Abstract
BACKGROUND
Antiviral therapy cannot completely block the progression of hepatitis B to hepatocellular carcinoma (HCC). Furthermore, there are few predictors of early HCC progression and limited strategies to prevent progression in patients with HBV-related cirrhosis who receive nucleos(t)ide analog (NA) therapy.
AIM
The study aim was to clarify risk factors and the diagnostic value of alpha-fetoprotein (AFP) for HCC progression in NA-treated hepatitis B virus (HBV)-related cirrhosis patients.
METHODS
In this retrospective cross-sectional study, we analyzed the clinical data of 266 patients with HBV-related cirrhosis who received NA treatment between February 2014 and April 2020 at Zhejiang Provincial People’s Hospital. The patients were divided into two groups, 145 who did not progress to HCC (No-HCC group), and 121 who progressed to HCC during NA treatment (HCC group). The logistic regression analysis was used to analyze the risk factors of HCC progression. The diagnostic value of AFP for HCC was evaluated by receiver operating characteristic (ROC) curve analysis.
RESULTS
Univariate analysis showed that age ≥ 60 years (P = 0.001), hepatitis B and alcoholic etiology (P = 0.007), smoking history (P < 0.001), family history of HBV-related HCC (P = 0.002), lamivudine resistance (P = 0.011), HBV DNA negative (P = 0.023), aspartate aminotransferase > 80 U/L (P = 0.002), gamma-glutamyl transpeptidase > 120 U/L (P = 0.001), alkaline phosphatase > 250 U/L (P = 0.001), fasting blood glucose (FBG) ≥ 6.16 (mmol/L) (P = 0.001) and Child-Pugh class C (P = 0.005) were correlated with HCC progression. In multivariate analysis, age ≥ 60 years [hazard ratio (HR) = 3.089, 95% confidence interval (CI): 1.437-6.631, P = 0.004], smoking history (HR = 4.001, 95%CI: 1.836-8.716, P < 0.01), family history of HBV-related HCC (HR = 6.763, 95%CI: 1.253-36.499, P < 0.05), lamivudine resistance (HR = 2.949, 95%CI: 1.207-7.208, P = 0.018), HBV DNA negative (HR = 0.026, 95%CI: 0.007-0.139, P < 0.01), FBG ≥ 6.16 mmol/L (HR = 7.219, 95%CI: 3.716-14.024, P < 0.01) were independent risk factors of HCC progression. ROC of AFP for diagnosis of HCC was 0.746 (95%CI: 0.674-0.818). A cutoff value of AFP of 9.00 ug/L had a sensitivity of 0.609, and specificity of 0.818 for diagnosing HCC.
CONCLUSION
Age ≥ 60 years, smoking history, family history of HCC, lamivudine resistance, HBV DNA negative, FBG ≥ 6.16 mmol/L were risk factors of HCC progression. Serum AFP had limited diagnostic value for HCC.
Keywords: Hepatitis B virus, Hepatocellular carcinoma, Cirrhosis, Risk factors, Nucleos(t)ide analogs, Progression
Core Tip: This retrospective cross-sectional study analyzed risk factors of hepatocellular carcinoma (HCC) progression in hepatitis B virus-related cirrhosis patients receiving nucleoside acid analog therapy for at least 6 mo. We discuss the diagnostic value of serum alpha-fetoprotein level in these patients. The results of the present study increase our understanding of HCC pathogenesis and help to provide HCC prevention and control strategies.
INTRODUCTION
Liver cancer has the third-highest cancer mortality rate worldwide[1]. Hepatocellular carcinoma (HCC) is one of the most common subtypes of liver cancer and is the sixth most prevalent cancer type. In most countries, the HCC mortality rates have increased in recent decades[2]. HCC is the fourth most common malignant tumor type in China, accounting for over 55% of the total number of HCC cases[3]. Infection with the hepatitis B virus (HBV) greatly increases the incidence of HCC because HBV causes chronic hepatitis B (CHB), liver cirrhosis, and ultimately HCC[4,5]. The estimated risk of developing HCC was observed to be 25 to 37-fold higher in hepatitis B surface antigen (HBsAg) carriers compared with noninfected patients HBV infection is one of the most important contributors to the pathogenesis of HCC[6-8]. Over the past 30 years, antiviral drugs, especially nucleos(t)ide analogs (NAs), have been widely used in clinical practice and have substantial long-term effects on the inhibition of HBV replication, namely, delaying and reducing the occurrence and development of hepatitis B-related events. Many studies have shown that antiviral therapy can considerably decrease the incidence of HCC, even for patients in whom CHB has progressed to cirrhosis[9,10]. However, antiviral therapy does not completely block the progression of CHB to liver cancer[11,12]. In the current study, we analyzed the risk factors of HCC progression in patients with HBV-related cirrhosis who received NAs therapy for at least 6 mo. The diagnostic value of the serum alpha-fetoprotein (AFP) level was evaluated in those patients by receiver operating characteristic (ROC) curve analysis. The study results increase our understanding of HCC pathogenesis and help to provide HCC prevention and control strategies.
MATERIALS AND METHODS
Patients and design
This cross-sectional study retrospectively enrolled 266 patients with HBV-related cirrhosis who were treated with NA antiviral therapy at Zhejiang Provincial People’s Hospital between February 2014 and April 2020. The 266 patients were divided into two groups, 145with cirrhosis who did not progress to HCC during the observation period (No-HCC group), and 121 with cirrhosis who progressed to HCC (HCC group). The inclusion criteria were: (1) Age ≥ 18 years; (2) Treatment with lamivudine (LAM), adefovir (ADV), telbivudine, entecavir (ENT), or tenofovir (TDF) nucleoside or NAs for at least 6 mo; (3) Diagnosis of cirrhosis established by either histology (progressive fibrosis, nodule formation, and loss of hepatic architecture) or clinical data (symptoms and signs of cirrhosis, abnormal liver function, and ultrasonic identification). Demographic, clinical, laboratory, imaging, and pathology data were collected during the patient’s hospital stay. The severity of cirrhosis was classified by the Child-Pugh criteria. Patients with HBV-related cirrhosis were diagnosed in accord with the guidelines for the prevention and treatment of chronic hepatitis B formulated by the Hepatology Branch and the Infectious Diseases Branch of the Chinese Medical Association[13,14]; and (4) HCC and hepatitis diagnoses confirmed by clinical and serological characteristics, ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), hepatic arteriography with digital subtraction angiography (DSA) and pathological examination consistent with the hepatitis and primary liver cancer clinical diagnosis criteria[15,16]. The exclusion criteria were: (1) Hepatitis C virus, hepatitis D virus, or human immunodeficiency virus coinfection; (2) Autoimmune hepatitis and drug hepatitis; and (3) Hepatocarcinoma prior to antiviral treatment or within 6 mo after antiviral treatment. The study received no support from any pharmaceutical company and was approved by the Ethics Committee of the Zhejiang Provincial People’s Hospital (2020QT155), Hangzhou, Zhejiang Province, China.
