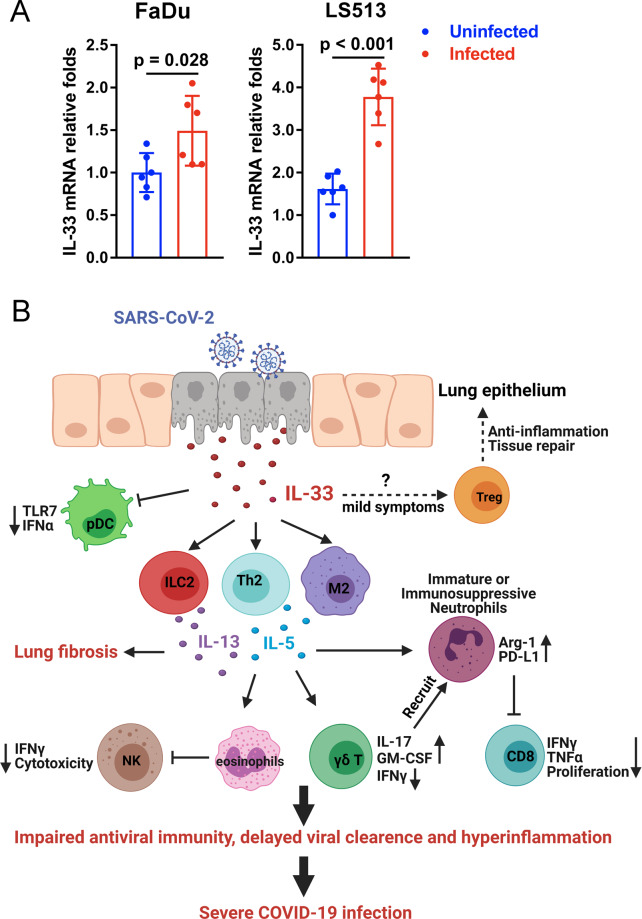
The COVID-19 pandemic, caused by the highly transmissible and pathogenic severe acute respiratory syndrome coronavirus 2 (SAR-CoV-2), has led to more than 2.7 million deaths worldwide as of March 2021. Although considerable efforts are underway to reveal the immunopathology of COVID-19, the key factors and processes that initiate hyperinflammatory responses and cause severe clinical outcomes in certain individuals remain unclear. The damage-associated molecular pattern (DAMP) molecule IL-33 belongs to the IL-1 family and has been recognized as an alarmin that indicates cellular damage or infection. Full-length IL-33 requires cleavage by proteases to generate its mature bioactive form, which can bind to the ST2 receptor (also known as IL-1RL1), leading to activation of the NF-κB pathway in various innate and adaptive immune cells. The relatively high abundance of IL-33 in epithelial and endothelial cells accounts for its proinflammatory role in respiratory diseases.1 Recent observations have revealed that serum IL-33 is upregulated in elderly patients with COVID-19 and associated with adverse outcomes.2,3 The increased IL-33 levels in severe infection could result from epithelial damage caused by strong interactions between the airway epithelium and activated immune cells. SARS-CoV-2-derived papain-like protease (PLpro), a powerful inducer of IL-33 in epithelial cells,4 may also trigger epithelium-derived IL-33 to initiate inflammatory responses in the lungs. To test whether SARS-CoV-2 infection induces IL-33 expression in epithelial cells, we infected two human epithelial cell lines, Fadu and LS513, with SARS-CoV-2 (viral strain: BetaCoV/JS02/Human/2019, isolated by Jiangsu Provincial Center for Disease Control and Prevention, China) in vitro. Our results demonstrated significant increases in IL-33 transcript levels in both cell lines at 72 h postinfection (Fig. 1A). Therefore, we provide evidence for the first time that SARS-CoV-2 infection promotes IL-33 expression in human epithelial cells. Clinically, transcriptomic analysis of bronchoalveolar lavage fluid from COVID-19 patients demonstrated strong upregulation of IL-33,5 indicating the induction of IL-33 in pulmonary recruited cells (e.g., macrophages) after infection. Moreover, a recent preprint reported that a SARS-CoV-2 spike peptide mixture could induce IL-33 secretion in the culture supernatant of PBMCs from seropositive individuals,6 suggesting that immune cells may also be a source of IL-33 in COVID-19. However, it is not clear whether IL-33 is secreted by activated immune cells or is directly released due to cell death.
