Abstract
OBJECTIVE:
To evaluate whether the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended influenza and tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccinations in pregnancy are associated with increased risk of stillbirth.
METHODS:
We performed a case–control study in the Vaccine Safety Datalink that was matched 1:4 on site, month, and year of last menstrual period, comparing the odds of vaccination in pregnancies that ended in stillbirth (defined as fetal loss at or after 20 weeks of gestation) compared with those that ended in live birth from January 1, 2012, to September 30, 2015. We included patients with singleton pregnancies that ended in stillbirth or live birth who had at least one prenatal care visit, pregnancy dating information, and continuous health plan enrollment for the duration of pregnancy. Medical records for all stillbirths were reviewed. We were statistically powered to detect an odds ratio (OR) of 1.37 when evaluating the association between influenza or Tdap vaccination and stillbirth. We also examined stillbirth rates in pregnant patients aged 14–49 years in the Vaccine Safety Datalink between 2007 and 2015.
RESULTS:
In our matched analysis of 795 confirmed stillbirths in the case group and 3,180 live births in the control group, there was no significant association between influenza vaccination during pregnancy and stillbirth (343/795 [43.1%] stillbirths in the case group vs 1,407/3,180 [44.3%] live births in the control group, OR 0.94, adjusted OR 0.95, 95% CI 0.79–1.14, P5.54) and no significant association between Tdap vaccination during pregnancy and stillbirth (184/795 [23.1%] stillbirths in the case group vs 746/3,180 [23.5%] live births in the control group, OR 0.97, aOR 0.96, 95% CI 0.76–1.28, P=.91). From 2007 to 2015, the stillbirth rate in the Vaccine Safety Datalink was 5.2 per 1,000 live births and stillbirths.
CONCLUSION:
No association was found between vaccination during pregnancy and the odds of stillbirth. These findings support the safety of ACIP recommendations for vaccination during pregnancy.
The Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices recommends that pregnant patients receive two vaccinations during each pregnancy: influenza vaccination to prevent maternal and infant influenza disease1 and tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccination to protect infants from pertussis.2 In addition, patients may receive vaccinations not routinely recommended during pregnancy (ie, hepatitis, pneumococcal, or meningococcal vaccinations) owing to high-risk situations. Furthermore, patients who are unaware of their pregnancy (inadvertent exposure) also may receive vaccinations that are contraindicated during pregnancy (such as measles–mumps–rubella or varicella vaccinations).
Despite overall reassuring safety data for recommended vaccinations during pregnancy, vaccination rates during pregnancy remains suboptimal.3 Concerns about vaccine safety among pregnant patients and health care professionals have been shown to be a leading cause of poor uptake of vaccinations during pregnancy.3–5 Multiple large epidemiologic studies have not found increased risks of pregnancy, fetal, or infant adverse outcomes associated with vaccination during pregnancy.6–17 Although studies to date have not found an increased risk of stillbirth after influenza,9–12,15 Tdap,6,7 human papillomavirus,16 or other vaccinations18–20 during pregnancy, most of these studies evaluated stillbirth as a secondary outcome and lacked statistical power. Moreover, these studies did not include detailed chart review to confirm the pregnancy timing and outcome or identify stillbirth risk factors. We conducted a large population-based study to evaluate the association between vaccination during pregnancy and risk of stillbirth based on chart review confirmation of the stillbirth outcome.
METHODS
The Vaccine Safety Datalink is a collaboration between the CDC and eight integrated health care systems with health care utilization, demographic, and vaccination data representing approximately 3% of the U.S. population.21,22 Seven sites (Kaiser Permanente Washington [Seattle, Washington], HealthPartners Institute [Minneapolis, Minnesota], Kaiser Permanente Northwest [Portland, Oregon], Kaiser Permanente Northern California [Oakland, California], Kaiser Permanente Colorado [Denver, Colorado], Marshfield Clinic Research Institute [Marshfield, Wisconsin], and Kaiser Permanente Southern California [Los Angeles, California]) had data available to contribute to this study. Data managers at each site prepare standardized files with demographics, health plan enrollment, birth information, hospitalizations, outpatient and inpatient encounters, and other data, including pregnancy information, from their site’s electronic medical records. Data are validated using weekly and annual data quality checks at the sites and the CDC. We used a validated algorithm to identify pregnancies in electronic health data.23 This algorithm uses diagnosis and procedure codes to identify pregnancies, pregnancy outcomes, and start and end dates of pregnancies and links patients whose pregnancies end in live births to their newborns.