Data collection and study design
The data collected from the electronic medical record system were age, sex, history of drinking and smoking, alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), glutamyltranspeptidase (GGT), alkaline phosphatase (ALP), total bilirubin, fasting blood glucose (FBG), AFP, prothrombin time (PT), presence of ascites, hepatic encephalopathy, Child-Pugh score and classification, and family history of HBV, hepatitis B, and HCC. Serological markers of hepatitis B, serum content of HBV DNA, and history of NA treatment were reported by the patients or their families. All patients in the cohort were followed-up for 3 years or until death.
Serum liver function was tested by routine automated techniques using an Olympus AU5400 automated analyzer (Olympus, Tokyo, Japan). HBsAg, hepatitis B e-antigen (HBeAg), and hepatitis B e-antibody were assessed at baseline by chemiluminescence immunoassay (Abbott ARCHITECT i2000 SR analyzer; Abbott Diagnostics, Chicago, IL, United States). The serum HBV DNA load was assessed by RT-PCR using a LightCycler PCR system (Roche LightCycler480 II fluorescence quantitative PCR) in strict accordance with the instructions provided with the reagent kit (Shenzheng PG Biotech Co. Ltd, China). The detection limit was approximately 100 viral genome IU/mL. Genotypic resistance to LAM and ADV were determined at baseline by direct sequencing of the PCR amplification products. The serum AFP tumor marker was assayed by electrochemiluminescence. The study protocol conformed to the 1975 Declaration of Helsinki ethical guidelines for clinical studies.
Statistical analysis
Descriptive data were expressed as means ± SD or n (%). Continuous variables were compared using Student’s t-test. Skewness distribution data were reported as the median with the range and were analyzed using the Mann–Whitney U test. Categorical variables were analyzed using Fisher’s exact test or Pearson’s χ2 test. A logistic regression model was used to analyze single factors, and multivariate analysis with stepwise regression was used to identify statistically significant variables in the single-factor analysis. The diagnostic performance of the serum AFP level was evaluated using ROC curves. The cutoff value, which was the maximum area under the ROC curve (AUROC), and accuracy were calculated with 95% confidence intervals (CIs). A P value of less than 0.05 was determined to indicate statistical significance. The SPSS statistical package (version 22.0; SPSS, Chicago, IL, United States) was used for the statistical analysis.
RESULTS
Clinical characteristics of HBV-related cirrhosis patients receiving NA therapy
The clinical features of the No-HCC and the HCC group were compared. There were no significant differences between the two groups in the sex ratio, duration of NA therapy, HBsAg level, HBeAg positivity, jaundice index, Child-Pugh class B ratio, and PT level (P > 0.05). The HCC group included significantly more patients older than 60 years of age and patients with increased levels of ALT, AST, GGT, ALP, and FBG, and decreased levels of ALB than the No-HCC group. The HCC group also contained more patients with mixed etiology (alcohol + HBV), history of smoking, family history of HBV-related HCC, LAM resistance, Child-Pugh class C status, and an AFP > 20 μg/L and fewer HBV DNA-negative and Child-Pugh class A patients than the No-HCC group (Table 1).
Table 1.
Comparison of the clinical characteristics of patients with and without hepatocellular carcinoma
|
Characteristic
|
no-HCC group (n = 145)
|
HCC group (n = 121)
|
P
value
|
| Age ≥ 60 yr, n (%) | 41 (28.3) | 64 (52.9) | < 0.001 |
| Male, n (%) | 99 (68.3) | 91 (75.2) | 0.133 |
| Etiology of liver cirrhosis, n (%) | |||
| HBV | 101 (60.7) | 76 (62.8) | 0.363 |
| HBV + alcohol | 31 (21.4) | 45 (37.2) | 0.006 |
| HBV + HEV | 1 (0.6) | 0 (0.0) | - |
| HBV + schistosome | 1 (0.6) | 0 (0.0) | - |
| Smoking history, n (%) | 21 (14.5) | 51 (42.1) | < 0.001 |
| Family history of HBV-related HCC, n (%) | 10 (6.9) | 23 (19.0) | 0.004 |
| Medication history | |||
| Duration of NA treatment, yr, median (P25, P75) | 3.9 (2.1, 5.8) | 5.4 (2.3, 6.9) | 0.067 |
| LAM resistance, n (%) | 18 (12.4) | 27 (22.3) | 0.021 |
| HBsAg level, IU/L, median (P25, P75) | 255.0 (56.0, 678.0) | 269.0 (67.0, 656.0) | 0.456 |
| HBeAg positive, n (%) | 37 (25.5) | 21 (17.4) | 0.136 |
| HBV DNA negative, n (%) | 67 (46.2) | 39 (32.2) | 0.033 |
| ALB, U/L | 36.29 ± 7.98 | 33.34 ± 6.62 | 0.002 |
| ALT, U/L, median (P25, P75) | 27.00 (18.00, 37.00) | 32.00 (21.27, 62.00) | 0.006 |
| AST, U/L, median (P25, P75) | 33.00 (23.00, 47.00) | 44.50 (31.65, 96.85) | < 0.001 |
| GGT, U/L, median (P25, P75) | 33.00 (20.00, 46.00) | 61.50 (32.75, 160.75) | < 0.001 |
| ALP, U/L, median (P25, P75) | 99.00 (73.00, 126.00) | 134.50 (92.00, 198.85) | < 0.001 |
| TB, μmol/L, median (P25, P75) | 20.41 (13.81, 44.60) | 24.46 (16.60, 42.80) | 0.192 |
| FBS, mmol/L, median (P25, P75) | 5.17 ± 0.68 | 6.99 ± 1.31 | 0.025 |
| Ascites, n (%) | 33 (20.0) | 43 (34.7) | 0.022 |
| Child-Pugh class, n (%) | |||
| A | 81 (55.9) | 55 (45.5) | 0.001 |
| B | 38 (26.2) | 26 (21.5) | 0.561 |
| C | 26 (17.9) | 40 (33.1) | 0.002 |
| AFP ≥ 20 μg/L, n (%) | 14 (9.7) | 56 (46.3) | < 0.001 |
| PT s, median (P25, P75) | 13.25 (11.80, 14.20) | 13.51 (12.41, 15.02) | 0.475 |
AFP: Alpha-fetoprotein; ALB: Albumin; ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; FBS: Fasting blood sugar; GGT: Glutamyl transpeptidase; HBeAb: Hepatitis B e-antibody; HBsAg: Hepatitis B surface antigen; HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma; LAM: Lamivudine; PT: Prothrombin time; SD: Standard deviation; TB: Total bilirubin.
