Abstract
This review briefly addresses the history of the discovery and elucidation of the three cloned 11β-hydroxysteroid dehydrogenase (11βHSD) enzymes in the human, 11βHSD1, 11βHSD2 and 11βHSD3, an NADP+-dependent dehydrogenase also called the 11βHSD1-like dehydrogenase (11βHSD1L), as well as evidence for yet identified 11βHSDs. Attention is devoted to more recently described aspects of this multi-functional family. The importance of 11βHSD substrates other than glucocorticoids including bile acids, 7-keto sterols, neurosteroids, and xenobiotics is discussed, along with examples of pathology when functions of these multi-tasking enzymes are disrupted. 11βHSDs modulate the intracellular concentration of glucocorticoids, thereby regulating the activation of the glucocorticoid and mineralocorticoid receptors, and 7β−27-hydroxycholesterol, an agonist of the retinoid-related orphan receptor gamma (RORγ). Key functions of this nuclear transcription factor include regulation of immune cell differentiation, cytokine production and inflammation at the cell level. 11βHSD1 expression and/or glucocorticoid reductase activity are inappropriately increased with age and in obesity and metabolic syndrome (MetS). Potential causes for disappointing results of the clinical trials of selective inhibitors of 11βHSD1 in the treatment of these disorders are discussed, as well as the potential for more targeted use of inhibitors of 11βHSD1 and 11βHSD2.
Keywords: 11β-hydroxysteroid dehydrogenase, 7-keto sterol, retinoid-related orphan receptor, mineralocorticoid, glucocorticoid
1.0. Introduction
Steroid hormone receptors are widely distributed nuclear transcription factors with a large variety of cell-specific functions. Phylogenetic analysis indicates that these receptors and the enzymes for synthesis and degradation of their steroid ligands evolved at about the same time in a primitive vertebrate about 540 million years ago (Baker, 2004). Discovery and isolation of steroids drove the study of their associated enzymes in the first half of the 20th century. Among these are short-chain dehydrogenase/reductases (SDR), including hydroxysteroid dehydrogenases (HSDs), which inactivate and/or activate specific steroids, often within their target cells. SDR may modulate concentrations of circulating steroids, but by altering the intracellular concentrations of ligands, they provide a degree of independence from the regulation of circulating levels of steroids. This function is particularly relevant for the mineralocorticoid and glucocorticoid receptors (MR and GR, respectively). The evolution of genes required for the separation between energy homeostasis, inflammation and repair functions and mineralocorticoid functions required for water and electrolyte homeostasis did not occur until animals started spending part of their lives out of water (Baker, 2010,Baker, Nelson and Studer, 2015). The MR and GR not only share cortisol and corticosterone as ligands, but bind most of the same DNA hormone response elements, often, but not always, resulting in opposite effects on gene transcription, as the GR is more likely to repress transcription (Gomez-Sanchez and Gomez-Sanchez, 2014). The affinity of the MR for cortisol and corticosterone is 10-fold that of the GR, thus the balance of MR:GR activity is regulated in a cell-specific manner by the 11βHSDs by modulating the intracellular concentrations of cortisol and corticosterone. Penning proposed that because SDR have a more cell-specific distribution than the steroid receptors, drugs targeting SDR could serve as “selective intracrine modulators” (SIMs) {Penning, 2003 #13217}. The therapeutic potential of SIMs has been a focus of attention for the last 20 years {Blum, 2003 #6654}. The ability of 11β-hydroxysteroid dehydrogenases, 11βHSD1, predominately a reductase, and 11βHSD2, a dehydrogenase, to interconvert the active glucocorticoids cortisol and corticosterone to their inactive metabolites cortisone and 11-dehydrocorticosterone earned them the name “molecular switches” by Monder et al {Monder, 1993 #3099}. They are also molecular switches for the RORγ by interconverting 7β,27-hydroxycholesterol (7β27OHC), an agonist of RORγ and 7,27-ketocholesterol (7k27OHC)(Beck, Inderbinen, Kanagaratnam et al., 2019).
This review emphasizes discoveries about the family of 11βHSDs of the last decade including the cloning of a 11βHSD3, substrates in addition to glucocorticoids, and the potential for 11βHSD inhibitors to treat age-related diseases and those associated with mertabolic syndrome (MetS).
1.1. Historical context
In his1855 monograph On the Constitutional and Local Effects of Disease of the Suprarenal Capsules Addison recognized that humors emanating from the adrenal glands were “Essential for Life”. It was a century before technology advanced enough to isolate and study the adrenocortical steroids essential for glucose, mineral and immune homeostasis (Steiger and Reichstein, 1937,Kendall, 1952,Simpson and Tait, 1955). Study of the enzymes that synthesize and degrade the steroids paralleled their isolation and within 2 years of its description Amelung et al demonstrated that an 11β-hydroxysteroid dehydrogenase (11βHSD) interconverted cortisol and cortisone in the liver (Amelung, Hubener, Roka et al., 1953). The 30-year delay in recognizing that there was more than one 11βHSD enzyme was due to the difficulty of purifying enzymes that are integrated into membranes without inactivating them (personal experience and reviewed in (Draper and Stewart, 2005)), as well as discrepant data from enzyme kinetic studies in whole cells and in vivo, compared to those done in homogenates of tissues comprising multiple cell types in which organelles were broken, mixing enzymes and cofactors that normally reside in separate sub-cellular compartments.
1.2. Apparent Mineralocorticoid Excess
Interest in 11βHSD was spurred by the search for the etiology of the inherited hypertensive syndrome Apparent Mineralocorticoid Excess (AME), first described by Maria New and colleagues in 1977 (New, Levine, Biglieri et al., 1977,Ulick, Ramirez and New, 1977). Patients with AME exhibit hallmarks of mineralocorticoid excess, hypertension and easily provoked hypokalemia that are ameliorated by mineralocorticoid receptor antagonist (spironolactone) treatment, yet their renin activities and angiotensin and aldosterone concentrations were suppressed. Attempts to isolate a new mineralocorticoid were in vain. Ulick demonstrated that a marked decrease in the conversion of cortisol to cortisone differentiated AME from all other hypertensive syndromes (Ulick, Levine, Gunczler et al., 1979). AME patients had normal circulating cortisol concentrations due to normal hypothalamic-pituitary-adrenal axis (HPA) regulation, high ratios of urinary cortisol and cortisol metabolites to cortisone and its primary metabolites, prolonged cortisol half-life, and decreased metabolism of cortisol tritiated at the 11α-position to tritiated water (Ulick et al., 1979). Suppression of the HPA and cortisol synthesis with dexamethasone, a poor agonist of the mineralocorticoid receptor, ameliorated AME; hydrocortisone treatment exacerbated it (Oberfield, Levine, Carey et al., 1983,Dimartino-Nardi, Stoner, Martin et al., 1987,Monder, Shackleton, Bradlow et al., 1986).
1.3. Cloning and Characterization of 11βHSD1
Monder’s group cloned the first 11βHSD cDNA, 11βHSD1, from rat liver (Lakshmi and Monder, 1985) (Agarwal, Monder, Eckstein et al., 1989). The expressed protein used NADPH and NADP+ as cofactors to catalyze the 11-oxoreduction of cortisone and 11-dehydrocorticosterone and 11β-dehydrogenation of cortisol & corticosterone, respectively. The Michaelis-Menten (Km) constants were high, in the 2–20 μM range. A year later this group cloned the human 11βHSD1 from testes (Tannin, Agarwal, Monder et al., 1991). It had similar kinetics and activity as the rat liver enzyme and a wide tissue distribution but was most prominently expressed in the liver where its affinity was 7-fold higher for cortisone (Km 0.3 μM) than for cortisol (Km 2.1 μM) (Tannin et al., 1991). The 11βHSD1 of other species have similarly low affinities for the glucocorticoids relative to their circulating concentrations, particularly as glucocorticoids are extensively bound to corticoid binding globulin and to a lesser extent, albumin (reviewed in Tomlinson et al (Tomlinson, Walker, Bujalska et al., 2004). Maser et al have proposed that the Km for 11βHSD1 is substantially lower under in vivo conditions that provide for 11βHSD1 dimerization and enzyme cooperativity (Maser, Volker and Friebertshauser, 2002), a hypothesis that has been contested (Tomlinson et al., 2004). Several splice variants were described for the human 11βHSD1 (Tannin et al., 1991) (White, Pascoe, Curnow et al., 1992,Krozowski, Provencher, Smith et al., 1994,Mercer, Obeyesekere, Smith et al., 1993,Yang, Yu and Han, 1995) (reviewed by Blum & Mercer (Blum and Maser, 2003), and studied for their possible involvement in AME (Stewart and Krozowski, 1994).
1.4. The cortisol-cortisone shuttle
Once cloned, the human mineralocorticoid receptor (MR) was found to have a similar affinity for cortisol, corticosterone and aldosterone and to be expressed in non-mineralocorticoid target tissues, as well as those tasked with water and ion transport such as the colon and kidney (Arriza, Weinberger, Cerelli et al., 1987). Moreover, the MR and the high affinity Type 1 Corticosteroid-binding Site prominent in the hippocampus were confirmed to be the same protein (Arriza, Simerly, Swanson et al., 1988). As circulating concentrations of aldosterone are 2–3 orders of magnitude less than those of glucocorticoids, the next issue to resolve was how aldosterone could bind the MR in mineralocorticoid target cells amid an abundance of competing glucocorticoids. Demonstration that over-indulgence in licorice, a known 11βHSD antagonist, caused reversible AME led Stewart et al to hypothesize that an 11β-hydroxysteroid dehydrogenase-driven “cortisol-cortisone shuttle” within aldosterone target cells of the kidney reduced active glucocorticoid concentrations in the immediate vicinity of the MR, allowing aldosterone to bind and activate the MR. Further, an inherited defect in this dehydrogenase was postulated to allow cortisol to activate aldosterone target MR, resulting in AME (Stewart, Corrie, Shackleton et al., 1988). Low affinity and primarily reductase activity made 11βHSD1 an unlikely candidate. 11βHSD2 had not yet been cloned, however evidence of a distinct NAD+-dependent 11β-hydroxysteroid dehydrogenase enzyme was strong (Abramovitz, Branchaud and Murphy, 1982) and it was soon confirmed that it conferred specificity for aldosterone binding to the MR through the local inactivation of glucocorticoids in the kidney (Edwards, Burt, McIntyre et al., 1988,Funder, Pearce, Smith et al., 1988).
1.5. Cloning of 11βHSD2
The purification and characterization of 11βHSD2 from human placenta (Brown, Chapman, Edwards et al., 1993) and 11βHSD2 from rat kidneys (Rusvai and Naray-Fejes-Toth, 1993) was published in 1993. Both groups confirmed that 11βHSD2 was distinct from 11βHSD1. Besides having a greater mass, it was a high affinity, NAD+ dependent 11β-dehydrogenase with no physiological reductase activity. Within a year a human 11βHSD2 cDNA was cloned (Agarwal, Mune, Monder et al., 1994) (Albiston, Obeyesekere, Smith et al., 1994); hsd11b2 from other species were cloned soonthereafter (Cole, 1995,Zhou, Gomez-Sanchez, Cox et al., 1995) (Brown, Chapman, Kotelevtsev et al., 1996). The correct sequence for HSD11B2 was determined by Brown et al, allowing them to produce an antibody to determine that the tissue distribution in the late mid-term fetus and adult by immunohistochemistry was similar (Brown et al., 1996).
As predicted, 11βHSD2 is expressed with MR in aldosterone target cells but not in the liver (Brown et al., 1996,Cole, 1995,Roland and Funder, 1996). A surprising number of mutations in the 11βHSD2 were found to cause AME of variable severity (Mune, Rogerson, Nikkilä et al., 1995,Obeyesekere, Ferrari, Andrews et al., 1995,Wilson, Harbison, Krozowski et al., 1995,Ferrari, Obeyesekere, Li et al., 1996,Stewart, Krozowski, Gupta et al., 1996,Rogoff, Smolenicka, Bergada et al., 1998,Krozowski, 1999,White, Agarwal, Nunez et al., 2000,Odermatt, Dick, Arnold et al., 2001,Alzahrani, Aljuhani, Qasem et al., 2014,Quinkler, Bappal, Draper et al., 2004). Non-classical AME due to an apparent partial 11βHSD2 insufficiency with no known 11βHSD2 mutation has been described in patients with systolic hypertension, low plasma renin activity, high urinary potassium excretion, elevated serum cortisol/cortisone ratio and low cortisone, as well as markers of renal and cardiac injury. Their hypertension responded well to MR antagonists (Tapia-Castillo, Baudrand, Vaidya et al., 2019).
The dearth of 11βHSD2 in the heart has remained a puzzle, as the MR in the cardiomyocyte appears to be responsive to inappropriate levels of aldosterone (Gomez-Sanchez, 2014). 11βHSD2 is expressed in the developing brain and is crucial for the normal patterning of mineralocorticoid and glucocorticoid receptor activity and normal behavior and cognition (Chapman, Holmes and Seckl, 2013). Expression of 11βHSD2 in the adult brain has been controversial; it is reported to be limited to the epithelial cells of the subcommissural organ and a small number of neurons (Zhou et al., 1995,Roland, Li and Funder, 1995,Chen, Gomez-Sanchez, Penman et al., 2014,Haque, Wilson, Sharma et al., 2015), primarily those of the nucleus tractus solitarius that co-express the mineralocorticoid receptor and 11βHSD2 (Geerling, Kawata and Loewy, 2006). Physiological and iatrogenic increases in aldosterone stimulate salt appetite through these MR. Involvement of the sympathetic nervous system was predicted by studies of mineralocorticoid hypertension by many investigators as reviewed by Gomez-Sanchez (Gomez-Sanchez, 2014). 11βHSD1 has been postulated to provide dehydrogenase activity in specific areas of the adult brain of the rat including the presympathetic neurons (Chen et al., 2014,Jellinck, Pavlides, Sakai et al., 1999). However targeted deletion of the 11βHSD2 gene in the brain increased salt appetite and salt-sensitive hypertension due to increased sympathetic nervous system drive which is abrogated treatment with a mineralocorticoid antagonist (Evans, Ivy, Wyrwoll et al., 2016). This model confirmed the importance of 11βHSD2 activity in a very few key aldosterone target neurons in the CNS that modulating the blood pressure (Gomez-Sanchez and Gomez-Sanchez, 1992).
1.6. Cloning of 11βHSD3
Other putative 11βHSDs have been described based on activity, kinetics, pyridine cofactor preference, and tissue and subcellular localization (Ge, Gao, Nacharaju et al., 1997,Gomez-Sanchez, Ganjam, Chen et al., 1997,Gomez-Sanchez and Gomez Sanchez, 1997,Robinzon and Prough, 2009,Ohno, Nakagawara, Honda et al., 2013), but no genes for another was cloned until recently. Application of new genomic, proteomic and protein structural analyses ushered in the modern era of 11βHSD enzymology. An 11βHSD3, also called 11βHSD1-like, was discovered by searching annotated sequences of the human genome (Bird, Greatorex, Reser et al., 2017). It is an NADP+-dependent dehydrogenase expressed primarily in pituitary gonadotrophs and gonads in human and several larger mammals, but not in the rat or mouse. This enzyme will be further discussed in section 4.
1.7. Relative tissue distribution of HSD11B1, HSD11B2 and HSD11B3 (HSD11B1L)
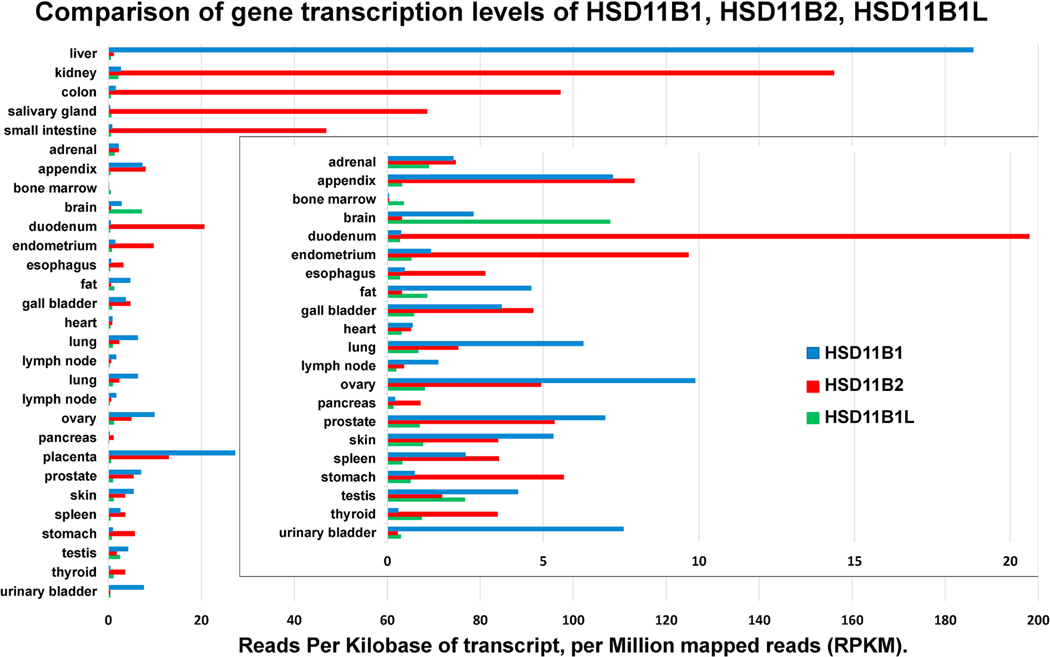
Figure 1 shows the tissue distribution of mRNA for HSD11B1, HSD11B2 and HSD11B3 expressed as Reads Per Kilobase of transcript (RPKM), per Million mapped reads (RPKM) updated November of 2020. Recently the name HSD11B1-Like (HSD11B1L) has become the preferred name for HSD11B3. Data for tissues with lower RPKM is repeated and superimposed with a shorter x-axis. (gene IDs 3290, 3291 and 374875; https://www.ncbi.nlm.nih.gov/gene). In general, MR mediated actions promote cell proliferation, while those of GR suppress proliferation and promote terminal differentiation. In the human 11βHSD1 expression is limited until 2–3 months of age (Gathercole, Lavery, Morgan et al., 2013), while that of 11βHSD2 is more widely distributed in the fetus and decreases after birth. The delicate balance between GR- and MR-mediated functions during ontogeny is discussed further in section 4. mRNA for HSDB1 is most highly expressed by far in the adult human liver at 7- and 18-fold higher RPKM greater than the placenta and ovary respectively, less in the urinary bladder, lung, appendix, and even less in other tissues. HSD11B2 expression is more limited than that of HSDB1 primarily in epithelial cells that mediate transport the vectorial transport of ions and water. 11βHSD2 is highest in the kidney, followed in order by the colon, salivary glands, small intestine, and other tissues where aldosterone is the physiological ligand for the MR. 11βHSD2 is also highly expressed in the placenta, fetal brain, and fetal and adult gonads. A low level of expression of mRNA in an organ does not necessarily reflect an insignificant role for an enzyme. Significant expression of a given protein in a limited number of crucial cells within a large organ may be below the limits of reliable detection by genomic or proteomic means. Though most studies fail to detect 11βHSD2 in the adult brain, 11βHSD2 in a very small number of aldosterone target neurons of the nucleus tractus solitarius that, despite their limited numbers, allow mineralocorticoids to stimulate a powerful increase in sodium appetite (Geerling et al., 2006).
Figure 1:
Comparison of gene transcription levels in Reads Per Kilobase of transcript, per Million mapped reads (RPKM). HSDB1: https://www.ncbi.nlm.nih.gov/gene/3290; HSD11B2: https://www.ncbi.nlm.nih.gov/gene/3291; HSD11B3: https://www.ncbi.nlm.nih.gov/gene/374875
Expression of human 11βHSD3/HSD11B1L is most abundant in the adult brain and bone marrow and is in greater abundance than 11βHSD2 in fat and testes where it may be responsible for most of the dehydrogenase formerly ascribed to 11βHSD2 in those species that have HSD11B1L.
2.0. General 11βHSD structure
11βHSD1, 11βHSD2 and 11βHSD3/11βHSD1-L are members of the short chain dehydrogenase/reductase (SDR) family that comprises roughly 3000 enzymes found in all living organisms, at least 63 of which are found in the human. The function of many are still unknown (Tomlinson et al., 2004,Bird et al., 2017). Homology of the gene and amino acid sequences of SDR enzymes, including the 11βHSDs, is low, however these enzymes share specific tertiary structures including the Rossmann fold comprising alternating α-helices and β-strands that form the NADH/NAD+ and NADPH/NADP+ binding domain. Among the conserved amino acid sequences are a GxxxGxG motif forming the turn between the helix and strand bordering the cofactor binding site and a triad of Ser, Tyr and Lys residues crucial for catalytic activity (Blum and Maser, 2003). Details of the protein sequence and tertiary structures of the SDRs are reviewed in by Blum and Maser (Blum and Maser, 2003) and Bird et al (Bird et al., 2017).