We conducted a matched case–control study of pregnancies in the Vaccine Safety Datalink that ended in a live birth or stillbirth from January 1, 2012, to September 30, 2015, to evaluate whether there was an association between vaccination and stillbirth. We chose the study time period after the recommendation that all pregnant patients receive Tdap during every pregnancy24 and before the United States’ initiation of the International Classification of Diseases, Tenth Revision, Clinical Modification, which allowed for consistency of coding of important covariates. We included patients enrolled at a participating Vaccine Safety Datalink site from 6 months before the start of pregnancy through 4 weeks after the pregnancy’s end date. These criteria were chosen to ensure we had complete information on baseline maternal comorbidities, vaccinations before and during pregnancy, and pregnancy outcome. We evaluated singleton pregnancies with known pregnancy outcomes and at least one prenatal visit during the pregnancy. We excluded patients with multifetal gestations (because they are at higher risk of stillbirth), those who did not have a live birth or stillbirth outcome (ie, spontaneous abortion, therapeutic abortion, molar pregnancy, ectopic pregnancy), those without prenatal care visits, and those without dating information. We also identified a cohort of pregnant patients aged 14–49 years with pregnancies that ended in live birth or stillbirth from January 1, 2007, through December 31, 2015, to describe rates of stillbirth in the Vaccine Safety Datalink.
The primary outcome was stillbirth, defined by the American College of Obstetricians and Gynecologists as a fetal death occurring at or after 20 weeks of gestation.25 We reviewed medical records of patients with pregnancies that ended in stillbirth, which were identified using the pregnancy algorithm, for confirmation of the outcome. Data on stillbirth dating, timing (antepartum vs intrapartum, according to the Brighton Collaboration definition),26 autopsy and pathology reports, laboratory data, maternal characteristics and underlying medical conditions, ultrasound reports, and health care encounter notes were abstracted by trained chart reviewers. A physician (L.P., H.S.L., V.G.) then reviewed all charts to determine the start and end dates of the pregnancy, timing of the fetal death, and potential causes of the stillbirth based on the information from the abstraction. Pregnancy start dates were confirmed based on a predetermined hierarchy, using the physician-determined gestational age at delivery, estimated delivery date as reported closest to the time of delivery, and last menstrual period date in cases where the first two were not available. Early ultrasound scans also were reviewed to assist with dating. All equivocal findings underwent a secondary adjudication by a trained obstetrician (N.K.T.).
Control-group pregnancies that ended in live birth were identified from the electronic pregnancy algorithm during the study period. Prior data on the validation of the electronic pregnancy algorithm have shown that it accurately determines start and ends dates for a pregnancy that ends in live birth.23 Furthermore, electronic gestational age and birth related data are supplemented with information from state vital records and hospital records when available.27 We matched each chart-confirmed stillbirth with four control-group live births based on site and month and year of last menstrual period to ensure all that pregnancies had similar start dates. We required all matched control-group live births to have pregnancy durations equal to or longer than those in the matched stillbirth case group to avoid capturing postpartum vaccinations. Furthermore, for each of the four matched control-group pregnancies that ended in live birth, we censored any time after the index date, defined as the outcome data of the matched stillbirth case. That is, vaccinations for live births in the control group were included in the analysis only if they occurred before the outcome date of the matched stillbirth in the case group to ensure that all analyzed pregnancies that ended in live birth or stillbirth had the same amount of time in the pregnancy to potentially receive a vaccination.
We included exposures to all vaccinations, including recommended vaccinations (influenza or Tdap), non–routinely recommended vaccinations (meningococcal, pneumococcal, inactivated polio, human papillomavirus, Japanese encephalitis, rabies, inactivated typhoid, yellow fever, hepatitis A, and hepatitis B vaccinations), and contraindicated vaccinations (live-attenuated influenza, measles–mumps–rubella, varicella, and herpes zoster vaccinations). Information on vaccinations and the date of administration are routinely collected in the Vaccine Safety Datalink data from electronic health record internal vaccination registries. Vaccination exposure during pregnancy was defined as all vaccinations from 14 days after the last menstrual period through 7 days before the index date. This end date was chosen to avoid mistakenly including vaccinations given in the postpartum period. To assess vaccinations administered in the periconception period, vaccinations from 30 days prior through 14 days after the last menstrual period were included. We also evaluated exposures from 120 days through 30 days before the last menstrual period as a negative control exposure, because this distant exposure should not affect the risk of stillbirth.
We determined a priori important confounders associated with stillbirth that could affect the propensity for vaccination. These confounders were identified based on demographic data and International Classification of Diseases, Ninth Revision (ICD-9) codes for patients in the control group and from demographic data, ICD-9 codes, and chart review (when unavailable from the first two) for those in the case group. Identified confounders included maternal age (35 years or older), obesity (prepregnancy body mass index [BMI, calculated as weight in kilograms divided by height in meters squared] 30 or higher), tobacco smoking during pregnancy, nulliparity, prior history of stillbirth, Black non-Hispanic race (self-reported, from administrative data), pregnancy complications occurring before vaccination or index date (defined as one inpatient or two outpatient ICD-9 codes for any maternal infections, placental complications, amniotic cavity or membrane problems, fetal growth restriction, maternal trauma, and uterine rupture during current pregnancy), maternal comorbidities before vaccination or index date (defined as one inpatient or two outpatient ICD-9 codes for maternal diabetes, systemic lupus erythematosus, thyroid disorders, hypertension, cardiovascular disease, cholestasis, renal disease, and sickle cell disease in the six months before or during pregnancy), and gestational age at prenatal care initiation. Covariates with more than 5% missing data were designated their own “missing” category in the model. All covariates included in the model were tested for interaction and collinearity owing to their potential overlap.
