Abstract
Lessons Learned
Palbociclib monotherapy demonstrated minimal clinical activity in patients with previously treated gastroesophageal cancers.
Further clinical evaluation of palbociclib monotherapy is not warranted in gastroesophageal cancers, but improved understanding of resistance mechanisms may permit rational combination approaches.
Background
Dysregulation of the cell cycle is a hallmark of cancer. Progression through the G1/S transition requires phosphorylation of retinoblastoma (RB) by cyclin‐dependent kinases (CDKs) 4 and 6, which are regulated by cyclins D and E. Amplifications of cyclin D loci and activating mutations in CDKs are frequent molecular aberrations in gastroesophageal malignancies. We conducted a phase II trial of the CDK4/6 inhibitor palbociclib as an initial test of efficacy.
Methods
Patients with previously treated metastatic gastroesophageal cancers with intact RB nuclear expression by immunohistochemistry were treated with 125 mg daily of palbociclib for days 1–21 of 28‐day cycles. The primary endpoint was overall response rate.
Results
We screened 29 patients and enrolled 21 patients: 5 with gastric adenocarcinoma, 3 with gastroesophageal junction adenocarcinoma, 8 with esophageal adenocarcinoma, and 5 with esophageal squamous cell carcinoma. All 29 tumors screened had intact nuclear RB expression, and four treated patients tested positive for CCND1 overexpression. No objective responses were seen. Median progression‐free survival was 1.8 months, and median overall survival was 3.0 months. All recurrent grade 3 or 4 toxicities were hematologic, with neutropenia in eight patients (38%), anemia in four patients (19%), and thrombocytopenia in two patients (10%).
Conclusion
Palbociclib has limited single‐agent activity in gastroesophageal tumors.
Discussion
Cell cycle dysregulation is a cancer hallmark, and alterations in the RB pathway controlling the G1 to S phase cell transition are among the most common in human cancer [1]. Palbociclib is a small molecule inhibitor of CDK4 and 6, which complex with cyclin D1 to phosphorylate RB [2]. Palbociclib improves overall survival in combination with hormonal therapy in metastatic breast cancer [3] and has demonstrated preclinical activity in a variety of tumor types [4].
Gastric and esophageal cancers display increased reliance on the RB pathway, with frequent overexpression of CCND1 and p16 loss [5, 6]. In this study, patients with advanced gastroesophageal cancer with at least one line of prior therapy were treated with palbociclib at standard doses until disease progression. Patients with HER2 overexpression were permitted to continue trastuzumab concurrently with palbociclib.
Palbociclib monotherapy failed to demonstrate relevant activity in this heavily pretreated population of patients with gastroesophageal cancer. No objective responses were observed, and only two patients had stable disease lasting for more than 4 months: one was treated concurrently with trastuzumab, and both had subsequent progression before 6 months. Median progression‐free survival was 1.8 months, and overall survival was similarly short, with a median of 3.0 months (Fig. 1).
Figure 1.
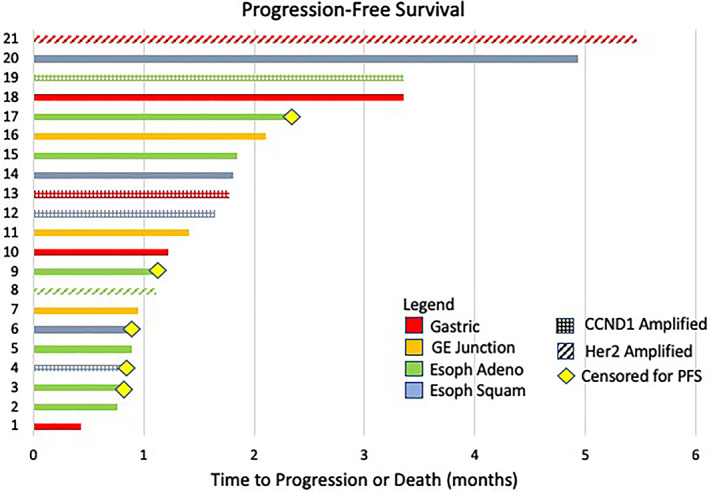
Swimmer plot: progression‐free survival. Abbreviations: Esoph Adeno, esophageal adenocarcinoma; Esoph Squam, esophageal squamous cell carcinoma; GE, gastroesophageal; PFS, progression‐free survival.
The toxicity of palbociclib was as expected, with cytopenias being the only recurring grade 3 and 4 events, with neutropenia in eight patients (38%), anemia in four patients (19%), and thrombocytopenia in two patients (10%). One patient with grade 4 thrombocytopenia experienced upper gastrointestinal bleeding from the tumor in the setting of therapeutic anticoagulation, and palbociclib was stopped. No other patients discontinued therapy because of toxicity, but five patients interrupted treatment because of cytopenias, and four patients had their dose reduced to 100 mg. No patients experienced neutropenic fever or other infectious complications attributable to palbociclib.
