Abstract
Pancreatic cancer remains a leading cause of cancer-related death with few available therapies for advanced disease. Recently, patients with germline BRCA mutations have received increased attention due to advances in the management of BRCA mutated ovarian and breast tumors. Germline BRCA mutations significantly increase risk of developing pancreatic cancer and can be found in up to 8% of patients with sporadic pancreatic cancer. In patients with germline BRCA mutations, platinum-based chemotherapies and poly (ADP-ribose) polymerase inhibitors are effective treatment options which may offer survival benefits. This review will focus on the molecular biology, epidemiology, and management of BRCA-mutated pancreatic cancer. Furthermore, we will discuss future directions for this area of research and promising active areas of research.
Keywords: Pancreatic cancer, Systemic therapy, Platinum chemotherapy, BRCA, Deoxyribonucleic acid repair, Poly (ADP-ribose) polymerase inhibitors
Core Tip: Recent advances in the field of BRCA-mutated pancreatic cancer suggest that these patients benefit from platinum-based chemotherapy regimens. In light of new findings from the Pancreas Cancer Olaparib Ongoing trial, patients with germline BRCA mutations may benefit from maintenance treatment with olaparib, a Poly (ADP-ribose) polymerase inhibitors following response to platinum-based chemotherapy. Based on these important findings, all pancreatic cancer patients should be offered early access to genetic screening in order to identify patients who will benefit from these therapies.
INTRODUCTION
Pancreatic cancer (PC) remains one of the most aggressive malignancies, with a 5-year survival rate of 8%[1,2]. Incidence of PC has increased over the past 4 decades, making it a leading cause of cancer-related mortality in North America[1-3]. The vast majority of pancreatic cancers are ductal adenocarcinomas (PDAC) of the exocrine pancreatic glands, occurring most commonly in the head of the pancreas[4]. Most cases of PDAC are considered sporadic, however 5%-10% are estimated to be familial with patients having a family history of PDAC[5]. Several genetic syndromes are known to cause familial PDAC including mutations of deoxyribonucleic acid (DNA) mismatch repair genes (Lynch syndrome), BRCA1 and BRCA2 (hereditary breast cancer syndrome); however, in the vast majority of cases a genetic cause cannot be identified[5-7].
Currently, the only potentially curative treatment for PC is surgical resection which is only possible in the early stages of the disease (locoregional) and highly dependent on the degree of invasion of surrounding critical structures such as vessels and bile ducts. Unfortunately, only 15%-20% of PDAC cases are considered resectable, and of these, over 75% will have recurrence within 5 years of their resection[4]. Recent data suggests that in patients with good performance status, treatment with a combination regimen of fluorouracil, oxaliplatin, leucovorin and irinotecan (FOLFIRINOX) is the optimal adjuvant therapy following resection[8]. Because early stage PC is usually asymptomatic, the vast majority of patients present with either locally advanced (involvement of local vasculature) or metastatic disease[4]. In these patients chemotherapy and occasionally radiotherapy form the backbone of treatment and are used to relieve symptoms and modestly prolong life.
In the advanced setting of disease, the two standard of care palliative chemotherapy options include gemcitabine plus albumin-bound paclitaxel (nab-paclitaxel) and FOLFIRINOX. In the first-line setting, both have been shown to prolong overall survival (OS) relative to gemcitabine monotherapy in prospective, randomized clinical trials[9,10]. Even with these treatments, 2-year survival remains at 10% and median OS ranges from 8-11 mo[4].
Recent genomic evidence suggests that PDAC is a genetically heterogenous disease with different molecular subtypes, potentially explaining the failure of many novel therapies when trialled in unselected populations[11,12]. Currently, efforts are ongoing to identify select PDAC patient populations who would benefit from targeted therapies. A patient group which has garnered much interest are those with mutations of BRCA1 and BRCA2. These genes are important players in the homologous DNA repair (HR) pathway and mutations of both genes are strong risk factors for the development of several cancers including, breast, ovarian, prostate and pancreatic cancer[13,14]. Importantly, BRCA mutations also have implications for treatment as they may increase tumor susceptibility to both DNA-damaging chemotherapies such as platinum chemotherapy (PtCh), as well as poly (ADP-ribose) polymerase (PARP) inhibitors in breast and ovarian cancers. More recently, work has been done to determine if these clinical features translate to BRCA-mutated pancreatic cancer. This review will discuss the biology, epidemiology and clinical implications of BRCA mutations in PDAC, and will discuss future directions for this area of research.
MOLECULAR BIOLOGY OF HOMOLOGOUS REPAIR
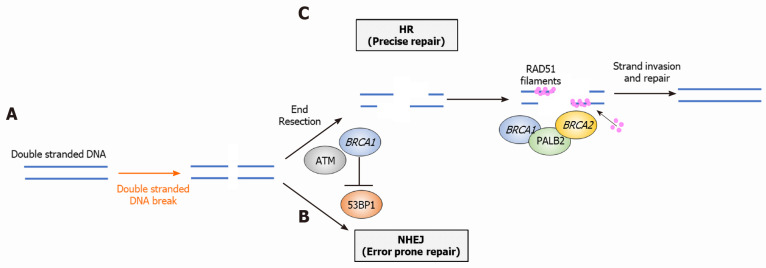
Several reviews have previously described the biology of the HR system and the specific roles of BRCA1/2[15,16]. Briefly, DNA damage can occur as either a single-stranded DNA break (SSB) or double-stranded DNA break (DSB). HR along with non-homologous end joining (NHEJ) are the two major pathways that respond to DSB. HR has the highest fidelity and precision of the DSB repair pathways, therefore defects in this pathway (homologous repair deficiency, HRD) lead to error-prone repair and genomic instability, increasing cancer risk. Important proteins in the HR system include BRCA1, BRCA2, PALB2, ATM and RAD51[15]. Following DSB, BRCA1 negatively regulates factors involved in the NHEJ pathway (53BP1) and promotes end resection, an important first step in the HR pathway. BRCA1 directly interacts with PALB2 to bind BRCA2 which facilitates formation of RAD51 filaments later in the pathway[15]. RAD51 filament form along ssDNA created earlier by BRCA1-mediated end resection, allowing formation of homologous DNA and repair of the DSB (Figure 1)[15]. Notably, other proteins involved in the HR pathways such as PALB2 and ATM are also mutated in PC, highlighting the importance of HR pathway integrity in determining PDAC risk[11,17].
Figure 1.
Overview of the homologous repair pathway and roles of key proteins. A: Following double strand break, BRCA 1 binds to the site of damage, mediating end resection and initiating homologous repair. This prevents repair via non-homologous end joining; B: BRCA1 binds with PALB2 and BRCA2 which facilitates assembly of RAD51 filaments; and C: RAD51 filaments form along ssDNA, subsequently leading to strand invasion and repair. DSB: Double strand break; HR: Homologous repair; NHEJ: Non-homologous end joining; BRCA: Breast cancer susceptibility gene.
While BRCA mutations confer increased cancer risk, emerging evidence suggests they also may be important markers for personalized medicine. In vitro and in vivo evidence suggests that both platinum-based chemotherapies and PARP inhibitors are more effective in patients harboring BRCA mutations[11].
EPIDEMIOLOGY AND DIAGNOSIS OF BRCA-MUTATED PDAC
Incidence of pathogenic BRCA mutations in sporadic and familial PDAC
Mutations of the BRCA1 and BRCA2 genes were first identified as breast and ovarian cancer risk factors in the mid-1990s during studies aimed at characterizing the genes responsible for familial clustering of breast and ovarian cancers[18,19]. Early studies by the Breast Cancer Linkage Consortium identified a 2.3-fold and 3.5-fold increased risk of PC in carriers of BRCA1 and BRCA2 gene mutations, respectively[13,14]. In the general population, germline BRCA mutations occur at a rate between 1/300 and 1/800[20]. However, incidence varies based on population as certain ethnic groups harbor founder mutations, increasing the incidence of BRCA mutations in these subgroups. The strongest example of the founder effect in BRCA is the Ashkenazi Jewish (AJ) population, where the presence of 3 founder mutations have increased rates of BRCA mutation to 1/40[21]. Other groups with founder BRCA mutations who are therefore at increased risk include Dutch, Norwegian and French-Canadian populations[22].