For our primary matched case–control analysis, we included chart confirmed stillbirths that occurred from January 1, 2012, to September 30, 2015. We described proportions of antepartum and intrapartum stillbirths. We identified stillbirths with abnormal placental pathology and reviewed fetal autopsy findings and maternal laboratory results. We performed a 1:4 matched case–control study using the optimal matching method,28 which is a nonlinear matching algorithm designed to find the overall closest matches between patients from the case and control groups. We compared maternal sociodemographic characteristics between stillbirths in the case group and matched live births in the control group using x2 tests for categorical variables and Wilcoxon median two-sample tests for continuous variables. We used a conditional logistic regression model to estimate the odds (95% CIs) of maternal vaccination in matched case–control pregnancies before and after the adjustment for multiple potential confounding factors.
We additionally conducted a retrospective cohort study of live births and stillbirths ending between January 1, 2007, through December 31, 2015, to estimate the stillbirth rate (number of stillbirths per 1,000 live births and stillbirths).29 We analyzed linear trends in rates by year over this study period using Poisson regression.
As a post hoc analysis, we conducted an additional 1:4 case–control match, matching on high-risk race (Black non-Hispanic [yes or no]) and age at pregnancy outcome (35 years or older [yes or no]) in addition to Vaccine Safety Datalink site and month and year of last menstrual period. We conducted comparisons of patients in the case and control groups and evaluated the odds of maternal vaccination in matched case–control pregnancies before and after adjusting for confounders.
Based on prior Vaccine Safety Datalink work,23 we expected 100,000 pregnancies per year and approximately 0.5% of these pregnancies to end in stillbirth. Assuming a 15% rate of influenza or Tdap vaccination during pregnancy and 1:4 matching of stillbirths in the case group and live births in the control group, 782 cases were needed to detect an odds ratio (OR) of 1.37, and 2,432 cases were needed to detect and OR of 1.20, with 80% power with an a level of less than 0.05 using two-tailed tests.
The study protocol was reviewed and approved by institutional review boards at the CDC and the seven participating Vaccine Safety Datalink sites and was determined as exempt from requiring participant consent. All analyses were conducted using SAS 9.4.
RESULTS
There were 225,700 pregnancies that ended in live birth or stillbirth from January 1, 2012, through September 30, 2015 (Fig. 1). After applying our inclusion and exclusion criteria, we matched 795 stillbirths to 3,180 live births. Both groups were similar in mean gestational age at prenatal care initiation, mean gestational age at first vaccination, and nulliparity status (Table 1). Patients with pregnancies that ended in stillbirth were more likely to be 35 years or older at time of delivery, have comorbid medical conditions, a history of stillbirth, obesity, and more likely to be of Black non-Hispanic race. Patients with pregnancies ending in live birth were more likely to have documented tobacco smoking during pregnancy. Missing data were rare (1% or less) for all variables of interest except tobacco smoking during pregnancy (3.9% stillbirths vs 6.9% live births) and obesity (3.5% stillbirths vs 13.4% live births).
Fig. 1.