Fig. 1.
The potential role of IL-33 in COVID-19. A Upregulation of IL-33 in human epithelial cells by SARS-CoV-2 infection. Human epithelial cell lines (FaDu and LS513) were infected with SARS-CoV-2 (0.01 TCID50/cell, viral strain: BetaCoV/JS02/Human/2019). Total RNA was extracted at 72 h postinfection, and IL-33 transcript levels were analyzed by real-time PCR. Primers: IL-33 Forward-GTGACGGTGTTGATGGTAAGAT, IL-33 Reverse-AGCTCCACAGAGTGTTCCTTG; and β-actin Forward-ACCAACTGGGACGACATGGAGAA, β-actin reverse-GTGGTGGTGAAGCTGTAGCC. A two-tailed Student’s t test was used for statistical analysis. B Immune modulation by IL-33 in COVID-19. SARS-CoV-2 infection triggers IL-33 release from damaged epithelial cells in the lung. IL-33 initiates type 2 immune responses via the activation of M2 macrophages, T helper 2 cells and type 2 innate lymphoid cells, leading to impaired antiviral activities of plasmacytoid dendritic cells, NK cells and CD8 T cells, as well as the dysregulation of neutrophils. Dampened antiviral immunity results in delayed viral clearance and hyperinflammation in patients with severe COVID-19
Upon binding with a receptor complex composed of ST2 and IL-1RAcP, mature IL-33 boosts type-2 immunity via the activation of eosinophils, mast cells, M2 macrophages, T helper 2 cells and group 2 innate lymphoid cells (ILC2s).1 Compared with severe acute respiratory syndrome coronavirus (SAR-CoV), SAR-CoV-2 favors a cytokine storm composed of low levels of type 1 cytokines (IL-12p70 and IL-15) but high expression of Th2/9 cytokines (IL-4, IL-9, IL-10, IL-13 and TGF-β).7 Elevated type 2 cytokine levels correlated with an increased number of ILC2s in COVID-19 patients3 and may contribute to the differentiation of pathogenic γδ T cells (IFN-γlow GM-CSFhigh). Therefore, an elevation in IL-33 in the lungs following SAR-CoV-2 infection might be the driving force of type 2 immune cytokines and account for respiratory immune dysregulation. Moreover, IL-33-dependent lung-resident ILC2s can modulate NK cell innate immunity by suppressing IFN-γ production and cytotoxic functions,8 leading to an impaired NK cell responses against SARS-CoV-2 infection. In addition to NK cells, IL-33 inhibits innate immunity in respiratory viral infection by degrading IL-1 receptor-associated kinase (IRAK1) and viperin in plasmacytoid dendritic cells, leading to TLR7 hyporesponsiveness.9 As TLR7 may be necessary for recognition of the SARS-CoV-2 genome and production of antiviral type I interferon,10 IL-33 may dampen innate antiviral immunity and delay viral clearance in COVID-19 patients.
Prominent neutrophil infiltration has been reported in severe COVID-19 patients, and a high neutrophil-to-lymphocyte ratio (NLR) is a predictor of in-hospital death.11 Notably, IL-33 promotes rapid neutrophil migration via macrophage-derived CXCL1 and CXCL2, whereas neutrophil elastase and cathepsin G further contribute to IL-33 processing and maturation to exacerbate inflammatory responses. It is plausible that pathogenic γδ17 T cells may also accelerate neutrophil recruitment to the lungs via IL-17 production. Furthermore, immature neutrophils have been reported in severe COVID-19 cases.12 Neutrophil dysregulation may be attributed to increased IL-33/ILC2 responses, since IL-33 can educate neutrophils towards a unique immunosuppressive phenotype via ILC2s and dampen the appropriate antiviral T cell immune response,13 which is potentially involved in the control of SARS-CoV-2 infection. Importantly, elevated IL-33 levels and the associated type 2 immunity in chronic viral infection are considered potential inducers of pulmonary fibrosis, which is a recognized sequelae of acute respiratory distress syndrome (ARDS) observed in approximately 40% of COVID-19 patients.14 Therefore, blockade of the IL-33/neutrophil feedback loop using IL-33- or ST2-neutralizing antibodies might be a novel therapeutic strategy for severe COVID-19 patients. Encouragingly, a phase II clinical trial of Astegolimab (anti-ST2) treatment in patients with severe COVID-19 pneumonia is close to completion (ClinicalTrials.gov Identifier: NCT04386616).