Limited biomarker work performed as part of this study confirmed continued RB expression in all patients and CCND1 overexpression by fluorescent in situ hybridization in four patients whose outcomes were similar to the overall population. In the eight patients who had next‐generation sequencing, the only gene mutated in more than one patient was TP53. No significant correlation was seen between any exploratory biomarker and disease outcome.
Based on the limited efficacy demonstrated in this study, further exploration of palbociclib monotherapy in advanced gastroesophageal cancer is not warranted. The disconnect between preclinical activity and the lack of clinical benefit observed in this study and others [7, 8, 9, 10] calls into question the validity of CDK4/6 pathway aberrations as broadly actionable targets for cancer therapy.
Trial Information
| Disease | Esophageal cancer |
| Disease | Gastric cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | No designated number of regimens |
| Type of Study | Phase II, single arm |
| Primary Endpoint | Overall response rate |
| Secondary Endpoints | Toxicity, progression‐free survival, overall survival |
| Additional Details of Endpoints or Study Design | |
| The response rate of interest in this trial was 15%. If one response was observed in the first 15 subjects, an additional 15 subjects would be enrolled for a total of 30. Observing zero responses in the first 15 subjects would exclude response rates as low as 15% with a 90% one‐sided upper confidence bound. If at least three responses were observed in a total of 30 evaluable patients, we would conclude that the drug is active and merited further study; if the true response rate was 15% or greater, three or more responses would be observed with a probability of at least 85%. If the true response rate was only 3%, three or more responses would be observed with a probability of only 6%. In addition to complete and partial responses, disease stabilization would be considered: if the proportion of patients with disease stabilization for 6 months exceeded 20%, we would conclude that the drug may warrant further investigation. With n = 30 subjects and a true response rate of 15%, the expected confidence interval width would be ±10.7% around the estimated proportion. Moreover, we would have 90% power to detect any unforeseen adverse effect that occurred in at least 7% of cases. | |
| Investigator's Analysis | Level of activity did not meet planned endpoint |
Drug Information
| Drug 1 | |
| Generic/Working Name | Palbociclib |
| Trade Name | Ibrance |
| Company Name | Pfizer |
| Drug Type | Small molecule |
| Drug Class | CDK |
| Dose | 125 milligrams (mg) per flat dose |
| Route | Oral (p.o.) |
| Schedule of Administration | Once daily for days 1–21 of a 28‐day cycle |
Patient Characteristics
| Number of Patients, Male | 15 |
| Number of Patients, Female | 6 |
| Stage | IV |
| Age | Median (range): 64 years (42–85 years) |
| Number of Prior Systemic Therapies | Median (range): 2 (1–6) |
| Performance Status: ECOG |
0 — 9 1 — 12 2 — 3 — Unknown — |
| Other | |
| Eligible patients were aged least 18 years with previously treated locally advanced or metastatic esophageal, gastric, or gastroesophageal junction cancer. All patients had measurable disease per RECIST criteria and an Eastern Cooperative Oncology Group performance status of 0 or 1. Standard laboratory eligibility parameters were used. Tumors must have retained RB nuclear expression by immunohistochemistry. Patients could not have received cytotoxic chemotherapy within 3 weeks or investigational therapy within 4 weeks of the first dose of palbociclib, and prior toxicities of treatment must have returned to baseline or grade 1. Patients with uncontrolled intercurrent illness, human immunodeficiency virus, and untreated brain metastases were not permitted. | |
| Cancer Types or Histologic Subtypes | Gastric adenocarcinoma, 5; gastroesophageal junction adenocarcinoma, 3; squamous cell carcinoma of the esophagus, 8; adenocarcinoma of the esophagus, 5. |
Primary Assessment Method
| Title | Overall Response Rate |
| Number of Patients Screened | 21 |
| Number of Patients Enrolled | 21 |
| Number of Patients Evaluable for Toxicity | 21 |
| Number of Patients Evaluated for Efficacy | 17 |
| Evaluation Method | RECIST version 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 0 (0%) |
| Response Assessment SD | n = 5 (29%) |