The 11βHSDs and 17βHSDs have similar N-terminal membrane spanning domains. The position of the catalytic domains, within the lumen or out, is dictated by charges on the amino acids adjacent to the membrane spanning domain. 11βHSD1 and 11βHSD2 are oriented in opposite directions. The N-terminus of the 11βHSD1 is cytoplasmic; its catalytic domain and C-terminus is in the lumen of the ER. The catalytic domain and C-terminus of 11βHSD2 are in the cytoplasm. Switching the N-terminal domains of 11βHSD1 and 11βHSD2 to reverse their orientation destroyed the enzyme activity for both (Odermatt, Arnold, Stauffer et al., 1999). Most such studies have been done in microsomes comprising nuclear and contiguous ER membranes (Tingey, Mudumbi, Schirmer et al., 2019). Both enzymes are also integrated into the outer nuclear membrane (Gomez-Sanchez et al., 1997,Brereton, van Driel, Suhaimi et al., 2001). The predicted structure of 11βHSD3 resembles those of 11βHSD1 and 11βHSD2 and includes an ER localization signal sequence at the N-terminus required for function and a cofactor domain like that of 11βHSD1 conforming to both using NADP+ and NDPH (Bird et al., 2017). Thus, 11βHSD3 is also called 11βHSD1-like or 11βHSD1L. The binding sites of the three 11βHSDs predict the accommodation of a wide variety of compounds. These include 7-keto and 7β,27-sterols for which the human 11βHSD1 and 11βHSD2 have greater affinity than for the glucocorticoids, as well as exogenous substrates, including known endocrine disrupter compounds (Beck et al., 2019,Odermatt and Klusonova, 2015). Discussion of other substrates will be continued in section 2.2–2.4.
The similarity of the Rossmann fold and ligand binding structures of 11βHSD1 and ACE2 and the inhibition of both by known antagonists of SAR-CoV-1 entry into cells, prompted an extensive in silico study and suggestion that 11βHSD1 as a pharmacophore for the identification and development of drugs to inhibit the entry, replication and maturation of SAR-CoV-2 (Robson, 2020).
2.1. 11β-hydroxysteroid dehydrogenases type 1 or SDR26C1
The human 11βHSD1 is the product of the gene HSDB1 on chromosome 1q32.2. It is a 34-kDa glycoprotein with a N-terminal membrane spanning domain that participates with other parts of the molecule in anchoring it to the ER membrane and nuclear envelope {Blum, 2001 #5137}(Brereton et al., 2001). Its orientation with the catalytic domain within the lumen is determined by the positive charge provided by two lysines on the cytosolic side of the membrane spanning domain and two glutamines on the luminal side that provide a negative charge (Odermatt et al., 1999). The milieu of the ER lumen promotes disulfide bond formation within the 11βHSD1 protein that enhance its activity (Ozols, 1995,Mziaut, Korza, Hand et al., 1999,Frick, Atanasov, Arnold et al., 2004). Cys272 appears to be a site for homodimer formation (Walker, Clark, Hewison et al., 2001) which has been proposed to significantly lower its Km (Maser et al., 2002). Glycosylation of the human 11βHSD1 is thought to stabilize the protein but is not required for activity; glycosylation sites are absent in the 11βHSD1 of some species (Tomlinson et al., 2004). A comprehensive review of the arduous work of many to characterize the structure and function of 11βHSD1, including a comparison of enzyme kinetics between species, is provided by Tomlinson et al (Tomlinson et al., 2004).
2.1.1. Lessons from Hsd11b1 manipulation in mice
Glucocorticoids regulate the HPA axis through GR in neurons of the hippocampus, hypothalamus and pituitary dearywhich also express 11βHSD1. Depending on the genetic background of the mouse, transgenic mice with global Hsd11b1 deletion have normal or high plasma corticosterone levels associated with increased or normal hypothalamic and pituitary GR levels, respectively (Carter, Paterson, Tworowska et al., 2009). This suggests that the increase in GR in some Hsd11b1−/− mice compensate for the loss of production of corticosterone from circulating 11-dehydrocorticosterone within cells of the HPA axis in some strains. Global deletion of Hsd11b1 in the mouse results in increased corticosterone synthesis and plasma levels, adrenal cortical hyperplasia, disruption of the circadian rhythm, and heightened HPA response to stress (Paterson, Holmes, Kenyon et al., 2007). Targeted expression of mouse Hsd11b1 in the liver rescued HPA abnormalities produced by its global deletion (Paterson et al., 2007). Information about energy status within the liver is relayed to the HPA axis through the vagus nerve (la Fleur, Manalo, Roy et al., 2005). Normalization of glucocorticoid activity in the liver increased hepatic metabolic fuels and appears to have suppressed the HPA axis (Paterson et al., 2007).
Hsd11b1−/− mice are protected from iatrogenic Cushing’s Syndrome, including glucose intolerance, hyperinsulinemia, hepatic steatosis, adiposity, hypertension, myopathy, and dermal atrophy, produced by the administration of corticosterone and 11-dehydrocorticosterone in the water (Tomlinson, Draper, Mackie et al., 2002,Morgan, McCabe, Gathercole et al., 2014). An analogous situation was reported for a patient with cortisone reductase deficiency who had Cushing’s disease without the expected phenotype (Tomlinson et al., 2002). Targeted deletion of Hsd11b1 in adipocytes protected mice consuming high doses of glucocorticoids from the expected metabolic dysfunction including hepatic triacylglycerol accumulation of (Morgan et al., 2014). However mice in which Hsd11b1 was deleted in the liver developed most of the expected phenotype upon receiving the same glucocorticoid excess (Morgan et al., 2014). The liver-specific deletion of Hsd11b1 did protect the mice from the effects of a lower 11-dehydrocorticosterone excess (Harno, Cottrell, Yu et al., 2013). These types of studies suggest that the high expression and activity of 11βHSD1 in the liver is responsible for more than the regeneration of 11-dehydrocorticosterone and that this task is done primarily 11βHSD1 in the adipose tissue. Other substrates of 11βHSD1 will be discussed in sections 2.2–2.2.4.
Targeted over-expression of Hsd11b1 in the liver or fat results in metabolic dysfunction in mice despite normal circulating corticosterone (Paterson, Morton, Fievet et al., 2004,Masuzaki, Paterson, Shinyama et al., 2001,Masuzaki, Yamamoto, Kenyon et al., 2003). Increased Hsd11b1 in the liver caused hepatic steatosis, insulin resistance, and hypertension. Hsd11b1 over-expression in adipose tissue resulted in a similar profile, plus visceral obesity, and dyslipidemia (Masuzaki et al., 2003).
2.1.2. Hexose-6-phosphate dehydrogenase, a requisite for 11βHSD1 reductase activity
11βHSD1 dehydrogenase activity is 2 orders of magnitude greater than reductase activity in artificial systems (Walker et al., 2001). However in intact cells the catalytic domain of 11βHSD1 resides within the ER where close association with hexose-6-phosphate dehydrogenase (H6PDH ) ensures efficient regeneration of NADPH from NADP+ for oxo-reductase activity (Draper, Walker, Bujalska et al., 2003,Bujalska, Draper, Michailidou et al., 2005,Banhegyi, Benedetti, Fulceri et al., 2004). H6PDH catalyzes the first two steps of the pentose phosphate pathway, the major source of intraluminal NADPH within the ER. While H6PDH can use several substrates, the primary is glucose-6-phosphate carried into the ER by a specific glucose-6-phosphate transporter (Odermatt and Klusonova, 2015,Atanasov, Nashev, Gelman et al., 2008). Interruption of the regeneration of NADPH within the ER results in 11βHSD1 dehydrogenase rather than a reductase activity (Odermatt and Klusonova, 2015,Draper et al., 2003,Bujalska et al., 2005,Atanasov, Nashev, Schweizer et al., 2004,Nashev, Chandsawangbhuwana, Balazs et al., 2007,Balazs, Nashev, Chandsawangbhuwana et al., 2009).
Glucocorticoids generally suppress cell proliferation and promote differentiation. At harvest human adipose stromal cells, which include preadipocytes, were reported to express low levels of 11βHSD1, no 11βHSD2, yet had 11βHSD dehydrogenase activity. Mature adipocytes had 11βHSD reductase activity (Bujalska, Walker, Hewison et al., 2002). The switch from 11βHSD1 dehydrogenase to oxo-reductase activity corresponded to a significant increase in H6PDH expression in the differentiating adipocytes (Bujalska et al., 2005). In contrast, mouse preadipocytes do exhibit 11βHSD1 reductase activity. Increased reductase activity was associated with visceral adipocyte maturation and lipid storage in mice fed a high fat diet (De Sousa Peixoto, Turban, Battle et al., 2008).
An example of H6PDH deficiency is the rare autosomal recessive disorder cortisol reductase deficiency (CRD). Reductase activity in adipose tissue explants from these patients is low or absent. The inability to convert cortisone to cortisol results in the increased metabolic clearance rate of cortisol, excreted primarily as cortisone and cortisone metabolites, and increased ACTH secretion to maintain normal circulating cortisol concentrations that results in ACTH-mediated androgen excess. Patients present with virilization in females that is ameliorated with dexamethasone suppression of ACTH (Draper et al., 2003). It was previously thought that some CRD patients had a partially deficient enzyme paired with a complex polymorphism of their HSDB1 gene (Draper et al., 2003). It has now been ascertained that an inactivating mutation of the H6PDH preventing normal 11βHSD1 reductase activity is the sole cause of CRD (Lavery, Walker, Tiganescu et al., 2008,Lavery, Idkowiak, Sherlock et al., 2013). Dehydrogenase activity of 11βHSD1 in early studies using whole kidney homogenates is now assumed to be an artifact of cell disruption and loss of the NADPH regenerating system. Though true, it may also reflect the mixing of different cells types. Intense 11βHSD1 immunoreactivity in the rat kidney is found in medullary interstitial cells and in tubular epithelial cells of the outer cortex; that of H6PDH is in the juxtomedullary cortex with little over-lap with the most dense 11βHSD1 staining in the renal cortex. Nor was H6PDH detected in the rat interstitial cells (Gomez-Sanchez, Romero, de Rodriguez et al., 2008) which express GR but not MR; dehydrogenase activity by 11βHSD1 may moderate activation of the glucocorticoid receptor in the rat renal interstitial cell. The balance between MR and GR activation is critical in diverse cell types including those in which the MR is normally activated by glucocorticoids (de Kloet, Oitzl and Joels, 1999,Meijer, 2002,Hoppmann, Perwitz, Meier et al., 2010,Harris, Holmes, de Kloet et al., 2013, de Kloet, Meijer, de Nicola et al., 2018,Oakley, Cruz-Topete, He et al., 2019). As the affinity of the MR for cortisol and corticosterone is about 10 times that of the GR, in addition to providing pre-receptor selectivity to the MR for aldosterone over glucocorticoids, the 11βHSDs modulate the balance of GR and MR activation by glucocorticoids. This is crucial because both bind many of the same DNA hormone response elements, but physiological outcomes are frequently opposite. In addition, there is some evidence that MR and GR form homodimers and heterodimers with different transcriptional efficacy depending on the cell type (Yang and Young, 2009,Farman and Rafestin-Oblin, 2001), Results from in vivo studies using physiological concentrations of ligands suggest that in tubular epithelial cells of nephron segments in which MR and GR are co-expressed, MR:GR heterodimers are more efficient than MR:MR homodimers in initiating the transcription of genes associated with ion channel activation, the primary mineralocorticoid activity in this prototypical aldosterone target tissue (Ackermann, Gresko, Carrel et al., 2010), confirming the premise that the appropriate balance of MR and GR activation is crucial for homeostasis (Gomez-Sanchez, 2014).
Dehydrogenase activity of 11βHSD1 in the absence of H6PDH may occur in other cells. 11βHSD2 expression in the adult brain is very limited (Chen et al., 2014,Geerling et al., 2006), while that of 11βHSD1 and H6PDH is extensive. Nonetheless, evidence that the MR in pre-sympathetic neurons of the paraventricular nucleus (PVN) of the hypothalamus are activated by aldosterone has accrued over the last 40 years (Gomez-Sanchez and Gomez-Sanchez, 2014,Gomez-Sanchez, 2014). Oral and central administration of 11βHSD inhibitors increase sympathetic nervous system activity and the blood pressure and the hypertension produced by oral 11βHSD inhibition is inhibited by the central infusion of an MR antagonist in the rat (Gomez-Sanchez and Gomez-Sanchez, 1992,Gomez-Sanchez and Gomez-Sanchez, 1999,Zhang, Kang, Yu et al., 2006). Moreover, rat brain tissue, including that of the hypothalamus, produces 11-dehydrocorticosterone from endogenous and tritiated substrates ex situ. Ample immunoreactivity for 11βHSD1, but not 11βHSD2 or H6PDH, was detected within rat pre-sympathetic neurons of the PVN identified by retrograde tracer, suggesting that 11βHSD1 may act as a dehydrogenase in these neurons and confer aldosterone specificity to their MR (Chen et al., 2014). (Rats do not express the obligatory dehydrogenase Hsd11B3 (Hsd11b1L)). However, recently others have detected
11βHSD2 immunoreactivity in neurons of the rat hypothalamus using more sophisticated antigen retrieval methods (Haque et al., 2015). Moreover, we have shown that relatively low expression of 11βHSD2 is enough to inactivate corticosterone and hinder its ability to activate the MR (unpublished results). The mechanisms regulating the balance of MR and GR activation in the central nervous system are still unclear.
2.2: 11βHSDs are multi-functional
The substrate binding domain of the 11βHSDs accommodate a variety of molecules having a 5α-conformation that provides a flat A/B ring junction. Rotational symmetry of steroid molecules allows 11βHSD1 to act on both positions 7 and 11. Steroids with 5β- conformation or bulky groups at C-21 are not substrates (Tomlinson et al., 2004, Banhegyi et al., 2004). 11-keto (11-oxo) sterols including 11-ketopregnenolones, 11-ketoprogesterones, 11 and 7-ketoandrogens, 7-keto sterols including 7-ketocholesterol (7kC), and bile acids are important endogenous substrates of 11βHSD1. Odermatt et al have provided an in depth review of substrates of 11βHSD1 in addition to glucocorticoids (Odermatt and Klusonova, 2015). In addition to modulating glucocorticoid activity in the CNS, 11βHSD1 in the presence of H6PDH reduces the 7-keto neurosteroids 7-keto-dehydroepiandrosterone (7kDHEA), and 7-keto-pregnenolone stereo-specifically to 7β-hydroxy-DHEA and 7β-hydroxy-pregnenolone (Nashev et al., 2007). These studies were conducted in a cell line isolated originally from the liver, however H6PDH is expressed widely and 11βHSD1 reductase activity is thought to be predominate in the brain.
Studies described in section 2.1.1 suggest that regeneration of active glucocorticoids may not be the only function of 11βHSD1 in the liver and Hsd11b1 expression in the liver is reported to be less sensitive to factors it in other tissues (Tomlinson et al., 2004). This may reflect its significant role in phase I biotransformation of many carbonyl bearing endogenous compounds and xenobiotics, a function it shares with cytosolic-reductases including carbonyl reductase 1 (CBR1) and the aldo-keto reductases (AKRs) (Odermatt and Klusonova, 2015). Conversion of a carbonyl to a hydroxyl group enables glucuronidation or sulfation and excretion of xenobiotics such as nitrosamine ketone, a primary metabolite of nicotine and carcinogen, triadimefon, a systemic agricultural fungicide and persistent environmental contaminant and endocrine disruptor, and 7-oxo-DHEA, a commonly used dietary supplement. Frequently used drugs may increase susceptibility to the toxic effects of drugs and pollutants (Robinzon and Prough, 2009). Among clinically important drugs reduced by 11βHSD1 are prednisone, bupropion, ketoprofen, metyrapone, the radiation therapy sensitizers para-nitroacetophenone and para-nitrobenzaldehyde, and the cancer therapeutic agents benfluron, oracin and doxorubicin (Wsol, Szotakova, Skalova et al., 2004,Maser, Wsol and Martin, 2006). Doxorubicin is an effective antineoplastic drug that is reduced to doxorubicinol by 11βHSD1, as well as CBR1 and AKRs. In addition to being inactive, doxorubicinol is cardiotoxic. Selective inhibitors of both 11βHSD1 and CBR1 are being developed to improve the therapeutic index of doxorubicin by decreasing the formation of the doxorubicinol metabolite (Yang, Hua, Ryu et al., 2018). Xenobiotic substrates of 11βHSD1 and 11βHSD2 and methods for their identification have been reviewed by Nashev et al (Nashev, Vuorinen, Praxmarer et al., 2012).
2.2.1: Bile acids
11βHSD1 is an important 7-oxo bile acid reductase and has other less well understood functions in bile acid homeostasis (Odermatt and Klusonova, 2015,Hult, Nobel, Abrahmsen et al., 2001,Odermatt, Da Cunha, Penno et al., 2011,Penno, Morgan, Rose et al., 2014). 7-Oxolithocholic acid (7-oxoLCA), is a secondary bile acid produced by gut microorganisms from the bile acids chenodeoxycholic (CDCA) and ursodeoxycholic acids (UDCA). Reduction of 7-oxoLCA in human and rat liver microsomes with NADPH as the preferred electron donor was reported (Fromm, Carlson, Hofmann et al., 1980) 3 decades before Odermatt’s team determined that 11βHSD1 was solely responsible for the reduction of 7-oxoLCA and its taurine- and glycine-conjugates to produce CDCA and UDCA (Odermatt et al., 2011). 11βHSD1 of different species differ in the proportion of CDCA and UDCA produced from 7-oxoLCA. CDCA is the primary product of the human 11βHSD1, thus it circulates in greater concentrations than UCDA in humans, while CDCA and UDCA are formed and circulate in similar concentrations in mice and rats.
2.2.2: Oxosterols
Oxosterols (ketosterols) are synthesized from cholesterol and cholesterol precursors. They participate in cholesterol, carbohydrate, and lipid homeostasis and are substrates for steroid hormone biosynthesis and some have significant intrinsic biological activities, particularly as inflammatory mediators, and disruption of their metabolism impacts multiple systems. Bjorkhem et al and Schroepfer et al have provided valuable reviews of the metabolism of oxosterols and pathological implications of its disruption (Schroepfer, 2000,Bjorkhem, 2013). 7kC is the most abundant cholesterol derivative in human circulation, with concentrations approximating those of cholesterol. It is derived from the diet and auto-oxidation of cholesterol in tissues and the accumulation of 7kC and its metabolites is implicated in the pathogenesis of atherosclerotic plaque and age-related disorders including macular degeneration and dementias including Alzheimer’s disease (Odermatt, Atanasov, Balazs et al., 2006,Mitic, Shave, Semjonous et al., 2013,Pariente, Pelaez, Perez-Sala et al., 2019).
The crucial role of 11βHSD1 in cholesterol metabolism was discovered while studying the pathogenesis of loss of function mutations of CYP27A1 which normally converts 7β- hydroxycholesterol (7βOHC) to 7β27OHC. The accumulation of 7α-hydroxylated bile acid precursors in CYP27A1 deficiency leads to the increase and deposition of 7kC and 7-hydroxylated cholesterol derivatives in many tissues, particularly the brain and tendons, causing cerebrotendinous xanthomatosis (Cali, Hsieh, Francke et al., 1991,Bjorkhem and Hansson, 2010,Tang, Liu, Li et al., 2020). These patients have a high incidence of atherosclerosis without significantly increased cholesterol levels. Deletion of CYP27A1 in mice indicated additional mechanisms for 7kC metabolism and excretion (Lyons, Maeda and Brown, 2002), leading to the discovery that the 11βHSD1 of many vertebrates reduces 7kC to 7 β-hydroxycholesterol (7βOHC) which is converted by CYP27A1 to 7β27OHC which is further metabolized for excretion in the bile (Odermatt et al., 2006).