Although increased IL-33 levels are considered a predictor of severe COVID-19, their precise roles in different stages of disease are still unclear (Fig. 1B). Individuals with relatively mild COVID-19 symptoms exhibit increased numbers of Treg cells, which are associated with the resolution of inflammation. In addition to proinflammatory activity, IL-33 may drive ST2+ regulatory T cell (Treg) expansion, inhibit innate γδ T cell responses, and restore respiratory tissue homoeostasis in patients who develop asymptomatic or mild disease. Notably, IL-33 is critical for antiviral CD8 T cell responses to persistent infection and may contribute to elimination of the virus.15 Additional animal studies and clinical trials are essential for us to better understand the precise role of IL-33 and how manipulation of the IL-33/ST2 axis could be an effective therapeutic strategy for COVID-19 treatment.
Acknowledgements
The authors of this work were supported by NIH grants, including EY028773 to J.S. and AI153586 to Y.L., and the UTMB Institute of Human Infections & Immunity Pilot grant to Y.L. We thank Dr. Sherry Haller and Dr. Hui Wang for their assistance with manuscript preparation. The image of Fig. 1B is created with BioRender.com.
Author contributions
Y.G. designed and completed the in vitro experimental work. Y.L. and J.S. wrote this manuscript. All authors approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yuejin Liang, Yiyue Ge
Contributor Information
Yuejin Liang, Email: yu2liang@utmb.edu.
Jiaren Sun, Email: jisun@utmb.edu.
References
- 1.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 2.Burke H, et al. Inflammatory phenotyping predicts clinical outcome in COVID-19. Respir. Res. 2020;21:245. doi: 10.1186/s12931-020-01511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez-Cadena A, et al. Severe COVID-19 patients exhibit an ILC2 NKG2D(+) population in their impaired ILC compartment. Cell Mol. Immunol. 2021;18:484–486. doi: 10.1038/s41423-020-00596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin D, et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020;587:657–662. doi: 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong Y, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michal, A. S. et al. IL-33 expression in response to SARS-CoV-2 correlates with seropositivity in COVID-19 convalescent individuals. Nat. Commun.21, 2133 (2021). [DOI] [PMC free article] [PubMed]
- 7.Zizzo G, Cohen PL. Imperfect storm: is interleukin-33 the Achilles heel of COVID-19? Lancet Rheumatol. 2020;2:e779–e790. doi: 10.1016/S2665-9913(20)30340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuijs MJ, et al. ILC2-driven innate immune checkpoint mechanism antagonizes NK cell antimetastatic function in the lung. Nat. Immunol. 2020;21:998–1009. doi: 10.1038/s41590-020-0745-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch JP, et al. Aeroallergen-induced IL-33 predisposes to respiratory virus-induced asthma by dampening antiviral immunity. J. Allergy Clin. Immunol. 2016;138:1326–1337. doi: 10.1016/j.jaci.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 10.van der Made CI, et al. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324:1–11. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020;81:e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulte-Schrepping J, et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182:1419–1440 e23. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang Y, et al. IL-33 induces immunosuppressive neutrophils via a type 2 innate lymphoid cell/IL-13/STAT6 axis and protects the liver against injury in LCMV infection-induced viral hepatitis. Cell Mol. Immunol. 2019;16:126–137. doi: 10.1038/cmi.2017.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spagnolo P, et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet. Respir. Med. 2020;8:750–752. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonilla WV, et al. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]