Abstract
Euglycemic diabetic ketoacidosis (DKA) is an acute life-threatening metabolic emergency characterized by ketoacidosis and relatively lower blood glucose (less than 11 mmol/L). The absence of hyperglycemia is a conundrum for physicians in the emergency department and intensive care units; it may delay diagnosis and treatment causing worse outcomes. Euglycemic DKA is an uncommon diagnosis but can occur in patients with type 1 or type 2 diabetes mellitus. With the addition of sodium/ glucose cotransporter-2 inhibitors in diabetes mellitus management, euglycemic DKA incidence has increased. The other causes of euglycemic DKA include pregnancy, fasting, bariatric surgery, gastroparesis, insulin pump failure, cocaine intoxication, chronic liver disease and glycogen storage disease. The pathophysiology of euglycemic DKA involves a relative or absolute carbohydrate deficit, milder degree of insulin deficiency or resistance and increased glucagon/insulin ratio. Euglycemic DKA is a diagnosis of exclusion and should be considered in the differential diagnosis of a sick patient with a history of diabetes mellitus despite lower blood glucose or absent urine ketones. The diagnostic workup includes arterial blood gas for metabolic acidosis, serum ketones and exclusion of other causes of high anion gap metabolic acidosis. Euglycemic DKA treatment is on the same principles as for DKA with correction of dehydration, electrolytes deficit and insulin replacement. The dextrose-containing fluids should accompany intravenous insulin to correct metabolic acidosis, ketonemia and to avoid hypoglycemia.
Keywords: Diabetic Ketoacidosis, Sodium/glucose co-transporter-2 inhibitors, Pregnancy with diabetic ketoacidosis, Diabetes complications, Pregnancy in diabetes, Ketosis, Metabolic acidosis
Core Tip: Euglycemia diabetic ketoacidosis (DKA) is an uncommon, life-threatening emergency with lower normal blood glucose. Euglycemic DKA can occur in both types of diabetes mellitus, and the absence of hyperglycemia may delay diagnosis with worse outcomes. The use of sodium/glucose cotransporter-2 (SGLT-2) inhibitors as a therapeutic option in the management of diabetes mellitus has increased the incidence of euglycemic DKA. Euglycemic DKA should be considered in any unexplained metabolic acidosis with a history of diabetes mellitus and associated risk factors. Patients on SGLT-2 inhibitors must be educated about potential risk factors for euglycemic DKA and dose adjustment for sick days.
INTRODUCTION
Diabetic ketoacidosis (DKA) is widely known as a life-threatening acute complication of diabetes mellitus (DM). It mainly occurs in patients with type 1 DM; however, any acute illness like infection, trauma or acute coronary syndrome may also trigger DKA in type 2 DM. Hyperglycemia (plasma glucose > 14 mmol/L) is a hallmark in the diagnosis of DKA completing the triad with metabolic acidosis and ketonemia[1,2].
Euglycemic DKA is defined as ketoacidosis (pH < 7.3 or serum bicarbonate < 18 mmol/L) with either near-normal plasma glucose or a milder degree of hyperglycemia (11-14 mmol/L)[3,4]. The absence of hyperglycemia can conceal the underlying DKA creating a diagnostic dilemma especially in the emergency department, which is associated with worse outcomes[3-5]. Dehydration in euglycemic DKA is also minor in the absence of polyuria and polydipsia. Patients may present instead with malaise, anorexia and tachypnoea because of ketonemia and accompanying ketoacidosis. The high index of suspicion with early testing for metabolic acidosis and blood ketones can identify these patients[4].
Euglycemic DKA was first described in 1973 by Munro et al[6] among type 1 DM. Euglycemic DKA is an uncommon diagnosis with an incidence ranging between 2.6% to 3.2% of admissions with DKA[7,8]. In a study by Munro et al[6], the incidence of euglycemic DKA had an incidence of 3.2%, using a plasma glucose cut-off of less than 16.7 mmol/L[8]. However, the cut-off of plasma glucose used for euglycemic DKA in recent reviews is lower (either 14 mmol/L[3] or 11 mmol/L[4]). Authors have also used other terminology for euglycemia, like near-normal or lower than anticipated plasma glucose[9,10]. The true incidence of euglycemic DKA is therefore unknown. With the introduction of sodium/glucose cotransporter-2 (SGLT-2) inhibitors in DM management, there is a definitive increase in the published case reports or series on euglycemic DKA[9,11]. The other common causes of euglycemic DKA are pregnancy and prolonged fasting. In this review, we will discuss the pathophysiology, diagnostic considerations and management of euglycemic DKA.