The reduction of 7kC by 11βHSD1 is stereospecific in most species studied; only 7β- hydroxycholesterol (7βOHC) is formed except in the hamster which converts 7kC to both 7βOHC and 7αOHC (Schweizer, Zurcher, Balazs et al., 2004). Studies in 11βHSD1- and H6PDH-null mice and their double knockout crosses demonstrated that 11βHSD1 alone is responsible for the oxoreduction of 7kC and that H6PDH is the source of microsomal NADPH for this conversion. Seckl and coworkers demonstrated that 7kC is a competitive inhibitor of 11βHSD1 in human adipocytes (Wamil, Andrew, Chapman et al., 2008). Conversely, competitive inhibition of 11βHSD1 by the long-term administration of prednisone has been implicated in 7kC accumulation and promotion of human atherosclerotic plaque formation (Kipari, Hadoke, Iqbal et al., 2013). There are significant species differences in the ability of 11βHSD1 and 11βHSD2 to interconvert 7kC and 7βOHC that should be considered when choosing models for oxosterol studies and the development of 11βHSD1 inhibitors. Reduction of 7kC to 7βOHC by the mouse 11βHSD1 is significantly higher than the reverse dehydrogenase activity, but lower than the reduction of 11-dehydrocorticosterone to corticosterone. Studies in the 11βHSD1- and/or H6PDH-null mice led to the conclusion that 7-keto sterols probably do not impact 11βHSD1 metabolism of glucocorticoids in healthy mice but that hyperlipidemia and the resulting oxidative stress in mice fed a “western diet” increase 7kC and its metabolites enough to effectively compete with glucocorticoids as substrates for the 11βHSD1 reductase activity in vivo (Mitic et al., 2013). In contrast to the mouse, affinity of the human 11βHSD1 and 11βHSD2 for 7kC metabolites was found to be greater than that for cortisone and 11-dehydrocorticosterone (Beck et al., 2019). This is another example of species differences in the affinities of the 11βHSDs for specific switch pair substrates to be considered in designing drugs to manipulate these enzymes.
11βHSD1 is expressed in macrophages and is particularly high in those that become foam cells in fatty streaks and atherosclerotic plaques in vessel walls, thus 7βOHC and 7kC are the most abundant oxysterols in atherosclerotic plaque (Kipari et al., 2013,Mitic, Andrew, Walker et al., 2013). Interruption of 11βHSD1 oxoreductase activity in vivo increases the 7KC to 7βOHC ratio within the ER and the accumulation of 7KC limits cholesterol transport from the ER, thus reducing endogenous cholesterol synthesis. This is may be a mechanism for some of the ameliorative effects of 11βHSD1 inhibitors in MetS (Mitic et al., 2013,Hardy, Botfield, Markey et al., 2020).
The human and mouse 11βHSD1 catalyzes the stereo-specific oxoreduction of 7k27OHC to 7β27OHC; 11βHSD2 mediates the reverse reactions. 7α27OHC was neither produced by 11βHSD1 nor was it a substrate for 11βHSD2 in these species. The reduction of 7k27OHC to 7β27OHC in HEK-293 cells stably expressing the human 11βHSD1 and H6PDH was 3 times more efficient than that for cortisone; the reverse reaction was not catalyzed in these cells (Beck et al., 2019). The IC50 for the competitive inhibition of cortisone reduction by 7k27OHC for the human 11βHSD1 was ~3μM. As a reminder of the significant differences in 11βHSD1 activity among species, the IC50 for the inhibition of cortisone reduction by 7k27OHC for the mouse 11βHSD1 was 66 nM (Beck et al., 2019).
2.2.3: Oxosterols interfere with the 11βHSD2-mediated cortisol-cortisone shuttle.
Decreased 11βHSD2 activity resulting in the inappropriate activation of the MR by glucocorticoids in several tissues is associated with MetS, hyperlipidemia and oxidative stress leading to inflammation, and repair including the replacement of damaged tissue with fibrosis reviewed by Chapman et al (Chapman et al., 2013) and Gomez-Sanchez et al (Gomez-Sanchez and Gomez-Sanchez, 2014). HEK-293 cells stably expressing the HSD11B2 converts 7β27OHC to 7k27OHC with slightly more efficiency than for conversion cortisol to cortisone. Human 11βHSD2 conversion of cortisol to cortisone was inhibited by both 7β27OHC and 7k27OHC with similar IC50 values in the nanomolar range; 7α27OHC had no effect (Beck et al., 2019). Use of an MR-dependent luciferase reporter gene in HEK-293 cells stably expressing 11βHSD2 demonstrated that 7β27OHC and 7k27OHC increased cortisol-dependent MR activation by 3.5-fold. In contrast, conversion of corticosterone to 11-dehydrocorticosterone by mouse kidney homogenates, which express both 11βHSD1 and 2, was not inhibited by either 7β27OHC or 7α27OHC and 7k27OHC was only a weak inhibitor of the mouse 11βHSD dehydrogenase activity. Docking studies with the predicted human and mouse 11βHSD2 found that the probability of binding to 7-and 27 sterols was significantly higher for the human enzyme commensurate with the kinetic studies (Beck et al., 2019). The affinity of the expressed human 11βHSD2 for 7-hydroxycholesterol metabolites suggests that increases in cholesterol metabolites could be relevant in vivo (Beck et al., 2019).
2.2.4: 11β-HSDs modulate RORγ agonist 7β27OHC
The Retinoic acid-related orphan receptor (ROR) family of ligand dependent transcription factors include isoforms of 3 different genes. Several cholesterol intermediates and metabolites are endogenous agonists or inverse agonists of the RORα and RORγ isoforms, among these are vitamin D derivatives not involved in calcium metabolism and triterpenoids found in plants and fungi (Jetten, Takeda, Slominski et al., 2018). RORγ is expressed in many tissues including fat, liver, kidney, and muscle and participates in the regulation circadian rhythm and the metabolism of glucose and lipids. Thymus related RORγ (RORγt) is expressed in select immune cells, including CD4+CD8+ thymocytes, Th17 cells, type 3 innate lymphoid cells (ILC3) that populate lymph nodes and Peyer’s patches, and immune cells of the colon induced by substances produced by the gut microbiome. Changes in cholesterol metabolism impacts immune and metabolic pathways regulated by these RORs and dysfunctional RORγ and RORγt activity is implicated in type 2 diabetes mellitus (T2DM), MetS, inflammation and several autoimmune diseases, neurological disorders, and cancers (Beck et al., 2019,Jetten et al., 2018). Jetten et al have provided a valuable review of the role of RORγ, sterol metabolites and immune function and dysfunction (Jetten et al., 2018).
Beck et al identified 7β27OHC as an agonist of RORγ and RORγt; 7k27OHC is not (Beck et al., 2019). Thus 11βHSD1 and 11βHSD2 have the potential to provide pre-receptor regulation of 7β27OHC for RORγ and RORγt with similarly diverse and important implications as their interconversion of glucocorticoids for GR and MR (Beck et al., 2019). A caveat to these studies is that the expression of 11βHSD2 in the cells studied so far, myeloid cells, hepatocytes and neurons is nil or extremely low. The tissue distribution of 11βHSD3 has not been extensively mapped, nor has its 7-oxosterol dehydrogenase activity been reported as yet, however its substrate pocket and catalytic domain are similar to those of 11βHSD1. 11βHSD3, rather than 11βHSD2, may be the switch pair with 11βHSD1 in the interconversion of sterol ligands for RORs.
2.2.5. Regulation of 11βHSD expression is cell-type specific
A review of studies of potential regulators of 11βHSD expression (Tomlinson et al., 2004) found that insulin was reported to decrease 11βHSD1 in the liver, while growth hormone (GH), transforming growth factor, basic fibroblast growth factor, hepatocyte growth factors, and insulin-like growth factor-1 (IGF-1) decrease or have no effect on 11βHSD1 transcription. Many of these studies were done in immortalized cell lines (Tomlinson et al., 2004). Normal GH and IGF-1 function is critical for energy homeostasis, however results of studies of their regulation of 11βHSD expression and activity have been discrepant, as reviewed by Huang et al (Huang, Li, Lin et al., 2010). Clinical studies in patients with pituitary dysfunctions suggested that GH and/or IGF decrease 11βHSD1 activity (Gelding, Taylor, Wood et al., 1998,Stewart, Toogood and Tomlinson, 2001,Moore, Monson, Kaltsas et al., 1999), as did a study done in hypophysectomized rats (Albiston, Smith and Krozowski, 1995). In contrast, comparison of tissues from wild type and IGF-1−/− mice found that IGF-1 deletion decreased mRNA, protein and activity of liver 11βHSD1 and kidney 11βHSD2 (Huang et al., 2010). The effect of IGF-1 deficiency was tissue specific; 11βHSD1 expression was not altered in the testes or kidney and 11βHSD2 was no different in the testes of the IGF-1−/− mice.
Glucocorticoids, progesterone, androgens, inflammatory cytokines, vitamin D, and gonadotropins are reported to increase basal 11βHSD1 expression and reductase activity except in the liver where they have less or no effect (Tomlinson et al., 2004). Estrogen was found to suppress 11βHSD1 mRNA and protein, as well as NADPH-dependent reductase activity in the liver and kidneys of intact and gonadectomized male and female rats, while testosterone had no significant effect (Albiston et al., 1995,Low, Assaad, Rajan et al., 1993,Gomez-Sanchez, Ganjam, Chen et al., 2003). Kidney 11βHSD1 mRNA correlated with estrogen levels throughout the estrus cycle (Albiston, Smith and Krozowski, 1995). Though estradiol decreased 11βHSD1 mRNA, it increased 11βHSD dehydrogenase activity in the kidneys, adding to evidence for a second enzyme (Low et al., 1993). Once it was cloned, 11βHSD2 mRNA and protein expression in the male rat kidney were demonstrated to be significantly increased by E2 treatment, however in vivo activity assessed as total and proportional corticosterone and 11-dehydrocorticosterone excretion did not differ between E2- and vehicle-treated rats. This was attributed to the formation of inactive dimers detected by western blot under non-denaturing conditions and in vitro NAD+-mediated dehydrogenase activity under reducing and non-reducing conditions (Gomez-Sanchez et al., 2003).
Isoforms of CAAAT/enhancer binding protein (C/EBP) are widely expressed transducers of cytokine and steroid hormone gene transcription signals, including those of glucocorticoids. Depending on the gene, C/EBPs promote transcription by steroid hormone receptors (SHR) bound to the DNA or initiate transcription of a gene that has no HRE for that SHR. The latter option requires the presence of the activated SHR and may require the recruitment by steroid receptor coactivators (SRCs) (Chen, Mishra, Yu et al., 2020). Glucocorticoids increase the transcription of several C/EBP isoforms depending on the cell type including C/EBPα, β and δ (Sai, Esteves, Kelly et al., 2008). In the presence of activated GR, C/EBPβ mediates glucocorticoid-induced transcription of genes that lack a GR HRE (Sai et al., 2008). The mouse Hsdb1 has two promoter sites. Use of the C/EBP-independent P1 produces an mRNA that includes exon 1A, while use of the C/EBP-dependent P2 includes exon 1B. Thus use of the P1 and P2 can be differentiated even though the 11βHSD1 protein sequence is the same regardless of the promoter used (Bruley, Lyons, Worsley et al., 2006). Glucocorticoid initiation of transcription of Hsd11b1 varies by cell type (Sai et al., 2008,Esteves, Kelly, Begay et al., 2012,Esteves, Verma, Rog-Zielinska et al., 2013). P1 is most important for Hsdb1 transcription in pulmonary fibroblasts. Use of the C/EBP-dependent P2 predominates in the less abundant pulmonary type II alveolar cells, and in liver, fat, and brain (Bruley et al., 2006). Of the C/EBPs studied, C/EBPα and C/EBPβ appear to be the most important for glucocorticoid promotion of 11βHSD1 gene expression (Sai et al., 2008). The work by Bruley et al (Bruley et al., 2006) and Sai et al (Sai et al., 2008) provides a more comprehensive description of complex mechanisms regulating the control of Hsd11b1 transcription.
2.2.6: Inflammatory cytokines promote 11βHSD1
transcription cell type specific The isolation of cortisone in the 1930s and discovery of its anti-inflammatory properties revolutionized medicine and merited a Nobel Prize (Kendall, 1952). The physiological role of the 11βHSDs in inflammation, repair and cell proliferation is complex and can go awry (Kipari et al., 2013,Hadoke, Kipari, Seckl et al., 2013,Chapman, Coutinho, Zhang et al., 2013), however the ability of 11βHSD1 to generate endogenous glucocorticoids at the site of inflammation is crucial for mounting a targeted response to injury (Hardy, Fenton, Croft et al., 2018). 11βHSD1 action provides sufficient concentrations of glucocorticoids induce the anti-inflammatory phenotype in macrophages (Zhang, Coutinho, Man et al., 2017,Desgeorges, Caratti, Mounier et al., 2019)and stabilize mast cells (Coutinho, Brown, Yang et al., 2013). Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κβ) promotes expression of IL-1β and TNFα (Tetsuka, Haines, Milne et al., 1999,Thieringer, Le Grand, Carbin et al., 2001) that induce the expression 11βHSD1 through the C/EBP-dependent P2 promoter (Chen et al., 2020,Chantong, Kratschmar, Nashev et al., 2012,Huang, Li, Xu et al., 2020). The resulting feed-forward production of glucocorticoids activates the GR and suppresses MR-stimulated transcription of inflammatory and pro-fibrotic genes as well as promotes GR-mediated anti-inflammatory effects. 11βHSD1 was found to be a key component of the anti- inflammatory and immunosuppressive functions induced in mesenchymal stem cells by interferonγ (INFγ) through a process called licensing by the anti-inflammatory factor TNF-stimulated gene 6 (TSG-6) in umbilical cord derived mesenchymal stem cells (Huang et al., 2020). INFγ was potentiated the NF-κB-mediated increase in TNF-α expression; TNF-α induced HSD11B1 transcription, which increased intracellular cortisol and activated GR-mediated expression of the TSG-6 (Huang et al., 2020). Chapman et al provide a valuable review of the role of 11βHSD1 in the regulation of inflammation (Chapman et al., 2013) and Desgeorges et al review the crucial role of glucocorticoids and 11βHSD1 in the induction of anti-inflammatory M2 macrophages (Desgeorges et al., 2019).
2.2.7. Bone and skin
11βHSD2 was demonstrated in the cartilage of mice until 1–3 days after birth but not adult mice using a Cre recombinase under the control of endogenous 11βHSD2 promoter and a LacZ reporter gene (Naray-Fejes-Toth and Fejes-Toth, 2007). The MR has not been reported in cartilage, suggesting that 11βHSD2 may decrease glucocorticoid suppression of osteoblast activity in rapidly ossifying perinatal cartilage. 11βHSD1 but not 11βHSD2 is present in osteoblasts along with GR. GR activation suppresses osteoblast functions, unbalancing the normal cycle of osteoclast remodeling of bone and resulting in bone loss. Decreased bone formation markers produced by prednisone treatment correlated with decreased 11βHSD1 activity rather than circulating cortisol or prednisolone levels after treatment, indicating that conversion of prednisone to prednisolone by 11βHSD1 within bone is more important for the deleterious glucocorticoid-meditated bone resorption than circulating prednisolone levels (Cooper, 2012). Cooper and colleagues have provided an extensive review of crucial 11βHSD1 function in bone (Cooper, 2012) and for the use of 11βHSD1 inhibitors in rheumatoid arthritis (Hardy, Seibel and Cooper, 2013) and potential problems with their use (Hardy et al., 2018). Increased expression of 11βHSD1 within bone is associated with age-related osteoporosis and has been cited as a target for the use of 11βHSD1 inhibitors (Hardy et al., 2013). Loss of estrogen-mediated suppression of 11βHSD1 after menopause and decreased androgens for aromatization to estrogens in tissues including bone and skin may be a driver of age-related osteoporosis in both sexes (Terao and Katayama, 2016).
The caveat to the use of 11βHSD1 inhibitors to promote bone health lies in its importance in targeting anti-inflammatory processes. Induction of 11βHSD1 in osteoblasts by inflammatory cytokines including TNFα and IL-1β accelerates glucocorticoid suppression of osteoblasts in the area of inflammation, allowing osteoclasts to phagocytize damaged bone at the site instead of their routine function of bone remodeling (Fenton, Doig, Fareed et al., 2019). However, global deletion of Hsd11b1 exacerbates synovial inflammation, joint destruction, and bone loss in a mouse model of severe chronic polyarthritis due to a decrease in M2 macrophages, a major anti-inflammatory effect mediated by GR, and relative increase in pro-inflammatory M1 macrophages (Hardy et al., 2018).
The skin is in a constant state of renewal and of inflammation and repair. 11βHSD2 is expressed in the sweat glands where the MR modulates water and electrolyte composition of the sweat. 11βHSD1 is expressed in all cells comprising the normal epidermis including keratinocytes, fibroblasts and melanocytes where the balance between proliferative and inflammatory responses are mediated in great measure by appropriate MR and GR activation by glucocorticoids is provided by 11βHSD1 (Terao and Katayama, 2016,Kenouch, Lombes, Delahaye et al., 1994,Smith, Maguire, Stein-Oakley et al., 1996,Farman, Maubec, Poeggeler et al., 2010,Bocchi, Kenouch, Lamarre-Cliche et al., 2004,Terao, Itoi, Murota et al., 2013). Epidermal 11βHSD1 expression increases with age and UV light skin damage and decreases keratinocyte and fibrocyte proliferation. There is ample evidence supporting the therapeutic use of 11βHSD1 inhibitors to suppress thinning of the skin due to aging (Terao and Katayama, 2016,Terao et al., 2013,Tiganescu, Tahrani, Morgan et al., 2013,Boudon, Heidl, Vuorinen et al., 2018). In contrast, expression of 11βHSD1 is decreased in psoriasis and seborrheic keratosis characterized by epidermal hyperproliferation. Whether the decrease in 11βHSD1 is a cause or is a consequence of hyperproliferative state of the cells is uncertain (Terao et al., 2013). 11βHSD2 is not expressed in normal dermal cells but is prominent in seborrheic keratosis and basal cell carcinoma. 11βHSD2 inhibitors including glycyrrhetinic acid have been proposed as suppressors of non-melanoma skin cancer (Mancha-Ramirez, Yang, Liang et al., 2019). The roles of 11βHSDs in cell proliferation, wound healing, inflammation, and aging of the skin have been reviewed by Terao et al (Terao and Katayama, 2016).
2.2.8. Rational for the therapeutic use of 11βHSD1 inhibitors
Selective 11βHSD1 inhibitors with minimal effects on carbonyl reductase 1, cytochrome P450 enzymes, and 11βHSD2 have been proposed to counter the excessive 11βHSD1 reductase activity and inappropriate glucocorticoid action occurring in aging, obesity, T2DM, MetS, osteoporosis, and neurocognitive impairment including Alzheimer’s disease (Boudon et al., 2018,Seckl, Kelly and Sharkey, 1991,Walker and Seckl, 2003,Sandeep, Yau, MacLullich et al., 2004,Sooy, Noble, McBride et al., 2015,Webster, McBride, Binnie et al., 2017). They have been developed as adjuvants to drugs that are metabolized by 11βHSD1, including those reduced to toxic metabolites (Yang et al., 2018). Excessive glucocorticoid activity in the brain such as occurs in chronic stress and major depression is neurotoxic. Early studies demonstrated that the non-selective 11βHSD inhibitor glycyrrhetinic acid increased glucose use in subregions of the hypothalamus, hippocampus, neocortex and subthalamus of the conscious rat suggesting that 11βHSD1 inhibitors might be of benefit in neurological conditions associated with chronic stress (Seckl et al., 1991), a premise supported by many studies on the effect of 11βHSD1 inhibition in age- and glucocorticoid excess related cognitive decline since (Walker and Seckl, 2003,Sandeep et al., 2004,Sooy et al., 2015,Webster et al., 2017,Yau, Noble, Kenyon et al., 2001,Holmes, Carter, Noble et al., 2010,Puigoriol-Illamola, Leiva, Vazquez-Carrera et al., 2020). Unfortunately larger clinical efficacy studies of 11βHSD1 inhibitors on neurocognitive disorders have been disappointing (Marek, Katz, Meier et al., 2014,Gregory, Hill, Grey et al., 2020), as recently reviewed by Watermeyer et al (Watermeyer, Robb, Gregory et al., 2020). Of ten genes related to glucocorticoid metabolism studied in two geographically separate cohorts of patients, only one polymorphism of HSD11B1 was identified that correlated with Alzheimer’s disease and it was found both groups of patients(de Quervain, Poirier, Wollmer et al., 2004). This mutation involves the recombination of rs846911 and rs860185 within the 5’ regulatory region which when studied in a human embryonic kidney cell line (HEK-293) decreased, rather than increased, HSD11B1 transcription (de Quervain et al., 2004). The authors suggested based on work in rats (Jellinck et al., 1999), that perhaps 11βHSD1 in key neurons of the hippocampus acts as a dehydrogenase that decreases cortisol (de Quervain et al., 2004). A subsequent study in a Scottish cohort found no correlation between cognitive decline and the HSD11B1 polymorphism rs846911, as well as two other previously reported potential candidates (Deary, Hayward, Permana et al., 2006). Moreover, in contrast to expectations from preclinical studies in animal surrogates, PET scans with a radio tracer for 11βHSD1 of obese and lean humans demonstrated that increased BMI correlated with decreased levels of 11βHSD1 in the caudate, hypothalamus, parietal lobe, and putamen (Bhatt, Nabulsi, Li et al., 2020). In similar studies increased age correlated with increased brain 11βHSD1 as expected (Bini, Bhatt, Hillmer et al., 2020), however the study cohort was too small to be conclusive.
