Abstract
Osteoarthritis of the knee impairs activities of daily living of those affected. Its irreversible degenerative changes to the knee joint induce functional disturbance and unpleasant arthralgia. The pain has inflammatory components and often is manifested with mechanical allodynia and hyperalgesia. Sustained weight bearing and joint movements increase pain sensitivity in knee osteoarthritis. Understanding the mechanisms underlying the mechanical allodynia and hyperalgesia might provide a therapeutical target for pain relief in patients with such symptoms. Piezo channel is a mechanically activated ion channel that may be involved in mechanical transduction in the articular cartilage. Although it has been shown that inflammation potentiates Piezo channel current induced by mechanical stimulation, whether Piezo expression levels are influenced by knee osteoarthritis has remained unknown. We measured Piezo mRNA in knee joints and dorsal root ganglia after establishing a model of knee osteoarthritis in rats using monosodium iodoacetate and found Piezo mRNA level is not upregulated. This finding raises a question as whether and how Piezo channels may be involved in mechanically induced pain in osteoarthritis.
Keywords: Osteoarthritis, mechanical allodynia, hyperalgesia, Piezo, knee, pain
Osteoarthritis of the knee impairs activities of daily living of those affected, especially the elderly.1 The principal pathology of knee osteoarthritis is irreversible degenerative change of the articular cartilage, subchondral bone, and soft tissue in joint components.1 Functional disturbance of the knee joint occurs by joint instability and capsular stiffness, which leads to increased mechanical loading stress and generates arthralgia.2 Sustained weight bearing and joint movements increase pain accompanied by inflammation in the chronic phase of knee osteoarthritis. This promotes negative emotions and reduces the quality of life of those affected.3
Piezo channels (Piezo1 and Piezo2) have been identified in vertebrates as mechanically activated ion channels that sense mechanical stimuli such as blood flow, light touch, and proprioception.4–7 Inflammation potentiates Piezo2 currents induced by mechanical stimuli in dorsal root ganglion (DRG) neurons.8 Piezo1 and Piezo2 may transduce information about injurious mechanical stress to chondrocytes in articular cartilage.9 Joint instability and arthralgia are increased as a result of knee osteoarthritis because of disrupted load-buffering systems such as cartilage and meniscus. However, it is currently unknown whether levels of Piezo mRNA expression are affected by knee osteoarthritis to cause mechanical allodynia and hyperalgesia. To address this issue, we measured Piezo mRNA in tissues of the knee joint and DRG after establishing a model of knee osteoarthritis in rats.
The rodent model of knee osteoarthritis was produced by intra-articular injections of monosodium iodoacetate. It is reliable and reproducible to induce degenerative changes of the knee joint as a result of chondrocyte necrosis by inhibition of glycolysis.10 The histological changes in the model are reported to mimic human knee osteoarthritis, in which decreased thickness of cartilage exposes the subchondral bone.11
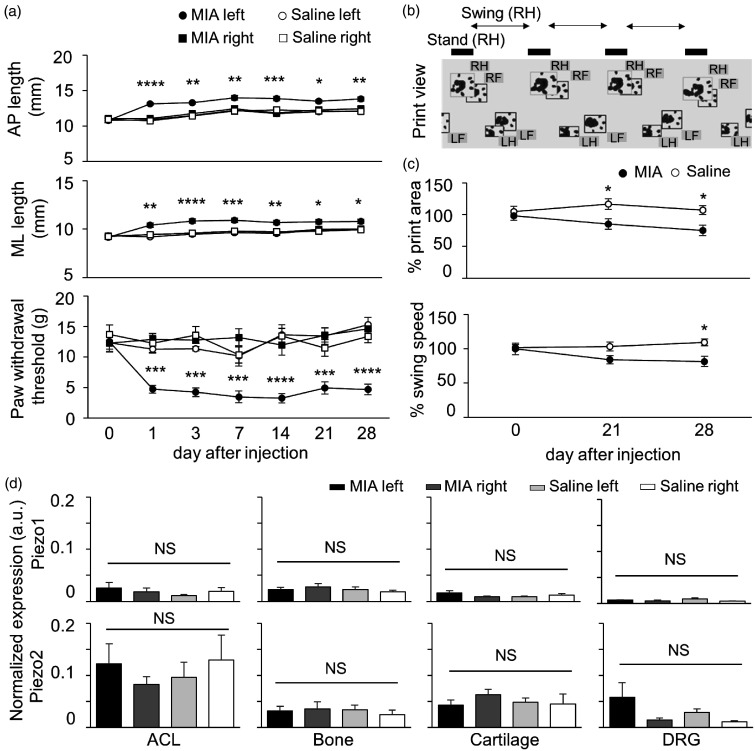
Male Sprague Dawley rats aged 6 weeks were used. Animal care and use conformed to the guidelines of the International Association for the Study of Pain and the Guiding Principles for the Care and Use of Animals in the Field of Physiological Sciences of the Physiological Society of Japan. Experimental protocols were approved by the Animal Experiment Committee at the Jikei University School of Medicine. Rats were anesthetized with 1.5%–2% isoflurane in 100% O2. To induce osteoarthritis, the left knee of each rat was injected intra-articularly with a single dose of 3 mg monosodium iodoacetate (Sigma-Aldrich) in 50 µL of physiological saline through the infrapatellar ligament using a 27 gauge needle (MIA group: n = 6). For control group, the left knees of rats were injected with 50 µL of physiological saline alone (saline group: n = 6). The right knee of every rat was not treated. All rats were housed in cages in an environmentally controlled room (temperature: around 25°C, humidity: around 50%). Body weight was measured throughout experiments and we noted no significant difference between groups (P = 0.22; two-way ANOVA). Development of arthritis was evaluated by caliper measurement of anterior–posterior and medial–lateral knee length (AP length and ML length, respectively). These values were acquired the day before injection (measured at day 0 after injection) and at 1, 3, 7, 14, 21, or 28 days after injection. MIA generated joint swelling of the ipsilateral knee (left), but not the contralateral knee (right) from day 1 to 28 days after injection (Figure 1(a) top and middle panels). On the other hand, saline alone did not cause joint swelling of the bilateral knee at any evaluation day after injection (Figure 1(a) top and middle panels).
Figure 1.
Piezo mRNA expression level in the rat model of osteoarthritis. (a) Summary of the knee joint changes and mechanical sensitivity in the model of osteoarthritis induced by injection of monosodium iodoacetate (MIA). Top and middle panels show anterior–posterior (AP) and medial–lateral (ML) length of the knee joint during the development of arthritis, respectively. The lowest panel shows a summary of paw withdrawal threshold in the rat model of osteoarthritis. Filled and open circles indicate the responses ipsilateral (left) to MIA and saline injection, respectively. Filled and open squares indicate the response contralateral (right) to MIA and saline injection, respectively. The values before injection were measured at day 0 after injection. Mean values ± standard error of the mean (SEM). *P < 0.05; **P < 0.01; ***P < 0.001 ****P < 0.0001 vs. saline left and non-injected knees (two-way ANOVA followed by a Bonferroni post hoc test). (b) Analysis of gait of rats treated to produce a model of osteoarthritis. This panel shows the print view of each paw, which was captured by CatWalk system. RH, LH, RF, and LF indicate right hind, left hind, right front, and left front, respectively. Stand (RH) and Swing (RH) show the duration in contact and no contact of a right hind paw on the glass plate, respectively. (c) Gait analysis was obtained by comparing values for the hind paw (LH) ipsilateral to the treatment to normalized values for the hind paw (RH) contralateral to the treatment. Top and bottom panel shows the ratio of print area and swing speed at each examination day. Filled and open circles indicate rats from the MIA- and saline-treated groups, respectively. Mean values ± SEM. *P < 0.05 vs. saline treated group (two-way ANOVA followed by a Bonferroni post hoc test). (d) Normalized expression levels of Piezo1 and Piezo2 mRNA were measured by quantitative polymerase chain reaction from the anterior cruciate ligament (ACL), subchondral bone (Bone), articular cartilage of medial compartment (Cartilage), and L3-4 dorsal root ganglia (DRG). Black and grey bars indicate Piezo expression levels ipsilateral (left) and contralateral (right) to MIA injection, respectively. Light grey and white bars indicate Piezo expression levels ipsilateral (left) and contralateral (right) to saline injection, respectively. Mean ± SEM. NS indicates no significant difference between groups (one-way ANOVA followed by a Bonferroni post hoc test).
Next, we examined behavioral signs of mechanical hypersensitivity and gait analysis that might result from arthralgia of the knee. Mechanical sensitivity was quantified by measuring paw withdrawal thresholds (PWTs). The PWT to mechanical stimuli was determined using calibrated von Frey filaments of different rigidities (0.4–15.0 g) as described previously.12 A 50% threshold was estimated according to an up and down method.13 The PWT of the left knee of rats in the MIA group decreased significantly compared with the right knee of rats in the MIA group, the left knee of rats in the saline group, or the right knee of rats in the saline group between day 1 after injection to the final examination (Figure 1(a) bottom panel).