PATHOPHYSIOLOGY OF EUGLYCEMIC DKA
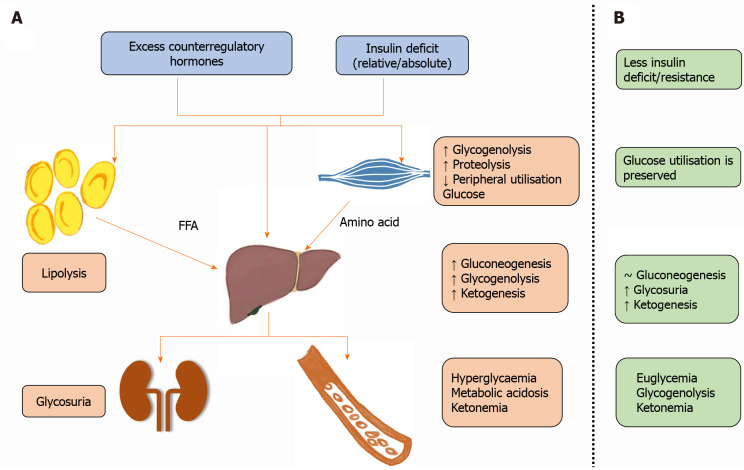
The pathophysiology of DKA is already very well-known, characterized by a relative or absolute deficiency of insulin and excess of counterregulatory (or counter responsive) hormones like glucagon, corticosteroids, catecholamines or growth hormones[1]. The hormonal imbalance causes hyperglycemia by increasing glycogenolysis, hepatic gluconeogenesis and decreased peripheral utilization of glucose. It also promotes gluconeogenesis and ketogenesis from free fatty acid mobilization by lipolysis in adipose tissue and proteolysis of amino acids[1]. Ketone bodies (beta-hydroxybutyrate, acetoacetate and acetone) are responsible for metabolic acidosis, while hyperglycemia through glycosuria and osmotic diuresis causes dehydration and hypovolemia (Figure 1).
Figure 1.
Ketone bodies (beta-hydroxybutyrate, acetoacetate and acetone) are responsible for metabolic acidosis, while hyperglycemia through glycosuria and osmotic diuresis causes dehydration and hypovolemia. A: Pathophysiology of diabetic ketoacidosis; B: Pathophysiology of euglycemic diabetic ketoacidosis. FFA: Free fatty acids; ↑: Increase; ↓: Decrease; ~: No change.
Carbohydrate deficit has a pivotal role in the pathophysiology of euglycemic DKA, while insulin deficit or insulin resistance is relatively minor and secondary (Figure 1B). However, the counterregulatory hormone production is unabated, causing an increased glucagon/insulin ratio and triggering ketogenesis with no significant change in hepatic gluconeogenesis and peripheral glucose utilization[3,4,12]. The precipitating causes for euglycemic DKA include fasting or prolonged physical activity with depleted hepatic glycogen stores and hence impaired glycogenolysis[3,12]. Increased glucagon also promotes lipid oxidation, generating acetyl-CoA and ketone bodies when glycolysis intermediates are unavailable due to reduced intracellular glucose oxidation. The unabated ketonemia and glycosuria (seen usually with SGLT-2 inhibitors) contribute to euglycemic (or hypoglycemic) DKA[4,9].
The three common causes of euglycemic DKA are SGLT-2 inhibitors, pregnancy and prolonged fasting.
SGLT-2 inhibitors
SGLT-2 inhibitors are the latest group of medications added to the arsenal to treat patients with DM. Their promotion in type 2 DM is due to clinical trials suggesting protection against major adverse cardiovascular events and reduced hospitalization for heart failure and deaths[13]. SGLT-2 inhibitors have also been shown to slow chronic kidney disease progression in type 2 DM[14,15]. The added advantages appeared to be modest weight reduction and lower systolic blood pressure aside from its effect on hyperglycemia[13,15]. SGLT-2 inhibitors act by blocking the SGLT-2 cotransporter located in the early proximal renal tubule, which is responsible for the reabsorption of most (80%-90%) of the glucose filtered by the glomerulus. It leads to glucosuria and resultant lowering of blood plasma glucose concentration[16,17]. The exact mechanism that can precipitate DKA in susceptible individuals includes, osmotic diuresis along with glucosuria (causing a state of carbohydrate deficit), volume depletion and dehydration[9,16]. Carbohydrate deficit and hypovolemia promote glucagon release, increase glucagon/insulin ratio and trigger ketogenesis with euglycemia. The other factors include the direct effect of SGLT-2 inhibitors on pancreatic alpha cells, causing glucagon release and inhibiting ketone bodies excretion by the kidneys[18,19].
There has been a steady increase in the published reports on DKA with the growing use of SGLT-2 inhibitors[9]. The exact incidence rate of SGLT-2 inhibitors associated with DKA is unknown. The clinical trials of SGLT-2 inhibitors with type 2 DM have reported an incidence of 0.16 to 0.76 events per 1000 patient-years[9,20]. In a sizeable multicentric cohort study by the Canadian Network for Observational Drug Effect Studies, the incidence of DKA with SGLT-2 inhibitors in type 2 DM was 1.40 (1.29-1.53) per 1000 patient-years. The risk of DKA was nearly three-fold higher with SGLT-2 inhibitors than dipeptidyl peptidase-4 inhibitors. The increased risk of DKA was observed with all three SGLT-2 inhibitors suggesting a class effect, with canagliflozin (hazard ratio 3.58) having the highest risk[21]. In an analysis of the Food and Drug Administration’s adverse event reporting system on DKA incidence with SGLT-2 inhibitors, there was a seven-fold increased risk, and around two-thirds of the reported DKA cases were euglycemic[22]. The risk is higher in patients with significant insulin insufficiency or type 1 DM (up to 9%)[9,16].
