Abstract
Background
Pressure ulcers (also known as pressure injuries, pressure sores, decubitus ulcers and bed sores) are localised injuries to the skin or underlying soft tissue, or both, caused by unrelieved pressure, shear or friction. Beds, overlays or mattresses are widely used with the aim of treating pressure ulcers.
Objectives
To assess the effects of beds, overlays and mattresses on pressure ulcer healing in people with pressure ulcers of any stage, in any setting.
Search methods
In November 2019, we searched the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations); Ovid Embase and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
We included randomised controlled trials that allocated participants of any age to pressure‐redistributing beds, overlays or mattresses. Comparators were any beds, overlays or mattresses that were applied for treating pressure ulcers.
Data collection and analysis
At least two review authors independently assessed studies using predetermined inclusion criteria. We carried out data extraction, 'Risk of bias' assessment using the Cochrane 'Risk of bias' tool, and the certainty of the evidence assessment according to Grading of Recommendations, Assessment, Development and Evaluations methodology.
Main results
We included 13 studies (972 participants) in the review. Most studies were small (median study sample size: 72 participants). The average age of participants ranged from 64.0 to 86.5 years (median: 82.7 years) and all studies recruited people with existing pressure ulcers (the baseline ulcer area size ranging from 4.2 to 18.6 cm2,median 6.6 cm2). Participants were recruited from acute care settings (six studies) and community and long‐term care settings (seven studies). Of the 13 studies, three (224 participants) involved surfaces that were not well described and therefore could not be classified. Additionally, six (46.2%) of the 13 studies presented findings which were considered at high overall risk of bias. We synthesised data for four comparisons in the review: alternating pressure (active) air surfaces versus foam surfaces; reactive air surfaces versus foam surfaces; reactive water surfaces versus foam surfaces, and a comparison between two types of alternating pressure (active) air surfaces. We summarise key findings for these four comparisons below.
(1) Alternating pressure (active) air surfaces versus foam surfaces: we are uncertain if there is a difference between alternating pressure (active) air surfaces and foam surfaces in the proportion of participants whose pressure ulcers completely healed (two studies with 132 participants; the reported risk ratio (RR) in one study was 0.97, 95% confidence interval (CI) 0.26 to 3.58). There is also uncertainty for the outcomes of patient comfort (one study with 83 participants) and adverse events (one study with 49 participants). These outcomes have very low‐certainty evidence. Included studies did not report time to complete ulcer healing, health‐related quality of life, or cost effectiveness.
(2) Reactive air surfaces versus foam surfaces: it is uncertain if there is a difference in the proportion of participants with completely healed pressure ulcers between reactive air surfaces and foam surfaces (RR 1.32, 95% CI 0.96 to 1.80; I2 = 0%; 2 studies, 156 participants; low‐certainty evidence). When time to complete pressure ulcer healing is considered using a hazard ratio, data from one small study (84 participants) suggests a greater hazard for complete ulcer healing on reactive air surfaces (hazard ratio 2.66, 95% CI 1.34 to 5.17; low‐certainty evidence). These results are sensitive to the choice of outcome measure so should be interpreted as uncertain. We are also uncertain whether there is any difference between these surfaces in patient comfort responses (1 study, 72 participants; very low‐certainty evidence) and in adverse events (2 studies, 156 participants; low‐certainty evidence). There is low‐certainty evidence that reactive air surfaces may cost an extra 26 US dollars for every ulcer‐free day in the first year of use (1 study, 87 participants). Included studies did not report health‐related quality of life.
(3) Reactive water surfaces versus foam surfaces: it is uncertain if there is a difference between reactive water surfaces and foam surfaces in the proportion of participants with healed pressure ulcers (RR 1.07, 95% CI 0.70 to 1.63; 1 study, 101 participants) and in adverse events (1 study, 120 participants). All these have very low‐certainty evidence. Included studies did not report time to complete ulcer healing, patient comfort, health‐related quality of life, or cost effectiveness.
(4) Comparison between two types of alternating pressure (active) air surfaces: it is uncertain if there is a difference between Nimbus and Pegasus alternating pressure (active) air surfaces in the proportion of participants with healed pressure ulcers, in patient comfort responses and in adverse events: each of these outcomes had four studies (256 participants) but very low‐certainty evidence. Included studies did not report time to complete ulcer healing, health‐related quality of life, or cost effectiveness.
Authors' conclusions
We are uncertain about the relative effects of most different pressure‐redistributing surfaces for pressure ulcer healing (types directly compared are alternating pressure air surfaces versus foam surfaces, reactive air surfaces versus foam surfaces, reactive water surfaces versus foam surfaces, and Nimbus versus Pegasus alternating pressure (active) air surfaces). There is also uncertainty regarding the effects of these different surfaces on the outcomes of comfort and adverse events. However, people using reactive air surfaces may be more likely to have pressure ulcers completely healed than those using foam surfaces over 37.5 days' follow‐up, and reactive air surfaces may cost more for each ulcer‐free day than foam surfaces.
Future research in this area could consider the evaluation of alternating pressure air surfaces versus foam surfaces as a high priority. Time‐to‐event outcomes, careful assessment of adverse events and trial‐level cost‐effectiveness evaluation should be considered in future studies. Further review using network meta‐analysis adds to the findings reported here.
Plain language summary
What are the benefits and risks of different types of beds, mattresses and mattress toppers for treating pressure ulcers?
Key messages
Due to a lack of robust evidence, the benefits and risks of most types of beds, mattresses and mattress toppers for treating pressure ulcers are unclear.
Beds with an air‐filled surface that apply constant pressure to the skin may be better than mattresses and toppers made of foam for ulcer healing if the evidence on the time needed to completely heal an ulcer is looked at, but may cost more.
Future research in this area should focus on options and effects that are important to decision‐makers, such as:
‐ foam or air‐filled surfaces that redistribute pressure under the body; and
‐ unwanted effects and costs.
What are pressure ulcers?
Pressure ulcers are also known as pressure sores or bed sores. They are wounds to the skin and underlying tissue caused by prolonged pressure or rubbing. They often occur on bony parts of the body, such as heels, elbows, hips and the bottom of the spine. People who have mobility problems or who lie in bed for long periods are at risk of developing pressure ulcers.
What did we want to find out?
There are beds, mattresses and mattress toppers specifically designed for people with pressure ulcers. These can be made from a range of materials (such as foam, air cells or water bags) and are divided into two groups:
‐ reactive (static) surfaces that apply a constant pressure to the skin, unless a person moves or is repositioned; and
‐ active (alternating pressure) surfaces that regularly redistribute the pressure under the body.
We wanted to find out if reactive and active surfaces:
‐ help ulcers to heal;
‐ are comfortable and improve people’s quality of life;
‐ have health benefits that outweigh their costs; and
‐ have any unwanted effects.
What did we do?
We searched the medical literature for studies that evaluated the effects of beds, mattresses and mattress toppers. We compared and summarised their results, and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 13 studies (972 people, average age: 83 years) that lasted between seven days and 18 months (average: 37.5 days).
In general, the studies did not provide sufficiently robust evidence for us to determine the effects of active and reactive surfaces.
Evidence from two studies suggests that, when compared with mattresses and mattress toppers made of foam, beds with a reactive air‐filled surface may:
‐ improve chances of pressure ulcers healing if the data on the time needed to completely heal an ulcer is looked at (1 study, 84 people);
‐ cost an extra 26 US dollars per person for every ulcer‐free day in the first year of use (1 study, 87 people).
The other benefits and risks of these and other surfaces are unclear.
What limited our confidence in the evidence?
Most studies were small (72 people on average) and nearly half of them (six studies) used methods likely to introduce errors in their results.
How up‐to‐date is this review?
The evidence in this Cochrane Review is current to November 2019.
Summary of findings
Background
Description of the condition
Pressure ulcers — also known as pressure injuries, pressure sores, decubitus ulcers and bed sores — are localised injuries to the skin or underlying soft tissue (or both), caused by unrelieved pressure, shear or friction (EPUAP/NPIAP/PPPIA 2019). Pressure ulcer severity is generally classified as follows, using the National Pressure Injury Advisory Panel (NPIAP) system (NPIAP 2016).
Stage 1: intact skin with a local appearance of non‐blanchable erythema
Stage 2: partial‐thickness skin loss with exposed dermis
Stage 3: full‐thickness skin loss
Stage 4: full‐thickness skin and tissue loss with visible fascia, muscle, tendon, ligament, cartilage or bone
Unstageable pressure injury: full‐thickness skin and tissue loss that is obscured by slough or eschar so that the severity of injury cannot be confirmed
Deep tissue pressure injury: local injury of persistent, non‐blanchable deep red, maroon, purple discolouration or epidermal separation revealing a dark wound bed or blood‐filled blister
The stages described above are consistent with those described in another commonly used system, the International Classification of Diseases for Mortality and Morbidity Statistics (World Health Organization 2019).
Pressure ulcers are complex wounds that are relatively common, affecting people across different care settings. A systematic review found that prevalence estimates for people affected by pressure ulcers in communities of the UK, USA, Ireland and Sweden ranged from 5.6 to 2300 per 10,000 depending on the nature of the population surveyed (Cullum 2016). A subsequent cross‐sectional survey of people receiving community health services in one city in the UK estimated that 1.8 people per 10,000 have a pressure ulcer (Gray 2018). Estimates of pressure ulcer prevalence in hospitals range from 470 to 3210 per 10,000 patients in the UK, USA and Canada (Kaltenthaler 2001).
Pressure ulcers confer a heavy burden in terms of personal impact and use of health‐service resources. Having a pressure ulcer may impair physical, social and psychological activities (Gorecki 2009). Ulceration impairs health‐related quality of life (Essex 2009); can result in longer institution stays (Graves 2005); and increases the risk of systemic infection (Livesley 2002). There is also substantial impact on health systems: a 2015 systematic review of 14 studies across a range of care settings in Europe and North America showed that costs related to pressure ulcer treatment ranged from EUR 1.71 to EUR 470.49 per person, per day (Demarré 2015). In the UK, the annual average cost to the National Health Service for managing one person with a pressure ulcer in the community was estimated to be GBP 1400 for a Stage 1 pressure ulcer and more than GBP 8500 for more severe stages (2015/2016 prices; Guest 2018). In Australia, the annual cost of treating pressure ulcers was estimated to be AUD 983 million (95% confidence interval (CI) 815 million to 1151 million) at 2012/2013 prices (Nguyen 2015). The serious consequences of pressure ulceration have led to an intensive focus on their prevention.
Description of the intervention
Pressure ulcers are considered treatable. Support surfaces are specialised medical devices designed to relieve or redistribute pressure on the body, or both, in order to prevent and treat pressure ulcers (NPIAP S3I 2007). Support surfaces are widely used for treating pressure ulcers. These include, but are not limited to, integrated bed systems, mattresses and overlays (NPIAP S3I 2007).
The NPIAP Support Surface Standards Initiative (S3I) terms and definitions related to support surfaces can be used to classify types of support surface (NPIAP S3I 2007). According to this system, beds, mattresses and overlays may:
be powered (i.e. require electrical power to function) or non‐powered;
passively redistribute body weight (i.e. reactive pressure redistribution), or mechanically alternate the pressure on the body (i.e. active pressure redistribution);
be made of a range of materials, including but not limited to: air cells, foam materials, fibre materials, gel materials, sheepskin for medical use and water bags; and
be constructed of air‐filled cells that have small holes on the surface for blowing out air to dry skin (i.e. low air‐loss feature) or have fluid‐like characteristics via forcing filtered air through ceramic beads (i.e. air‐fluidised feature), or have neither of these features.
Full details of bed, overlay and mattress classifications are listed in Appendix 1. Various types of beds, overlays and mattresses can be used for treating pressure ulcers, including alternating pressure (active) air surfaces, reactive air surfaces, high‐specification reactive foam surfaces, and alternative reactive support surfaces that are made of neither foam materials or air cells.
How the intervention might work
The aim of using support surfaces to treat pressure ulceration is to redistribute pressure beneath the body, thereby facilitating blood flow to tissues and preventing distortion of the skin and soft tissue (Wounds International 2010). Active support surfaces achieve pressure redistribution by frequently changing the points of contact between the surface and body, reducing the duration of the pressure applied to each anatomical site (Clark 2011;NPIAP S3I 2007). This contrasts with the mode of action of reactive support surfaces, which is more passive and includes immersion (i.e. 'sinking' of the body into a support surface) and envelopment (i.e. conforming of a support surface to the irregularities in the body). These devices distribute the pressure over a greater area, thereby reducing the magnitude of the pressure at specific sites (Clark 2011).
Why it is important to do this review
Beds, overlays and mattresses are the focus of recommendations in international and national guidelines (EPUAP/NPIAP/PPPIA 2019; NICE 2014). Since the publication of the Cochrane Review, 'Support surfaces for treating pressure ulcers' (McInnes 2018), there has been international recognition of the NPIAP S3I terms and definitions related to support surfaces (NPIAP S3I 2007). It is important to update the evidence base to ensure that it is contemporaneous with current guidelines and other reviews in the field.
In this evidence update, we will consider all types of beds and mattresses (instead of including other types of support surfaces such as cushions, as in McInnes 2018) because beds and mattresses are the primary focus in pressure ulcer guidelines (EPUAP/NPIAP/PPPIA 2019; NICE 2014). We have therefore changed the title of this review to 'Beds, overlays and mattresses for treating pressure ulcers' (Differences between protocol and review).
Objectives
To assess the effects of beds, overlays and mattresses on pressure ulcer healing in people with pressure ulcers of any stage, in any setting.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs), including multi‐armed studies, cluster‐RCTs and cross‐over trials, regardless of the language of publication. We excluded studies using quasi‐random allocation methods (e.g. alternation).
Types of participants
We included studies in people with a diagnosis of pressure ulcer of any stage (EPUAP/NPIAP/PPPIA 2019), managed in any care setting. We accepted study authors' definitions of pressure ulcer stage. Where study authors used grading scales other than NPIAP, we mapped these to the NPIAP scale (EPUAP/NPIAP/PPPIA 2019).
Types of interventions
We included studies that assessed beds and mattresses (i.e. integrated bed systems, mattresses and overlays) (see Description of the intervention). The types of bed and mattress support surfaces we planned to cover included:
alternating pressure (active) air surfaces (e.g. alternating pressure air mattress, dynamic low‐air‐loss mattresses, Softform Premier Active air mattresses);
foam surfaces;
reactive air surfaces (e.g. Sofflex static air overlay);
reactive fibre surfaces (e.g. Silicore fibre overlay);
reactive gel surfaces (e.g. a gel pad used on an operating table);
reactive sheepskin surfaces (e.g. Australian Medical Sheepskins overlay); and
reactive water surfaces.
We planned to include studies where two or more bed and mattress support surfaces were used sequentially over time or in combination, where the beds or mattresses of interest would have been included in one of the study arms. However, we did not identify such studies.
We included studies comparing eligible beds, overlays and mattresses against any comparator defined as a bed, overlay or mattress. Comparators were not limited to any specific type of support surfaces. They could be either different from, or the same type as, the eligible bed, overlay or mattress of interest. We included studies in which co‐interventions (e.g. repositioning) were delivered, provided that the co‐interventions were the same in all arms of the study (i.e. interventions randomised were the only systematic difference).
Types of outcome measures
Primary outcomes
The primary outcome of this review was complete pressure ulcer healing. We included studies that measured complete pressure ulcer healing. Trialists used a range of different methods for measuring and reporting this outcome. RCTs that reported one or more of the following were considered as providing the most relevant and rigorous measures of ulcer healing.
Time to complete pressure ulcer healing (correctly analysed using survival, time‐to‐event approaches or median (or mean) time to healing, if it was clear that all ulcers were healed at follow‐up).
Proportion of participants with pressure ulcers completely healed during follow‐up.
We used the study authors' definitions of complete pressure ulcer healing, and reported these where possible. Where both the complete‐healing outcome measures listed above were reported for a study, we considered the proportion of participants with pressure ulcers healed as the primary outcome for this review. Our preferred measure was time to pressure ulcer healing; however, we did not expect it to be reported in many studies. We extracted and analysed time‐to‐event data but focused on the binary outcome in our conclusions. If an included study had only recruited people with Stage 1 ulcers and reported the outcome of the resolution of Stage 1 ulcers, we planned to term the resolution outcome as complete pressure ulcer healing in this review. We planned to use the same method to consider the resolution outcome where an included study had recruited participants with pressure ulcers of Stage 1 and those with more severe ulcers. However, we did not identify these types of studies.
Note that we recorded any other healing outcome measures reported in the included studies, such as the rate of change in the area or volume of the ulcers. However, we did not consider them as primary outcome measures and these data were not analysed because these measures are more difficult to measure accurately and are less clinically relevant than complete healing.
Secondary outcomes
Patient support‐surface‐associated comfort. We considered patient comfort outcome data in this review only if the evaluation of patient comfort was pre‐planned and was systematically conducted across all participants in the same way in a study. The definition and measurement of this outcome varied from one study to another; for example, the proportion of participants who reported comfort, or comfort measured by a scale with continuous (categorical) numbers. We included these data with different measurements in separate meta‐analyses.
All reported adverse events (measured using surveys or questionnaires, other data capture process or visual analogue scale). We included data where study authors specified a clear method for collecting adverse event data. Where available, we extracted data on all serious and all non‐serious adverse events as outcomes. We recorded where it was clear that events were reported at the participant level or whether multiple events per person were reported, in which case appropriate adjustments were required for data clustering (Peryer 2019). We considered the assessment of any event in general defined as adverse by participants, health professionals, or both.
Health‐related quality of life (measured using a standardised generic questionnaire such as EQ‐5D (Herdman 2011), 36‐item Short Form (SF‐36; Ware 1992), or pressure ulcer‐specific questionnaires such as the PURPOSE Pressure Ulcer Quality of Life (PU‐QOL) questionnaire (Gorecki 2013), at noted time points). We did not include ad hoc measures of quality of life because these measures were unlikely to be validated.
Cost effectiveness: within‐trial cost‐effectiveness analysis comparing mean differences in effects with mean cost differences between the two arms. We extracted data on incremental mean cost per incremental gain in benefit (incremental cost‐effectiveness ratio (ICER)). We also considered other measures of relative cost‐effectiveness (e.g. net monetary benefit, net health benefit).
Other outcome considerations
If a study did not report any review‐relevant outcomes but was otherwise eligible (i.e. eligible study design, participants and interventions), we planned to contact the study authors (where possible) to clarify whether they measured a relevant outcome but did not report it. We did not contact study authors for this purpose, however, because the relevant studies (see Characteristics of excluded studies) appeared to focus on the topic of ulcer prevention. We expected that the study authors did not measure any review‐relevant outcomes and excluded these studies.
If a study measured an outcome at multiple time points, we considered outcome measures at three months as of primary interest to this review (Bergstrom 2008), regardless of the time points specified as being of primary interest by the study. If the study did not report three‐month outcome measures, we considered those closest to three months.
Where a study only reported a single time point, we considered that time point in this review. Where the study did not specify a time point for their outcome measurement, we assumed this was the final duration of follow‐up noted.
Search methods for identification of studies
Electronic searches
We searched the following databases to identify reports of relevant clinical trials:
the Cochrane Wounds Specialised Register (searched 14 November 2019);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 10) in the Cochrane Library (searched 14 November 2019);
MEDLINE Ovid, including In‐Process & Other Non‐Indexed Citations (1946 to 14 November 2019);
Embase Ovid (1974 to 14 November 2019);
EBSCO Cumulative Index to Nursing and Allied Health Literature (CINAHL Plus; 1937 to 14 November 2019).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, MEDLINE Ovid, Embase Ovid and EBSCO CINAHL Plus can be found in Appendix 2. We combined the MEDLINE Ovid search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2019). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2019). We combined the CINAHL Plus search with the trial filter developed by Glanville 2019. There were no restrictions with respect to language, date of publication or study setting.
We also checked all RCTs included in the Cochrane Review McInnes 2018 against our eligibility criteria.
We also searched the following clinical trials registries:
US National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov (clinicaltrials.gov) (searched 20 November 2019);
World Health Organization (WHO) International Clinical Trials Registry Platform (https://www.who.int/clinical-trials-registry-platform) (searched 20 November 2019).
Search strategies for clinical trials registries can be found in Appendix 2.
Searching other resources
For previous versions of McInnes 2018, the review authors of McInnes 2018 contacted experts in the field of wound care to enquire about potentially relevant studies that were ongoing or recently published. In addition, the review authors of McInnes 2018 contacted manufacturers of support surfaces for details of any studies manufacturers were conducting. This approach did not yield any additional studies; therefore, we did not repeat it for this review.
We identified other potentially eligible studies or ancillary publications by searching the reference lists of retrieved included studies, as well as relevant systematic reviews, meta‐analyses and health technology assessment reports.
We did not perform a separate search for adverse events of interventions used. We considered adverse events described in included studies only.
Data collection and analysis
We carried out data collection and analysis according to the methods stated in the published protocol (Shi 2020), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Li 2019). Changes from the protocol or previous published versions of the review are documented in Differences between protocol and review.
Selection of studies
One review author (CS) re‐checked the RCTs included in McInnes 2018 for eligibility. Two review authors (CS and AJB) independently assessed the titles and abstracts of the new search results for relevance using Rayyan (Ouzzani 2016) (Differences between protocol and review), and then independently inspected the full text of all potentially eligible studies. The two review authors resolved disagreements through discussion or by involving a third review author (JCD), if necessary.
Data extraction and management
One review author (CS) checked data from the studies included in McInnes 2018, and extracted additional data where necessary. A second review author or researcher (SR, AJB, VR, EM, Zhenmi Liu, Gill Norman, or Melanie Stephens) checked any new data extracted. For new included studies, one review author (CS) independently extracted data and another review author or researcher (SR, AJB, VR, EM, Zhenmi Liu, Gill Norman, or Melanie Stephens) checked all data (Differences between protocol and review). Any disagreements were resolved through discussion or, if necessary, by involving another review author (JCD). Where necessary, we contacted the authors of included studies (or referred to relevant publications) to clarify data.
We extracted these data using a pre‐prepared data extraction form:
basic characteristics of studies (first author, publication type, publication year and country);
funding sources;
care setting;
characteristics of participants (trial eligibility criteria, average age in each arm or in a study, proportions of participants by gender and the stage of pressure ulcers at baseline);
bed and mattress support surfaces being compared (including their descriptions);
details on any co‐interventions;
duration of follow‐up;
the number of participants enrolled;
the number of participants randomised to each arm;
the number of participants analysed;
participant withdrawals, with reasons;
proportion of participants with pressure ulcers healed;
data on time to pressure ulcer healing (e.g. Kaplan Meier plot, hazard ratio (HR) and 95% confidence interval (CI));
comfort/discomfort outcome data;
adverse event outcome data;
health‐related quality of life outcome data; and
cost‐effectiveness outcome data.
We (CS and NC) classified specific beds and mattresses in the included studies into intervention groups using the NPIAP S3I terms and definitions related to support surfaces (NPIAP S3I 2007). Therefore, to accurately assign specific beds and mattresses to intervention groups, we extracted full descriptions of support surfaces from included studies, and when necessary, supplemented the information with that from external sources, such as other publications about the same support surface, manufacturers’ or product websites and expert clinical opinion (Shi 2018b). If we were unable to define any specific support surfaces evaluated in an included study, we extracted available data and reported these as additional data outside the main review results.
Assessment of risk of bias in included studies
Two review authors or researchers (CS and SR, AJB, VR, EM, Zhenmi Liu, Gill Norman, or Melanie Stephens) independently assessed risk of bias of each included study using the Cochrane 'Risk of bias' tool (see Appendix 3). This tool has seven specific domains: sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete data (attrition bias), selective outcome reporting (reporting bias), and other issues (Higgins 2017). We assessed performance bias, detection bias and attrition bias separately for each of the review outcomes (Higgins 2017). We noted that it is often impossible to blind participants and personnel in device trials. In this case, performance bias may be introduced if knowledge of treatment allocation results in deviations from intended interventions, differential use of co‐interventions or care between groups not specified in the study protocol that may influence outcomes. We attempted to understand if, and how, included studies compensated for challenges in blinding; for example, by implementing strict protocols to maximise consistency of co‐interventions between groups to reduce the risk of performance bias. We also noted that complete pressure ulcer healing is a subjective outcome. Compared with blinded assessment, non‐blinded assessment of subjective outcomes tends to be associated with more optimistic effect estimates of experimental interventions in RCTs (Hróbjartsson 2012). Therefore, we judged non‐blinded outcome assessment as being at high risk of detection bias. In this review, we included factors such as extreme baseline imbalance and unit of analysis under the domain of 'other issues' (see Appendix 3). For example, unit of analysis issues occurred where a cluster‐randomised trial had been undertaken but analysed at the individual level in the study report.
For the studies included in McInnes 2018, one review author (CS) checked the 'Risk of bias' judgements and, where necessary, updated them. A second review author or researcher (SR, AJB, VR, EM, Zhenmi Liu, Gill Norman, or Melanie Stephens) checked any updated judgement. We assigned each 'Risk of bias' domain a judgement of high, low or unclear risk of bias. We resolved any discrepancy through discussion, or by involving another review author (JCD) where necessary. Where possible, useful and feasible, when a lack of reported information resulted in a judgement of unclear risk of bias, we planned to contact study authors for clarification.
We present our assessment of risk of bias using two 'Risk of bias' summary figures: one is a summary of bias for each item across all studies, and the second shows a cross‐tabulation of each trial by all of the 'Risk of bias' items. Once judgements had been given for all domains, the overall risk of bias for each study was judged as:
low risk of bias, if we judged all domains to be at low risk of bias;
unclear risk of bias, if we judged one or more domains to be at unclear risk of bias but no domain was at high risk of bias; or
high risk of bias, as long as we judged one or more domains as being at high risk of bias, or all domains had unclear 'Risk of bias' judgements, as this could substantially reduce confidence in the result.
We resolved any discrepancy between review authors through discussion, or by involving another review author (JCD) where necessary. For studies using cluster randomisation, we planned to consider the risk of bias in relation to: recruitment bias, baseline imbalance, loss of clusters, incorrect analysis and comparability with individually randomised studies (Eldridge 2016; Higgins 2019) (Appendix 3). However, we did not include any studies with a cluster design.
Measures of treatment effect
For meta‐analysis of data on the proportion of participants with pressure ulcers healed, we present the risk ratio (RR) with its 95% confidence interval (CI). For continuous outcome data (e.g. healing rate in terms of change in the area of the ulcers), we present the mean difference (MD) with 95% CIs for studies that used the same assessment scale. If studies reporting continuous data used different assessment scales, we planned to report the standardised mean difference (SMD) with 95% CIs. However, this was not undertaken in the review.
For time‐to‐event data (e.g. time to pressure ulcer healing), we present the hazard ratio (HR) with its 95% CI. If included studies reporting time‐to‐event data did not report an HR, then, when feasible, we estimated this using other reported outcomes, such as numbers of events, through employing available statistical methods (Parmar 1998; Tierney 2007).
Unit of analysis issues
We noted whether studies presented outcomes at the level of the pressure ulcer or at the level of participants. We also recorded whether the same participant was reported as having multiple pressure ulcers. Where studies randomised at the participant level and outcomes were measured at the level of the ulcer, we considered the participant as the unit of analysis if the number of ulcers observed appeared to be equal to the number of participants (e.g. one pressure ulcer per person).
Unit of analysis issues may occur if studies randomise at the participant level but the healing of multiple pressure ulcers is observed and data are presented and analysed at the level of the ulcer (clustered data). We noted whether data regarding multiple ulcers on a participant were (incorrectly) treated as independent within a study, or were analysed using within‐participant analysis methods. If clustered data were incorrectly analysed, we recorded this as part of the 'Risk of bias' assessment.