Among unselected PC patient cohorts, multiple studies have aimed to estimate the incidence of germline pathogenic BRCA mutations. Prevalence estimates ranged from 0.7%-5.7% for BRCA2 and 0.3%-2.3% for BRCA1 (Summarized in Table 1)[6,23-26]. Notably, the cohorts in these studies varied widely based on several factors which could influence estimates of prevalence, including, number of AJ PC patients included, the number of patients with family histories of cancer, and median patient age[23]. For example, in AJ PDAC patients, studies have found that up to 19% of patients harbour germline BRCA mutations[23,27,28].
Table 1.
Summary of studies of incidence of germline BRCA mutations in unselected pancreatic cancer cohorts
|
Ref.
|
Year
|
Population
|
Cohort size (Number AJ)
|
Germline BRCA1 pathogenic mutation incidence (%)
|
Germline BRCA2 pathogenic mutation incidence (%)
|
Combined germline BRCA mutation Incidence
|
| Holter et al[23] | 2015 | North American | 306 (33) | 1.0% | 3.6% | 4.6% |
| Brand et al[24] | 2018 | North American | 298 (26) | 1.3% | 1.3% | 2.6% |
| Mizukami et al[25] | 2020 | Japanese | 1005 (-) | 1.7% | 2.5% | 4.2% |
| Grant et al[6] | 2015 | North American | 290 (13) | 0.3% | 0.7% | 1% |
| Lowery et al[26] | 2018 | North American | 615 (111) | 2.3% | 5.7% | 8% |
AJ: Ashkenazi Jewish; BRCA: Breast cancer susceptibility gene.
In familial PC, BRCA mutations, especially BRCA2 are also at increased frequency. In the case of BRCA2 mutations, studies have found germline mutations in 3.7%-19% of patients with strong familial histories of PDAC[29-32]. This range in estimates is likely a result of different criteria for familial pancreatic cancer (FPC), and different studies methodologies. Studies finding higher rates of BRCA2 mutation tended to have smaller sample sizes and included patients with three or more first- or second-degree relatives with PC, therefore included higher risk patients. Conversely, more recent studies have included larger sample sizes of patients, who met the more moderate FPC case definition (two first- or second- degree relatives with PC), finding more conservative estimates of prevalence (3.7% and 6%)[31,32]. Therefore, in patients with a stronger family history of PC, BRCA carrier status is more likely. The incidence of BRCA1 mutations in FPC has not been studied as well as BRCA2, however a recent study by Zhen et al[31] found that germline BRCA1 mutations were present in 1.2% of patients with FPC.
Diagnosis of BRCA-mutated PDAC and screening guidelines
While the identification of patients carrying BRCA mutations has been important in determining cancer risk, the discovery of personalized medicine options for this population has increased the clinical importance of identifying BRCA carriers. Genetic testing guidelines vary by region however, are primarily based on cancer phenotype which includes family history of breast, ovarian, prostate and pancreatic cancer, AJ ancestry and clinical presentation. Recently, genetic testing guidelines are being increasingly questioned as evidence accumulates to suggest that they would miss a large proportion of patients harboring BRCA mutations who may benefit from PARP inhibitors or platinum chemotherapies. In 2007, a Norwegian study tested breast and ovarian cancer patients for germline mutations in BRCA1 and BRCA2 and identified that 50% of patients with germline BRCA mutations do not have family histories of BRCA-associated cancers[33]. Since then, multiple studies in different populations including patients with PDAC have confirmed these findings, showing poor associations between presence of BRCA mutations and expected family histories [23,34-38]. Furthermore, a recent study using data from 23&Me, a direct-to-consumer genetic test identified that 20% of carriers of the AJ founder variants don’t identify as AJ, and therefore would be excluded from screening criteria that include AJ ancestry[39]. They also found that of 393 BRCA mutation carriers with available data on family cancer history, 44% had no family history of BRCA-associated cancers, and therefore, given a diagnosis of PDAC, would not meet screening requirements. The recent IMPACT trial by the Memorial Sloan-Kettering Cancer Centre provided strong evidence in favour of increased testing access. Investigators tested 1040 patients (176 PDAC) with advanced cancer and identified germline mutations in 21.5% of the PDAC patients. Notably, they found that across all cancers, 55% of clinically actionable mutations would not have been detected under current phenotype-based screening guidelines[40]. Together, this evidence strongly supports calls for increased access to genetic testing for PC patients. In early 2020, the National Comprehensive Cancer Network updated their recommendations to suggest universal genetic testing for all PC patients as early as possible due to the rapid progression of the disease, and potential for early personalized therapy[41].
CLINICAL FEATURES OF BRCA-MUTATED PDAC AND PROGNOSTIC IMPLICATIONS
While the ability of BRCA mutations to increase risk of PDAC is well established, their impact on the clinical features of the disease is less clear. Multiple cohort studies have shown in PDAC patients with germline mutations including BRCA1, BRCA2, PALB2, CDKN2A and ATM, are diagnosed earlier with PDAC than PDAC patients without germline mutations[31,42]. Conversely, a 2009 study comparing Jewish PDAC patients with and without germline BRCA mutations found no significant differences between age at diagnosis or any other clinicopathologic feature studied[28]. From a prognostic perspective, studies have shown mixed results. The largest cohort study to date including 71 BRCA-positive PDAC patients found a median OS of 14 mo for the whole cohort and 12 mo for patients with stage 3/4 disease. At time of publication, the median OS for early stage disease had not been reached as 52% of patients were still alive at 60 mo[43]. These findings suggest that BRCA-mutated PDAC patients may have a considerably better prognosis than the general PDAC population. On the contrary, more recent case-control studies by Blair et al[44] compared PDAC patients with BRCA1 and BRCA2 mutations to age-matched controls and showed that both OS and disease-free survival (DFS) were lower in carriers than controls. Another case-control study comparing BRCA mutation-positive, early-stage PDAC patients undergoing surgical resection to age-matched BRCA-wildtype controls found no significant differences in median OS or DFS between the groups and concluded that BRCA mutations were not prognostic in early PDAC[45]. Authors have suggested that early findings of improved prognosis in this population may have been a result of ascertainment bias as patients surviving longer were more likely to receive genetic testing and participate in the study. Another factor that may lead to improved prognosis in this patient population is increased susceptibility to treatments such as PtCh. Most recently, a study using data from the Know Your Tumor program aimed to assess whether mutations of HRD and other DNA-damage response (DDR) genes conferred a survival benefit or whether observed benefits were a result of increased PtCh-sensitivity[46]. The authors found that patients with advanced PDAC and HR/DDR mutations had improved survival but only if treated with PtCh. In PtCh-naïve patients, there was no survival benefit in this patient population[46].
Overall, identifying clinical differences between BRCA-mutated PDAC and wildtype PDAC has been difficult due to the relative rarity of these patients. Furthermore, the increasing use of personalized therapies (PARP inhibitors and platinum chemotherapy) in this population will make determining the prognostic implications of BRCA mutations more challenging.
MANAGEMENT OF BRCA-MUTATED PDAC: SYSTEMIC THERAPY
Platinum chemotherapy
While both FOLFIRINOX and gemcitabine/nab-paclitaxel chemotherapy regimens are more effective than gemcitabine monotherapy, there is yet to be a comparative randomized clinical trial to provide data on which regimen is more effective. In the locally advanced setting, a recent case series of 485 consecutive patients suggested that FOLFIRINOX was associated with a higher response rate (19% vs 6%, P = 0.001), however OS was not different with either treatment[47]. Retrospective studies in metastatic PDAC are inconclusive, with some studies reporting survival improvement on FOLFIRINOX while others report no difference between the two regimens[47,48]. Given the increased toxicity associated with FOLFIRINOX and potential survival benefits, identifying subsets of patients who are more likely to benefit from this regimen will be an important advancement in PC management.
The HRD phenotype of BRCA-mutated cancers appears to render them more sensitive to chemotherapies that induce DNA damage, such as PtCh. Early studies found that cells lacking BRCA1 are more sensitive to treatment with cisplatin[49]. In the presence of HRD, these cells are unable to appropriately repair the DNA damage, leading to genomic instability and cell death[50]. Clinical studies in breast cancer have found that platinum-chemotherapy improves objective response rates (ORRs) for metastatic breast cancer patients only in BRCA-mutated cancers. Based on genomic studies in PDAC, it appears that tumors with BRCA-mutations have “unstable” molecular phenotypes and are more likely to be sensitive to genotoxic therapies such as PtCh[11].