11βHSD1 and glucocorticoids are significantly increased in the adipose tissue of obese humans and animals, a phenomenon dubbed “Cushing’s Disease of the omentum” (Bujalska, Kumar and Stewart, 1997,Bujalska, Quinkler, Tomlinson et al., 2006). Masuzaki et al produced a transgenic mouse in which the aP2 gene promoter was used to targeted over-expression of Hsd11b1 in adipose tissue to a level that increased 11βHSD1 protein in their fat to levels found in the fat of obese humans. GR and its transcriptional targets required for lipid accumulation in adipocytes were increased in these mice, they developed hyperlipidemia, insulin resistance, hyperglycemia, and hypertension when fed standard chow, and had a greater response to an obesogenic diet than control mice (Masuzaki et al., 2001). The mice were hyperphagic, but as aP2 is expressed in the hypothalamus, it is not clear whether this was due to the disruption of adipocyte-hypothalamic circuits controlling appetite or of a direct transcriptional effect in the hypothalamus. Global Hsd11b1 deletion increased the expression of adipocyte genes associated with insulin sensitization and decreased those mediating insulin resistance (Morton, Paterson, Masuzaki et al., 2004). The abundant evidence in support of the use of selective antagonists of 11βHSD1 for the treatment of obesity and its dire complications has been the subject of several reviews (Chapman et al., 2013,Gathercole et al., 2013,Vuorinen, Nashev, Odermatt et al., 2014,Lee, Pramyothin, Karastergiou et al., 2014).
In contrast, a recent systematic analysis of a large number of reports on the clinical use of 11βHSD1 inhibitors to mitigate obesity, type 2 diabetes, MetS, and Alzheimer’s disease found overall disappointing results in the 29 trials meeting the criteria for inclusion in the study (Gregory et al., 2020). Full details are juxtaposed in an extensive table (Gregory et al., 2020). While disappointing, the results of the review of clinical trials provided clues to which patients are most likely to benefit from an inhibitor (Gregory et al., 2020). HSD11B1 was increased in adipose tissue of obese patients, but not consistently in patients with MetS, T2DM, or Alzheimer’s disease who were not obese. Increased 11βHSD1 activity as assessed by plasma steroid levels correlated with increased BMI in humans with T2DM (Shukla, Basu, Mandal et al., 2019). In contrast, in another study basal skeletal muscle expression and activity of 11βHSD1 was found to be less in T2DM than controls. However, skeletal muscle 11βHSD1 increased in the diabetics when study participants were challenged with dexamethasone (Jang, Obeyesekere, Dilley et al., 2007). Nor did increased 11βHSD1 expression in the fat of obese subjects correlate with T2DM in a study of Pima and Tohono O’Odham People of Arizona (Nair, Lee, Lindsay et al., 2004). However, two 11βHSD1 polymorphisms, rs 846910 G/A and rs 12086634 G/T, were significantly associated with diabetes in this cohort (Nair et al., 2004). The same polymorphisms were identified in a group of South Indian patients with T2DM and treatment with licorice at a dose that did not increase blood pressure or serum potassium over 3 weeks ameliorated the hyperinsulinemia in these patients (Devang, Adhikari, Nandini et al., 2020). Despite the lack of significant efficacy of 11βHSD1 inhibitors in earlier clinical trials, interest in the development of selective antagonists prevails. Recent reviews of the structural details of the natural and most promising synthetic inhibitors of hsd1 abound (Hardy et al., 2020,Hollis and Huber, 2011,Chuanxin, Shengzheng, Lei et al., 2020).
3.0. 11βHSD2 or SDR9C2
The human HSD11B2 is located on chromosome 16q22.1. Its molecular mass differs somewhat between species; that of the human 11βHSD2 is 44 kDa. The structure of 11βHSD2 is similar to that of 11βHSD1 but with the catalytic domain in the cytosol. Like 11βHSD1 there are species differences in 11βHSD2 that must be considered, as reviewed by Heussner (Heussner, Ruebner, Huebner et al., 2016). In all species 11βHSD2 is an NAD+ dehydrogenase that is inhibited by its products 11-dehydrocorticosterone, cortisone and the 7-keto sterols (Beck et al., 2019,Odermatt and Klusonova, 2015,Brem, Matheson, Barnes et al., 1991,Brem, Matheson, Latif et al., 1993). Estrogens were found to significantly increase hsd11b2 expression and 11ßHSD2 protein in the rat kidney, however the formation of inactive dimers was also increased and the increase in expression was not associated with an increase dehydrogenase activity(Gomez-Sanchez et al., 2003,Gomez-Sanchez, Ganjam, Chen et al., 2001). The mechanism for control of 11ßHSD2 dimerization has not been clarified. As described in more detail in section 4.2, 11βHSD2 is more highly expressed during ontogeny when cell proliferation is most active, disappearing in many adult cells due to epigenetic silencing (Alikhani-Koopaei, Fouladkou, Frey et al., 2004). Inappropriate expression of 11βHSD2 occurs in several types of cancers, including more than half of mammary tumors and osteosarcomas, and is associated with resistance to glucocorticoid suppression of neoplastic cell proliferation (Sasano, Frost, Saitoh et al., 1997,Sai, Nakagawa, Yamaguchi et al., 2011) (Terao and Katayama, 2016,Mancha-Ramirez et al., 2019,Sai, Esteves, Kelly et al., 2020). Inhibitors of 11βHSD2 have been suggested as treatment of hyperproliferative diseases including cancer, however appropriate targeting of an inhibitor will be crucial.
3.1. GALFs
Licorice, from the root of Glycyrrhiza glabra, has been used for several thousand years as flavoring and anti-inflammatory agents and was recognized as having a mineralocorticoid effect in 1950 (Molhuysen, Gerbrandy, De Vries et al., 1950). Recognition of its ability to inhibit 11βHSD led to the discovery of the cause of AME (Stewart, Wallace, Valentino et al., 1987) (see section 1.2). The term glycyrrhetinic acid-like factor (GALF) was coined over 30 years ago to describe circulating inhibitors of 11βHSD2 that might be responsible for hypertension and other manifestations of inappropriate glucocorticoid action. The anti-inflammatory effects of the primary active components of licorice, glycyrrhetinic and glycyrrhizic acids, are due to inhibition of both 11βHSD2 and 15-hydroxyprostaglandin dehydrogenase which are related structurally and phylogenetically (Baker, 1994). Chronic consumption of licorice, most often in herbal nostrums and tobacco products, continue to cause toxicity related to 11βHSD2 inhibition (Yoshino, Yanagawa and Watanabe, 2014). A recently reported AME syndrome was attributed to the over-use of mumijo, a hardened oily substance from petrel stomachs also used in as a traditional anti-inflammatory nostrum (Stavropoulos, Sotiriadis, Patoulias et al., 2018). Naringenin, another ubiquitous 11βHSD2 inhibitor, is found in several commonly consumed foods including grapefruit, bergamot (consumed widely in tea), tomatoes, and oregano, as well as herbal medicines (Zhang, Lorenzo and Reidenberg, 1994,Palermo, Armanini and Delitala, 2003). Gossypol, found in high concentrations in unrefined cotton seed oil, inhibits 11βHSD2 as well as spermatogenesis and is responsible for the severe hypokalemic and hypertensive effects secondary to use of gossypol as a contraceptive (Ohno et al., 2013,Zhang et al., 1994,Michael, 1998,Ma, Lian, Dong et al., 2011).
The list of GALFs has increased progressively (Morris, Semafuko, Latif et al., 1992,Walker, Aggarwal, Stewart et al., 1995,Morris, Latif and Brem, 2014). Among these are products of the gut microbiome including 11β-OH-progesterone and 11β-OH-(allo)-5alpha-preganolones which increase to pathogenic concentrations in patients with 17α-hydroxylase deficiency causing the accumulation corticosterone and its 5alpha-ring A-reduced metabolites to be excreted in the bile (Morris and Ridlon, 2017). Biliary obstruction resulting in the accumulation of the relatively weak 11βHSD2 inhibitors chenodeoxycholic acid (CDCA) and deoxycholic acid (DCA) causes inappropriate activation of the MR, renal sodium retention and potassium loss in patients with liver cirrhosis (Quattropani, Vogt, Odermatt et al., 2001,Stauffer, Rochat, Dick et al., 2002). 7-Hydroxysterol substrates were added to the list of GALFs due to their effective competition with glucocorticoids for 11βHSD2 (Beck et al., 2019).
Two of the azole antifungal agents inhibit steroidogenic enzymes with variable affinities. Itraconazole and Posaconazole cause hypertension and hypokalemia with suppressed aldosterone, iatrogenic AME, by different mechanisms. Itraconozole and its main active metabolite are potent inhibitors of 11βHSD2. Posaconazole is also a more potent inhibitor of CYP11B1 than of 11βHSD2. Its inhibition of CYP11B1 produces a syndrome akin to congenital adrenal hyperplasia whereby deficient cortisol synthesis and failure to suppress the hypothalamus-pituitary-adrenal axis (HPA) and leads to the accumulation of upstream substrates including the mineralocorticoids 11- deoxycorticosterone and 11-deoxycortisol. Molecular modeling calculations predicted binding of posaconazole, itraconazole and its metabolite to 11βHSD2 and CYP11B1 (Beck, Bachler, Vuorinen et al., 2017,Beck, Telisman, van Koppen et al., 2020).
4.0. HSD11B3 or SCDR10B
HSD11B3 (11βHSD1-like; HSDB1L) is located on chromosome 19p13.3, comprises 315 amino acids and has a molecular mass of 34.8 kDa (genecards.org). Despite compelling evidence for the existence of another 11βHSD based on enzyme activity and kinetics, cofactor dependence, and sensitivity to substrate and product inhibition (Ge et al., 1997,Gomez-Sanchez et al., 1997,Gomez-Sanchez and Gomez-Sanchez, 1997,Robinzon and Prough, 2009), a distinct protein and gene for a third enzyme had not been isolated until Huang et al used the sequence of the human 11βHSD3 to search GenBank for ESTs with no homologies with either 11βHSD1 or 2 (Huang, Wan, Gao et al., 2009). Assembly of the ESTs led to the cloning of a gene that encodes a protein with a predicted molecular mass of 30.8 kDa, slightly less than that of 11βHSD1 & 2, with the transmembrane helixes characteristic of the 11βHSDs, and a cofactor binding domain like that of 11βHSD1 that accommodates NADP+. The gene was cloned from a human brain cDNA library and submitted to GenBank as SCDR10B and HSD11B3. In the November 2020 update of GenBank it is listed as HSD11B1-L. Phylogenetic analysis indicates that the genetic sequence of HSD11B3 is highly conserved across kingdoms, including those of plants, and it precedes 11βHSD1 and 2 in animals (Baker, 2004,Huang et al., 2009). It is found in the human, several other primates, cow, sheep, pig, fish, and frogs, but not rats or mice (Baker, 2010,Baker et al., 2015,Gohin, Bobe and Chesnel, 2010,Feswick, Ings, Doyle et al., 2014).
Bird et al further characterized the 11βHSD3 and protein and compared its predicted structure to that of the human 11βHSD1 and 11βHSD2 (Bird et al., 2017). HSD11B3 has an ER localization signal sequence at the N-terminus, confirmed in transfected cells. HSD11B3/HSD11BL-6 is most highly expressed in the primate brain, especially the hippocampus and cortex, much less in the lungs, spleen, stomach, testes, ovaries, and skeletal muscle, and none in the heart and liver (Bird et al., 2017,Huang et al., 2009). 11βHSD3 was not found to colocalize with 11βHSD2 in the kidney and colon (Bird et al., 2017). Strong 11βHSD3 immunoreactivity was found in ovarian granulosa cells, Leydig cells and gonadotrophs (Bird et al., 2017). Ohno et al demonstrated that 11βHSD3 is responsible for the high affinity NADP+-dependent dehydrogenase in pig Leydig cells (Ohno et al., 2013). It is yet unknow whether 11βHSD3 modulates sterol ligands for RORs.
Hsd11b3 expression is highly regulated in the maturing oocyte and spermatocyte production in the testes of fish and amphibians (Gohin et al., 2010,Feswick et al., 2014). 11βHSD3 appears to modulate the pituitary gonadal axis and prevent glucocorticoid suppression of gonadal function rather than provide of MR selectivity for aldosterone in in mammal. Its primary localization in the hippocampus where there is ample evidence that the MR is a high affinity glucocorticoid receptor suggests that 11βHSD3 is likely to dampen acute surges in glucocorticoids at the level of the neuronal GR. The existence of an enzyme like it was predicted sheep 2 decades ago (Gomez-Sanchez et al., 1997). The inability to detect 11βHSD2 or 3 in the heart leaves us with the current mystery about how inappropriate aldosterone levels have such devastating effects in the heart (Gomez-Sanchez and Gomez-Sanchez, 2014,Gomez-Sanchez, 2014). 11βHSD3 is not expressed in the rat so the third 11βHSD described by Ge e al 30 years ago remains unidentified (Ge et al., 1997,Ge, Dong, Sottas et al., 2005). Nor has the enzyme responsible for the formation of 11-dehydrocorticosterone from tritiated precursors by incubated rat brain minces been identified (Gomez-Sanchez, Zhou, Cozza et al., 1997) or the mechanism for the protection of MR in aldosterone-sensitive neurons that modulate hemodynamic functions in the adult brain been verified (Gomez-Sanchez, 2014,Chen et al., 2014,Jellinck et al., 1999). Perhaps there is yet another 11βHSD3 for the rat and mouse.
5.0. 11βHSD1 and 11βHSD2: reproduction and ontogeny.
11βHSD2 diminishes activation of the GR by circulating glucocorticoids, thereby reducing glucocorticoid mediated suppression of androgen synthesis and Leydig cell apoptosis in the testes (Monder, Miroff, Marandici et al., 1994,Ge, Hardy, Catterall et al., 1997,Ge, Dong, Niu et al., 2005,Honda, Ohno and Nakajin, 2008), as well as steroidogenesis and follicular maturation in the ovary (Michael, Pester, Curtis et al., 1993,Yong, Thong, Andrew et al., 2000,Tetsuka, Yamamoto, Hayashida et al., 2003,Yazawa, Uesaka, Inaoka et al., 2008). GR- and MR-mediated functions in the gonads are orchestrated throughout development and reproductive life by both 11βHSD1 and 11βHSD2 (reviewed in (Michael, Thurston and Rae, 2003)). 11βHSD3 (or 1βHSD1L) appears to cooperate with 11βHSD2 in the testes of the pig to prevent cortisol suppression of reproductive function (Ohno et al., 2013) and is regulated during gametogenesis in at least some teleosts and ranidae (Gohin et al., 2010,Feswick et al., 2014).
5.1. 11βHSDs modulate androgen activity
As introduced in sections 2.2, the 11βHSDs are multi-functional carbonyl reductases, that include the C11-hydroxy and C11-keto derivatives of the C19 and C21 steroids produced by the adrenal cortex (Bloem, Storbeck, Schloms et al., 2013,Storbeck, Bloem, Africander et al., 2013). 11βHSD2 catalyzes the formation of the 11-keto metabolites of 11β-hydroxyandrostenedione, 11β-hydroxytestosterone, and 11β-hydroxyprogesterone produced in the adrenal more efficiently than 11βHSD1 is in reducing their 11-keto forms (Gent, du Toit, Bloem et al., 2019). While the 11-keto glucocorticoids are inactive, 11-ketotestosterone and 11-ketodihydrotestosterone, a metabolite of both 11-ketoandrostenedione and 11-ketotestosterone, have similar androgenic potency as dihydrotestosterone (DHT) (Storbeck et al., 2013). Adrenal production of C11-hydroxy androgens remain stable with age in men and have an important role in castration resistant prostatic cancers (Gent et al., 2019,Davio, Woolcock, Nanba et al., 2020). The adrenal is the primary source of androgens in women. While testosterone, dehydroepiandrosterone, and dehydroepiandrosterone sulfate decrease with age in women, the C11-hydroxyandrogens and their active keto derivatives do not (Nanba, Rege, Ren et al., 2019). Adrenal androgens are elevated in PCOS, implicating a disequilibrium in 11βHSD activity (Gent et al., 2019,Michael, Glenn, Wood et al., 2013,Chin, Shackleton, Prasad et al., 2000). While the ratio of urinary 11-keto to 11-OH- androgen metabolites were unchanged in a cohort of women with and without PCOS (Chin et al., 2000), androgen metabolites in circulation and the glomerular filtrate may not reflect those in the relevant peripheral tissues.
5.2. ontogeny
Overexposure to glucocorticoids during early development, whether from 11βHSD2 inhibition, Hsd11b2 deletion, glucocorticoid excess, or severe calorie or protein deprivation, produces intrauterine growth restriction and similar long-term effects in the progeny (Cottrell, Holmes, Livingstone et al., 2012). A similar developmental problems occur with maternal obesity (Correia-Branco, Keating and Martel, 2015). GR and 11βHSD1 are increased and 11βHSD2 suppressed, disrupting the development of organs and homeostatic systems and predisposing the progeny to MetS, hypertension, cardiovascular disease, and cognitive and mood disorders (Cottrell et al., 2012,Correia-Branco et al., 2015,McTernan, Draper, Nicholson et al., 2001,Shams, Kilby, Somerset et al., 1998,Whorwood, Firth, Budge et al., 2001).
Changes in 11βHSD1 and 11βHSD2 expression within the different layers of the placental offer “exquisite local control of glucocorticoid action” and were shown to persist even after fetectomy (Waddell, Benediktsson, Brown et al., 1998) and to be regulated in part by estrogens (Albrecht and Pepe, 1999). 11βHSD2 expression in the placenta and in fetal tissues including the heart, kidneys and brain protect the developing cells from the high amounts of glucocorticoids required by the dam during pregnancy (Shearer, Wyrwoll and Holmes, 2019). Excessive glucocorticoid action in the placenta suppresses the angiogenesis required to sustain the fetus and results in a smaller placenta and abnormal blood flow in the umbilical vessels that contribute to abnormal fetal heart development in addition to the direct effects of glucocorticoid excess on cardiac development (Shearer et al., 2019) and has been implicated in pre-eclampsia (Shallie, Margolis, Shallie et al., 2020). Women with gestational diabetes treated with diet alone express greater than normal 11βHSD2 and activity at term, presumably to overcome the effects of hyperglycemia and hypercortisolemia (Ma, Liu, Wu et al., 2012). 11βHSD2 also decreases the inappropriate activation of placental progesterone receptors by glucocorticoids (Burton and Waddell, 1999).
In the human and baboon, 11βHSD1 expression is primarily in the membranes of placental microvilli adjacent to the maternal circulation and exceeds that of 11βHSD2 throughout gestation. 11βHSD2 is expressed throughout the syncytiotrophoblast including in the basal membranes facing the fetal vasculature. Its levels increase after midterm, ensuring normal activation and maturation of the fetal pituitary-adrenocortical axis (Albrecht and Pepe, 1999,Pepe, Burch and Albrecht, 2001). The role of the 11βHSDs in the protection of other substrates including environmental endocrine disrupting compounds is also important for the placenta and developing fetus (Ma et al., 2011).
The maternal decidua is an example of cell-specific regulation of 11βHSD reductase and dehydrogenase activity by steroids within the same tissue. The human endometrium expresses 11βHSD1 in stromal cells only, and 11βHSD2 in both stromal and glandular cells (Kuroda, Venkatakrishnan, Salker et al., 2013). Decidualization of endometrial stromal cells (ESC) is initiated upon conception by progesterone. Microarray studies demonstrated that HSD11B1 was among the genes with the greatest increase in transcription produced by treatment of primary human ESC with progesterone. GR expression also increased; MR and 11βHSD2 mRNA levels were unchanged (Kuroda et al., 2013). The resulting increase in cortisol production in stromal cells activated GR, resulting in a GR-mediated increase in 11βHSD1 expression and surge in cortisol which enhanced activation of the GR. Genes related to DNA methylation mechanisms were the most increased of the GR-regulated genes. Among the MR-regulated genes most increased were those for lipid droplet formation and retinoid metabolism, hallmarks of decidua formation. As in other tissues, GR activation produced more transcriptional repression than promotion; MR activation stimulated gene transcription (Kuroda et al., 2013).