If a cluster‐RCT was not correctly analysed, we planned to use the following information (see below) to adjust for clustering ourselves where possible, in accordance with guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
The number of clusters randomly assigned to each intervention, or the average (mean) number of participants per cluster.
Outcome data ignoring the cluster design for the total number of participants.
Estimate of the intra‐cluster (or intra‐class) correlation coefficient (ICC).
However, we did not identify any cluster‐RCTs in this review.
Cross‐over trials
For cross‐over trials, we only considered outcome data at the first intervention phase (i.e. prior to cross‐over) as eligible.
Studies with multiple treatment groups
If a study had more than two eligible study groups, where appropriate we combined results across these arms to make single pair‐wise comparisons (Higgins 2019).
Dealing with missing data
Data are commonly missing from study reports. Reasons for missing data could be the exclusion of participants after randomisation, withdrawal of participants from a study, or loss to follow‐up. The exclusion of these data from analysis may break the randomisation and potentially introduce bias.
Where there were missing data and where relevant, we contacted study authors to pose specific queries about these data. In the absence of other information, for the proportion of participants with pressure ulcers healed we assumed that participants with missing data had ulcers healed for the main analysis (i.e. we added missing data to the denominator but not the numerator). We examined the impact of this assumption through undertaking a sensitivity analysis (see Sensitivity analysis). Where a study did not specify the number of randomised participants prior to dropout, we used the available number of participants as the number randomised.
Assessment of heterogeneity
Assessing heterogeneity can be a complex, multifaceted process. Firstly, we considered clinical and methodological heterogeneity; that is, the extent to which the included studies varied in terms of participant, intervention, outcome and other characteristics, including duration of follow‐up, clinical settings and overall study‐level 'Risk of bias' judgement (Deeks 2019). In terms of the duration of follow‐up, in order to assess the relevant heterogeneity, we recorded and categorised assessment of outcome measures as follows:
up to eight weeks (short‐term);
more than eight weeks to 24 weeks (medium‐term); and
more than 24 weeks (long‐term).
We supplemented this assessment of clinical and methodological heterogeneity with information regarding statistical heterogeneity assessed using the Chi2 test. We considered a P value less than 0.10 to indicate statistically significant heterogeneity given that the Chi2 test has low power, particularly in the case where studies included in a meta‐analysis have small sample sizes. We carried out this statistical assessment in conjunction with the I2 statistic (Higgins 2003), and the use of prediction intervals for random‐effects meta‐analyses (Borenstein 2017; Riley 2011).
The I2 statistic is the percentage of total variation across studies due to heterogeneity rather than chance (Higgins 2003). Very broadly, we considered that I2 values of 25% or less may indicate a low level of heterogeneity and values of 75% or more may indicate very high heterogeneity (Higgins 2003). For random‐effects models where the meta‐analysis had more than 10 included studies and no clear funnel plot asymmetry, we also planned to present 95% prediction intervals (Deeks 2019). We planned to calculate prediction intervals following methods proposed by Borenstein 2017.
Random‐effects analyses produce an average treatment effect, with 95% confidence intervals indicating where the true population average value is likely to lie. Prediction intervals quantify variation away from this average due to between‐study heterogeneity. The interval conveys where a future study treatment effect estimate is likely to fall based on the data analysed to date (Riley 2011). Prediction intervals are always wider than confidence intervals (Riley 2011).
It is important to note that prediction intervals will reflect heterogeneity of any source, including from methodological issues as well as clinical variation. For this reason, some authors have suggested that prediction intervals are best calculated for studies at low risk of bias to ensure intervals that have meaningful clinical interpretation (Riley 2011). We had planned to calculate prediction intervals for all analyses to assess heterogeneity and then to explore the impact of risk of bias in subgroup analysis stratified by study risk of bias assessment as detailed below. However, we did not calculate any prediction intervals because all conducted meta‐analyses contained fewer than 10 studies.
Assessment of reporting biases
We followed the systematic framework recommended by Page 2019 to assess risk of bias due to missing results (non‐reporting bias) in the meta‐analysis of data on the proportion of participants with pressure ulcers healed. To make an overall judgement about risk of bias due to missing results, we:
identified whether the missing outcome data were unavailable by comparing the details of outcomes in trials registers, protocols or statistical analysis plans (if available) with reported results. If the above information sources were unavailable, we compared outcomes in the conference abstracts or in the methods section of the publication, or both, with the reported results. If we found non‐reporting of study results, we then judged whether the non‐reporting was associated with the nature of findings by using the 'Outcome Reporting Bias In Trials' (ORBIT) system (Kirkham 2018).
assessed the influence of definitely missing outcome data on meta‐analysis.
assessed the likelihood of bias where a study had been conducted but not reported in any form. For this assessment, we considered whether the literature search was comprehensive and planned to produce a funnel plot for meta‐analysis for seeking more evidence about the extent of missing results, provided there were at least 10 included studies (Peters 2008; Salanti 2014).
However, we did not produce a funnel plot for any meta‐analysis because all analyses in this review had fewer than 10 included studies.
Data synthesis
We summarised the included studies narratively and synthesised included data by using meta‐analysis where applicable. We structured comparisons according to type of comparator and then by outcomes, ordered by follow‐up period.
We considered clinical and methodological heterogeneity and undertook pooling when studies appeared appropriately similar in terms of participants, beds and mattresses and outcome type. Where statistical synthesis of data from more than one study was not possible or considered inappropriate, we conducted a narrative review of eligible studies.
Once the decision to pool was made, we used a random‐effects model, which estimated an underlying average treatment effect from studies. Conducting meta‐analysis with a fixed‐effect model in the presence of even minor heterogeneity may provide overly narrow confidence intervals. We used the Chi2 test and I2 statistic to quantify heterogeneity but not to guide choice of model for meta‐analysis (Borenstein 2009). We exercised caution when meta‐analysed data were at risk of small‐study effects because use of a random‐effects model may be unsuitable in this situation. In this case, or where there were other reasons to question the choice of a fixed‐effect or random‐effects model, we assessed the impact of the approach using sensitivity analyses to compare results from alternate models (Thompson 1999).
We performed meta‐analyses largely using Review Manager 5.4 (Review Manager 2020). We presented data using forest plots where possible. For dichotomous outcomes, we presented the summary estimate as a RR with 95% CI. Where continuous outcomes were measured, we presented the MD with 95% CIs; we planned to report SMD estimates where studies measured the same outcome using different methods. For time‐to‐event data, we presented the summary estimates as HRs with 95% CIs.
Subgroup analysis and investigation of heterogeneity
Investigation of heterogeneity
When important heterogeneity occurred, we planned to follow steps proposed by Cipriani 2013 and Deeks 2019 to investigate further:
check the data extraction and data entry for errors and possible outlying studies;
if outliers exist, perform sensitivity analysis by removing them; and
if heterogeneity was still present, we planned to perform subgroup analyses for study‐level characteristics (see below) in order to explain heterogeneity as far as possible. However, we did not undertake any subgroup analysis because meta‐analyses in this review included fewer than 10 studies.
Subgroup analysis
We investigated heterogeneity using the methods described in Section 10.11 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019). We planned to perform subgroup analyses to determine whether the size of treatment effects was influenced by these two study‐level characteristics:
risk of bias (binary: low or unclear risk of bias; and high risk of bias (Schulz 1995)); and
settings (categorical: acute care and other hospital settings; long‐term care settings; operating theatre setting; and intensive care unit).
We planned to compare subgroup findings using the 'Test for Subgroup Differences’ in Review Manager 5.4 (Review Manager 2020). We did not perform subgroup analysis when the number of studies included in the meta‐analysis was not reasonable (i.e. fewer than 10).
Sensitivity analysis
We conducted sensitivity analyses for the following factors, to assess the robustness of meta‐analysis of data on the proportion of participants with pressure ulcers healed.
Impact of the selection of healing outcome measure. The proportion of pressure ulcers completely healed was the primary outcome measure for this review but we also analysed time to pressure ulcer healing, where data were available.
Impact of missing data. The primary analysis assumed that the ulcers of participants with missing data had healed; we also analysed healing by only including data for the participants for whom we had endpoint data (complete cases).
Impact of altering the effects model used. We used a random‐effects model for the main analysis followed by a fixed‐effect analysis.
Summary of findings and assessment of the certainty of the evidence
We presented the main, pooled results of the review in 'Summary of findings' tables, which we created using GRADEpro GDT software. These tables present key information concerning the certainty of evidence, the magnitude of the effects of the interventions examined and the sum of available data for the main outcomes (Schünemann 2019). The tables also include an overall grading of the certainty of the evidence associated with each of the main outcomes that we assessed using the GRADE approach. The GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest.
The GRADE assessment involves consideration of five factors: within‐trial risk of bias, directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias (Schünemann 2019). The certainty of evidence can be assessed as being: high, moderate, low or very low. RCT evidence has the potential to be high‐certainty. We did not downgrade the certainty of evidence for the risk of bias factor in a specific circumstance. That is, if the blinding of participants and personnel was the only domain resulting in our judgement of overall high risk of bias for the included studies; however, for these studies, it was impossible to blind participants and personnel.
When downgrading for imprecision, we followed the methods described in Guyatt 2011: either considering both the optimal information size (OIS) and the 95% CI of each meta‐analysis if they were estimable; or considering the sample size, the number of events and other effectiveness indicators if the calculation of OIS and undertaking a meta‐analysis were not applicable. Where necessary, we used the GRADE 'default' minimum important difference values (RR = 1.25 and 0.75 for binary outcome data) as the thresholds to judge if a 95% CI was wide (imprecise) so as to include the possibility of clinically important harm and benefit (Guyatt 2011).
We presented a separate 'Summary of findings' table for all but one comparison evaluated in this review. The exception was the comparison of alternating pressure (active) air surfaces versus the another type of alternating pressure (active) air surfaces (Differences between protocol and review). We presented these outcomes in the 'Summary of findings' tables:
proportion of participants with pressure ulcers healed;
time to pressure ulcer healing;
patient support‐surface‐associated comfort;
all reported adverse events;
health‐related quality of life; and
cost effectiveness.
We prioritised the time points and method of outcome measurement specified in Types of outcome measures for presentation in ‘Summary of findings’ tables. Where we did not pool data for some outcomes within a comparison, we conducted a GRADE assessment for each of these outcomes and presented these assessments in a narrative format in 'Summary of findings' tables (Differences between protocol and review).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies.
Results of the search
The electronic searches identified 1624 records, including 1164 from electronic databases and 460 from trials registries. We excluded 218 duplicate records and screened 1406 records, of which 233 were identified as potentially eligible and obtained as full‐text. Following full‐text screening, we considered 13 records of 11 studies eligible for inclusion in this review (Allman 1987; Cassino 2013a; Day 1993; Devine 1995; Evans 2000a; Evans 2000b; Ferrell 1993; Ferrell 1995; Groen 1999; Russell 2000a; Strauss 1991).
From other resources, we identified eight potentially eligible records by scanning reference lists of the 14 systematic reviews or meta‐analyses that were identified from electronic searches (Chou 2013; Huang 2013; McGinnis 2011; McInnes 2015; McInnes 2018; Mistiaen 2010a; De Oliveira 2017; Rae 2018; Reddy 2006; Reddy 2008; Serraes 2018; Shi 2018a; Smith 2013; Yao 2018), as well as clinical practice guidelines listed in Searching other resources. Following full‐text screening of three full‐text reports, we considered two studies (Mulder 1994; Munro 1989) eligible for inclusion in this review.
In total we included 13 studies (15 publications) in the review, of which two separate studies were reported in the same publication (Evans 2000a; Evans 2000b). See Figure 1.
1.
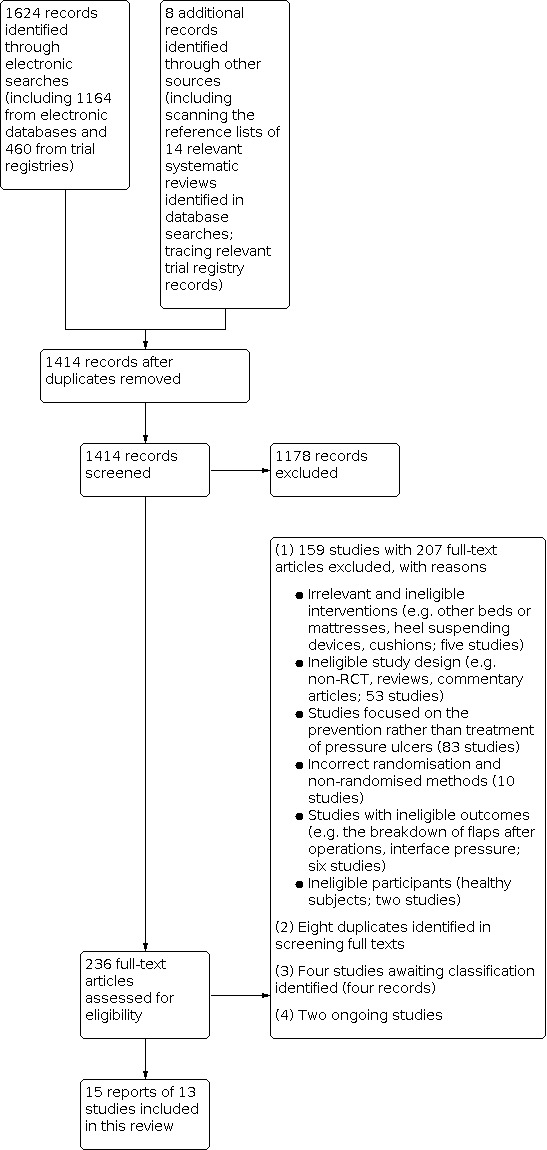
Study flow diagram
Included studies
Types of studies
Of the 13 included studies, 12 were two‐armed RCTs using a parallel group design, and one (Ferrell 1995) was a trial‐based economic evaluation associated with Ferrell 1993.
Of all included studies, five were conducted at more than one research site (Cassino 2013a; Ferrell 1993; Ferrell 1995; Groen 1999; Strauss 1991). All of the included studies were conducted in high‐income and upper‐middle‐income economies in the regions of Europe and North America, including: Italy (Cassino 2013a), the Netherlands (Groen 1999), the UK (Devine 1995; Evans 2000a; Evans 2000b; Russell 2000a), and the USA (Allman 1987; Day 1993; Ferrell 1993; Ferrell 1995; Mulder 1994; Munro 1989; Strauss 1991).
In the 13 studies, the median duration of follow‐up was 37.5 days (range: 7 days to 18 months).
Types of participants
Age and sex at baseline
The 13 studies enrolled a total of 972 participants (median study sample size: 72 participants; range: 12 to 183). The average participant age was specified for 11 studies and ranged from 64.0 to 86.5 years (median: 82.7 years). Sex was specified for 10 studies; and within these 284 (46.3%) of participants were male and 329 (53.7%) were female.
Pressure ulcer characteristics at baseline
All 13 studies (972 participants) recruited people with existing pressure ulcers, of which Cassino 2013a recruited people with ulcers of stage I to IV; six recruited those with ulcers of stage II to IV or stage III to IV (Day 1993; Devine 1995; Evans 2000a; Evans 2000b; Mulder 1994; Strauss 1991); but five did not specify the stage of ulcers included (Allman 1987; Ferrell 1993; Ferrell 1995; Groen 1999; Russell 2000a). Six studies specified the pressure ulcer stage systems used, including the Shea criteria (Allman 1987; Ferrell 1993; Strauss 1991), the Torrance criteria (Russell 2000a), and the early versions of the EPUPA/NPUAP stage system (Cassino 2013a; Day 1993).
The average size of pressure ulcers at baseline was specified for seven studies (353 participants; Allman 1987; Devine 1995; Evans 2000a; Evans 2000b; Ferrell 1993; Ferrell 1995; Munro 1989) and ranged from 4.2 to 18.6 cm2 (median: 6.6 cm2). Six studies did not specify the average pressure ulcer size at baseline (Cassino 2013a; Day 1993; Groen 1999; Mulder 1994; Russell 2000a; Strauss 1991).
Care settings
Participants were from two types of settings, including:
acute care settings (including hospitals in general) (Allman 1987; Day 1993; Devine 1995; Evans 2000a; Munro 1989; Russell 2000a); and
community and long‐term care settings (including community, nursing homes, long‐term facilities, geriatric units) (Cassino 2013a; Ferrell 1993; Ferrell 1995; Evans 2000b; Groen 1999; Mulder 1994; Strauss 1991).
Types of interventions
Beds and mattresses evaluated in included studies are summarised in Appendix 4. The studies investigated a wide range of support surfaces, including alternating pressure (active) air surfaces, reactive air surfaces, foam surfaces, reactive gel surfaces, reactive water surfaces and a type of reactive surface that we could not define using NPIAP S3I 2007 support surface terms.
In terms of comparator surfaces, 10 of the 13 studies used surfaces that could be classified using the NPIAP S3I support surfaces terms and definitions. The following control surfaces could not be classified further:
the 'standard bed' evaluated in Munro 1989 (40 participants) as the control surface was not specified;
the ‘conventional therapy’ evaluated in Strauss 1991 (112 participants) as the control surface was one of many options from 'alternating pressure pads, air support mattresses, water mattresses, and high‐density foam pads”; and
the Aiartex® overlay evaluated in one study (72 participants; Cassino 2013a) as the surface did not match any of the NPIAP S3I support surfaces terms.
We defined the control surfaces used in Munro 1989 and Strauss 1991 as 'standard hospital surfaces'.
Nine studies specified co‐interventions (e.g. repositioning, cushions) (Allman 1987; Devine 1995; Evans 2000a; Evans 2000b; Ferrell 1993; Groen 1999; Mulder 1994; Russell 2000a; Strauss 1991); all stated or indicated that the same co‐interventions were applied in all study groups.
Funding sources
All but one of the 13 included studies specified the details of funding sources. All of these 12 studies were completely or partly funded by industry or received mattresses under evaluation from industries (Allman 1987; Cassino 2013a; Day 1993; Devine 1995; Evans 2000a; Evans 2000b; Ferrell 1993; Ferrell 1995; Mulder 1994; Munro 1989; Russell 2000a; Strauss 1991).
Excluded studies
We excluded 159 studies (with 207 records). The main reasons for these 159 exclusions were: irrelevant and ineligible interventions (five studies); ineligible study design (e.g. non‐RCT, reviews, commentary articles; 53 studies); studies focused on the prevention rather than treatment of pressure ulcers (83 studies); incorrect randomisation and non‐randomised methods (10 studies); studies with ineligible outcomes (six studies); and ineligible participants (healthy subjects; two studies). We also identified eight duplicates in screening full texts (see Figure 1).
Ongoing studies
We identified two ongoing studies from the trials registry search (ACTRN12618000319279; JPRN‐UMIN000029680). The two studies randomised participants who had existing pressure ulcers into two study arms using different support surfaces. They both measured pressure ulcer healing outcomes and other outcomes (e.g. participant support‐surface‐associated comfort).
Studies awaiting classification
There were four studies (four records) about which we could not make eligibility decisions because we were unable to obtain them in full text despite extensive efforts (in part due to more limited access to intra‐library loans during the COVID‐19 period) (Chaloner 2000b; Henn 2004; Knight 1999; Melland 1998).
Risk of bias in included studies
We summarise 'Risk of bias' assessments for the primary outcome of this review in Figure 2 and Figure 3.
2.
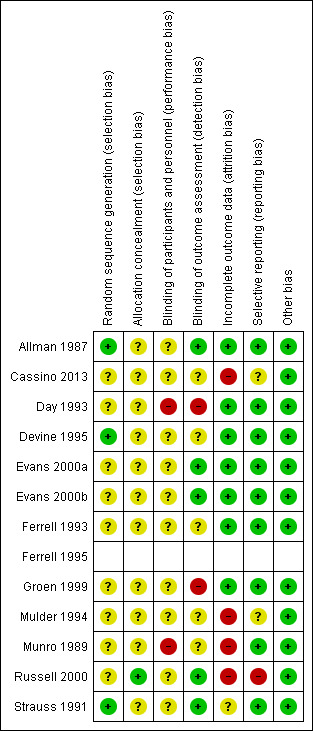
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.
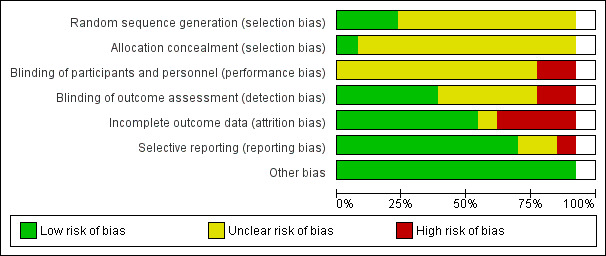
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
We judged six of the 12 RCTs (of the 13 studies) as having unclear overall risk of bias for the primary outcome (Allman 1987; Devine 1995; Evans 2000a; Evans 2000b; Ferrell 1993; Strauss 1991). We judged another six studies as being at high overall risk of bias as they had one or more domains with high risk of bias judgement (Cassino 2013a; Day 1993; Groen 1999; Mulder 1994; Munro 1989; Russell 2000a). Of these six studies, three had high risk of bias judgement for the primary outcome in domains of blinding of participants and personnel, blinding of outcome assessment, or both (Day 1993; Groen 1999; Munro 1989).
Publication bias
We ran a comprehensive search and were able to locate two eligible studies from other resources. We considered the risk of having missed published reports to be low. We were unable to assess for the risk of non‐publication of studies with negative findings as we could not present funnel plots given the small number of included studies in each analysis.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Alternating pressure (active) air surfaces compared with foam surfaces for treating pressure ulcers.
| Alternating pressure (active) air surfaces compared with foam surfaces for treating pressure ulcers | ||||||
| Patient or population: people with pressure ulcers Setting: acute care setting and nursing home Intervention: alternating pressure (active) air surfaces Comparison: foam surfaces | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with foam surfaces | Risk with alternating pressure (active) air surfaces | |||||
| Proportion of participants with pressure ulcers completely healed Follow‐up: range 7 days to 12 weeks | Two studies reported this outcome: Mulder 1994 reported analysable data and the RR was 0.97 (95% CI 0.26 to 3.58). Day 1993 did not report analysable data but stated that the analysis of covariance showed no statistically significant difference in the healing of pressure ulcers between alternating pressure (active) air surfaces and foam surfaces (F[1,78] = 0.35, P value > 0.05). | not estimable | 132 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain if there is a difference in the proportion of participants with healed pressure ulcers between alternating pressure (active) air surfaces and foam surfaces. | |
| Time to pressure ulcer healing | Included studies did not report this outcome. | |||||
| Support surface‐associated patient comfort (assessed with the visual analogue scale, ranging from very comfortable at one end of the scale to very uncomfortable at the other end of the scale; however, the range of scores was not specified) Follow‐up: 7 days | The mean support surface associated patient comfort was 0 | MD 0.4 higher (0.42 lower to 1.22 higher) | ‐ | 39 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain whether there is any difference between alternating pressure (active) air surfaces and foam surfaces in patient comfort responses. |
| All reported adverse events Follow‐up: 12 weeks | Mulder 1994 (49 participants) reported there was no major adverse events that could be attributed to the support surfaces used. | not estimable | 49 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain if there is a difference in adverse events between alternating pressure (active) air surfaces and foam surfaces. | |
| Health‐related quality of life | Included studies did not report this outcome. | |||||
| Cost effectiveness | Included studies did not report this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded twice for high risk of detection bias or attrition bias. bDowngraded twice for substantial imprecision due to the small sample size and the reported very wide confidence interval and null effect.
Summary of findings 2. Reactive air surfaces compared with foam surfaces for treating pressure ulcers.
| Reactive air surfaces compared with foam surfaces for treating pressure ulcers | ||||||
| Patient or population: people with pressure ulcers Setting: acute care setting and nursing home Intervention: reactive air surfaces Comparison: foam surfaces | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with foam surfaces | Risk with reactive air surfaces | |||||
| Proportion of participants with pressure ulcers completely healed Follow‐up: 13.0 days and 37.5 days | Study population | RR 1.32 (0.96 to 1.80) | 156 (2 RCTs) | ⊕⊕⊝⊝ Lowa | It is uncertain if there is a difference in the proportion of participants with pressure ulcers completely healed between reactive air surfaces and foam surfaces. | |
| 442 per 1000 | 583 per 1000 (424 to 795) | |||||
| Time to complete pressure ulcer healing Follow‐up: 37.5 days |
Study population | HR 2.66 (1.34 to 5.17) | 84 (1 RCT) | ⊕⊕⊝⊝ Lowb | People using reactive air surfaces may be more likely to have healed pressure ulcers compared with using foam surfaces. | |
| 463 per 1000 | 809 per 1000 (566 to 960) | |||||
| Support surface associated patient comfort Follow‐up: 13 days | The only included study (Allman 1987; 72 participants) defined this outcome as the number of participants having changes in comfort from baseline with the level of comfort measured by asking participants: “Which of the following best describes the bed you are using here in the hospital: very comfortable, comfortable, uncomfortable, or very uncomfortable?”. Allman 1987 reported 8 participants using reactive air surfaces had increased comfort, 4 without change, and 1 with decreased comfort whilst 3 participants using foam surfaces had increased comfort, 4 had no change and 6 reported decreased comfort (P value = 0.04). | ‐ | 72 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c | We are uncertain whether there is any difference between reactive air surfaces and foam surfaces in patient comfort responses. | |
| All reported adverse events Follow‐up: range 13.0 days to 37.5 days | Two studies (156 participants) reported this outcome (Allman 1987; Ferrell 1993). We did not pool these data as the definitions of adverse events varied between studies. | ‐ | 156 (2 RCTs) | ⊕⊕⊝⊝ Lowd,e | It is uncertain if there is a difference in adverse events between reactive air surfaces and foam surfaces | |
| Health‐related quality of life | Included studies did not report this outcome. | |||||
| Cost‐effectiveness Follow‐up: 37.5 days | Ferrell 1995 (87 participants) reported the additional cost due to the use of reactive air surfaces divided by the additional days without an ulcer and suggested that people using reactive air surfaces may cost an extra 26 US dollars for every ulcer‐free day in the first year. | ‐ | 87 (1 RCT) | ⊕⊕⊝⊝ Lowf | Reactive air surfaces may cost an extra 26 US dollars for every ulcer‐free day in the first year | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded twice for imprecision as the optimal information size (OIS) was not met and the wide confidence interval crossed RR = 1.25. bDowngraded twice for imprecision due to the very small sample size. cDowngraded twice for high risk of attrition bias for this outcome. dDowngraded once for imprecision due to the small sample size. eDowngraded once for indirectness as the outcome of "all reported adverse events" as a whole was not used in the included studies. fDowngraded twice for imprecision for the time to ulcer healing outcome from which the cost effectiveness was evaluated.
Summary of findings 3. Reactive water surfaces compared with foam surfaces for treating pressure ulcers.
| Reactive water surfaces compared with foam surfaces for treating pressure ulcers | ||||||
| Patient or population: people with pressure ulcers Setting: nursing home Intervention: reactive water surfaces Comparison: foam surfaces | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with foam surfaces | Risk with reactive water surfaces | |||||
| Proportion of participants with pressure ulcers completely healed Follow‐up: 4 weeks | Study population | RR 1.07 (0.70 to 1.63) | 101 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain if there is a difference in the proportion of participants with healed pressure ulcers between reactive water surfaces and foam surfaces. | |
| 449 per 1000 | 480 per 1000 (314 to 732) | |||||
| Time to complete pressure ulcer healing | Included studies have not reported this outcome. | |||||
| Support surface associated patient comfort | Included studies have not reported this outcome. | |||||
| All reported adverse events Follow‐up: 4 weeks | Groen 1999 (120 participants) reported this outcome, which was defined as the percentages of participants with one or more of the following types of adverse events: eczema, maceration and pain (Table 4). | ‐ | 120 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c,d | It is uncertain if there is any difference in adverse events between reactive water surfaces and foam surfaces. | |
| Health‐related quality of life | Included studies did not report this outcome. | |||||
| Cost‐effectiveness | Included studies did not report this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded twice for high risk of detection bias in the only study. bDowngraded twice for imprecision as the optimal information size (OIS) was unmet and the very wide confidence interval crossed RRs = 0.75 and 1.25. cDowngraded once for imprecision due to the small sample size. dDowngraded once for indirectness as the study reported specific adverse events rather than all reported adverse events.