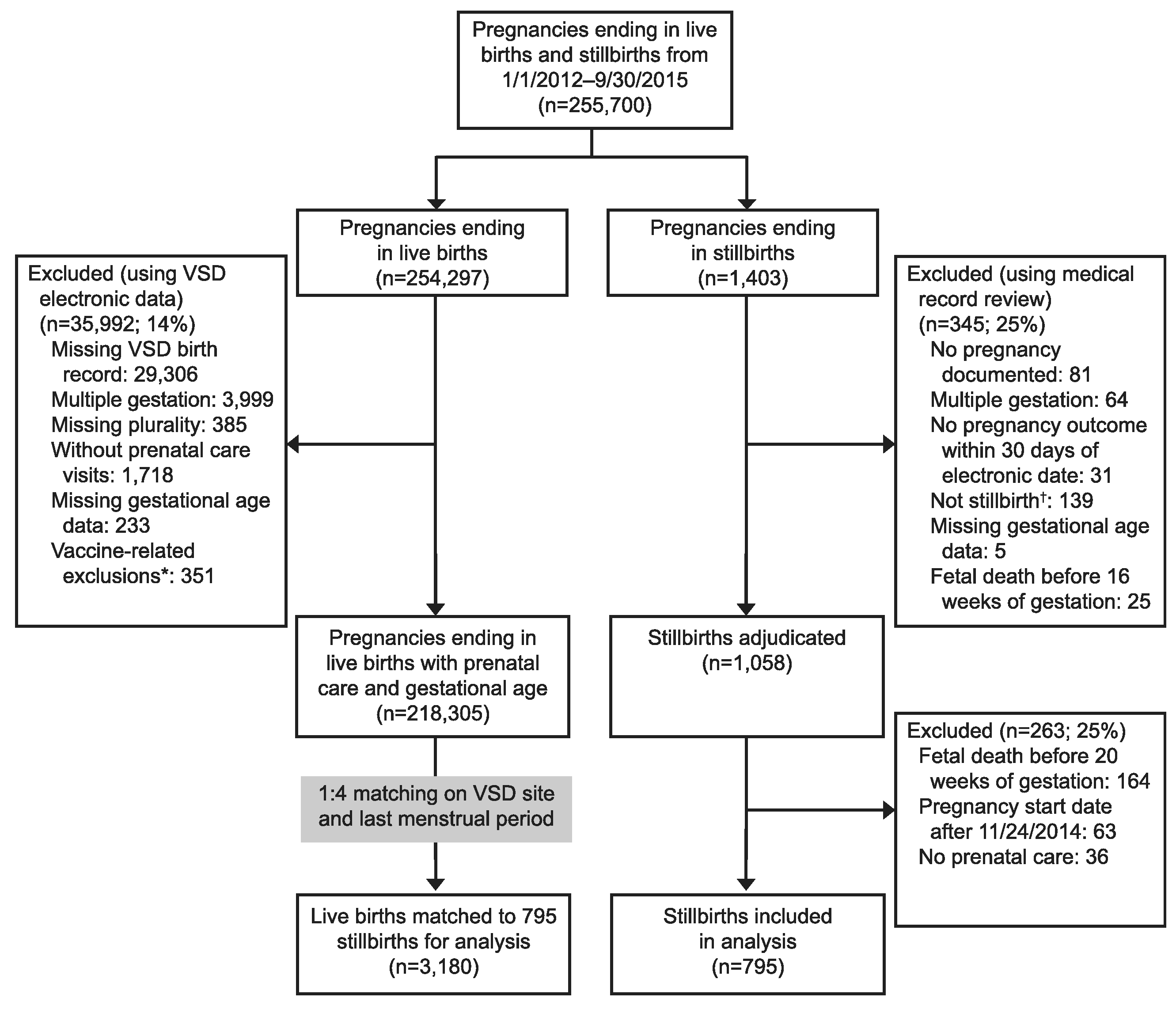
Pregnant patients aged 14–49 years in the Vaccine Safety Datalink (VSD) with pregnancies ending in live births and stillbirths, 2012–2015: case and control flow diagram. *Vaccine-related exclusions include exposure to foreign vaccine formulations, experimental vaccines, vaccines licensed only for infants, and vaccines where unknown whether live or inactivated formulation administered. †Includes live births, ectopic pregnancies, early spontaneous abortions, and therapeutic abortions miscoded as stillbirths.
Table 1.
Selected Demographic Characteristics of Patients With Pregnancies Ending in Stillbirth (n = 795) and Matched Live Births (n=3,180)
| Characteristic | Stillbirths | Live Births | P* |
|---|---|---|---|
| Age at delivery (y) | 30.6 (14–49) | 30.4 (14–46) | .01 |
| Maternal age 35 y or older | 218 (27.4) | 633 (19.9) | <.001 |
| Gestational age at prenatal care initiation (wk) | 7.5 (5–30) | 7.4 (5–37) | .59 |
| Maternal comorbidity† | 108 (13.6) | 123 (3.9) | <.001 |
| Pregnancy complication‡ | 365 (45.9) | 218 (6.86) | <.001 |
| Gestational age at 1st vaccination (wk) | 17.6 (2–39)§ | 17.4 (2–39)∥ | .64 |
| Tobacco smoking during pregnancy | 57 (7.2) | 279 (8.8) | .002 |
| Nulliparous | 238 (29.9) | 974 (30.6) | .38 |
| History of stillbirth | 40 (5.0) | 54 (1.7) | <.001 |
| Black non-Hispanic | 107 (13.5) | 234 (7.4) | <.001 |
| Prepregnancy obesity | 279 (35.1) | 584 (18.4) | <.001 |
Data are mean (range) or n (%) unless otherwise specified.
P-values calculated by χ2 tests for categorical variables and Wilcoxon median two-sample test for continuous variables.
International Classification of Diseases, Ninth Revision codes for maternal diabetes, systemic lupus erythematosus, thyroid disorders, hypertension, cardiovascular disease cholestasis, renal disease, and sickle cell disease in the 6 months before pregnancy through vaccination date or index date (stillbirth outcome date or reference date for matched control).
International Classification of Diseases, Ninth Revision codes for maternal infections, placental complications, amniotic cavity or membrane problems, fetal growth restriction, maternal trauma, and uterine rupture during current pregnancy and before vaccination date or index date.
Vaccinations received in 51.7% of pregnancies ending in stillbirth.
Vaccinations received in 52.9% of pregnancies ending in live birth.
Of 795 stillbirths, 622 (78%) were antepartum, 161 (20%) were intrapartum, and 12 (2%) could not be classified as antepartum or intrapartum stillbirths based on information available. In 783 cases (98.5%), gestational age data were determined by the physician at the time of delivery. The gestational age at stillbirth ranged from 20 to 41 weeks of gestation, with peaks occurring at 22 weeks (n=65, 8%) and 39 weeks (n=57, 7%). Of the 757 stillbirths with pathology available (placenta or fetal autopsy) for review, 582 (77%) had abnormal pathologic findings; 194 stillbirths had more than one abnormal finding. The most common pathologic abnormality was chorioamnionitis in 264 stillbirths (33%). The next most common pathologic abnormality was a placental event (ie, impaired uteroplacental perfusion, placental abruption, and placenta accreta) (n=194, 24%). On reviewing laboratory findings, 248 (31%) of pregnancies that ended in stillbirth had abnormal maternal or fetal laboratory results, most commonly maternal Group B Streptococcus in 91 (11%), followed by abnormal fetal genetic tests in 64 (8%).