Risk factors for HCC progression in HBV-related cirrhosis patients receiving NA therapy
We analyzed factors associated with HCC progression in HBV-related cirrhosis patients who received NA therapy. Univariate analysis found age ≥ 60 years (P = 0.002), HBV + alcohol mixed etiology (P = 0.007), smoking history (P < 0.001), family history of HBV-related HCC (P = 0.002), LAM resistance (P = 0.046), HBV DNA negativity (P = 0.023), AST > 80 U/L (P = 0.002), GGT > 120 U/L (P = 0.001), ALP > 250 U/L (P = 0.001), FBG ≥ 6.16 mmol/L (P = 0.001), and Child-Pugh class C (P = 0.005) to be significantly related to HCC (Table 2). Multivariate analysis showed that age ≥ 60 years [hazard ratio (HR) = 3.089, 95%CI: 1.437-6.631, P = 0.004], smoking history (HR = 4.001 95%CI: 1.836-8.716, P < 0.01), family history of HBV-related HCC (HR = 6.763, 95%CI: 1.253-36.499, P < 0.05), LAM resistance (HR = 2.949, 95%CI: 1.207-7.208, P = 0.018), HBV DNA negative (HR = 0.026, 95%CI: 0.007-0.139, P < 0.01), and FBG ≥ 6.16 mmol/L (HR = 7.219, 95%CI: 3.716-14.024, P < 0.01) independently predicted HCC progression in patients with HBV-related cirrhosis who received NAs therapy (Table 3).
Table 2.
Univariate logistic regression analysis of hepatitis B-related cirrhosis progressing to hepatocellular carcinoma in patients treated with nucleos(t)ide analogs
|
Characteristic
|
No-HCC group (n = 145)
|
HCC group (n = 121)
|
Univariate adjusted HR (95%CI)
|
P
value
|
| Age, yr | ||||
| ≥ 60 | 40 (27.6) | 65 (52.9) | 2.664 (1.606-4.418) | 0.001 |
| < 60 | 105 (72.4) | 56 (46.3) | ||
| HBV + alcohol | ||||
| Yes | 31 (21.4) | 45 (37.2) | 2.384 (1.271-4.473) | 0.007 |
| No | ||||
| Smoking history | ||||
| Yes | 21 (14.5) | 51 (42.1) | 4.073 (2.281-7.273) | < 0.001 |
| No | ||||
| Family history of HBV-related HCC | ||||
| Yes | 10 (6.9) | 23 (19.0) | 3.546 (1.573-7.998) | 0.002 |
| No | ||||
| LAM resistance | ||||
| Yes | 18 (12.4) | 27 (22.3) | 2.284 (1.214-4.297) | 0.011 |
| No | ||||
| HBV DNA negative | ||||
| Yes | 67 (46.2) | 39 (32.2) | 0.559 (0.339-0.922) | 0.023 |
| No | ||||
| ALB (g/L) | ||||
| < 35 | 67(46.2) | 62(51.2) | 1.223 (0.754-1.984) | 0.414 |
| ≥ 35 | 78 (53.8) | 59 (48.8) | ||
| ALT (U/L) | ||||
| 50-100 | 13 (9.0) | 15 (12.4) | 1.324 (0.612-2.866) | 0.476 |
| > 100 | 10 (7.0) | 14 (11.6) | 1.138(0.482-2.688) | 0.768 |
| AST (U/L) | ||||
| 40-80 | 32 (22.1) | 24 (19.8) | 0.919 (−0.713-0.514) | 0.783 |
| > 80 | 16 (10.3) | 31 (29.8) | 2.899 (0.436-1.767) | 0.002 |
| GGT (U/L) | ||||
| 60-120 | 15 (10.3) | 20 (16.5) | 1.853 (0.892-3.847) | 0.098 |
| > 120 | 8 (5.1) | 28 (23.1) | 5.663 (1.075-2.573) | 0.001 |
| ALP (U/L) | ||||
| 125-250 | 36(24.8) | 45 (38.8) | 1.609 (−0.062-1.028) | 0.073 |
| > 250 | 6 (1.4) | 21 (17.4) | 4.865 (0.667-2.993) | 0.001 |
| FBG (mmol/L) | ||||
| ≥ 6.16 | 19 (13.1) | 37 (30.6) | 3.3179 (0.587-1.902) | 0.001 |
| < 6.16 | ||||
| Ascites class | ||||
| Yes | 33 (22.8) | 43 (35.5) | 0.834 (−0.412-0.060) | 0.142 |
| No | ||||
| Child-Pugh class | ||||
| A | 81 (58.6) | 55 (37.9) | 0.658 (−0.938-0.064) | 0.091 |
| B | 38 (26.2) | 26 (21.5) | 0.671 (−0.981-0.112) | 0.165 |
| C | 26 (17.9) | 40 (33.1) | 2.260 (0.247-1.427) | 0.005 |
Data are n (%) or mean ± SD as shown. AFP: Alpha-fetoprotein; ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CI: Confidence interval; FBG: Fasting blood glucose; GGT: Glutamyl transpeptidase; HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma; HR: Hazard ratio; LAM: Lamivudine. .
Table 3.
Multivariate analysis of factors associated with hepatitis B-related cirrhosis progression to hepatocellular carcinoma in nucleos(t)ide analog-treated patients
|
Risk factor
|
β
|
SE
|
Wald
|
P
value
|
OR (95%CI)
|
| Age ≥ 60 yr | 1.127 | 0.390 | 8.347 | 0.004 | 3.089 (1.437-6.631) |
| Smoking history | 1.387 | 0.397 | 12.180 | < 0.01 | 4.001 (1.836-8.716) |
| Family history of HBV-related HCC | 1.911 | 0.860 | 4.938 | < 0.05 | 6.763 (1.253-36.499) |
| LAM resistance | 1.082 | 0.456 | 5.638 | 0.018 | 2.949 (1.207-7.208) |
| HBV DNA negative | -3.479 | 0.816 | 19.427 | < 0.01 | 0.026 (0.007-0.139) |
| FBG ≥ 6.16 mmol/L | 1.977 | 0.339 | 34.030 | < 0.01 | 7.219 (3.716-14.024) |
CI: Confidence interval; FBG: Fasting blood glucose; HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma; LAM: Lamivudine OR: Odds ratio;; SE: Standard error.