See Table 1; Table 2; Table 3.
Unless otherwise stated, random‐effects analysis was used throughout. Each pooled result presented is an average effect, rather than a common effect, and should be interpreted as such.
We have not reported data from the three studies with surfaces that were not classified in the main body of the results (Cassino 2013a; Munro 1989; Strauss 1991). For completeness, we summarise the results of these studies in Appendix 5 and Appendix 6.
We performed data analyses for the following comparisons and outcomes.
Comparison 1: Alternating pressure (active) air surfaces versus foam surfaces (two studies, 132 participants)
Two studies compared alternating pressure (active) air surfaces with foam surfaces (Day 1993; Mulder 1994).
Primary outcomes
Proportion of participants with pressure ulcers completely healed (follow‐up duration 7 days and 12 weeks)
Both studies (132 participants) reported this outcome. It is uncertain if there is a difference in the proportion of participants with healed pressure ulcers between alternating pressure (active) air surfaces and foam surfaces. Of the two studies, Mulder 1994 (49 participants) reported analysable data and the RR is 0.97 (95% CI 0.26 to 3.58; Analysis 1.1). Day 1993 (83 participants) did not report analysable data but stated that an analysis of covariance showed no statistically significant difference in the healing of pressure ulcers between alternating pressure (active) air surfaces versus foam surfaces (F[1,78] = 0.35, P value > 0.05). Evidence is of very low certainty, downgraded twice for high risk of detection or attrition bias in the two included studies, and twice for imprecision due to the small sample sizes and the wide confidence interval reported in one study and the null effect reported in another study.
1.1. Analysis.

Comparison 1: Alternating pressure (active) air surfaces compared with foam surfaces, Outcome 1: Proportion of participants with pressure ulcers completely healed
Subgroup analysis
We considered the studies included for this outcome heterogeneous in terms of care settings and follow‐up durations. Because Analysis 1.1 included only one study, however, we did not undertake a subgroup analysis.
Sensitivity analyses
We did not perform any pre‐specified sensitivity analyses because Analysis 1.1 included only one study with available data. In addition, the included studies did not report data on time to pressure ulcer healing.
Secondary outcomes
Support‐surface‐associated patient comfort (follow‐up duration 7 days)
It is uncertain whether there is any difference between alternating pressure (active) air surfaces and foam surfaces in patient comfort responses. Only Day 1993 (83 participants) reported this outcome, defined as the participant self‐rated perception of comfort using a visual analogue scale ranging from 'Very comfortable' to 'Very uncomfortable'. There were only outcome data for 39 participants: the MD is 0.40 (95% CI ‐0.42 to 1.22; Analysis 1.2). Evidence is of very low certainty, downgraded twice for high risk of attrition bias for this outcome, and twice for substantial imprecision due to the small sample size and very wide confidence interval.
1.2. Analysis.

Comparison 1: Alternating pressure (active) air surfaces compared with foam surfaces, Outcome 2: Support surface associated patient comfort
All reported adverse events (follow‐up duration 12 weeks)
It is uncertain if there is a difference in adverse events between alternating pressure (active) air surfaces and foam surfaces. Only Mulder 1994 (49 participants) reported this outcome but stated there were no major adverse events that could be attributed to the support surfaces used. Evidence is of very low certainty, downgraded twice for high risk of attrition bias, and twice for imprecision due to the small sample sizes.
Health‐related quality of life
Not reported.
Cost effectiveness
Not reported.
Comparison 2: Reactive air surfaces versus foam surfaces (three studies, 156 participants)
Three studies (156 participants) compared reactive air surfaces with foam surfaces (Allman 1987; Ferrell 1993; Ferrell 1995). Ferrell 1995 was an economic evaluation based on the trial of Ferrell 1993.
Primary outcomes
Proportion of participants with pressure ulcers completely healed (follow‐up duration 13 days and 37.5 days)
Two studies (156 participants) reported data for this outcome that were pooled (Allman 1987; Ferrell 1993). It is uncertain if there is a difference in the proportion of participants with completely healed pressure ulcers between reactive air surfaces (46/79 (58.2%)) and foam surfaces (34/77 (44.2%)). The RR is 1.32 (95% CI 0.96 to 1.80; I2 = 0%; Analysis 2.1). Evidence is of low certainty, downgraded twice for imprecision due to the OIS being unmet and the wide confidence interval that crossed RR = 1.25.
2.1. Analysis.

Comparison 2: Reactive air surfaces compared with foam surfaces, Outcome 1: Proportion of participants with pressure ulcers completely healed
Subgroup analysis
We considered the studies included in Analysis 2.1 heterogeneous in terms of care settings. As noted in Subgroup analysis and investigation of heterogeneity, because there were fewer than 10 studies, however, a subgroup analysis was not undertaken.
Sensitivity analyses
Sensitivity analysis with fixed‐effect (rather than random‐effects) model . The use of fixed‐effect model resulted in the same RR of 1.32 (95% CI 0.96 to 1.80; I2 = 0%). This remained consistent with the main analysis.
Sensitivity analysis with time to complete pressure ulcer healing as the primary outcome (follow‐up duration of 37.5 days) . One study (84 participants) reported this outcome measure (Ferrell 1993). The reported hazard ratio from the Cox regression (adjusted for fecal continence) is 2.66 (95% CI 1.34 to 5.17). Low‐certainty evidence suggests that people using reactive air surfaces may be more likely to have healed pressure ulcers compared with those on foam surfaces. Evidence certainty was downgraded twice for imprecision due to the very small sample size. These results are sensitive to the choice of format for the primary outcome measure so the results of Analysis 2.1 should be interpreted cautiously.
Secondary outcomes
Support‐surface‐associated patient comfort (follow‐up duration 13 days)
We are uncertain whether there is any difference between reactive air surfaces and foam surfaces in patient comfort responses. Only Allman 1987 (72 participants) reported this outcome, defined by the study authors as the number of participants having changes in comfort from baseline, with the level of comfort measured by asking participants: “Which of the following best describes the bed you are using here in the hospital: very comfortable, comfortable, uncomfortable, or very uncomfortable?” Allman 1987 reported eight participants using reactive air surfaces had increased comfort, four without change, and one with decreased comfort whilst three participants using foam surfaces had increased comfort, four had no change and six reported decreased comfort (P value = 0.04). Evidence is of very low certainty, downgraded twice for high risk of attrition bias for this outcome, and twice for imprecision due to the very small sample size.
All reported adverse events (follow‐up duration 13.0 and 37.5 days)
Two studies (156 participants) reported this outcome (Allman 1987; Ferrell 1993). We did not pool these data as the definitions of adverse events varied between studies (Table 4). It is uncertain if there is a difference in adverse events between reactive air surfaces and foam surfaces (low‐certainty evidence). Evidence certainty was downgraded once for indirectness as the outcome of "all reported adverse events" as a whole was not used in the included studies, and once for imprecision due to the small sample sizes of both studies.
1. All reported adverse events.
| Study ID | Results | Comment | |
| Comparison: reactive air surfaces versus foam surfaces | |||
| Allman 1987 | 8 died, 2 pneumonia, 10 urinary tract infections, 6 hypotension, 5 hypernatraemia, 5 oliguria, 7 sepsis, 16 fever, and 3 heart failure | 7 died, 4 pneumonia, 7 urinary tract infections, 7 hypotension, 5 hypernatraemia, 8 oliguria, 6 sepsis, 22 fever, and 6 heart failure on conventional therapy | Some participants may have had multiple adverse events. |
| Ferrell 1993 | 11 of 43 participants died | 7 of 41 died | The deaths were unlikely related to the use of specific support surfaces. |
| Comparison: foam surfaces versus reactive water surfaces | |||
| Groen 1999 | Percentage of participants with complications at week 4
|
Percentage of participants with complications at week 4
|
|
| Comparison: alternating pressure (active) air surfaces (Nimbus system) versus alternating pressure (active) air surfaces (Pegasus system) | |||
| Devine 1995 | 5 of 22 participants died | 4 of 19 participants died | The deaths were unlikely related to the use of specific support surfaces. |
| Evans 2000a | 1 of 7 participants developed methicillin‐resistant Staphylococcus aureus (MRSA) | 2 of 5 participants died | The deaths were unlikely related to the use of specific support surfaces. |
| Evans 2000b | 7 of 10 participants died | 1 of 10 participants died | The deaths were unlikely related to the use of specific support surfaces. |
| Russell 2000a | 16 of 57 participants died | 10 of 55 participants died | The deaths were unlikely related to the use of specific support surfaces. |
Health‐related quality of life
Not reported.
Cost effectiveness (follow‐up duration 37.5 days)
Only Ferrell 1995 (87 participants) reported this outcome, which considered standard care costs, support surfaces cost and additional nursing time for pressure ulcer care in base‐case cost‐effectiveness analysis. Ferrell 1995 reported the additional cost due to the use of reactive air surfaces divided by the additional days without an ulcer. Low‐certainty evidence suggests that reactive air surfaces may cost an extra 26 US dollars for every ulcer‐free day in the first year of use. Evidence certainty was downgraded twice for imprecision for the time to ulcer healing outcome from which the cost effectiveness was evaluated.
Comparison 3: Reactive water surfaces versus foam surfaces (one study, 120 participants)
Primary outcomes
Proportion of participants with pressure ulcers completely healed (follow‐up duration four weeks)
Groen 1999 (101 participants) reported this outcome. It is uncertain if there is a difference in the proportion of participants with completely healed pressure ulcers between reactive water surfaces (25/52 (48.1%)) and foam surfaces (22/49 (44.9%)). The RR is 1.07 (95% CI 0.70 to 1.63; Analysis 3.1). The evidence is of very low certainty, downgraded twice for high risk of detection bias and twice for imprecision due to the OIS being unmet and a very wide confidence interval that crossed RRs = 0.75 and 1.25.
3.1. Analysis.

Comparison 3: Reactive water surfaces compared with foam surfaces, Outcome 1: Proportion of participants with pressure ulcers completely healed
Sensitivity analyses
We did not perform any pre‐specified sensitivity analyses because Analysis 3.1 included only one study with available data. In addition, the included study did not report data on time to pressure ulcer healing.
Secondary outcomes
Support‐surface‐associated patient comfort
Not reported.
All reported adverse events (follow‐up duration four weeks)
Groen 1999 (120 participants) reported this outcome, defined as the percentage of participants with one or more of the following adverse events: eczema, maceration and pain (Table 4). It is uncertain if there is any difference in adverse events between reactive water surfaces and foam surfaces. Evidence is of very low certainty, downgraded twice for high risk of detection bias, once for indirectness as the study reported specific adverse events rather than all reported adverse events, and once for imprecision due to the small sample size (120 participants).
Health‐related quality of life
Not reported.
Cost effectiveness
Not reported.
Comparison 4: Comparison between two types of alternating pressure (active) air surface (four studies, 256 participants)
We included four studies (256 participants) comparing different types of alternating pressure (active) air surfaces: all used distinct Nimbus alternating pressure air systems in one study arm and different Pegasus systems in the other study arm (Devine 1995; Evans 2000a; Evans 2000b; Russell 2000a).
We did not pool data from the four studies but instead summarised study findings narratively below with outcome data presented in Table 4, Table 5, and Table 6.
2. Pressure ulcer healing outcome results reported in studies that compared different types of alternating pressure (active) air surfaces.
| Study ID | Results | Comment | |
| Comparison: alternating pressure (active) air surfaces compared with other types of alternating pressure (active) air surfaces | |||
| Devine 1995 | Alternating pressure (active) air surfaces (Nimbus I DFS)
|
Alternating pressure (active) air surfaces (Pegasus Airwave)
|
|
| Evans 2000a | Alternating pressure (active) air surfaces (Nimbus 3)
|
Alternating pressure (active) air surfaces (Pegasus Airwave, Pegasus Biwave and AlphaXcell, or Pegasus Cairwave)
|
|
| Evans 2000b | Alternating pressure (active) air surfaces (Nimbus 3)
|
Alternating pressure (active) air surfaces (Pegasus AlphaXcell, and Quattro)
|
|
| Russell 2000a | Alternating pressure (active) air surfaces (Nimbus 3)
|
Alternating pressure (active) air surfaces (Pegasus Cairwave therapy system)
|
|
3. Support‐surface‐associated patient comfort.
| Study ID | Results | Comment | |
| Comparison: alternating pressure (active) air surface type (Nimbus system) versus alternating pressure (active) air surface type (Pegasus system) | |||
| Devine 1995 | Median 8 (range 5 to 10) | Median 8 (range 3 to 10) |
|
| Evans 2000a | Median 5 (range 5 to 5) | Median 4 (range 4 to 5) |
|
| Evans 2000b | Median 5 (range 3 to 5) | Median 4 (2 to 5) |
|
| Russell 2000a | Participants in both study arms were equally comfortable (no statistical difference). | Participants in both study arms were equally comfortable (no statistical difference). |
|
Primary outcomes
Proportion of participants with pressure ulcers completely healed (follow‐up period minimum 20.5 days maximum 18.0 months)
Four studies (256 participants) reported data for this outcome (Devine 1995; Evans 2000a; Evans 2000b; Russell 2000a). It is uncertain if there is a difference in the proportion of participants with completely healed pressure ulcers between Nimbus alternating pressure (active) air surfaces (38/88 (43.2%)) and Pegasus alternating pressure (active) air surfaces (27/87 (31.0%)). See Table 5. Evidence is of very low certainty, downgraded once for risk of bias (one large study was at high risk of attrition and reporting bias and three small studies were at unclear risk of bias), twice for imprecision due to small sample size, and once for inconsistency.
The included studies did not report data on time to pressure ulcer healing.
Secondary outcomes
Support‐surface‐associated patient comfort (follow‐up duration minimum 20.5 days maximum 18.0 months)
Four studies (256 participants) reported this outcome (Devine 1995; Evans 2000a; Evans 2000b; Russell 2000a). It is uncertain if there is a difference in patient comfort responses between different types of alternating pressure (active) air surface. The studies reported a range of different outcome measurement methods (see Table 6): two small studies reported greater comfort with the Nimbus system whilst two larger studies found no difference. Evidence is of very low certainty, downgraded once for risk of bias (one large study was at high risk of attrition and reporting bias and three small studies were at unclear risk of bias), once for inconsistency, and once for imprecision due to small sample sizes.
All reported adverse events (follow‐up duration minimum 20.5 days maximum 18.0 months)
Four studies (256 participants) reported this outcome (Devine 1995; Evans 2000a; Evans 2000b; Russell 2000a). We are uncertain if there is a difference in adverse events between different types of alternating pressure (active) air surfaces. The studies largely report death data but did not state if there were other adverse events (see Table 4). Evidence is of very low certainty, downgraded once for risk of bias (one large study was at high risk of attrition and reporting bias and three small studies were at unclear risk of bias), once for indirectness as the study reported specific adverse events rather than all reported adverse events, and once for imprecision due to small sample sizes.
Health‐related quality of life
Not reported.
Cost effectiveness
Not reported.
Discussion
Summary of main results
We report evidence from 13 RCTs on the effects of a range of beds, overlays and mattresses on the healing of pressure ulcers in any setting and population. We did not analyse data reported in the three studies with surfaces that we could not classify. We synthesised evidence for the following four comparisons and summarise their key findings below.
Alternating pressure air surfaces versus foam surfaces: it is uncertain if there is a difference between alternating pressure (active) air surfaces and foam surfaces in the proportion of participants with healed pressure ulcers (two studies with 132 participants), in patient comfort (one study with 83 participants), or in adverse events (one study with 49 participants).
Reactive air surfaces versus foam surfaces: it is uncertain if there is a difference in the proportion of participants with pressure ulcers completely healed between reactive air surfaces and foam surfaces (two studies with 156 participants, low‐certainty evidence). However, this should be interpreted cautiously because there is low‐certainty evidence that people using reactive air surfaces may be more likely to have an ulcer heal over 37.5 days' follow‐up when the time to complete pressure ulcer healing is considered using a hazard ratio (one study with 84 participants). We are uncertain whether there is any difference between reactive air surfaces and foam surfaces in patient comfort (one study with 72 participants; very low‐certainty evidence) and in the risk of adverse events (two studies with 156 participants, low‐certainty evidence). Reactive air surfaces may cost an extra 26 US dollars for every ulcer‐free day in the first year of use (one study with 87 participants, low‐certainty evidence).
Reactive water surfaces versus foam surfaces: it is uncertain if there is a difference in pressure ulcer healing between reactive water surfaces and foam surfaces (one study with 101 participants) and in adverse events (one study with 120 participants).
Alternating pressure (active) air surfaces (Nimbus systems) compared with the another type of alternating pressure (active) air surfaces (Pegasus systems): it is uncertain if there is a difference between Nimbus systems and Pegasus systems in pressure ulcer healing, in patient comfort and in adverse events: each of the outcomes included four studies (256 participants) but very low‐certainty evidence.
Overall completeness and applicability of evidence
As detailed in Search methods for identification of studies, we ran a comprehensive set of literature searches to maximise the relevant research included here.
Whilst use of support surfaces is relevant to adults and children, all participants in the included studies were adults (with the reported average age ranging from 64.0 to 86.5 years, median of 82.7 years). Across the included studies, more than half (53.7%) of enrolled participants were female. All of the studies enrolled people with existing pressure ulcers: seven studies (353 participants) reported the average size of pressure ulcers ranging from 4.2 to 18.6 cm2 (median: 6.6 cm2).
Most of the included studies were small (half had fewer than 72 participants) whilst only three studies enrolled more than 100 participants (Groen 1999; Russell 2000a; Strauss 1991). The geographical scope of the included studies was limited, and all the studies were from Europe and North America.
Included studies recruited participants from two types of care settings: acute care settings (six studies); and community and long‐term care settings (seven studies). There were no specific data for operating rooms or intensive care units. Whilst two of the four comparisons included studies from these two different care settings, due to the limited number of included studies for these comparisons, we could not perform pre‐specified subgroup analysis by different care settings. Thus, for these two comparisons, we are unable to drawn conclusions about potential modification of treatment effects in different care settings.
Included studies compared a wide range of support surfaces, including alternating pressure (active) air surfaces, reactive air surfaces, foam surfaces, reactive water surfaces and reactive gel surfaces. However, there were no data for reactive fibre surfaces or reactive sheepskin surfaces.
In this review, we considered all specific types of alternating pressure air surfaces (e.g. alternating pressure air overlays, and "hybrid" air mattresses like Nimbus 3) in the broad term of "alternating pressure (active) air surfaces". This is because all these specific air surfaces have the same underlying mechanism of redistributing pressure activity (i.e. mechanically alternating pressure). Some health professionals have expressed an interest in the effectiveness of air‐filled support surfaces defined as "hybrid", based on having a mixed composition of alternating pressure mode and reactive mode as opposed to only alternating pressure mode. Exploring the evidence on “hybrid” surfaces and alternating pressure air surfaces separately may be important for future work if deemed a clinical priority.
We included three studies which had surfaces that could not be classified. We did not perform any analysis for these studies. However, for completeness of all relevant evidence, we reported the data from these studies in Appendix 5 and Appendix 6.
Another limitation in the included studies was the large variation in durations of follow‐up (ranging from seven days to 18 months, median of 37.5 days). This variation arises partly because different durations of follow‐up are appropriate in different care settings. For example, participants in acute care settings are more likely to be discharged after a short‐term hospital stay whilst those staying at community and long‐term care settings are often available for longer‐term follow‐up. The short median duration of follow‐up may contribute to an under‐estimation of pressure ulcer healing across study groups of the included studies because the median healing time for all ulcers could be 63 days (Öien 2013). Some healed pressure ulcers may have been missed in these studies.
Quality of the evidence
We implemented the GRADE approach for assessing the certainty of the evidence and found that most included evidence from our 13 meta‐analyses or syntheses across the four comparisons was of low or very low certainty: downgrading of evidence was largely due to the high risk of bias of findings and imprecision due to small study sizes. There was also some indirectness for adverse event outcomes.
Limitations in study design
We downgraded once or twice for study limitations for evidence in nine syntheses. We assessed risk of bias according to seven domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, selective outcome reporting, incomplete follow‐up, and other potential biases. Of the 13 studies, one was an economic evaluation and we judged six of the remaining 12 studies as being at high overall risk of bias and only six at unclear overall risk of bias. The prevalence of high overall risk of bias is partly due to incomplete outcome data for most of the comparisons. Two studies had high risk of performance bias (Day 1993; Munro 1989), and were related to two comparisons: alternating pressure (active) air surfaces versus foam surfaces, and reactive air surfaces versus standard hospital surfaces. We acknowledged that the blinding of participants and personnel was impractical for these two comparisons. Therefore, we did not downgrade certainty of evidence for studies at high overall risk of bias that was solely due to the possible presence of performance bias.
Two studies were also at high risk of bias due to unblinded outcome assessment (Day 1993; Groen 1999). Unblinded assessment has been found to exaggerate odds ratios (from subjective binary outcomes) by, on average, 36% (Hróbjartsson 2012). The outcome assessment of complete pressure ulcer healing relies on the subjective judgement of investigators, and blinded assessment ‐ whilst operationally challenging ‐ can be undertaken (e.g. masked adjudication of photographs of pressure areas) (Baumgarten 2009). Therefore, we considered unblinded pressure ulcer healing assessment could substantially bias effect estimates in the included studies and downgraded the certainty of evidence for detection bias on a study by study basis.
Indirectness of evidence
We downgraded for indirectness for three pieces of adverse events evidence. This was because we considered that the reported adverse event outcomes (e.g. deaths) in the included studies could not directly represent all reported adverse events (i.e. the outcome of interest for this review).
Inconsistency of results and unexplained heterogeneity
Statistical heterogeneity was low for all but two syntheses we performed and we did not downgrade for inconsistency for this evidence. The low statistical heterogeneity was partly because seven of the 13 syntheses included only one study. The exceptions are the outcomes 'pressure ulcer incidence' and 'support‐surface‐associated patient comfort' for the comparison of alternating pressure (active) air surfaces (Nimbus systems) versus another type of alternating pressure (active) air surfaces (Pegasus systems). This is because results of included studies were inconsistent for the pressure ulcer incidence and comfort outcomes for this comparison.
We have to note that although we planned to calculate prediction intervals to understand the implications of heterogeneity, none of these analyses included more than 10 studies so further analysis was not possible.
Imprecision of results
We downgraded once or twice for imprecision for evidence in all 13 syntheses. Study sample sizes were mainly small (median sample size: 72; range: 12 to 183) with small numbers of events and wide confidence intervals. Confidence intervals often crossed the line of null effect and/or RRs = 0.75 and 1.25 so we could not discern whether the true population effect was likely to be beneficial or harmful.
Publication bias
We did not downgrade the certainty of evidence for publication bias because (1) we have confidence in the comprehensiveness of our literature searches; and (2) we did not find any clear evidence of non‐reporting bias of study results. Although we planned to perform funnel plots for meta‐analysis to visually inspect for publication bias, there was no analysis including more than 10 studies.
Potential biases in the review process
We followed pre‐specified methods to review evidence in order to prevent potential bias in the review process. For example, we ran comprehensive electronic searches, searched trials registries and checked references of systematic reviews identified in electronic searches.
This review also has limitations. Firstly, some included studies may have considered co‐interventions as 'usual care' but did not fully describe them. We assumed that all studies had provided co‐interventions equally to participants in their study groups if there was nothing to indicate that this was not the case. Secondly, we did not implement pre‐specified subgroup analyses as we mentioned above, mainly because no analysis included more than 10 studies. Thirdly, we were not able to pre‐specify the comparisons included in this review. This is because specific support surfaces applied could only be known and defined once eligible studies were included. However, we used the NPIAP S3I 2007 support surface terms and definitions to define specific support surfaces as we pre‐planned in order to avoid any potential bias. Finally, we included three studies that evaluated surfaces we could not classify (Cassino 2013a; Munro 1989; Strauss 1991). Future classification of these surfaces using the NPIAP S3I support surfaces terms may change the evidence base.
Agreements and disagreements with other studies or reviews
To our knowledge, among the systematic reviews or meta‐analyses we identified in electronic searches of this review (McGinnis 2011; McInnes 2018; Rae 2018; Reddy 2008; Smith 2013), the Cochrane Review 'Support surfaces for treating pressure ulcers' (McInnes 2018) is the most recent comprehensive review.
This review is different from McInnes 2018 in eligibility criteria. In this review, we considered studies enrolling and randomising participants with existing pressure ulcers as eligible whilst McInnes 2018 included some studies that randomised participants at risk of having a new pressure ulcer and simultaneously measured ulcer healing outcome (Ewing 1964; Keogh 2001; Nixon 2006).
We included studies that reported the measure of ulcer healing but excluded studies that measured intermediate outcomes (e.g. the change in ulcer sizes), whilst McInnes 2018 included some studies with intermediate outcomes. As a result, we excluded five studies (Branom 2001; Caley 1994; Clark 1998; Osterbrink 2005; Russell 2003a) that were included in McInnes 2018. Evans 2000a and Evans 2000b were considered as one study in McInnes 2018 because they were both reported in a single publication; however, we considered them as two separate studies. We added a new study: Ferrell 1995.
The Cochrane Review McInnes 2018 followed the terms of support surfaces used in original studies. This review used the NPIAP S3I terms to define the specific support surfaces included. McInnes 2018 grouped some interventions under the term 'standard/conventional therapy' or 'standard hospital mattress' but acknowledged that the types of surfaces labelled in this way varied over time. In this review, we made great efforts to define surfaces, where these surfaces were described as a 'standard hospital surface' in the included studies, to ensure they were placed in the correct comparisons. We defined those 'standard hospital mattresses' used in the included studies as undefined surfaces if we could not collect sufficient information to accurately define them.
This review is inconsistent with McInnes 2018 in terms of the methods used for assessing risk of bias in the included studies. We applied seven risk of bias domains and the criteria of Higgins 2017 to assess the risk of bias whilst McInnes 2018 used the Cochrane tool for assessing risk of bias (Higgins 2011).
Both this review and McInnes 2018 consistently suggest that there is some uncertain evidence with the use of pairwise meta‐analysis methods. Further review work using network meta‐analysis adds to the findings reported here (Shi 2021).
Authors' conclusions
Implications for practice.
Current evidence is uncertain about the relative effects of alternating pressure (active) air surfaces versus foam surfaces, reactive water surfaces versus foam surfaces, or Nimbus versus Pegasus alternating pressure (active) air surfaces in complete pressure ulcer healing, adverse events and patient comfort responses. For people in acute care or long‐term care settings, those using reactive air surfaces may be more likely to have pressure ulcers completely healed than those using foam surfaces up to 37.5 days' follow‐up. However, people using reactive air surfaces may cost more for each ulcer‐free day than people using foam surfaces.
Implications for research.
Given the large number of different support surfaces available, future studies should prioritise which support surfaces to evaluate on the basis of the priorities of decision‐makers; for example, alternating pressure air surfaces versus foam surfaces could be a high priority for evaluation. All interventions used should be clearly described using the current classification system, and researchers should clearly specify the surfaces evaluated (avoiding use of generic terms such as 'standard hospital mattress'). Limitations in included studies are largely due to small sample size and sub‐optimal RCT design. Under‐recruitment or over‐estimation of event rates that then fail to occur, or both, can lead to imprecision and less robust effect estimates.