In the main analysis, 411 of 795 (52%) of pregnancies that ended in a stillbirth and 1,682 of 3,180 (53%) of matched pregnancies that ended in a live birth were exposed to one or more vaccinations during pregnancy (Table 2). There was no significant association between stillbirth and receipt of any vaccination (51.7% among stillbirths in the case group vs 52.9% among live births in the control group, crude OR 0.94, adjusted odds ratio [aOR] 0.95, 95% CI 0.78–1.14, P5.56) or antepartum stillbirths and receipt of any vaccination (55.5% among stillbirths in the case group vs 55.8% among live births in the control group, OR 0.98, aOR 0.96, 95% CI 0.78–1.20, P=.74). The vaccination rate for recommended vaccinations (influenza or Tdap) during pregnancy was 406 of 795 (51%) in pregnancies that ended in stillbirths compared with 1,667 of 3,180 (52%) for pregnancies that ended in live birth (OR 0.93, aOR 0.94, 95% CI 0.78–1.14, P=.54). We found no significant association between stillbirths and exposure to influenza and Tdap vaccinations when analyzed separately or to non–routinely recommended vaccinations during pregnancy. There were no stillbirths after contraindicated vaccinations in pregnancy. When evaluating stillbirths after receipt of any vaccination in the periconception period (Table 3), the vaccination rate in stillbirths was 51 of 795 (6%) compared with 202 of 3,180 (6%) in matched live births (OR 1.01, aOR 1.05, 95% CI 0.75–1.47, P5.77). The vaccination rate for recommended vaccinations during the periconception period was 46 of 795 (6%) in pregnancies that ended in stillbirths, compared with 176 of 3,180 (6%) for pregnancies that ended in live birth (OR 1.05, aOR 1.08, 95% CI 0.76–1.53, P5.68). We also found no associations between Tdap, influenza, non-routinely recommended vaccinations, and contraindicated vaccinations and stillbirth. There was no association between the odds of vaccination and stillbirth in the negative control prepregnancy exposure period (Appendix 1, available online at http://links.lww.com/AOG/C114). There was no interaction or collinearity of covariates used in the analyses. Results of the post hoc analysis were similar to the main results (Appendices 2 and 3, available online at http://links.lww.com/AOG/C114).
Table 2.
Matched Case–Control Analysis of Stillbirths After Maternal Vaccination During Pregnancy*
| Exposure During Pregnancy | Vaccination Rate in Stillbirths (n=795) | Vaccination Rate in Live Births (n=3,180) | Crude OR (95% CI) | Adjusted† OR (95% CI) | P |
|---|---|---|---|---|---|
| Any vaccination | 411 (51.7) | 1,682 (52.9) | 0.94 (0.78–1.12) | 0.95 (0.78–1.14) | .56 |
| Influenza vaccination‡ | 343 (43.1) | 1,407 (44.3) | 0.94 (0.79–1.13) | 0.95 (0.79–1.14) | .54 |
| Tdap vaccination§ | 184 (23.1) | 746 (23.5) | 0.97 (0.76–1.24) | 0.96 (0.76–1.28) | .91 |
| Non–routinely recommended vaccinations∥ | 9 (1.1) | 35 (1.1) | 1.03 (0.49–2.17) | 0.92 (0.42–2.00) | .84 |
OR, odds ratio; Tdap, tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis.
Data are n (%) unless otherwise specified.
Fourteen days after the last menstrual period through 7 days before the index date (stillbirth outcome date or reference date for matched control).
Adjusted for maternal age, smoking during pregnancy, obesity, nulliparity, history of stillbirth, Black non-Hispanic race, and maternal comorbidities and pregnancy complications occurring before vaccination.
Inactivated influenza vaccination with or without Tdap vaccination.
Tdap vaccination with or without inactivated influenza vaccination.
Human papillomavirus, meningococcal, pneumococcal, inactivated polio, Japanese encephalitis, rabies, inactivated typhoid, yellow fever, hepatitis A, hepatitis B.
Table 3.
Matched Case–Control Analysis of Stillbirths After Maternal Vaccination in the Periconception* Period
| Periconception Exposure | Vaccination Rate in Stillbirths (n=795) | Vaccination Rate in Live Births (n=3,180) | Crude OR (95% CI) | Adjusted† OR (95% CI) | P |
|---|---|---|---|---|---|
| Any vaccination | 51 (6.4) | 202 (6.4) | 1.01 (0.74–1.40) | 1.05 (0.75–1.47) | .77 |
| Influenza vaccination‡ | 37 (4.7) | 151 (4.8) | 0.98 (0.67–1.43) | 1.02 (0.69–1.50) | .94 |
| Tdap vaccination§ | 12 (1.5) | 35 (1.1) | 1.38 (0.71–2.66) | 1.27 (0.65–2.50) | .49 |
| Non–routinely recommended vaccinations∥ | 8 (1.0) | 29 (0.9) | 1.11 (0.50–2.45) | 1.30 (0.57–2.93) | .53 |
| Contraindicated vaccinations¶ | 2 (0.3) | 5 (0.2) | 1.60 (0.31–8.25) | 1.38 (0.24–7.81) | .71 |
OR, odds ratio; Tdap, tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis.