Serum AFP levels in the No-HCC and HCC groups
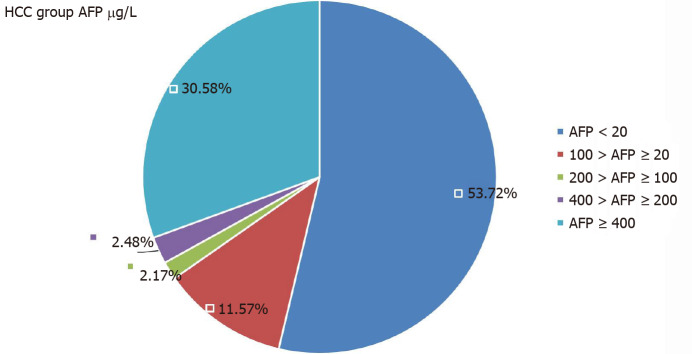
In the HCC group, there were 56 patients with serum AFP levels > 20 μg/L, but that was seen in only 17 patients in the No-HCC group (P < 0.001). In the HCC group, 65 patients had AFP levels < 20 μg/L. The AFP levels of 37 patients in the HCC group were > 400 μg/L, but only two patients in the No-HCC group levels > 400 μg/L (P < 0.001, Table 4). The 17 patients in the No-HCC group with AFP levels > 20 µg/L were followed for 1 year. Their AFP level was determined every month and showed a dynamic decline, returning to normal within 1 year. All patients underwent enhanced MRI of imaging of the liver. We believe that hepatitis B activity, rather than HCC, led to the AFP abnormality. The AFP level distributions in the two groups are shown in Figures 1 and 2.
Table 4.
Comparison of the alpha-fetoprotein level distributions in patients with and without hepatocellular carcinoma, n (%)
|
AFP (μg/L)
|
No-HCC group (n = 145) | HCC group (n = 121) |
P
value
|
| < 20 | 128 (88.27) | 65 (53.72) | < 0.001 |
| 20-100 | 12 (8.27) | 14 (11.57) | 0.51 |
| 100-200 | 1 (0.69) | 2 (2.17) | 0.231 |
| 200-400 | 2 (1.38) | 3 (2.48) | 1 |
| ≥ 400 | 2 (1.38) | 37 (30.58) | < 0.001 |
AFP: Alpha-fetoprotein; HCC: Hepatocellular carcinoma.
Figure 1.
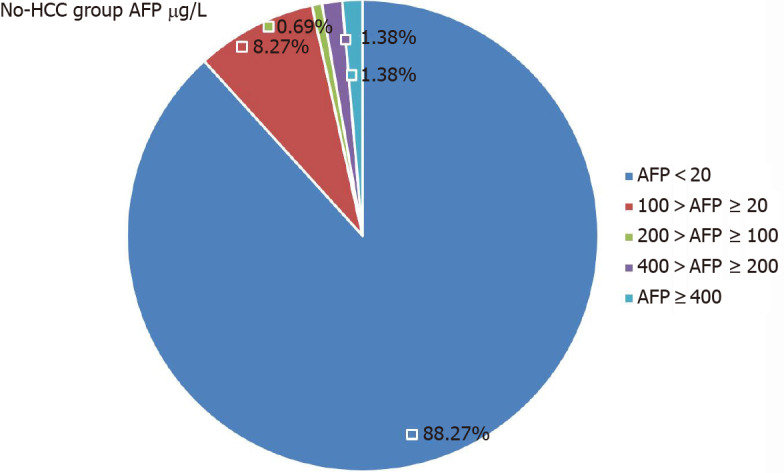
Distribution of alpha-fetoprotein levels in patients without hepatocellular carcinoma. AFP: Alpha-fetoprotein; HCC: Hepatocellular carcinoma.
Figure 2.
Distribution of alpha-fetoprotein level in patients with hepatocellular carcinoma. AFP: Alpha-fetoprotein; HCC: Hepatocellular carcinoma.
Serum AFP has limited ability to diagnose HBV-related HCC
We investigated the value of using serum AFP to diagnose HCC in patients who had HBV-related cirrhosis and were receiving NAs therapy. The AUROC of serum AFP for the diagnosis of HCC was 0.746 (95%CI: 0.674-0.818). The sensitivity of serum AFP in diagnosing HCC in those patients was 0.609, and the specificity was 0.818. The positive predictive value of HCC was 22.51%, the negative predictive value of HCC was 46.07%, the cutoff was 9.00, and the Youden index was 0.427 (Figure 3).
Figure 3.
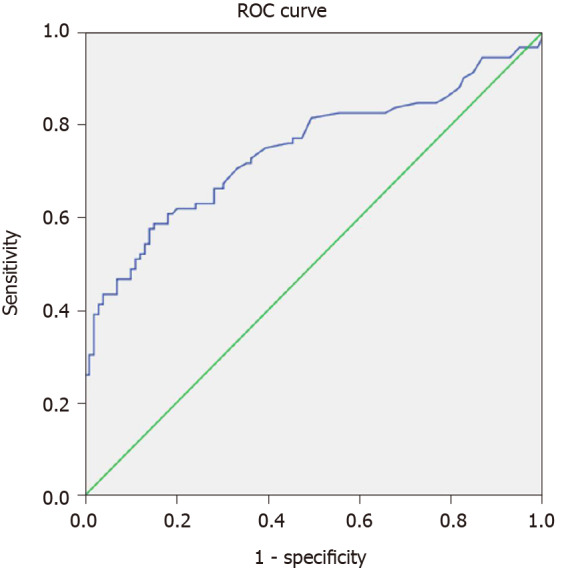
Receiver operating characteristic curve analysis of alpha-fetoprotein to diagnose hepatocellular carcinoma in patients with hepatitis B virus-related cirrhosis receiving nucleos(t)ide analog therapy. ROC: Receiver operating characteristic.
DISCUSSION
HBV infection remains a major risk factor for the development of cirrhosis and HCC[17]. Patients with chronic HBV are at risk of developing liver-related complications, namely, cirrhosis and HCC. In China, 77% of cirrhosis cases and 84% of HCC cases are caused by HBV infection[18]. Antiviral therapy can reduce but not eliminate the risk of developing HCC[19-21]. The annual incidence of HCC ranges from 0.01% to 5.4% in patients with CHB who are treated with ENT or TDF[19,22]. NAs antiviral treatments can markedly inhibit viral replication and improve liver necrosis, inflammation, and fibrosis. However, NAs cannot eliminate covalently closed template DNA (cccDNA) produced during hepatitis B viral replication or clear the integrated HBV genome; therefore, NAs cannot completely block hepatitis B cirrhosis from developing into HCC[12,21]. The persistence of cccDNA and an integrated HBV genome is the basis for hepatitis B cirrhosis progressing to primary liver cancer[23,24]. In this study, we investigated the clinical characteristics of HCC progression in patients with HBV-related cirrhosis who received antiviral therapy with NAs.