Future studies should also consider carefully the choice of outcomes they report; time to pressure ulcer complete healing data should be used in studies. Careful and consistent assessment and reporting of adverse events needs to be undertaken to generate meaningful data that can be compared between studies. Likewise, patient comfort is an important outcome but it is poorly defined and reported, and this needs to be considered in future research studies. Further studies should aim to collect and report health‐related quality of life using validated measures. Finally, future studies should nest cost‐effectiveness analysis in their conduct where possible.
Any future studies must be undertaken to the highest standard possible. Whilst it is challenging to avoid the risk of performance bias in trials of support surfaces as blinding of participants and personnel is seldom possible, stringent protocols ‐ for example, in terms of encouraging consistent care and blinded decision‐making ‐ can help to minimise risk. It is also important to fully describe co‐interventions (e.g. repositioning) and ensure protocols mandate balanced use of these co‐interventions across trial arms. The risk of detection bias can also be minimised with the use of digital photography and adjudicators of the photographs being masked to support surfaces (Baumgarten 2009). Follow‐up periods should be for as long as possible and clinically relevant in different settings. Where possible and useful, data collection after discharge from acute settings may be considered.
What's new
| Date | Event | Description |
|---|---|---|
| 18 August 2021 | Amended | Minor amendment to include link to overview and network meta‐analysis. |
History
Protocol first published: Issue 5, 2020 Review first published: Issue 5, 2021
Acknowledgements
The authors would like to thank Jessica Sharp for copy‐editing the protocol of this review, to Denise Mitchell for additional copy‐edit feedback, to Faith Armitage for copy‐editing the review and to Nicole Pitcher for writing the Plain Language Summary. Thanks are also due to peer reviewers Julie Bruce and Zena Moore for providing feedback on the review and to Cochrane Musculoskeletal, Oral, Skin and Sensory Network Editors Peter Tugwell and Jennifer Hilgart for their feedback and final approval of the review for publication.
We also would like to thank Zhenmi Liu, Gill Norman, and Melanie Stephens for double‐checking data extraction and risk of bias assessment for this review.
Appendices
Appendix 1. Full details of support surfaces classifications
| Overarching class of support surface (as used in this review) | Corresponding subclasses of support surfaces used inShi 2018b | Descriptions of support surfaces | Selected examples (with example brands where possible) |
| Reactive air surfaces | Powered/non‐powered reactive air surfaces | A group of support surfaces constructed of air cells, which redistribute body weight over a maximum surface area (i.e. has reactive pressure redistribution mode), with or without the requirement for electrical power. | Static air mattress overlay, dry flotation mattress (e.g. Roho, Sofflex), static air mattress (e.g. EHOB), and static mode of Duo 2 mattress. |
| Powered/non‐powered reactive low‐air‐loss air surfaces | A group of support surfaces made of air cells, which have reactive pressure redistribution modes and a low‐air‐loss function, with or without the requirement for electrical power. | Low‐air‐loss hydrotherapy. | |
| Powered reactive air‐fluidised surfaces | A group of support surfaces made of air cells, which have reactive pressure redistribution modes and an air‐fluidised function, with the requirement for electrical power. | Air‐fluidised bed (e.g. Clinitron). | |
| Foam surfaces | Non‐powered reactive foam surfaces | A group of support surfaces made of foam materials, which have a reactive pressure redistribution function, without the requirement for electrical power. | Convoluted foam overlay (or pad), elastic foam overlay (e.g. microfluid static overlay), polyether foam pad, foam mattress replacement (e.g. MAXIFLOAT), solid foam overlay, viscoelastic foam mattress/overlay (e.g. Tempur, CONFOR‐Med, Akton, Thermo). |
| Alternative reactive support surfaces (non‐foam or air‐filled): reactive fibre surfaces | Non‐powered reactive fibre surfaces | A group of support surfaces made of fibre materials, which have a reactive pressure redistribution function, without the requirement for electrical power. | Silicore (e.g. Spenco) overlay/pad. |
| Alternative reactive support surfaces (non‐foam or air‐filled): reactive gel surfaces | Non‐powered reactive gel surfaces | A group of support surfaces made of gel materials, which have a reactive pressure redistribution function, without the requirement for electrical power. | Gel mattress, gel pad used in operating theatre. |
| Alternative reactive support surfaces (non‐foam or air‐filled): reactive sheepskin surfaces | Non‐powered reactive sheepskin surfaces | A group of support surfaces made of sheepskin, which have a reactive pressure redistribution function, without the requirement for electrical power. | Australian Medical Sheepskins overlay. |
| Alternative reactive support surfaces (non‐foam or air‐filled): reactive water surfaces | Non‐powered reactive water surfaces | A group of support surfaces based on water, which has the capability of a reactive pressure redistribution function, without the requirement for electrical power. | Water mattress. |
| Alternating pressure (active) air surfaces | Powered active air surfaces | A group of support surfaces made of air cells, which mechanically alternate the pressure beneath the body to reduce the duration of the applied pressure (mainly via inflating and deflating to alternately change the contact area between support surfaces and the body; i.e. alternating pressure, or active, mode), with the requirement for electrical power. | Alternating pressure‐relieving air mattress (e.g. Nimbus II, Cairwave, Airwave, MicroPulse), large‐celled ripple. |
| Powered active low‐air‐loss air surfaces | A group of support surfaces made of air cells, which have the capability of alternating pressure redistribution as well as low air loss for drying local skin, with the requirement for electrical power. | Alternating pressure low‐air‐loss air mattress. | |
| Powered hybrid system air surfaces | A group of support surfaces made of air cells, which offer both reactive and active pressure redistribution modes, with the requirement for electrical power. | Foam mattress with dynamic and static modes (e.g. Softform Premier Active). | |
| Powered hybrid system low‐air‐loss air surfaces | A group of support surfaces made of air cells, which offer both reactive and active pressure redistribution modes as well as a low‐air‐loss function, with the requirement for electrical power. | Stand‐alone bed unit with alternating pressure, static modes and low air‐loss (e.g. TheraPulse). | |
| Standard hospital surfaces | Standard hospital surfaces | A group of support surfaces made of any materials, used as‐usual in a hospital and without reactive nor active pressure redistribution capabilities, nor any other functions (e.g. low‐air‐loss, or air‐fluidised). | Standard hospital (foam) mattress, NHS Contract hospital mattress, standard operating theatre surface configuration, standard bed unit and usual care. |
Appendix 2. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR beds EXPLODE ALL AND INREGISTER
2 mattress* AND INREGISTER
3 (foam or transfoam) AND INREGISTER
4 overlay* AND INREGISTER
5 (pad or pads) AND INREGISTER
6 gel AND INREGISTER
7 (pressure NEXT relie*) AND INREGISTER
8 (pressure NEXT reduc*) AND INREGISTER
9 (pressure NEXT alleviat*) AND INREGISTER
10 ("low pressure" near2 device*) AND INREGISTER
11 ("low pressure" near2 support) AND INREGISTER
12 (constant near2 pressure) AND INREGISTER
13 "static air" AND INREGISTER
14 (alternat* next pressure) AND INREGISTER
15 (air next suspension*) AND INREGISTER
16 (air next bag*) AND INREGISTER
17 (water next suspension*) AND INREGISTER
18 sheepskin AND INREGISTER
19 (turn* or tilt*) next (bed* or frame*) AND INREGISTER
20 kinetic next (therapy or table*) AND INREGISTER
21 (net next bed*) AND INREGISTER
22 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 AND INREGISTER
23 MESH DESCRIPTOR Pressure Ulcer EXPLODE ALL AND INREGISTER
24 (pressure next (ulcer* or sore* or injur*)) AND INREGISTER
25 (decubitus next (ulcer* or sore*)) AND INREGISTER
26 ((bed next sore*) or bedsore*) AND INREGISTER
27 #23 OR #24 OR #25 OR #26 AND INREGISTER
28 #22 AND #27 AND INREGISTER
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Beds] explode all trees
#2 mattress*:ti,ab,kw
#3 (foam or transfoam):ti,ab,kw
#4 overlay*:ti,ab,kw
#5 "pad" or "pads":ti,ab,kw
#6 "gel":ti,ab,kw
#7 (pressure next relie*):ti,ab,kw
#8 (pressure next reduc*):ti,ab,kw
#9 (pressure next alleviat*):ti,ab,kw
#10 ("low pressure" near/2 device*):ti,ab,kw
#11 ("low pressure" near/2 support):ti,ab,kw
#12 (constant near/2 pressure):ti,ab,kw
#13 "static air":ti,ab,kw
#14 (alternat* next pressure):ti,ab,kw
#15 (air next suspension*):ti,ab,kw
#16 (air next bag*):ti,ab,kw
#17 (water next suspension*):ti,ab,kw
#18 sheepskin:ti,ab,kw
#19 (turn* or tilt*) next (bed* or frame*):ti,ab,kw
#20 kinetic next (therapy or table*):ti,ab,kw
#21 (net next bed*):ti,ab,kw
#22 {or #1‐#21}
#23 MeSH descriptor: [Pressure Ulcer] explode all trees
#24 (pressure next (ulcer* or sore* or injur*)):ti,ab,kw
#25 (decubitus next (ulcer* or sore*)):ti,ab,kw
#26 ((bed next sore*) or bedsore*):ti,ab,kw
#27 {or #23‐#26}
#28 (#22 and #27) in Trials
Ovid MEDLINE
1 exp Beds/
2 mattress*.mp.
3 (foam or transfoam).mp.
4 overlay*.mp.
5 (pad or pads).ti,ab.
6 gel.ti,ab.
7 pressure relie*.mp.
8 pressure reduc*.mp.
9 pressure alleviat*.mp.
10 (low pressure adj2 device*).mp.
11 (low pressure adj2 support).mp.
12 (constant adj2 pressure).mp.
13 static air.mp.
14 (alternat* adj pressure).mp.
15 air suspension*.mp.
16 air bag*.mp.
17 water suspension*.mp.
18 sheepskin.mp.
19 ((turn* or tilt*) adj (bed* or frame*)).mp.
20 (kinetic adj (therapy or table*)).mp.
21 net bed*.mp.
22 or/1‐21
23 exp Pressure Ulcer/
24 (pressure adj (ulcer* or sore*)).mp.
25 (decubitus adj (ulcer* or sore*)).mp.
26 (bed adj (ulcer* or sore*)).mp.
27 or/23‐26
28 and/22,27
29 randomized controlled trial.pt.
30 controlled clinical trial.pt.
31 randomi?ed.ab.
32 placebo.ab.
33 clinical trials as topic.sh.
34 randomly.ab.
35 trial.ti.
36 or/29‐35
37 exp animals/ not humans.sh.
38 36 not 37
39 28 and 38
Ovid Embase
1 exp Bed/
2 mattress*.mp.
3 (foam or transfoam).mp.
4 overlay*.mp.
5 (pad or pads).ti,ab.
6 gel.ti,ab.
7 pressure relie*.mp.
8 pressure reduc*.mp.
9 pressure alleviat*.mp.
10 (low pressure adj2 device*).mp.
11 (low pressure adj2 support).mp.
12 (constant adj2 pressure).mp.
13 static air.mp.
14 (alternat* adj pressure).mp.
15 air suspension*.mp.
16 air bag*.mp.
17 water suspension*.mp.
18 sheepskin.mp.
19 ((turn* or tilt*) adj (bed* or frame*)).mp.
20 (kinetic adj (therapy or table*)).mp.
21 net bed*.mp.
22 or/1‐21
23 exp Decubitus/
24 (pressure adj (ulcer* or sore*)).mp.
25 (decubitus adj (ulcer* or sore*)).mp.
26 (bed adj (ulcer* or sore*)).mp.
27 or/23‐26
28 and/22,27
29 Randomized controlled trials/
30 Controlled clinical study/
31 Single‐Blind Method/
32 Double‐Blind Method/
33 Crossover Procedure/
34 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab.
35 (doubl* adj blind*).ti,ab.
36 (singl* adj blind*).ti,ab.
37 or/29‐36
38 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
39 human/ or human cell/
40 and/38‐39
41 38 not 40
42 37 not 41
43 28 and 42
EBSCO CINAHL Plus
S50 S26 AND S49
S49 S48 NOT S47
S48 S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41
S47 S45 NOT S46
S46 MH (human)
S45 S42 OR S43 OR S44
S44 TI (animal model*)
S43 MH (animal studies)
S42 MH animals+
S41 AB (cluster W3 RCT)
S40 MH (crossover design) OR MH (comparative studies)
S39 AB (control W5 group)
S38 PT (randomized controlled trial)
S37 MH (placebos)
S36 MH (sample size) AND AB (assigned OR allocated OR control)
S35 TI (trial)
S34 AB (random*)
S33 TI (randomised OR randomized)
S32 MH cluster sample
S31 MH pretest‐posttest design
S30 MH random assignment
S29 MH single‐blind studies
S28 MH double‐blind studies
S27 MH randomized controlled trials
S26 S20 AND S25
S25 S21 OR S22 OR S23 OR S24
S24 TI decubitus or AB decubitus
S23 TI ( bed sore* or bedsore* ) or AB ( bed sore* or bedsore* )
S22 TI ( pressure ulcer* or pressure sore* ) or AB ( pressure ulcer* or pressure sore* )
S21 (MH "Pressure Ulcer")
S20 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19
S19 TI net bed* or AB net bed*
S18 TI ( kinetic therapy or kinetic table* ) or AB ( kinetic therapy or kinetic table* )
S17 TI ( turn* bed* or tilt* bed* ) or AB ( turn* frame* or tilt* frame* )
S16 TI sheepskin OR AB sheepskin
S15 TI water suspension or AB water suspension
S14 TI air bag* or AB air bag*
S13 TI air suspension or AB air suspension
S12 TI alternat* pressure or AB alternat* pressure
S11 TI static air or AB static air
S10 TI constant N2 pressure or AB constant N2 pressure
S9 TI low pressure N2 support or AB low pressure N2 support
S8 TI low pressure N2 device* or AB low pressure N2 device*
S7 TI pressure alleviat* or AB pressure alleviat*
S6 TI pressure reduc* or AB pressure reduc*
S5 TI pressure relie* or AB pressure relie*
S4 TI ( overlay* or pad or pads or gel ) or AB ( overlay* or pad or pads or gel )
S3 TI ( foam or transfoam ) or AB ( foam or transfoam )
S2 TI mattress* or AB mattress*
S1 (MH "Beds and Mattresses+")
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air | Pressure Ulcer
bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air | Pressure Injury
bed OR mattress OR sheepskin OR gel OR pad OR foam OR pressure OR support OR air | Pressure Ulcers buttock
bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air | Ulcer, Pressure
bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air | Pressure Ulcer Stage 1
bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air | Pressure Ulcers Stage II
bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air | Pressure Ulcers Stage III
World Health Organization International Clinical Trials Registry Platform
pressure ulcer [title] and bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air [intervention]
pressure ulcer [condition] and bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air [intervention]
pressure injury [title] and bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air [intervention]
pressure injury [condition] and bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air [intervention]
Appendix 3. Risk of bias
1 'Risk of bias' assessment in individually randomised controlled trials
1. Was the allocation sequence randomly generated?
Low risk of bias
The study authors describe a random component in the sequence generation process, such as referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots.
High risk of bias
The study authors describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example, sequence generated by odd or even date of birth, sequence generated by some rule based on date (or day) of admission, sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and study authors enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or study authors enrolling participants could possibly foresee assignments and thus introduce selection bias, e.g. allocation was based on using an open random allocation schedule (e.g. a list of random numbers), assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non opaque or not sequentially numbered), alternation or rotation, date of birth, case record number, any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding: was knowledge of the allocated interventions by participants and personnel adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others is likely to introduce bias.
Unclear
Any one of the following.
Insufficient information to permit a judgement of low or high risk of bias.
The study did not address this outcome.
4. Blinding: was knowledge of the allocated interventions by outcome assessors adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding.
Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome measurement is likely to be influenced by lack of blinding.
Blinding of outcome assessment attempted, but likely that the blinding could have been broken.
Unclear
Any one of the following.
Insufficient information to permit a judgement of low or high risk of bias.
The study did not address this outcome.
5. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is not sufficient to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes is not sufficient to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data is likely to be related to the true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is sufficient to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes is sufficient to induce clinically relevant bias in the observed effect size.
‘As‐treated’ analysis done, with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following.
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated; no reasons for missing data provided).
The study did not address this outcome.
6. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Any of the following.
The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
7. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
2 'Risk of bias' assessment in cluster‐randomised controlled trials (cluster‐RCTs)
1. Recruitment bias
Recruitment bias (or identification bias) is the bias that occurs in cluster‐RCTs if the personnel recruiting participants know individuals’ allocation, even when the allocation of clusters has been concealed appropriately. The knowledge of the allocation of clusters may lead to bias because the individuals' recruitment in cluster trials is often behind the clusters' allocation to different interventions; and the knowledge of allocation can determine whether individuals are recruited selectively.
This bias can be judged through considering the following questions.
Were all the individual participants identified/recruited before randomisation of clusters?
Is it likely that selection of participants was affected by knowledge of the intervention?
Were there baseline imbalances that suggest differential identification or recruitment of individual participants between arms?
2. Baseline imbalance
Baseline imbalance between intervention groups can occur due to chance, problems with randomisation, or identification/recruitment bias. The issue of recruitment bias has been considered above.
In terms of study design, the risk of chance baseline imbalance can be reduced by the use of stratified or pair‐matched randomisation. Minimisation — an equivalent technique to randomisation — can be used to achieve better balance in cluster characteristics between intervention groups if there is a small number of clusters.
Concern about the influence of baseline imbalance can be reduced if studies report the baseline comparability of clusters, or statistical adjustment for baseline characteristics.
3. Loss of clusters
Similar to missing outcome data in individually randomised trials, bias can occur if clusters are completely lost from a cluster‐RCT, and are omitted from the analysis.
The amount of missing data, the reasons for missingness and the way of analysing data given the missingness should be considered in assessing the possibility of bias.
4. Incorrect analysis
Data analyses, which do not take the clustering into account, in cluster‐RCTs will be incorrect. Such analyses lead to a 'unit of analysis error' and over‐precise results (overly small standard error) and overly small P values. Though these analyses will not result in biased estimates of effect, they (if not correctly adjusted) will lead to too much weight allocated to cluster trials in a meta‐analysis.
Note that the issue of analysis may not lead to concern any more and will not be considered substantial if approximate methods are used by review authors to address clustering in data analysis.
5. Comparability with individually randomised trials
In the case that a meta‐analysis includes, for example, both cluster‐randomised and individually randomised trials, potential differences in the intervention effects between different trial designs should be considered. This is because the 'contamination' of intervention effects may occur in cluster‐RCTs, which would lead to underestimates of effect. The contamination could be known as a 'herd effect': that is, within clusters, individuals' compliance with using an intervention may be enhanced, which in return affects the estimation of effect.
Appendix 4. Interventions used in the included studies
| Study ID | Specific support surfaces | Specific comparators |
| Comparison: alternating pressure (active) air surfaces versus alternating pressure (active) air surfaces | ||
| Devine 1995 | Nimbus I DFS (HNE Healthcare, Luton, UK), 2 alternative sets of cells are inflated and deflated over a 10 minute cycle.
|
Airwave mattress (Pegasus, Ltd. Waterlooville), a double layer mattress with a 3 cell alternating cycle lasting 7 ½ minutes.
|
| Evans 2000a | Nimbus 3 alternating pressure mattress replacement system.
|
Alternating pressure mattress overlay (Pegasus Airwave, Pegasus Biwave and AlphaXcell, and the Pegasus Cairwave).
|
| Evans 2000b | Nimbus 3 alternating pressure mattress replacement system.
|
Alternating pressure mattress overlay (Pegasus Airwave, Pegasus Biwave and AlphaXcell, and the Pegasus Cairwave).
|
| Russell 2000a | Huntleigh Nimbus 3 with the Aura cushion (Group A).
|
Pegasus Cairwave therapy system with the Proactive cushion (Group B).
|
| Comparison: alternating pressure (active) air surfaces versus foam surfaces | ||
| Day 1993 | Air‐suspension bed (TheraPulse, Kinetic Concepts).
|
Foam overlay (GeoMatt, SpanAmerica).
|
| Mulder 1994 | Therapulse low air loss bed (Kinetic Concepts, Inc., San Antonio, TX).
|
Geomatt mattress overlay (Span‐America Inc., Greenville, SC).
|
| Comparison: reactive air surfaces versus foam surfaces | ||
| Allman 1987 | Air‐fluidised bed (Clinitron Therapy, Support Systems International, Inc.) … contain ceramic beads … warm, pressurized air is forced up through the beads, on the characteristics of a fluid.
|
Conventional therapy used a vinyl alternating air‐mattress covered by a 19‐mm thick foam pad (Lapidus Air Float System, American Pharmaceal Company) on a regular bed.
|
| Ferrell 1993; Ferrell 1995 | Low‐air‐loss bed (Kinair bed, Kinetic Concepts International, San Antonio, Tex).
|
10‐cm convoluted foam mattress overlying a regular hospital mattress.
|
| Comparison: reactive air surfaces versus undefined 'standard hospital surfaces' | ||
| Munro 1989 | Clinitron bed air‐fluidised support system.
|
Standard bed, unspecified.
|
| Strauss 1991 | Air‐fluidised bed therapy (CLINITRON Therapy Unit, Support Systems International, Charleston, SC).
|
Conventional therapy (including alternating pressure pads, air support mattresses, water mattresses, and high‐density foam pads).
|
| Comparison: foam surfaces versus reactive water surfaces | ||
| Groen 1999 | Foam replacement mattress (TheraRest).
|
Water mattress (Secutex) placed on top of the standard hospital mattress.
|
| Comparison: reactive gel surfaces versus undefined reactive surfaces | ||
| Cassino 2013a | Akton gel overlay (Akton® Overlay, Action products) (15.9 mm thick).
|
Aiartex®: the three‐dimensional overlay (Aiartex®, Herniamesh srl).
|
Appendix 5. Results of studies with surfaces that were not classified
| Outcomes | Results |
| Comparison: reactive air surfaces compared with undefined 'standard hospital surfaces' | |
| Support surface associated patient comfort (median follow‐up duration 15 days) | Only Munro 1989 (40 participants) reported this outcome defined as self‐rated participant satisfaction measured using an 8‐item scale with a totality of 11 points ranging from 'total dissatisfaction' to 'complete satisfaction'. In total, 18 of 40 participants responded on the scale and we analysed these data. The MD is 8.90 (95% CI 0.18 to 17.62; I2 = 0%). |
| All reported adverse events (follow‐up duration 15 days and 36 weeks) | Two studies (152 participants) reported this outcome (Munro 1989; Strauss 1991). We did not pool these data as the definitions of adverse events varied between studies (Appendix 6). It is uncertain if there is any difference in adverse events between reactive air surfaces and standard hospital surfaces. |
| Comparison: reactive gel surfaces versus undefined reactive surfaces | |
| Proportion of participants with pressure ulcers completely healed (follow‐up duration 12 weeks) | Cassino 2013a (72 participants) reported this outcome and the proportions of participants with pressure ulcers completely healed were 13.5% (5/37) in people using reactive gel surfaces and 8.6% (3/35) in those using undefined reactive surfaces. The RR is 1.58 (95% CI 0.41 to 6.11). |
| Support surface associated patient comfort (follow‐up duration 12 weeks) | Cassino 2013a (72 participants) reported this outcome which was defined as patient comfort with 4 responses: 'Poor', 'Fair', 'Good' and 'Excellent'. Eighteen participants using reactive gel surfaces responded with 'Poor', 12 with 'Fair', 6 with 'Good', and 1 with 'Excellent' whilst 8 participants using the undefined reactive surfaces responded with 'Poor', 13 with 'Fair', 10 with 'Good', and 4 with 'Excellent'. |
| All reported adverse events (follow‐up duration 12 weeks) | Cassino 2013a (72 participants) reported this outcome. We did not pool these data (Appendix 6). It is uncertain if there is any difference in adverse events between reactive gel surfaces and undefined reactive surfaces. |
Appendix 6. All reported adverse events in the studies involving undefined surfaces
| Study ID | Results | Comment | |
| Comparison: reactive air surfaces versus undefined 'standard hospital surfaces' | |||
| Munro 1989 | Patients' perception of pain scores reduced over time. | Patients' perception of pain scores reduced over time. | Patients' perception of pain rated using an adaptation of the Levitt and Derogatis scale and 13 of 40 participants responded. No statistical difference in pain scores between groups (F = 0.87, P = 0.359). |
| Strauss 1991 | 14 died; several patients noted dry skin, and 1 experienced mild dehydration. | 19 died. | The deaths were unlikely related to the use of specific support surfaces. |
| Comparison: reactive gel surfaces versus undefined reactive surfaces | |||
| Cassino 2013a | 14 participants suspended in reactive gel surfaces group; majority suspended due to worsening of lesions; 7 died. Safety assessment was mentioned but not reported. | 19 suspended in undefined reactive surfaces group; majority suspended due to worsening of lesions; 3 died. Safety assessment was mentioned but not reported. | |
Data and analyses
Comparison 1. Alternating pressure (active) air surfaces compared with foam surfaces.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Proportion of participants with pressure ulcers completely healed | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.26, 3.58] |
| 1.2 Support surface associated patient comfort | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.42, 1.22] |
Comparison 2. Reactive air surfaces compared with foam surfaces.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Proportion of participants with pressure ulcers completely healed | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.96, 1.80] |
Comparison 3. Reactive water surfaces compared with foam surfaces.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Proportion of participants with pressure ulcers completely healed | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.70, 1.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allman 1987.
| Study characteristics | ||
| Methods |
Study objective: to compare the effectiveness and adverse effects of air‐fluidised beds and conventional therapy for patients with pressure sores. Study design including the number of centres: randomised controlled trial, single centre. Study grouping: parallel group Duration of follow‐up: median 13 days Number of arms: 2 Study start date and end date: recruited between October 1984 and March 1986. Care setting: urban, academic referral, and primary care medical centre. |
|
| Participants |
Baseline characteristics Inclusion criteria: age greater than 18 years old; presence of a pressure sore on the sacrum, buttocks, trochanters, or back; activity expected to be limited to bed or chair in the hospital for at least 1 week; patient expected to live at least 1 week; informed consent obtained. Exclusion criteria: had been in the trial previously or a skin graft or flap planned for the pressure sore within 1 week. Sex (M/F): 27/38 overall. 11/20 in air‐fluidised bed; 16/18 in conventional therapy. Age (years): mean 65.5 (SD 15.6) in air‐fluidised bed, 67.6 (18.3) in conventional therapy; estimated overall 66.6 (17.0). The stage of pressure ulcers at baseline: using Shea criteria; 16 superficial and 15 deep ulcers on air‐fluidised bed; 20 superficial and 14 deep ulcers on conventional therapy. Median total surface area 7.8 cm2 (range 0.3 to 83.2) on air‐fluidised bed, 10.8 cm2 (0.4 to 180.3) on conventional therapy. Group difference: no difference Total number of participants: 140 patients met inclusion criteria, 72 patients consent and randomised (65 completed the study). Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Air‐fluidised bed
Conventional therapy
|
|
| Outcomes |
Proportion of participants with pressure ulcers healed
Time to pressure ulcer healing
Support‐surface‐associated patient comfort
All reported adverse events
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly allocated to treatment groups in two strata in balanced blocks of six with stratification … The randomization sequence was determined using a table of random numbers …” Comment: low risk of bias due to the use of a proper randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “… treatment allocations were placed in envelopes sealed and numbered sequentially. After establishing eligibility, one of the investigators selected the unopened envelope with the lowest number in the appropriate strata and allocated the patient to the treatment indicated on the enclosed card” Comment: unclear risk of bias because information is still insufficient to ensure if concealment is performed properly. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcome group: ulcer healing Quote: “The masked assessment included review of serial photographs of all pressure sores present …” Comment: low risk of bias. Outcome group: comfort Comment: high risk of bias because patients self‐rated comfort. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: all outcomes Comment: low risk of bias because of the low rate of attrition (7/72, 9.7%). |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Cassino 2013a.
