a curvilinear structure hypointense on T2-weighted images located at the superior margin of the lateral ventricles (18). Milder forms of dysgenesis are more difficult to diagnose, because of the normally thin appearance of the fetal corpus callosum in fetal life, especially during the second trimester (18). In the future, DTI will possibly assume much more relevance for detecting uncertain commissural abnormalities (1).
In utero MRI is helpful also in detecting associated CNS abnormalities (Figure 7), such as abnormal sulcation, posterior fossa abnormalities, periventricular nodular heterotopia, dysplastic ventricles, abnormal deep grey nuclei, and parenchymal haemorrhage and/or injury, with a crucial prognostic impact. Moreover, the identification of additional findings may suggest a specific disorder or syndrome associated with callosal agenesis with important implications for patient counselling and for recurrence risk in future pregnancies (18).
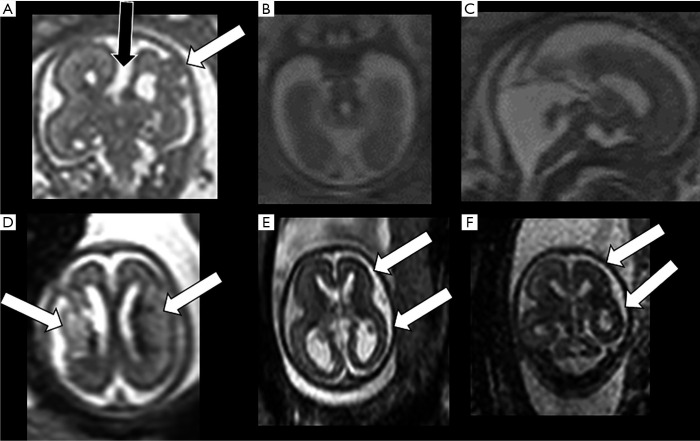
Figure 7.
In (A) an example of coronal ss-FSE T2-weighted section in a 21-week gestation fetus depicting complete agenesis of corpus callosum (black arrow) associated with cortical formation anomalies in one hemisphere (white arrow) and normal contralateral hemisphere. In (B and C) example in a 21-week gestation foetus of cerebellar-brainstem anomalies on axial and sagittal T2-weighted sections (molar-tooth malformation with small vermis and cerebellum). In (D) an example of a 21-week gestation foetus with bilateral areas of increased parenchymal T2-weighted signal and swelling compatible with recent bilateral infarction (arrows). In (E and F) an example of a 21-week gestation foetus affected by CMV infection with consequent parenchymal atrophy-thinning and with subtle irregular brain surface especially in one hemisphere (arrows). ss-FSE, single-shot fast spin echo.
On the other hand, fetal MRI reveals normal corpus callosum in approximately 20% of cases of suspected callosal abnormality at US examination (21).
Cortical formation anomalies
Despite significant advances in knowledge and issues being raised, the most recent update of the classification of malformations of cortical development (MCD) is still based on the concept that cortical formation is schematically composed of three largely overlapping stages: cell proliferation and apoptosis, cell migration, and post-migrational development (cortical “organization”) (22). Among all the possible malformative features detectable by prenatal imaging, cortical formation anomalies (CFA) are the most difficult (or even impossible, in some cases) to detect with the US. In such instances, MRI offers clear advantages over the US (Figure 7), but its accuracy has not yet been clearly established and significant issues remain (23). First, CFA are especially hard to detect in very immature brains. Secondly, the morphological appearance of CFA in fetal brains is quite different from their “post-natal” appearance and changes over time showing different features during brain maturation (24,25). Therefore, once again, to correctly recognize CFA it is crucial to be familiar with the timing of normal brain lamination, sulcation, and gyration. With this in mind, focal CFA can be identified by MRI even at a very early stage of brain development (<24 week), when the gyration process has not yet started and the brain is “physiologically lissencephalic” (peculiar patterns of cortical plate irregularities such as “wart-like”, “abnormal invaginating sulcus”, “sawtooth” and “bumps” have been described) (24). Despite this, some CFA are still quite hard to depict; for an example the sensitivity of MRI for gray matter heterotopias has been reported to be as low as 44% below the 24th week and 73% later on (23). On the other hand, other anomalies such as polymicrogyria and schizencephaly seem to be more easily detectable (23).
