Abstract
Background
Iron deficiency anaemia in pregnancy is common and is a major cause of maternal and neonatal morbidity worldwide. Serum ferritin is the current gold standard test for identifying iron depletion, with a cut-off value of 30 µg/L. Recent studies in low- and middle-income countries have identified mean cell haemoglobin concentration as a surrogate marker for the prediction of iron depletion.
Methods
We studied values from 786 antenatal blood results from 2018 in Oxford, UK, and correlated the red cell indices with serum ferritin measurements.
Results
Haemoglobin, mean cell volume, mean cell haemoglobin and mean cell haemoglobin concentration have low specificity and sensitivity for the identification of iron depletion.
Conclusions
We found that haemoglobin, mean cell volume, mean cell haemoglobin and mean cell haemoglobin concentration do not have sufficient predictive value in this population to be used as a screening test for non-anaemic iron depletion.
Keywords: Iron deficiency, iron depletion, pregnancy, iron deficiency anaemia
Background
Iron deficiency is considered to be the most common cause of anaemia worldwide, with estimates of the prevalence in preschool children and pregnant women of at least 30%–40% in industrialised countries when anaemia is used as an indirect indicator.1 There is a lack of evidence for the prevalence of iron depletion (ID) in the absence of anaemia, which is likely due to difficulties in the assessment of iron status with currently available tests. In pregnancy, the prevalence of iron deficiency anaemia (IDA) rises with contributory factors including increased demands for iron for the expansion of maternal and fetal red blood cell production and for fetoplacental growth in addition to usual dietary requirements, so identifying ID in the early stages of pregnancy could help to prevent IDA.2
IDA is an important cause of maternal and neonatal morbidity throughout the world. Effects on the mother include fatigue, decreased work capacity and performance, increased severity and susceptibility to infections, risk of postpartum haemorrhage and negative effects on postpartum cognition and emotions. In addition, a haemoglobin (Hb) less than 70 g/L has been shown to be independently associated with a twofold increased risk of maternal mortality. Effects of IDA on the fetus include low birth weight, prematurity, and potentially negative effects on psychomotor and mental development, as well as emotional and social behaviours.3
Current UK guidelines from the British Society of Haematology3 advise empirical treatment with oral iron replacement for pregnant women who are found to be anaemic, defined as Hb less than 110 g/L in first trimester, less than 105 g/L in second/third trimesters and less than 100 g/L post-partum. The response should be assessed after two weeks and a diagnosis of IDA made if there is an a rise in haemoglobin. These measures ensure prompt treatment of anaemia, but the opportunity to identify ID before the development of IDA is being missed.
Serum ferritin (SF) is the current gold standard for the assessment of iron status; a low level is diagnostic of ID but as it is an acute phase reactant, a normal or raised SF does not exclude it. Other markers such as transferrin saturation, serum iron or total iron binding capacity are influenced by diurnal fluctuation, recent iron ingestion or inflammation. The World Health Organization (WHO) recommendations from 20114 suggest an SF less than 15 µg/L as indicative of depleted iron stores in patients older than five years, correlating with a study from 1993 which showed a high specificity and sensitivity when SF less than 15 µg/L is used to identify ID, defined as no stainable bone marrow iron.5 In further studies, van den Broek et al.6 have found a high sensitivity and specificity of SF using a cut-off of less than 30 µg/L to assess iron stores, and Crispin et al.7 found that first trimester SF less than 30 µg/L or reduced transferrin saturation (less than 20%) is predictive of IDA at delivery. This level is used for the treatment of ID in the British Society for Haematology (BSH) guidelines pragmatically as early ID at this level ‘will worsen unless treated’.3 However, the known limitations of these tests bring into question the cost-effectiveness of routine use as a screening test on a large scale. In the UK, guidelines recommend that only non-anaemic women at increased risk of ID (see Table 1) should have SF checked. Researchers in low- and middle-income countries (LMICs) have therefore assessed whether red cell indices can be used to identify ID before anaemia ensues. Rabindrakumar et al. found that a mean cell haemoglobin concentration (MCHC) of 33.2 g/dL or less had a sensitivity of 74.6% and 51.9% specificity for the detection of ID when correlated with SF in a study of 90 women in Colombo, Sri Lanka.8 In this study, mean cell volume (MCV) of 83.2 fl or less also had a high sensitivity of 74.1% with specificity of 57.1%, while mean cell haemoglobin (MCH) of 26.9 pg or less had a sensitivity of 88.9% but a low specificity of 41.3%. A study of women in the first 20 weeks of pregnancy in Bangladesh9 found a high sensitivity and specificity of red cell distribution width (RDW) to detect IDA of 82.3% and 97.4%, respectively, when correlated with a SF less than 12ug/l.