The United States Food and Drug Administration has warned against the risk of DKA with SGLT-2 inhibitors and so far has not approved its use for type 1 DM[23]. The risk of DKA with SGLT-2 inhibitors in type 1 DM varies widely across the published data of different randomized controlled trials, and factors responsible for such variation are not well understood. In a trial of dapagliflozin evaluation in patients with inadequately controlled type 1 diabetes[24], a significant number of patients in the dapagliflozin groups had DKA as compared to placebo at 52 weeks of follow-up. The risk of DKA was 4.0%, 3.4% and 1.9% in the dapagliflozin 5 mg, 10 mg and placebo groups, respectively. The DKA rate was also higher in the empagliflozin 10 mg and 25 mg groups compared with placebo in the empagliflozin as adjunctive to insulin therapy program[24]. The DKA rate was 4.3% and 3.3% with empagliflozin 25 mg and 10 mg groups, respectively, compared to 1.2% in the placebo group. It corresponds to an incidence of 5.9, 5.1 and 1.8 per 1000 patient-years[25], respectively. A similar rate of DKA has been observed in clinical trials of sotagliflozin and canagliflozin[26,27].
The duration of SGLT-2 inhibitor treatment before a diagnosis of DKA onset is hugely variable in the literature (0.3-420 days)[28]. In a recent meta-analysis by Musso et al[29], the risk factors of DKA with SGLT-2 inhibitors in type 1 DM included (1) baseline body mass index > 27 kg/m2; (2) insulin resistance calculated by estimated glucose disposal rate < 8.3 mg/kg/min; (3) the ratio of total insulin dose reduction-to-baseline insulin sensitivity; and (4) degree of volume depletion. These risk factors should be considered by clinicians while using SGLT-2 inhibitors in type 1 DM to reduce the risk of DKA. Recently, the National Institute for Health and Care Excellence revised its guidance and recommended SGLT-2 inhibitors for the treatment of type 1 DM[30]. The patients with a body mass index of 27 kg/m2 or more, insulin requirement of 0.5 units/kg of body weight/day or more and inadequate glycemic control despite optimal insulin therapy can be considered for the addition of dapagliflozin with insulin under supervision of a physician. However, the patient should receive education on the risk, signs and symptoms of DKA. They should also be trained on home monitoring of blood ketones and on appropriate action-plan in case of elevated blood ketones[30].
DKA in patients on SGLT-2 inhibitors can be precipitated by one of these causes (Table 1). The excessive reduction (> 50%) or omission of insulin doses, insulin pump failure or malfunction, a low carbohydrate diet, nausea and vomiting induced by other drug combination like glucagon-like peptide 1 agonists, excessive alcohol intake, acute stressful conditions like myocardial infarction, heart failure, infections or fever, trauma and surgery[9,10]. The SGLT-2 inhibitor prescription in a new-onset DM without establishing the mechanism of hyperglycemia can also precipitate DKA in undiagnosed type 1 DM[28].
Table 1.
Precipitating causes for euglycemic diabetic ketoacidosis and their mechanisms
|
Risk factors
|
Pathophysiology
|
| Infection | Insulin resistance due to counterregulatory hormones (adrenaline, glucagon, etc.), increased peripheral glucose utilization, decreased intake (nausea, vomiting) |
| Surgery | Perioperative fasting, gastrointestinal surgery has increased incidence as fasting is prolonged and/or gut absorption is slow |
| Fasting | Decreased glycogen stores, increased risk with SGLT-2 inhibitors and type 1 DM |
| Alcohol intake | Deceased carbohydrate intake, osmotic diuresis, increased ketogenesis (beta hydroxybutyrate) due to altered NADH/NAD ratio, increased risk in patients on SGLT-2 inhibitors |
| Acute vascular events (ACS or stroke) | Increased counterregulatory hormones, decreased oral intake |
| Trauma | Decreased oral intake, increased counterregulatory hormone, blood glucose dilution by large fluid shifts during resuscitation |
| Prolonged physical activity or exercise | Increased counterregulatory hormones, increased peripheral glucose utilization, decreased carbohydrate intake |
ACS: Acute coronary syndrome; DM: Diabetes mellitus; NAD: Nicotinamide adenine dinucleotide; NADH: Nicotinamide adenine dinucleotide hydrogen; SGLT2: Sodium/glucose cotransporter-2.