As a result, it seems quite challenging to classify CFA using official paediatric nomenclature (26). Therefore, a recent multicentric study proposed a new classification of CFA detectable on iuMRI no more based on the nomenclature used in neonates or children, but on characteristics of laterality (bilateral asymmetric, bilateral symmetric or unilateral) and morphology (25). This hopefully will improve not only CFA detection but also prognostication of possible long-term neurodevelopmental sequelae. However, post-natal follow-up or post-mortem MR-autopsy are still strongly advised, when possible (27).
Posterior fossa anomalies
Fetal posterior fossa anomalies are numerous, extremely heterogeneous, and include both malformative and clastic (disruptive) lesions. Unfortunately, posterior fossa examination can be difficult at US, especially in the third trimester, due to the progressive ossification of the skull base. Conversely, MRI allows direct visualization of the cerebellar hemispheres, vermis, and brainstem in three orthogonal planes, with satisfactory anatomic detail (Figure 7) (1). Furthermore, it simultaneously provides information about associated supratentorial and even extracranial anomalies that can influence prognosis (28). On the other hand, it must be noted that iuMRI showed some limitations in the evaluation of the posterior fossa, particularly when performed early in gestation, when compared to postnatal MRI; therefore, despite recent advances in accuracy, postnatal MRI follow-up is advised (29). In addition, knowledge of normal posterior fossa development and maturation is essential for a correct interpretation of MRI findings (Figure 5) (30).
A universally accepted classification scheme for posterior fossa malformations is still lacking, and the range of midbrain-hindbrain abnormalities that can be diagnosed with fetal MRI is vast (rhombencephalosynapsis, diencephalic-mesencephalic junction dysplasia, pontocerebellar hypoplasia etc.) (31). In such cases, often challenging at early gestational ages, careful evaluation of posterior fossa biometry and comparison with normative measures is pivotal. A subset of such anomalies, the so-called “cystic malformations of the posterior fossa”, constitute one of the most frequent indications for fetal MRI of the CNS. They are Mega Cisterna Magna (MCM), Dandy-Walker malformation (DWM), Persistent Blake’s Pouch (PBP) and Inferior Vermian Hypoplasia (IVH). Interestingly, they constitute a range of conditions that often have common imaging appearances, but sometimes quite different outcomes (32). At fetal MRI, DWM is characterised by hypoplastic and upwardly rotated vermis, upward displacement of the tentorium and torcula, and cystic dilation of the fourth ventricle. In PBP, a lack of or delayed fenestration of Blake’s pouch leads to an enlargement of the inferior portion of the fourth ventricle and, in some cases, hydrocephalus. In IVH, there is a reduced cranio-caudal vermian diameter due to a global or localised deficiency in its development. Interestingly, a common “early” second trimester feature of these anomalies is an upward (anticlockwise) rotation of the cerebellar vermis: predictive features of delayed cerebellar vermis de-rotation have been described and could be helpful in pregnancy management of questionable cases (32).
Chiari II malformation is another frequent indication for both spinal and brain fetal imaging (in fact, Chiari I deformity is extremely rarely reported in utero) (33). It is characterized by a sacral myelomeningocele and a small posterior fossa with herniation of the inferior part of the vermis (especially the nodulus) and tonsils, partial dislocation of the fourth ventricle, pons, and medulla into the cervical spinal canal, hydrocephalus (ventriculomegaly) and several supratentorial associated malformations. Chiari II can be easily diagnosed by ultrasound (the so-called “lemon sign”, ventriculomegaly etc.): however, iuMRI better evaluates the degree of downward displacement of cerebellum, the presence of signal changes within the brain, the type of neural tube defect (open or skin covered) and the improvement of hindbrain herniation after in utero repair of the myelomeningocele (18,33).
