Abstract
Due to their immunomodulatory potential and release of trophic factors that promote healing, mesenchymal stromal cells (MSCs) are considered important players in tissue homeostasis and regeneration. MSCs have been widely used in clinical trials to treat multiple conditions associated with inflammation and tissue damage. Recent evidence suggests that most of the MSC therapeutic effects are derived from their secretome, including the extracellular vesicles, representing a promising approach in regenerative medicine application to treat organ failure as a result of inflammation/fibrosis. The recent outbreak of respiratory syndrome coronavirus, caused by the newly identified agent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has forced scientists worldwide to use all available instruments to fight the infection, including the inflammatory cascade caused by this pandemic disease. The use of MSCs is a valid approach to combat organ inflammation in different compartments. In addition to the lungs, which are considered the main inflammatory target for this virus, other organs are compromised by the infection. In particular, the liver is involved in the inflammatory response to SARS-CoV-2 infection, which causes organ failure, leading to death in coronavirus disease 2019 (COVID-19) patients. We herein summarize the current implications derived from the use of MSCs and their soluble derivatives in COVID-19 treatment, and emphasize the potential of MSC-based therapy in this clinical setting.
Keywords: Mesenchymal stromal cell, COVID-19, SARS-CoV-2, Organ failure, Inflammation, Transplantation
Core Tip: The recent coronavirus disease 2019 (COVID-19) pandemic outbreak has forced scientists worldwide to use all available options to fight this disease, in particular the inflammatory cascade caused by this infection. Mesenchymal stromal cells, for their immunomodulatory potential, represent a valid approach to combat organ inflammation. The main targets for this virus are the lungs, while other organs such as the liver are compromised by the infection. Evaluation of the albumin role in COVID-19 patients, and the connection to the “capillary leak syndrome” have focused attention on liver dysfunction correlated with the infection.
INTRODUCTION
The liver can be damaged by various factors, including cytotoxic molecules, ischemia, metabolic alterations, or viral infections[1], which result in inflammatory responses contributing to further liver damage[2]. If the inflammation persists, a transition from acute to chronic injury can occur, inducing hepatic fibrosis[2]. Therefore, therapies that can reduce liver inflammation/fibrosis are crucial in order to avoid organ failure and the need for transplantation.
In recent years, the use of mesenchymal stromal cells (MSCs) has been considered a promising therapeutic approach to treat liver injuries[3]. MSCs can be isolated from different compartments including adipose tissue[4], umbilical cord[5], bone marrow[6], or placenta[7,8]. These cells have been successfully used in different therapeutic applications aimed at reducing inflammatory responses[9]. Moreover, the infusion of MSCs immediately after liver transplantation promotes organ regeneration and prolonged recipient survival by reducing acute inflammation[10].
Despite their beneficial properties, there are several limitations to the use of MSCs for cellular therapies; for example, their plasticity causes the potential risk of differentiation into undesired tissues and the possibility of malignant transformation is under debate[11,12]. To overcome these issues, the use of cell-free therapy is gaining considerable attention as a treatment for liver injury, an alternative to conventional cell transplantation[13]. Indeed, the regenerative properties of the MSC secretome include immunomodulatory effects mediated by growth factors and cytokines, such as transforming growth factor beta (TGF-β), prostaglandin E2, indoleamine 2,3-dioxygenase, hepatocyte growth factor (HGF), interleukin-10 (IL-10), and tumor necrosis factor alpha (TNF-α)[14,15], which can also attenuate fibrogenesis. In addition, the MSC therapeutic effects could also result from the released extracellular vesicles (EVs). EVs include a highly heterogeneous group of vesicles of different size able to modulate the immune responses[16,17]. Indeed, MSC-derived EVs can be selectively enriched with anti-fibrotic[18] and anti-apoptotic[19] factors, as well as specific non-coding RNA with therapeutic potential[20].
In December 2019, several cases of death from pneumonia were reported in Wuhan, later related to a new coronavirus-related disease called coronavirus disease 2019 (COVID-19). Analysis of its genome revealed it to be phylogenetically related to severe acute respiratory syndrome coronavirus (SARS-CoV)[21], and for this reason it was named SARS-CoV-2 by the World Health Organization (WHO). Due to its worldwide spread, the WHO declared COVID-19 a pandemic in March 2020. Angiotensin-converting enzyme 2 receptor (ACE2), highly expressed in the respiratory tract, was considered the main SARS-CoV-2 viral attachment for animal cells. Most likely for this reason, the lungs are the principal target organs for SARS-CoV-2[22,23]. This virus triggers an exacerbated immune reaction because large amounts of different inflammatory factors, including cytokines and chemokines, are produced by immune reactive cells.
It has been hypothesized that MSC-based therapy for COVID-19 patients can prevent the development of a cytokine storm by activating the immune system and promoting organ repair[24,25]. Intravenously injected MSCs reach the lungs, where they engraft and secrete a variety of soluble factors including anti-inflammatory factors, angiogenic factors, and EVs[26,27]. Studies aimed toward reversing COVID-19 side effects through MSC treatment are ongoing. In this review, we summarize the therapeutic potentials of the MSC secretome for treating liver injuries associated with COVID-19.