Pregnancy
DKA incidence in pregnancy is significantly higher than in nonpregnant females (8.9% vs 3.1%) and associated with lower blood glucose levels and increased perinatal morbidity and mortality[31,32]. There are various case reports of euglycemic DKA in pregnancy with type 1 DM, type 2 DM and gestational DM[32-36]. The physiological changes of pregnancy include hypoinsulinemia and carbohydrate deficit to match the glucose requirement of the fetus and placenta[31,35,36]. The respiratory alkalosis seen with pregnancy and compensatory urinary loss of bicarbonate reduces the body reserves to buffer metabolic acidosis. There is also an insulin resistance caused by counterregulatory pregnancy hormones (progesterone, estrogen, human placental lactogen and tumor necrosis factor-α) seen during the second and third trimester of pregnancy. Euglycemia DKA is also common during pregnancy due to physiological hemodilution of blood glucose and increased glomerular filtration rate with glucosuria[31,35,36]. Any acute illness like infection, vomiting, fasting or short starvation can trigger ketogenesis in pregnancy. Ketogenesis and metabolic acidosis during pregnancy occur faster than when not pregnant and at lower blood sugar levels[31,35,36]. Any unexplained acidosis with a history of nausea, vomiting and decreased intake in a pregnant should raise a suspicion of euglycemic DKA[4].
Fasting
Low-calorie intake, especially with intercurrent illness in patients with type 2 DM, can precipitate DKA with euglycemia[37,38]. Patients with type 1 DM who do not adjust their insulin to low carbohydrate intake while fasting or ill can also develop euglycemic DKA[38,39]. Fasting produces a carbohydrate deficit and depletion of glycogen stores leading to alternative energy sources like free fatty acids and lipolysis[39]. Continued intake of insulin and depleted glycogen stores maintain a euglycemic state while lipolysis and ketogenesis remain unabated, triggering euglycemic DKA. A very restricted carbohydrate diet or starvation can also cause euglycemic DKA in patients without DM[4]. The fasting-induced euglycemic DKA must be differentiated from starvation ketosis in which metabolic acidosis is not present (serum bicarbonate > 18 mmol/L)[4,40]. However, euglycemic DKA during fasting or starvation is familiar with type 1 DM vs nondiabetic patients.
The keto diet, characterized by a low carbohydrate and high fat diet, is promoted as a popular weight-loss method and other physical or metabolic benefits[41,42]. The carbohydrate deficit and excess of fatty acids promote ketogenesis and divert ketones bodies as a source of nutrition. The weight loss is caused by reduced insulin requirement, ketone-induced osmotic diuresis and decreased oral intake because of ketonemia. The keto diet has been tried effectively in type 2 DM with weight loss benefits, better glycemic control and medication reduction for a short duration[42-45]. The benefit is found more in patients with obesity and rigorous compliance with the diet. However, the long-term effects on glycemic control, adherence and safety in patients with DM are unproven[45]. The keto diet can precipitate DKA in type 2 DM, especially during pregnancy or SGLT-2 inhibitors with a higher incidence of euglycemic DKA[44-49]. The safety of the keto diet has not been demonstrated in type 1 DM due to the risk of ketonemia and hypoglycemia[42,50].
Other causes
Euglycemic DKA has been rarely reported with other conditions like bariatric surgery[51-53], acute pancreatitis[54], sepsis[36,55], cocaine intoxication[56], insulin pump failure[56] and gastroparesis[57]. The patients undergoing bariatric surgery are prone to DKA because of perioperative deficient carbohydrate diet and prolonged fasting[4,53]. Euglycemic DKA risk is higher in type 1 DM, patients on SGLT-2 inhibitors and prolonged perioperative fasting during bariatric surgery[51,52]. Exogenous insulin administration in patients with DKA while en route to the hospital can also present lower blood glucose on admission[1].
DIAGNOSIS
Euglycemic DKA is an acute life-threatening medical emergency. The absence of hyperglycemia delays euglycemic DKA diagnosis in the emergency department or intensive care unit[3,4]. However, euglycemic DKA is a diagnosis of exclusion, and other causes of high anion gap metabolic acidosis must be excluded[3]. The common causes of high anion gap metabolic acidosis are alcoholic intoxication (excessive ethanol or toxic alcohols like methanol or polyethylene glycol), sepsis, lactic acidosis, drug overdoses (salicylate and tricyclic antidepressants) and renal failure. Other differential diagnoses include alcoholic ketoacidosis, chronic liver disease, starvation ketosis and glycogen storage disease.
Alcoholic ketoacidosis is seen in patients with chronic alcoholism[3,7]. The patient is in a state of chronic carbohydrate deficit and is dependent on alcohol for calories. Any acute illness that can cause an inability to consume alcohol triggers ketonemia and ketoacidosis. The presentation is similar to euglycemic DKA with gastrointestinal symptoms (nausea, vomiting or abdominal pain), metabolic acidosis and ketonemia. Some authors consider alcoholic ketoacidosis as a subtype of euglycemic DKA[3,7]. The pathophysiology is also similar with an increased glucagon/insulin ratio. However, a history of binge alcohol consumption, nondiabetic and hypoglycemia instead of euglycemia helps diagnose alcoholic ketoacidosis[58]. The ketone bodies in alcoholic ketoacidosis are predominantly β-hydroxybutyrate (instead of acetoacetate), which could not be detected on routine urine strip testing[59]. Serum ketones must be used for detection of ketonemia in the cases of suspicion of alcoholic ketoacidosis.