Medical and healthcare progress, improved living standards, and decreased population fertility have led to population aging in China. Data from 22 tumor registration centers in China have shown that the average age of liver cancer onset increased from 58.80 to 62.35 years for men and from 64.02 to 68.99 years for women between 2000 and 2014[25]. Our study found that age ≥ 60 years was an independent risk factor for the progression of hepatitis B-related cirrhosis to HCC while receiving NAs antiviral therapy. An aging population and the burden caused by HCC mortality could be a future challenge for China. Previous studies have confirmed that poor lifestyle habits are related to a high incidence of liver cancer; in particular, a history of smoking and drinking increases the risk of HCC[26,27]. Tobacco smoke contains various carcinogens, 11 of which are classified as International Agency for Research on Cancer Group 1 human carcinogens. Epidemiologic evidence from a recent meta-analysis showed a positive association between current tobacco smoking and liver cancer risk (risk ratio: 1.55, 95%CI: 1.46-1.65), suggesting a causal role of smoking in liver cancer development[28]. Liu et al[29] found that tobacco smoking and HBV infection positively interact in the development of liver cancer. Our results revealed smoking to be an independent risk factor of HCC progression (95%CI: 1.836-8.716, P < 0.01) even if the patients were receiving antiviral treatment. Studies have frequently reported that a family history of liver cancer increases HCC risk independent of hepatitis. The combination of a family history of liver cancer and hepatitis B serum markers is associated with a greater than 70-fold increase in HCC risk[30]. Super-additive and super-multiplicative interactions may exist between a family history of liver cancer and HBV infection that increase the risk of the development of liver cancer[31]. Our study found that a family history of HBV-related HCC is a risk factor of progression in cirrhotic patients receiving antiviral therapy (95%CI: 1.253-36.499, P < 0.05). Our results suggest that patients with HBV-related cirrhosis who smoke should quit smoking and cultivate a healthy lifestyle to reduce the risk of developing HCC[32,33]. Furthermore, close monitoring should be carried out if a patient’s first-degree relative develops HBV-related HCC during antiviral treatment.
LAM has been used as an antiviral treatment for hepatitis B for the past 20 years in China. Some Chinese patients with hepatitis B-related cirrhosis have previously undergone primary LAM monotherapy. Studies have shown that long-term LAM antiviral therapy can delay disease progression, reduce liver function decompensation, and reduce the incidence of HCC[34,35]. The inflammation seen in liver histology can improve in patients with hepatitis B-related cirrhosis when they are treated with LAM, but with a prolonged treatment time, the incidence of drug-resistant viral mutation increases[34,36]. The clinical benefit of LAM is limited by the emergence of resistant mutant strains and viral breakthrough. Although some LAM-resistant CHB patients have received ADV combination therapy or sequential ENT or TDF monotherapy, some of these patients did not experience a beneficial treatment effect and still had continuous replication of HBV in the liver[37,38]. In patients with LAM resistance, those with cirrhosis had a higher HCC risk than non-cirrhotic patients. Rescue treatment with ADV in patients who developed viral breakthrough did not appear to reduce the risk of HCC compared with untreated patients without remission[11]. In our study of patients treated with antiviral therapy for more than 6 mo, the number of HBV DNA-negative patients in the HCC group was lower than that in the No-HCC group. Many studies have found a correlation between serum HBV DNA levels and the occurrence of HCC in patients with hepatitis B. Kaneko et al[39] reported that detectable HBV DNA was significantly associated with a higher risk of HCC development compared with continuously undetectable HBV DNA. Chen et al[40] found that the incidence of HCC increased with serum HBV DNA level at study entry in a dose-response relationship ranging from 108/100,000 person-years for an HBV DNA level of < 300 copies/mL to 1152/100,000 person-years for an HBV DNA level of 1 million copies/mL or more. The corresponding cumulative incidence rates of HCC were 1.3% and 14.9%.
Even if the serum HBV DNA level in patients with hepatitis B-related cirrhosis is kept at a low level (< 2000 IU/mL), the risk of HCC is still high[41]. As shown in our study, HBV DNA negativity (HR = 0.026, 95%CI: 0.007-0.139, P < 0.01) independently predicted HCC progression in patients with HBV-related cirrhosis who received NAs therapy. Thus, to help avoid HCC progression, hepatitis B patients should continue to maintain an HBV DNA-negative status. Therefore, drugs with a high genetic barrier to resistance are suggested as first-line antiviral drugs for HBV therapy, and are recommended by current guidelines. Drugs such as ETV, TDF, and TDF alafenamide fumarate should be selected to generate a sustained antiviral treatment response and to reduce the occurrence of HBV resistance and the incidence of HCC[42,43].
As an important metabolic organ, the liver plays a key role in maintaining glucose homeostasis. Studies have found a positive relationship between liver cancer and diabetes mellitus[44,45]. Cell and animal experiments have shown that type 2 diabetes and male sex are associated with HCC development. Gao et al[46] demonstrated that heterozygous deletion of the Ncoa5 gene caused spontaneous development of HCC exclusively in male mice, and NCOA5 deficiency increased susceptibility to both glucose intolerance and HCC. In a prospective cohort study, adjusted multivariable analysis showed that participants with 4.82 mmol/L ≤ FBG ≤ 5.49 mmol/L had a 47% increased risk of HCC, and those with an FBG > 5.49 mmol/L had a 69% increased risk[47]. In our study, the FBG level in the HCC group was higher than that in the No-HCC group (Table 1), and FBG ≥ 6.16 mmol/L was an independent risk factor for the HCC progression in patients with hepatitis B-related cirrhosis receiving NA antiviral treatment. Controlling blood sugar concentrations might be a way to decrease the risk of HCC in the Chinese population.