| Study characteristics | ||
| Methods |
Study objective: to evaluate the performance and effectiveness of an anti‐bedsore, three‐dimensional overlay (Aiartex®, Herniamesh) compared with a commonly‐used gel overlay (Akton® Overlay). Study design including the number of centres: randomised controlled trial, open label, multi‐sites. Study grouping: parallel group Duration of follow‐up: observation period of 12 weeks and subsequent follow‐up at 28 (+/‐ 3) days. Number of arms: 2 Study start date and end date: patients recruited between May and July 2012. Care setting: long‐term care wards |
|
| Participants |
Baseline characteristics Inclusion criteria: informed consent; age > 18 years; Braden score between 6 and 14; Norton Score between 5 and 12; patients with EPUAP‐NPUAP Stages I to IV decubitus; BMI > 16 and < 40; absence of infection. Exclusion criteria: patients without decubitus; terminal patients; immunosuppressive or antiblastic therapies; pregnant women; patients who need different aids; allergies to overlay materials; AIDS, HCV; patients enrolled in other studies in the 3 preceding months. Sex (M/F): 17:55 overall; 7:28 in Aiartex and 10:27 in Akton gel overlay. Age (years): mean 84.9 (SD 9.1) in Aiartex and 85.9 (9.1) in Akton; estimated overall 85.4 (9.0). The stage of pressure ulcers at baseline: all with EPUAP‐NPUAP Stages I to IV decubitus; mean Norton score 9.4 (SD 1.9) in Aiartex and 10.1 (1.7) in Akton gel overlay. Braden Q score 12 (SD 1.5) Aiartex and 12.5 (SD 1.5) in Akton gel overlay. Group difference: no difference Total number of participants: 72 patients, 86 lesions with a mean 1.19 lesions per patient and a range of 1‐3. Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Aiartex®
Akton gel overlay
|
|
| Outcomes |
Proportion of participants with pressure ulcers healed
Time to pressure ulcer healing
Support‐surface‐associated patient comfort
All reported adverse events
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Assignment to one aid or the other was randomised using closed envelopes which were opened at the moment of assignment. In the randomization lists the two aids were balanced at a ratio of 1:1” Comment: unclear risk of bias because the method of generating random numbers is not described. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Assignment to one aid or the other was randomised using closed envelopes which were opened at the moment of assignment.” Comment: unclear risk of bias because it is not state if the envelopes are opaque and sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Outcome group: all outcomes Quote: “Clinical evaluation was performed by previously trained medical staff of the facilities involved” Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Outcome group: all outcomes Comment: high risk of bias because suspensions due to worsening lesions make this 50% withdrawal rate. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available but it is unclear whether safety assessments have been reported as pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Day 1993.
| Study characteristics | ||
| Methods |
Study objective: not given Study design including the number of centres: randomised controlled trial, single centre. Study grouping: parallel group Duration of follow‐up: at least 7 days Number of arms: 2 Study start date and end date: not given Care setting: acute care setting |
|
| Participants |
Baseline characteristics Inclusion criteria: hospitalised patients older than 18 years of age with Stage II, III, or IV pressure ulcers; life expectancy of at least 1 week; activity limited to chair or bed during hospitalisation; informed consent signed by the patient, or patient’s family or guardian, and permission of the attending physician. Exclusion criteria: patients previously enrolled in the study; patient hospitalised for less than 7 days; patient having undergone skin grafting or flap within 7 days of enrolment in the study. Sex (M/F): 17/27 in air‐suspension bed; 18/21 in foam overlay. Age (years): mean 75.09 (SD 15.37; range 32 to 102) years in air‐suspension bed; mean 77.13 (SD 10.76; range 54 to 93) in foam overlay; estimated overall 76.0 (13.4). The stage of pressure ulcers at baseline: using 1989 NPUAP stage criteria; 25 Stage II, 6 Stage III, 11 Stage IV, 2 unable to stage in air‐suspension bed (modified Norton mean 8.84, SD 2.84); 23 Stage II, 8 Stage III, 4 Stage IV, 4 unable to stage in foam overlay (Norton mean 9.03, SD 3.19). Group difference: no difference in other variables but the initial ulcer size (t[81] = 2.13, P = 0.036) with more severe wounds in the air‐suspension group. Total number of participants: 118 enrolled, 83 patients randomised/analysed. Unit of analysis: individuals Unit of randomisation (per patient): individuals (1 ulcer per patient selected) |
|
| Interventions |
Intervention characteristics Air‐suspension bed
Foam overlay
|
|
| Outcomes |
Proportion of participants with pressure ulcers healed
Time to pressure ulcer healing
Support‐surface‐associated patient comfort
All reported adverse events
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | 118 patients were enrolled in this study. Of these, 35 patients (19 from the air‐suspension group and 16 from foam overlay group) did not complete the study due to either death or discharge earlier (i.e. not meeting inclusion criteria). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomised to either the air‐suspension bed or the foam mattress overlay” Quote: "In spite of randomisation there was a statistically significant difference in the initial ulcer size between the two groups (t[81] = 2.13, p=.036) with more severe wounds in the air‐suspension group" Comment: unclear risk of bias because the method of generating random numbers is unspecified. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “An equal distribution of labels marked foam mattress overlay or air‐suspension bed were sealed in envelops” Comment: unclear risk of bias because no sufficient information on the allocation process. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Outcome group: ulcer healing Comment: high risk of bias because the report seemed to indicate that the allocation was unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Outcome group: ulcer healing Comment: high risk of bias because the report seemed to indicate that randomisation groups were unblinded to outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Outcome group: ulcer healing Comment: it appeared to include all 83 patients in analysis. However, the authors enrolled 118 patients in the study but excluded 35 based on inclusion criterion. It was unclear if the exclusion was prior to randomisation. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Devine 1995.
| Study characteristics | ||
| Methods |
Study objective: to assess the role of this type of mattress [large celled alternating pressure mattress] in the treatment of existing pressure sores and to compare the more established Pegasus Airwave version with the newer Nimbus I Dynamic Flotation System. Study design including the number of centres: randomised controlled trial, single centre Study grouping: parallel group Duration of follow‐up: 4 weeks Number of arms: 2 Study start date and end date: not described Care setting: hospital |
|
| Participants |
Baseline characteristics Inclusion criteria: patients in the Geriatric Unit who were admitted with or who developed sores of grade 2 or above and who agreed to being included in the study. Exclusion criteria: not described Sex (M/F): 17:24 overall; 12:10 in NIMBUS I and 5:14 in Airwave. Age (years): mean 82.5 (range 69 to 98) overall; mean 81 (SD 5) in NIMBUS I and 84 (8) in Airwave. The stage of pressure ulcers at baseline: sores of grade 2 or above (2 having grade 2 sores, 26 having grade 3, 8 having grade 4 sores and 5 having the most severe grade 5); initial sore size (cm2) 13.5 in NIMBUS I and 12 in Airwave (estimated overall 12.8); mean Norton score 10 (range 7 to 14) in NIMBUS I and 10 (6 to 14) in Airwave. Group difference: no difference Total number of participants: 41 Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Nimbus I
Airwave mattress
|
|
| Outcomes |
Proportion of participants with pressure ulcers healed
Time to pressure ulcer healing
Support‐surface‐associated patient comfort
All reported adverse events
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Allocation to each group was achieved using a computer generated list of random numbers kept separately from the trial co‐ordinator” Comment: low risk of bias because of the use of a proper randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Allocation to each group was achieved using a computer generated list of random numbers kept separately from the trial co‐ordinator” Comment: unclear risk of bias because the method of concealing allocation is not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: ulcer healing Quote: “it was thought reasonable to include these individuals on an intention to treat basis” Comment: low risk of bias because despite it being unclear if ITT analysis was performed as was mentioned in the Discussion, reasons of exclusions from analysis are mainly related to deaths and these data can be re‐included in the analysis by review authors. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Evans 2000a.
| Study characteristics | ||
| Methods |
Study objective: assessed the clinical effectiveness of the Nimbus 3 alternating pressure mattress replacement system (APMRS) on pressure ulcer healing and comfort in subjects > 65 years, with at least a Grade 2 ulcer and some mobility problems. Study design including the number of centres: randomised controlled trial, single centre. Study grouping: parallel group Duration of follow‐up: not described; median days in trial 20.5 (range 10 to 41) overall; 20 (13 to 41) in Nimbus 3 and 28 (10 to 37) in control. Number of arms: 2 Study start date and end date: not described Care setting: hospital |
|
| Participants |
Baseline characteristics Inclusion criteria: aged ≥ 65 years with a Grade 3 pressure ulcer, or ≥ 65 years with a Grade 2 pressure ulcer and 1 or more of the following: difficulty repositioning in bed and unable to tolerate a 30° tilt; unable to move in bed; in bed for more than 20h in 24h; weight ≥ 108kg (17 stone) and bed bound; undergone spinal anaesthetic. Exclusion criteria: spinal metastases; exudating wounds that may lead to hygiene or infection control problems; weight > 250kg (39 stone). Sex (M/F): hospital setting – 4:3 in Nimbus 3; 2:3 in control. Age (years): hospital setting – median 68 (range 66 to 91) in Nimbus 3; 78 (65 to 91) in control; estimated overall 72.2. The stage of pressure ulcers at baseline: hospital setting – 3 reference ulcers of Grade 2 and 4 reference ulcers of Grade 3; with median reference ulcer size at baseline (cm2) 3.1 (range 1.6 to 12.4) in Nimbus 3; 2 reference ulcer of Grade 2 and 3 ulcers of Grade 3; with median baseline size 5.7 (range 2.4 to 11.5); estimated overall size 4.2. Group difference: no difference Total number of participants: 12 patients with 12 reference ulcers Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics NIMBUS 3
Alternating pressure mattress overlay
|
|
| Outcomes |
Proportion of participants with pressure ulcers healed
Time to pressure ulcer healing
Support‐surface‐associated patient comfort
All reported adverse events
Health‐related quality of life (HRQOL)
Cost‐effectiveness
|
|
| Notes | The authors conducted trials at 2 different settings separately but used the same methods and reported both trials in the same paper. Data for each trial were extracted for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Treatments were randomly allocated to sequentially‐labelled sealed envelopes” Comment: unclear risk of bias because the method of generating random numbers is not described. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Treatments were randomly allocated to sequentially‐labelled sealed envelopes. After baseline assessment, the TVN opened the top envelope that indicated which surface a subject would be nursed on.” Comment: unclear risk of bias because it is unclear if envelopes are opaque and numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Outcome group: Quote: “Both groups were cared for in a similar manner, except for the PR device used” Comment: unclear risk of bias because it is challenging to blind participants and personnel but the attempts are made to keep deviations from interventions fewer. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcome group: Quote: “Two research team members, blind to the surface used, carried out the WSA measurements” Comment: low risk of bias as the quotation states. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: Quote: “Even when in the study for a short time subjects were included on an intention‐to‐treat basis” Comment: low risk of bias because ITT analysis was performed. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Evans 2000b.
| Study characteristics | ||
| Methods |
Study objective: assessed the clinical effectiveness of the Nimbus 3 alternating pressure mattress replacement system (APMRS) on pressure ulcer healing and comfort in subjects > 65 years, with at least a Grade 2 ulcer and some mobility problems. Study design including the number of centres: randomised controlled trial, single centre Study grouping: parallel group Duration of follow‐up: not described; median days in trial 53.5 (range 4 to 467) overall; 33 (7 to 217) in Nimbus 3 and 87 (4 to 467) in control. Number of arms: 2 Study start date and end date: not described Care setting: nursing home |
|
| Participants |
Baseline characteristics Inclusion criteria: aged ≥ 65 years with a Grade 3 pressure ulcer, or ≥ 65 years with a Grade 2 pressure ulcer and 1 or more of the following: difficulty repositioning in bed and unable to tolerate a 30° tilt; unable to move in bed; in bed for more than 20h in 24h; weight ≥ 108kg (17 stone) and bed bound; undergone spinal anaesthetic. Exclusion criteria: spinal metastases; exudating wounds that may lead to hygiene or infection control problems; weight > 250kg (39 stone). Sex (M/F): 0:10 in Nimbus 3; and 1:9 in control. Age (years): median 84.5 (range 71 to 99) in Nimbus 3; and 88.5 (72 to 94) in control; estimated overall 86.5. The stage of pressure ulcers at baseline: 1 reference ulcer of Grade 2, 7 Grade 3 and 2 reference ulcers of Grade 4; with median reference ulcer size at baseline (cm2) 6.9 (range 2.2 to 21.0) in Nimbus 3; 2 reference ulcers of Grade 2, 4 ulcers of Grade 3 and 4 Grade 4; with median baseline size 6.3 (range 0.1 to 37.4); overall estimated ulcer size 6.6. Group difference: no difference Total number of participants: 20 patients with 20 reference ulcers. Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics NIMBUS 3
Alternating pressure mattress overlay
|
|
| Outcomes |
Proportion of participants with pressure ulcers healed
Time to pressure ulcer healing
Support‐surface‐associated patient comfort
All reported adverse events
Health‐related quality of life (HRQOL)
Cost‐effectiveness
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Treatments were randomly allocated to sequentially‐labelled sealed envelopes” Comment: unclear risk of bias because the method of generating random numbers is not described. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Treatments were randomly allocated to sequentially‐labelled sealed envelopes. After baseline assessment, the TVN opened the top envelope that indicated which surface a subject would be nursed on.” Comment: unclear risk of bias because it is unclear if envelopes are opaque and numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Outcome group: Quote: “Both groups were cared for in a similar manner, except for the PR device used” Comment: unclear risk of bias because it is challenging to blind participants and personnel but the attempts are made to keep deviations from interventions fewer. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcome group: Quote: “Two research team members, blind to the surface used, carried out the WSA measurements” Comment: low risk of bias as the quotation states. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: Quote: “Even when in the study for a short time subjects were included on an intention‐to‐treat basis” Comment: low risk of bias because ITT analysis was performed. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Ferrell 1993.
| Study characteristics | ||
| Methods |
Study objective: to assess the effectiveness of low‐air‐loss beds for the treatment of pressure ulcers in nursing homes. Study design including the number of centres: randomised controlled trial, multiple centres. Study grouping: parallel group Duration of follow‐up: median 37.5 days Number of arms: 2 Study start date and end date: recruited between November 1987 and March 1991. Care setting: nursing homes |
|
| Participants |
Baseline characteristics Inclusion criteria: presence of pressure ulcers on the trunk, buttocks, or trochanters; informed consent from the patient or patient’s proxy for health care decisions; and approval of the primary care physician. Ulcers were defined by the presence of abrasion, bulla, skin necrosis, or ulcer formation as a result of pressure over a bony prominence (stage 2 or greater by the Shea scale). The largest ulcer was chosen as the index ulcer among those with multiple ulcers. Exclusion criteria: expected survival less than 1 month; previous participation in the study; or previous or planned surgical excision of the pressure ulcer. Sex (M/F): 22/21 in low‐air‐loss bed; 20/21 in foam mattress. Age (years): median 85 (IQR 71 and 92) in low‐air‐loss; 84 (88 and 91) in foam mattress; estimated overall 84.5. The stage of pressure ulcers at baseline: using Shea criteria: 25 superficial ulcers and 18 deep ulcers in low‐air‐loss; 27 superficial and 14 deep ulcers in foam mattress. Initial surface area (cm2) median 4.3 (IQR 2.6 to 14.0) in low‐air‐loss bed; 4.1 (0.97 and 8.95) in foam mattress; estimated overall ulcer size 4.2. Group difference: no difference Total number of participants: 84 patients analysed Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Low‐air‐loss bed
10‐cm corrugated foam mattress
|
|
| Outcomes |
Proportion of participants with pressure ulcers healed
Time to pressure ulcer healing
Support‐surface‐associated patient comfort
All reported adverse events
Health‐related quality of life (HRQOL)
Cost‐effectiveness
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “At each facility, groups of 10 subjects were separately randomised, five to each treatment” Comment: unclear risk of bias because the method of generating random sequence is unspecified. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Assignment were sealed in individual envelopes and opened sequentially on establishment of subject criteria” Comment: unclear risk of bias because the available information on using envelopes is not sufficient for judging the allocation is concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Outcome group: ulcer healing Quote: “The treatment protocol was similar between groups and consistent with each facility’s routine policy and procedure for pressure ulcer management” Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: ulcer healing Comment: all patients included in analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Ferrell 1995.
| Study characteristics | ||
| Methods |
Study objective: to estimate cost‐effectiveness based on patient and ulcer characteristics. Study design including the number of centres: cost‐effectiveness analysis. See Ferrell 1993 for the details of the trial associated with this cost‐effectiveness analysis. |
|
| Participants | See Ferrell 1993 for the details of the trial associated with this cost‐effectiveness analysis. From 3 nursing homes, 84 subjects with trunk or trochanter pressure ulcers (Shea stage II or greater) were randomly allocated. |
|
| Interventions | See Ferrell 1993 for the details of the trial associated with this cost‐effectiveness analysis. Briefly, a low‐air‐loss bed (Kinair Bed; Kinetic Concepts International, San Antonio, TX) (n = 43) compared with a 4‐inch corrugated foam mattress overlying a conventional hospital mattress (n = 41). |
|
| Outcomes |
Cost‐effectiveness
|
|
| Notes | This cost‐effectiveness analysis is related to Ferrell 1993 trial. Risk of bias judgements of Ferrell 1993 are applied for this cost‐effectiveness analysis. | |
Groen 1999.
| Study characteristics | ||
| Methods |
Study objective: to determine the effectiveness of high‐quality foam replacement mattresses (TheraRest) in the treatment of pressure ulcers compared with a water mattress (Secutex). Study design including the number of centres: randomised controlled trial, 3 sites. Study grouping: parallel group Duration of follow‐up: 4 weeks Number of arms: 2 Study start date and end date: not given Care setting: nursing home |
|
| Participants |
Baseline characteristics Inclusion criteria: aged 60 years or over and had pressure ulcers on the trunk that had been classified Grade III (superficial cutaneous or subcutaneous necrosis) or IV (deep subcutaneous necrosis). Exclusion criteria: patients with severe or terminal illness. Sex (M/F): not described Age (years): average age 81.9 years in foam mattress and 83.5 in water mattress; estimated overall 82.7. The stage of pressure ulcers at baseline: pressure ulcer severity 4.8 in foam mattress and 5.5 in water mattress. Group difference: higher number of patients with occasional incontinence of urine in water mattress. Total number of participants: 120 randomised (101 analysed) Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Foam replacement mattress
Water mattress
|
|
| Outcomes |
Proportion of participants with pressure ulcers healed
Time to pressure ulcer healing
Support‐surface‐associated patient comfort
All reported adverse events
Health‐related quality of life (HRQOL)
Cost‐effectiveness
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Subjects were randomly divided into two groups of 60 by selection of sealed envelopes.” Comment: unclear risk of bias because the method of generating random numbers is not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Outcome group: all outcomes Quote: “The outcome assessment was not blinded because the disturbance caused by moving patients from the mattresses was thought to be undesirable” Comment: high risk of bias because unblinded assessment is stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: all outcomes Quote: “All patients who took part in the trial for 14 days or longer were included” Quote: “Of the 120 patients in the trial, 19 withdrew – 11 from Group A and eight from Group B” Comment: low risk of bias because of the low rate of missing data. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Mulder 1994.
| Study characteristics | ||
| Methods |
Study objective: to determine the effectiveness of the use of the low air loss therapy versus a standard support surface in the treatment of patients with Stage III or Stage IV pressure ulcers. Study design including the number of centres: randomised controlled trial, single site. Study grouping: parallel group Duration of follow‐up: 12 weeks Number of arms: 2 Study start date and end date: not given Care setting: nursing home |
|
| Participants |
Baseline characteristics Inclusion criteria: patients with full thickness pressure ulcers (Stages III, IV) within a range of 1.5 cm x 1.5 cm to 10.0 cm x 20.0 cm. Exclusion criteria: patients with carcinomatosis, osteomyelitis affecting the target ulcer, uncontrolled target ulcer infection, immune deficiency disorders, and inadequate nutritional status. Sex (M/F): not given Age (years): not given The stage of pressure ulcers at baseline: all Stage III or IV Group difference: no difference Total number of participants: 49 patients analysed Unit of analysis: individuals (1 ulcer per individual) Unit of randomisation (per patient): ulcers |
|
| Interventions |
Intervention characteristics Therapulse beds
Conventional treatment
|
|
| Outcomes |
Proportion of participants with pressure ulcers healed
Time to pressure ulcer healing
Support‐surface‐associated patient comfort
All reported adverse events
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Patients were seen on a weekly basis. During each visit, wounds were photographed and volume and area measured. Wound tracings were taken using an acetate film, which was then analyzed by computerized photoplanimetry" Comment: unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "A total of 49 evaluable patients were entered into the study. Ten individuals were dropped from the study and not included in the data analysis. Eight patients died, one was lost to follow‐up and one patient was dropped due to a break in the protocol" Comment: high risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | Comment: unclear risk of bias because pain and care cost were both seemingly mentioned but it is unclear if they were measured. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Munro 1989.
| Study characteristics | ||
| Methods |
Study objective: to examine if air fluidised bed is better than standard beds with usual pressure‐relieving devices. Study design including the number of centres: randomised controlled trial, single centre. Study grouping: parallel group Duration of follow‐up: 15 days Number of arms: 2 Study start date and end date: not described Care setting: Veterans’ Administration Medical Center. |
|
| Participants |
Baseline characteristics Inclusion criteria: eligible, consenting patients with Stage II or III ulcers, who were expected to remain in the hospital for at least 15 days. Exclusion criteria: patients with Stage IV ulcers; those who weigh more than 250 pounds; extremely malnourished patients – those at less than 70 percent of ideal body weight or with serum albumin less than 2.1 g/100 mL. Sex (M/F): 40:0 overall; 20:0 in each group. Age (years): mean 67.2 (range 48 to 88). The stage of pressure ulcers at baseline: 21 (52.5%) with Stage II ulcers and 19 (47.5%) with Stage III ulcers; average ulcer size at day 1 (1464 mm2 in standard bed and 2660 mm2 in Clinitron; estimated overall 18.62 cm2). Group difference: no difference Total number of participants: 40 patients analysed Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Clinitron bed
Standard bed
|
|
| Outcomes |
Proportion of participants with pressure ulcers healed
Time to pressure ulcer healing
Support‐surface‐associated patient comfort
All reported adverse events
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | The study authors stated “No attempt was made to standardise the treatment because we wanted to measure the results with common nursing practice versus those with the Clinitron bed”. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: those who “were expected to remain in the hospital for at least 15 days were randomly assigned …” Comment: unclear risk of bias because the method of generating random numbers is not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Outcome group: all outcomes Quote: “No attempt was made to standardize the treatment …” Comment: high risk of bias because it is understandably challenging to blind participants and personnel for a trial of non‐drug interventions and there is a clear statement above. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Outcome group: adverse events and comfort Comment: high risk of bias because more than half of included participants did not respond to outcome assessment. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available and it is unclear if the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Russell 2000a.
| Study characteristics | ||
| Methods |
Study objective: to compare the efficacy of the Pegasus Cairwave mattress and Proactive seating cushion and the Huntleigh Nimbus 3 mattress and Aura cushion. Study design including the number of centres: randomised controlled trial, single centre Study grouping: parallel group Duration of follow‐up: 18 months Number of arms: 2 Study start date and end date: recruitment between August 1997 and December 1998 Care setting: care of the elderly unit at a hospital |
|
| Participants |
Baseline characteristics Inclusion criteria: all patients admitted to the Health Care of the Elderly Unit with a pressure sore of grade 2 and above on the Torrance grading system. Exclusion criteria: unwilling to participate; randomised equipment not available; had previously been included in the trial and were re‐admitted with a pressure ulcer; or weighed more than 25 stone. Sex (M/F): not described Age (years): mean 83.9 (SD 5.91) in Nimbus 3 and 84.6 (6.21) in Cairwave (n = 112 completed cases); estimated overall 84.2 (SD 6.0). The stage of pressure ulcers at baseline: using Torrance stage criteria; average severity of ulcers 2.46 (SD 0.49) in Nimbus 3; 2.57 (0.48) in Cairwave (n = 183 all cases). Group difference: a statistical significant difference between patient groups for incontinence, with a greater proportion of patients on the Nimbus Bed incontinent. Total number of participants: n = 183 patients; 112 analysed Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Cairwave
Nimbus 3
|
|
| Outcomes |
Proportion of participants with pressure ulcers healed
Time to pressure ulcer healing
Support‐surface‐associated patient comfort
All reported adverse events
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “All patients … were randomly allocated a Pegasus Cairwave or Huntleigh Nimbus 3 mattress and matching seat cushion” (Russell 1999) Quote: “On admission to the study, subjects were randomly allocated to trial equipment.” Comment: unclear risk of bias because the method of generating random numbers is not described. |
| Allocation concealment (selection bias) | Low risk | Quote: “To prevent bias, randomisation of bed allocation and interim statistical monitoring to ensure ethical compliance coordination was undertaken by a member of the team who had no responsibility for direct patient care or knowledge of pressure‐relieving systems” (Russell 2000a) Comment: low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “No additional pressure‐relieving equipment was used during the trial.” Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcome group: ulcer healing Quote: “ulcers were graded during the trial by one of three designated nurses who regularly work together” (Russell 2000a) Quote: “All pressure sores were photographed digitally on a weekly basis and the images stored on CD‐ROM.” (Russell 1999) Quote: “Images were stored on compact discs, using codes that ensured image analysis could be carried out ‘blind’ to treatment group” (Russell 2000a) Comment: low risk of bias given this is likely to blind ulcer outcome assessment. Outcome group: comfort Quote: “patients with dementia were not included in the comfort survey” (Russell 2000a) Comment: low risk of bias because patients’ opinions on the comfort of the bed are measured by an impartial observer from the clinical audit team using a questionnaire. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Outcome group: ulcer healing Quote: “Data from patients who did not complete the trial are not included in the statistical analysis” Quote: “183 subjects were recruited … Of the 112 who completed the trial …” Comment: high risk of bias given the rate of dropouts is more than 20%. Outcome group: comfort Quote: “patients with dementia were not included in the comfort survey” and Table 6 Comment: high risk of bias because Table 6 shows only 29 in Nimbus 3 and 24 in Cairwave completed comfort outcome assessment. |
| Selective reporting (reporting bias) | High risk | Comment: the study protocol is not available but it is clear that the published reports do not include all expected outcomes, e.g. economic evaluation was specified in Russell 1999 but not in Russell 2000a. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Strauss 1991.