In PC, several large retrospective studies have investigated the efficacy of PtCh such as FOLFIRINOX in patients with BRCA mutations or other genetic mutations leading to HRD (Table 2). To date, the largest cohort study was conducted by Golan et al[43] This multi-institution cohort study included 71 PC patients with germline BRCA mutations and found that among patients with advanced PDAC, OS was significantly longer in patients treated with PtCh (22 vs 9 mo). Since this study, several other retrospective cohort studies have reported improved outcomes [ORR, progression free survival (PFS)] in patients with germline mutations to HR-related genes who were treated with PtCh in both resectable and non-resectable PDAC[35,44,51,52]. For example, Blair et al[44] showed that median survival was significantly improved in resected PDAC patients with germline BRCA mutations who were treated with adjuvant PtCh compared to non-PtCh (31.0 vs 17.8 mo). Reiss et al[52] showed significant improvement in mOS in patients with unresectable PDAC and mutations in BRCA1, BRCA2 or PALB2 who were treated with PtCh compared to patients treated with non-PtCh (median follow-up of 20.1 mo vs mOS of 15.5 mo). Several studies have also compared the effectiveness of PtCh between patients with and without HRD mutations. In a cohort study of platinum-treated PDAC patients, patients found to have tumor-level mutations to 12 HR-related genes (including BRCA1, BRCA2, ATM and PALB2) had significantly improved median PFS compared to platinum-treated patient without HR-related gene mutations[35]. Similarly, two recent case-control studies reported improved PFS and ORR in platinum-treated patients who carried mutations to BRCA1, BRCA2 and PALB2[53,54]. Wattenberg et al[53] showed an ORR of 58% in mutation carriers treated with PtCh compared to 21% non-mutated PDAC patients. In resected PDAC treated with perioperative PtCh, Yu et al[54] reported that mutation carriers had significantly greater survival (mOS not met vs 23.1 mo, HR = 0.12).
Table 2.
Retrospective studies of platinum-chemotherapies in BRCA-mutated pancreatic ductal adenocarcinoma
|
Ref.
|
Year
|
Study design
|
Patient population
|
Findings
|
| Golan et al[43] | 2014 | Multi-institution cohort study | 71 patients with germline BRCA mutations (21 BRCA1, 49 BRCA2, 1 both) | Superior mOS in stage 3/4 patients treated with platinum compared to non-platinum chemotherapy (22 vs 9 mo, P = 0.039) |
| Vyas et al[51] | 2015 | Cohort study | 10 patients with BRCA2 mutation and known PDAC | Duration of response on platinum agents ranged from 8-32 wk, mean of 19.3 wk |
| Blair et al[44] | 2018 | Combined case control cohort study | 22 patients with resected sporadic PDAC and germline BRCA mutations (1 BRCA1, 18 BRCA2) | Improved OS in BRCA-mutated patients treated with adjuvant PtCh compared to patients treated with alternative chemotherapies or no adjuvant therapy (31.0 vs 17.8 vs 9.3 mo, P < 0.001) |
| Reiss et al[52] | 2018 | Cohort study | 29 patients with unresectable PDAC and germline mutations of BRCA1, BRCA2 or PALB2(12 BRCA1, 15 BRCA2, 2 PALB2) | Superior mOS in platinum-treated patients (undefined mOS (median follow up 21 mo) vs 15.5 mo, P = 0.02) |
| Kondo et al[35] | 2018 | Cohort study | 28 patients with advanced PDAC (13 had HR-related gene mutations, 15 without mutations to HR-related genes) | Superior median PFS in HR-mutated PDAC patients treated with platinum chemotherapy compared to PDAC patients without HR mutations treated with platinum therapy (20.8 mo vs 1.7 mo, P = 0.049) |
| Yu et al[54] | 2019 | Case control study | 32 resected PC patients with germline BRCA1, BRCA2, or PALB2 mutation, 64 resected PC patient controls without germline mutations | With peri-operative platinum exposure, mOS was longer in mutation-positive group that mutation negative group (mOS not yet met vs 23.1 mo, HR= 0.12) |
| Wattenberg et al[53] | 2020 | Case control study | 26 platinum-treated patients with advanced stage PDAC and mutations of BRCA1, BRCA2 or PALB2, 52 platinum-treated, wildtype, age-matched controls | Improved ORR in patients with mutations compared to controls (58% vs 21%, P = 0.0022). Improved real world PFS in mutation carriers (10.1 mo vs 6.9 mo, HR = 0.43, P = 0.0068) |
HR: Homologous Repair; mOS: Median overall survival; ORR: Objective response rate; PDAC: Pancreatic adenocarcinoma; PtCh: Platinum chemotherapy; BRCA: Breast cancer susceptibility gene.
While these studies are promising, the retrospective nature introduces several limitations. Firstly, outcomes are widely subdivided as PtCh vs non-PtCh, however the PtCh groups generally include a variety of regimens such as gemcitabine + cisplatin, gemcitabine + oxaliplatin, FOLFOX and FOLFIRINOX. Seeing as oxaliplatin and cisplatin exert DNA damage through different mechanisms of action, it is unclear how well these findings will translate to modern clinics where patients are typically treated with FOLFIRINOX as a first-line therapy[52]. One study reported that there was no significant difference in survival for mutation-positive patients on different PtCh regimens, however in the mutation-negative group, patients only responded to FOLFIRINOX[53]. This suggests that there is potentially a role for PtCh regimens in BRCA-mutated patients that did not show benefit when tested in unselected PDAC populations, in situations when FOLFIRINOX cannot be tolerated. Another limitation is the current practices with respect to treatment selection. Because of the toxicity associated with PtCh such as FOLFIRINOX, these regimens are generally used in younger patients with better performance status. Therefore, in retrospective analyses of BRCA-mutated PDAC cohorts, it is unclear whether survival benefits seen are because of increased activity of PtCh in this patient population or because the patients treated with PtCh are younger and have better performance status. Few studies have reported data on patient age in these analyses and none have reported patient performance status. In light of this, these retrospective analyses are difficult to interpret. Lastly, retrospective studies may be affected by survival bias. Most studies compared confirmed mutation carriers to untested cohorts. It is possible that patients who survive longer are more likely to undergo genetic testing and be classified as carriers. In light of these limitations, a recent meta-analysis concluded that the current available evidence suggests PtCh is more effective in BRCA-mutated patients, however the quality of evidence is low[55].
To date, there have been few prospective studies assessing the effectiveness of platinum-chemotherapies in this population. A recent phase II randomized controlled trial investigated cisplatin and gemcitabine with or without Veliparib, a PARP inhibitor in patients with untreated advanced PDAC and a germline mutation of BRCA or PALB2[56]. While the primary endpoint (response rate) was not significantly different with Veliparib, the authors reported unprecedented survival rates, with a 2-year survival rate of 30.6% and a 3-year survival rate of 17.8%[56]. Response rates were also high for both arms of the study (74% with Veliparib, 65.2% without veliparib)[56]. While this data provides compelling evidence for the use of PtCh in this patient population, the study lacks a control group treated with non-PtCh for comparison. This study adds to the literature as all patients were on the same PtCh regimen (gemcitabine + cisplatin) which showed impressive responses and survival rates. Notably, the patients included in this study all had a good performance status (ECOG 0-1) and therefore these results may not translate as well to real-world PDAC patients where performance status may be lower.
Overall, there is evidence in favour of the use of PtCh as a first-line treatment for BRCA-mutated PDAC, however, most data is retrospective and the quality of the evidence in favour of this treatment is low. There is yet to be a randomized controlled trial confirming the observations that PtCh is preferable to other chemotherapy regimens in this population, however enrollment to such a study may be difficult due to current management practice. Furthermore, it is unclear whether or not FOLFIRINOX or gemcitabine plus cisplatin should be used for this patient population.
PARP inhibitors
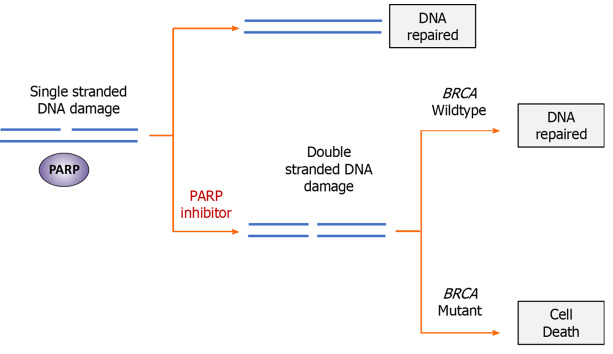
The sensitivity of BRCA-deficient cancers to PARP inhibition was first reported in 2005, in which researchers identified that loss of function of both BRCA and PARP is synthetically lethal[57,58]. PARP is an important family of enzymes involved in responding to SSB the other prominent form of DNA damage other than DSB. This combined loss of SSB repair in HRD cells is thought to lead to synthetic lethality (Figure 2). While the exact mechanism of action is still unclear, the earliest theory was that PARP inhibition prevents the repair of single-stranded DNA breaks (SSBs), leading to accumulation of replication-associated DSBs[59]. In HRD cells which have defective DSB repair, DSBs are repaired via error-prone NHEJ, leading to genomic instability and cell death. More recent evidence suggests that the biology of BRCA and PARP deficient synthetic lethality is more complex, however the detailed mechanisms are outside the scope of this review[60].