Knee osteoarthritis can lead to limping because of the pain and instability of the joint. Gait was analyzed using the CatWalk system (Noldus Information Technology, Wageningen, Netherlands) described in detail elsewhere.14 Treated and control rats were placed on a glass plate located in a dark room and allowed to walk freely. When each paw touches the glass plate, light beams are reflected downwards (Figure 1(b)). The entire run image was recorded 3 times with a video camera installed below the glass plate. Acquired data were compressed and analyzed using CatWalk software. We normalized two ipsilateral hind paw values with contralateral hind paw values. One of the parameters is print area (surface area of the complete print) and the other is swing speed (speed of the paw during swing: stride length/swing time) on the day before and after injection. Consistent with recent reports, the area of ground contact on the affected side decreased from 21 days after injection, and significant limping appeared 28 days after injection (Figure 1(c)).15,16 This measurement might correspond with pain assessments in patients with knee osteoarthritis.
Finally, to detect Piezo mRNA expression in the tissues related to knee osteoarthritis, we applied quantitative RT-PCR. Bilateral anterior cruciate ligament (ACL), femoral subchondral bone (Bone), articular cartilage of medial compartment (Cartilage), and L3-4 dorsal root ganglion (DRG) innervating the knee joint were obtained from rats of each group under 2%–3% isoflurane anesthesia on the day after the final behavioral test. ACL, Bone, and Cartilage were selected as target tissues because they play a critical role in proprioception, pain sensation, and modulation of mechanical loading in the knee joint, respectively.17–19 Resected tissues were placed into microtubes that contained TRIzol Reagent (Thermo Fisher Scientific, Cat. No 15596018) and stored at –20°C. Extracted RNA was used for complementary DNA synthesis with PrimeScript RT Master Mix kit (TaKaRa, catalog No. RR036A). The total RNA of each sample was reverse transcribed with Fast SYBR Green Master Mix (Thermo Fisher Scientific, catalog No. 4385612) according to the manufacturer’s instructions, under the following thermal cycler conditions: 15 min at 37°C, 5 s at 85°C, and held at 4°C using an ABI Step One Plus apparatus (version 2.1; Thermo Fisher Scientific). The reference gene chosen was glyceraldehyde-3-phosphate dehydrogenase (GAPDH): forward GGCACAGTCAAGGCTGAGAATG, and reverse ATGGTGGTGAAGACGCCAGTA. Piezo primers were designed as described previously:6 Piezo1 forward ACAGGTCGCCTGCTTCGTGC, reverse TGCCACCAGCACTCCCAGGT; Piezo2 forward TTCGGAAGTGGTGTGCGGGC, and reverse GTAAGCGGGTGCGATGCGGT. Each sample was run in sets of three technical replicates, then averaged values were normalized by GAPDH.
We detected Piezo1 mRNA in ACL, Bone, Cartilage, and DRG. Normalized expression levels of Piezo1 mRNA were not significantly different between left and right knees in either the MIA or saline groups (Figure 1(d) top panels). Piezo2 mRNA was detected in the same target tissues as Piezo1 mRNA. In comparison with the saline left and non-injected right knees, normalized expression levels of Piezo2 mRNA in the MIA left knee were not changed significantly (Figure 1(d) bottom panels).
Our results in the present study show knee osteoarthritis did not affect the expression levels of Piezo1 or Piezo2 mRNA. However, recent paper reported that the density of Piezo2 nerve endings increased in osteoarthritic subchondral bone, which is related to pain in knee osteoarthritis.15 Synergistic action of Piezo1 and Piezo2 expression on chondrocytes is related to high strain-induced Ca2+ influx, and apoptotic cell death induced by Piezo1 mechanotransduction is suppressed through urocortin1 chondroprotection.9,20 Moreover, inflammatory mediator bradykinin increased Piezo2 currents through protein kinase A and protein kinase C.8 Cell swelling, which is induced by pathological conditions such as tissue inflammation and chemical treatment, enhances Piezo2 currents reversibly via increase of static plasma membrane tension.21 These previous findings suggest that Piezo channel functions may be potentiated without changes of the level of mRNA expression in knee osteoarthritis. Alternatively, Piezo channels may not be involved in the development of mechanical allodynia and hyperalgesia in knee osteoarthritis. Further studies will need to be performed to test these possibilities.