MSC SECRETOME AND EVS FOR ORGAN INJURY
The use of MSC-based therapy for regenerative medicine applications counts in the hundreds of registered clinical trials (www.clinicaltrial.gov) because of the ability of these cells to promote immunomodulation and organ regeneration[28]. The release of trophic factors has demonstrated that their action is in part attributable to their secretome and, in particular, to secreted EVs[29]. Because of their intrinsic therapeutic potential, EVs are a powerful tool of regenerative medicine for the treatment of a wide range of diseases[30]. Due to heterogeneity in size and contents, as well as lack of specific markers, distinguishing the various EV subtypes is an ongoing challenge. According to the International Society for Extracellular Vesicles (ISEV), the generic term EVs includes nano-sized particles naturally released into the extracellular space by all cell types; they are delimited by a lipid bilayer and cannot replicate[31]. The ISEV consensus suggests considering physical parameters (e.g., size or density) to distinguish “small” EVs, often referred as “exosomes” (< 100-200 nm in diameter) from “medium/large” EVs or “microvesicles” (> 200 nm). EVs are replete with diverse proteins, lipids, carbohydrates, and nucleic acids, and exert many of their functions of intercellular communicators by transferring their cargo molecules among cells. The specific cargo composition of EVs is largely defined by the tissue/cell type from which they originate[32]. Similarly to EVs from other cell types, MSC-EVs can be characterized according to the guidelines indicated by the ISEV. The available data suggest that EVs may significantly contribute to the paracrine effects of MSCs on tissue regeneration[33]. Because of EVs’ broad biological functions, as well as their ability to transfer molecules between cells, MSC-EV-based therapy represents an attractive alternative to cell-based therapy. Application of MSC-EVs as a cell-free therapy has several advantages over conventional cell therapy. Primarily, EV injection carries lower safety risks because of their minimal reactivity to the immune system, and seem to be generally well tolerated, even when used xenogenically[34]. Then, because of their small size compared to MSCs, the intravenous delivery of EVs presents lower risk of vascular obstructions. Finally, EVs can also be genetically manipulated to carry desired therapeutic cargo for a broad, expanding range of potential clinical applications. The number of studies demonstrating the therapeutic potential of MSC-EVs in different disease models is growing rapidly. The beneficial effects of MSC-EV-based treatment are evidenced especially in cardioprotection and angiogenesis[35].
Understanding the mechanisms of action behind the therapeutic effects of MSC-EVs are crucial in view of their future clinical applications. Despite increasing interest, this field is still in its infancy in identifying the relevant bioactive molecules released by MSC-EVs that play a role in tissue repair. Efforts to identify these molecules lead to the conclusion that MSC-EVs preferentially contain mRNAs and microRNAs (miRNAs) targeting genes that participate in several cellular pathways involved in tissue repair, such as angiogenesis, migration, proliferation, self-renewal, differentiation, cellular transport, and apoptosis[36,37]. The overexpression of certain miRNAs can contribute to enhancing the therapeutic efficacy of MSC-EVs. For example, MSC-EVs over-expressing miR-21 have neuroprotective effects by targeting several genes involved in the inhibition of cell apoptosis[38,39]. The list of miRNAs known to increase the therapeutic potential of MSC-EVs in numerous disease models is long, and their therapeutic effects range from tumor modulation, immune suppression, and angiogenesis to tissue regeneration[40].
In addition to miRNAs, the beneficial effect of EV-derived proteins has been explored in terms of tissue repair and anti-inflammatory effects as a treatment for liver fibrosis, ischemia, myocardial infarction, acute renal injury, neural regeneration, or in the context of bone and cartilage regeneration[40]. Proteins identified in MSC-EVs and linked to tissue repair include glial-derived neurotrophic factor, vascular endothelial growth factor, fibroblast growth factor, HGF, and angiotensin 1[41].
Although the number of clinical studies is limited, growing evidence shows the beneficial effects of MSC-EVs on tissue injuries. The impact of MSC-EVs on tissue regeneration has been investigated in several animal models of neuronal, cardiac, bone, cartilage, kidney, muscle, wound healing, respiratory injury, and liver regeneration[41,42]. Interestingly, data from animal models indicate that MSC-EVs can exert therapeutic potential similar to their cellular origin[41,43-46]. The list of registered clinical trials (https://clinicaltrials.gov) reporting tissue injuries-treated with MSC-EVs is shown in Table 1.
Table 1.
List of registered clinical trials on the use of mesenchymal stromal cell-derived extracellular vesicles for tissue injury
|
Tissue injury disease
|
Condition
|
Treatment
|
Trial ID
|
Status
|
| Chronic lung disease | Pediatric bronchopulmonary dysplasia | BM-MSC-derived EVs | NCT03857841 | Phase I |
| Lung disease | Pneumonia, COVID-19 | BM-MSC-derived EVs | NCT04493242 | Not yet recruiting |
| Lung disease | Pneumonia, COVID-19 | Inhalation of mesenchymal stem cell exosomes | NCT04276987 | Phase I |
| Multiple organ failure | Multiple organ dysfunction syndrome | MSC exosomes | NCT04356300 | Not yet recruiting |
| Lung disease | Pulmonary infection | MSC exosomes | NCT04544215 | Recruiting |
| Dry eye | GVHD | UC-MSC exosomes | NCT04213248 | Recruiting |
| Cartilage injury | Osteoarthritis | Secretome or EVs from adipose MSCs | NCT04223622 | Not yet recruiting |
| Skin disease | Dystrophic epidermolysis bullosa | BM-MSC EVs | NCT04173650 | Phase II |
| Brain | Cerebrovascular disorders | Allogenic MSCs enriched with miR-124 | NCT03384433 | Phase II |
BM: Bone marrow; COVID-19: Coronavirus disease 2019; EV: Extracellular vesicle; GVHD: Graft-vs-host disease; MSC: Mesenchymal stromal cell; UC: Umbilical cord.
MSC-EVs show great potential as a regenerative medicine treatment for liver diseases. The benefits of MSC-EVs in liver diseases are documented in animal models of both acute[20] and chronic[47] liver injuries. MSC-EVs exert a beneficial effect by alleviating fibrosis and improving regeneration of hepatocytes[46]. In particular, EVs from fetal MSCs promote hepatocyte proliferation and decrease hepatocyte apoptosis in liver injury induced by carbon tetrachloride[48], or ameliorate oxidative stress in ischemia reperfusion injury (IRI) models in rats[49] and mice[50]. Similarly, EVs of MSC-derived induced pluripotent stem cells have hepatoprotective effects on a rat model of IRI by inducing hepatocyte proliferation[51,52]. Finally, the anti-fibrotic effects of hydrogel-embedded MSC-EVs are documented in chronic liver failure[53]. The results from in vivo studies indicate EVs as essential contributors to MSC therapeutic efficacy, and suggest that MSC-EV-based therapy may be a successful alternative to cell-based treatments. Nevertheless, there are still many important questions to be answered before MSC-EVs can become a fully realized cell-free therapy. These challenges comprise studies establishing the exact contribution of EVs to MSC-based therapy, including the underlying molecule mechanisms, or identifying which EV population is the most therapeutically effective. In addition, a major ongoing debate in the field of MSC EV-based therapy concerns the purity of the obtained vesicles due to contamination of the samples with non-EV proteins, RNAs, and lipoproteins[41].