Data are n (%) unless otherwise specified.
Thirty days before through 14 days after last menstrual period.
Adjusted for maternal age, smoking during pregnancy, obesity, nulliparity, history of stillbirth, Black non-Hispanic race, maternal comorbidities, pregnancy complications before vaccination or index date.
Inactivated influenza vaccination with or without Tdap vaccination.
Tdap vaccination with or without inactivated influenza vaccination.
Human papillomavirus, meningococcal, pneumococcal, inactivated polio, Japanese encephalitis, rabies, inactivated typhoid, yellow fever, hepatitis A, hepatitis B.
Live attenuated influenza vaccination, measles–mumps–rubella, varicella, zoster.
From January 1, 2007, through December 31, 2015, 604,671 pregnancies ended in either live birth (n5601,528) or stillbirth (n53,143). The stillbirth rate was 5.2 per 1,000 live births and stillbirths. The yearly rate ranged from 4.8 (2013) to 5.7 (2010) (Appendix 4, available online at http://links.lww.com/AOG/C114). There were no statistically significant linear trends by year over the entire study period (P=.92).
DISCUSSION
Concerns about vaccine safety by patients and health care professionals have been shown to be a leading reason for suboptimal rates of vaccination during pregnancy.4,5 We did not detect an association between the occurrence of stillbirth and the receipt of vaccinations given during pregnancy or in the peri-conception period. These findings inform the evidence for the safety of vaccinations recommended by the Advisory Committee on Immunization Practices and should provide reassurance to pregnant patients and their health care professionals.
There has been limited information on vaccinations during pregnancy and risk of stillbirth. Our study adds critical information on exposures to both recommended and nonrecommended vaccinations and stillbirth. In contrast to many prior studies that relied exclusively on registries, ICD codes, or insurance claims,6,9,12,14 we included a comprehensive chart review and clinician adjudication process of all stillbirth cases. This increased the accuracy of stillbirth identification and the precision of timing of vaccination in relation to pregnancy. Furthermore, we were well-powered for our main analyses, including both the association with any vaccination and stillbirth and influenza and Tdap vaccinations and stillbirth. Post hoc, we determined that we had 80% power to detect an OR of 1.27 given the vaccination rates in this study.
The stillbirth rate in our study was generally consistent with published national estimates. The most recent rate from the National Vital Statistics Report on fetal death30 was 6.1 stillbirths per 1,000 live births and stillbirths, compared with our rate of 5.2 per 1,000 live births and stillbirths in the Vaccine Safety Datalink. The lower rate we observed may be a result of better case ascertainment and a result of studying an insured population with better access to prenatal care than in the general population. In addition, when calculating proportions of stillbirths by gestational age, we observed increases at 22 and 39 weeks of gestation, similar to stillbirth outcome by gestational week data from national estimates.29
Our results are similar to prior studies with a primary outcome of stillbirth, in that we did not find an increased risk of stillbirth after vaccination.6,12 Although our estimate is consistent with a possibility of a protective effect, the CI also includes the null and some values greater than 1. This differs from other studies that have shown statistically decreased risks of stillbirth after maternal influenza vaccination. These differences may be related to differences in immunization practices and rates of influenza in the study populations, unmeasured confounding, or chance. A prior U.S. study that evaluated the risk of stillbirth among 8,690 pregnant patients who received trivalent inactivated influenza vaccination in pregnancy at a single hospital system13 found that stillbirth occurred in 0.3% of vaccinated pregnancies, compared with 0.6% of unvaccinated pregnancies (P=.006). Another study of 5,076 pregnant woman who received trivalent influenza vaccination in Western Australia showed that stillbirths were 51% less likely among influenza-vaccinated mothers compared with unvaccinated mothers (adjusted hazard ratio 0.49, 95% CI 0.29–0.84). A study of 7,062 patients vaccinated with the AS03-adjuvanted H1N1pdm09 vaccination in Denmark found that the adjusted hazard ratio for stillbirth that compared vaccinated with unvaccinated patients was 0.44 (95% CI 0.20–0.94).9 Another study of 23,340 patients receiving the H1N1 influenza vaccination in Canada also showed stillbirths were less likely among vaccinated patients (adjusted relative risk 0.66, 95% CI 0.47–0.91).31 The methods of these prior studies differed from ours; the matched case–control design of our study allowed us to closely match the exposure period of patients in the case group and those in the control group and ensure that all patients in the case group and matched patients in the control group had the same opportunity for exposure to vaccinations during pregnancy. Our design also allowed us to account for potential seasonality in birth outcomes, differential practices across participating health care sites, and temporal differences in immunization recommendations and practices, especially as related to influenza vaccination, which may explain the differences in our study’s findings from that of these earlier studies. Furthermore, by studying vaccination exposures both as a composite (ie, all vaccinations) and stratified exposure (ie, recommended, contraindicated), we were able to assess the safety of any vaccination as well as specific types of vaccinations and the risk of stillbirth. Because the Tdap vaccination during pregnancy recommendation is more recent, there are fewer safety studies and data on Tdap vaccination and risk of stillbirth. Furthermore, this study adds to the limited existing evidence on the safety of non–routinely recommended vaccinations, which is important for high-risk patients who have other vaccination indications during pregnancy apart from influenza and Tdap vaccinations (ie, hepatitis A vaccination).