Cerebellar destructive lesions are also quite common in fetal imaging, including both infectious and ischemic-haemorrhagic lesions (34-36). The cerebellum is exquisitely vulnerable to abnormal events from early fetal age, and isolated cerebellar haemorrhages (not associated with supratentorial bleeding) before the 26th week of gestation can be noted on iuMRI, uncertain in nature (venous engorgement?). Such lesions may appear as malformations in post-natal scans, and unilateral cerebellar hemisphere dysplasia is a common finding. Bilateral cerebellar hypo/dysplasia is a common feature of early CMV infection (34).
Primary neurulation anomalies/neural tube defects
Errors in primary neurulation can lead to a large spectrum of spine defects, classified in two big groups: open and closed neural tube defects. The former is characterized by the exposure of a neural placode at the back of the fetus with leakage of CSF and a variable degree of neural tissue herniation from meningocele to myelomeningocele. The latter are less severe spinal dysraphism, no neural tissue is exposed because it is covered by mesoderm and ectoderm, there is no leakage of CSF and are further divided into two classes based on the presence or the absence of a subcutaneous mass.
Myelomeningocele is the most common and severe open neural tube defect, and it is associated with a range of CNS abnormalities, including the Chiari II malformation, which in turn is almost invariably associated with a myelomeningocele (33,37). The outcome is variable, including dysphagia, poor motor function, bladder dysfunction, and is related to the level and the extension of the lesion and to the presence or absence of a covering membrane (37). Although the US prenatal detection of neural tube defects is reported to be excellent (as high as 100% for myelomeningocele), iuMRI provides additional information and it is especially useful in characterizing associated CNS lesions and spinal cord status with greater detail, useful for prenatal counselling, especially in cases of questionable spinal cord integrity. MR examination could be helpful also in establishing if fetal surgery is a worthy option and for delivery planning or postnatal management (38).
CNS infections
In utero MRI is extremely helpful in detecting CNS anomalies associated with TORCH infections (Figure 7) (Toxoplasmosis, Other Agents, Rubella, Cytomegalovirus, Herpes Simplex virus). Among them, Cytomegalovirus is by far the most common in utero infection and constitutes a common cause of mental retardation and non-genetic sensorineural hearing loss, possibly associated with significant neurological impairment. Despite 90% of infants affected by congenital CMV infection being asymptomatic at birth, 10–15% of these and almost all neonates with symptomatic infection at birth will develop persistent neurologic deficits (34).
In utero MRI can depict features of CNS CMV infection with high sensitivity and specificity, even when ultrasound is normal. Among them, cortical malformations (especially polymicrogyria), white matter lesions, cerebellar hypoplasia, and temporal lobe lesions are considered quite specific. Additional common findings, but less specific, are ventriculomegaly, intraventricular septa (especially in the occipital horns), ependymal cysts, calcifications, microcephaly and microencephaly. Admitting that calcifications are better detected by ultrasound, MRI has higher sensitivity than ultrasound in the detection of polar temporal lesions, microencephaly and cortical malformations, even at early gestational age (<24 weeks). Findings like microencephaly and cortical malformations have a strong prognostic value, being related to a higher incidence of mental impairment and epilepsy (34).
Complications of monochorionic twin pregnancy
Monochorionic twin pregnancy has a higher risk of complications than dichorionic twin pregnancy, since monochorionic twins share a common placenta that often contains abnormal intertwin vascular connections. In case of complications of monochorionic twin pregnancy, such as co-twin demise and twin-to-twin transfusion syndrome (TTTS), iuMRI should be performed as early as possible to identify acute injuries and a follow-up may be necessary to detect subacute/chronic sequelae (Figure 8).
Figure 8.
Example of coronal sections from a 20-week gestation foetus with acute, probably ischemic, lesion identified by diffusion-weighting (B) and ADC map (C) (arrows), but hardly visible on T2-weighting (A). ADC, apparent diffusion coefficient.