Table 1.
Risk factors for iron depletion during pregnancy (adapted from Pavord et al.3).
| Previous anaemia |
| Multiparity (more than 3 births) |
| Consecutive pregnancy less than 1 year following delivery |
| Diets excluding animal products |
| Teenage pregnancy |
| Recent history of bleeding |
The aim of our study was to assess whether red cell indices can predict ID in pregnant women in the UK.
Methods
All antenatal booking blood requests from 1 January until 31 December 2018 from Oxford University Hospitals Maternity Service were identified. These are taken at the first contact with antenatal services, usually at 16 weeks and in some women, repeated at 28 weeks. Blood sets were identified using a laboratory database and no unique identifiers were recorded. Each blood set was recorded separately, but some may represent follow-up blood tests for women with results already recorded. Those without a concurrent SF value were excluded. Values were recorded for Hb, MCV, MCH, MCHC and SF. ID was defined as SF less than 30 µg/dL.
Statistical analysis was performed using GraphPad Prism version 6. SF was log transformed using the Normalise function, and antilogarithms were used to present the relevant data. Mean, standard deviation (SD), standard error of the mean (SEM) and 95% confidence interval (CI) of the mean were calculated using the Column Statistics function. Correlations between SF and red cell indices (Hb, MCV, MCH, MCHC) were derived with Correlation, Pearson’s correlation coefficients. Red cell indices were compared using t test (unpaired) between iron deplete (SF less than 30 µg/dL) and iron replete (SF greater than 30 µg/dL) women. Statistical significance was set at p < 0.001(*) or p < 0.0001(**).
Receiver operating characteristic (ROC) curves were constructed to identify the optimal cut-off for red cell indices (Hb, MCV, MCH, MCHC) with the most effective measure of sensitivity and specificity in diagnosing ID during early pregnancy. The red cell indices were compared against ID indicated by a SF less than 30 µg/L. Youden’s Index was calculated as (sensitivity + specificity) −1 using the ROC curves Sensitivity and Specificity tables.
Results
There were 5854 antenatal booking bloods requested in Oxford University Hospitals in 2018, and these were screened to identify those with both full blood count (FBC) and SF. There were 786 antenatal blood samples with FBC and SF identified from 625 patients. Of these, 450 samples (57.3%) had confirmed ID with SF less than 30 µg/L; mean SF was 14.6 µg/L and mean Hb 111.2 g/L. Those samples with a normal SF (n = 336) had an average SF value of 70.6 µg/L and a mean Hb of 116.3 g/L.
Blood test sets with ID were then analysed to assess the correlation between the red cell indices and SF. All of the observed indices showed a statistically significant, though weak, positive correlation with SF levels. The correlation coefficient between SF level and Hb was low, at an r value of 0.12. MCHC showed a weaker correlation, at an r value of 0.16. However, both MCV and MCH had a stronger correlation with SF levels, with r values of 0.20 (95% CI: 0.13–0.27) and 0.22 (95% CI: 0.16–0.29), respectively.