Our study has limitations. There is potential for unmeasured confounding in our analysis. Although we adjusted for race in our main analysis and matched on race in the post hoc analysis, the use of race in this study is a social construct and not a biological one, and likely is a proxy for other factors, related to systemic racism, bias, and access to care, that are unmeasurable within our database. The patients in the case and control groups differed by several covariates, which is expected when comparing pregnancies that ended in live birth with those that ended in stillbirth. We did perform a post hoc analysis adding race and maternal age to the match, which resulted in more similar case and control groups. Results of the post hoc analysis were similar to the main analysis. The Vaccine Safety Datalink does not routinely collect some pregnancy-related data on pregnancies that do not end in live birth (ie, smoking during pregnancy, BMI). Therefore, in some cases, we compared covariates from different sources in pregnancies ending in live birth or stillbirth. We may have underestimated the number of pregnant patients with live births who were smokers or had prepregnancy obesity because this information was obtained electronically and not confirmed in chart review. Finally, we may have underestimated vaccinations during pregnancy if they were not captured in our data. However, in addition to vaccinations given at Vaccine Safety Datalink sites, our data include certain state immunization registries and vaccinations entered afterwards by health care professionals. By requiring all pregnancies to have prenatal care and prepregnancy and postpregnancy enrollment in the Vaccine Safety Datalink system, we believe that we have near-complete capture of vaccinations during pregnancy. Although requiring insurance enrollment for this study may limit the generalizability of our results, it was important for us to ensure the pregnant patients had prenatal care, which would give them the opportunity for vaccination and would allow us to assess differences in the risk of stillbirth among patients with similar characteristics and determine any potential effects from vaccination.
Our large population-based study that used chart confirmation of stillbirths found that influenza and Tdap vaccination during pregnancy do not increase the risk of stillbirth. These findings help strengthen the existing safety evidence and may help bolster confidence in vaccination during pregnancy.
Supplementary Material
Acknowledgments
The Vaccine Safety Datalink Project is funded by the Centers for Disease Control and Prevention. The funder of this study had no role in the design of the study, data collection, data analysis, data interpretation, or writing of the report. The manuscript was approved by the Centers for Disease Control and Prevention clearance process.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors thank Maria Mascola and James G. Donahue, from Marshfield Clinic, and James D. Nordin from HealthPartners Institute for their assistance with the planning of the study; Dhavan P. Pasumarthi, from HealthPartners Institute, for his assistance with data management through REDCap; and Yi Mu and Charles Licata from the Centers for Disease Control and Prevention for their assistance with the statistical analyses. None of the mentioned individuals received compensation for their contributions.
Financial Disclosure
Allison Naleway reports research support from Pfizer. Nicola Klein reports research support from GlaxoSmithKline, Sanofi Pasteur, Merck, Pfizer, and Protein Science. Jennifer Nelson reports receiving funding from GlaxoSmithKline for statistical consulting on a Shingrix vaccine safety study, and Elsevier Publishing for service as an Associate Editor for the journal Vaccine. The other authors did not report any potential conflicts of interest.
REFERENCES
- 1.Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019;68:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women— Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013;62:131–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Lindley MC, Kahn KE, Bardenheier BH, D’Angelo DV, Da-wood FS, Fink RV, et al. Vital signs: burden and prevention of influenza and pertussis among pregnant women and infants— United States. MMWR Morb Mortal Wkly Rep 2019;68:885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Böhm S, Röbl-Mathieu M, Scheele B, Wojcinski M, Wichmann O, Hellenbrand W. Influenza and pertussis vaccination during pregnancy—attitudes, practices and barriers in gynaecological practices in Germany. BMC Health Serv Res 2019;19:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panda B, Stiller R, Panda A. Influenza vaccination during pregnancy and factors for lacking compliance with current CDC guidelines. J Matern Fetal Neonatal Med 2011;24:402–6. [DOI] [PubMed] [Google Scholar]
- 6.Donegan K, King B, Bryan P. Safety of pertussis vaccination in pregnant women in UK: observational study. BMJ 2014;349: g4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan JL, Baggari SR, McIntire DD, Sheffield JS. Pregnancy outcomes after antepartum tetanus, diphtheria, and acellular pertussis vaccination. Obstet Gynecol 2015;125:1433–8. [DOI] [PubMed] [Google Scholar]