Figure 2.
Mechanism of synthetic lethality in BRCA-mutated cells treated with poly (ADP-ribose) polymerase inhibitors. While neither a breast cancer susceptibility (BRCA) mutation or treatment with Poly (ADP-ribose) polymerase (PARP) inhibitors alone is lethal to cancer cells, dual-inhibition of both systems through mutation and pharmacological inhibition is incompatible with survival. Following PARP inhibition, single-stranded deoxyribonucleic acid (DNA) breaks are unable to be repaired. During replication, replication forks stall at unrepaired DNA damage, resulting in formation of double-stranded DNA break. In cells with defective homologous repair (BRCA mutations), double-stranded damage is repaired through non-homologous end joining, resulting in genomic instability and cell death. Poly (ADP-Ribose) Polymerase. PARP: Poly (ADP-ribose) polymerase; BRCA: Breast cancer susceptibility gene.
Therapeutic inhibitors of this pathway were evaluated in a phase I study of olaparib and confirmed activity in several different tumor types harboring BRCA mutations[61]. In ovarian cancer, PARP inhibitors are FDA-approved for use as a maintenance therapy in patients with recurrent ovarian cancer who demonstrated a complete or partial response to PtCh, regardless of HRD biomarker status[62]. This approval came following three phase III trials which demonstrated significant improvements in PFS in patients treated with oral PARP inhibitors as maintenance therapy following chemotherapy[63-65]. More recently, emerging data from several randomized clinical trials reporting efficacy of PARP inhibitors as a front-line treatment for newly diagnosed ovarian cancer[62]. In advanced breast cancer, PARP inhibitors have demonstrated improvements in PFS relative to chemotherapy in patients with HER2-negative, BRCA-mutation positive tumors[66,67]. However, there is yet to be a clinical trial demonstrating improvements in OS with PARP inhibitor use in advanced breast cancer[68]. Recently, PARP inhibitors have also demonstrated effectiveness in metastatic prostate cancer[69].
With the success of PARP inhibitors in other BRCA-associated cancers, focus has shifted to translating these findings to BRCA-associated PDAC. To date multiple phase II studies have evaluated the efficacy of PARP inhibitors in PDAC patients with germline BRCA mutations[56,70,71]. In a phase II study by Kaufman et al[71], 298 patients with advanced cancer (23 with pancreas cancer) and germline BRCA1/2 mutations were treated with oral olaparib. The response rate among PC patients was 21.7% in patients who had received two prior lines of chemotherapy[71]. Conversely, another phase II study evaluated the efficacy of Veliparib in 16 advanced PDAC patients with known germline mutations of BRCA1/2 or PALB2 who had undergone 1-2 previous lines of treatment, finding no objective responses[70]. Authors suggested potential differences between olaparib and veliparib as a potential explanation for the difference in response rates between the two trials. Furthermore, the high rates of pre-treatment with PtCh (88% of study population) coupled with a high disease progression rate (64% of those on PtCh) may indicate a high-level of platinum-resistance in this study population, which may in turn lead to PARP inhibitor resistance[70]. This is a plausible explanation given the known association between platinum-sensitivity and PARP inhibitor sensitivity seen in ovarian cancer. Due to the tendency of cancers to develop resistance to PARP inhibitors, another approach that has been tried is combination regimens involving chemotherapy and PARP inhibitors. A recent phase II trial compared a combination regimen of gemcitabine plus cisplatin with or without veliparib as first line therapy for advanced PDAC patients with germline mutations of BRCA1/2 or PALB2[56]. Veliparib did not improve response rates over gemcitabine plus cisplatin alone (74.1% vs 65.2%, P = 0.55), however as discussed earlier, the response rates in both arms both exceeded pre-study thresholds of efficacy and therefore, the high response rate to gemcitabine plus cisplatin may have obscured any signal of benefit from veliparib.
With the relative success of combination chemotherapy regimens in PDAC (FOLFIRINOX, Gemcitabine-Abraxane), focus has been placed on the development of maintenance therapies which can prolong PFS and improve quality of life (QOL) in responders. Most recently, data from the Pancreas Cancer Olaparib Ongoing (POLO) trial has supported the use of PARP inhibitors as a maintenance therapy in this patient population following response to platinum-chemotherapy[72]. The POLO trial was an international phase III, double-blind, placebo-controlled randomized clinical trial investigating oral olaparib maintenance therapy in metastatic PDAC patients with germline BRCA1/2 mutations who had not progressed during first-line PtCh (minimum of 16 wk of chemotherapy). Patients were randomized to either olaparib or placebo maintenance therapy. PFS was significantly longer in the olaparib group (7.4 vs 3.8 mo). At the time of publication, data on OS was not yet mature but preliminary results indicated no significant difference in OS between the two groups (18.9 vs 18.1 mo)[72]. 18 patients (20%) in the olaparib and 6 patients (11%) in the placebo group achieved a tumor response, and the median duration of responses were 24.9 mo and 3.7 mo, respectively. Other evidence for maintenance therapy comes from the phase II study by O’Reilly et al[56] who reported exploratory analyses for 10 patients with germline BRCA or PALB2 mutations who underwent at least 4 mo of PtCh without progression and subsequently were switched to a PARP inhibitor as maintenance therapy, finding a median PFS of 23.4 mo in this subset of patients.
In the context of maintenance therapy, preservation of quality of life and minimization of adverse effects are important goals of treatment. In the POLO trial, Grade ≥ 3 adverse events occurred in 40% of the olaparib group and 23% of the placebo group[43]. The most frequently reported adverse events in the treatment group were fatigue or anesthesia, nausea and anemia, with the majority of these cases being low grade. Only 15% and 5% of patients on olaparib underwent dose reductions or discontinued treatment because of adverse events, respectively. More recently, secondary outcomes of health-related QOL were reported, showing that olaparib treatment did not lead to a reduction in quality of life scores, a concern in the context of maintenance therapy meant to preserve functioning and QOL[73].
In light of these findings, the FDA has approved olaparib for maintenance therapy in patients with metastatic PDAC patients with germline mutations of BRCA1/2 who have not progressed on at least 16 wk of first-line PtCh. This approval is not without controversy as there are several criticisms of the POLO trial and unanswered questions in regards to this therapy[74]. For example, the lack of improvement in OS puts the validity of the finding of improved PFS into question[74]. However, this may be because of the high rates of therapy in the placebo group following disease progression, including 15% of the patients who received a PARP inhibitor. In addition, it should be stated that the OS results were from an interim analysis with only 46% data maturity. Furthermore, concern has been raised that the discontinuation of PtCh after 16 wk in patients who were responding is incongruent with clinical practice guidelines for first-line platinum chemotherapy[74]. However, in the POLO trial, the majority of patients received FOLFIRINOX (> 80%) with a median duration of first line PtCh of 5 mo and 33% of patients receiving > 6 mo prior to randomization[72]. In addition, the PRODIGE 4/ACCORD 11 trial recommended a total of 6 mo of palliative chemotherapy[10], therefore, the duration of therapy of 1st line PtCh may not be out of keeping with other clinical trials in this setting of disease. Furthermore, use of placebo alone in the control group has come under criticism as evidence has emerged in favour of the continuation of 5-FU as maintenance therapy in patients who respond to FOLFIRINOX[75]. That being said, the accumulating side effects of > 4 mo of FOLFIRINOX may justify a treatment break, especially if there is no evidence of progression on imaging. Lastly, POLO only included patients with germline mutations of BRCA1/BRCA2, therefore it remains unclear if there is a broader population of PDAC patients who would benefit from olaparib as well, such as patients with germline mutations to other components of the HR system (PALB2, ATM) or patients with other positive biomarkers of HRD.