Euglycemic ketoacidosis because of either fasting or any intercurrent illness with reduced calorie intake needs to be differentiated from starvation ketosis[4,40]. No previous history of DM, no intercurrent illness and hypoglycemia differentiate starvation ketosis. The metabolic acidosis is also not profound, with bicarbonate levels usually more than 18 mmol/L.
Sepsis[36,55] with or without associated lactic acidosis is a common presentation in an emergency that can conceal euglycemic DKA. High lactate levels with the absence of serum ketones help in the diagnosis of sepsis.
Unexplained high anion gap metabolic acidosis in a patient with DM and associated risk factors should raise suspicion of euglycemic DKA. A detailed history of risk factors like pregnancy, surgery, fasting, infections and SGLT-2 inhibitors should be evaluated (Table 1). The laboratory tests include serum and urine ketones, electrolytes (including calcium and magnesium), glucose, renal function (creatinine, blood urea nitrogen), blood gas analysis (venous or arterial), lactic acid, chest radiograph and electrocardiogram. Wide osmolar gap (the difference between measured and calculated serum osmolarity), inebriate state and multiorgan involvement help to diagnose toxic alcohol ingestion. History and symptoms of infection with laboratory tests showing leukocytosis, procalcitonin, organ dysfunction and lactate help diagnose sepsis and septic shock.
TREATMENT
The treatment is straightforward once the diagnosis is made. The treatment is based on the same principles as in DKA[1]: Insulin to correct metabolic acidosis and anion gap and correction of electrolytes and dehydration. The fluid resuscitation is similar to DKA with correction of dehydration and starts with balanced crystalloids. Insulin replacement using a fixed rate intravenous insulin infusion calculated on 0.1 units/per kilogram body weight should be continued until anion gap (metabolic acidosis) correction, and the patient can accept orally. However, an early glucose requirement (for prevention of hypoglycemia) allows concomitant insulin infusion to suppress ketogenesis[3]. Dextrose (10% or 5%) and intravenous infusion of insulin must be used until the anion gap and metabolic acidosis is corrected. The resolution of DKA is defined as pH > 7.3 units, bicarbonate > 15.0 mmol/L and blood ketone level < 0.6 mmol/L[1]. Patients may require intensive care unit admission and monitoring for hemodynamic and electrolyte disturbances. The laboratory monitoring for acidosis, glucose and electrolytes must be similar to DKA management.
PREVENTION OF EUGLYCEMIC DKA
The patients who are prescribed SGLT-2 inhibitors should be explained the risk factors of DKA (Table 1). The off-label use of SGLT-2 inhibitors in type 1 DM should be done with close monitoring, starting with a lower dose, personalized insulin reduction regimen and patient education on carbohydrate intake[9,10]. Patient education on “sick days” and other lifestyle modifications are essential and should be done in patients with type 1 and type 2 DM[9]. Education about stopping SGLT-2 inhibitors when feeling ill or feverish, prolonged exercise, fasting or excessive alcohol intake must be done. The drug should also be stopped 3-4 days before planned surgery and adjust the insulin regimen accordingly[9,10].
CONCLUSION
Euglycemic DKA can be a missed diagnosis in the emergency department with worse outcomes. The presence of metabolic acidosis in a patient with DM and risk factors should be assessed for ketonemia, even in the absence of hyperglycemia. Patients on SGLT-2 inhibitors must be educated about risk factors and dose adjustment for sick days.
Footnotes
Conflict-of-interest statement: Prashant Nasa has received fees for serving as an advisory board member for Edward Life Sciences. Other authors declare no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: January 22, 2021
First decision: February 25, 2021
Article in press: March 25, 2021
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United Arab Emirates
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Patoulias DI S-Editor: Zhang L L-Editor: Filipodia P-Editor: Ma YJ
Contributor Information
Prashant Nasa, Department of Critical Care Medicine, NMC Specialty Hospital, Dubai 7832, United Arab Emirates. dr.prashantnasa@hotmail.com.
Sandeep Chaudhary, Department of Endocrinology, NMC Specialty Hospital, Dubai 7832, United Arab Emirates.
Pavan Kumar Shrivastava, Department of Internal Medicine, NMC Specialty Hospital, Dubai 7832, United Arab Emirates.
Aanchal Singh, Department of Critical Care Medicine, NMC Specialty Hospital, Dubai 7832, United Arab Emirates.