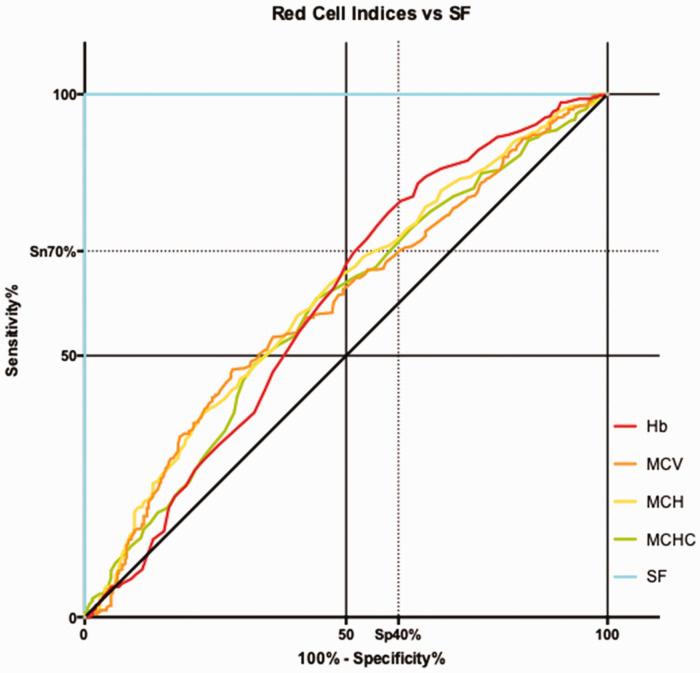
The area under the ROC curve was highest for MCH, at 0.6096. Hb, MCV and MCHC produced lower values at 0.5975, 0.5952 and 0.5864, respectively. Hb gave the highest sensitivity at 77.33% but was associated with a low specificity of 41.96%. MCH gave an optimal cut off sensitivity of 57.78% with a specificity of 59.23%. MCV had low sensitivity for ID at 47.33%, and the mean MCV of the ID samples was in the middle of the normal range at 87.81 fl. These values are shown in Table 2, and the ROC curves are illustrated in Figure 1.
Table 2.
Values for area under ROC curves and for optimum sensitivity and specificity for red cell indices for iron depletion.
| r Coefficient vs. ferritin | Area under ROC curve | Optimal cut off sensitivity (%) | Optimal cut off specificity (%) | |
|---|---|---|---|---|
| Hb | 0.12* | 0.59 | 77.3 | 41.9 |
| MCV | 0.20* | 0.59 | 47.3 | 71.7 |
| MCH | 0.22* | 0.60 | 57.8 | 59.2 |
| MCHC | 0.15* | 0.58 | 60.9 | 55.6 |
ROC: receiver operating characteristic; Hb: haemoglobin; MCV: mean cell volume; MCH: mean cell haemoglobin; MCHC: mean cell haemoglobin concentration.
*p < 0.001.
Figure 1.
ROC curves. Hb: haemoglobin; MCV: mean cell volume; MCH: mean cell haemoglobin; MCHC: mean cell haemoglobin concentration; SF: serum ferritin.
Conclusion
This study confirmed the poor specificity of Hb for the detection of iron depletion in the absence of anaemia, consistent with studies from LMIC.8–10 This is because iron stores are depleted in response to rising iron requirements for the expansion of red cell mass, so a drop in haemoglobin is a late indicator of ID.11 The low sensitivity of the MCV is not surprising, given the usual rise in MCV as pregnancy advances; correlation with gestation may improve the sensitivity of this test.
Although results from studies in LMICs have been promising, MCH was not found to be as sensitive in our population as in the study of Sri Lankan patients by Rabindrakumar et al.8 (57.78% vs. 88.9%), and so it would not be a reliable marker to identify non-anaemic patients with ID, supported by the smaller area under the ROC curve (0.64 vs. 0.61). This is despite the use of the same SF cut-off value of lesser than 30 µg/L, as per UK guidelines.
Limitations to our study are that this was a retrospective study with only laboratory values collected and no socioeconomic or demographic background data included. Therefore, patients with haemoglobinopathies, inflammatory disease and anaemia of other causes were not excluded and results were not correlated with gestation. A future prospective study could include these demographic data and exclusion criteria and could consider other measurements of iron status such as transferrin saturation, serum transferrin receptor, reticulocyte-He, RDW and percentage of hypochromic cells, which are not routinely measured in antenatal screening blood tests and may correlate with ID better than the red cell indices studied here. Reticulocyte-He and the percentage of hypochromic cells have been used to monitor iron status in patients receiving continuous ambulatory peritoneal dialysis,12 and their use in pregnancy has not been evaluated.