Immunotherapies
While immunotherapies such as checkpoint inhibitors (anti-PD1/PDL1 and CTLA-4) have revolutionized the management of many cancers, they have had limited efficacy in PDAC. The genomic instability and increased total mutational load of BRCA-mutated and other HRD tumors results in neoantigens which may increase efficacy of immunotherapy in these tumors[11]. Recent translational studies have showed that specifically BRCA2-mutated tumors show increased sensitivity to immune checkpoint blockade as a result of their effect on the tumor immune microenvironment[76]. This is in line with previous findings of associations between BRCA mutations and PD-L1 expression in PDAC, a predictive marker for immunotherapy[77,78].
An emerging strategy for BRCA-mutated cancers is combination therapy with immune check point inhibitors and PARP inhibitors[79]. Given that treatment with PARP inhibitors also increases expression of PD-L1 and total mutational burden (potential biomarkers of response), combining these two therapies may act synergistically against HRD tumors[79]. In BRCA-mutated ovarian and breast cancers, several clinical trials are currently exploring the clinical efficacy of PARP inhibitor/immune checkpoint blockade combination therapy with early trials showing promising results[80]. In the maintenance setting, the ATHENA trial is currently testing a combination therapy consisting of rucaparib with nivolumab as a therapy for ovarian cancer following response to PtCh (NCT03522246). In PDAC, there are several ongoing Phase II trials investigating combination regimens involving PARP inhibitors and immune checkpoint inhibitors (Table 3). The PARPVAX study is investigating combination therapy of niraparib + either ipilimumab or nivolumab as maintenance therapy following response to PtCh (NCT03404960). Another phase II study is investigating combination therapy regimens including olaparib plus durvalumab in PDAC with a primary outcomes of changes in genomic and immune markers (NCT03851614). Most recently, a study has been initiated comparing olaparib with and without pembrolizumab as maintenance therapy for BRCA1/BRCA2 mutated-PDAC patients who responded to first-line PtCh (NCT04548752). Given the recent evidence for PARP inhibitors in PDAC, the use of immune checkpoint blockade for PDAC remains an active field of research.
Table 3.
Ongoing phase II clinical trials investigating poly (ADP-ribose) polymerase inhibitor/Immune Checkpoint blockade combination therapy in pancreatic ductal adenocarcinoma
|
Study identifier
|
Patient population
|
Immunotherapy
|
PARP inhibitor
|
Phase and design
|
Estimated completion date
|
| NCT03404960 | Advanced PDAC patients who did not progress on PtCh | Nivolumab or Ipilimumab | Niraparib | Phase Ib/II trial evaluating effectiveness of olaparib with either nivolumab or ipilimumab | June 2021 |
| NCT03851614 | Advanced PDAC, leiomyosarcoma or mismatch repair-proficient colorectal cancer | Durvalumab | Olaparib | Phase II trial evaluating impact of combination therapy on genomic and immune biomarkers | March 2022 |
| NCT04493060 | Metastatic PDAC with mutations of BRCA1/2 or PALB2, previously treated with 1-2 lines of chemotherapy including a PtCh agent | Dostarlimab | Niraparib | Phase II, evaluating the disease control rate at 12 weeks (DCR12) with combination therapy | December 2022 |
| NCT04548752 | Metastatic PDAC with germline BRCA1 or BRCA2 mutation treated with first-line PtCh | Pembrolizumab | Olaparib | Phase II trial comparing combination therapy to olaparib alone as maintenance therapy | March 2025 |
PDAC: Pancreatic adenocarcinoma; PtCh: Platinum chemotherapy; BRCA: Breast cancer susceptibility gene.
BIOMARKERS OF HRD
In the context of both PtCh and PARP inhibitors, the development of biomarkers for HRD will be an important step in implementing these therapies broadly in clinical practice. While most research to date has focused on germline mutations of BRCA1/2 and PALB2, combined these represent less than 10% of all PDAC cases. While this is an important mechanism of HRD, HRD can also arise through somatic mutations or epigenetic modification of DDR genes potentially resulting in sensitivity to PtCh and PARP inhibitors. Therefore, relying solely on germline mutations of these three genes for treatment selection will likely miss patients who would otherwise benefit from targeted therapy. For example, in advanced pancreatic cancer, tumor-level mutations to HRR genes such as BRCA1/2, ATM, PALB2, RAD51 were highly predictive of response to PtCh[35]. Recently a meta-analysis compared outcomes (ORR, survival) in PARP inhibitor trials and found that similar outcomes between patients with germline and patients with somatic BRCA mutations[81]. Interestingly, out of 99 studies of PARP inhibitors screened, only 18 included patients with somatic mutations, indicating that this is an understudied area of research[81]. Specifically in PDAC, only two studies investigated PARP inhibitors in patients with somatic BRCA mutations and both reported a non-significant increase in response rate in patients with somatic mutations, relative to germline[81]. No trials to date have evaluated the efficacy of maintenance olaparib, the only FDA-approved PARP inhibitor indication in PDAC in patients with somatic HR mutations. Two active trials of olaparib in PDAC are including patients with BRCA-associated family history or somatic HRD mutations, but explicitly excluding patients with germline BRCA mutations (NCT02677038, NCT02511223). However, these trials are not using olaparib in the maintenance setting. Given the efficacy of PARP inhibitors and PtCh in somatic BRCA-mutated ovarian cancer[63,82] this is an important area for future investigation in PDAC.
In addition to mutations of BRCA and other HR-related genes, genomic signatures of HRD have emerged as a promising biomarker of the HRD phenotype and subsequent treatment response[11]. These biomarkers will allow the identification sub-populations of PDAC patients who would benefit from PtCh or PARP inhibitors, and therefore expand the scope of use for these agents in PDAC. Multiple commercial assays now exist which can assess tumor tissues and assign an HRD score[62]. Examples of these assays include MyChoice CDx Assay (Myriad Genetics) and the FoundationOne CDx (Foundation Medicine) which are both FDA-approved for the evaluation of HRD. These tests combine loss-of heterozygosity scores with other markers of genomic instability (telomeric-allelic imbalance, large-scale transition) in order to quantify HRD and identify patients who would benefit from HRD-targeting therapies. These assays have been used in several clinical trials in breast and ovarian cancer and have been validated as useful biomarkers for response to PARP inhibitors[64,83,84]. Confirmation of HRD by assay is now an FDA-approved biomarker for the use of several treatment regimens including combined olaparib with bevacizumab for ovarian cancer. Furthermore, olaparib was recently approved for metastatic prostate cancer in patients with BRCA mutations or HRD. Investigating these biomarkers in PDAC will aid in identifying BRCA-wildtype patients who may benefit from PARP inhibitors and PtCh, an important prospect considering the poor prognosis in advanced PC.
CONCLUSION
The field of HRD in PDAC is in its infancy relative to ovarian and breast cancers, however promising advances have been made in recent years. Currently, the available data from retrospective studies suggests that first-line PtCh is preferred however the PtCh regimen is yet to be defined. Olaparib maintenance therapy is a standard of care option in patients with BRCA1/2 mutations and offers the benefit of ongoing anti-cancer therapy without traditional cytotoxic therapy toxicities. Important next steps include investigating these PtCh regimens and PARP inhibitors in the neoadjuvant setting, and determining if patients with somatic HR mutations or HRD as detected by genomic assays will also benefit from these treatments.
Footnotes
Conflict-of-interest statement: Michael N Rosen and Michael M Vickers declare no conflict of interest for this topic. Rachel A Goodwin has received compensation for an advisory role with AstraZeneca.
Manuscript source: Invited manuscript
Peer-review started: January 25, 2021
First decision: February 27, 2021
Article in press: April 13, 2021
Specialty type: Oncology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Bree E S-Editor: Zhang L L-Editor: A P-Editor: Ma YJ
Contributor Information
Michael N Rosen, Faculty of Medicine, The University of Ottawa, Ottawa K1H 8L6, Ontario, Canada.
Rachel A Goodwin, Faculty of Medicine, The University of Ottawa, Ottawa K1H 8L6, Ontario, Canada.
Michael M Vickers, The Ottawa Hospital Cancer Center, The University of Ottawa, Ottawa K1H 8L6, Ontario, Canada. mvickers@toh.ca.
References
- 1.Canadian Cancer Society. Canadian Cancer Statistics 2019 [cited 1 March 2021]. Available from: https://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2019-EN.pdf?la=en .
- 2.American Cancer Society. Cancer Facts and Figures 2020 [cited 1 March 2021]. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf .
- 3.Saad AM, Turk T, Al-Husseini MJ, Abdel-Rahman O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer. 2018;18:688. doi: 10.1186/s12885-018-4610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 5.Petersen GM. Familial pancreatic cancer. Semin Oncol. 2016;43:548–553. doi: 10.1053/j.seminoncol.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant RC, Selander I, Connor AA, Selvarajah S, Borgida A, Briollais L, Petersen GM, Lerner-Ellis J, Holter S, Gallinger S. Prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Gastroenterology. 2015;148:556–564. doi: 10.1053/j.gastro.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, Griffin C, Cameron JL, Yeo CJ, Kern S, Hruban RH. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–2638. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 8.Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 9.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX vs gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 11.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MC, Robertson AJ, Fadlullah MZ, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Wilson PJ, Markham E, Cloonan N, Anderson MJ, Fink JL, Holmes O, Kazakoff SH, Leonard C, Newell F, Poudel B, Song S, Taylor D, Waddell N, Wood S, Xu Q, Wu J, Pinese M, Cowley MJ, Lee HC, Jones MD, Nagrial AM, Humphris J, Chantrill LA, Chin V, Steinmann AM, Mawson A, Humphrey ES, Colvin EK, Chou A, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Pettitt JA, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Jamieson NB, Graham JS, Niclou SP, Bjerkvig R, Grützmann R, Aust D, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Falconi M, Zamboni G, Tortora G, Tempero MA Australian Pancreatic Cancer Genome Initiative. Gill AJ, Eshleman JR, Pilarsky C, Scarpa A, Musgrove EA, Pearson JV, Biankin AV, Grimmond SM. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall BR, Cannon A, Atri P, Wichman CS, Smith LM, Ganti AK, Are C, Sasson AR, Kumar S, Batra SK. Advanced pancreatic cancer: a meta-analysis of clinical trials over thirty years. Oncotarget. 2018;9:19396–19405. doi: 10.18632/oncotarget.25036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 14.Thompson D, Easton DF Breast Cancer Linkage Consortium. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 15.Chen CC, Feng W, Lim PX, Kass EM, Jasin M. Homology-Directed Repair and the Role of BRCA1, BRCA2, and Related Proteins in Genome Integrity and Cancer. Annu Rev Cancer Biol. 2018;2:313–336. doi: 10.1146/annurev-cancerbio-030617-050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prakash R, Zhang Y, Feng W, Jasin M. Homologous recombination and human health: the roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb Perspect Biol. 2015;7:a016600. doi: 10.1101/cshperspect.a016600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu C, Hart SN, Polley EC, Gnanaolivu R, Shimelis H, Lee KY, Lilyquist J, Na J, Moore R, Antwi SO, Bamlet WR, Chaffee KG, DiCarlo J, Wu Z, Samara R, Kasi PM, McWilliams RR, Petersen GM, Couch FJ. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA. 2018;319:2401–2409. doi: 10.1001/jama.2018.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 19.Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C, Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 20.Paluch-Shimon S, Cardoso F, Sessa C, Balmana J, Cardoso MJ, Gilbert F, Senkus E ESMO Guidelines Committee. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27:v103–v110. doi: 10.1093/annonc/mdw327. [DOI] [PubMed] [Google Scholar]
- 21.Roa BB, Boyd AA, Volcik K, Richards CS. Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat Genet. 1996;14:185–187. doi: 10.1038/ng1096-185. [DOI] [PubMed] [Google Scholar]
- 22.Janavičius R. Founder BRCA1/2 mutations in the Europe: implications for hereditary breast-ovarian cancer prevention and control. EPMA J. 2010;1:397–412. doi: 10.1007/s13167-010-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holter S, Borgida A, Dodd A, Grant R, Semotiuk K, Hedley D, Dhani N, Narod S, Akbari M, Moore M, Gallinger S. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients With Pancreatic Adenocarcinoma. J Clin Oncol. 2015;33:3124–3129. doi: 10.1200/JCO.2014.59.7401. [DOI] [PubMed] [Google Scholar]
- 24.Brand R, Borazanci E, Speare V, Dudley B, Karloski E, Peters MLB, Stobie L, Bahary N, Zeh H, Zureikat A, Hogg M, Lee K, Tsung A, Rhee J, Ohr J, Sun W, Lee J, Moser AJ, DeLeonardis K, Krejdovsky J, Dalton E, LaDuca H, Dolinsky J, Colvin A, Lim C, Black MH, Tung N. Prospective study of germline genetic testing in incident cases of pancreatic adenocarcinoma. Cancer. 2018;124:3520–3527. doi: 10.1002/cncr.31628. [DOI] [PubMed] [Google Scholar]
- 25.Mizukami K, Iwasaki Y, Kawakami E, Hirata M, Kamatani Y, Matsuda K, Endo M, Sugano K, Yoshida T, Murakami Y, Nakagawa H, Spurdle AB, Momozawa Y. Genetic characterization of pancreatic cancer patients and prediction of carrier status of germline pathogenic variants in cancer-predisposing genes. EBioMedicine. 2020;60:103033. doi: 10.1016/j.ebiom.2020.103033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowery MA, Wong W, Jordan EJ, Lee JW, Kemel Y, Vijai J, Mandelker D, Zehir A, Capanu M, Salo-Mullen E, Arnold AG, Yu KH, Varghese AM, Kelsen DP, Brenner R, Kaufmann E, Ravichandran V, Mukherjee S, Berger MF, Hyman DM, Klimstra DS, Abou-Alfa GK, Tjan C, Covington C, Maynard H, Allen PJ, Askan G, Leach SD, Iacobuzio-Donahue CA, Robson ME, Offit K, Stadler ZK, O'Reilly EM. Prospective Evaluation of Germline Alterations in Patients With Exocrine Pancreatic Neoplasms. J Natl Cancer Inst. 2018;110:1067–1074. doi: 10.1093/jnci/djy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas AL, Shakya R, Lipsyc MD, Mitchel EB, Kumar S, Hwang C, Deng L, Devoe C, Chabot JA, Szabolcs M, Ludwig T, Chung WK, Frucht H. High prevalence of BRCA1 and BRCA2 germline mutations with loss of heterozygosity in a series of resected pancreatic adenocarcinoma and other neoplastic lesions. Clin Cancer Res. 2013;19:3396–3403. doi: 10.1158/1078-0432.CCR-12-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrone CR, Levine DA, Tang LH, Allen PJ, Jarnagin W, Brennan MF, Offit K, Robson ME. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol. 2009;27:433–438. doi: 10.1200/JCO.2008.18.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy KM, Brune KA, Griffin C, Sollenberger JE, Petersen GM, Bansal R, Hruban RH, Kern SE. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Res. 2002;62:3789–3793. [PubMed] [Google Scholar]
- 30.Hahn SA, Greenhalf B, Ellis I, Sina-Frey M, Rieder H, Korte B, Gerdes B, Kress R, Ziegler A, Raeburn JA, Campra D, Grützmann R, Rehder H, Rothmund M, Schmiegel W, Neoptolemos JP, Bartsch DK. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214–221. doi: 10.1093/jnci/95.3.214. [DOI] [PubMed] [Google Scholar]