| Study characteristics | ||
| Methods |
Study objective: to compare air‐fluidised bed therapy with conventional home therapy in terms of costs and cost savings. Study design including the number of centres: randomised controlled trial, multi‐centres alongside a trial‐based economic evaluation Study grouping: parallel group Duration of follow‐up: 36 weeks Number of arms: 2 Study start date and end date: not given Care setting: community |
|
| Participants |
Baseline characteristics Inclusion criteria: (1) had at least one 3rd stage or 4th stage pressure sore; (2) had an attending physician who believed that the patient would probably require future hospitalisation for pressure‐sore‐related care; (3) had severely limited mobility; (4) had adequate social support to use home air‐fluidised bed therapy (usually the assistance of a relative, friend, or paid caregiver); (5) was likely to comply with the home care regimen; (6) was likely to live at least 1 year; (7) was at least 16 years of age; (8) had been out of the hospital for at least 3 weeks; and (9) had a personal physician who was willing to closely manage care in the patient’s home. Exclusion criteria: were febrile or septic or otherwise required immediate hospitalisation; had pressure sores on radiated skin. Sex (M/F): 29/29 in air‐fluidised bed and 28/26 in control. Age (years): mean 65 in air‐fluidised bed and 63 in control; estimated overall 64. The stage of pressure ulcers at baseline: using Shea stage criteria; patients with Stage III or IV; ulcer size and stages not reported. Group difference: no difference Total number of participants: n = 112 Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Air‐fluidised bed therapy
Conventional therapy
|
|
| Outcomes |
Proportion of participants with pressure ulcers healed
Time to pressure ulcer healing
Support‐surface‐associated patient comfort
All reported adverse events
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Using forms created by a computerized random number‐generating system, the study physician or nurse would assign the patient to either the air‐fluidized bed therapy group or the control group” Comment: low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Outcome group: ulcer healing Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcome group: ulcer healing Quote: “assessed clinical outcome through reviews by two independent nurses … who were blinded to treatment category” Comment: low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Outcome group: ulcer healing Quote: "Excluding patients in the 'completely dropped' category, there were 47 patients in the group that received air‐fluidized bed therapy and 50 patients in the control group who were receiving conventional therapy" Comment: unclear risk of bias due to the moderate level of dropout and the unspecified dropout reasons. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersen 1982 | Prevention study |
| Andrews 1988 | Ineligible study design ‐ not an RCT |
| Anonymous 2006 | Ineligible study design ‐ review article |
| Aronovitch 1999 | Prevention study |
| Ballard 1997 | Prevention study |
| Beeckman 2019 | Prevention study |
| Bell 1993 | Ineligible study design ‐ not an RCT |
| Bennett 1998 | Prevention study |
| Berthe 2007 | Prevention study |
| Bliss 1966 | Ineligible study design ‐ not an RCT |
| Bliss 1967 | Prevention study |
| Bliss 1993 | Ineligible study design ‐ review article |
| Bliss 1995a | Prevention study |
| Bliss 1995b | Ineligible study design ‐ review article |
| Bliss 2003 | Reproduction of previous work |
| Bliss 2004 | Commentary on a trial |
| Branom 1999 | Incorrect randomisation (treatment study; alternate randomisation) |
| Branom 2001 | Incorrect randomisation (treatment study; alternate randomisation) |
| Brown 2001 | Summary of the Cochrane Review McInnes 2015 |
| Bueno de Camargo 2018 | Prevention study |
| Cadue 2008 | This RCT was to compare heel suspending device with the package of interventions |
| Caley 1994 | Ineligible outcomes (did not report any relevant outcome) |
| Cassino 2013b | Prevention study |
| Cavicchioli 2007 | Prevention study |
| Chaloner 2000a | Incorrect randomisation method (quasi‐randomisation) |
| ChiCTR1800017466 | Ineligible interventions |
| Chou 2013 | Review articles |
| Cobb 1997 | Prevention study |
| Collier 1996 | Prevention study |
| Conine 1990 | Prevention study |
| Cooper 1998 | Prevention study |
| Cummins 2019 | Ineligible study design ‐ quality improvement project without RCT design |
| Daechsel 1985 | Prevention study |
| Defloor 2005 | Ineligible interventions ‐ different combinations of turning and support surfaces under evaluations |
| Demarre 2012 | Prevention study |
| De Oliveira 2017 | Review article |
| Economides 1995 | This RCT was to observe the breakdown of flaps after operations rather than the incidence of new ulcers |
| Ewing 1964 | Prevention study |
| Exton‐Smith 1982 | This trial used alternation to allocate patients into groups. Proper randomisation not completed. |
| Feuchtinger 2006 | Prevention study |
| Finnegan 2008 | Prevention study |
| Fleischer 1997 | Ineligible study design |
| García Fernández 2004 | Commentary on an RCT |
| Gardner 2008 | Prevention study |
| Gazzerro 2008 | Ineligible outcome (wound healing of flap surgery) |
| Gebhardt 1994a | Incorrect randomisation method (randomisation based on participants' hospital numbers) |
| Gebhardt 1994b | Incorrect randomisation method (randomisation based on participants' hospital numbers) |
| Gebhardt 1996 | Incorrect randomisation method |
| Geelkerken 1994 | Commentary |
| Goldstone 1982 | Incorrect randomisation method |
| Gray 1994 | Prevention study |
| Gray 2000 | Prevention study |
| Gray 2008 | Prevention study |
| Greer 1988 | Ineligible study design (treatment study; case control design) |
| Grindley 1996 | Prevention study |
| Gunningberg 2000 | Prevention study |
| Gunningberg 2001 | Ineligible study design (cross sectional design) |
| Haalboom 1994 | Commentary |
| Hale 1990 | Ineligible study design (cost analysis without RCT data) |
| Hampton 1997 | Prevention study |
| Hampton 1998 | Ineligible study design (not an RCT) |
| Hampton 1999 | Ineligible study design (not an RCT) |
| Hawkins 1997 | Ineligible study design (not an RCT) |
| Hofman 1994 | Prevention study |
| Holzgreve 1993 | Ineligible study design (not an RCT) |
| Hommel 2008 | Ineligible study design (not an RCT) |
| Hoshowsky 1994 | Prevention study |
| Hoskins 2007a | Summary of findings of Nixon 2006 |
| Hoskins 2007b | Summary of findings of Nixon 2006 |
| Huang 2013 | Review article |
| Huang 2018 | Ineligible interventions (head pad rather than beds or mattresses) |
| Hungerford 1998 | Commentary on a RCT |
| Iglesias 2006 | Prevention study |
| Inman 1993 | Prevention study |
| IRCT2015110619919N3 | Prevention study |
| IRCT2016091129781N1 | Prevention study |
| Ismail 2001 | Prevention study |
| Jiang 2014 | Prevention study |
| Jolley 2004 | Prevention study |
| Kemp 1993 | Prevention study |
| Keogh 2001 | Prevention study |
| Klein 1989 | Review article |
| Laurent 1998 | Prevention study |
| Lazzara 1991 | Prevention study |
| Lee 1974 | Ineligible study design (not an RCT) |
| Maklebust 1988 | Prevention study |
| Malbrain 2010 | Prevention study |
| Marutani 2019 | Incorrect randomisation method |
| Mastrangelo 2010a | Incorrect randomisation (treatment study; alternate randomisation) |
| Mastrangelo 2010b | Prevention study |
| McGinnis 2011 | Review article |
| McGowan 2000 | Prevention study |
| McInnes 2015 | Review article |
| McInnes 2018 | Review article |
| Mendoza 2019 | Ineligible participants and outcome (flap closure) |
| Mistiaen 2010a | Prevention study |
| Mistiaen 2010b | Prevention study |
| Nakahara 2012 | Ineligible study design (not an RCT) |
| NCT01402765 | Ineligible outcome (interface pressure) |
| NCT02565797 | Ineligible study design (case control design) |
| NCT02634892 | Prevention study |
| NCT02735135 | Prevention study |
| NCT03048357 | Prevention study |
| NCT03211910 | Prevention study |
| NCT03351049 | Prevention study |
| Nixon 1998 | Prevention study |
| Nixon 2006 | Prevention study |
| Nixon 2019 | Prevention study |
| Ooka 1995 | Ineligible study design (not an RCT) |
| Osterbrink 2005 | Ineligible outcome (not reported any relevant outcome) |
| Ozyurek 2015 | Prevention study |
| Park 2017 | Prevention study |
| Phillips 1999 | Prevention study |
| Price 1999 | Prevention study |
| Pring 1998 | Prevention study |
| Rae 2018 | Review article |
| Rafter 2011 | Prevention study |
| Reddy 2006 | Review article |
| Reddy 2008 | Review article |
| Ricci 2013a | Prevention study |
| Ricci 2013b | Duplicate record |
| Rithalia 1995 | Ineligible participants (healthy people) |
| Rosenthal 2003 | Prevention study |
| Russell 2000b | Prevention study |
| Russell 2003a | Ineligible outcome (did not report any relevant outcome) |
| Russell 2003b | Prevention study |
| Sanada 2003 | Prevention study |
| Santy 1994 | Prevention study |
| Santy 1995 | Review article |
| Sauvage 2017 | Prevention study |
| Scheffel 2011 | Summary of a review |
| Schultz 1999 | Prevention study |
| Scott 2000 | Prevention study |
| Scott‐Williams 2006 | Ineligible study design (not an RCT) |
| Serraes 2018 | Review article |
| Shakibamehr 2019 | Ineligible interventions (cushions rather than beds or mattresses) |
| Sharp 2007 | Ineligible study design |
| Shi 2018a | Review article |
| Sideranko 1992 | Prevention study |
| Smith 2013 | Review article |
| Stannard 1993 | Commentary on an RCT |
| Stapleton 1986 | Prevention study |
| Sterzi 2003 | Ineligible study design (not an RCT) |
| Takala 1994 | Ineligible study design (not an RCT) |
| Takala 1996 | Prevention study |
| Taylor 1999 | Prevention study |
| Tewes 1993 | Review article |
| Theaker 2005 | Prevention study |
| Vanderwee 2005 | Prevention study |
| Van Leen 2011 | Prevention study |
| Van Leen 2013 | Prevention study |
| Van Leen 2018 | Prevention study |
| Van Rijswijk 1994 | Commentary |
| Vermette 2012 | Prevention study |
| Vyhlidal 1997 | Prevention study |
| Wallace 2009 | Review article |
| Whitney 1984 | Prevention study |
| Whittingham 1999 | Prevention study |
| Yao 2018 | Review article |
Characteristics of studies awaiting classification [ordered by study ID]
Chaloner 2000b.
| Methods | Not available |
| Participants | Not available |
| Interventions | Two types of alternating pressure air surfaces. |
| Outcomes | Not available |
| Notes | Unable to obtain its full text. |
Henn 2004.
| Methods | Not available |
| Participants | Not available |
| Interventions | Alternating pressure air surfaces and a type of surface that cannot be defined. |
| Outcomes | Not available |
| Notes | Unable to obtain its full text. |
Knight 1999.
| Methods | Not available |
| Participants | Not available |
| Interventions | Pressure relieving surfaces that cannot be defined. |
| Outcomes | Not available |
| Notes | Unable to obtain its full text. |
Melland 1998.
| Methods | Not available |
| Participants | Not available |
| Interventions | 'Freedom bed' that cannot be defined. |
| Outcomes | Not available |
| Notes | Unable to obtain its full text. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12618000319279.
| Study name | Comparison of active and reactive pressure mattresses in the treatment of Stage 2, unstageable pressure injuries and Suspected Deep Tissue Injuries for people over 50 years residing in the community |
| Methods | Randomised controlled trial Randomisation methods: central randomisation by computer; eligible participants will be allocated to groups by an online random number generator between 1 and 2. Blinding: the people assessing the outcomes, the people analysing the results/data blinded. |
| Participants | Eligibility criteria: 1. aged 50 years or older 2. residing in a community setting i.e. in a private home 3. existing Stage 2 pressure injury, unstageable pressure injury or Suspected Deep Tissue Injury ‐ any location or aetiology 4. have a bed that is appropriate for the support surfaces 5. inability to re‐position off the pressure injury Sample size: a priori sample was determined for a total sample of n = 80 to aim for a minimum final total sample of n = 60 (n = 30 for each group, utilising the central limit theorem) after allowing for withdrawals. |
| Interventions | Interventions: Provision of a reactive pressure mattress chosen from a range of mattresses (following list is not exhaustive): ‐ ROHO overlay (3x ROHO sections and 1x foam section) ‐ Curocell AreaZone mattress replacement ‐ Curocell SAM mattress overlay ‐ Softform Premier mattress replacement ‐ Pressureguard CFT mattress replacement ‐ BetterLiving Triple layer mattress replacement Provision of an active pressure mattress chosen from a range of mattresses (following list is not exhaustive): ‐ Curocell Uno mattress replacement ‐ Viruoso mattress replacement ‐ Salsbury mattress overlay ‐ Premium Digital 9 mattress replacement ‐ Premium Digital 5 mattress overlay |
| Outcomes | 1. Time to complete healing of the primary pressure injury as determined by the Revised Photographic Wound Assessment Tool (RevPWAT). 2. Rate of healing will be used as determined by changes in RevPWAT points over time (RevPWAT baseline score ‐ RevPWAT 8 week score)/8 (no. of weeks in study). 3. User acceptability of the provided mattress as determined by a survey designed specifically for this study. 4. Changes in sleeping habits as determined by a survey designed specifically for this study. 5. Changes in pain levels as determined by a 10‐point pain scale and subjective comments from a survey designed specifically for this study. |
| Starting date | 26 March 2018 |
| Contact information | Mrs Katherine E Rae Address Occupational Therapist, ACT Health Directorate: Canberra, Australian PhD Candidate, University of Canberra; Canberra, Australia Village Creek Health Centre, 37 Kingsmill St, Kambah, ACT, 2905, AustraliaCountry AustraliaPhone +61 2 5124 1057Fax Email katherine.rae@act.gov.au |
| Notes |
JPRN‐UMIN000029680.
| Study name | Effects of robotic mattress on pressure ulcer healing, comfort level among pressure ulcer patients, and nursing work load: a randomised controlled trial |
| Methods | randomised controlled trial |
| Participants | Eligibility criteria: 1. More than 20 years old 2. Male and female 3. Patients with pressure ulcers in their sacral, coccyx, ischial tuberosity, greater trochanter, or heel |
| Interventions | Not specified |
| Outcomes | 1. Wound healing rate within 3 weeks 2. Patient comfort level 3. Nursing work load |
| Starting date | 1 November 2017 |
| Contact information | Aya Kitamura Address Faculty of Medicine Bldg. 5‐301, 7‐3‐1, Hongo, Bunkyo‐ku, Tokyo,Japan Japan Telephone 03‐5841‐3451 E‐mail ktmr‐tky@umin.ac.jp Affiliation The University of Tokyo Graduate School of Medicine |
| Notes |
Differences between protocol and review
We changed the title of this review to 'Beds, overlays and mattresses for treating pressure ulcers' whilst the title of the published protocol was 'Beds and mattresses for treating pressure ulcers' (Shi 2020).
Two review authors independently assessed the titles and abstracts of the new search results for relevance using Rayyan rather than using Covidence.
For new included studies, one review author independently extracted data and another review author checked all data, rather two review authors carrying out independent data extraction.
We presented separate 'Summary of findings' tables for three of the four comparisons evaluated in this review. We did not present the table for the comparison between different types of alternating pressure (active) air surfaces.
Where we did not pool data, we conducted a GRADE assessment and presented these assessments in a narrative format in 'Summary of findings' tables. This was not previously planned.
Contributions of authors
Chunhu Shi: conceived the review; designed the review; coordinated the review; extracted data; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; performed statistical analysis; produced the first draft of the review; contributed to writing or editing the review; wrote to study authors/experts/companies; approved the final review prior to publication; is guarantor of the review.
Jo Dumville: conceived the review; designed the review; coordinated the review; checked quality of data extraction; analysed or interpreted data; checked quality of statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; secured funding; approved the final review prior to publication.
Nicky Cullum: conceived the review; designed the review; coordinated the review; checked quality of data extraction; analysed or interpreted data; contributed to writing or editing the review; advised on the review; secured funding; approved the final review prior to publication.
Sarah Rhodes: conceived the review; designed the review; checked quality of data extraction; analysed or interpreted data; checked quality assessment; checked quality of statistical analysis; contributed to writing or editing the review; advised on the review; approved the final review prior to publication.
Asmara Jammali‐Blasi: checked quality of data extraction; checked quality assessment; advised on the review; performed previous work that was the foundation of the current review; approved final review prior to publication.
Victoria Ramsden: checked quality of data extraction; checked quality assessment; advised on the review; performed previous work that was the foundation of the current review; approved the final review prior to publication.
Elizabeth McInnes: conceived the review; designed the review; coordinated the review; checked quality of data extraction; analysed or interpreted data; checked quality assessment; contributed to writing or editing the review; advised on the review; performed previous work that was the foundation of the current review; approved the final review prior to publication.
Contributions of the editorial base
Gill Norman (Editor): edited the protocol; advised on methodology, interpretation and content; approved the final protocol prior to publication. Gill Rizzello (Managing Editor): coordinated the editorial process; advised on content; edited the protocol and the review. Sophie Bishop (Information Specialist): designed the search strategy and edited the search methods section. Tom Patterson (Editorial Assistant): edited the reference sections of the protocol and the review.
Sources of support
Internal sources
Division of Nursing, Midwifery and Social Work, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, UK
External sources
-
National Institute for Health Research, UK
This project is funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB‐PG‐1217‐20006). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
-
NIHR Manchester Biomedical Research Centre (BRC), UK
This research was co‐funded by the NIHR Manchester BRC. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
-
National Institute for Health Research (NIHR), UK
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
-
National Institute for Health Applied Research Collaboration, Greater Manchester, UK
Nicky Cullum and Jo Dumville’s work on this project was partially funded by the National Institute for Health Applied Research Collaboration, Greater Manchester. The views expressed in this publication are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
Declarations of interest
Chunhu Shi: I received research funding from the National Institute for Health Research (NIHR; Research for Patient Benefit, Evidence synthesis for pressure ulcer prevention and treatment, PB‐PG‐1217‐20006). I received support from the Tissue Viability Society to attend conferences unrelated to this work. The Doctoral Scholar Awards Scholarship and Doctoral Academy Conference Support Fund (University of Manchester) also supported a PhD and conference attendance respectively; both were unrelated to this work.
Jo Dumville: I am Chief Investigator on a National Institute for Health Research grant that funded the conduct of this review (Research for Patient Benefit, Evidence synthesis for pressure ulcer prevention and treatment, PB‐PG‐1217‐20006). This research was co‐funded by the NIHR Manchester Biomedical Research Centre, and partly funded by the National Institute for Health Research Applied Research Collaboration Greater Manchester. Nicky Cullum: I am Co‐investigator on a National Institute for Health Research grant that funded the conduct of this review (Research for Patient Benefit, Evidence synthesis for pressure ulcer prevention and treatment, PB‐PG‐1217‐20006). This research was co‐funded by the NIHR Manchester Biomedical Research Centre, and partly funded by the National Institute for Health Research Applied Research Collaboration Greater Manchester.
My previous and current employers received research grant funding from the NHS Research and Development Programme and subsequently the NIHR for previous versions of this review. The funders had no role in the conduct of the review. My previous employer received research grant funding for an RCT comparing a mattress and an overlay. This RCT was not eligible for inclusion in this review as it was not a treatment study.
Sarah Rhodes: my salary is funded from three NIHR grants and a grant from Greater Manchester Cancer.
Asmara Jammali‐Blasi: none known.
Victoria Ramsden: none known.
Elizabeth McInnes: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Allman 1987 {published data only}
- Allman RM, Walker JM, Hart MK, Laprade CA, Noel LB, Smith CR. Air-fluidized beds or conventional therapy for pressure sores. A randomized trial. Annals of Internal Medicine 1987;107(5):641-8. [DOI] [PubMed] [Google Scholar]
Cassino 2013a {published data only}
- Cassino R, Ippolito AM, Cuffaro C, Corsi A, Ricci E. A controlled, randomized study on the effectiveness of two overlays in the treatment of decubitus ulcers. Minerva Chirurgica 2013;68(1):105-16. [PubMed] [Google Scholar]
Day 1993 {published data only}
- Day A, Leonard F. Seeking quality care for patients with pressure ulcers. Decubitus 1993;6(1):32-43. [PMID: ] [PubMed] [Google Scholar]
Devine 1995 {published data only}
- Devine B. Alternating pressure air mattresses in the management of established pressure sores. Journal of Tissue Viability 1995;5(3):94-8. [Google Scholar]
Evans 2000a {published data only}
- Evans D, Land L, Geary A. A clinical evaluation of the Nimbus 3 alternating pressure mattress replacement system. Journal of Wound Care 2000;9(4):181-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Evans 2000b {published data only}
- Evans D, Land L, Geary A. A clinical evaluation of the Nimbus 3 alternating pressure mattress replacement system. Journal of Wound Care 2000;9(4):181-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ferrell 1993 {published data only}
- Ferrell BA, Osterweil D, Christenson P. A randomized trial of low-air-loss beds for treatment of pressure ulcers. JAMA 1993;269(4):494-7. [PMID: ] [PubMed] [Google Scholar]
Ferrell 1995 {published data only}
- Ferrell BA, Keeler E, Siu AL, Ahn SH, Osterweil D. Cost-effectiveness of low-air-loss beds for treatment of pressure ulcers. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 1995;50(3):M141-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Groen 1999 {published data only}
- Groen HW, Groenier KH, Schuling J. Comparative study of a foam mattress and a water mattress. Journal of Wound Care 1999;8(7):333-5. [DOI] [PubMed] [Google Scholar]
Mulder 1994 {published data only}
- Mulder GD, Taro N, Seeley J, Andrews K. A study of pressure ulcer response to low air loss beds vs. conventional treatment. Journal of Geriatric Dermatology 1994;2(3):87-91. [Google Scholar]
Munro 1989 {published data only}
- Munro BH, Brown L, Heitman BB. Pressure ulcers: one bed or another? How does an air-fluidized bed compare with pads and other devices on a standard bed? Geriatric Nursing 1989;10:190-2. [DOI] [PubMed] [Google Scholar]
Russell 2000a {published data only}
- Russell L, Reynolds T, Carr J, Evans A, Holmes M. A comparison of healing rates on two pressure-relieving systems. British Journal of Nursing (Mark Allen Publishing) 2000;9(22):2270-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Russell L, Reynolds TM, Carr J, Evans A, Holmes M. Randomised controlled trial of two pressure-relieving systems. Journal of Wound Care 2000;9(2):52-5. [DOI] [PubMed] [Google Scholar]
- Russell L. Randomized comparative clinical trial of Pegasus Cairwave mattress and Proactive seating cushion and Huntleigh Nimbus 3 & Aura seating cushion. Journal of Tissue Viability 1999;9(3):103-4. [Google Scholar]
- Russell L. Randomized comparative clinical trial of the Pegasus Cairwave mattress and Proactive seating cushion and the Huntleigh Nimbus III mattress and Alpha Transcell seating cushion. In: European Wound Management Association and Journal of Wound Care Autumn Conference; 1998 November; Harrogate (UK). 1998:4.
Strauss 1991 {published data only}
- Strauss MJ, Gong J, Gary BD, Kalsbeek WD, Spear S. The cost of home air-fluidized therapy for pressure sores. A randomized controlled trial. Journal of Family Practice 1991;33(1):52-9. [PMID: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Andersen 1982 {published data only}
- Andersen KE, Jensen O, Kvorning SA, Bach E. Decubitus prophylaxis: a prospective trial on the efficacy of alternating-pressure air-mattresses and water-mattresses. Acta Dermatovener (Stockholm) 1982;63:227-30. [PubMed] [Google Scholar]
Andrews 1988 {published data only}
- Andrews J, Balai R. The prevention and treatment of pressure sores by use of pressure distributing mattresses. Care Science and Practice 1989;7(3):72-6. [PubMed] [Google Scholar]
- Andrews J, Balai R. The prevention and treatment of pressure sores by use of pressure distributing mattresses. Decubitus 1988;1(4):14-21. [PubMed] [Google Scholar]
Anonymous 2006 {published data only}
- Anonymous. Effective methods for preventing pressure ulcers. Journal of Family Practice 2006;55(11):942. [PMID: ] [PubMed] [Google Scholar]
Aronovitch 1999 {published data only}
- Aronovitch SA, Wilber M, Slezak S, Martin T, Utter D. A comparative study of an alternating air mattress for the prevention of pressure ulcers in surgical patients. Ostomy/Wound Management 1999;45(3):34-44. [PubMed] [Google Scholar]
- Aronovitch SA. A comparative, randomized, controlled study to determine safety and efficacy of preventive pressure ulcer systems: preliminary analysis. Advances in Wound Care 1998;11:15-6. [PubMed] [Google Scholar]
Ballard 1997 {published data only}
- Ballard K. Pressure-relief mattresses and patient comfort. Professional Nurse 1997;13(1):27-32. [PubMed] [Google Scholar]
Beeckman 2019 {published data only}
- Anrys C, Van Tiggelen H, Verhaeghe S, Van Hecke A, Beeckman D. Independent risk factors for pressure ulcer development in a high-risk nursing home population receiving evidence-based pressure ulcer prevention: results from a study in 26 nursing homes in Belgium. International Wound Journal 2019;16(2):325-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeckman D, Serraes B, Anrys C, Van Tiggelen H, Van Hecke A, Verhaeghe S. A multicentre prospective randomised controlled clinical trial comparing the effectiveness and cost of a static air mattress and alternating air pressure mattress to prevent pressure ulcers in nursing home residents. International Journal of Nursing Studies 2019;97:105-13. [DOI] [PubMed] [Google Scholar]
- NCT03597750. Comparison of static air support devices (Repose®) and alternating-pressure devices in the prevention of pressure ulcers. ClinicalTrials.gov/show/NCT03597750.
Bell 1993 {published data only}
- Bell JC, Matthews SD. Results of a clinical investigation of four pressure-reduction replacement mattresses. Journal of ET Nursing 1993;20(5):204-10. [PMID: ] [PubMed] [Google Scholar]
Bennett 1998 {published data only}
- Bennett RG, Baran PJ, DeVone LV, Bacetti H, Kristo B, Tayback M, et al. Low airloss hydrotherapy versus standard care for incontinent hospitalized patients. Journal of the American Geriatrics Society 1998;46(5):569-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Berthe 2007 {published data only}
- Berthe JV, Bustillo A, Mélot C, De Fontaine D. Does a foamy-block mattress system prevent pressure sores? A prospective randomised clinical trial in 1729 patients. Acta Chirurgica Belgica 2007;107(2):155-61. [PubMed] [Google Scholar]
Bliss 1966 {published data only}
- Bliss MR, McLaren R, Exton-Smith AN. Mattresses for preventing pressure sores in geriatric patients. Monthly Bulletin of the Ministry of Health and the Public Health Laboratory Service 1966;25:238-68. [PMID: ] [PubMed] [Google Scholar]
Bliss 1967 {published data only}
- Bliss MR, McLaren R, Exton-Smith AN. Preventing pressure sores in hospital: controlled trial of a large-celled ripple mattress. British Medical Journal 1967;1(5537):394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bliss 1993 {published data only}
- Bliss MR, Thomas JM. An investigative approach. An overview of randomised controlled trials of alternating pressure supports. Professional Nurse (London, England) 1993;8(7):437-44. [PMID: ] [PubMed] [Google Scholar]
Bliss 1995a {published data only}
- Bliss MR. Preventing pressure sores in elderly patients: a comparison of seven mattress overlays. Age and Ageing 1995;24:297-302. [DOI] [PubMed] [Google Scholar]
- Bliss MR. Randomised controlled trial of seven pressure relieving mattress overlays for preventing pressure sores in elderly patients. In: Conference of the Tissue Viability Society; 1994; Harrogate (UK). 1994:5.
Bliss 1995b {published data only}
- Bliss MR. Managing pressure ulcers. Advances in Wound Care 1995;8(5):6, 8. [PMID: ] [PubMed] [Google Scholar]
Bliss 2003 {published data only}
- Bliss MR. Clinical research in patient support systems. Journal of Tissue Viability 2003;13(4):154-6, 158, 160. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bliss 2004 {published data only}
- Bliss M. Trials of alternating pressure mattresses. Journal of Tissue Viability 2004;14(1):32-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Branom 1999 {published data only}
- Branom R, Knox L. Low-air-loss vs non-powered/dynamic surfaces in wound management. In: 31st Annual Wound, Ostomy and Continence Conference: Minneapolis (MN). 1999:483.