LIVER FAILURE IN COVID-19 PATIENTS
SARS-CoV-2 is the etiological agent of the pandemic COVID-19, characterized by respiratory distress and/or hypoxemia, fever, fatigue, dry cough and, in severe cases, septic shock, metabolic acidosis, and death[54]. SARS-CoV-2, as with other corona viruses, enters the host cells by binding to the ACE-2 receptor[55], while the serine protease transmembrane serine protease 2 is required for S protein priming[56]. Despite the higher tropism for the respiratory tract, SARS-CoV-2 also targets other tissues, given that the ACE2 receptor is widely distributed in other tissues[57-61]. To shed light on the SARS-CoV-2 tropism, Nardo et al[62] analyzed, on the Human Protein Atlas, the expression levels of two proteins, ACE-2 receptor and TMPRSS2, in different human tissues, thus revealing a higher expression in the intestine and gall bladder, but their absence in the liver. A single-cell analysis, performed on healthy human liver samples, showed that while ACE-2 expression level in cholangiocytes is comparable to that of alveolar cells in the lungs, it is barely detectable in hepatocytes[63]. Interestingly, the liver cell line HuH7 is an established permissive cell type for both SARS-CoV and SARS-CoV-2 infection, and has recently been extensively used as a model in SARS-CoV-2 studies[64,65]. In addition, an in vitro study found that SARS-CoV-2 infection leads to a decrease of cholangiocellular tight junction protein claudin 1 mRNA expression, implying a reduced barrier function of cholangiocytes[66]. The presence of SARS-CoV-2 receptors in the gastrointestinal (GI) tract suggests an important role of the hepatobiliary tract in viral replication and excretion[67]. In fact, the virus has also been isolated from stool samples[68]. The involvement of the GI tract in COVID-19 disease is confirmed by the GI symptoms occurring in more than 60% of infected patients, as la ack of appetite, loss of smell and taste, anorexia, diarrhea, abdominal pain, nausea, and vomiting[69-74]. Moreover, post-mortem biopsies of SARS-CoV-2-infected patients showed the presence of the viral genome in hepatocytes and the GI tract by reverse transcription polymerase chain reaction (RT-PCR)[75-77].
Though liver failure in COVID-19 patients has been considered marginal, the incidence of hepatic tissue injury in these patients ranges from 14.8% to 53%[78], while mortality ranges from 58.06% to 78%[79,80]. The liver is a key organ in nearly all metabolic processes, has immunologic functions, and is the main detoxifying organ. Moreover, because of the production of albumin, acute phase reactants and coagulation factors, the liver can strongly affect the multisystem manifestations of COVID-19[62]. In fact, modified levels of hepatic function indicators such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, gamma-glutamyl transferase, and bilirubin have been observed in patients with COVID-19, and principally in severe diseases[59,81-84]. Many studies have shown that liver injury occurs in the early stage of the disease, with mild or moderate increase of ALT, AST, or bilirubin together with a decrease in albumin levels[79,85,86]. High AST levels have been associated with the highest mortality risk[57,87], while decreased albumin levels have been associated with severe infection and poor prognosis[88,89]. Since the specific pathogenetic mechanism by which the virus causes liver injury is still unclear, many hypotheses have been offered, including immune-mediated damage. The triggering of an exacerbated immune response to the viral infection leads to a massive release of cytokines and inflammation mediators known as cytokine storm, which is responsible for immune-mediated liver damage[89] (Figure 1).
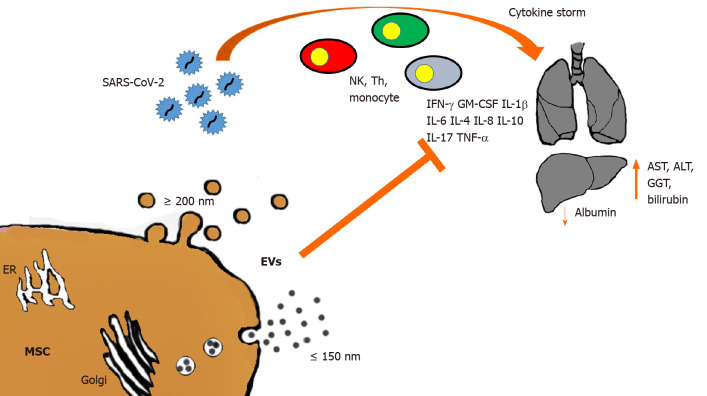
Figure 1.
Schematic representation of severe acute respiratory syndrome coronavirus 2 impact on lungs and liver. Cytokine storm with the cascade triggered by natural killer (NK) cells, T helper (Th) cell and monocytes, and the production of inflammatory cytokines (interleukin 1 beta [IL-1b], Il-2, IL-6, IL-8, IL-10, Il-17, interferons [IFNs], IFN-induced protein 10, tumor necrosis factor alpha, granulocyte-macrophage colony-stimulating factor [GM-CSF]). The infection in the liver causes an increase in aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT) and bilirubin, and a decrease in albumin. Mesenchymal stromal cells (MSCs) can reduce the inflammatory response by extracellular vesicle (EV) release (large ≥ 200 nm and small ≤ 150 nm). ER: Endoplasmic reticulum; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
High levels of cytokines and chemokines (i.e. IL-1β, Il-2, IL-6, IL-8, IL-10, Il-17, interferons [IFNs], IFN-induced protein 10, TNF-α, granulocyte-macrophage colony-stimulating factor [GM-CSF], monocyte chemoattractant protein-1, macrophage inflammatory protein-1α) and other inflammatory molecules (PCR, ferritin, lactate dehydrogenase, D-dimer) have been observed in severe SARS-CoV-2-infected patients[54,57,88,90-92]. This highly inflammatory milieu leads to multiorgan damage, including liver failure, and is strictly linked to poor prognosis and death in COVID-19 patients[88,90]. As confirmation, liver samples from COVID-19 patient autopsies have revealed micro-vesicular steatosis and inflammation[93-95]. In fact, SARS-CoV-2 infects both hepatic cells and bile duct epithelium, causing liver impairment by direct cytopathic effect, as demonstrated by high transaminase levels and post-mortem liver biopsy specimens showing moderate micro-vesicular steatosis and mild lobular and portal activity[79]. Furthermore, the presence of SARS-CoV-2 has been found in parenchymal cells and vascular endothelium of the liver in COVID-19 patients[76,77].