References
- 1.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Islam T, Sherani K, Surani S, Vakil A. Guidelines and controversies in the management of diabetic ketoacidosis - A mini-review. World J Diabetes. 2018;9:226–229. doi: 10.4239/wjd.v9.i12.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modi A, Agrawal A, Morgan F. Euglycemic Diabetic Ketoacidosis: A Review. Curr Diabetes Rev. 2017;13:315–321. doi: 10.2174/1573399812666160421121307. [DOI] [PubMed] [Google Scholar]
- 4.Barski L, Eshkoli T, Brandstaetter E, Jotkowitz A. Euglycemic diabetic ketoacidosis. Eur J Intern Med. 2019;63:9–14. doi: 10.1016/j.ejim.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Plewa MC, Bryant M, King-Thiele R. Euglycemic Diabetic Ketoacidosis 2021. [PubMed] [Google Scholar]
- 6.Munro JF, Campbell IW, McCuish AC, Duncan LJ. Euglycaemic diabetic ketoacidosis. Br Med J. 1973;2:578–580. doi: 10.1136/bmj.2.5866.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu X, Zhang S, Zhang L. Newer Perspectives of Mechanisms for Euglycemic Diabetic Ketoacidosis. Int J Endocrinol. 2018;2018:7074868. doi: 10.1155/2018/7074868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins D, Close CF, Krentz AJ, Nattrass M, Wright AD. Euglycaemic diabetic ketoacidosis: does it exist? Acta Diabetol. 1993;30:251–253. doi: 10.1007/BF00569937. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg RM, Berard LD, Cheng AYY, Gilbert JD, Verma S, Woo VC, Yale JF. SGLT2 Inhibitor-associated Diabetic Ketoacidosis: Clinical Review and Recommendations for Prevention and Diagnosis. Clin Ther 2016; 38: 2654-2664. :e1. doi: 10.1016/j.clinthera.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, Ferrannini E, Fonseca VA, Garber AJ, Grunberger G, LeRoith D, Umpierrez GE, Weir MR. American association of clinical endocrinologists and american college of endocrinology position statement on the association of sglt-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016;22:753–762. doi: 10.4158/EP161292.PS. [DOI] [PubMed] [Google Scholar]
- 11.Burke KR, Schumacher CA, Harpe SE. SGLT2 Inhibitors: A Systematic Review of Diabetic Ketoacidosis and Related Risk Factors in the Primary Literature. Pharmacotherapy. 2017;37:187–194. doi: 10.1002/phar.1881. [DOI] [PubMed] [Google Scholar]
- 12.Bonora BM, Avogaro A, Fadini GP. Euglycemic Ketoacidosis. Curr Diab Rep. 2020;20:25. doi: 10.1007/s11892-020-01307-x. [DOI] [PubMed] [Google Scholar]
- 13.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 14.Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, Bompoint S, Levin A, Jardine MJ. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7:845–854. doi: 10.1016/S2213-8587(19)30256-6. [DOI] [PubMed] [Google Scholar]
- 15.Toyama T, Neuen BL, Jun M, Ohkuma T, Neal B, Jardine MJ, Heerspink HL, Wong MG, Ninomiya T, Wada T, Perkovic V. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes Metab. 2019;21:1237–1250. doi: 10.1111/dom.13648. [DOI] [PubMed] [Google Scholar]
- 16.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment With Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care. 2015;38:1687–1693. doi: 10.2337/dc15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packer M. SGLT2 Inhibitors Produce Cardiorenal Benefits by Promoting Adaptive Cellular Reprogramming to Induce a State of Fasting Mimicry: A Paradigm Shift in Understanding Their Mechanism of Action. Diabetes Care. 2020;43:508–511. doi: 10.2337/dci19-0074. [DOI] [PubMed] [Google Scholar]
- 18.Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thévenet J, Beaucamps C, Delalleau N, Popescu I, Malaisse WJ, Sener A, Deprez B, Abderrahmani A, Staels B, Pattou F. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21:512–517. doi: 10.1038/nm.3828. [DOI] [PubMed] [Google Scholar]
- 20.Erondu N, Desai M, Ways K, Meininger G. Diabetic Ketoacidosis and Related Events in the Canagliflozin Type 2 Diabetes Clinical Program. Diabetes Care. 2015;38:1680–1686. doi: 10.2337/dc15-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douros A, Lix LM, Fralick M, Dell'Aniello S, Shah BR, Ronksley PE, Tremblay É, Hu N, Alessi-Severini S, Fisher A, Bugden SC, Ernst P, Filion KB. Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Diabetic Ketoacidosis: A Multicenter Cohort Study. Ann Intern Med. 2020;173:417–425. doi: 10.7326/M20-0289. [DOI] [PubMed] [Google Scholar]
- 22.Blau JE, Tella SH, Taylor SI, Rother KI. Ketoacidosis associated with SGLT2 inhibitor treatment: Analysis of FAERS data. Diabetes Metab Res Rev. 2017;33 doi: 10.1002/dmrr.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. December 2015 [cited 17 January 2021]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about-too-much-acid-blood-and-serious .