Branom 2001 {published data only}
- Branom R, Rappl LM. "Constant force technology" versus low-air-loss therapy in the treatment of pressure ulcers. Ostomy/Wound Management 2001;47(9):38-46. [PMID: ] [PubMed] [Google Scholar]
Brown 2001 {published data only}
- Brown SJ. Bed surfaces and pressure sore prevention: an abridged report. Orthopedic Nursing 2001;20(4):38-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bueno de Camargo 2018 {published data only}
- Bueno de Camargo WH, Pereira RD, Tanita MT, Heko L, Grion IC, Festti J, et al. The effect of support surfaces on the incidence of pressure injuries in critically ill patients: a randomized clinical trial. Critical Care Research and Practice 2018;2018:Article ID 3712067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02844166. Support surfaces to prevent pressure injuries. clinicaltrials.gov/show/NCT02844166.
Cadue 2008 {published data only}
- Cadue JF, Karolewicz S, Tardy C, Barrault C, Robert R, Pourrat O. Prevention of heel pressure sores with a foam body-support device. A randomized controlled trial in a medical intensive care unit [Efficacite de supports anatomiques en mousse pour la prevention des escarres de talons. Etude controlee randomisee en reanimation medicale]. Presse Medicale (Paris, France: 1983) 2008;37(1 Pt 1):30-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Caley 1994 {published data only}
- Caley L, Jones S, Freer J, Muller JS. Randomized prospective trial: treatment outcomes and cost-effectiveness of two types of low-air-loss therapy. In: Proceedings of the 9th Annual Clinical Symposium on Pressure Ulcer and Wound Management. 1994:1-2.
Cassino 2013b {published data only}
- Cassino R, Ippolito AM, Ricci E. Comparison of two mattress overlays in the prevention of pressure ulcers. Acta Vulnologica 2013;11(1):15-21. [Google Scholar]
Cavicchioli 2007 {published data only}
- Cavicchioli A, Carella G. Clinical effectiveness of a low-tech versus high-tech pressure-redistributing mattress. Journal of Wound Care 2007;16(7):285-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chaloner 2000a {published data only}
- Chaloner D, Cave J. Should weaker study designs ever be preferred over randomised controlled trials. Journal of Tissue Viability 2000;10(3 suppl):7-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
ChiCTR1800017466 {published data only}
- ChiCTR1800017466. The study on the application of fluidized positioner in preventing intraoperative pressure injury. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1800017466 2018.
Chou 2013 {published data only}
- Chou R, Dana T, Bougatsos C, Blazina I, Starmer AJ, Reitel K, et al. Pressure ulcer risk assessment and prevention: a systematic comparative effectiveness review. Annals of Internal Medicine 2013;159(1):28-38. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cobb 1997 {published data only}
- Cobb GA, Yoder LH, Warren JB. Pressure ulcers: patient outcomes on a KinAir bed or EHOB waffle mattress. TriService Nursing Research Program (TSNRP) 1997.
Collier 1996 {published data only}
- Collier ME. Pressure-reducing mattresses. Journal of Wound Care 1996;5(5):207-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Conine 1990 {published data only}
- Conine TA, Daechsel D, Choi AK, Lau MS. Costs and acceptability of two special overlays for the prevention of pressure sores. Rehabilitation Nursing 1990;15(3):133-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Conine TA, Daechsel D, Lau MS. The role of alternating air and Silicore overlays in preventing decubitus ulcers. International Journal of Rehabilitation Research 1990;13(1):133-7. [DOI] [PubMed] [Google Scholar]
Cooper 1998 {published data only}
- Cooper PJ, Gray DG, Mollison J. A randomised controlled trial of two pressure-reducing surfaces. Journal of Wound Care 1998;7(8):374-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cummins 2019 {published data only}
- Cummins KA, Watters R, Leming-Lee T. Reducing pressure injuries in the pediatric intensive care unit. Nursing Clinics of North America 2019;54(1):127-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Daechsel 1985 {published data only}
- Daechsel D, Conine TA. Special mattresses: effectiveness in preventing decubitus ulcers in chronic neurologic patients. Archives of Physical Medicine and Rehabilitation 1985;66(4):246-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Defloor 2005 {published data only}
- Defloor T, De Bacquer D, Grypdonck MH. The effect of various combinations of turning and pressure reducing devices on the incidence of pressure ulcers. International Journal of Nursing Studies 2005;42(1):37-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
Demarre 2012 {published data only}
- Demarre L, Beeckman D, Vanderwee K, Defloor T, Grypdonck M, Verhaeghe S. Multi-stage versus single-stage inflation and deflation cycle for alternating low pressure air mattresses to prevent pressure ulcers in hospitalised patients: a randomised-controlled clinical trial. International Journal of Nursing Studies 2012;49(4):416-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Demarre L, Vanderwee K, Beeckman D, Defloor T. Pressure ulcer prevention: randomized controlled trial comparing the effect of a standard alternating pressure air mattress and a alternating low pressure air mattress with gradual inflation and deflation. EWMA Journal 2010;10(2):44. [Google Scholar]
- Demarre L, Vanderwee K, Beeckman D, Defloor T. The effectiveness of a multistage low pressure air mattress in pressure ulcer prevention: an RCT Fourth European Nursing Congress. Journal of Clinical Nursing 2010;19:41.
- Demarre L, Verhaeghe S, Van Hecke A, Grypdonck M, Clays E, Vanderwee K, et al. The effectiveness of three types of alternating pressure air mattresses in the prevention of pressure ulcers in Belgian hospitals. Research in Nursing & Health 2013;36(5):439-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
De Oliveira 2017 {published data only}
- De Oliveira KF, Nascimento KG, Nicolussi AC, Chavaglia SR, De Araujo CA, Barbosa MH. Support surfaces in the prevention of pressure ulcers in surgical patients: an integrative review. International Journal of Nursing Practice 2017;23(4):doi: 10.1111/ijn.12553. [DOI] [PubMed] [Google Scholar]
Economides 1995 {published data only}
- Economides NG, Skoutakis VA, Carter CA, Smith VH. Evaluation of the effectiveness of two support surfaces following myocutaneous flap surgery. Advances in Wound Care 1995;8(1):49-53. [PMID: ] [PubMed] [Google Scholar]
Ewing 1964 {published data only}
- Ewing MR, Garrow C, Presley TA, Ashley C, Kisella NM. Further experiences in the use of sheep skins as an aid in nursing. Australian Nurses' Journal 1964;1964 September:215-9. [Google Scholar]
Exton‐Smith 1982 {published data only}
- Exton-Smith AN, Overstall PW, Wedgwood J, Wallace G. Use of the 'air wave system' to prevent pressure sores in hospital. Lancet 1982;1(8284):1288-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Feuchtinger 2006 {published data only}
- Feuchtinger J, De Bie R, Dassen T, Halfens R. A 4-cm thermoactive viscoelastic foam pad on the operating room table to prevent pressure ulcer during cardiac surgery. Journal of Clinical Nursing 2006;15(2):162-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Feuchtinger J. Preventing decubitus ulcer in heart surgery interventions: visco-elastic foam layer on the operating room table--a study [Dekubituspravention wahrend kardiochirurgischer Eingriffe: Viskoelastische Schaumstoffauflage auf dem Operationstisch--eine Studie]. Pflege Zeitschrift 2006;59(8):498-501. [PMID: ] [PubMed] [Google Scholar]
Finnegan 2008 {published data only}
- Finnegan MJ, Gazzerro L, Finnegan JO, Lo P. Comparing the effectiveness of a specialized alternating air pressure mattress replacement system and an air-fluidized integrated bed in the management of post-operative flap patients: a randomized controlled pilot study. Journal of Tissue Viability 2008;17(1):2-9. [DOI] [PubMed] [Google Scholar]
Fleischer 1997 {published data only}
- Fleischer I, Bryant D. Evaluating replacement mattresses. Nursing Management 1997;28(8):38-41. [PMID: ] [PubMed] [Google Scholar]
García Fernández 2004 {published data only}
- García Fernández FP, Pancorbo Hidalgo PL, Rodríguez Torres M. Utility and cost-effectiveness of air suspension bed in the prevention of pressure ulcers. Gerokomos 2004;15(3):162-7. [Google Scholar]
Gardner 2008 {published data only}
- Gardner A, Dunk AM, Gardner G. Which mattress works best? A clinical trial of the comparative effectiveness of constant low pressure and alternating pressure devices in hospital patients deemed at risk of pressure injury. In: Australian Wound Management Association 7th National Conference. 2008:52.
Gazzerro 2008 {published data only}
- Gazzerro L, Finnegan M. Comparing the effectiveness of alternating air pressure mattress replacement systems and air fluidized integrated beds in the management of flap and graft patients: ten case studies. Scientific and Clinical Abstracts from the 40th Annual Wound, Ostomy and Continence Nurses Annual Conference. Journal of Wound, Ostomy, and Continence Nursing 2008;35(3):S63. [Google Scholar]
Gebhardt 1994a {published data only}
- Gebhardt K, Bliss MR. A controlled study to compare the efficacy, practicability and cost of pressure relieving supports to prevent and heal pressure sores. In: 2nd European Conference on Advances in Wound Management; 1992 October 20-23; Harrogate (UK). 1993:166.
- Gebhardt K. A randomized trial of alternating pressure (AP) and constant low pressure (CLP) supports for the prevention of pressure sores. Journal of Tissue Viability 1994;4(3):93. [Google Scholar]
Gebhardt 1994b {published data only}
- Gebhardt KS, Bliss MR, Winwright PL. A randomised controlled trial to compare the efficacy of alternating and constant low pressure supports for preventing pressure sores in an intensive care unit (ICU). Clinical Science 1994;86(S30):39P. [Google Scholar]
Gebhardt 1996 {published data only}
- Gebhardt KS, Bliss MR, Winwright PL, Thomas J. Pressure-relieving supports in an ICU. Journal of Wound Care 1996;5(3):116-21. [DOI] [PubMed] [Google Scholar]
Geelkerken 1994 {published data only}
- Geelkerken RH, Breslau PJ, Hermans J, Wille J, Hofman A, Hamming JJ. Anti-decubitus mattress [Anti-decubitusmatrassen]. Nederlands Tijdschrift voor Geneeskunde 1994;138(36):1834; author reply 1834-5. [PMID: ] [PubMed]
Goldstone 1982 {published data only}
- Goldstone LA, Norris M, O'Reilly M, White J. A clinical trial of a bead bed system for the prevention of pressure sores in elderly orthopaedic patients. Journal of Advanced Nursing 1982;7(6):545-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gray 1994 {published data only}
- Gray D. A randomised clinical trial of two foam mattresses. Aberdeen Royal Hospitals NHS Trust. Medical Support System 1994.
- Gray D. A randomised controlled trial of two foam mattresses. Journal of Tissue Viability 1994;4(3):92. [Google Scholar]
- Gray DG, Campbell M. A randomised clinical trial of two types of foam mattresses. Journal of Tissue Viability 1994;4(4):128-32. [Google Scholar]
- Gray DG, Cooper PJ, Campbell M. A study of the performance of a pressure reducing foam mattress after three years of use. Journal of Tissue Viability 1998;8(3):9-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gray 2000 {published data only}
- Gray D, Smith M. A randomized controlled trial of two pressure-reducing foam mattresses. In: European Wound Management Association Conference; 1998 November; Harrogate (UK). 1998:4.
- Gray DG, Smith M. Comparison of a new foam mattress with the standard hospital mattress. Journal of Wound Care 2000;9(1):29-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gray 2008 {published data only}
- Gray D, Cooper P, Bertram M, Duguid K, Pirie G. A clinical audit of the Softform Premier Active™ mattress in two acute care of the elderly wards. Wounds UK 2008;4(4):124-8. [Google Scholar]
Greer 1988 {published data only}
- Greer DM, Morris J, Walsh NE, Glenn AM, Keppler J. Cost-effectiveness and efficacy of air-fluidized therapy in the treatment of pressure ulcers. Journal of Enterostomal Therapy 1988;15(6):247-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Grindley 1996 {published data only}
- Grindley A, Acres J. Alternating pressure mattresses: comfort and quality of sleep. British Journal of Nursing (Mark Allen Publishing) 1996;5(21):1303-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gunningberg 2000 {published data only}
- Gunningberg L, Lindholm C, Carlsson M, Sjoden PO. Effect of visco-elastic foam mattresses on the development of pressure ulcers in patients with hip fractures. Journal of Wound Care 2000;9(10):455-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gunningberg 2001 {published data only}
- Gunningberg L, Lindholm C, Carlsson M, Sjoden PO. Reduced incidence of pressure ulcers in patients with hip fractures: a 2-year follow-up of quality indicators. International Journal for Quality in Health Care 2001;13(5):399-407. [PMID: ] [DOI] [PubMed] [Google Scholar]
Haalboom 1994 {published data only}
- Haalboom JR. Anti-decubitus mattresses [Anti-decubitusmatrassen]. Nederlands Tijdschrift voor Geneeskunde 1994;138(26):1309-10. [PMID: ] [PubMed] [Google Scholar]
Hale 1990 {published data only}
- Hale JS, Smith SH. Pressure reduction mattresses versus pressure reduction overlays: a cost analysis. Journal of Enterostomal Therapy 1990;17(6):241-3. [PMID: ] [PubMed] [Google Scholar]
Hampton 1997 {published data only}
- Hampton S. Evaluation of the new Cairwave Therapy System in one hospital trust. British Journal of Nursing (Mark Allen Publishing) 1997;6(3):167-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hampton 1998 {published data only}
- Hampton S. Can electric beds aid pressure sore prevention in hospitals? British Journal of Nursing (Mark Allen Publishing) 1998;7(17):1010-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hampton 1999 {published data only}
- Hampton S. Efficacy and cost-effectiveness of the Thermo contour mattress. British Journal of Nursing (Mark Allen Publishing) 1999;8(15):990-6. [DOI] [PubMed] [Google Scholar]
Hawkins 1997 {published data only}
- Hawkins JE. The effectiveness of pressure-reducing table pads as an intervention to reduce the risk of intraoperatively acquired pressure sores. Military Medicine 1997;162(11):759-61. [PMID: ] [PubMed] [Google Scholar]
Hofman 1994 {published data only}
- Hofman A, Geelkerken RH, Wille J, Hamming JJ, Hermans J, Breslau PJ. Pressure sores and pressure-decreasing mattresses: controlled clinical trial. Lancet 1994;343(8897):568-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Holzgreve 1993 {published data only}
- Holzgreve A, Waldner M, Waldner PW, Hohlbach G. Bed sore prophylaxis in patients with chronic arterial occlusive disease with a new thermoactive pressure alternating mattress. Langenbecks Archiv fur Chirurgie 1993:1117.
Hommel 2008 {published data only}
- Hommel A, Thorngre KG, Ulander K. How to prevent patients with a hip fracture from developing pressure ulcers. EWMA Journal 2008;8(2):157. [Google Scholar]
Hoshowsky 1994 {published data only}
- Hoshowsky VM, Schramm CA. Intraoperative pressure sore prevention: an analysis of bedding materials. Research in Nursing & Health 1994;17(5):333-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hoskins 2007a {published data only}
- Hoskins A. Alternating pressure mattresses were more cost effective than alternating pressure overlays for preventing pressure ulcers. Evidence-Based Nursing 2007;10(1):23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hoskins 2007b {published data only}
- Hoskins A. Similar proportions of patients developed pressure ulcers on alternating pressure overlays and alternating pressure mattresses. Evidence-Based Nursing 2007;10(1):22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Huang 2013 {published data only}
- Huang HY, Chen HL, Xu XJ. Pressure-redistribution surfaces for prevention of surgery-related pressure ulcers: a meta-analysis. Ostomy/Wound Management 2013;59(4):36-8, 42, 44, 46, 48. [PMID: ] [PubMed] [Google Scholar]
Huang 2018 {published data only}
- Huang W, Zhu Y, Qu H. Use of an alternating inflatable head pad in patients undergoing open heart surgery. Medical Science Monitor 2018;24:970-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hungerford 1998 {published data only}
- Hungerford K. A specially designed foam mattress replacement reduced pressure ulcers in nursing home residents. Evidence-Based Nursing 1998;1(2):51. [Google Scholar]
Iglesias 2006 {published data only}
- Iglesias C, Nixon J, Cranny G, Nelson EA, Hawkins K, Phillips A, et al. Pressure relieving support surfaces (PRESSURE) trial: cost effectiveness analysis. BMJ 2006;332(7555):1416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias CP, Nixon J, Cranny G, Nelson A, Hawkins K, Philips A, et al. The NHS Health Technology Assessment Programme. Pressure trial: cost effectiveness analysis of two alternating pressure surfaces for the prevention of pressure ulcers. EWMA Journal 2006;6(1):38. [Google Scholar]
- Iglesias CP, Nixon J, Cranny G. Pressure trial: cost effectiveness analysis of two alternating pressure surfaces for the prevention of pressure ulcers. In: European Wound Management Association Conference; 2005 September 15-17; Stuttgart (Germany). 2005:156.
Inman 1993 {published data only}
- Inman KJ, Sibbald WJ, Rutledge FS, Clark BJ. Clinical utility and cost-effectiveness of an air suspension bed in the prevention of pressure ulcers. JAMA 1993;269(9):1139-43. [PMID: ] [PubMed] [Google Scholar]
IRCT2015110619919N3 {published data only}
- IRCT2015110619919N3. The effect of silicone protective pad on pressure ulcer. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015110619919N3 2016.
IRCT2016091129781N1 {published data only}
- IRCT2016091129781N1. Comparison gum gel and foam cushions to prevent bedsores. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016091129781N1.
Ismail 2001 {published data only}
- Ismail ZB. Comparative study between the use of a pressure relieving overlay mattress and other mattresses commonly used by homebound patients in the community. Singapore Nursing Journal 2001;28(2):13-6. [Google Scholar]
Jiang 2014 {published data only}
- Jiang Q, Li X, Zhang A, Guo Y, Liu Y, Liu H, et al. Multicenter comparison of the efficacy on prevention of pressure ulcer in postoperative patients between two types of pressure-relieving mattresses in China. International Journal of Clinical and Experimental Medicine 2014;7(9):2820-7. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Jolley 2004 {published data only}
- Jolley DJ, Wright R, McGowan S, Hickey MB, Campbell DA, Sinclair RD, et al. Preventing pressure ulcers with the Australian Medical Sheepskin: an open-label randomised controlled trial. Medical Journal of Australia 2004;180(7):324-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Jolley DJ. A multilevel analysis of three randomised controlled trials of the Australian Medical Sheepskin in the prevention of sacral pressure ulcers. Medical Journal of Australia 2011;194(2):104. [DOI] [PubMed] [Google Scholar]
Kemp 1993 {published data only}
- Kemp MG, Kopanke D, Tordecilla L, Fogg L, Shott S, Matthiesen V, et al. The role of support surfaces and patient attributes in preventing pressure ulcers in elderly patients. Research in Nursing & Health 1993;16(2):89-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Keogh 2001 {published data only}
- Keogh A, Dealey C. Profiling beds versus standard hospital beds: effects on pressure ulcer incidence outcomes. Journal of Wound Care 2001;10(2):15-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Klein 1989 {published data only}
- Klein L, Gilroy K. Evaluating mattress overlays and pressure relieving systems: a question of perception or reality? Journal of Enterostomal Therapy 1989;16(2):58-60. [PMID: ] [PubMed] [Google Scholar]
Laurent 1998 {published data only}
- Laurent S. Effectiveness of pressure decreasing mattresses in cardiovascular surgery patients: a controlled clinical trial. In: 3rd European Conference for Nurse Managers, 1997 Oct; Brussels (Belgium). 1998.
Lazzara 1991 {published data only}
- Lazzara DJ, Buschmann MT. Prevention of pressure ulcers in elderly nursing home residents: are special support surfaces the answer? Decubitus 1991;4(4):42-4, 46, 48. [PMID: ] [PubMed] [Google Scholar]
Lee 1974 {published data only}
- Lee EO, Kim MJ. A comparative study on the effect of gel pad, sheepskin and sponge on prevention and treatment of decubitus ulcers. Journal of Nurses Academic Society 1974;4(3):93-104. [Google Scholar]
Maklebust 1988 {published data only}
- Maklebust J, Brunckhorst L, Cracchiolo-Caraway A, Ducharme MA, Dundon R, Panfilli R, et al. Pressure ulcer incidence in high-risk patients managed on a special three-layered air cushion. Decubitus 1988;1(4):30-40. [PMID: ] [PubMed] [Google Scholar]
Malbrain 2010 {published data only}
- Malbrain M, Hendriks B, Wijnands P, Denie D, Jans A, Vanpellicom J, et al. A pilot randomised controlled trial comparing reactive air and active alternating pressure mattresses in the prevention and treatment of pressure ulcers among medical ICU patients. Journal of Tissue Viability 2010;19(1):7-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Marutani 2019 {published data only}
- JPRN-UMIN000035568. Evaluation of pressure ulcer prevention and QOL for using dual-fit-air-cell-mattresses. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000035568.
- Marutani A, Okuwa M, Sugama J. Use of 2 types of air-cell mattresses for pressure ulcer prevention and comfort among patients with advanced-stage cancer receiving palliative care: an interventional study. Ostomy Wound Management 2019;65(5):24-32. [PubMed] [Google Scholar]
Mastrangelo 2010a {published data only}
- Mastrangelo D, Farina E, Gallicchio V, De Anna D, Bresadola F. Observational study of the use of antidecubitus mattress covers in the prevention and care of pressure ulcers. Acta Vulnologica 2010;8(2):87-92. [Google Scholar]
Mastrangelo 2010b {published data only}
- Mastrangelo D, Farina E, Gallicchio V, De Anna D, Bresadola F, Farina MA. Initial analyses of the prospective study randomized on the anti-decubitis lesion mattress cover. In: SAWC Spring: the Symposium on Advanced Wounds Care and the Wound Healing Society; 2010 April 17-20; Orlando (FL). 2010:S32.
McGinnis 2011 {published data only}
- McGinnis E, Stubbs N. Pressure-relieving devices for treating heel pressure ulcers. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD005485. [DOI: 10.1002/14651858.CD005485.pub2] [DOI] [PubMed] [Google Scholar]
McGowan 2000 {published data only}
- McGowan S, Montgomery K, Jolley D, Wright R. The role of sheepskins in preventing pressure ulcers in elderly orthopaedic patients. Primary Intention 2000:127-34.
McInnes 2015 {published data only}
- McInnes E, Jammali-Blasi A, Bell-Syer SE, Dumville JC, Middleton V, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD001735. [DOI: 10.1002/14651858.CD001735.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
McInnes 2018 {published data only}
- McInnes E, Jammali-Blasi A, Bell-Syer SE, Leung V. Support surfaces for treating pressure ulcers. Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD009490. [DOI: 10.1002/14651858.CD009490.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendoza 2019 {published data only}
- Mendoza RA, Lorusso GA, Ferrer DA, Helenowski IB, Liu J, Soriano RH, et al. A prospective, randomised controlled trial evaluating the effectiveness of the fluid immersion simulation system vs an air-fluidised bed system in the acute postoperative management of pressure ulcers: a midpoint study analysis. International Wound Journal 2019;16(4):989-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mistiaen 2010a {published data only}
- Mistiaen P, Achterberg W, Ament A, Halfens R, Huizinga J, Montgomery K, et al. Cost-effectiveness of the Australian Medical Sheepskin for the prevention of pressure ulcers in somatic nursing home patients: study protocol for a prospective multi-centre randomised controlled trial (ISRCTN17553857). BMC Health Services Research 2008;8:4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistiaen P, Achterberg W, Ament A, Halfens R, Huizinga J, Montgomery K, et al. The effectiveness of the Australian Medical Sheepskin for the prevention of pressure ulcers in somatic nursing home patients: a prospective multicenter randomized-controlled trial (ISRCTN17553857). Wound Repair and Regeneration 2010;18(6):572-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mistiaen P, Ament A, Francke AL, Achterberg W, Halfens R, Huizinga J, et al. An economic appraisal of the Australian Medical Sheepskin for the prevention of sacral pressure ulcers from a nursing home perspective. BMC Health Services Research 2010;10:226. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistiaen P, Francke A, Achterberg W, Ament A, Halfens R, Huizinga J. Australian Medical Sheepskin is effective for the prevention of pressure ulcers. Tijdschrift voor Ouderengeneeskunde 2009;5:186-90.
Mistiaen 2010b {published data only}
- Mistiaen P, Jolley D, McGowan S, Hickey M, Spreeuwenberg P, Francke A. The Australian Medical Sheepskin prevents pressure ulcers: a combined multilevel analysis of three RCTs. Fourth European Nursing Congress. Journal of Clinical Nursing 2010;19:20.
- Mistiaen P, Jolley DJ, McGowan S, Hickey MB, Spreeuwenberg P, Francke AL. 'Australian Medical Sheepskin' in the prevention of decubitus: meta-analysis with individual patient data shows efficacy ['Australische Medische Schapenvacht' ter preventie van decubitus: Meta-analyse met individuele patientendata toont effectiviteit]. Nederlands Tijdschrift voor Geneeskunde 2011;155(15):686-91. [PMID: ] [PubMed] [Google Scholar]
- Mistiaen P, Jolley DJ, McGowan S, Hickey MB, Spreeuwenberg P, Francke AL. [Australian Medical Sheepskin for prevention of pressure ulcers: individual patient data meta-analysis shows effectiveness] ['Australische Medische Schapenvacht' ter preventie van decubitus: meta-analyse met individuele patientendata toont effectiviteit]. Nederlands Tijdschrift voor Geneeskunde 2011;155(18):A3034. [PMID: ] [PubMed] [Google Scholar]
- Mistiaen PJ, Jolley DJ, McGowan S, Hickey MB, Spreeuwenberg P, Francke AL. A multilevel analysis of three randomised controlled trials of the Australian Medical Sheepskin in the prevention of sacral pressure ulcers. Medical Journal of Australia 2010;193(11-12):638-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NTR878. Cost-effectiveness of the Australian Medical Sheepskin for the prevention of pressure ulcers in somatic nursing home clients. trialregister.nl/trial/864.
Nakahara 2012 {published data only}
- Nakahara R, Ohto S, Kato H. Treating pressure ulcers using alternating pressure replacement mattresses. In: 4th Congress of the World Union of Wound Healing Societies; 2012 September 2-6; Yokohama (Japan). 2012.
NCT01402765 {published data only}
- NCT01402765. Interface pressure measures for mattresses: Nimbus 3 versus Summit. clinicaltrials.gov/show/NCT01402765.
NCT02565797 {published data only}
- NCT02565797. Comparative prevention-effectiveness trial of DabirAIR overlay system. ClinicalTrials.gov/show/NCT02565797.
NCT02634892 {published data only}
- NCT02634892. Pressure redistributing overlay with targeted cooling technology (PRO-TECT) for pressure ulcer prevention. ClinicalTrials.gov/show/NCT02634892.
NCT02735135 {published data only}
- NCT02735135. Comparison of 2 mattresses for the prevention of bedsores by measuring skin pressure in the sacral area. clinicaltrials.gov/show/NCT02735135.
NCT03048357 {published data only}
- NCT03048357. Effectiveness of Freedom Bed compared to manual turning in prevention of pressure injuries in persons with limited mobility due to traumatic brain injury and/or spinal cord injury. clinicaltrials.gov/show/NCT03048357.
NCT03211910 {published data only}
- NCT03211910. Sacral Savers: study of prevention and enhanced healing of sacral and trochenteric ulcers. ClinicalTrials.gov/show/NCT03211910.
NCT03351049 {published data only}
- NCT03351049. An RCT on support surfaces for pressure ulcer prevention. ClinicalTrials.gov/show/NCT03351049.
Nixon 1998 {published data only}
- Bridel-Nixon J, McElvenny D, Brown J, Mason S. A randomized controlled trial using a double-triangular sequential design: methodology and management issues. In: European Wound Management Association Conference; 1997 April 27-29; Milan (Italy). 1997:65-6.