Additional causes of liver injury can include hypoxia, hypovolemia, and microvascular thrombosis. The hypoxic state associated with COVID-19 can induce ischemic/hypoxic liver injury[87-89]. Considering that COVID-19 patients suffer from severe hypoxia, with the induction of ACE2 receptor expression on hepatocytes[96], a direct infection of hepatocytes by SARS-CoV-2 in hypoxic conditions has been suggested[25]. Liver injury can also be drug-induced. Most of the drugs used against SARS-CoV-2 are potentially hepatotoxic: Antivirals (lopinavir/ritonavir, remdesivir, umifenovir), antibiotics (macrolides, quinolones), chloroquine, tocilizumab, and steroids as well as antipyretic drugs used for fever in COVID-19[79,90,97,98]. Moreover, it must be considered that the majority of COVID-19 patients developing liver complications have a pre-existing chronic liver disease, rendering them more susceptible to liver injury. Interestingly, it has been reported that liver fibrotic/ cirrhotic conditions lead to an increase of ACE-2 receptor expression in hepatocytes[96], thus suggesting again a possible role of pre-existing pathological liver conditions in exacerbating SARS-CoV-2 hepatic tropism.
MSCS AND IMMUNOMODULATION IN COVID-19
The immunomodulatory properties of MSCs represent a promising therapeutic approach for the treatment of autoimmune and inflammatory diseases[99]. The anti-inflammatory and regenerative properties of MSCs have been established in numerous preclinical models of immune-related disorders including graft-vs-host disease, sepsis, inflammatory bowel disease, and allergic airway disease[100-103]. Recent phase I/II clinical trials have shown that the infusion of MSCs immediately after liver transplantation promoted organ regeneration and prolonged recipient survival by reducing acute inflammation, thus suggesting that MSCs can be a promising candidate for cell-based immunotherapy in solid organ transplantation[10,104]. In addition, murine models of liver fibrosis showed that human MSC-derived EVs are able to reduce hepatic inflammation and fibrosis through a decrease of TGF-β, IFN-γ, IL-1, IL-2 and TNF-α levels, an increase of Treg numbers, and a reduction of collagen deposition, all acting together to combat necrosis in the liver[105-107] (Figure 1). Among others, liver injury has been reported as a common complication in SARS-CoV-2 infection, with the degree of liver damage strictly related to the severity of COVID-19[92,108-110]. Although the exact mechanism of liver injury in COVID-19 patients is still unknown, it has been suggested that either the progression of pre-existing hepatic diseases or a direct damage of the liver can be associated with the systemic inflammation caused by SARS-CoV-2 infection, toxicity of anti-viral drugs, or hypoxia-reperfusion injury[109,110].
Pathogenic T cells are rapidly activated after SARS-CoV-2 infection, thus producing GM-CSF, IL-6, and other proinflammatory factors. GM-CSF will further activate inflammatory monocytes (CD14+CD16+), which in turn produce a larger amount of IL-6 and other pro-inflammatory factors, triggering the cytokine storm, which is the main cause of the organ damage, such as in the lungs, kidney, and liver[110]. Recently, the use of MSCs has been proposed as a promising therapeutic approach for COVID-19 patients. The effectiveness and safety of MSC-based treatment are supported by several clinical studies, suggesting that MSC therapy may improve the clinical outcomes of COVID-19 patients through immunomodulation, regulation of inflammatory response, and promotion of tissue repair[111-115]. Moreover, a vast number of clinical trials that use MSCs to treat COVID-19 have already been registered (http://www.chictr.org.cn; https://clinicaltrials.gov). According to their immuno-modulatory properties, the use of MSC-based therapies could be a novel strategy to counteract the harmful effects on the liver caused by SARS-CoV-2 infection.
DISCUSSION
Among the numerous drug treatments, which include antiviral therapy, cytokine inhibitors (e.g., IL-6), and specific antibody treatment (serum/monoclonal)[116], MSCs represent a potential option for critical cases[117]. As discussed above, SARS-CoV-2 infection induces a cytokine storm, causing acute respiratory distress syndrome and multiple-organ failure. IL-6 inhibition by tocilizumab was positively tested in a randomized clinical trial (http://www.chictr.org.cn/showprojen.aspx?proj=49409). Likewise, in this inhibition MSCs can represent a valid alternative, and it has been shown that EV administration counteracts IL-6-induced acute liver injury (ALI) in rat models through the presence of miR-455-3p[118]. MSC treatment showed that the symptomatology of patients was relieved within 2-4 d after MSC infusion, with oxygen saturation increasing to 95% at rest[119]. Another study involved critically ill COVID-19 patients treated with an infusion of human umbilical cord MSCs. In this case, the patients were treated with three different infusions of cells at an interval of 3 d, displayed no observable side effects, and were able to walk within 4 d[115]. Leng et al[119] showed that after infusion of MSCs in COVID-19 patients, the number of peripheral lymphocytes increased, while the levels of C-reactive protein decreased. In addition, in MSC-treated COVID-19 patients compared with those treated with conventional therapy a clear reduction of the major pro-inflammatory cytokine TNF-α, and an increase of IL-10 concentration were observed[119]. Therefore, in an immune-mediated disease condition like COVID-19 infection, the anti-inflammatory activities of MSCs could contribute to improving the conditions of patients after their infusion.
Despite the limited published data, and based on various studies, it could be speculated that SARS-COV-2 induces ALI[79]. SARS-CoV-2 could insult the liver either directly, by the cytopathic effect of the virus after infections of the hepatocytes, or indirectly, by induction of uncontrolled immune reaction, oxidative stress, and/or by pharmacological treatments for COVID-19 that induce liver injury. However, the mechanisms underlying liver impairment in COVID-19 patients are still unknown. Tian et al[94] found sinusoidal dilatation and focal macrovesicular steatosis in liver biopsies obtained post-mortem from four patients with COVID-19 and, in one of these, SARS-CoV-2 RNA was isolated from liver tissue. Wang et al[73] found that four patients (2.9% of 138 patients hospitalized for COVID-19) had chronic liver disease. In another study, cases of ALI were reported in 13 of 274 patients (4.7%)[120]. Interestingly, Richardson et al[121] showed that, in a study including 5700 COVID-19 patients, 58.4% and 39% developed higher levels of ALT and AST, respectively. In addition, among these patients, 56 (1%) developed acute hepatic injury (32320003). Therefore, many COVID-19 patients showed higher levels of both ALT and AST, and mainly in patients with severe disease, liver impairment can occur[54,59,120].