AFP is a glycoprotein that exists in a variety of different glycotypes. AFP has been used in the screening, diagnosis, efficacy evaluation, and prognosis evaluation of HCC. AFP elevation is commonly seen in active hepatitis, pregnancy, liver cancer, and embryonic tumors[48,49]. Previous studies reported that there was a significant correlation between serum AFP levels and the tumor size in liver cancer, and that the sensitivity and specificity of AFP depended on the selected serum level threshold[20,50]. Liu et al[51] found that approximately one-third of patients with HCC had normal serum AFP levels and that the level was related to the volume of liver cancer lesions, vascular invasion, and differentiation. In our study, we found 56 patients with a serum AFP > 20 µg/L, and 19 with serum AFP levels between 20 and 400 µg/L and with HCC confirmed by imaging and histopathological examination of liver masses. However, an AFP level of < 20 μg/L did not exclude the possibility of HCC. This study showed that 65 patients with a serum AFP of < 20 μg/L had space-occupying lesions that were confirmed as HCC by MRI enhancement, histopathology, and liver DSA examination. That indicates that more sensitive diagnostic markers of HCC must be developed. In addition, more sensitive serum tumor markers such as milk fat globule-EGF factor 8, osteopontin, miRNA classifier, glypican-3[52-54], and others should be actively investigated or combined to identify HCC at an early stage[55]. However, their clinical sensitivity and specificity must first be confirmed.
This study had some limitations. First, it was not prospective. The impact of antiviral treatment time and the amount of smoking and drinking on the development of HCC require confirmation in prospective studies with larger sample sizes. Second, longer follow-up and surveillance of HBV-related cirrhosis patients receiving NA therapy is necessary to observe whether they progress to HCC in their lifetime, even though the 145 cirrhotic patients with NA therapy did not progress to HCC during the observation period. Third, additional molecular markers should be assessed for their ability to provide an early diagnosis of HCC in patients with HBV-related cirrhosis. Some data indicate that the currently used potent NAs can reduce but not eliminate the risk of HCC. The inability to eliminate HCC risk might persist because of risk factors that are not amenable to change by antiviral therapy or because of events that may have taken place before treatment initiation.
CONCLUSION
In conclusion, age ≥ 60 years, a history of smoking, family history of HBV-related HCC, LAM resistance, HBV DNA negativity, and FBG ≥ 6.16 mmol/L were risk factors for HCC progression in patients with HBV-related cirrhosis who received NAs therapy. Patients with HBV-related cirrhosis should be treated with NA antiviral therapy that has a high genetic barrier to resistance[7] in order to improve the long-term response to antiviral therapy, to maintain an HBV DNA-negative status, and to prevent subsequent hepatitis activity. The early identification of HCC in patients with HBV-related cirrhosis remains difficult. Patients who receive antiviral therapy with NAs, especially those older than 60 years of age, should avoid smoking, control their blood sugar at a reasonable level, and undergo routine imaging examination of liver biochemistry and serum AFP evaluation. If space-occupying lesions are identified, the patient should undergo liver CT or MRI enhancement, or even liver DSA examination to identify HCC as promptly as possible, even if the AFP level is within the normal range.
ARTICLE HIGHLIGHTS
Research background
Antiviral therapy cannot completely block the progression of hepatitis B to hepatocellular carcinoma (HCC). Furthermore, there are few early predictors of HCC progression and early identification is difficult in patients with HBV-related cirrhosis who receive nucleos(t)ide analog (NA) therapy. The study is helpful to provide HCC prevention and control strategies by analyzing the risk factors of HCC progression and the diagnostic value of AFP for HCC in those people.
Research motivation
The study objective was to identify factors that affect the occurrence of HCC and how to identify early HCC in patients with hepatitis B virus (HBV)-related cirrhosis who receive NA therapy. The results can improve the understanding of the development of HCC in those patients so as to improve the early detection and prevention of HCC.
Research objectives
The study objectives were to clarify risk factors and the diagnostic value of alpha-fetoprotein (AFP) for HCC progression in patients with HBV-related cirrhosis treated with NAs and to provide new strategies for prevention and control of HCC in those patients.
Research methods
Logistic regression analysis was used to analyze the risk factors of HCC progression. The diagnostic value of AFP for HCC was evaluated by receiver operating chara-cteristic curve analysis.
Research results
The study showed that age ≥ 60 years, smoking history, family history of HCC, lamivudine resistance, HBV DNA negativity, fasting blood sugar ≥ 6.16 mmol/L were independent risk factors of HCC progression. Serum AFP had limited diagnostic value for HCC. The results provide a meaningful strategy for early prediction and identification for HCC in those patients.
Research conclusions
A retrospective cross-sectional study was conducted to analyze risk factors of HCC progression in HBV-related cirrhosis patients receiving NA therapy. Metabolic effects of fasting blood sugar levels on the progress of HCC were seen during the receipt of NA therapy. The diagnostic value of the serum AFP level was evaluated in those patients.
Research perspectives
The study results will change the strategies used to prevent HCC in patients with HBV-related cirrhosis an receive NA therapy.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of the Zhejiang Provincial People’s Hospital (2020QT155, Hangzhou, Zhejiang Province, China).
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: January 31, 2021
First decision: March 14, 2021
Article in press: April 2, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rodrigues AT S-Editor: Yan JP L-Editor: Filipodia P-Editor: Liu JH
Contributor Information
Dan-Hong Yang, Department of Infectious Diseases, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Hangzhou 310014, Zhejiang Province, China. ydh-11@163.com.
Wei-Ping Wang, Postgraduate Department, Bengbu Medical College, Bengbu 233030, Anhui Province, China.
Qiang Zhang, Postgraduate Department, Bengbu Medical College, Bengbu 233030, Anhui Province, China.
Hong-Ying Pan, Department of Infectious Diseases, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Hangzhou 310014, Zhejiang Province, China.
Yi-Cheng Huang, Department of Infectious Diseases, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Hangzhou 310014, Zhejiang Province, China.
Jia-Jie Zhang, Department of Infectious Diseases, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Hangzhou 310014, Zhejiang Province, China.
Data sharing statement
No additional data are available.