- Bridel-Nixon J, McElvenny D, Brown J, Mason S. Findings from a double-triangular sequential-design randomized clinical trial of a dry polymer gel pad. In: European Wound Management Association Conference; 1997 April 27-29; Milan (Italy). 1997:20-1.
- Brown J, McElvenny D, Nixon J, Bainbridge J, Mason S. Some practical issues in the design, monitoring and analysis of a sequential randomized trial in pressure sore prevention. Statistics in Medicine 2000;19(24):3389-400. [PMID: ] [DOI] [PubMed] [Google Scholar]
- ISRCTN43076542. Pressure sore risk in the operating department. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN43076542.
- Nixon J, McElvenny D, Mason S, Brown J, Bond S. A sequential randomised controlled trial comparing a dry visco-elastic polymer pad and standard operating table mattress in the prevention of post-operative pressure sores. International Journal of Nursing Studies 1998;35(4):193-203. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nixon 2006 {published data only}
- ISRCTN78646179. Randomised controlled trial comparing alternating pressure overlays with alternating pressure mattresses for pressure sore prevention and treatment. isrctn.com/ISRCTN78646179.
- Nelson EA, Nixon J, Mason S, Barrow H, Phillips A, Cullum N. A nurse-led randomised trial of pressure-relieving support surfaces. Professional Nurse 2003;18(9):513-6. [PMID: ] [PubMed] [Google Scholar]
- Nixon J, Cranny G, Iglesias C, Nelson EA, Hawkins K, Phillips A, et al. Randomised, controlled trial of alternating pressure mattresses compared with alternating pressure overlays for the prevention of pressure ulcers: PRESSURE (pressure relieving support surfaces) trial. BMJ 2006;332(7555):1413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon J, Cranny G, Nelson A, Iglesias C, Phillips A, Hawkins k, et al. Pressure trial clinical and patient outcomes. European Wound Management Association; 1998 November; Harrogate (UK) 2005:157.
- Nixon J, Cranny G, Nelson A, Iglesias C, Phillips A, Hawkins K, et al. The NHS Health Technology Assessment Programme. Pressure trial clinical and patient outcomes. EWMA Journal 2006;6(1):38. [Google Scholar]
- Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al. Pressure relieving support surfaces: a randomised evaluation. Health Technology Assessment (Winchester, England) 2006;10(22):1-163. [DOI] [PubMed] [Google Scholar]
- Nixon J, Thorpe H, Barrow H, Phillips A, Nelson EA, Mason SA, et al. Reliability of pressure ulcer classification and diagnosis. Journal of Advanced Nursing 2005;50(6):613-23. [DOI] [PubMed] [Google Scholar]
- Nixon J. Erratum: randomised, controlled trial of alternating pressure mattresses compared with alternating pressure overlays for the prevention of pressure ulcers: PRESSURE (pressure relieving support surfaces) trial (British Medical Journal (July 1, 2006) 333 (30)). BMJ 2006;333:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nixon 2019 {published data only}
- Brown S, Smith I, Brown J, Hulme C, Nixon J. Pressure relieving support surfaces: a randomised evaluation 2 (PRESSURE 2). Trials 2013;14(Suppl 1):P68. [Google Scholar]
- Brown S, Smith IL, Brown JM, Hulme C, McGinnis E, Stubbs N, et al. Pressure RElieving Support SUrfaces: a Randomised Evaluation 2 (PRESSURE 2): study protocol for a randomised controlled trial. Trials 2016;17(1):604. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN01151335. Pressure RElieving Support SUrfaces: a Randomised Evaluation 2. isrctn.com/ISRCTN01151335.
- McGinnis E, Brown S, Collier H, Faulks P, Gilberts R, Greenwood C, et al. Pressure RElieving Support SUrfaces: a Randomised Evaluation 2 (PRESSURE 2) photographic validation sub-study: study protocol for a randomised controlled trial. Trials 2017;18(1):132. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon J, Brown S, Smith IL, McGinnis E, Vargas-Palacios A, Nelson EA, et al. Comparing alternating pressure mattresses and high-specification foam mattresses to prevent pressure ulcers in high-risk patients: the PRESSURE 2 RCT. Health Technology Assessment (Winchester, England) 2019;23(52):1-176. [PMID: ] [DOI] [PMC free article] [PubMed]
- Nixon J, Smith IL, Brown S, McGinnis E, Vargas-Palacios A, Nelson EA, et al. Pressure Relieving Support Surfaces for Pressure Ulcer Prevention (PRESSURE 2): clinical and health economic results of a randomised controlled trial. EClinicalMedicine 2019;14:42-52. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ooka 1995 {published data only}
- Ooka M, Kemp MG, McMyn R, Shott S. Evaluation of three types of support surfaces for preventing pressure ulcers in patients in a surgical intensive care unit. Journal of Wound, Ostomy, and Continence Nursing 1995;22(6):271-9. [DOI] [PubMed] [Google Scholar]
Osterbrink 2005 {published data only}
- Osterbrink J, Mayer H, Schroder G. Clinical evaluation of the effectiveness of a multimodal static pressure relieving device. In: 8th European Pressure Ulcer Advisory Panel Open Meeting; 2005 May 5-7; Aberdeen (Scotland). 2005:73.
Ozyurek 2015 {published data only}
- Ozyurek P, Yavuz M. Prevention of pressure ulcers in the intensive care unit: a randomized trial of 2 viscoelastic foam support surfaces. Clinical Nurse Specialist CNS 2015;29(4):210-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Park 2017 {published data only}
- Park KH, Park J. The efficacy of a viscoelastic foam overlay on prevention of pressure injury in acutely ill patients: a prospective randomized controlled trial. Journal of Wound, Ostomy, and Continence Nursing 2017;44(5):440-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Phillips 1999 {published data only}
- Phillips L. Providing correct pressure-relieving devices for optimum outcome. British Journal of Nursing 1999;8(21):1447-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Price 1999 {published data only}
- Price P, Bale S, Newcombe R, Harding K. Challenging the pressure sore paradigm. Journal of Wound Care 1999;8(4):187-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pring 1998 {published data only}
- Pring J, Millman P. Evaluating pressure-relieving mattresses. Journal of Wound Care 1998;7(4):177-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rae 2018 {published data only}
- Rae KE, Isbel S, Upton D. Support surfaces for the treatment and prevention of pressure ulcers: a systematic literature review. Journal of Wound Care 2018;27(8):467-74. [DOI] [PubMed] [Google Scholar]
Rafter 2011 {published data only}
- Rafter L. Evaluation of patient outcomes: pressure ulcer prevention mattresses. British Journal of Nursing 2011;20(11):32. [DOI] [PubMed] [Google Scholar]
Reddy 2006 {published data only}
- Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA 2006;296(8):974-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Reddy 2008 {published data only}
- Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Treatment of pressure ulcers: a systematic review. JAMA 2008;300(22):2647-62. [DOI] [PubMed] [Google Scholar]
Ricci 2013a {published data only}
- Ricci E, Roberto C, Ippolito A, Bianco A, Scalise MT. A randomized study on the effectiveness of a new pressure-relieving mattress overlay for the prevention of pressure ulcers in elderly patients at risk. EWMA Journal 2013;13(1):27-32. [Google Scholar]
Ricci 2013b {published data only}
- Ricci E, Cassino R, Ippolito A. A randomized study on efficacy on 2 overlays in pressure sores treatment. EWMA Journal 2013;13(1):302.
Rithalia 1995 {published data only}
- Rithalia SV. Comparison of performance characteristics of the Nimbus and Airwave mattresses. International Journal of Rehabilitation Research 1995;18(2):182-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rosenthal 2003 {published data only}
- Rosenthal MJ, Felton RM, Nastasi AE, Naliboff BD, Harker J, Navach JH. Healing of advanced pressure ulcers by a generic total contact seat: 2 randomized comparisons with low air loss bed treatments. Archives of Physical Medicine and Rehabilitation 2003;84(12):1733-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Russell 2000b {published data only}
- Dunlop V. Preliminary results of a randomized, controlled study of a pressure ulcer prevention system. Advances in Wound Care 1998;11(3 Suppl):14. [PMID: ] [PubMed] [Google Scholar]
- Lichtenstein S. A 7 day comparative randomized parallel single centre study to determine the safety and efficacy of the Micropulse system for the prevention of pressure ulcers. Micropulse 1997.
- Russell JA, Lichtenstein SL. Randomized controlled trial to determine the safety and efficacy of a multi-cell pulsating dynamic mattress system in the prevention of pressure ulcers in patients undergoing cardiovascular surgery. Ostomy/Wound Management 2000;46(2):46-51, 54-5. [PMID: ] [PubMed] [Google Scholar]
Russell 2003a {published data only}
- Russell L, Reynolds TM, Towns A, Worth W, Greenman A, Turner R. Randomized comparison trial of the RIK and the Nimbus 3 mattresses. British Journal of Nursing 2003;12(4):254, 256-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Russell 2003b {published data only}
- Russell LJ, Reynolds TM, Park C, Rithalia S, Gonsalkorale M, Birch J, et al. Randomized clinical trial comparing 2 support surfaces: results of the prevention of pressure ulcers study. Advances in Skin & Wound Care 2003;16(6):317-27. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sanada 2003 {published data only}
- Matsui Y, Miyake S, Kawasaki T, Konya C, Sugama J, Sanada H. Randomized controlled trial of a two layer type air cell mattress in the prevention of pressure ulcers. Japanese Journal of Pressure Ulcers 2001;3(3):331-7. [Google Scholar]
- Sanada H, Sugama J, Matsui Y, Konya C, Kitagawa A, Okuwa M, et al. Randomised controlled trial to evaluate a new double-layer air-cell overlay for elderly patients requiring head elevation. Journal of Tissue Viability 2003;13(3):112-4, 116, 118. [PMID: ] [DOI] [PubMed] [Google Scholar]
Santy 1994 {published data only}
- Santy JE, Butler MK, Whyman JD. A comparison study of 6 types of hospital mattress to determine which most effectively reduces the incidence of pressure sores in elderly patients with hip fractures in a District General Hospital. Report to Northern & Yorkshire Regional Health Authority 1994.
Santy 1995 {published data only}
- Santy J. Hospital mattresses and pressure sore prevention. Journal of Wound Care 1995;4(7):329-32. [DOI] [PubMed] [Google Scholar]
Sauvage 2017 {published data only}
- Sauvage P, Touflet M, Pradere C, Portalier F, Michel JM, Charru P, et al. Pressure ulcers prevention efficacy of an alternating pressure air mattress in elderly patients: E(2)MAO a randomised study. Journal of Wound Care 2017;26(6):304-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Scheffel 2011 {published data only}
- Scheffel S, Panfil EM. Mattresses and Co. for prevention of decubitus ulcer. What measures are effective? [Matratzen und Co. zur Dekubituspravention. Welche Massnahmen sind wirksam?]. Pflege Zeitschrift 2011;64(3):162-3. [PubMed] [Google Scholar]
Schultz 1999 {published data only}
- Schultz A, Bien M, Dumond K, Brown K, Myers A. Etiology and incidence of pressure ulcers in surgical patients. AORN Journal 1999;70(3):434, 437-40, 443-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Schultz AA. Study results: prediction and prevention of pressure ulcers in surgical patients. Advances in Wound Care 1998;11(3):11. [PubMed] [Google Scholar]
Scott 2000 {published data only}
- Scott EM. The prevention of pressure ulcers in the operating department. Journal of Wound Care 2000;9(1):18-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Scott‐Williams 2006 {published data only}
- Scott-Williams S, Lummas AC. Perioperative pressure ulcer assessment and prevention efficacy study of a multilayered pad for the operating room. Ostomy/Wound Management 2006;52(4):110-1. [Google Scholar]
Serraes 2018 {published data only}
- Serraes B, Van Leen M, Schols J, Van Hecke A, Verhaeghe S, Beeckman D. Prevention of pressure ulcers with a static air support surface: a systematic review. International Wound Journal 2018;15(3):333-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shakibamehr 2019 {published data only}
- Shakibamehr J, Rad M, Akrami R, Rad M. Effectiveness of Tragacanth gel cushions in prevention of pressure ulcer in traumatic patients: a randomized controlled trial. Journal of Caring Sciences 2019;8(1):45-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharp 2007 {published data only}
- Sharp A. Pilot study to compare the incidence of pressure ulceration on two therapeutic support surfaces in elderly care. In: 17th Conference of the European Wound Management Association; 2007 May 2-4; Glasgow (Scotland). 2007:Oral presentation 114, 73.
Shi 2018a {published data only}
- Shi C, Dumville JC, Cullum N. Support surfaces for pressure ulcer prevention: a network meta-analysis. PLOS One 2018;13(2):e0192707. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sideranko 1992 {published data only}
- Sideranko S, Quinn A, Burns K, Froman RD. Effects of position and mattress overlay on sacral and heel pressures in a clinical population. Research in Nursing & Health 1992;15(4):245-51. [DOI] [PubMed] [Google Scholar]
Smith 2013 {published data only}
- Smith ME, Totten A, Hickam DH, Fu R, Wasson N, Rahman B, et al. Pressure ulcer treatment strategies: a systematic comparative effectiveness review. Annals of Internal Medicine 2013;159(1):39-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stannard 1993 {published data only}
- Stannard D. Commentary on the role of support surfaces and patient attributes in preventing pressure ulcers in elderly patients. AACN Nursing Scan In Critical Care 1993;3(6):2-3. [DOI] [PubMed] [Google Scholar]
Stapleton 1986 {published data only}
- Stapleton M. Preventing pressure sores--an evaluation of three products. Geriatric Nursing 1986;6(2):23-5. [PMID: ] [PubMed] [Google Scholar]
Sterzi 2003 {published data only}
- Sterzi S, Selvaggi G, Romanelli A, Valente P, Bertolini C. Evaluation of prevalence and incidence of pressure ulcers and their relationship with mattresses used in a general hospital intensive care unit. European Journal of Plastic Surgery 2003;25(7):401-4. [Google Scholar]
Takala 1994 {published data only}
- Takala J, Soini HO, Soppi E, Kataja M, Olkkonen K. Can risk factors for pressure sores be decreased with a special mattress? Duodecim; Laaketieteellinen Aikakauskirja 1994;110(4):407-14. [PubMed] [Google Scholar]
Takala 1996 {published data only}
- Takala J, Varmavuo S, Soppi E. Prevention of pressure sores in acute respiratory failure: a randomised controlled trial. Clinical Intensive Care 1996;7(5):228-35. [Google Scholar]
Taylor 1999 {published data only}
- Taylor L. Evaluating the Pegasus Trinova: a data hierarchy approach. British Journal of Nursing 1999;8(12):771-4, 776-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tewes 1993 {published data only}
- Tewes M. Prevention and treatment of pressure sores--a neglected research subject? An overview of clinically controlled studies in the period 1987-91 [Tryksarforebyggelse og -behandling--et forsomt forskningsomrade? En gennemgang af klinisk kontrollerede undersogelser i perioden 1987-91]. Vard i Norden 1993;13(2):4-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Theaker 2005 {published data only}
- Theaker C, Kuper M, Soni N. Pressure ulcer prevention in intensive care - a randomised control trial of two pressure-relieving devices. Anaesthesia 2005;60(4):395-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vanderwee 2005 {published data only}
- Vanderwee K, Grypdonck MH, Defloor T. Effectiveness of an alternating pressure air mattress for the prevention of pressure ulcers. Age and Ageing 2005;34(3):261-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Leen 2011 {published data only}
- Van Leen M, Hovius S, Neyens J, Halfens R, Schols J. Pressure relief, cold foam or static air? A single center, prospective, controlled randomized clinical trial in a Dutch nursing home. Journal of Tissue Viability 2011;20(1):30-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Leen 2013 {published data only}
- Van Leen M, Hovius S, Halfens R, Neyens J, Schols J. Pressure relief with visco-elastic foam or with combined static air overlay? A prospective, crossover randomized clinical trial in a Dutch nursing home. Wounds 2013;25(10):287-92. [PMID: ] [PubMed] [Google Scholar]
Van Leen 2018 {published data only}
- NTR4557. Is lowering of shear and friction forces (cost)effective for prevention of pressure ulcers? trialregister.nl/trial/4435.
- Van Leen M, Halfens R, Schols J. Preventive effect of a microclimate-regulating system on pressure ulcer development: a prospective, randomized controlled trial in Dutch nursing homes. Advances in Skin & Wound Care 2018;31(1):1-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Rijswijk 1994 {published data only}
- Van Rijswijk L. Pressure sores and pressure-decreasing mattresses: controlled clinical trial. Ostomy Wound Management 1994;40(6):12. [Google Scholar]
Vermette 2012 {published data only}
- Vermette S, Reeves I, Lemaire J. Cost effectiveness of an air-inflated static overlay for pressure ulcer prevention: a randomized, controlled trial. Wounds 2012;24(8):207-14. [PMID: ] [PubMed] [Google Scholar]
Vyhlidal 1997 {published data only}
- Vyhlidal SK, Moxness D, Bosak KS, Van Meter FG, Bergstrom N. Mattress replacement or foam overlay? A prospective study on the incidence of pressure ulcers. Applied Nursing Research 1997;10(3):111-20. [DOI] [PubMed] [Google Scholar]
Wallace 2009 {published data only}
- Wallace M. Review: alternative-foam mattresses and some operating-table overlays reduce pressure ulcers more than standard surfaces. Evidence-Based Nursing 2009;12(3):81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Whitney 1984 {published data only}
- Whitney JD, Fellows BJ, Larson E. Do mattresses make a difference? Journal of Gerontological Nursing 1984;10(9):20-1, 24-5. [DOI] [PubMed] [Google Scholar]
Whittingham 1999 {published data only}
- Whittingham K. Randomized control trial of six pressure-redistributing foam mattresses. Journal of Tissue Viability 1999;9(3):104. [Google Scholar]
Yao 2018 {published data only}
- Yao L, Ding N, Yang L, Han C, Jiang L, Jiang B, et al. Effects of different decompression device in the prevention of pressure sore: a network meta-analysis. Chinese Journal of Evidence-Based Medicine 2018;18(10):1086-92. [Google Scholar]
References to studies awaiting assessment
Chaloner 2000b {published data only}
- Chaloner D. A prospective, controlled comparison between two dynamic alternating mattress replacement systems within a community setting. In: 9th European Conference on Advances in Wound Management; 1999 November 9-11; Harrogate (UK). 2000.
Henn 2004 {published data only}
- Henn G, Russell L, Towns A, Taylor H. A two-centre prospective study to determine the utility of a dynamic mattress and mattress overlay. In: 2nd World Union of Wound Healing Societies Meeting; 2004 July 8-13; Paris (France). 2004:54.
Knight 1999 {published data only}
- Knight M, Simpson J, Fear-Price M. An evaluation of pressure-reducing overlay mattresses in the community situation. In: 9th European Conference on Advances in Wound Management; 1999 November 9-11; Harrogate (UK). 1999:25.
Melland 1998 {published data only}
- Melland HI, Langemo D, Hanson D, Olson B, Hunter S. Clinical trial of the Freedom Bed. Prairie Rose 1998;67(2):11-2a. [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12618000319279 {published data only}
- ACTRN12618000319279. Testing the effectiveness of pressure mattresses for people over 65 years residing in the community. www.anzctr.org.au/AnzctrAttachments/374599-Research%20Proposal%20Ethics%20V2%20(FINAL).pdf 2018.
JPRN‐UMIN000029680 {published data only}
- JPRN-UMIN000029680. Effects of robotic mattress on pressure ulcer healing, comfort level among pressure ulcer patients, and nursing work load: a randomized controlled trial. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000029680.
Additional references
Baumgarten 2009
- Baumgarten M, Margolis DJ, Selekof JL, Moye N, Jones PS, Shardell M. Validity of pressure ulcer diagnosis using digital photography. Wound Repair and Regeneration 2009;17:287-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergstrom 2008
- Bergstrom N, Smout R, Horn S, Spector W, Hartz A, Limcangco MR. Stage 2 pressure ulcer healing in nursing homes. Journal of the American Geriatrics Society 2008;56(7):1252-8. [DOI] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta-Analysis. West Sussex (UK): John Wiley & Sons, Ltd, 2009. [Google Scholar]
Borenstein 2017
- Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Research Synthesis Methods 2017;8(1):5-18. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cipriani 2013
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013;159(2):130-7. [DOI] [PubMed] [Google Scholar]
Clark 1998
- Clark M, Donald IP. A randomised controlled trial comparing the healing of pressure sores upon two pressure‐redistributing seat cushions. In: 7th European Conference on Advances in Wound Management; 1997 November 18‐20; Harrogate (UK). 1998:122-5.
Clark 2011
- Clark M. Technology update: understanding support surfaces. Wounds International 2011;2(3):17-21. [Google Scholar]
Cullum 2016
- Cullum N, Buckley H, Dumville J, Hall J, Lamb K, Madden M, et al. Wounds Research for Patient Benefit: A 5-year Programme of Research. Southampton (UK): NIHR Journals Library, 2016. [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Demarré 2015
- Demarré L, Van Lancker A, Van Hecke A, Verhaeghe S, Grypdonck M, Lemey J, et al. The cost of prevention and treatment of pressure ulcers: a systematic review. International Journal of Nursing Studies 2015;52(11):1754-74. [DOI] [PubMed] [Google Scholar]
Eldridge 2016
- Eldridge S, Campbell M, Campbell M, Dahota A, Giraudeau B, Higgins J, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0): additional considerations for cluster-randomized trials. www.bristol.ac.uk/media-library/sites/social-community-medicine/images/centres/cresyda/RoB2-0_cluster_parallel_guidance.pdf (accessed 01 October 2019).
EPUAP/NPIAP/PPPIA 2019
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel, and Pan Pacific Pressure Injury Alliance (EPUAP/NPIAP/PPPIA). Prevention and Treatment of Pressure Ulcers/Injuries: Quick Reference Guide. EPUAP/NPIAP/PPPIA, 2019. [Google Scholar]
Essex 2009
- Essex HN, Clark M, Sims J, Warriner A, Cullum N. Health-related quality of life in hospital inpatients with pressure ulceration: assessment using generic health-related quality of life measures. Wound Repair and Regeneration 2009;17(6):797-805. [DOI] [PubMed] [Google Scholar]
Glanville 2019
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel-Storr A. Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information and Libraries Journal 2019;36(1):73-90. [DOI] [PubMed] [Google Scholar]
Gorecki 2009
- Gorecki C, Brown JM, Nelson EA, Briggs M, Schoonhoven L, Dealey C, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. Journal of the American Geriatrics Society 2009;57(7):1175-83. [DOI] [PubMed] [Google Scholar]
Gorecki 2013
- Gorecki C, Brown JM, Cano S, Lamping DL, Briggs M, Coleman S, et al. Development and validation of a new patient-reported outcome measure for patients with pressure ulcers: the PU-QOL instrument. Health and Quality of Life Outcomes 2013;11:95. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 30 September 2019. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Graves 2005
- Graves N, Birrell F, Whitby M. Effect of pressure ulcers on length of hospital stay. Infection Control and Hospital Epidemiology 2005;26(3):293-7. [DOI] [PubMed] [Google Scholar]
Gray 2018
- Gray TA, Rhodes S, Atkinson RA, Rothwell K, Wilson P, Dumville JC, et al. Opportunities for better value wound care: a multiservice, cross-sectional survey of complex wounds and their care in a UK community population. BMJ Open 2018;8(3):e019440. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guest 2018
- Guest JF, Fuller GW, Vowden P, Vowden KR. Cohort study evaluating pressure ulcer management in clinical practice in the UK following initial presentation in the community: costs and outcomes. BMJ Open 2018;8(7):e021769. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence - study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64:407-15. [DOI] [PubMed] [Google Scholar]
Herdman 2011
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research 2011;21(10):1727-36. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.training.cochrane.org/handbook.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2019
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hróbjartsson 2012
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non-blinded outcome assessors. BMJ 2012;344:e1119. [DOI] [PubMed] [Google Scholar]
Kaltenthaler 2001
- Kaltenthaler E, Whitfield MD, Walters SJ, Akehurst RL, Paisley S. UK, USA and Canada: how do their pressure ulcer prevalence and incidence data compare? Journal of Wound Care 2001;10(1):530-5. [DOI] [PubMed] [Google Scholar]
Kirkham 2018
- Kirkham JJ, Altman DG, Chan AW, Gamble C, Dwan KM, Williamson PR. Outcome reporting bias in trials: a methodological approach for assessment and adjustment in systematic reviews. BMJ 2018;362:k3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Li 2019
- Li T, Higgins JP, Deeks JJ (editors). Chapter 5: Collecting data. In Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Livesley 2002
- Livesley NJ, Chow AW. Infected pressure ulcers in elderly individuals. Clinical Infectious Diseases 2002;35(11):1390-6. [DOI] [PubMed] [Google Scholar]
McGinnis 2014
- McGinnis E, Stubbs N. Pressure‐relieving devices for treating heel pressure ulcers. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No: CD005485. [DOI: 10.1002/14651858.CD005485.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2015
- Nguyen KH, Chaboyer W, Whitty JA. Pressure injury in Australian public hospitals: a cost-of-illness study. Australian Health Review 2015;39(3):329-36. [DOI] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence (NICE). Pressure ulcers: prevention and management. www.nice.org.uk/guidance/cg179 (accessed 08 October 2019). [PubMed]
NPIAP 2016
- National Pressure Injury Advisory Panel (NPIAP). NPUAP Pressure Injury Stages. Available at cdn.ymaws.com/npuap.site-ym.com/resource/resmgr/npuap_pressure_injury_stages.pdf (accessed 19 March 2020).
NPIAP S3I 2007
- National Pressure Injury Advisory Panel (NPIAP) Support Surface Standards Initiative (S3I). Terms and definitions related to support surfaces. Available at cdn.ymaws.com/npiap.com/resource/resmgr/website_version_terms_and_de.pdf (accessed 18 February 2020).
Öien 2013
- Öien RF, Forssell HW. Ulcer healing time and antibiotic treatment before and after the introduction of the Registry of Ulcer Treatment: an improvement project in a national quality registry in Sweden. BMJ open 2013;3:e003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ouzzani 2016
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan — a web and mobile app for systematic reviews. Systematic Reviews 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2019
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Peryer 2019
- Peryer G, Golder S, Junqueira DR, Vohra S, Loke YK (editors). Chapter 19: Adverse effects. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Peters 2008
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991-6. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. The Cochrane Collaboration, 2020.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [PMID: ] [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Giovane CD, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLOS One 2014;9(7):e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Shi 2018b
- Shi C, Westby M, Norman G, Dumville J, Cullum N. Node-making processes in network meta-analysis of non-pharmacological interventions should be well planned and reported. Journal of Clinical Epidemiology 2018;101:124-5. [DOI] [PubMed] [Google Scholar]
Shi 2021
- Shi C, Dumville JC, Cullum N, Rhodes S, McInnes E, Goh EL, et al. Beds, overlays and mattresses for preventing and treating pressure ulcers: an overview of Cochrane Reviews and network meta-analysis. Cochrane Database of Systematic Reviews 2021, Issue 8. Art. No: CD013761. [DOI: 10.1002/14651858.CD013761.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693-708. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):472-83. [PubMed] [Google Scholar]
World Health Organization 2019
- World Health Organization. EH90 Pressure ulceration. ICD-11 for Mortality and Morbidity Statistics (Version: 04/2019). icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f45533017 (accessed 17 February 2020).
Wounds International 2010
- Wounds International. International Review. Pressure Ulcer Prevention: Pressure, Shear, Friction and Microclimate in Context. A Consensus Document. London (UK): Wounds International, 2010. [Google Scholar]
References to other published versions of this review
Shi 2020
- Shi C, Dumville JC, Cullum N, Rhodes S, McInnes E. Beds and mattresses for treating pressure ulcers. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD013624. [DOI: 10.1002/14651858.CD013624] [DOI] [PMC free article] [PubMed] [Google Scholar]