The intravenous administration of MSCs lowered the elevated serum levels of AST and ALT, and increased the amount of HGF, resulting in reduction of ALI[122]. Moreover, in a rat model of ALI, MSCs inhibited neutrophil infiltration, oxidative stress, and hepatocyte apoptosis[123], showing that MSC treatment had significant systemic anti-inflammatory effects and reduction of hepatic inflammation. Moreover, MSCs can prevent lung damage not only directly, with anti-inflammatory activity, but also indirectly by supporting liver function in maintaining the plasma level of albumin (Figure 1). Johnson et al[124] recently underscored the interplay between albumin and SARS-CoV-2, while the importance of albumin in COVID-19 patients has also been strongly stressed by several research teams, who describe a “capillary leak syndrome” in infected patients. This extravascular leakage of intravascular fluids is induced by hypoglobulinemia[125]. A histological analysis of COVID-19 lungs in SARS-CoV-2-infected patients confirmed the presence of pulmonary vascular permeability where the endothelial cells appear swollen[126]. Hypoalbuminemia is an indication of liver dysfunction in the elderly, where it is, per se, an index of increased mortality[127]. The large amounts of extravascular fluid due to the resulting vascular permeability, require mechanical ventilation to overcome the problem.
CONCLUSION
At present, there is no standardized therapy for COVID-19 patients. Though many innovative treatments have been rapidly approved, additional experimental therapies are necessary to treat the worse cases of infection. Despite the fact that all MSC clinical trials for COVID-19 treatment are currently focused on lung/respiratory function, and some of the exclusion criteria are liver disease/insufficiency, we believe, on the basis of current studies, that MSC-based therapy can also help liver dysfunction correlated with SARS-CoV-2 infection.
Footnotes
Conflict-of-interest statement: The authors declare having no conflicts of interest.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Ordine Nazionale dei Biologi, No. AA_074528.
Peer-review started: January 27, 2021
First decision: February 24, 2021
Article in press: April 5, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li Q, Suzuki YJ S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
Contributor Information
Cinzia Maria Chinnici, Department of Research, Mediterranean Institute for Transplantation and Advanced Specialized Therapies (IRCCS-ISMETT), Palermo 90127, Italy; Department of Regenerative Medicine, Fondazione Ri.MED, Palermo 90127, Italy.
Giovanna Russelli, Department of Research, Mediterranean Institute for Transplantation and Advanced Specialized Therapies (IRCCS-ISMETT), Palermo 90127, Italy.
Matteo Bulati, Department of Research, Mediterranean Institute for Transplantation and Advanced Specialized Therapies (IRCCS-ISMETT), Palermo 90127, Italy.
Vitale Miceli, Department of Research, Mediterranean Institute for Transplantation and Advanced Specialized Therapies (IRCCS-ISMETT), Palermo 90127, Italy.
Alessia Gallo, Department of Research, Mediterranean Institute for Transplantation and Advanced Specialized Therapies (IRCCS-ISMETT), Palermo 90127, Italy.
Rosalia Busà, Department of Research, Mediterranean Institute for Transplantation and Advanced Specialized Therapies (IRCCS-ISMETT), Palermo 90127, Italy.
Rosaria Tinnirello, Neuroscience Unit, CNR Institute of Biomedicine and Molecular Immunology, Palermo 90146, Italy.
Pier Giulio Conaldi, Department of Research, Mediterranean Institute for Transplantation and Advanced Specialized Therapies (IRCCS-ISMETT), Palermo 90127, Italy.
Gioacchin Iannolo, Department of Research, Mediterranean Institute for Transplantation and Advanced Specialized Therapies (IRCCS-ISMETT), Palermo 90127, Italy. giannolo@ismett.edu.
References
- 1.Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Campo JA, Gallego P, Grande L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J Hepatol. 2018;10:1–7. doi: 10.4254/wjh.v10.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang YH, Wu DB, Chen B, Chen EQ, Tang H. Progress in mesenchymal stem cell-based therapy for acute liver failure. Stem Cell Res Ther. 2018;9:227. doi: 10.1186/s13287-018-0972-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miranda JP, Filipe E, Fernandes AS, Almeida JM, Martins JP, De la Fuente A, Abal M, Barcia RN, Cruz P, Cruz H, Castro M, Santos JM. The Human Umbilical Cord Tissue-Derived MSC Population UCX(®) Promotes Early Motogenic Effects on Keratinocytes and Fibroblasts and G-CSF-Mediated Mobilization of BM-MSCs When Transplanted In Vivo. Cell Transplant. 2015;24:865–877. doi: 10.3727/096368913X676231. [DOI] [PubMed] [Google Scholar]
- 6.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miceli V, Pampalone M, Vella S, Carreca AP, Amico G, Conaldi PG. Comparison of Immunosuppressive and Angiogenic Properties of Human Amnion-Derived Mesenchymal Stem Cells between 2D and 3D Culture Systems. Stem Cells Int. 2019;2019:7486279. doi: 10.1155/2019/7486279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magatti M, De Munari S, Vertua E, Gibelli L, Wengler GS, Parolini O. Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities. Stem Cells. 2008;26:182–192. doi: 10.1634/stemcells.2007-0491. [DOI] [PubMed] [Google Scholar]