- 31.Zhen DB, Rabe KG, Gallinger S, Syngal S, Schwartz AG, Goggins MG, Hruban RH, Cote ML, McWilliams RR, Roberts NJ, Cannon-Albright LA, Li D, Moyes K, Wenstrup RJ, Hartman AR, Seminara D, Klein AP, Petersen GM. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med. 2015;17:569–577. doi: 10.1038/gim.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Couch FJ, Johnson MR, Rabe KG, Brune K, de Andrade M, Goggins M, Rothenmund H, Gallinger S, Klein A, Petersen GM, Hruban RH. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:342–346. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- 33.Møller P, Hagen AI, Apold J, Maehle L, Clark N, Fiane B, Løvslett K, Hovig E, Vabø A. Genetic epidemiology of BRCA mutations--family history detects less than 50% of the mutation carriers. Eur J Cancer. 2007;43:1713–1717. doi: 10.1016/j.ejca.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 34.Shindo K, Yu J, Suenaga M, Fesharakizadeh S, Cho C, Macgregor-Das A, Siddiqui A, Witmer PD, Tamura K, Song TJ, Navarro Almario JA, Brant A, Borges M, Ford M, Barkley T, He J, Weiss MJ, Wolfgang CL, Roberts NJ, Hruban RH, Klein AP, Goggins M. Deleterious Germline Mutations in Patients With Apparently Sporadic Pancreatic Adenocarcinoma. J Clin Oncol. 2017;35:3382–3390. doi: 10.1200/JCO.2017.72.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondo T, Kanai M, Kou T, Sakuma T, Mochizuki H, Kamada M, Nakatsui M, Uza N, Kodama Y, Masui T, Takaori K, Matsumoto S, Miyake H, Okuno Y, Muto M. Association between homologous recombination repair gene mutations and response to oxaliplatin in pancreatic cancer. Oncotarget. 2018;9:19817–19825. doi: 10.18632/oncotarget.24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabai-Kapara E, Lahad A, Kaufman B, Friedman E, Segev S, Renbaum P, Beeri R, Gal M, Grinshpun-Cohen J, Djemal K, Mandell JB, Lee MK, Beller U, Catane R, King MC, Levy-Lahad E. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci USA. 2014;111:14205–14210. doi: 10.1073/pnas.1415979111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manchanda R, Loggenberg K, Sanderson S, Burnell M, Wardle J, Gessler S, Side L, Balogun N, Desai R, Kumar A, Dorkins H, Wallis Y, Chapman C, Taylor R, Jacobs C, Tomlinson I, McGuire A, Beller U, Menon U, Jacobs I. Population testing for cancer predisposing BRCA1/BRCA2 mutations in the Ashkenazi-Jewish community: a randomized controlled trial. J Natl Cancer Inst. 2015;107:379. doi: 10.1093/jnci/dju379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metcalfe KA, Poll A, Royer R, Llacuachaqui M, Tulman A, Sun P, Narod SA. Screening for founder mutations in BRCA1 and BRCA2 in unselected Jewish women. J Clin Oncol. 2010;28:387–391. doi: 10.1200/JCO.2009.25.0712. [DOI] [PubMed] [Google Scholar]
- 39.Tennen RI, Laskey SB, Koelsch BL, McIntyre MH, Tung JY. Identifying Ashkenazi Jewish BRCA1/2 founder variants in individuals who do not self-report Jewish ancestry. Sci Rep. 2020;10:7669. doi: 10.1038/s41598-020-63466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandelker D, Zhang L, Kemel Y, Stadler ZK, Joseph V, Zehir A, Pradhan N, Arnold A, Walsh MF, Li Y, Balakrishnan AR, Syed A, Prasad M, Nafa K, Carlo MI, Cadoo KA, Sheehan M, Fleischut MH, Salo-Mullen E, Trottier M, Lipkin SM, Lincoln A, Mukherjee S, Ravichandran V, Cambria R, Galle J, Abida W, Arcila ME, Benayed R, Shah R, Yu K, Bajorin DF, Coleman JA, Leach SD, Lowery MA, Garcia-Aguilar J, Kantoff PW, Sawyers CL, Dickler MN, Saltz L, Motzer RJ, O'Reilly EM, Scher HI, Baselga J, Klimstra DS, Solit DB, Hyman DM, Berger MF, Ladanyi M, Robson ME, Offit K. Mutation Detection in Patients With Advanced Cancer by Universal Sequencing of Cancer-Related Genes in Tumor and Normal DNA vs Guideline-Based Germline Testing. JAMA. 2017;318:825–835. doi: 10.1001/jama.2017.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daly MB, Pilarski R, Yurgelun MB, Berry MP, Buys SS, Dickson P, Domchek SM, Elkhanany A, Friedman S, Garber JE, Goggins M, Hutton ML, Khan S, Klein C, Kohlmann W, Kurian AW, Laronga C, Litton JK, Mak JS, Menendez CS, Merajver SD, Norquist BS, Offit K, Pal T, Pederson HJ, Reiser G, Shannon KM, Visvanathan K, Weitzel JN, Wick MJ, Wisinski KB, Dwyer MA, Darlow SD. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J Natl Compr Canc Netw. 2020;18:380–391. doi: 10.6004/jnccn.2020.0017. [DOI] [PubMed] [Google Scholar]
- 42.Smith AL, Wong C, Cuggia A, Borgida A, Holter S, Hall A, Connor AA, Bascuñana C, Asselah J, Bouganim N, Poulin V, Jolivet J, Vafiadis P, Le P, Martel G, Lemay F, Beaudoin A, Rafatzand K, Chaudhury P, Barkun J, Metrakos P, Marcus V, Omeroglu A, Chong G, Akbari MR, Foulkes WD, Gallinger S, Zogopoulos G. Reflex Testing for Germline BRCA1 , BRCA2 , PALB2 , and ATM Mutations in Pancreatic Cancer: Mutation Prevalence and Clinical Outcomes From Two Canadian Research Registries. JCO Precis Oncol. 2018;2:1–16. doi: 10.1200/PO.17.00098. [DOI] [PubMed] [Google Scholar]
- 43.Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S, Aderka D, Paluch-Shimon S, Kaufman B, Gershoni-Baruch R, Hedley D, Moore MJ, Friedman E, Gallinger S. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 2014;111:1132–1138. doi: 10.1038/bjc.2014.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blair AB, Groot VP, Gemenetzis G, Wei J, Cameron JL, Weiss MJ, Goggins M, Wolfgang CL, Yu J, He J. BRCA1/BRCA2 Germline Mutation Carriers and Sporadic Pancreatic Ductal Adenocarcinoma. J Am Coll Surg 2018; 226: 630-637. :e1. doi: 10.1016/j.jamcollsurg.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golan T, Sella T, O'Reilly EM, Katz MH, Epelbaum R, Kelsen DP, Borgida A, Maynard H, Kindler H, Friedmen E, Javle M, Gallinger S. Overall survival and clinical characteristics of BRCA mutation carriers with stage I/II pancreatic cancer. Br J Cancer. 2017;116:697–702. doi: 10.1038/bjc.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pishvaian MJ, Blais EM, Brody JR, Lyons E, DeArbeloa P, Hendifar A, Mikhail S, Chung V, Sahai V, Sohal DPS, Bellakbira S, Thach D, Rahib L, Madhavan S, Matrisian LM, Petricoin EF 3rd. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020;21:508–518. doi: 10.1016/S1470-2045(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perri G, Prakash L, Qiao W, Varadhachary GR, Wolff R, Fogelman D, Overman M, Pant S, Javle M, Koay EJ, Herman J, Kim M, Ikoma N, Tzeng CW, Lee JE, Katz MHG. Response and Survival Associated With First-line FOLFIRINOX vs Gemcitabine and nab-Paclitaxel Chemotherapy for Localized Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2020;155:832–839. doi: 10.1001/jamasurg.2020.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williet N, Saint A, Pointet AL, Tougeron D, Pernot S, Pozet A, Bechade D, Trouilloud I, Lourenco N, Hautefeuille V, Locher C, Desrame J, Artru P, Thirot Bidault A, Le Roy B, Pezet D, Phelip JM, Taieb J. Folfirinox versus gemcitabine/nab-paclitaxel as first-line therapy in patients with metastatic pancreatic cancer: a comparative propensity score study. Therap Adv Gastroenterol. 2019;12:1756284819878660. doi: 10.1177/1756284819878660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 50.Mylavarapu S, Das A, Roy M. Role of BRCA Mutations in the Modulation of Response to Platinum Therapy. Front Oncol. 2018;8:16. doi: 10.3389/fonc.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vyas O, Leung K, Ledbetter L, Kaley K, Rodriguez T, Garcon MC, Saif MW. Clinical outcomes in pancreatic adenocarcinoma associated with BRCA-2 mutation. Anticancer Drugs. 2015;26:224–226. doi: 10.1097/CAD.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 52.Reiss KA, Yu S, Judy R, Symecko H, Nathanson KL, Domchek SM. Retrospective Survival Analysis of Patients With Advanced Pancreatic Ductal Adenocarcinoma and Germline BRCA or PALB2 Mutations. JCO Precis Oncol . 2018;2:1–9. doi: 10.1200/PO.17.00152. [DOI] [PubMed] [Google Scholar]
- 53.Wattenberg MM, Asch D, Yu S, O'Dwyer PJ, Domchek SM, Nathanson KL, Rosen MA, Beatty GL, Siegelman ES, Reiss KA. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br J Cancer. 2020;122:333–339. doi: 10.1038/s41416-019-0582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu S, Agarwal P, Mamtani R, Symecko H, Spielman K, O’Hara M, O’Dwyer PJ, Schneider C, Teitelbaum U, Nathanson KL, Domchek SM, Reiss KA. Retrospective Survival Analysis of Patients With Resected Pancreatic Ductal Adenocarcinoma and a Germline BRCA or PALB2 Mutation. JCO Precis Oncol . 2019;2:1–11. doi: 10.1200/PO.18.00271. [DOI] [PubMed] [Google Scholar]