In utero death of a co-twin is associated with an increased risk of brain injuries in the surviving co-twin: they are usually ischemic, involving the cerebral hemispheres and usually sparing the posterior fossa. Brain lesions include encephalomalacia, periventricular white matter injury, germinal matrix hemorrhages, intraventricular hemorrhage, intraparenchymal hemorrhage, and cortical malformations (36).
Twin-to-twin transfusion syndrome is characterized by abnormal blood flow from the donor twin to the recipient twin via placental vascular connections. The recipient twin develops polyhydramnios due to volume overload, and the donor twin develops oligohydramnios. Brain injuries rate is high with hemorrhagic or non-hemorrhagic lesions: they have been classified as focal and non-focal, with focal lesions occurring more frequently in pregnancies complicated by twin-twin transfusion syndrome or in which an obstetric intervention has been performed, and possibly related to thromboembolic events (36). Laser ablation of the vascular connections can be performed in cases of TTTS.
Head and neck
Common indications for fetal head and neck (H&N) imaging include masses of the H&N district (such as lymphatic malformations, hemangiomas, teratomas), abnormal shape of calvarium and face (facial cleft, craniosynostosis, micro/macrocephaly), orbital and petrous bone anomalies. In addition, iuMRI can be helpful in assessing airway obstruction, which may impact prenatal management and delivery planning (39). The detailed description of MRI findings related to such pathologies goes beyond the scope of this review.
Conclusions
Fetal MRI represents a safe and effective tool for in utero CNS evaluation. Indeed, while ultrasound remains the method of choice for fetal screening, MRI may add crucial diagnostic and prognostic information, which impact on parent counselling and pregnancy management.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editor (Felice D’Arco) for the series “Pediatric Neuroradiology for Trainees and Fellows: An Updated Practical Guide” published in Translational Pediatrics. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-293). The series “Pediatric Neuroradiology for Trainees and Fellows: An Updated Practical Guide” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Weisstanner C, Kasprian G, Gruber GM, et al. MRI of the Fetal Brain. Clin Neuroradiol 2015;25:189-96. 10.1007/s00062-015-0413-z [DOI] [PubMed] [Google Scholar]
- 2.Manganaro L, Bernardo S, Antonelli A, et al. Fetal MRI of the central nervous system: State-of-the-art. Eur J Radiol 2017;93:273-83. 10.1016/j.ejrad.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 3.Craven I, Bradburn MJ, Griffiths PD. Antenatal diagnosis of agenesis of the corpus callosum. Clin Radiol 2015;70:248-53. 10.1016/j.crad.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 4.Conte G, Parazzini C, Falanga G, et al. Diagnostic value of prenatal MR imaging in the detection of brain malformations in fetuses before the 26th week of gestational age. AJNR Am J Neuroradiol 2016;37:946-51. 10.3174/ajnr.A4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prayer D, Malinger G, Brugger PC, et al. ISUOG Practice Guidelines: performance of fetal magnetic resonance imaging. Ultrasound Obstet Gynecol 2017;49:671-80. 10.1002/uog.17412 [DOI] [PubMed] [Google Scholar]
- 6.Chartier AL, Bouvier MJ, McPherson DR, et al. The safety of maternal and fetal MRI at 3 T. AJR Am J Roentgenol 2019;213:1170-3. 10.2214/AJR.19.21400 [DOI] [PubMed] [Google Scholar]
- 7.Patenaude Y, Pugash D, Lim K, et al. The Use of Magnetic Resonance Imaging in the Obstetric Patient. J Obstet Gynaecol Can 2014; 36:349-63. 10.1016/S1701-2163(15)30612-5 [DOI] [PubMed] [Google Scholar]