Co-expression of 11βHSD2 and MR occurs early in the developing human fetal upper gastrointestinal tract, kidney, skin, and lung suggesting their role in water and electrolyte transport between fetus and amniotic fluid, fetal urine production, and lung development. Co-expression of 11βHSD2 and MR in colon epithelial and pancreatic exocrine duct cells, where they are highly expressed in the adult, did not occur until near term (Hirasawa, Sasano, Suzuki et al., 1999).
Precise regulation of 11βHSD1 and 2 activities is also crucial for normal development of the brain and is evolutionarily conserved in mammals (Shearer et al., 2019). Brain-specific 11βHSD2 deletion in mice produced adults with depressive-like behavior and memory deficits (Shearer et al., 2019). By late gestation 11βHSD2 expression is negligible in the brain except for a few aldosterone target neurons. 11βHSD1 and GR in the hypothalamus and pituitary decrease, damping the negative feedback of cortisol on the hypothalamic-pituitary-adrenal (HPA) axis and allowing the HPA axis to mature and integrate signals from the hippocampus and other parts of the brain (Sloboda, Moss, Li et al., 2008). Maturation of the liver including production of corticosteroid-binding globulin is also critical to normal HPA development (Toews, Hammond and Viau, 2020). Tightly controlled 11βHSD1 and 11βHSD2 activity is essential throughout gestation and early development of the hypothalamic-pituitary-gonadal axes (Toews et al., 2020) as well as in the developing gonads (Ge et al., 1997,Ge et al., 2005,Wagner and Claus, 2008,Koeva, Bakalska, Atanassova et al., 2009). 11βHSD3 is also expressed in the developing testes (Ohno et al., 2013,Gohin et al., 2010,Feswick et al., 2014). Its function during ontogeny has not yet been clarified. h
While excessive GR-mediated activity suppresses proliferation, cell migration and growth, it is crucial for maturation and function of cells. Among these are the surfactant producing type II alveolar epithelial cells (Gomez-Sanchez and Gomez-Sanchez, 2014). MR and 11βHSD2 co-expression in the columnar epithelium of fetal terminal bronchiolar buds and ciliated tracheal and bronchial epithelial cells occurs early and persists throughout in ontogeny (Hirasawa et al., 1999). However, pulmonary alveoli must mature and quickly expand with air at birth. Near term both GR activation and inflammatory cytokines stimulate Hsd11b1 transcription through its C/EBPα-dependent P2 promoter. The increase in intracellular glucocorticoids resulting from 11βHSD1 action provides a feed-forward increase in 11βHSD1 and tissue glucocorticoid production just prior to parturition required for lung maturation and surfactant production by type II alveolar epithelial cells. Double deletion of SRC1 and SRC2 reduced GR-mediated expression of C/EBPs, NF-kB-stimulated expression of the transcription factors IL-β1 and TNF-α and C/EBP-mediated increase in Hsd11b1 transcription required for normal development and surfactant production in the fetal mouse lung (Chen et al., 2020).
Conclusion:
Over the last decade the family of 11β-hydroxysteroid dehydrogenases has grown with the addition of a high affinity NADP+-dependent dehydrogenase, 11βHSD3, also called 11βHSD1-like (11βHSD1L). 11βHSD3 is expressed in humans and several other species but not in rats, despite evidence for such another 11βHSD. The list of endogenous substrates for which 11βHSD1 and 11βHSD2 have a similar or greater activity as for glucocorticoids has expanded. Among these are 7- and 11- keto sterols which are pro-inflammatory and major components of atherosclerotic lesions and C19 and C21 steroids that include the adrenal androgens. 7β27OHC is an agonist for RORγ, an important modulator of immune cell differentiation and function. The interconversion of 7k27OHC and 7β27OHC by the 11βHSDs modulates RORγ activity. Carbonyl reduction of endogenous and exogenous substrates by 11βHSD1 accelerates their entry into phase 1 biotransformation; competing substrates and inhibitors lead to their accumulation and untoward effects. Significant differences between the mouse and human 11βHSD kinetics for key substrates have been discovered. A better understanding of mechanisms responsible for the failure for inhibitors of 11βHSD1 in clinical trials to slow or reverse the effects of excessive metabolic activity should lead to strategies for their use. Among these would be how to separate the crucial role 11βHSD1 has in targeting an effective anti-inflammatory response by the glucocorticoid receptor from.
Acknowledgements:
These studies were supported by grants R01 HL144847 from the National Heart, Lung and Blood Institute, 1U54GM115428 from the National Institute of General Medical Sciences, and BX004681 from the Department of Veteran Affairs.
Abbreviations
- 11βHSD
11β-hydroxysteroid dehydrogenase
- 11βHSD1
11β-hydroxysteroid dehydrogenase 1
- 11βHSD2
11β-hydroxysteroid dehydrogenase 2
- 11βHSD3
11β-hydroxysteroid dehydrogenase 3 also called 11βHSD1-like dehydrogenase
- 11βHSD1L
11βHSD1-like dehydrogenase
- AME
Apparent Mineralocorticoid Excess
- SDR
short-chain dehydrogenase/reductases
- H6PDH
hexose-6-phosphate dehydrogenase
- SHR
steroid hormone receptor
- HRE
hormone receptor binding element on DNA
- GR
glucocorticoid receptor
- MR
mineralocorticoid receptor
- RORγ
retinoid-related orphan receptor gamma
- HPA
hypothalamic-pituitary-adrenal axis
- 7-kC
7-ketocholesterol
- 7-oxoLCA
7-Oxolithocholic acid
- 7βOHC
7β-hydroxycholesterol
- 7αOHC
7α-hydroxycholesterol
- 7β27OHC
7β, 27-dihydroxycholesterol
- SIMs
selective intracrine modulators
- MetS
metabolic syndrome
- T2DM
Diabetes Mellitus type 2
- INFγ
interferonγ
- TSG-6
TNF-stimulated gene 6
- GALF
glycyrrhetinic acid-like factor
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker ME, 2004. Co-evolution of steroidogenic and steroid-inactivating enzymes and adrenal and sex steroid receptors, Mol Cell Endocrinol. 215, 55–62. [DOI] [PubMed] [Google Scholar]
- Baker ME, 2010. 11Beta-hydroxysteroid dehydrogenase-type 2 evolved from an ancestral 17beta-hydroxysteroid dehydrogenase-type 2, Biochem Biophys Res Commun. 399, 215–20. [DOI] [PubMed] [Google Scholar]
- Baker ME, Nelson DR and Studer RA, 2015. Origin of the response to adrenal and sex steroids: Roles of promiscuity and co-evolution of enzymes and steroid receptors, J Steroid Biochem Mol Biol. 151, 12–24. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez E. and Gomez-Sanchez CE, 2014. The multifaceted mineralocorticoid receptor, Compr Physiol. 4, 965–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KR, Inderbinen SG, Kanagaratnam S, Kratschmar DV, Jetten AM, Yamaguchi H. and Odermatt A, 2019. 11beta-Hydroxysteroid dehydrogenases control access of 7beta,27-dihydroxycholesterol to retinoid-related orphan receptor gamma, J Lipid Res. 60, 1535–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger M. and Reichstein T, 1937. Desoxy-corticosterone (21-oxy-progesterone). Aus d-3-oxo-etiocholensäure, Helv Chim Acta. 20, 1164. [Google Scholar]
- Kendall EC, 1952. Hormones of the adrenal cortex in clinical medicine, Edinb Med J. 59, 1–12. [PMC free article] [PubMed] [Google Scholar]
- Simpson SA and Tait JF, 1955. Recent progress in methods of isolation, chemistry, and physiology of aldosterone, Recent Prog Horm Res. 11, 183–219. [Google Scholar]
- Amelung D, Hubener HJ, Roka L. and Meyerheim G, 1953. Conversion of cortisone to compound F, J Clin Endocrinol Metab. 13, 1125–6. [DOI] [PubMed] [Google Scholar]
- Draper N. and Stewart PM, 2005. 11beta-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action, J Endocrinol. 186, 251–71. [DOI] [PubMed] [Google Scholar]
- New MI, Levine LS, Biglieri EG, Pareira J. and Ulick S, 1977. Evidence for an unidentified steroid in a child with apparent mineralocorticoid hypertension, J Clin Endocrinol Metab. 44, 924–33. [DOI] [PubMed] [Google Scholar]
- Ulick S, Ramirez LC and New MI, 1977. An abnormality in steroid reductive metabolism in a hypertensive syndrome, J Clin Endocrinol Metab. 44, 799–802. [DOI] [PubMed] [Google Scholar]
- Ulick S, Levine LS, Gunczler P, Zanconato G, Ramirez LCR, Rosler A, Bradlow HL and New MI, 1979. A syndrome of apparent mineralocorticoid excess associated with defects in the peripheral metabolism of cortisol, J Clin Endocrinol Metab. 49, 757–764. [DOI] [PubMed] [Google Scholar]
- Oberfield SE, Levine LS, Carey RM, Greig F, Ulick S. and New MI, 1983. Metabolic and blood pressure responses to hydrocortisone in the syndrome of apparent mineralocorticoid excess, J Clin Endocrinol Metab. 56, 332–339. [DOI] [PubMed] [Google Scholar]
- Dimartino-Nardi J, Stoner E, Martin K, Balfe JW, Jose PA and New MI, 1987. New findings in apparent mineralocorticoid excess, Clin Endocrinol. 27, 49–62. [DOI] [PubMed] [Google Scholar]
- Monder C, Shackleton CH, Bradlow HL, New MI, Stoner E, Iohan F. and Lakshmi V, 1986. The syndrome of apparent mineralocorticoid excess: its association with 11 beta-dehydrogenase and 5 beta-reductase deficiency and some consequences for corticosteroid metabolism, J Clin Endocrinol Metab. 63, 550–7. [DOI] [PubMed] [Google Scholar]
- Lakshmi V. and Monder C, 1985. Extraction of 11 beta-hydroxysteroid dehydrogenase from rat liver microsomes by detergents, J Steroid Biochem. 22, 331–40. [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Monder C, Eckstein B. and White PC, 1989. Cloning and expression of rat cDNA encoding corticosteroid 11 beta-dehydrogenase*, J Biol Chem. 264, 18939–18943. [PubMed] [Google Scholar]
- Tannin GM, Agarwal AK, Monder C, New MI and White PC, 1991. The human gene for 11 beta-hydroxysteroid dehydrogenase. Structure, tissue distribution, and chromosomal localization, J Biol Chem. 266, 16653–8. [PubMed] [Google Scholar]
- Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M. and Stewart PM, 2004. 11{beta}-hydroxysteroid dehydrogenase type 1: A tissue-specific regulator of glucocorticoid response, Endocr Rev. [DOI] [PubMed] [Google Scholar]
- Maser E, Volker B. and Friebertshauser J, 2002. 11 Beta-hydroxysteroid dehydrogenase type 1 from human liver: dimerization and enzyme cooperativity support its postulated role as glucocorticoid reductase, Biochemistry. 41, 2459–65. [DOI] [PubMed] [Google Scholar]
- White PC, Pascoe L, Curnow KM, Tannin G. and Rösler A, 1992. Molecular biology of 11β-hydroxylase and 11β-hydroxysteroid dehydrogenase enzymes, J Steroid Biochem Mol Biol. 43, 32767–32760. [DOI] [PubMed] [Google Scholar]
- Krozowski ZS, Provencher PH, Smith RE, Obeyesekere VR, Mercer WR and Albiston AL, 1994. Isozymes of 11β-hydroxysteroid dehydrogenase: Which enzyme endows mineralocorticoid specificity?, Steroids. 59, 116–120. [DOI] [PubMed] [Google Scholar]
- Mercer W, Obeyesekere V, Smith R. and Krozowski Z, 1993. Characterization of 11 beta-HSD1B gene expression and enzymatic activity, Mol Cell Endocrinol. 92, 247–51. [DOI] [PubMed] [Google Scholar]
- Yang K, Yu M. and Han VK, 1995. Identification and tissue distribution of a novel variant of 11 beta-hydroxysteroid dehydrogenase 1 transcript, J Steroid Biochem Mol Biol. 55, 247–253. [DOI] [PubMed] [Google Scholar]
- Blum A. and Maser E, 2003. Enzymology and molecular biology of glucocorticoid metabolism in humans, Prog Nucleic Acid Res Mol Biol. 75, 173–216. [DOI] [PubMed] [Google Scholar]
- Stewart PM and Krozowski Z, 1994. Lessons from Appenberg: 11 beta hydroxysteroid dehydrogenase 1B or 2?, J Endocrinol. 141, 191–3. [DOI] [PubMed] [Google Scholar]
- Arriza JW, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DEand Evans RM, 1987. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor, Science. 237, 268–275. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Simerly RB, Swanson LW and Evans RM, 1988. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response, Neuron. 1, 887–900. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Corrie JET, Shackleton CHL and Edwards CRW, 1988. Syndrome of apparent mineralocorticoid excess. A defect in the cortisol-cortisone shuttle, J Clin Invest. 82, 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramovitz M, Branchaud CL and Murphy BE, 1982. Cortisol-cortisone interconversion in human fetal lung: contrasting results using explant and monolayer cultures suggest that 11 beta-hydroxysteroid dehydrogenase (EC 1.1.1.146) comprises two enzymes, J Clin Endocrinol Metab. 54, 563–8. [DOI] [PubMed] [Google Scholar]
- Edwards CRW, Burt D, McIntyre MA, De Kloet ER, Stewart PM, Brett L, Sutanto WS and Monder C, 1988. Localisation of 11β-hydroxysteroid dehydrogenase-tissue specific protector of the mineralocorticoid receptor, Lancet. ii, 986–989. [DOI] [PubMed] [Google Scholar]
- Funder JW, Pearce PT, Smith R. and Smith AI, 1988. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated, Science. 242, 583–585. [DOI] [PubMed] [Google Scholar]
- Brown RW, Chapman KE, Edwards CRW and Seckl JR, 1993. Human placental 11β-hydroxysteroid dehydrogenase: Evidence for and partial purification of a distinct NAD-dependent isoform, Endocrinology. 132, 2614–2621. [DOI] [PubMed] [Google Scholar]
- Rusvai E. and Naray-Fejes-Toth A, 1993. A new isoform of 11β-hydroxysteroid dehydrogenase in aldosterone target cells, J Biol Chem. 268, 10717–10720. [PubMed] [Google Scholar]
- Agarwal AK, Mune T, Monder C. and White PC, 1994. NAD(+)-dependent isoform of 11 beta-hydroxysteroid dehydrogenase. Cloning and characterization of cDNA from sheep kidney, J Biol Chem. 269, 25959–62. [PubMed] [Google Scholar]
- Albiston AL, Obeyesekere VR, Smith RE and Krozowski ZS, 1994. Cloning and tissue distribution of the human 11β-hydroxysteroid dehydrogenase type 2 enzyme, Mol Cell Endocrinol. 105, R11–R17. [DOI] [PubMed] [Google Scholar]
- Cole TJ, 1995. Cloning of the mouse 11β-hydroxysteroid dehydrogenase type 2 gene: tissue specific expression and localization in distal convoluted tubules and collecting ducts of the kidney, Endocrinology. 136, 4693–4696. [DOI] [PubMed] [Google Scholar]
- Zhou MY, Gomez-Sanchez EP, Cox DL, Cosby D. and Gomez-Sanchez CE, 1995. Cloning, expression and tissue distribution of the rat NAD+-dependent 11β-hydroxysteroid dehydrogenase, Endocrinology. 136, 3729–3734. [DOI] [PubMed] [Google Scholar]
- Brown RW, Chapman KE, Kotelevtsev Y, Yau JL, Lindsay RS, Brett L, Leckie C, Murad P, Lyons V, Mullins JJ, Edwards CR and Seckl JR, 1996. Cloning and production of antisera to human placental 11 beta-hydroxysteroid dehydrogenase type 2, Biochem J. 313 ( Pt 3), 1007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, 1995. Cloning of the mouse 11 beta-hydroxysteroid dehydrogenase type 2 gene: tissue specific expression and localization in distal convoluted tubules and collecting ducts of the kidney, Endocrinology. 136, 4693–4696. [DOI] [PubMed] [Google Scholar]
- Roland BL and Funder JW, 1996. Localization of 11beta-hydroxysteroid dehydrogenase type 2 in rat tissues: in situ studies, Endocrinology. 137, 1123–1128. [DOI] [PubMed] [Google Scholar]
- Mune T, Rogerson FM, Nikkilä H, Agarwal AK and White PC, 1995. Human hypertension caused by mutations in the kidney isozyme of 11β-hydroxysteroid dehydrogenase, Nature Genetics. 10, 394–399. [DOI] [PubMed] [Google Scholar]
- Obeyesekere VR, Ferrari P, Andrews RK, Wilson RC, New MI and Funder JWK, 1995. The R337C mutation generates a high Km 11 beta-hydroxysteroid dehydrogenase type II enzyme in a family with apparent mineralocorticoid excess, J Clin Endocrinol Metab. 80, 3381–3383. [DOI] [PubMed] [Google Scholar]
- Wilson RC, Harbison MD, Krozowski ZS, Funder JWS, Hanauske-Abel HM, Wei JQ, Hertecant J, Moran A, Neiberger RE. and al., e., 1995. Several homozygous mutations in the gene for 11 beta-hydroxysteroid dehydrogenase type 2 in patients with apparent mineralocorticoid excess, J Clin Endocrinol Metab. 80, 3145–3150. [DOI] [PubMed] [Google Scholar]
- Ferrari P, Obeyesekere VR, Li K, Wilson RC, New MI and Funder JW, 1996. Point mutations abolish 11 beta-hydroxysteroid dehydrogenase type II activity in three families with the congenital syndrome of apparent mineralocorticoid excess, Mol Cell Endocrinol. 119, 21–24. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Krozowski ZS, Gupta A, Milford DV, Howie AJ and Sheppard MCW, 1996. Hypertension in the syndrome of apparent mineralocorticoid excess due to mutation of the 11 beta-hydroxysteroid dehydrogenase type 2 gene, Lancet. 347, 88–91. [DOI] [PubMed] [Google Scholar]
- Rogoff D, Smolenicka Z, Bergada I, Vallejo G, Barontini M. and Heinrich JJ, 1998. The codon 213 of the 11 beta-hydroxysteroid dehydrogenase type 2 gene is a hot spot for mutations in apparent mineralocorticoid excess, J Clin Endocrinol Metab. 83, 4391–4393. [DOI] [PubMed] [Google Scholar]
- Krozowski Z, 1999. The 11beta-hydroxysteroid dehydrogenases: functions and physiological effects. [Review] [70 refs], Molecular & Cellular Endocrinology. 151, 121–7. [DOI] [PubMed] [Google Scholar]
- White PC, Agarwal AK, Nunez BS, Giacchetti G, Mantero F. and Stewart PM, 2000. Genotype-phenotype correlations of mutations and polymorphisms in HSD11B2, the gene encoding the kidney isozyme of 11beta-hydroxysteroid dehydrogenase., Endocrine Research. 26, 771–80. [DOI] [PubMed] [Google Scholar]
- Odermatt A, Dick B, Arnold P, Zaehner T, Plueschke V, Deregibus MN, Repetto H, Frey BM, Frey FJ and Ferrari P, 2001. A mutation in the cofactor-binding domain of 11beta-hydroxysteroid dehydrogenase type 2 associated with mineralocorticoid hypertension, J Clin Endocrinol Metab. 86, 1247–52. [DOI] [PubMed] [Google Scholar]
- Alzahrani AS, Aljuhani N, Qasem E, Almohanna M, Hamoudh E, AlOmair A. and Alharbi N, 2014. Apparent Mineralocorticoid Excess Caused by a Novel Mutation in 11-beta Hydroxysteroid Dehydrogenase Type 2 Enzyme: Its Genetics and Response to Therapy, Endocr Pract. 1–19. [DOI] [PubMed] [Google Scholar]
- Quinkler M, Bappal B, Draper N, Atterbury AJ, Lavery GG, Walker EA, DeSilva V, Taylor NF, Hala S, Rajendra N. and Stewart PM, 2004. Molecular basis for the Apparent Mineralocorticoid Excess Syndrome in the Oman population, Mol Cell Endocrinol. 217, 143–9. [DOI] [PubMed] [Google Scholar]