- 9.Sargent A, Miller RH. MSC Therapeutics in Chronic Inflammation. Curr Stem Cell Rep. 2016;2:168–173. doi: 10.1007/s40778-016-0044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Du Z, Yan J, Ma D, Shi M, Zhang M, Peng C, Li H. Mesenchymal stem cells promote liver regeneration and prolong survival in small-for-size liver grafts: involvement of C-Jun N-terminal kinase, cyclin D1, and NF-κB. PLoS One. 2014;9:e112532. doi: 10.1371/journal.pone.0112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15:36–45. doi: 10.7150/ijms.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med. 2011;9:29. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu C, Zhao L, Zhang L, Bao Q, Li L. Mesenchymal stem cell-based cell-free strategies: safe and effective treatments for liver injury. Stem Cell Res Ther. 2020;11:377. doi: 10.1186/s13287-020-01895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfaifi M, Eom YW, Newsome PN, Baik SK. Mesenchymal stromal cell therapy for liver diseases. J Hepatol. 2018;68:1272–1285. doi: 10.1016/j.jhep.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 15.Parekkadan B, van Poll D, Megeed Z, Kobayashi N, Tilles AW, Berthiaume F, Yarmush ML. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;363:247–252. doi: 10.1016/j.bbrc.2007.05.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulati M, Miceli V, Gallo A, Amico G, Carcione C, Pampalone M, Conaldi PG. The Immunomodulatory Properties of the Human Amnion-Derived Mesenchymal Stromal/Stem Cells Are Induced by INF-γ Produced by Activated Lymphomonocytes and Are Mediated by Cell-To-Cell Contact and Soluble Factors. Front Immunol. 2020;11:54. doi: 10.3389/fimmu.2020.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 18.Rong X, Liu J, Yao X, Jiang T, Wang Y, Xie F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10:98. doi: 10.1186/s13287-019-1204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49:e346. doi: 10.1038/emm.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haga H, Yan IK, Takahashi K, Matsuda A, Patel T. Extracellular Vesicles from Bone Marrow-Derived Mesenchymal Stem Cells Improve Survival from Lethal Hepatic Failure in Mice. Stem Cells Transl Med. 2017;6:1262–1272. doi: 10.1002/sctm.16-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Xiao X, Wei X, Li J, Yang J, Tan H, Zhu J, Zhang Q, Wu J, Liu L. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 2020;92:595–601. doi: 10.1002/jmv.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells. 2014;6:526–539. doi: 10.4252/wjsc.v6.i5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lizardo-Thiebaud MJ, Cervantes-Alvarez E, Limon-de la Rosa N, Tejeda-Dominguez F, Palacios-Jimenez M, Méndez-Guerrero O, Delaye-Martinez M, Rodriguez-Alvarez F, Romero-Morales B, Liu WH, Huang CA, Kershenobich D, Navarro-Alvarez N. Direct or Collateral Liver Damage in SARS-CoV-2-Infected Patients. Semin Liver Dis. 2020;40:321–330. doi: 10.1055/s-0040-1715108. [DOI] [PubMed] [Google Scholar]
- 26.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu S, Park J, Liu A, Lee J, Zhang X, Hao Q, Lee JW. Mesenchymal Stem Cell Microvesicles Restore Protein Permeability Across Primary Cultures of Injured Human Lung Microvascular Endothelial Cells. Stem Cells Transl Med. 2018;7:615–624. doi: 10.1002/sctm.17-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinnici CM, Pietrosi G, Iannolo G, Amico G, Cuscino N, Pagano V, Conaldi PG. Mesenchymal stromal cells isolated from human fetal liver release soluble factors with a potential role in liver tissue repair. Differentiation. 2019;105:14–26. doi: 10.1016/j.diff.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Tsiapalis D, O'Driscoll L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells. 2020;9 doi: 10.3390/cells9040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8 doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang B, Tian X, Hao J, Xu G, Zhang W. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Tissue Regeneration. Cell Transplant. 2020;29:963689720908500. doi: 10.1177/0963689720908500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleh AF, Lázaro-Ibáñez E, Forsgard MA, Shatnyeva O, Osteikoetxea X, Karlsson F, Heath N, Ingelsten M, Rose J, Harris J, Mairesse M, Bates SM, Clausen M, Etal D, Leonard E, Fellows MD, Dekker N, Edmunds N. Extracellular vesicles induce minimal hepatotoxicity and immunogenicity. Nanoscale. 2019;11:6990–7001. doi: 10.1039/c8nr08720b. [DOI] [PubMed] [Google Scholar]
- 35.Amosse J, Martinez MC, Le Lay S. Extracellular vesicles and cardiovascular disease therapy. Stem Cell Investig. 2017;4:102. doi: 10.21037/sci.2017.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O'Brien D, van Wijnen AJ, Lerman LO. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551:55–64. doi: 10.1016/j.gene.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chinnici CM, Amico G, Gallo A, Iannolo G, Cuscino N, Vella S, Carcione C, Nascari D, Conaldi PG. Small Extracellular Vesicles from Human Fetal Dermal Cells and Their MicroRNA Cargo: KEGG Signaling Pathways Associated with Angiogenesis and Wound Healing. Stem Cells Int. 2020;2020:8889379. doi: 10.1155/2020/8889379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Wang Y, Lv Q, Gao J, Hu L, He Z. MicroRNA-21 Overexpression Promotes the Neuroprotective Efficacy of Mesenchymal Stem Cells for Treatment of Intracerebral Hemorrhage. Front Neurol. 2018;9:931. doi: 10.3389/fneur.2018.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang J, Li Z, Zhi Z, Wang S, Xu G. MiR-21 derived from the exosomes of MSCs regulates the death and differentiation of neurons in patients with spinal cord injury. Gene Ther. 2019;26:491–503. doi: 10.1038/s41434-019-0101-8. [DOI] [PubMed] [Google Scholar]
- 40.Park KS, Bandeira E, Shelke GV, Lässer C, Lötvall J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:288. doi: 10.1186/s13287-019-1398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varderidou-Minasian S, Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. 2020;10:5979–5997. doi: 10.7150/thno.40122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abraham A, Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9:28–38. doi: 10.1002/sctm.19-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci USA. 2016;113:170–175. doi: 10.1073/pnas.1522297113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stone ML, Zhao Y, Robert Smith J, Weiss ML, Kron IL, Laubach VE, Sharma AK. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir Res. 2017;18:212. doi: 10.1186/s12931-017-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rostom DM, Attia N, Khalifa HM, Abou Nazel MW, El Sabaawy EA. The Therapeutic Potential of Extracellular Vesicles Versus Mesenchymal Stem Cells in Liver Damage. Tissue Eng Regen Med. 2020;17:537–552. doi: 10.1007/s13770-020-00267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mardpour S, Hassani SN, Mardpour S, Sayahpour F, Vosough M, Ai J, Aghdami N, Hamidieh AA, Baharvand H. Extracellular vesicles derived from human embryonic stem cell-MSCs ameliorate cirrhosis in thioacetamide-induced chronic liver injury. J Cell Physiol. 2018;233:9330–9344. doi: 10.1002/jcp.26413. [DOI] [PubMed] [Google Scholar]