- 8.Weisstanner C, Gruber GM, Brugger PC, et al. Fetal MRI at 3T-ready for routine use? Br J Radiol 2017;90:20160362. 10.1259/bjr.20160362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shellock FG, Crues JV. MR procedures: Biologic effects, safety, and patient care. Radiology 2004;232:635-52. 10.1148/radiol.2323030830 [DOI] [PubMed] [Google Scholar]
- 10.Huynh K, Baghdanian AH, Baghdanian AA, et al. Updated guidelines for intravenous contrast use for CT and MRI. Emerg Radiol 2020:27:115-26. 10.1007/s10140-020-01751-y [DOI] [PubMed] [Google Scholar]
- 11.Falanga G, Moscatelli M, Izzo G, et al. Single-Shot Version of FLAIR Sequence in the Detection of Intraventricular Anomalies: Preliminary Experience in Fetal MR Imaging. J Comput Assist Tomogr 2018;42:487-91. 10.1097/RCT.0000000000000701 [DOI] [PubMed] [Google Scholar]
- 12.Jarvis DA, Griffiths PD. Current state of MRI of the fetal brain in utero. J Magn Reson Imaging 2019;49:632-46. 10.1002/jmri.26316 [DOI] [PubMed] [Google Scholar]
- 13.Glenn OA, Barkovich AJ. Magnetic resonance imaging of the fetal brain and spine: An increasingly important tool in prenatal diagnosis, part 1. AJNR Am J Neuroradiol 2006;27:1604-11. [PMC free article] [PubMed] [Google Scholar]
- 14.Garel C, Chantrel E, Elmaleh M, et al. Fetal MRI: Normal gestational landmarks for cerebral biometry, gyration and myelination. Childs Nerv Syst 2003;19:422-5. 10.1007/s00381-003-0767-4 [DOI] [PubMed] [Google Scholar]
- 15.Kyriakopoulou V, Vatansever D, Davidson A, et al. Normative biometry of the fetal brain using magnetic resonance imaging. Brain Struct Funct 2017; 222:2295-307. 10.1007/s00429-016-1342-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conte G, Milani S, Palumbo G, et al. Prenatal brain MR imaging: Reference linear biometric centiles between 20 and 24 gestational weeks. AJNR Am J Neuroradiol 2018;39:963-7. 10.3174/ajnr.A5574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scelsa B, Rustico M, Righini A, et al. Mild ventriculomegaly from fetal consultation to neurodevelopmental assessment: A single center experience and review of the literature. Eur J Paediatr Neurol 2018;22:919-28. 10.1016/j.ejpn.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 18.Glenn OA. MR imaging of the fetal brain. Pediatr Radiol 2010; 40:68-81. 10.1007/s00247-009-1459-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouahba J, Luton D, Vuillard E, et al. Prenatal isolated mild ventriculomegaly: Outcome in 167 cases. BJOG 2006;113:1072-9. 10.1111/j.1471-0528.2006.01050.x [DOI] [PubMed] [Google Scholar]
- 20.Parazzini C, Righini A, Doneda C, et al. Is fetal magnetic resonance imaging indicated when ultrasound isolated mild ventriculomegaly is present in pregnancies with no risk factors? Prenat Diagn 2012;32:752-7. 10.1002/pd.3896 [DOI] [PubMed] [Google Scholar]
- 21.Glenn OA, Goldstein RB, Li KC, et al. Fetal Magnetic Resonance Imaging in the Evaluation of Fetuses Referred for Sonographically Suspected Abnormalities of the Corpus Callosum. J Ultrasound Med 2005;24:791-804. 10.7863/jum.2005.24.6.791 [DOI] [PubMed] [Google Scholar]
- 22.Barkovich AJ, Guerrini R, Kuzniecky RI, et al. A developmental and genetic classification for malformations of cortical development: Update 2012. Brain 2012;135:1348-69. 10.1093/brain/aws019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glenn OA, Cuneo AA, Barkovich AJ, et al. Malformations of cortical development: Diagnostic accuracy of fetal MR imaging. Radiology 2012;263:843-55. 10.1148/radiol.12102492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Righini A, Parazzini C, Doneda C, et al. Early formative stage of human focal cortical gyration anomalies: Fetal MRI. AJR Am J Roentgenol 2012;198:439-47. 10.2214/AJR.11.6662 [DOI] [PubMed] [Google Scholar]