- Tapia-Castillo A, Baudrand R, Vaidya A, Campino C, Allende F, Valdivia C, Vecchiola A, Lagos CF, Fuentes CA, Solari S, Martinez-Aguayo A, Garcia H, Carvajal CA and Fardella CE, 2019. Clinical, Biochemical, and Genetic Characteristics of “Nonclassic” Apparent Mineralocorticoid Excess Syndrome, J Clin Endocrinol Metab. 104, 595–603. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, 2014. Brain mineralocorticoid receptors in cognition and cardiovascular homeostasis, Steroids. 91, 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K, Holmes M. and Seckl J, 2013. 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action, Physiol Rev. 93, 1139–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland BL, Li KX and Funder JW, 1995. Hybridization histochemical localization of 11 beta-hydroxysteroid dehydrogenase type 2 in rat brain, Endocrinology. 136, 4697–700. [DOI] [PubMed] [Google Scholar]
- Chen J, Gomez-Sanchez CE, Penman A, May PJ and Gomez-Sanchez E, 2014.Expression of mineralocorticoid and glucocorticoid receptors in preautonomic neurons of the rat paraventricular nucleus, Am J Physiol Regul Integr Comp Physiol. 306, R328–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M, Wilson R, Sharma K, Mills NJ and Teruyama R, 2015. Localisation of 11beta-Hydroxysteroid Dehydrogenase Type 2 in Mineralocorticoid Receptor Expressing Magnocellular Neurosecretory Neurones of the Rat Supraoptic and Paraventricular Nuclei, J Neuroendocrinol. 27, 835–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Kawata M. and Loewy AD, 2006. Aldosterone-sensitive neurons in the rat central nervous system, J Comp Neurol. 494, 515–27. [DOI] [PubMed] [Google Scholar]
- Jellinck PH, Pavlides C, Sakai RR and McEwen BS, 1999. 11beta-hydroxysteroid dehydrogenase functions reversibly as an oxidoreductase in the rat hippocampus in vivo, J Steroid Biochem Mol Biol. 71, 139–44. [DOI] [PubMed] [Google Scholar]
- Evans LC, Ivy JR, Wyrwoll C, McNairn JA, Menzies RI, Christensen TH, Al-Dujaili EA, Kenyon CJ, Mullins JJ, Seckl JR, Holmes MC and Bailey MA, 2016. Conditional Deletion of Hsd11b2 in the Brain Causes Salt Appetite and Hypertension, Circulation. 133, 1360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez EP and Gomez-Sanchez CE, 1992. Central Hypertensinogenic effects of glycyrrhizic acid and carbenoxolone, Am J Physiol. 263, E1125–E1130. [DOI] [PubMed] [Google Scholar]
- Ge R-S, Gao HB, Nacharaju VL, Gunsalus GL and Hardy MP, 1997. Identification of a kinetically distinct activity of 11-beta-hydroxysteroid dehydrogenase in rat leydig cells, Endocrinology. 138, 2435–2442. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Ganjam V, Chen YJ, Cox DL, Zhou MY, Thanigaraj S. and Gomez-Sanchez CE, 1997. The sheep kidney contains a novel unidirectional, high affinity NADP+-dependent 11β-hydroxysteroid dehydrogenase (11-HSD3), Steroids. 62, 444–450. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP and Gomez-Sanchez CE, 1997. First there was one, then two...why more 11beta-hydroxysteroid dehydrogenases?, Endocrinology. 138, 5087–8. [DOI] [PubMed] [Google Scholar]
- Robinzon B. and Prough RA, 2009. A novel NADP(+)-dependent dehydrogenase activity for 7alpha/beta- and 11beta-hydroxysteroids in human liver nuclei: A third 11beta-hydroxysteroid dehydrogenase, Arch Biochem Biophys. 486, 170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S, Nakagawara S, Honda Y. and Nakajin S, 2013. Evidence for expression of 11beta-hydroxysteroid dehydrogenase type3 (HSD11B3/HSD11B1L) in neonatal pig testis, Mol Cell Biochem. 381, 145–56. [DOI] [PubMed] [Google Scholar]
- Bird AD, Greatorex S, Reser D, Lavery GG and Cole TJ, 2017. Hydroxysteroid dehydrogenase HSD1L is localised to the pituitary-gonadal axis of primates, Endocr Connect. 6, 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole LL, Lavery GG, Morgan SA, Cooper MS, Sinclair AJ, Tomlinson JW and Stewart PM, 2013. 11beta-Hydroxysteroid dehydrogenase 1: translational and therapeutic aspects, Endocr Rev. 34, 525–55. [DOI] [PubMed] [Google Scholar]
- Odermatt A, Arnold P, Stauffer A, Frey BM and Frey FJ, 1999. The N-terminal anchor sequences of 11beta-hydroxysteroid dehydrogenases determine their orientation in the endoplasmic reticulum membrane, J Biol Chem. 274, 28762–70. [DOI] [PubMed] [Google Scholar]
- Tingey M, Mudumbi KC, Schirmer EC and Yang W, 2019. Casting a Wider Net: Differentiating between Inner Nuclear Envelope and Outer Nuclear Envelope Transmembrane Proteins, Int J Mol Sci. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brereton PS, van Driel RR, Suhaimi F.b., Koyama K, Dilley R. and Krozowski Z, 2001. Light and electron microscopy localization of the 11beta-hydroxysteroid dehydrogenase type I enzyme in the rat, Endocrinology. 142, 1644–51. [DOI] [PubMed] [Google Scholar]
- Odermatt A. and Klusonova P, 2015. 11beta-Hydroxysteroid dehydrogenase 1: Regeneration of active glucocorticoids is only part of the story, J Steroid Biochem Mol Biol. 151, 85–92. [DOI] [PubMed] [Google Scholar]
- Robson B, 2020. COVID-19 Coronavirus spike protein analysis for synthetic vaccines, a peptidomimetic antagonist, and therapeutic drugs, and analysis of a proposed achilles’ heel conserved region to minimize probability of escape mutations and drug resistance, Comput Biol Med. 121, 103749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols J, 1995. Lumenal orientation and post-translational modifications of the liver microsomal 11 beta-hydroxysteroid dehydrogenase, J Biol Chem. 270, 10360. [PubMed] [Google Scholar]
- Mziaut H, Korza G, Hand AR, Gerard C. and Ozols J, 1999. Targeting proteins to the lumen of endoplasmic reticulum using N-terminal domains of 11beta-hydroxysteroid dehydrogenase and the 50-kDa esterase, J Biol Chem. 274, 14122–9. [DOI] [PubMed] [Google Scholar]
- Frick C, Atanasov AG, Arnold P, Ozols J. and Odermatt A, 2004. Appropriate function of 11beta-hydroxysteroid dehydrogenase type 1 in the endoplasmic reticulum lumen is dependent on its N-terminal region sharing similar topological determinants with 50-kDa esterase, J Biol Chem. 279, 31131–8. [DOI] [PubMed] [Google Scholar]
- Walker EA, Clark AM, Hewison M, Ride JP and Stewart PM, 2001. Functional expression, characterization, and purification of the catalytic domain of human 11-beta -hydroxysteroid dehydrogenase type 1, J Biol Chem. 276, 21343–50. [DOI] [PubMed] [Google Scholar]
- Carter RN, Paterson JM, Tworowska U, Stenvers DJ, Mullins JJ, Seckl JR and Holmes MC, 2009. Hypothalamic-pituitary-adrenal axis abnormalities in response to deletion of 11beta-HSD1 is strain-dependent, J Neuroendocrinol. 21, 879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson JM, Holmes MC, Kenyon CJ, Carter R, Mullins JJ and Seckl JR, 2007. Liver-selective transgene rescue of hypothalamic-pituitary-adrenal axis dysfunction in 11beta-hydroxysteroid dehydrogenase type 1-deficient mice, Endocrinology. 148, 961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fleur SE, Manalo SL, Roy M, Houshyar H. and Dallman MF, 2005. Hepatic vagotomy alters limbic and hypothalamic neuropeptide responses to insulin-dependent diabetes and voluntary lard ingestion, Eur J Neurosci. 21, 2733–42. [DOI] [PubMed] [Google Scholar]
- Tomlinson JW, Draper N, Mackie J, Johnson AP, Holder G, Wood P. and Stewart PM, 2002. Absence of Cushingoid phenotype in a patient with Cushing’s disease due to defective cortisone to cortisol conversion, J Clin Endocrinol Metab. 87, 57–62. [DOI] [PubMed] [Google Scholar]
- Morgan SA, McCabe EL, Gathercole LL, Hassan-Smith ZK, Larner DP, Bujalska IJ, Stewart PM, Tomlinson JW and Lavery GG, 2014. 11beta-HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess, Proc Natl Acad Sci U S A. 111, E2482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harno E, Cottrell EC, Yu A, DeSchoolmeester J, Gutierrez PM, Denn M, Swales JG, Goldberg FW, Bohlooly YM, Andersen H, Wild MJ, Turnbull AV, Leighton B. and White A, 2013. 11beta-Hydroxysteroid dehydrogenase type 1 (11beta-HSD1) inhibitors still improve metabolic phenotype in male 11beta-HSD1 knockout mice suggesting off-target mechanisms, Endocrinology. 154, 4580–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson JM, Morton NM, Fievet C, Kenyon CJ, Holmes MC, Staels B, Seckl JR and Mullins JJ, 2004. Metabolic syndrome without obesity: Hepatic overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in transgenic mice, Proc Natl Acad Sci U S A. 101, 7088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR and Flier JS, 2001. A transgenic model of visceral obesity and the metabolic syndrome, Science. 294, 2166–70. [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Yamamoto H, Kenyon CJ, Elmquist JK, Morton NM, Paterson JM, Shinyama H, Sharp MG, Fleming S, Mullins JJ, Seckl JR and Flier JS, 2003. Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice, J Clin Invest. 112, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper N, Walker EA, Bujalska IJ, Tomlinson JW, Chalder SM, Arlt W, Lavery GG, Bedendo O, Ray DW, Laing I, Malunowicz E, White PC, Hewison M, Mason PJ, Connell JM, Shackleton CH and Stewart PM, 2003. Mutations in the genes encoding 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase interact to cause cortisone reductase deficiency, Nat Genet. 34, 434–9. [DOI] [PubMed] [Google Scholar]
- Bujalska IJ, Draper N, Michailidou Z, Tomlinson JW, White PC, Chapman KE, Walker EA and Stewart PM, 2005. Hexose-6-phosphate dehydrogenase confers oxo-reductase activity upon 11 beta-hydroxysteroid dehydrogenase type 1, J Mol Endocrinol. 34, 675–84. [DOI] [PubMed] [Google Scholar]
- Banhegyi G, Benedetti A, Fulceri R. and Senesi S, 2004. Cooperativity between 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase in the lumen of the endoplasmic reticulum, J Biol Chem. 279, 27017–21. [DOI] [PubMed] [Google Scholar]
- Atanasov AG, Nashev LG, Gelman L, Legeza B, Sack R, Portmann R. and Odermatt A, 2008. Direct protein-protein interaction of 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase in the endoplasmic reticulum lumen, Biochim Biophys Acta. 1783, 1536–43. [DOI] [PubMed] [Google Scholar]
- Atanasov AG, Nashev LG, Schweizer RA, Frick C. and Odermatt A, 2004. Hexose-6-phosphate dehydrogenase determines the reaction direction of 11beta-hydroxysteroid dehydrogenase type 1 as an oxoreductase, FEBS Lett. 571, 129–33. [DOI] [PubMed] [Google Scholar]
- Nashev LG, Chandsawangbhuwana C, Balazs Z, Atanasov AG, Dick B, Frey FJ, Baker ME and Odermatt A, 2007. Hexose-6-phosphate dehydrogenase modulates 11beta-hydroxysteroid dehydrogenase type 1-dependent metabolism of 7-keto- and 7beta-hydroxy-neurosteroids, PLoS ONE. 2, e561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs Z, Nashev LG, Chandsawangbhuwana C, Baker ME and Odermatt A, 2009. Hexose-6-phosphate dehydrogenase modulates the effect of inhibitors and alternative substrates of 11beta-hydroxysteroid dehydrogenase 1, Mol Cell Endocrinol. 301, 117–22. [DOI] [PubMed] [Google Scholar]
- Bujalska IJ, Walker EA, Hewison M. and Stewart PM, 2002. A switch in dehydrogenase to reductase activity of 11 beta-hydroxysteroid dehydrogenase type 1 upon differentiation of human omental adipose stromal cells, J Clin Endocrinol Metab.87, 1205–10. [DOI] [PubMed] [Google Scholar]
- De Sousa Peixoto RA, Turban S, Battle JH, Chapman KE, Seckl JR and Morton NM, 2008. Preadipocyte 11beta-hydroxysteroid dehydrogenase type 1 is a keto-reductase and contributes to diet-induced visceral obesity in vivo, Endocrinology. 149, 1861–8. [DOI] [PubMed] [Google Scholar]
- Lavery GG, Walker EA, Tiganescu A, Ride JP, Shackleton CH, Tomlinson JW, Connell JM, Ray DW, Biason-Lauber A, Malunowicz EM, Arlt W. and Stewart PM, 2008. Steroid biomarkers and genetic studies reveal inactivating mutations in hexose-6-phosphate dehydrogenase in patients with cortisone reductase deficiency, J Clin Endocrinol Metab. 93, 3827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery GG, Idkowiak J, Sherlock M, Bujalska I, Ride JP, Saqib K, Hartmann MF, Hughes B, Wudy SA, De Schepper J, Arlt W, Krone N, Shackleton CH, Walker EA and Stewart PM, 2013. Novel H6PDH mutations in two girls with premature adrenarche: ‘apparent’ and ‘true’ CRD can be differentiated by urinary steroid profiling, Eur J Endocrinol. 168, K19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Romero DG, de Rodriguez AF, Warden MP, Krozowski Z. and Gomez-Sanchez CE, 2008. Hexose-6-Phosphate Dehydrogenase and 11{beta}-Hydroxysteroid Dehydrogenase-1 Tissue Distribution in the Rat, Endocrinology. 149, 525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS and Joels M, 1999. Stress and cognition: are corticosteroids good or bad guys?, Trends Neurosci. 22, 422–6. [DOI] [PubMed] [Google Scholar]
- Meijer OC, 2002. Coregulator proteins and corticosteroid action in the brain, J Neuroendocrinol. 14, 499–505. [DOI] [PubMed] [Google Scholar]
- Hoppmann J, Perwitz N, Meier B, Fasshauer M, Hadaschik D, Lehnert H. and Klein J, 2010. The balance between gluco- and mineralo-corticoid action critically determines inflammatory adipocyte responses, J Endocrinol. 204, 153–64. [DOI] [PubMed] [Google Scholar]
- Harris AP, Holmes MC, de Kloet ER, Chapman KE and Seckl JR, 2013. Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behaviour, Psychoneuroendocrinology. 38, 648–58. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Meijer OC, de Nicola AF, de Rijk RH and Joels M, 2018. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation, Front Neuroendocrinol. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Cruz-Topete D, He B, Foley JF, Myers PH, Xu X, Gomez-Sanchez CE, Chambon P, Willis MS and Cidlowski JA, 2019. Cardiomyocyte glucocorticoid and mineralocorticoid receptors directly and antagonistically regulate heart disease in mice, Sci Signal. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. and Young MJ, 2009. The mineralocorticoid receptor and its coregulators, J Mol Endocrinol. 43, 53–64. [DOI] [PubMed] [Google Scholar]
- Farman N. and Rafestin-Oblin ME, 2001. Multiple aspects of mineralocorticoid selectivity, Am J Physiol Renal Physiol. 280, F181–92. [DOI] [PubMed] [Google Scholar]
- Ackermann D, Gresko N, Carrel M, Loffing-Cueni D, Habermehl D, Gomez-Sanchez C, Rossier BC and Loffing J, 2010. In vivo nuclear translocation of mineralocorticoid and glucocorticoid receptors in rat kidney: differential effect of corticosteroids along the distal tubule, Am J Physiol Renal Physiol. 299, F1473–85. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP and Gomez-Sanchez CE, 1999. Maternal hypertension and progeny blood pressure: role of aldosterone and 11β-HSD, Hypertension. 33, 1369–1373. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Kang YM, Yu Y, Wei SG, Schmidt TJ, Johnson AK and Felder RB, 2006. 11beta-hydroxysteroid dehydrogenase type 2 activity in hypothalamic paraventricular nucleus modulates sympathetic excitation, Hypertension. 48, 127–33. [DOI] [PubMed] [Google Scholar]
- Wsol V, Szotakova B, Skalova L. and Maser E, 2004. The novel anticancer drug oracin: different stereospecificity and cooperativity for carbonyl reduction by purified human liver 11beta-hydroxysteroid dehydrogenase type 1, Toxicology. 197, 253–61. [DOI] [PubMed] [Google Scholar]
- Maser E, Wsol V. and Martin HJ, 2006. 11Beta-hydroxysteroid dehydrogenase type 1: purification from human liver and characterization as carbonyl reductase of xenobiotics, Mol Cell Endocrinol. 248, 34–7. [DOI] [PubMed] [Google Scholar]
- Yang X, Hua W, Ryu S, Yates P, Chang C, Zhang H. and Di L, 2018. 11beta-Hydroxysteroid Dehydrogenase 1 Human Tissue Distribution, Selective Inhibitor, and Role in Doxorubicin Metabolism, Drug Metab Dispos. 46, 1023–1029. [DOI] [PubMed] [Google Scholar]
- Nashev LG, Vuorinen A, Praxmarer L, Chantong B, Cereghetti D, Winiger R, Schuster D. and Odermatt A, 2012. Virtual screening as a strategy for the identification of xenobiotics disrupting corticosteroid action, PLoS One. 7, e46958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hult M, Nobel CS, Abrahmsen L, Nicoll-Griffith DA, Jornvall H. and Oppermann UC, 2001. Novel enzymological profiles of human 11beta-hydroxysteroid dehydrogenase type 1, Chem Biol Interact. 130–132, 805–14. [DOI] [PubMed] [Google Scholar]
- Odermatt A, Da Cunha T, Penno CA, Chandsawangbhuwana C, Reichert C, Wolf A, Dong M. and Baker ME, 2011. Hepatic reduction of the secondary bile acid 7-oxolithocholic acid is mediated by 11beta-hydroxysteroid dehydrogenase 1, Biochem J. 436, 621–9. [DOI] [PubMed] [Google Scholar]
- Penno CA, Morgan SA, Rose AJ, Herzig S, Lavery GG and Odermatt A, 2014. 11beta-Hydroxysteroid dehydrogenase-1 is involved in bile acid homeostasis by modulating fatty acid transport protein-5 in the liver of mice, Mol Metab. 3, 554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm H, Carlson GL, Hofmann AF, Farivar S. and Amin P, 1980. Metabolism in man of 7-ketolithocholic acid: precursor of cheno- and ursodeoxycholic acids, Am J Physiol. 239, G161–6. [DOI] [PubMed] [Google Scholar]
- Schroepfer GJ Jr., 2000. Oxysterols: modulators of cholesterol metabolism and other processes, Physiol Rev. 80, 361–554. [DOI] [PubMed] [Google Scholar]
- Bjorkhem I, 2013. Five decades with oxysterols, Biochimie. 95, 448–54. [DOI] [PubMed] [Google Scholar]
- Odermatt A, Atanasov AG, Balazs Z, Schweizer RA, Nashev LG, Schuster D. and Langer T, 2006. Why is 11beta-hydroxysteroid dehydrogenase type 1 facing the endoplasmic reticulum lumen? Physiological relevance of the membrane topology of 11beta-HSD1, Mol Cell Endocrinol. 248, 15–23. [DOI] [PubMed] [Google Scholar]
- Mitic T, Shave S, Semjonous N, McNae I, Cobice DF, Lavery GG, Webster SP, Hadoke PW, Walker BR and Andrew R, 2013. 11beta-Hydroxysteroid dehydrogenase type 1 contributes to the balance between 7-keto- and 7-hydroxy-oxysterols in vivo, Biochem Pharmacol. 86, 146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariente A, Pelaez R, Perez-Sala A. and Larrayoz IM, 2019. Inflammatory and cell death mechanisms induced by 7-ketocholesterol in the retina. Implications for age-related macular degeneration, Exp Eye Res. 187, 107746. [DOI] [PubMed] [Google Scholar]