- 48.Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao J, Zheng J, Cai J, Zeng K, Zhou C, Zhang J, Li S, Li H, Chen L, He L, Chen H, Fu H, Zhang Q, Chen G, Yang Y, Zhang Y. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2019;33:1695–1710. doi: 10.1096/fj.201800131RR. [DOI] [PubMed] [Google Scholar]
- 50.Haga H, Yan IK, Borrelli DA, Matsuda A, Parasramka M, Shukla N, Lee DD, Patel T. Extracellular vesicles from bone marrow-derived mesenchymal stem cells protect against murine hepatic ischemia/reperfusion injury. Liver Transpl. 2017;23:791–803. doi: 10.1002/lt.24770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nong K, Wang W, Niu X, Hu B, Ma C, Bai Y, Wu B, Wang Y, Ai K. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy. 2016;18:1548–1559. doi: 10.1016/j.jcyt.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Du Y, Li D, Han C, Wu H, Xu L, Zhang M, Zhang J, Chen X. Exosomes from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells (hiPSC-MSCs) Protect Liver against Hepatic Ischemia/ Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway. Cell Physiol Biochem. 2017;43:611–625. doi: 10.1159/000480533. [DOI] [PubMed] [Google Scholar]
- 53.Mardpour S, Ghanian MH, Sadeghi-Abandansari H, Mardpour S, Nazari A, Shekari F, Baharvand H. Hydrogel-Mediated Sustained Systemic Delivery of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improves Hepatic Regeneration in Chronic Liver Failure. ACS Appl Mater Interfaces. 2019;11:37421–37433. doi: 10.1021/acsami.9b10126. [DOI] [PubMed] [Google Scholar]
- 54.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. :e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20–32. doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. 2020 Preprint. Available from: bioRxiv:2020.2002.2003.931766.
- 64.Chu H, Chan JF, Yuen TT, Shuai H, Yuan S, Wang Y, Hu B, Yip CC, Tsang JO, Huang X, Chai Y, Yang D, Hou Y, Chik KK, Zhang X, Fung AY, Tsoi HW, Cai JP, Chan WM, Ip JD, Chu AW, Zhou J, Lung DC, Kok KH, To KK, Tsang OT, Chan KH, Yuen KY. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1:e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X, Zhu J, Zhao Z, Jaffré F, Zhang T, Kim TW, Harschnitz O, Redmond D, Houghton S, Liu C, Naji A, Ciceri G, Guttikonda S, Bram Y, Nguyen DT, Cioffi M, Chandar V, Hoagland DA, Huang Y, Xiang J, Wang H, Lyden D, Borczuk A, Chen HJ, Studer L, Pan FC, Ho DD, tenOever BR, Evans T, Schwartz RE, Chen S. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell 2020; 27: 125-136. :e7. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020; 158: 1831-1833. :e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo S, Zhang X, Xu H. Don't Overlook Digestive Symptoms in Patients With 2019 Novel Coronavirus Disease (COVID-19) Clin Gastroenterol Hepatol. 2020;18:1636–1637. doi: 10.1016/j.cgh.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of Gastrointestinal Symptoms in Patients With COVID-19. Gastroenterology. 2020;158:2294–2297. doi: 10.1053/j.gastro.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, Shen L, Chan WW. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology 2020; 159: 765-767. :e2. doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmulson M, Dávalos MF, Berumen J. Beware: Gastrointestinal symptoms can be a manifestation of COVID-19. Rev Gastroenterol Mex. 2020;85:282–287. doi: 10.1016/j.rgmx.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin L, Jiang X, Zhang Z, Huang S, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 76.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, Shen H, Qiu L, Li Z, Geng J, Cai J, Han H, Li X, Kang W, Weng D, Liang P, Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farcas GA, Poutanen SM, Mazzulli T, Willey BM, Butany J, Asa SL, Faure P, Akhavan P, Low DE, Kain KC. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J Infect Dis. 2005;191:193–197. doi: 10.1086/426870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J, Wang S, Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J Med Virol. 2020;92:680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, Song Q, Jia Q, Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15:e0235458. doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584–599. doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yadav DK, Singh A, Zhang Q, Bai X, Zhang W, Yadav RK, Zhiwei L, Adhikari VP, Liang T. Involvement of liver in COVID-19: systematic review and meta-analysis. Gut. 2021;70:807–809. doi: 10.1136/gutjnl-2020-322072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kumar-M P, Mishra S, Jha DK, Shukla J, Choudhury A, Mohindra R, Mandavdhare HS, Dutta U, Sharma V. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol Int. 2020;14:711–722. doi: 10.1007/s12072-020-10071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu C, Jiang ZC, Shao CX, Zhang HG, Yue HM, Chen ZH, Ma BY, Liu WY, Huang HH, Yang J, Wang Y, Liu HY, Xu D, Wang JT, Yang JY, Pan HQ, Zou SQ, Li FJ, Lei JQ, Li X, He Q, Gu Y, Qi XL. [Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study] Zhonghua Gan Zang Bing Za Zhi. 2020;28:107–111. doi: 10.3760/cma.j.issn.1007-3418.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 85.Duan ZP, Chen Y, Zhang J, Zhao J, Lang ZW, Meng FK, Bao XL. [Clinical characteristics and mechanism of liver injury in patients with severe acute respiratory syndrome] Zhonghua Ganzang Bing Zazhi. 2003;11:493–496. [PubMed] [Google Scholar]
- 86.Duan XF, Liu Z, Hao R, Luo L, Zhang YN. [The dynamic change of liver injury in patients with severe acute respiratory syndrome] Zhonghua Ganzang Bing Zazhi. 2004;12:439. [PubMed] [Google Scholar]