References
- 1.Sayiner M, Golabi P, Younossi ZM. Disease Burden of Hepatocellular Carcinoma: A Global Perspective. Dig Dis Sci. 2019;64:910–917. doi: 10.1007/s10620-019-05537-2. [DOI] [PubMed] [Google Scholar]
- 2.Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, Negri E. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67:302–309. doi: 10.1016/j.jhep.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Llovet IM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 4.McClune AC, Tong MJ. Chronic hepatitis B and hepatocellular carcinoma. Clin Liver Dis. 2010;14:461–476. doi: 10.1016/j.cld.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Ou DP, Yang LY, Huang GW, Tao YM, Ding X, Chang ZG. Clinical analysis of the risk factors for recurrence of HCC and its relationship with HBV. World J Gastroenterol. 2005;11:2061–2066. doi: 10.3748/wjg.v11.i14.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu CM, Liaw YF. Hepatitis B virus-related cirrhosis: natural history and treatment. Semin Liver Dis. 2006;26:142–152. doi: 10.1055/s-2006-939752. [DOI] [PubMed] [Google Scholar]
- 7.Chen QY, Dong BQ, Yang JY, Wei SC, Fang KX, Wang XY, Fang ZL. [A prospective study of the relationship between serum hepatitis B virus DNA and the risk of primary liver cancer] Zhonghua Gan Zang Bing Za Zhi. 2009;17:930–934. [PubMed] [Google Scholar]
- 8.Chen YC, Chu CM, Yeh CT, Liaw YF. Natural course following the onset of cirrhosis in patients with chronic hepatitis B: a long-term follow-up study. Hepatol Int. 2007;1:267–273. doi: 10.1007/s12072-007-5001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu T, Seto WK, Zhu RX, Lai CL, Yuen MF. Prevention of hepatocellular carcinoma in chronic viral hepatitis B and C infection. World J Gastroenterol. 2013;19:8887–8894. doi: 10.3748/wjg.v19.i47.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu CY, Lin JT, Ho HJ, Su CW, Lee TY, Wang SY, Wu C, Wu JC. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology 2014; 147: 143-151. :e5. doi: 10.1053/j.gastro.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 11.Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348–356. doi: 10.1016/j.jhep.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 12.Papatheodoridis GV, Manolakopoulos S, Touloumi G, Vourli G, Raptopoulou-Gigi M, Vafiadis-Zoumbouli I, Vasiliadis T, Mimidis K, Gogos C, Ketikoglou I, Manesis EK HEPNET. Greece Cohort Study Group. Virological suppression does not prevent the development of hepatocellular carcinoma in HBeAg-negative chronic hepatitis B patients with cirrhosis receiving oral antiviral(s) starting with lamivudine monotherapy: results of the nationwide HEPNET. Greece cohort study. Gut. 2011;60:1109–1116. doi: 10.1136/gut.2010.221846. [DOI] [PubMed] [Google Scholar]
- 13.Hepatology Branch of Chinese Medical Association. Infectious diseases branch of Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (2015 Edition) Zhonghua Shiyan He linchuang Ganranbing Zazhi. 2015;9:570–589. [Google Scholar]
- 14.Hepatology Branch of Chinese Medical Association. Infectious diseases branch of Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (2019 version) Zhonghua Shiyan He linchuang Ganranbing Zazhi. 2019;37:711–736. [Google Scholar]
- 15.Ministry of Health of the People's Republic of China. Standardization for diagnosis and treatment for hepatocellular carcinoma. Zhonghua Zhongliu Linchunag. 2011;16:929–946. [Google Scholar]
- 16.Health and Bureau of Medical Administration, National Health and Family Planning Commission of the People's Republic of China. Standardization of diagnosis and treatment for hepatocellular carcinoma (2017 edition) Zhonghua Waike Xiaohua Zazhi. 2017;16:635–647. [Google Scholar]
- 17.Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, Su J, Sun CA, Liaw YF, Chen CJ Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HBV (REVEAL-HBV) Study Group. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138:1747–1754. doi: 10.1053/j.gastro.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Lin SX, Tao J, Wei XQ, Liu YT, Chen YM, Wu B. Study of liver cirrhosis over ten consecutive years in Southern China. World J Gastroenterol. 2014;20:13546–13555. doi: 10.3748/wjg.v20.i37.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arends P, Sonneveld MJ, Zoutendijk R, Carey I, Brown A, Fasano M, Mutimer D, Deterding K, Reijnders JG, Oo Y, Petersen J, van Bömmel F, de Knegt RJ, Santantonio T, Berg T, Welzel TM, Wedemeyer H, Buti M, Pradat P, Zoulim F, Hansen B, Janssen HL VIRGIL Surveillance Study Group. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: limited role for risk scores in Caucasians. Gut. 2015;64:1289–1295. doi: 10.1136/gutjnl-2014-307023. [DOI] [PubMed] [Google Scholar]
- 20.Vlachogiannakos J, Papatheodoridis G. Hepatocellular carcinoma in chronic hepatitis B patients under antiviral therapy. World J Gastroenterol. 2013;19:8822–8830. doi: 10.3748/wjg.v19.i47.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loomba R, Liu J, Yang HI, Lee MH, Lu SN, Wang LY, Iloeje UH, You SL, Brenner D, Chen CJ REVEAL–HBV Study Group. Synergistic effects of family history of hepatocellular carcinoma and hepatitis B virus infection on risk for incident hepatocellular carcinoma. Clin Gastroenterol Hepatol 2013; 11: 1636-45. :e1–3. doi: 10.1016/j.cgh.2013.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62:956–967. doi: 10.1016/j.jhep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Yan H, Yang Y, Zhang L, Tang G, Wang Y, Xue G, Zhou W, Sun S. Characterization of the genotype and integration patterns of hepatitis B virus in early- and late-onset hepatocellular carcinoma. Hepatology. 2015;61:1821–1831. doi: 10.1002/hep.27722. [DOI] [PubMed] [Google Scholar]