- Cali JJ, Hsieh CL, Francke U. and Russell DW, 1991. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis, J Biol Chem. 266, 7779–83. [PMC free article] [PubMed] [Google Scholar]
- Bjorkhem I. and Hansson M, 2010. Cerebrotendinous xanthomatosis: an inborn error in bile acid synthesis with defined mutations but still a challenge, Biochem Biophys Res Commun. 396, 46–9. [DOI] [PubMed] [Google Scholar]
- Tang Y, Liu Y, Li D, Guo D. and Xing Y, 2020. A novel mutation in the CYP27A1 gene in a family with cerebrotendinous xanthomatosis, Int J Neurosci. 130, 972–975. [DOI] [PubMed] [Google Scholar]
- Lyons MA, Maeda N. and Brown AJ, 2002. Paradoxical enhancement of hepatic metabolism of 7-ketocholesterol in sterol 27-hydroxylase-deficient mice, Biochim Biophys Acta. 1581, 119–26. [DOI] [PubMed] [Google Scholar]
- Schweizer RA, Zurcher M, Balazs Z, Dick B. and Odermatt A, 2004. Rapid hepatic metabolism of 7-ketocholesterol by 11beta-hydroxysteroid dehydrogenase type 1: species-specific differences between the rat, human, and hamster enzyme, J Biol Chem.279, 18415–24. [DOI] [PubMed] [Google Scholar]
- Wamil M, Andrew R, Chapman KE, Street J, Morton NM and Seckl JR, 2008. 7-oxysterols modulate glucocorticoid activity in adipocytes through competition for 11beta-hydroxysteroid dehydrogenase type, Endocrinology. 149, 5909–18. [DOI] [PubMed] [Google Scholar]
- Kipari T, Hadoke PW, Iqbal J, Man TY, Miller E, Coutinho AE, Zhang Z, Sullivan KM, Mitic T, Livingstone DE, Schrecker C, Samuel K, White CI, Bouhlel MA, Chinetti-Gbaguidi G, Staels B, Andrew R, Walker BR, Savill JS, Chapman KE and Seckl JR, 2013. 11beta-hydroxysteroid dehydrogenase type 1 deficiency in bone marrow-derived cells reduces atherosclerosis, Faseb J. 27, 1519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitic T, Andrew R, Walker BR and Hadoke PW, 2013. 11beta-Hydroxysteroid dehydrogenase type 1 contributes to the regulation of 7-oxysterol levels in the arterial wall through the inter-conversion of 7-ketocholesterol and 7beta-hydroxycholesterol, Biochimie. 95, 548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RS, Botfield H, Markey K, Mitchell JL, Alimajstorovic Z, Westgate CSJ, Sagmeister M, Fairclough RJ, Ottridge RS, Yiangou A, KH HS, Taylor AE, Gilligan LC, Arlt W, Stewart PM, Tomlinson JW, Mollan SP, Lavery GG and Sinclair AJ, 2020. 11betaHSD1 inhibition with AZD4017 improves lipid profiles and lean muscle mass in Idiopathic intracranial hypertension, J Clin Endocrinol Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten AM, Takeda Y, Slominski A. and Kang HS, 2018. Retinoic acid-related Orphan Receptor gamma (RORgamma): connecting sterol metabolism to regulation of the immune system and autoimmune disease, Curr Opin Toxicol. 8, 66–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Li X, Lin H, Chu Y, Chen B, Lian Q. and Ge RS, 2010. Regulation of 11beta-hydroxysteroid dehydrogenase 1 and 2 by IGF-1 in mice, Biochem Biophys Res Commun. 391, 1752–6. [DOI] [PubMed] [Google Scholar]
- Gelding SV, Taylor NF, Wood PJ, Noonan K, Weaver JU, Wood DF and Monson JP, 1998. The effect of growth hormone replacement therapy on cortisol-cortisone interconversion in hypopituitary adults: evidence for growth hormone modulation of extrarenal 11 beta-hydroxysteroid dehydrogenase activity, Clin Endocrinol (Oxf). 48, 153–62. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Toogood AA and Tomlinson JW, 2001. Growth hormone, insulin-like growth factor-I and the cortisol-cortisone shuttle, Horm Res. 56, 1–6. [DOI] [PubMed] [Google Scholar]
- Moore JS, Monson JP, Kaltsas G, Putignano P, Wood PJ, Sheppard MC, Besser GM, Taylor NF and Stewart PM, 1999. Modulation of 11beta-hydroxysteroid dehydrogenase isozymes by growth hormone and insulin-like growth factor: in vivo and in vitro studies, Journal of Clinical Endocrinology & Metabolism. 84, 4172–7. [DOI] [PubMed] [Google Scholar]
- Albiston AL, Smith RE and Krozowski ZS, 1995. Sex- and tissue- specific regulation of 11 beta-hydroxysteroid dehydrogenase mRNA, Mol Cell Endocrinol. 109, 183–188. [DOI] [PubMed] [Google Scholar]
- Low SC, Assaad SN, Rajan V, Chapman KE, Edwards CR and Seckl JR, 1993. Regulation of 11 beta-hydroxysteroid dehydrogenase by sex steroids in vivo: further evidence for the existence of a second dehydrogenase in rat kidney, J Endocrinol. 139, 27–35. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Ganjam V, Chen YJ, Liu Y, Zhou MY, Toroslu C, Romero DG, Hughson MD, de Rodriguez A. and Gomez-Sanchez CE, 2003. Regulation of 11 beta-hydroxysteroid dehydrogenase enzymes in the rat kidney by estradiol, Am J Physiol Endocrinol Metab. 285, E272–9. [DOI] [PubMed] [Google Scholar]
- Albiston AL, Smith RE and Krozowski ZS, 1995. Changes in the levels of 11 beta hydroxysteroid dehydrogenase mRNA over the oestrous cycle in the rat, J Steroid Biochem Mol Biol. 52, 45–48. [DOI] [PubMed] [Google Scholar]
- Chen J, Mishra R, Yu Y, McDonald JG, Eckert KM, Gao L. and Mendelson CR, 2020. Decreased 11beta-hydroxysteroid dehydrogenase 1 in lungs of steroid receptor coactivator (Src)-1/−2 double-deficient fetal mice is caused by impaired glucocorticoid and cytokine signaling, FASEB J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai S, Esteves CL, Kelly V, Michailidou Z, Anderson K, Coll AP, Nakagawa Y, Ohzeki T, Seckl JR and Chapman KE, 2008. Glucocorticoid regulation of the promoter of 11beta-hydroxysteroid dehydrogenase type 1 is indirect and requires CCAAT/enhancer-binding protein-beta, Mol Endocrinol. 22, 2049–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruley C, Lyons V, Worsley AG, Wilde MD, Darlington GD, Morton NM, Seckl JR and Chapman KE, 2006. A novel promoter for the 11beta-hydroxysteroid dehydrogenase type 1 gene is active in lung and is C/EBPalpha independent, Endocrinology. 147, 2879–85. [DOI] [PubMed] [Google Scholar]
- Esteves CL, Kelly V, Begay V, Man TY, Morton NM, Leutz A, Seckl JR and Chapman KE, 2012. Regulation of adipocyte 11beta-hydroxysteroid dehydrogenase type 1 (11beta-HSD1) by CCAAT/enhancer-binding protein (C/EBP) beta isoforms, LIP and LAP, PLoS One. 7, e37953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves CL, Verma M, Rog-Zielinska E, Kelly V, Sai S, Breton A, Donadeu FX, Seckl JR and Chapman KE, 2013. Pro-inflammatory cytokine induction of 11beta-hydroxysteroid dehydrogenase type 1 in A549 cells requires phosphorylation of C/EBPbeta at Thr235, PLoS One. 8, e75874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadoke PW, Kipari T, Seckl JR and Chapman KE, 2013. Modulation of 11beta-hydroxysteroid dehydrogenase as a strategy to reduce vascular inflammation, Curr Atheroscler Rep. 15, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KE, Coutinho AE, Zhang Z, Kipari T, Savill JS and Seckl JR, 2013. Changing glucocorticoid action: 11beta-Hydroxysteroid dehydrogenase type 1 in acute and chronic inflammation, J Steroid Biochem Mol Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RS, Fenton C, Croft AP, Naylor AJ, Begum R, Desanti G, Buckley CD, Lavery G, Cooper MS and Raza K, 2018. 11 Beta-hydroxysteroid dehydrogenase type 1 regulates synovitis, joint destruction, and systemic bone loss in chronic polyarthritis, J Autoimmun. 92, 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Coutinho AE, Man TY, Kipari TMJ, Hadoke PWF, Salter DM, Seckl JR and Chapman KE, 2017. Macrophage 11beta-HSD-1 deficiency promotes inflammatory angiogenesis, J Endocrinol. 234, 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgeorges T, Caratti G, Mounier R, Tuckermann J. and Chazaud B, 2019. Glucocorticoids Shape Macrophage Phenotype for Tissue Repair, Front Immunol. 10, 1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho AE, Brown JK, Yang F, Brownstein DG, Gray M, Seckl JR, Savill JS and Chapman KE, 2013. Mast cells express 11beta-hydroxysteroid dehydrogenase type 1: a role in restraining mast cell degranulation, PLoS One. 8, e54640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsuka M, Haines LC, Milne M, Simpson GE and Hillier SG, 1999. Regulation of 11beta-hydroxysteroid dehydrogenase type 1 gene expression by LH and interleukin-1beta in cultured rat granulosa cells, J Endocrinol. 163, 417–23. [DOI] [PubMed] [Google Scholar]
- Thieringer R, Le Grand CB, Carbin L, Cai TQ, Wong B, Wright SD and Hermanowski-Vosatka A, 2001. 11 Beta-hydroxysteroid dehydrogenase type 1 is induced in human monocytes upon differentiation to macrophages, J Immunol. 167, 30–5. [DOI] [PubMed] [Google Scholar]
- Chantong B, Kratschmar DV, Nashev LG, Balazs Z. and Odermatt A, 2012. Mineralocorticoid and glucocorticoid receptors differentially regulate NF-kappaB activity and pro-inflammatory cytokine production in murine BV-2 microglial cells, J Neuroinflammation. 9, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Li Y, Xu C, Melino G, Shao C. and Shi Y, 2020. HSD11B1 is upregulated synergistically by IFNgamma and TNFalpha and mediates TSG-6 expression in human UC-MSCs, Cell Death Discov. 6, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naray-Fejes-Toth A. and Fejes-Toth G, 2007. Novel mouse strain with Cre recombinase in 11beta-hydroxysteroid dehydrogenase-2-expressing cells, Am J Physiol Renal Physiol. 292, F486–94. [DOI] [PubMed] [Google Scholar]
- Cooper MS, 2012. Glucocorticoids in bone and joint disease: the good, the bad and the uncertain, Clin Med (Lond). 12, 261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RS, Seibel MJ and Cooper MS, 2013. Targeting 11beta-hydroxysteroid dehydrogenases: a novel approach to manipulating local glucocorticoid levels with implications for rheumatic disease, Curr Opin Pharmacol. 13, 440–4. [DOI] [PubMed] [Google Scholar]
- Terao M. and Katayama I, 2016. Local cortisol/corticosterone activation in skin physiology and pathology, J Dermatol Sci. 84, 11–16. [DOI] [PubMed] [Google Scholar]
- Fenton CG, Doig CL, Fareed S, Naylor A, Morrell AP, Addison O, Wehmeyer C, Buckley CD, Cooper MS, Lavery GG, Raza K. and Hardy RS, 2019. 11beta-HSD1 plays a critical role in trabecular bone loss associated with systemic glucocorticoid therapy, Arthritis Res Ther. 21, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenouch S, Lombes M, Delahaye F, Eugene E, Bonvalet J-P and Farman N, 1994. Human skin as target for aldosterone: coexpression of mineralocorticoid receptors and 11β-hydroxysteroid dehydrogenase, J Clin Endocrinol Metab. 79, 1334–1341. [DOI] [PubMed] [Google Scholar]
- Smith RE, Maguire JA, Stein-Oakley AN, Sasano H, Takahashi K, Fukushima K. and Krozowski ZS, 1996. Localization of 11 beta-hydroxysteroid dehydrogenase type II in human epithelial tissues, J Clin Endocrinol Metab. 81, 3244–3248. [DOI] [PubMed] [Google Scholar]
- Farman N, Maubec E, Poeggeler B, Klatte JE, Jaisser F. and Paus R, 2010. The mineralocorticoid receptor as a novel player in skin biology: beyond the renal horizon?, Exp Dermatol. 19, 100–7. [DOI] [PubMed] [Google Scholar]
- Bocchi B, Kenouch S, Lamarre-Cliche M, Muffat-Joly M, Capron MH, Fiet J, Morineau G, Azizi M, Bonvalet JP and Farman N, 2004. Impaired 11-beta hydroxysteroid dehydrogenase type 2 activity in sweat gland ducts in human essential hypertension, Hypertension. 43, 803–8. [DOI] [PubMed] [Google Scholar]
- Terao M, Itoi S, Murota H. and Katayama I, 2013. Expression profiles of cortisol-inactivating enzyme, 11beta-hydroxysteroid dehydrogenase-2, in human epidermal tumors and its role in keratinocyte proliferation, Exp Dermatol. 22, 98–101. [DOI] [PubMed] [Google Scholar]
- Tiganescu A, Tahrani AA, Morgan SA, Otranto M, Desmouliere A, Abrahams L, Hassan-Smith Z, Walker EA, Rabbitt EH, Cooper MS, Amrein K, Lavery GG and Stewart PM, 2013. 11beta-Hydroxysteroid dehydrogenase blockade prevents age-induced skin structure and function defects, J Clin Invest. 123, 3051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudon S, Heidl M, Vuorinen A, Wandeler E, Campiche R, Odermatt A. and Jackson E, 2018. Design, synthesis, and biological evaluation of novel selective peptide inhibitors of 11beta-hydroxysteroid dehydrogenase 1, Bioorg Med Chem. 26, 5128–5139. [DOI] [PubMed] [Google Scholar]
- Mancha-Ramirez AM, Yang X, Liang H, Junco J, Lee KP, Bovio SF, Espinoza M, Wool J, Slaga A, Glade DC, Hanes M, Malik G, Kim DJ, DiGiovanni J. and Slaga TJ, 2019. Harnessing the gatekeepers of glucocorticoids for chemoprevention of non-melanoma skin cancer, Mol Carcinog. 58, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR, Kelly PAT and Sharkey J, 1991. Glycyrrhetinic acid, an inhibitor of 11β-hydroxysteroid dehydrogenase, alters local cerebral glucose utilization in vivo, J Steroid Biochem Molec Biol. 39, 777–779. [DOI] [PubMed] [Google Scholar]
- Walker BR and Seckl JR, 2003. 11beta-Hydroxysteroid dehydrogenase Type 1 as a novel therapeutic target in metabolic and neurodegenerative disease, Expert Opin Ther Targets. 7, 771–83. [DOI] [PubMed] [Google Scholar]
- Sandeep TC, Yau JL, MacLullich AM, Noble J, Deary IJ, Walker BR and Seckl JR, 2004. 11Beta-hydroxysteroid dehydrogenase inhibition improves cognitive function in healthy elderly men and type 2 diabetics, Proc Natl Acad Sci U S A. 101, 6734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooy K, Noble J, McBride A, Binnie M, Yau JL, Seckl JR, Walker BR and Webster SP, 2015. Cognitive and Disease-Modifying Effects of 11beta-Hydroxysteroid Dehydrogenase Type 1 Inhibition in Male Tg2576 Mice, a Model of Alzheimer’s Disease, Endocrinology. 156, 4592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster SP, McBride A, Binnie M, Sooy K, Seckl JR, Andrew R, Pallin TD, Hunt HJ, Perrior TR, Ruffles VS, Ketelbey JW, Boyd A. and Walker BR, 2017. Selection and early clinical evaluation of the brain-penetrant 11beta-hydroxysteroid dehydrogenase type 1 (11beta-HSD1) inhibitor UE2343 (Xanamem), Br J Pharmacol. 174, 396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, Noble J, Kenyon CJ, Hibberd C, Kotelevtsev Y, Mullins JJ and Seckl JR, 2001. Lack of tissue glucocorticoid reactivation in 11beta -hydroxysteroid dehydrogenase type 1 knockout mice ameliorates age-related learning impairments, Proc Natl Acad Sci U S A. 98, 4716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MC, Carter RN, Noble J, Chitnis S, Dutia A, Paterson JM, Mullins JJ, Seckl JR and Yau JL, 2010. 11beta-hydroxysteroid dehydrogenase type 1 expression is increased in the aged mouse hippocampus and parietal cortex and causes memory impairments, J Neurosci. 30, 6916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigoriol-Illamola D, Leiva R, Vazquez-Carrera M, Vazquez S, Grinan-Ferre C. and Pallas M, 2020. 11beta-HSD1 Inhibition Rescues SAMP8 Cognitive Impairment Induced by Metabolic Stress, Mol Neurobiol. 57, 551–565. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Katz DA, Meier A, Greco N.t., Zhang W, Liu W. and Lenz RA, 2014. Efficacy and safety evaluation of HSD-1 inhibitor ABT-384 in Alzheimer’s disease, Alzheimers Dement. 10, S364–73. [DOI] [PubMed] [Google Scholar]
- Gregory S, Hill D, Grey B, Ketelbey W, Miller T, Muniz-Terrera G. and Ritchie CW, 2020. 11beta-hydroxysteroid dehydrogenase type 1 inhibitor use in human disease-a systematic review and narrative synthesis, Metabolism. 108, 154246. [DOI] [PubMed] [Google Scholar]
- Watermeyer T, Robb C, Gregory S. and Udeh-Momoh C, 2020. Therapeutic implications of hypothalamic-pituitaryadrenal-axis modulation in Alzheimer’s disease: A narrative review of pharmacological and lifestyle interventions, Front Neuroendocrinol. 60, 100877. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Poirier R, Wollmer MA, Grimaldi LM, Tsolaki M, Streffer JR, Hock C, Nitsch RM, Mohajeri MH and Papassotiropoulos A, 2004. Glucocorticoid-related genetic susceptibility for Alzheimer’s disease, Hum Mol Genet. 13, 47–52. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Hayward C, Permana PA, Nair S, Whalley LJ, Starr JM, Chapman KE, Walker BR and Seckl JR, 2006. Polymorphisms in the gene encoding 11B-hydroxysteroid dehydrogenase type 1 (HSD11B1) and lifetime cognitive change, Neurosci Lett. 393, 74–7. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Nabulsi NB, Li S, Cai Z, Matuskey D, Bini J, Najafzadeh S, Kapinos M, Ropchan JR, Carson RE, Cosgrove KP, Huang Y. and Hillmer AT, 2020. First in-human PET study and kinetic evaluation of [(18)F]AS2471907 for imaging 11beta-hydroxysteroid dehydrogenase type 1, J Cereb Blood Flow Metab. 40, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bini J, Bhatt S, Hillmer AT, Gallezot JD, Nabulsi N, Pracitto R, Labaree D, Kapinos M, Ropchan J, Matuskey D, Sherwin RS, Jastreboff AM, Carson RE, Cosgrove K. and Huang Y, 2020. Body Mass Index and Age Effects on Brain 11beta-Hydroxysteroid Dehydrogenase Type 1: a Positron Emission Tomography Study, Mol Imaging Biol. 22, 1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujalska IJ, Kumar S. and Stewart PM, 1997. Does central obesity reflect “Cushing’s disease of the omentum”?, Lancet. 349, 1210–1213. [DOI] [PubMed] [Google Scholar]
- Bujalska IJ, Quinkler M, Tomlinson JW, Montague CT, Smith DM and Stewart PM, 2006. Expression profiling of 11beta-hydroxysteroid dehydrogenase type-1 and glucocorticoid-target genes in subcutaneous and omental human preadipocytes, J Mol Endocrinol. 37, 327–40. [DOI] [PubMed] [Google Scholar]
- Morton NM, Paterson JM, Masuzaki H, Holmes MC, Staels B, Fievet C, Walker BR, Flier JS, Mullins JJ and Seckl JR, 2004. Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11 beta-hydroxysteroid dehydrogenase type 1-deficient mice, Diabetes. 53, 931–8. [DOI] [PubMed] [Google Scholar]