- 55.Rebelatto TF, Falavigna M, Pozzari M, Spada F, Cella CA, Laffi A, Pellicori S, Fazio N. Should platinum-based chemotherapy be preferred for germline BReast CAncer genes (BRCA) 1 and 2-mutated pancreatic ductal adenocarcinoma (PDAC) patients? Cancer Treat Rev. 2019;80:101895. doi: 10.1016/j.ctrv.2019.101895. [DOI] [PubMed] [Google Scholar]
- 56.O'Reilly EM, Lee JW, Zalupski M, Capanu M, Park J, Golan T, Tahover E, Lowery MA, Chou JF, Sahai V, Brenner R, Kindler HL, Yu KH, Zervoudakis A, Vemuri S, Stadler ZK, Do RKG, Dhani N, Chen AP, Kelsen DP. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin With or Without Veliparib in Patients With Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J Clin Oncol. 2020;38:1378–1388. doi: 10.1200/JCO.19.02931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 58.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 59.Keung MYT, Wu Y, Vadgama JV. PARP Inhibitors as a Therapeutic Agent for Homologous Recombination Deficiency in Breast Cancers. J Clin Med. 2019;8 doi: 10.3390/jcm8040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011;5:387–393. doi: 10.1016/j.molonc.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 62.Mirza MR, Coleman RL, González-Martín A, Moore KN, Colombo N, Ray-Coquard I, Pignata S. The forefront of ovarian cancer therapy: update on PARP inhibitors. Ann Oncol. 2020;31:1148–1159. doi: 10.1016/j.annonc.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, Ben-Baruch NE, Marth C, Mądry R, Christensen RD, Berek JS, Dørum A, Tinker AV, du Bois A, González-Martín A, Follana P, Benigno B, Rosenberg P, Gilbert L, Rimel BJ, Buscema J, Balser JP, Agarwal S, Matulonis UA ENGOT-OV16/NOVA Investigators. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 64.Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp A, Scambia G, Leary A, Holloway RW, Gancedo MA, Fong PC, Goh JC, O'Malley DM, Armstrong DK, Garcia-Donas J, Swisher EM, Floquet A, Konecny GE, McNeish IA, Scott CL, Cameron T, Maloney L, Isaacson J, Goble S, Grace C, Harding TC, Raponi M, Sun J, Lin KK, Giordano H, Ledermann JA ARIEL3 investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, Korach J, Huzarski T, Poveda A, Pignata S, Friedlander M, Colombo N, Harter P, Fujiwara K, Ray-Coquard I, Banerjee S, Liu J, Lowe ES, Bloomfield R, Pautier P SOLO2/ENGOT-Ov21 investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 66.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, Wu W, Goessl C, Runswick S, Conte P. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 67.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee KH, Fehrenbacher L, Yerushalmi R, Mina LA, Martin M, Roché H, Im YH, Quek RGW, Markova D, Tudor IC, Hannah AL, Eiermann W, Blum JL. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robson ME, Tung N, Conte P, Im SA, Senkus E, Xu B, Masuda N, Delaloge S, Li W, Armstrong A, Wu W, Goessl C, Runswick S, Domchek SM. OlympiAD final overall survival and tolerability results: Olaparib vs chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30:558–566. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Mehra N, Goessl C, Kang J, Burgents J, Wu W, Kohlmann A, Adelman CA, Hussain M. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382:2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 70.Lowery MA, Kelsen DP, Capanu M, Smith SC, Lee JW, Stadler ZK, Moore MJ, Kindler HL, Golan T, Segal A, Maynard H, Hollywood E, Moynahan M, Salo-Mullen EE, Do RKG, Chen AP, Yu KH, Tang LH, O'Reilly EM. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur J Cancer. 2018;89:19–26. doi: 10.1016/j.ejca.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, Mitchell G, Fried G, Stemmer SM, Hubert A, Rosengarten O, Steiner M, Loman N, Bowen K, Fielding A, Domchek SM. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hammel P, Kindler HL, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Joo S, Yoo HK, Patel N, Golan T POLO Investigators. Health-related quality of life in patients with a germline BRCA mutation and metastatic pancreatic cancer receiving maintenance olaparib. Ann Oncol. 2019;30:1959–1968. doi: 10.1093/annonc/mdz406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishikawa G, Booth C, Prasad V. Olaparib for BRCA mutant pancreas cancer: Should the POLO trial change clinical practice? Cancer. 2020;126:4087–4088. doi: 10.1002/cncr.32979. [DOI] [PubMed] [Google Scholar]
- 75.Hammel P, Vitellius C, Boisteau É, Wisniewski M, Colle E, Hilmi M, Dengremont C, Granier S, Turpin A, de Mestier L, Neuzillet C. Maintenance therapies in metastatic pancreatic cancer: present and future with a focus on PARP inhibitors. Ther Adv Med Oncol. 2020;12:1758835920937949. doi: 10.1177/1758835920937949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Samstein RM, Krishna C, Ma X, Pei X, Lee KW, Makarov V, Kuo F, Chung J, Srivastava RM, Purohit TA, Hoen DR, Mandal R, Setton J, Wu W, Shah R, Qeriqi B, Chang Q, Kendall S, Braunstein L, Weigelt B, Blecua Carrillo Albornoz P, Morris LGT, Mandelker DL, Reis-Filho JS, de Stanchina E, Powell SN, Chan TA, Riaz N. Mutations in BRCA1 and BRCA2 differentially affect the tumor microenvironment and response to checkpoint blockade immunotherapy. Nat Cancer . 2020;1:1188–1203. doi: 10.1038/s43018-020-00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sato H, Niimi A, Yasuhara T, Permata TBM, Hagiwara Y, Isono M, Nuryadi E, Sekine R, Oike T, Kakoti S, Yoshimoto Y, Held KD, Suzuki Y, Kono K, Miyagawa K, Nakano T, Shibata A. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. 2017;8:1751. doi: 10.1038/s41467-017-01883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seeber A, Zimmer K, Kocher F, Puccini A, Xiu J, Nabhan C, Elliott A, Goldberg RM, Grothey A, Shields AF, Battaglin F, El-Deiry WS, Philip PA, Marshall JL, Hall M, Korn WM, Lenz HJ, Wolf D, Feistritzer C, Spizzo G. Molecular characteristics of BRCA1/2 and PALB2 mutations in pancreatic ductal adenocarcinoma. ESMO Open. 2020;5:e000942. doi: 10.1136/esmoopen-2020-000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peyraud F, Italiano A. Combined PARP Inhibition and Immune Checkpoint Therapy in Solid Tumors. Cancers (Basel) 2020;12 doi: 10.3390/cancers12061502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vikas P, Borcherding N, Chennamadhavuni A, Garje R. Therapeutic Potential of Combining PARP Inhibitor and Immunotherapy in Solid Tumors. Front Oncol. 2020;10:570. doi: 10.3389/fonc.2020.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohyuddin GR, Aziz M, Britt A, Wade L, Sun W, Baranda J, Al-Rajabi R, Saeed A, Kasi A. Similar response rates and survival with PARP inhibitors for patients with solid tumors harboring somatic vs Germline BRCA mutations: a Meta-analysis and systematic review. BMC Cancer. 2020;20:507. doi: 10.1186/s12885-020-06948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, Thornton A, Norquist BM, Casadei S, Nord AS, Agnew KJ, Pritchard CC, Scroggins S, Garcia RL, King MC, Swisher EM. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoppe MM, Sundar R, Tan DSP, Jeyasekharan AD. Biomarkers for Homologous Recombination Deficiency in Cancer. J Natl Cancer Inst. 2018;110:704–713. doi: 10.1093/jnci/djy085. [DOI] [PubMed] [Google Scholar]
- 84.Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, Fujiwara K, Vergote I, Colombo N, Mäenpää J, Selle F, Sehouli J, Lorusso D, Guerra Alía EM, Reinthaller A, Nagao S, Lefeuvre-Plesse C, Canzler U, Scambia G, Lortholary A, Marmé F, Combe P, de Gregorio N, Rodrigues M, Buderath P, Dubot C, Burges A, You B, Pujade-Lauraine E, Harter P PAOLA-1 Investigators. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med. 2019;381:2416–2428. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]