In conclusion, Hb level is poorly sensitive and specific for ID so is not a useful test in the detection of non-anaemic ID. MCV, MCH and MCHC show some correlation with SF less than 30 µg/L in pregnant women, but the correlation is not strong enough for these red cell indices to be used as a valid screening test for ID in pregnancy in the UK. Recommended management therefore should not change from the current BSH guidelines for the assessment of iron status with SF at booking for women at risk of ID. Outside these groups, a trial of oral iron therapy is used as the most cost and time effective response to anaemia in pregnancy, with a haemoglobin rise after two weeks of treatment considered diagnostic of IDA.3 WHO recommendations are for universal supplementation of 60 mg elemental iron/day in pregnant women,2 but this takes into account the severely depleted iron stores prevalent in women in developing countries. There is an increased risk of accidental iron poisoning in children with this strategy, so universal supplementation is not recommended in the UK.3 An individualised approach to iron supplementation based on Hb and SF should be undertaken.
Acknowledgements
The authors thank Kevin Paddon for providing the data.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This was discussed with Oxford University Hospitals Joint Research Office and NHS ethics approval was not required.
Informed consent: Informed written consent was not required: anonymised laboratory data was used.
Guarantor: Oxford University Hospitals NHS Foundation Trust.
Contributorship: This manuscript was written by SMV and SP with statistical analysis by GM.
References
- 1.Daru J, Zamora J, Fernández-Félix BM, et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: a multilevel analysis. Lancet Glob Health 2018; 6: e548–e554. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Iron deficiency anaemia: assessment, prevention, and control – a guide for programme managers. Geneva: World Health Organization, 2001. [Google Scholar]
- 3.Pavord S, Myers B, Robinson S, et al. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol 2012; 156: 588–600. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Serum ferritin concentrations for assessment of iron status and iron deficiency in populations. Vitam Miner Nutr Inf Syst Geneva, World Health Organization, 2011 (WHO/NMH/NHD/MNM/11.2). Available at: http://www.who.int/vmnis/indicators/serum_ferritin.pdf (accessed 30 April 2019). [Google Scholar]
- 5.Hallberg L, Bengtsson C, Lapidus L, et al. Screening for iron deficiency: an analysis based on bone-marrow examinations and serum ferritin determinations in a population sample of women. Br J Haematol 1993; 85: 787–798. [DOI] [PubMed] [Google Scholar]
- 6.van den Broek V, Letsky EA, White SA, et al. Iron status in pregnant women: which measurements are valid? Br J Haematol 1998; 103: 817–824. [DOI] [PubMed] [Google Scholar]
- 7.Crispin P, Stephens B, McArthur E, et al. First trimester ferritin screening for pre-delivery anaemia as a patient blood management strategy. Transfus Apher Sci 2019; 58: 50–57. [DOI] [PubMed] [Google Scholar]
- 8.Rabindrakumar MSK, Pujitha Wickramasinghe V, Gooneratne L, et al. The role of haematological indices in predicting early iron deficiency among pregnant women in an urban area of Sri Lanka. BMC Hematol 2018; 18: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sultana GS, Haque SA, Sultana T, et al. Role of red cell distribution width (RDW) in the detection of iron deficiency anaemia in pregnancy within the first 20 weeks of gestation. Bangladesh Med Res Counc Bull 2011; 37: 102–105. [DOI] [PubMed] [Google Scholar]
- 10.Tiwari M, Kotwal J, Kotwal A, et al. Correlation of haemoglobin and red cell indices with serum ferritin in Indian women in second and third trimester of pregnancy. Med J Armed Forces India 2013; 69: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiss JE, Birch RJ, Steele WR, et al. Quantification of body iron and iron absorption in the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion 2017; 57: 1656–1664. [DOI] [PubMed] [Google Scholar]
- 12.Richardson D, Bartlett C, Jolly H, et al. Intravenous iron for CAPD populations: proactive or reactive strategies? Nephrol Dial Transplant 2001; 16: 115–119. [DOI] [PubMed] [Google Scholar]