- 24.Zeng HM, Cao MM, Zheng RS, Zhang SC, Cai JQ, Qu CF, Bi XY, Zou XN, Chen WQ, He J. Trend analysis of mean age of diagnosis for liver cancer in cancer registry are as in China, 2000-2014. Zhonghua Yufan Yixue Zazhi. 2018;52:573–578. doi: 10.3760/cma.j.issn.0253-9624.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64:S84–S101. doi: 10.1016/j.jhep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Pascual S, Herrera I, Irurzun J. New advances in hepatocellular carcinoma. World J Hepatol. 2016;8:421–438. doi: 10.4254/wjh.v8.i9.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baecker A, Liu X, La Vecchia C, Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev. 2018;27:205–212. doi: 10.1097/CEJ.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdel-Rahman O, Helbling D, Schöb O, Eltobgy M, Mohamed H, Schmidt J, Giryes A, Mehrabi A, Iype S, John H, Tekbas A, Zidan A, Oweira H. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: An updated systematic review of 81 epidemiological studies. J Evid Based Med. 2017;10:245–254. doi: 10.1111/jebm.12270. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Baecker A, Wu M, Zhou JY, Yang J, Han RQ, Wang PH, Jin ZY, Liu AM, Gu X, Zhang XF, Wang XS, Su M, Hu X, Sun Z, Li G, Mu L, He N, Li L, Zhao JK, Zhang ZF. Interaction between tobacco smoking and hepatitis B virus infection on the risk of liver cancer in a Chinese population. Int J Cancer. 2018;142:1560–1567. doi: 10.1002/ijc.31181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turati F, Edefonti V, Talamini R, Ferraroni M, Malvezzi M, Bravi F, Franceschi S, Montella M, Polesel J, Zucchetto A, La Vecchia C, Negri E, Decarli A. Family history of liver cancer and hepatocellular carcinoma. Hepatology. 2012;55:1416–1425. doi: 10.1002/hep.24794. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Baecker A, Wu M, Zhou JY, Yang J, Han RQ, Wang PH, Jin ZY, Liu AM, Gu X, Zhang XF, Wang XS, Su M, Hu X, Sun Z, Li G, Fu A, Jung SY, Mu L, He N, Li L, Zhao JK, Zhang ZF. Family history of liver cancer may modify the association between HBV infection and liver cancer in a Chinese population. Liver Int. 2019;39:1490–1503. doi: 10.1111/liv.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol. 2018;113:175–194. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24:1073274817729245. doi: 10.1177/1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J Cirrhosis Asian Lamivudine Multicentre Study Group. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 35.Su MH, Lu AL, Li SH, Zhong SH, Wang BJ, Wu XL, Mo YY, Liang P, Liu ZH, Xie R, He LX, Fu WD, Jiang JN. Long-term lamivudine for chronic hepatitis B and cirrhosis: A real-life cohort study. World J Gastroenterol. 2015;21:13087–13094. doi: 10.3748/wjg.v21.i46.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao GB, Zhu M, Cui ZY, Wang BE, Yao JL, Zeng MD. A 7-year study of lamivudine therapy for hepatitis B virus e antigen-positive chronic hepatitis B patients in China. J Dig Dis. 2009;10:131–137. doi: 10.1111/j.1751-2980.2009.00375.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen CH, Lin CL, Hu TH, Hung CH, Tseng PL, Wang JH, Chang JY, Lu SN, Chien RN, Lee CM. Entecavir vs. lamivudine in chronic hepatitis B patients with severe acute exacerbation and hepatic decompensation. J Hepatol. 2014;60:1127–1134. doi: 10.1016/j.jhep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Liu S, Chen YU, Zheng S, Zhou LI, Lu F, Duan Z. Lamivudine-resistant rtL180M and rtM204I/V are persistently dominant during combination rescue therapy with entecavir and adefovir for hepatitis B. Exp Ther Med. 2016;11:2293–2299. doi: 10.3892/etm.2016.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaneko S, Kurosaki M, Joko K, Marusawa H, Kondo M, Kojima Y, Uchida Y, Kimura H, Tsuji K, Yagisawa H, Kusakabe A, Kobashi H, Akahane T, Tamaki N, Kirino S, Abe T, Yoshida H, Matsushita T, Hasebe C, Izumi N. Detectable HBV DNA during nucleos(t)ide analogues stratifies predictive hepatocellular carcinoma risk score. Sci Rep. 2020;10:13021. doi: 10.1038/s41598-020-69522-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Sinn DH, Kang W, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology. 2017;66:335–343. doi: 10.1002/hep.28916. [DOI] [PubMed] [Google Scholar]
- 42.European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Ghany MG. Current treatment guidelines of chronic hepatitis B: The role of nucleos(t)ide analogues and peginterferon. Best Pract Res Clin Gastroenterol. 2017;31:299–309. doi: 10.1016/j.bpg.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 44.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 45.Shang RZ, Qu SB, Wang DS. Reprogramming of glucose metabolism in hepatocellular carcinoma: Progress and prospects. World J Gastroenterol. 2016;22:9933–9943. doi: 10.3748/wjg.v22.i45.9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao S, Li A, Liu F, Chen F, Williams M, Zhang C, Kelley Z, Wu CL, Luo R, Xiao H. NCOA5 haploinsufficiency results in glucose intolerance and subsequent hepatocellular carcinoma. Cancer Cell. 2013;24:725–737. doi: 10.1016/j.ccr.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu T, Wang W, Cui H, Sun M, Wang Y, Liu X, Cao L, Liu H, Liu S. Elevated fasting serum glucose levels increase the risk of hepatocellular carcinoma: A prospective cohort study. Medicine (Baltimore) 2019;98:e16369. doi: 10.1097/MD.0000000000016369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng W, Bai B, Bai Z, Li Y, Yue P, Li X, Qiao L. The immunosuppression role of alpha-fetoprotein in human hepatocellular carcinoma. Discov Med. 2016;21:489–494. [PubMed] [Google Scholar]
- 49.Zhou L, Liu C, Meng FD, Qu K, Tian F, Tai MH, Wei JC, Wang RT. Long-term prognosis in hepatocellular carcinoma patients after hepatectomy. Asian Pac J Cancer Prev. 2012;13:483–486. doi: 10.7314/apjcp.2012.13.2.483. [DOI] [PubMed] [Google Scholar]
- 50.Abbasi A, Bhutto AR, Butt N, Munir SM. Corelation of serum alpha fetoprotein and tumor size in hepatocellular carcinoma. J Pak Med Assoc. 2012;62:33–36. [PubMed] [Google Scholar]
- 51.Liu S, Wang M, Zheng C, Zhong Q, Shi Y, Han X. Diagnostic value of serum glypican-3 alone and in combination with AFP as an aid in the diagnosis of liver cancer. Clin Biochem. 2020;79:54–60. doi: 10.1016/j.clinbiochem.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Shimagaki T, Yoshio S, Kawai H, Sakamoto Y, Doi H, Matsuda M, Mori T, Osawa Y, Fukai M, Yoshida T, Ma Y, Akita T, Tanaka J, Taketomi A, Hanayama R, Yoshizumi T, Mori M, Kanto T. Serum milk fat globule-EGF factor 8 (MFG-E8) as a diagnostic and prognostic biomarker in patients with hepatocellular carcinoma. Sci Rep. 2019;9:15788. doi: 10.1038/s41598-019-52356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu M, Zheng J, Wu F, Kang B, Liang J, Heskia F, Zhang X, Shan Y. OPN is a promising serological biomarker for hepatocellular carcinoma diagnosis. J Med Virol. 2020 doi: 10.1002/jmv.25704. [DOI] [PubMed] [Google Scholar]
- 54.Lin XJ, Chong Y, Guo ZW, Xie C, Yang XJ, Zhang Q, Li SP, Xiong Y, Yuan Y, Min J, Jia WH, Jie Y, Chen MS, Chen MX, Fang JH, Zeng C, Zhang Y, Guo RP, Wu Y, Lin G, Zheng L, Zhuang SM. A serum microRNA classifier for early detection of hepatocellular carcinoma: a multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study. Lancet Oncol. 2015;16:804–815. doi: 10.1016/S1470-2045(15)00048-0. [DOI] [PubMed] [Google Scholar]
- 55.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.