- 24.Dandona P, Mathieu C, Phillip M, Hansen L, Tschöpe D, Thorén F, Xu J, Langkilde AM DEPICT-1 Investigators. Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes: The DEPICT-1 52-Week Study. Diabetes Care. 2018;41:2552–2559. doi: 10.2337/dc18-1087. [DOI] [PubMed] [Google Scholar]
- 25.Rosenstock J, Marquard J, Laffel LM, Neubacher D, Kaspers S, Cherney DZ, Zinman B, Skyler JS, George J, Soleymanlou N, Perkins BA. Empagliflozin as Adjunctive to Insulin Therapy in Type 1 Diabetes: The EASE Trials. Diabetes Care. 2018;41:2560–2569. doi: 10.2337/dc18-1749. [DOI] [PubMed] [Google Scholar]
- 26.Sands AT, Zambrowicz BP, Rosenstock J, Lapuerta P, Bode BW, Garg SK, Buse JB, Banks P, Heptulla R, Rendell M, Cefalu WT, Strumph P. Sotagliflozin, a Dual SGLT1 and SGLT2 Inhibitor, as Adjunct Therapy to Insulin in Type 1 Diabetes. Diabetes Care. 2015;38:1181–1188. doi: 10.2337/dc14-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and Safety of Canagliflozin, a Sodium-Glucose Cotransporter 2 Inhibitor, as Add-on to Insulin in Patients With Type 1 Diabetes. Diabetes Care. 2015;38:2258–2265. doi: 10.2337/dc15-1730. [DOI] [PubMed] [Google Scholar]
- 28.Bonora BM, Avogaro A, Fadini GP. Sodium-glucose co-transporter-2 inhibitors and diabetic ketoacidosis: An updated review of the literature. Diabetes Obes Metab. 2018;20:25–33. doi: 10.1111/dom.13012. [DOI] [PubMed] [Google Scholar]
- 29.Musso G, Sircana A, Saba F, Cassader M, Gambino R. Assessing the risk of ketoacidosis due to sodium-glucose cotransporter (SGLT)-2 inhibitors in patients with type 1 diabetes: A meta-analysis and meta-regression. PLoS Med. 2020;17:e1003461. doi: 10.1371/journal.pmed.1003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Institute for Health and Care Excellence (NICE) Guidance. Dapagliflozin with insulin for treating type 1 diabetes. Technology appraisal guidance [TA597]. 28 August 2019 [cited 28 February 2021]. Available from: https://www.nice.org.uk/guidance/ta597/chapter/1-Recommendations .
- 31.Sibai BM, Viteri OA. Diabetic ketoacidosis in pregnancy. Obstet Gynecol. 2014;123:167–178. doi: 10.1097/AOG.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 32.Guo RX, Yang LZ, Li LX, Zhao XP. Diabetic ketoacidosis in pregnancy tends to occur at lower blood glucose levels: case-control study and a case report of euglycemic diabetic ketoacidosis in pregnancy. J Obstet Gynaecol Res. 2008;34:324–330. doi: 10.1111/j.1447-0756.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- 33.Jaber JF, Standley M, Reddy R. Euglycemic Diabetic Ketoacidosis in Pregnancy: A Case Report and Review of Current Literature. Case Rep Crit Care. 2019;2019:8769714. doi: 10.1155/2019/8769714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madaan M, Aggarwal K, Sharma R, Trivedi SS. Diabetic ketoacidosis occurring with lower blood glucose levels in pregnancy: a report of two cases. J Reprod Med. 2012;57:452–455. [PubMed] [Google Scholar]
- 35.Karpate SJ, Morsi H, Shehmar M, Dale J, Patel C. Euglycemic ketoacidosis in pregnancy and its management: case report and review of literature. Eur J Obstet Gynecol Reprod Biol. 2013;171:386–387. doi: 10.1016/j.ejogrb.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 36.Lucero P, Chapela S. Euglycemic Diabetic Ketoacidosis in the ICU: 3 Case Reports and Review of Literature. Case Rep Crit Care. 2018;2018:1747850. doi: 10.1155/2018/1747850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joseph F, Anderson L, Goenka N, Vora J. Starvation-induced true diabetic euglycemic ketoacidosis in severe depression. J Gen Intern Med. 2009;24:129–131. doi: 10.1007/s11606-008-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burge MR, Hardy KJ, Schade DS. Short-term fasting is a mechanism for the development of euglycemic ketoacidosis during periods of insulin deficiency. J Clin Endocrinol Metab. 1993;76:1192–1198. doi: 10.1210/jcem.76.5.8496310. [DOI] [PubMed] [Google Scholar]
- 39.Burge MR, Garcia N, Qualls CR, Schade DS. Differential effects of fasting and dehydration in the pathogenesis of diabetic ketoacidosis. Metabolism. 2001;50:171–177. doi: 10.1053/meta.2001.20194. [DOI] [PubMed] [Google Scholar]
- 40.Larroumet A, Camoin M, Foussard N, Alexandre L, Mesli S, Redonnet I, Baillet-Blanco L, Rigalleau V, Mohammedi K. Euglycemic ketoacidosis induced by therapeutic fasting in a non-diabetic patient. Nutrition. 2020;72:110668. doi: 10.1016/j.nut.2019.110668. [DOI] [PubMed] [Google Scholar]