- 87.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhong P, Xu J, Yang D, Shen Y, Wang L, Feng Y, Du C, Song Y, Wu C, Hu X, Sun Y. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5:256. doi: 10.1038/s41392-020-00373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lozano-Sepulveda SA, Galan-Huerta K, Martínez-Acuña N, Arellanos-Soto D, Rivas-Estilla AM. SARS-CoV-2 another kind of liver aggressor, how does it do that? Ann Hepatol. 2020;19:592–596. doi: 10.1016/j.aohep.2020.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13–17. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 Autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hanley B, Lucas SB, Youd E, Swift B, Osborn M. Autopsy in suspected COVID-19 cases. J Clin Pathol. 2020;73:239–242. doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 96.Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, Davern TJ 2nd, Murray NG, McCashland T, Reisch JS, Robuck PR Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009; 137: 856-864, 864. :e1. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rad F, Ghorbani M, Mohammadi Roushandeh A, Habibi Roudkenar M. Mesenchymal stem cell-based therapy for autoimmune diseases: emerging roles of extracellular vesicles. Mol Biol Rep. 2019;46:1533–1549. doi: 10.1007/s11033-019-04588-y. [DOI] [PubMed] [Google Scholar]
- 100.Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, Bartholomew A. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft vs host disease. Eur J Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 102.Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy. 2011;66:523–531. doi: 10.1111/j.1398-9995.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 103.Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.English K, Wood KJ. Mesenchymal stromal cells in transplantation rejection and tolerance. Cold Spring Harb Perspect Med. 2013;3:a015560. doi: 10.1101/cshperspect.a015560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tamura R, Uemoto S, Tabata Y. Immunosuppressive effect of mesenchymal stem cell-derived exosomes on a concanavalin A-induced liver injury model. Inflamm Regen. 2016;36:26. doi: 10.1186/s41232-016-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bulut Ö, GÜrsel İ. Mesenchymal stem cell derived extracellular vesicles: promising immunomodulators against autoimmune, autoinflammatory disorders and SARS-CoV-2 infection. Turk J Biol. 2020;44:273–282. doi: 10.3906/biy-2002-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Portincasa P, Krawczyk M, Machill A, Lammert F, Di Ciaula A. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med. 2020;77:18–24. doi: 10.1016/j.ejim.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang C, Li Q, Xu W, Chen L. Molecular and cellular mechanisms of liver dysfunction in COVID-19. Discov Med. 2020;30:107–112. [PubMed] [Google Scholar]
- 110.Zhao JN, Fan Y, Wu SD. Liver injury in COVID-19: A minireview. World J Clin Cases. 2020;8:4303–4310. doi: 10.12998/wjcc.v8.i19.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Golchin A, Farahany TZ, Khojasteh A, Soleimanifar F, Ardeshirylajimi A. The Clinical Trials of Mesenchymal Stem Cell Therapy in Skin Diseases: An Update and Concise Review. Curr Stem Cell Res Ther. 2019;14:22–33. doi: 10.2174/1574888X13666180913123424. [DOI] [PubMed] [Google Scholar]
- 112.Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, McVerry BJ, Ortiz LA, Exline M, Christman JW, Abbott J, Delucchi KL, Caballero L, McMillan M, McKenna DH, Liu KD. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X, Gao H, Lu X, Yu L, Dai X, Xiang C, Li L. Clinical Study of Mesenchymal Stem Cell Treatment for Acute Respiratory Distress Syndrome Induced by Epidemic Influenza A (H7N9) Infection: A Hint for COVID-19 Treatment. Engineering (Beijing) 2020;6:1153–1161. doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Namba F. Mesenchymal stem cells for the prevention of bronchopulmonary dysplasia. Pediatr Int. 2019;61:945–950. doi: 10.1111/ped.14001. [DOI] [PubMed] [Google Scholar]
- 115.Liang B, Chen J, Li T, Wu H, Yang W, Li Y, Li J, Yu C, Nie F, Ma Z, Yang M, Xiao M, Nie P, Gao Y, Qian C, Hu M. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine (Baltimore) 2020;99:e21429. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shah TG, Predescu D, Predescu S. Mesenchymal stem cells-derived extracellular vesicles in acute respiratory distress syndrome: a review of current literature and potential future treatment options. Clin Transl Med. 2019;8:25. doi: 10.1186/s40169-019-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shao M, Xu Q, Wu Z, Chen Y, Shu Y, Cao X, Chen M, Zhang B, Zhou Y, Yao R, Shi Y, Bu H. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther. 2020;11:37. doi: 10.1186/s13287-020-1550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW the Northwell COVID-19 Research Consortium. Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA . 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hwang Y, Kim JC, Tae G. Significantly enhanced recovery of acute liver failure by liver targeted delivery of stem cells via heparin functionalization. Biomaterials. 2019;209:67–78. doi: 10.1016/j.biomaterials.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 123.Wang Y, Wang JL, Ma HC, Tang ZT, Ding HR, Shi XL. Mesenchymal stem cells increase heme oxygenase 1-activated autophagy in treatment of acute liver failure. Biochem Biophys Res Commun. 2019;508:682–689. doi: 10.1016/j.bbrc.2018.11.146. [DOI] [PubMed] [Google Scholar]
- 124.Johnson AS, Fatemi R, Winlow W. SARS-CoV-2 Bound Human Serum Albumin and Systemic Septic Shock. Front Cardiovasc Med. 2020;7:153. doi: 10.3389/fcvm.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu MA, Fossali T, Pandolfi L, Carsana L, Ottolina D, Frangipane V, Rech R, Tosoni A, Lopez G, Agarossi A, Cogliati C, Meloni F, Marchini B, Nebuloni M, Catena E, Colombo R. Hypoalbuminemia in COVID-19: assessing the hypothesis for underlying pulmonary capillary leakage. J Intern Med. 2021 doi: 10.1111/joim.13208. [DOI] [PubMed] [Google Scholar]
- 126.Bahloul M, Ketata W, Lahyeni D, Mayoufi H, Kotti A, Smaoui F, Kallel N, Daoud E, Bouaziz M, Kammoun S. Pulmonary capillary leak syndrome following COVID-19 virus infection. J Med Virol. 2021;93:94–96. doi: 10.1002/jmv.26152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Corti MC, Guralnik JM, Salive ME, Sorkin JD. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA. 1994;272:1036–1042. [PubMed] [Google Scholar]