- 8.Sheffield JS, Greer LG, Rogers VL, Roberts SW, Lytle H, McIntire DD, et al. Effect of influenza vaccination in the first trimester of pregnancy. Obstet Gynecol 2012;120:532–7. [DOI] [PubMed] [Google Scholar]
- 9.Pasternak B, Svanström H, Mølgaard-Nielsen D, Krause TG, Emborg HD, Melbye M, et al. Vaccination against pandemic A/H1N1 2009 influenza in pregnancy and risk of fetal death: cohort study in Denmark. BMJ 2012;344:e2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallen B, Olausson PO. Vaccination against H1N1 influenza with Pandemrix(I) during pregnancy and delivery outcome: a Swedish register study. BJOG 2012;119:1583–90. [DOI] [PubMed] [Google Scholar]
- 11.Heikkinen T, Young J, van Beek E, Franke H, Verstraeten T, Wei JG, et al. Safety of MF59-adjuvanted A/H1N1 influenza vaccine in pregnancy: a comparative cohort study. Am J Obstet Gynecol 2012;207:177.e1–8. [DOI] [PubMed] [Google Scholar]
- 12.Ludvigsson JF, Ström P, Lundholm C, Cnattingius S, Ekbom A, Örtqvist Å, et al. Maternal vaccination against H1N1 influenza and offspring mortality: population based cohort study and sibling design. BMJ 2015;351:h5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wortman AC, Casey BM, McIntire DD, Sheffield JS. Association of influenza vaccination on decreased stillbirth rate. Am J Perinatol 2015;32:571–6. [DOI] [PubMed] [Google Scholar]
- 14.Regan AK, Moore HC, de Klerk N, Omer SB, Shellam G, Mak DB, et al. Seasonal trivalent influenza vaccination during pregnancy and the incidence of stillbirth: population-based retrospective cohort study. Clin Infect Dis 2016;62:1221–7. [DOI] [PubMed] [Google Scholar]
- 15.Huang WT, Tang FW, Yang SE, Chih YC, Chuang JH. Safety of inactivated monovalent pandemic (H1N1) 2009 vaccination during pregnancy: a population-based study in Taiwan. Vaccine 2014;32:6463–8. [DOI] [PubMed] [Google Scholar]
- 16.Scheller NM, Pasternak B, Mølgaard-Nielsen D, Svanström H, Hviid A. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med 2017;376:1223–33. [DOI] [PubMed] [Google Scholar]
- 17.Getahun D, Fassett MJ, Peltier MR, Takhar HS, Shaw SF, Im TM, et al. Association between seasonal influenza vaccination with pre- and postnatal outcomes. Vaccine 2019;37:1785–91. [DOI] [PubMed] [Google Scholar]
- 18.Suzano CE, Amaral E, Sato HK, Papaiordanou PM; Campinas Group on Yellow Fever Immunization during Pregnancy. The effects of yellow fever immunization (17DD) inadvertently used in early pregnancy during a mass campaign in Brazil. Vaccine 2006;24:1421–6. [DOI] [PubMed] [Google Scholar]
- 19.Badilla X, Morice A, Avila-Aguero ML, Saenz E, Cerda I, Reef S, et al. Fetal risk associated with rubella vaccination during pregnancy. Pediatr Infect Dis J 2007;26:830–5. [DOI] [PubMed] [Google Scholar]
- 20.Goss MA, Lievano F, Buchanan KM, Seminack MM, Cunningham ML, Dana A. Final report on exposure during pregnancy from a pregnancy registry for quadrivalent human papillomavirus vaccine. Vaccine 2015;33:3422–8. [DOI] [PubMed] [Google Scholar]
- 21.McNeil MM, Gee J, Weintraub ES, Belongia EA, Lee GM, Glanz JM, et al. The Vaccine Safety Datalink: successes and challenges monitoring vaccine safety. Vaccine 2014;32:5390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Vaccine safety datalink (VSD). Available at: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html. Retrieved April 1, 2020.
- 23.Naleway AL, Gold R, Kurosky S, Riedlinger K, Henninger ML, Nordin JD, et al. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine 2013;31:2898–903. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and old—r —Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2012;61:468–70. [PubMed] [Google Scholar]
- 25.Management of stillbirth. Obstetric Care Consensus No. 10. American College of Obstetricians and Gynecologists. Obstet Gynecol 2020;135:e110–32. [DOI] [PubMed] [Google Scholar]
- 26.Tavares Da Silva F, Gonik B, McMillan M, Keech C, Dellicour S, Bhange S, et al. Stillbirth: case definition and guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2016;34:6057–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrade SE, Scott PE, Davis RL, Li DK, Getahun D, Chee-tham TC, et al. Validity of health plan and birth certificate data for pregnancy research. Pharmacoepidemiol Drug Saf 2013;22: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenbaum PR. Optimal matching for observational studies. J Am Stat Assoc 1989;84:1024–32. [Google Scholar]
- 29.MacDorman MF, Gregory EC. Fetal and perinatal mortality: United States, 2013. Natl Vital Stat Rep 2015;64:1–24. [PubMed] [Google Scholar]
- 30.Hoyert DL, Gregory EC. Cause of fetal death: data from the fetal death report, 2014. Natl Vital Stat Rep 2016;65:1–25. [PubMed] [Google Scholar]
- 31.Fell DB, Sprague AE, Liu N, Yasseen AS, Wen SW, Smith G, et al. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health 2012;102:e33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