- 41.Mohorko N, Černelič-Bizjak M, Poklar-Vatovec T, Grom G, Kenig S, Petelin A, Jenko-Pražnikar Z. Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr Res. 2019;62:64–77. doi: 10.1016/j.nutres.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Bolla AM, Caretto A, Laurenzi A, Scavini M, Piemonti L. Low-Carb and Ketogenic Diets in Type 1 and Type 2 Diabetes. Nutrients. 2019;11 doi: 10.3390/nu11050962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goday A, Bellido D, Sajoux I, Crujeiras AB, Burguera B, García-Luna PP, Oleaga A, Moreno B, Casanueva FF. Short-term safety, tolerability and efficacy of a very low-calorie-ketogenic diet interventional weight loss program vs hypocaloric diet in patients with type 2 diabetes mellitus. Nutr Diabetes. 2016;6:e230. doi: 10.1038/nutd.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saslow LR, Kim S, Daubenmier JJ, Moskowitz JT, Phinney SD, Goldman V, Murphy EJ, Cox RM, Moran P, Hecht FM. A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PLoS One. 2014;9:e91027. doi: 10.1371/journal.pone.0091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sainsbury E, Kizirian NV, Partridge SR, Gill T, Colagiuri S, Gibson AA. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;139:239–252. doi: 10.1016/j.diabres.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 46.Shaikh S, Mohamed MM, Mujeeb A, Shaikh F, Harris B. Euglycemic Diabetic Ketoacidosis Precipitated by a Keto Diet: Importance of Dietary History in Diagnosis. Cureus. 2020;12:e10199. doi: 10.7759/cureus.10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukuyama Y, Numata K, Yoshino K, Santanda T, Funakoshi H. Euglycemic diabetic ketoacidosis due to a strict low-carbohydrate diet during treatment with sodium-glucose cotransporter 2 inhibitors. Acute Med Surg. 2020;7:e480. doi: 10.1002/ams2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garay PS, Zuniga G, Lichtenberg R. A Case of Euglycemic Diabetic Ketoacidosis Triggered by a Ketogenic Diet in a Patient With Type 2 Diabetes Using a Sodium-Glucose Cotransporter 2 Inhibitor. Clin Diabetes. 2020;38:204–207. doi: 10.2337/cd19-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yaron M, Shalit R, Kreiser D, Cukierman-Yaffe T, Yoeli R. Very restricted carbohydrate (ketogenic) diet: A rare cause of a recurrent hypoglycemic-euglycemic diabetic ketoacidosis in the pregnancy. Eur J Obstet Gynecol Reprod Biol. 2020;248:257–258. doi: 10.1016/j.ejogrb.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 50.McClean AM, Montorio L, McLaughlin D, McGovern S, Flanagan N. Can a ketogenic diet be safely used to improve glycaemic control in a child with type 1 diabetes? Arch Dis Child. 2019;104:501–504. doi: 10.1136/archdischild-2018-314973. [DOI] [PubMed] [Google Scholar]
- 51.Dowsett J, Humphreys R, Krones R. Normal Blood Glucose and High Blood Ketones in a Critically Unwell Patient with T1DM Post-Bariatric Surgery: a Case of Euglycemic Diabetic Ketoacidosis. Obes Surg. 2019;29:347–349. doi: 10.1007/s11695-018-3548-6. [DOI] [PubMed] [Google Scholar]
- 52.Iqbal QZ, Mishiyev D, Zia Z, Ruggiero RA, Aftab G. Euglycemic Diabetic Ketoacidosis With Sodium-Glucose Cotransporter-2 Inhibitor Use Post-Bariatric Surgery: A Brief Review of the Literature. Cureus. 2020;12:e10878. doi: 10.7759/cureus.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ashrafian H, Harling L, Toma T, Athanasiou C, Nikiteas N, Efthimiou E, Darzi A, Athanasiou T. Type 1 Diabetes Mellitus and Bariatric Surgery: A Systematic Review and Meta-Analysis. Obes Surg. 2016;26:1697–1704. doi: 10.1007/s11695-015-1999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thawabi M, Studyvin S. Euglycemic Diabetic Ketoacidosis, a Misleading Presentation of Diabetic Ketoacidosis. N Am J Med Sci. 2015;7:291–294. doi: 10.4103/1947-2714.157490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abu-Abed Abdin A, Hamza M, Khan MS, Ahmed A. Euglycemic Diabetic Ketoacidosis in a Patient with Cocaine Intoxication. Case Rep Crit Care. 2016;2016:4275651. doi: 10.1155/2016/4275651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rawla P, Vellipuram AR, Bandaru SS, Pradeep Raj J. Euglycemic diabetic ketoacidosis: a diagnostic and therapeutic dilemma. Endocrinol Diabetes Metab Case Rep. 2017;2017 doi: 10.1530/EDM-17-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Legaspi R, Narciso P. Euglycemic Diabetic Ketoacidosis Due to Gastroparesis, A Local Experience. J Ark Med Soc. 2015;112:62–63. [PubMed] [Google Scholar]
- 58.Suzuki K, Tamai Y, Urade S, Ino K, Sugawara Y, Katayama N, Hoshino T. Alcoholic ketoacidosis that developed with a hypoglycemic attack after eating a high-fat meal. Acute Med Surg. 2014;1:109–114. doi: 10.1002/ams2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGuire LC, Cruickshank AM, Munro PT. Alcoholic ketoacidosis. Emerg Med J. 2006;23:417–420. doi: 10.1136/emj.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]