Footnotes
Author Contributions: RI and DA designed and performed the experiments and then acquired the data. RI and MS contributed to data analysis and interpretation. All authors read and approved the final manuscript.
Acknowledgments: We thank Drs. Fusao Kato and Keishi Marumo for giving the continuous encouragement, and Hiroko Takizawa for providing expert assistance in the experiments.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by JSPS KAKENHI Grant Number JP 17K09041 to RI, the MEXT-Supported Program for the Strategic Research Foundation at Private Universities (no. S1311009) to FK.
ORCID iD: Ryo Ikeda https://orcid.org/0000-0001-6603-7146
References
- 1.Sharma L. Osteoarthritis of the knee. N Engl J Med 2021; 384: 51–59. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz DE, Ryals AR, Block JA, Sharma L, Schnitzer TJ, Andriacchi TP. Knee pain and joint loading in subjects with osteoarthritis of the knee. J Orthop Res 2000; 18: 572–579. [DOI] [PubMed] [Google Scholar]
- 3.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. Pain 2010; 149:573–581. [DOI] [PubMed] [Google Scholar]
- 4.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010; 330: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DA, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad RK, Evans PC, Ainscough JF, Beech DJ. Piezo1 integration of vascular architecture with physiological force. Nature 2014; 515: 279–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell 2014; 157: 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA, Patapoutian A. Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci 2015; 18: 1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubin AE, Schmidt M, Mathur J, Petrus MJ, Xiao B, Coste B, Patapoutian A. Inflammatory signals enhance Piezo2-mediated mechanosensitive currents. Cell Rep 2012; 2: 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL, Wu J, Beicker KN, Coles J, Zauscher S, Grandl J, Sachs F, Guilak F, Liedtke WB. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci U S A 2014; 111: E5114–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Sousa Valente J. The pharmacology of pain associated with the monoiodoacetate model of osteoarthritis. Front Pharmacol 2019; 10: 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzman RE, Evans MG, Bove S, Morenko B, Kilgore K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol 2003; 31: 619–624. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda R, Takahashi Y, Inoue K, Kato F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain 2007; 127: 161–172. [DOI] [PubMed] [Google Scholar]
- 13.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 14.Hamers FP, Lankhorst AJ, van Laar TJ, Veldhuis WB, Gispen WH. Automate quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J Neurotrauma 2001; 18: 187–201. [DOI] [PubMed] [Google Scholar]
- 15.Zhu S, Zhu J, Zhen G, Hu Y, An S, Li Y, Zheng Q, Chen Z, Yang Y, Wan M, Skolasky RL, Cao Y, Wu T, Gao B, Yang M, Gao M, Kuliwaba J, Ni S, Wang L, Wu C, Findlay D, Eltzschig HK, Ouyang HW, Crane J, Zhou FQ, Guan Y, Dong X, Cao X. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J Clin Invest 2019; 129: 1076–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyagi M, Ishikawa T, Kamoda H, Suzuki M, Inoue G, Sakuma Y, Oikawa Y, Orita S, Uchida K, Takahashi K, Takaso M, Ohtori S. Efficacy of nerve growth factor antibody in a knee osteoarthritis pain model in mice. BMC Musculoskelet Disord 2017; 18: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma L, Pai YC. Impaired proprioception and osteoarthritis. Curr Opin Rheumatol 1997; 9: 253–258. [DOI] [PubMed] [Google Scholar]
- 18.Ogino S, Sasho T, Nakagawa K, Suzuki M, Yamaguchi S, Higashi M, Takahashi K, Moriya H. Detection of pain-related molecules in the subchondral bone of osteoarthritic knees. Clin Rheumatol 2009; 28: 1395–1402. [DOI] [PubMed] [Google Scholar]
- 19.Paranjape CS, Cutcliffe HC, Grambow SC, Utturkar GM, Collins AT, Garrett WE, Spritzer CE, DeFrate LE. A new stress test for knee joint cartilage. Sci Rep 2019; 9: 2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence KM, Jones RC, Jackson TR, Baylie RL, Abbott B, Bruhn-Olszewska B, Board TN, Locke IC, Richardson SM, Townsend PA. Chondroprotection by urocortin involves blockade of the mechanosensitive ion channel Piezo1. Sci Rep 2017; 7: 5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia Z, Ikeda R, Ling J, Viatchenko-Karpinski V, Gu JG. Regulation of Piezo2 mechanotransduction by static plasma membrane tension in primary afferent neurons. J Biol Chem 2016; 291: 9087–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]