- 25.Righini A, Genovese M, Parazzini C, et al. Cortical formation abnormalities on foetal MR imaging: a proposed classification system trialled on 356 cases from Italian and UK centres. Eur Radiol 2020;30:5250-60. 10.1007/s00330-020-06899-2 [DOI] [PubMed] [Google Scholar]
- 26.Severino M, Geraldo AF, Utz N, et al. Definitions and classification of malformations of cortical development: practical guidelines. Brain 2020;143:2874-94. 10.1093/brain/awaa174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izzo G, Talenti G, Falanga G, et al. Intrauterine fetal MR versus postmortem MR imaging after therapeutic termination of pregnancy: evaluation of the concordance in the detection of brain abnormalities at early gestational stage. Eur Radiol 2019;29:2740-50. 10.1007/s00330-018-5878-0 [DOI] [PubMed] [Google Scholar]
- 28.Patek KJ, Kline-Fath BM, Hopkin RJ, et al. Posterior fossa anomalies diagnosed with fetal MRI: Associated anomalies and neurodevelopmental outcomes. Prenat Diagn 2012;32:75-82. 10.1002/pd.2911 [DOI] [PubMed] [Google Scholar]
- 29.Limperopoulos C, Robertson RL, Khwaja OS, et al. How accurately does current fetal imaging identify posterior fossa anomalies? AJR Am J Roentgenol 2008;190:1637-43. 10.2214/AJR.07.3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinto J, Paladini D, Severino M, et al. Delayed rotation of the cerebellar vermis: a pitfall in early second-trimester fetal magnetic resonance imaging. Ultrasound Obstet Gynecol 2016;48:121-4. 10.1002/uog.15782 [DOI] [PubMed] [Google Scholar]
- 31.Jissendi-Tchofo P, Severino M, Nguema-Edzang B, et al. Update on neuroimaging phenotypes of mid-hindbrain malformations. Neuroradiology 2015;57:113-38. 10.1007/s00234-014-1431-2 [DOI] [PubMed] [Google Scholar]
- 32.Conte G, Caschera L, Parazzini C, et al. Prenatal magnetic resonance imaging within the 26th week of gestation may predict the fate of isolated upward rotation of the cerebellar vermis: insights from a multicentre study. Eur Radiol 2020;30:2161-70. 10.1007/s00330-019-06538-5 [DOI] [PubMed] [Google Scholar]
- 33.Righini A, Parazzini C, Doneda C, et al. Fetal MRI features related to the Chiari malformations. Neurol Sci 2011;32 Suppl 3:S279-81. 10.1007/s10072-011-0694-1 [DOI] [PubMed] [Google Scholar]
- 34.Doneda C, Parazzini C, Righini A, et al. Early cerebral lesions in cytomegalovirus infection: Prenatal MR imaging. Radiology 2010;255:613-21. 10.1148/radiol.10090749 [DOI] [PubMed] [Google Scholar]
- 35.Martino F, Malova M, Cesaretti C, et al. Prenatal MR imaging features of isolated cerebellar haemorrhagic lesions. Eur Radiol 2016;26:2685-96. 10.1007/s00330-015-4053-0 [DOI] [PubMed] [Google Scholar]
- 36.Conte G, Righini A, Griffiths PD, et al. Brain-injured survivors of monochorionic twin pregnancies complicated by single intrauterine death: MR findings in a multicenter study. Radiology 2018;288:582-90. 10.1148/radiol.2018171267 [DOI] [PubMed] [Google Scholar]
- 37.Chao TT, Dashe JS, Adams RC, et al. Fetal spine findings on MRI and associated outcomes in children with open neural tube defects. AJR Am J Roentgenol 2011;197:W956-61. 10.2214/AJR.11.6745 [DOI] [PubMed] [Google Scholar]
- 38.Talenti G, Giussani C, Parazzini C, et al. Early Prenatal MRI of Cervical “Abortive” Myelocystocele: Case Report and Review of the Literature. Neuropediatrics 2017;48:104-7. [DOI] [PubMed] [Google Scholar]
- 39.Mirsky DM, Shekdar K v., Bilaniuk LT. Fetal MRI. Head and Neck. Magn Reson Imaging Clin N Am 2012;20:605-18. 10.1016/j.mric.2012.06.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as