- Vuorinen A, Nashev LG, Odermatt A, Rollinger JM and Schuster D, 2014. Pharmacophore Model Refinement for 11beta-Hydroxysteroid Dehydrogenase Inhibitors: Search for Modulators of Intracellular Glucocorticoid Concentrations, Mol Inform. 33, 15–25. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Pramyothin P, Karastergiou K. and Fried SK, 2014. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity, Biochim Biophys Acta. 1842, 473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla R, Basu AK, Mandal B, Mukhopadhyay P, Maity A, Chakraborty S. and Devrabhai PK, 2019. 11beta Hydroxysteroid dehydrogenase - 1 activity in type 2 diabetes mellitus: a comparative study, BMC Endocr Disord. 19, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang C, Obeyesekere VR, Dilley RJ, Krozowski Z, Inder WJ and Alford FP, 2007. Altered activity of 11beta-hydroxysteroid dehydrogenase types 1 and 2 in skeletal muscle confers metabolic protection in subjects with type 2 diabetes, J Clin Endocrinol Metab. 92, 3314–20. [DOI] [PubMed] [Google Scholar]
- Nair S, Lee YH, Lindsay RS, Walker BR, Tataranni PA, Bogardus C, Baier LJ and Permana PA, 2004. 11beta-Hydroxysteroid dehydrogenase Type 1: genetic polymorphisms are associated with Type 2 diabetes in Pima Indians independently of obesity and expression in adipocyte and muscle, Diabetologia. 47, 1088–95. [DOI] [PubMed] [Google Scholar]
- Devang N, Adhikari P, Nandini M, Satyamoorthy K. and Rai PS, 2020. Effect of licorice on patients with HSD11B1 gene polymorphisms- a pilot study, J Ayurveda Integr Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis G. and Huber R, 2011. 11beta-Hydroxysteroid dehydrogenase type 1 inhibition in type 2 diabetes mellitus, Diabetes Obes Metab. 13, 1–6. [DOI] [PubMed] [Google Scholar]
- Chuanxin Z, Shengzheng W, Lei D, Duoli X, Jin L, Fuzeng R, Aiping L. and Ge Z, 2020. Progress in 11beta-HSD1 inhibitors for the treatment of metabolic diseases: A comprehensive guide to their chemical structure diversity in drug development, Eur J Med Chem. 191, 112134. [DOI] [PubMed] [Google Scholar]
- Heussner K, Ruebner M, Huebner H, Rascher W, Menendez-Castro C, Hartner A, Fahlbusch FB and Rauh M, 2016. Species differences of 11beta-hydroxysteroid dehydrogenase type 2 function in human and rat term placenta determined via LC-MS/MS, Placenta. 37, 79–84. [DOI] [PubMed] [Google Scholar]
- Brem AS, Matheson KL, Barnes JL and Morris DJ, 1991. 11-Dehydrocorticosterone, a glucocorticoid metabolite, inhibits aldosterone action in toad bladder, Am J Physiol. 261, F873–9. [DOI] [PubMed] [Google Scholar]
- Brem AS, Matheson KL, Latif S. and Morris DJ, 1993. Activity of 11 beta-hydroxysteroid dehydrogenase in toad bladder: effects of 11-dehydrocorticosterone, Am J Physiol. 264, F854–8. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Ganjam V, Chen YJ, Liu Y, Clark SA and Gomez-Sanchez CE, 2001. The 11beta hydroxysteroid dehydrogenase 2 exists as an inactive dimer, Steroids. 66, 845–8. [DOI] [PubMed] [Google Scholar]
- Alikhani-Koopaei R, Fouladkou F, Frey FJ and Frey BM, 2004. Epigenetic regulation of 11 beta-hydroxysteroid dehydrogenase type 2 expression, J Clin Invest. 114, 1146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasano H, Frost AR, Saitoh R, Matsunaga G, Nagura H, Krozowski ZS and Silverberg SG, 1997. Localization of mineralocorticoid receptor and 11 beta-hydroxysteroid dehydrogenase type II in human breast and its disorders, Anticancer Res. 17, 2001–7. [PubMed] [Google Scholar]
- Sai S, Nakagawa Y, Yamaguchi R, Suzuki M, Sakaguchi K, Okada S, Seckl JR, Ohzeki T. and Chapman KE, 2011. Expression of 11beta-hydroxysteroid dehydrogenase 2 contributes to glucocorticoid resistance in lymphoblastic leukemia cells, Leuk Res. 35, 1644–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai S, Esteves C, Kelly V, Sakaguchi K, McAndrew R, Chudleigh S, Spence A, Gibson B, Thomas A. and Chapman KE, 2020. Reciprocal Regulation of HSD11B1 and HSD11B2 Predicts Glucocorticoid Sensitivity in Childhood Acute Lymphoblastic Leukemia, J Pediatr. 220, 249–253. [DOI] [PubMed] [Google Scholar]
- Molhuysen JA, Gerbrandy J, De Vries LA, Jong JC and Borst JGG, 1950. A liquorice extract with deoxycortone like action, Lancet. ii, 381–386. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Wallace AM, Valentino R, Burt D, Shackleton CHL and Edwards CRW, 1987. Mineralocorticoid activity of liquorice: 11-Beta-hydroxysteroid dehydrogenase deficiency comes of age, Lancet. ii, 821–824. [DOI] [PubMed] [Google Scholar]
- Baker ME, 1994. Licorice and enzymes other than 11β-hydroxysteroid dehydrogenase: An evolutionary perspective, Steroids. 59, 136–141. [DOI] [PubMed] [Google Scholar]
- Yoshino T, Yanagawa T. and Watanabe K, 2014. Risk factors for pseudoaldosteronism with rhabdomyolysis caused by consumption of drugs containing licorice and differences between incidence of these conditions in Japan and other countries: case report and literature review, J Altern Complement Med. 20, 516–20. [DOI] [PubMed] [Google Scholar]
- Stavropoulos K, Sotiriadis A, Patoulias D, Imprialos K, Dampali R, Athyros V. and Dinas K, 2018. Pseudohyperaldosteronism due to mumijo consumption during pregnancy: a licorice-like syndrome, Gynecol Endocrinol. 34, 1019–1021. [DOI] [PubMed] [Google Scholar]
- Zhang YD, Lorenzo B. and Reidenberg MM, 1994. Inhibition of 11 betahydroxysteroid dehydrogenase obtained from guinea pig kidney by furosemide, naringenin and some other compounds, J Steroid Biochem Mol Biol. 49, 81–5. [DOI] [PubMed] [Google Scholar]
- Palermo M, Armanini D. and Delitala G, 2003. Grapefruit juice inhibits 11beta-hydroxysteroid dehydrogenase in vivo, in man, Clin Endocrinol (Oxf). 59, 143–4. [DOI] [PubMed] [Google Scholar]
- Michael AE, 1998. 11 beta HSD and the mechanism of gossypol-induced hypokalemia, Int J Androl. 21, 313. [DOI] [PubMed] [Google Scholar]
- Ma X, Lian QQ, Dong Q. and Ge RS, 2011. Environmental inhibitors of 11beta-hydroxysteroid dehydrogenase type 2, Toxicology. 285, 83–9. [DOI] [PubMed] [Google Scholar]
- Morris DJ, Semafuko WEB, Latif SA, Vogel B, Grimes CA and Sheff MF, 1992. Detection of glycyrrhetinic acid-like factors (GALFs) in human urine, Hypertension. 20, 356–360. [DOI] [PubMed] [Google Scholar]
- Walker BR, Aggarwal I, Stewart PM, Padfield PL and Edwards CR, 1995. Endogenous inhibitors of 11 beta-hydroxysteroid dehydrogenase in hypertension, J Clin Endocrinol Metab. 80, 529–533. [DOI] [PubMed] [Google Scholar]
- Morris DJ, Latif SA and Brem AS, 2014. An alternative explanation of hypertension associated with 17alpha-hydroxylase deficiency syndrome, Steroids. 79, 44–8. [DOI] [PubMed] [Google Scholar]
- Morris DJ and Ridlon JM, 2017. Glucocorticoids and gut bacteria: “The GALF Hypothesis” in the metagenomic era, Steroids. 125, 1–13. [DOI] [PubMed] [Google Scholar]
- Quattropani C, Vogt B, Odermatt A, Dick B, Frey BM and Frey FJ, 2001. Reduced activity of 11 beta-hydroxysteroid dehydrogenase in patients with cholestasis, J Clin Invest. 108, 1299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer AT, Rochat MK, Dick B, Frey FJ and Odermatt A, 2002. Chenodeoxycholic acid and deoxycholic acid Inhibit 11beta -hydroxysteroid dehydrogenase type 2 and cause cortisol-induced transcriptional activation of the mineralocorticoid receptor, J Biol Chem. 15, 15. [DOI] [PubMed] [Google Scholar]
- Beck KR, Bachler M, Vuorinen A, Wagner S, Akram M, Griesser U, Temml V, Klusonova P, Yamaguchi H, Schuster D. and Odermatt A, 2017. Inhibition of 11beta-hydroxysteroid dehydrogenase 2 by the fungicides itraconazole and posaconazole, Biochem Pharmacol. 130, 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KR, Telisman L, van Koppen CJ, Thompson GR 3rd and Odermatt A, 2020. Molecular mechanisms of posaconazole- and itraconazole-induced pseudohyperaldosteronism and assessment of other systemically used azole antifungals, J Steroid Biochem Mol Biol. 199, 105605. [DOI] [PubMed] [Google Scholar]
- Huang C, Wan B, Gao B, Hexige S. and Yu L, 2009. Isolation and characterization of novel human short-chain dehydrogenase/reductase SCDR10B which is highly expressed in the brain and acts as hydroxysteroid dehydrogenase, Acta Biochim Pol. 56, 279–89. [PubMed] [Google Scholar]
- Gohin M, Bobe J. and Chesnel F, 2010. Comparative transcriptomic analysis of follicle-enclosed oocyte maturational and developmental competence acquisition in two non-mammalian vertebrates, BMC Genomics. 11, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feswick A, Ings JS, Doyle MA, Bosker T, Munkittrick KR and Martyniuk CJ, 2014. Transcriptomics profiling and steroid production in mummichog (Fundulus heteroclitus) testes after treatment with 5alpha-dihydrotestosterone, Gen Comp Endocrinol. 203, 106–19. [DOI] [PubMed] [Google Scholar]
- Ge RS, Dong Q, Sottas CM, Chen H, Zirkin BR and Hardy MP, 2005. Gene expression in rat leydig cells during development from the progenitor to adult stage: a cluster analysis, Biol Reprod. 72, 1405–15. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez CE, Zhou MY, Cozza EN, Morita H, Foecking MF and Gomez-Sanchez EP, 1997. Aldosterone biosynthesis in the rat brain, Endocrinology. 138, 3369–3373. [DOI] [PubMed] [Google Scholar]
- Monder C, Miroff Y, Marandici A. and Hardy MP, 1994. 11β-hydroxysteroid dehydrogenase alleviates glucocorticoid-mediated inhibition of steroidogenesis in rat Leydig cells, Endocrinology. 134, 1199–1204. [DOI] [PubMed] [Google Scholar]
- Ge R-S, Hardy DO, Catterall JF and Hardy MP, 1997. Developmental changes in glucocorticoid receptor and 11β-hydroxysteroid dehydrogenase oxidative and reductive activities in rat leydig cells, Endocrinology. 138, 5089–5095. [DOI] [PubMed] [Google Scholar]
- Ge RS, Dong Q, Niu EM, Sottas CM, Hardy DO, Catterall JF, Latif SA, Morris DJ and Hardy MP, 2005. 11{beta}-Hydroxysteroid dehydrogenase 2 in rat leydig cells: its role in blunting glucocorticoid action at physiological levels of substrate, Endocrinology. 146, 2657–64. [DOI] [PubMed] [Google Scholar]
- Honda Y, Ohno S. and Nakajin S, 2008. Leydig cells from neonatal pig testis abundantly express 11beta-hydroxysteroid dehydrogenase (11beta-HSD) type 2 and effectively inactivate cortisol to cortisone, J Steroid Biochem Mol Biol. 108, 91–101. [DOI] [PubMed] [Google Scholar]
- Michael AE, Pester LA, Curtis P, Shaw RW, Edwards CR and Cooke BA, 1993. Direct inhibition of ovarian steroidogenesis by cortisol and the modulatory role of 11 beta-hydroxysteroid dehydrogenase, Clin Endocrinol (Oxf). 38, 641–4. [DOI] [PubMed] [Google Scholar]
- Yong PY, Thong KJ, Andrew R, Walker BR and Hillier SG, 2000. Development-related increase in cortisol biosynthesis by human granulosa cells, J Clin Endocrinol Metab. 85, 4728–33. [DOI] [PubMed] [Google Scholar]
- Tetsuka M, Yamamoto S, Hayashida N, Hayashi KG, Hayashi M, Acosta TJ and Miyamoto A, 2003. Expression of 11beta-hydroxysteroid dehydrogenases in bovine follicle and corpus luteum, J Endocrinol. 177, 445–52. [DOI] [PubMed] [Google Scholar]
- Yazawa T, Uesaka M, Inaoka Y, Mizutani T, Sekiguchi T, Kajitani T, Kitano T, Umezawa A. and Miyamoto K, 2008. Cyp11b1 is induced in the murine gonad by luteinizing hormone/human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen: conservation and evolution of the androgen metabolic pathway, Endocrinology. 149, 1786–92. [DOI] [PubMed] [Google Scholar]
- Michael AE, Thurston LM and Rae MT, 2003. Glucocorticoid metabolism and reproduction: a tale of two enzymes, Reproduction. 126, 425–41. [DOI] [PubMed] [Google Scholar]
- Bloem LM, Storbeck KH, Schloms L. and Swart AC, 2013. 11beta-hydroxyandrostenedione returns to the steroid arena: biosynthesis, metabolism and function, Molecules. 18, 13228–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storbeck KH, Bloem LM, Africander D, Schloms L, Swart P. and Swart AC, 2013. 11beta-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer?, Mol Cell Endocrinol. 377, 135–46. [DOI] [PubMed] [Google Scholar]
- Gent R, du Toit T, Bloem LM and Swart AC, 2019. The 11beta-hydroxysteroid dehydrogenase isoforms: pivotal catalytic activities yield potent C11-oxy C19 steroids with 11betaHSD2 favouring 11-ketotestosterone, 11-ketoandrostenedione and 11-ketoprogesterone biosynthesis, J Steroid Biochem Mol Biol. 189, 116–126. [DOI] [PubMed] [Google Scholar]
- Davio A, Woolcock H, Nanba AT, Rege J, O’Day P, Ren J, Zhao L, Ebina H, Auchus R, Rainey WE and Turcu AF, 2020. Sex Differences in 11-Oxygenated Androgen Patterns Across Adulthood, J Clin Endocrinol Metab. 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba AT, Rege J, Ren J, Auchus RJ, Rainey WE and Turcu AF, 2019. 11-Oxygenated C19 Steroids Do Not Decline With Age in Women, J Clin Endocrinol Metab. 104, 2615–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael AE, Glenn C, Wood PJ, Webb RJ, Pellatt L. and Mason HD, 2013. Ovarian 11beta-Hydroxysteroid Dehydrogenase (11betaHSD) Activity Is Suppressed in Women With Anovulatory Polycystic Ovary Syndrome (PCOS): Apparent Role for Ovarian Androgens, J Clin Endocrinol Metab. 98, 3375–83. [DOI] [PubMed] [Google Scholar]
- Chin D, Shackleton C, Prasad VK, Kohn B, David R, Imperato-McGinley J, Cohen H, McMahon DJ and Oberfield SE, 2000. Increased 5alpha-reductase and normal 11beta-hydroxysteroid dehydrogenase metabolism of C19 and C21 steroids in a young population with polycystic ovarian syndrome, Journal of Pediatric Endocrinology & Metabolism. 13, 253–9. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Holmes MC, Livingstone DE, Kenyon CJ and Seckl JR, 2012. Reconciling the nutritional and glucocorticoid hypotheses of fetal programming, FASEB J. 26, 1866–74. [DOI] [PubMed] [Google Scholar]
- Correia-Branco A, Keating E. and Martel F, 2015. Maternal undernutrition and fetal developmental programming of obesity: the glucocorticoid connection, Reprod Sci. 22, 138–45. [DOI] [PubMed] [Google Scholar]
- McTernan CL, Draper N, Nicholson H, Chalder SM, Driver P, Hewison M, Kilby MD and Stewart PM, 2001. Reduced placental 11beta-hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms, J Clin Endocrinol Metab. 86, 4979–83. [DOI] [PubMed] [Google Scholar]
- Shams M, Kilby MD, Somerset DA, Howie AJ, Gupta A, Wood PJ, Afnan M. and Stewart PM, 1998. 11β-hydroxysteroid dehydrogenase type 2 in human pregnancy and reduced expression in intrauterine growth restriction, Human Reproduction. 13, 799–804. [DOI] [PubMed] [Google Scholar]
- Whorwood CB, Firth KM, Budge H. and Symonds ME, 2001. Maternal undernutrition during early to midgestation programs tissue-specific alterations in the expression of the glucocorticoid receptor, 11beta-hydroxysteroid dehydrogenase isoforms, and type 1 angiotensin ii receptor in neonatal sheep, Endocrinology. 142, 2854–64. [DOI] [PubMed] [Google Scholar]
- Waddell BJ, Benediktsson R, Brown RW and Seckl JR, 1998. Tissue-specific messenger ribonucleic acid expression of 11beta-hydroxysteroid dehydrogenase types 1 and 2 and the glucocorticoid receptor within rat placenta suggests exquisite local control of glucocorticoid action, Endocrinology. 139, 1517–23. [DOI] [PubMed] [Google Scholar]
- Albrecht ED and Pepe GJ, 1999. Central integrative role of oestrogen in modulating the communication between the placenta and fetus that results in primate fetal-placental development, Placenta. 20, 129–139. [DOI] [PubMed] [Google Scholar]
- Shearer FJG, Wyrwoll CS and Holmes MC, 2019. The Role of 11beta-Hydroxy Steroid Dehydrogenase Type 2 in Glucocorticoid Programming of Affective and Cognitive Behaviours, Neuroendocrinology. 109, 257–265. [DOI] [PubMed] [Google Scholar]
- Shallie PD, Margolis D, Shallie OF and Naicker T, 2020. Placental 11beta-HSD2 downregulated in HIV associated preeclampsia, J Reprod Immunol. 142, 103185. [DOI] [PubMed] [Google Scholar]
- Ma R, Liu J, Wu L, Sun J, Yang Z, Yu C, Yuan P. and Xiao X, 2012. Differential expression of placental 11beta-hydroxysteroid dehydrogenases in pregnant women with diet-treated gestational diabetes mellitus, Steroids. 77, 798–805. [DOI] [PubMed] [Google Scholar]
- Burton PJ and Waddell BJ, 1999. Dual function of 11 beta-hydroxysteroid dehydrogenase in placenta: Modulating placental glucocorticoid passage and local steroid action, Biol Reprod. 60, 234–240. [DOI] [PubMed] [Google Scholar]
- Pepe GJ, Burch MG and Albrecht ED, 2001. Estrogen regulates 11 beta-hydroxysteroid dehydrogenase-1 and −2 localization in placental syncytiotrophoblast in the second half of primate pregnancy, Endocrinology. 142, 4496–503. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Venkatakrishnan R, Salker MS, Lucas ES, Shaheen F, Kuroda M, Blanks A, Christian M, Quenby S. and Brosens JJ, 2013. Induction of 11beta-HSD 1 and activation of distinct mineralocorticoid receptor- and glucocorticoid receptor-dependent gene networks in decidualizing human endometrial stromal cells, Mol Endocrinol. 27, 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa G, Sasano H, Suzuki T, Takeyama J, Muramatu Y, Fukushima K,Hiwatashi N, Toyota T, Nagura H. and Krozowski ZS, 1999. 11Beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor in human fetal development, Journal of Clinical Endocrinology & Metabolism. 84, 1453–8. [DOI] [PubMed] [Google Scholar]
- Sloboda DM, Moss TJ, Li S, Matthews SG, Challis JR and Newnham JP, 2008. Expression of glucocorticoid receptor, mineralocorticoid receptor, and 11beta- hydroxysteroid dehydrogenase 1 and 2 in the fetal and postnatal ovine hippocampus: ontogeny and effects of prenatal glucocorticoid exposure, J Endocrinol. 197, 213–20. [DOI] [PubMed] [Google Scholar]
- Toews JN, Hammond GL and Viau V, 2020. Liver at the nexus of rat postnatal HPA axis maturation and sexual dimorphism, J Endocrinol. [DOI] [PubMed] [Google Scholar]
- Wagner A. and Claus R, 2008. Aromatase and 11beta-hydroxysteroid dehydrogenase 2 localisation in the testes of pigs from birth to puberty linked to changes of hormone pattern and testicular morphology, Reprod Fertil Dev. 20, 505–12. [DOI] [PubMed] [Google Scholar]
- Koeva Y, Bakalska M, Atanassova N, Georgieva K. and Davidoff M, 2009. Age- related changes in the expression of 11beta-hydroxysteroid dehydrogenase type 2 in rat Leydig cells, Folia Histochem Cytobiol. 47, 281–7. [DOI] [PubMed] [Google Scholar]