| Response Assessment PD | n = 12 (71%) |
| (Median) Duration Assessments PFS | 1.8 months; confidence interval, 1.1–4.9 |
| (Median) Duration Assessments OS | 3.0 months; confidence interval, 1.9–6.6 |
| Outcome Notes | |
| Overall response rate and progression‐free survival were assessed using RECIST criteria version 1.1. Subjects were assessed at baseline and approximately every 9 weeks (±4 days) from the first dose of palbociclib until documented progression or withdrawal from the study. | |
Adverse Events
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA, % | Grade 1, % | Grade 2, % | Grade 3, % | Grade 4, % | Grade 5, % | All grades, % |
| Platelet count decreased | 85 | 5 | 5 | 0 | 5 | 0 | 15 |
| Neutrophil count decreased | 57 | 0 | 5 | 24 | 14 | 0 | 43 |
| Lymphocyte count decreased | 81 | 0 | 0 | 14 | 5 | 0 | 19 |
| White blood cell decreased | 90 | 5 | 0 | 5 | 0 | 0 | 10 |
| Anemia | 67 | 0 | 14 | 14 | 5 | 0 | 33 |
| Headache | 95 | 0 | 0 | 5 | 0 | 0 | 5 |
| Aspartate aminotransferase increased | 95 | 0 | 0 | 5 | 0 | 0 | 5 |
| Alanine aminotransferase increased | 95 | 0 | 0 | 5 | 0 | 0 | 5 |
| Gait disturbance | 95 | 0 | 0 | 5 | 0 | 0 | 5 |
| Thromboembolic event | 90 | 0 | 10 | 0 | 0 | 0 | 10 |
| Dysgeusia | 95 | 0 | 5 | 0 | 0 | 0 | 5 |
| Fatigue | 86 | 0 | 14 | 0 | 0 | 0 | 14 |
| Anorexia | 90 | 5 | 5 | 0 | 0 | 0 | 10 |
| Diarrhea | 95 | 0 | 5 | 0 | 0 | 0 | 5 |
| Rash maculopapular | 95 | 0 | 5 | 0 | 0 | 0 | 5 |
| Cough | 95 | 5 | 0 | 0 | 0 | 0 | 5 |
Five patients required dose interruption and four patients required dose reduction to 100 mg of palbociclib because of thrombocytopenia and/or neutropenia. All nonhematologic toxicities were managed without modification of palbociclib dosage.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Serious Adverse Events
| Name | Grade | Attribution |
|---|---|---|
| GI bleed | 3 | Possible |
| Small bowel obstruction | 3 | Unrelated |
| Esophageal obstruction | 3 | Unrelated |
| Confusion | 3 | Unrelated |
| Atrial fibrillation | 3 | Unrelated |
Abbreviation: GI, gastrointestinal.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Level of activity did not meet planned endpoint |
At the time this trial was developed, gastric and esophageal cancers were targeted based on near universal expression of retinoblastoma (RB), frequent overexpression of CCND1, and a positive feedback loop between CCND1 and apoptosis signal‐regulating kinase 1 [5]. Later large‐scale sequencing efforts through The Cancer Genome Atlas confirmed very low levels of RB pathway mutations, high levels of CCND1 amplification, and frequent epigenetic silencing of p16, all of which were expected to increase reliance on the cyclin‐dependent kinase 4 and 6 (CDK4/6) pathway [6]. Importantly, p16 loss was later shown not to correlate with palbociclib sensitivity in breast cancer [11]. A more recent comprehensive mutational analysis of 551 esophageal adenocarcinoma samples demonstrated sensitizing mutations to CDK4/6 inhibitors in more than 50% of samples, with corresponding sensitivity to CDK4/6 inhibitors in cell culture and organoid models [12].
Despite this strong preclinical evidence suggesting a reliance of many gastroesophageal cancers on the CDK4/6 pathway, clinical activity with palbociclib was very limited in this study, a result similar to that seen with palbociclib in a variety of nonbreast tumor types [7, 8, 9, 10]. Recent insights into the complex picture of the actions of CDK4/6 inhibitors in cancer may enhance mechanistic understanding and guide future rational combinations [1, 13]. One promising strategy is the combination of CDK4/6 inhibition and immune checkpoint blockade, with multiple publications showing synergy through effects on regulatory T cells, myeloid derived suppressor cells, antigen expression, and interferon production [14, 15, 16]. An ongoing trial of abemaciclib with the PD‐1 inhibitor pembrolizumab in gastroesophageal cancers may allow for further clinical elucidation of relevant mechanisms of action and resistance (NCT03997448).
Disclosures
Thomas Benjamin Karasic: Pfizer (C/A); Nevena Damjanov: Merck (C/A, RF); Peter J O'Dwyer: Array, Genentech, (C/A), Pfizer, Genentech, Bristol‐Myers Squibb, GlaxoSmithKline, Five Prime, FortySeven, BBI, Novartis, Celgene, Incyte, Lilly/Imclone, Array, H3Biomedicine, Taiho, Pharmacyclics/Abbvie (RF), Eli Lilly & Co. (ET). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT01037790
- Sponsor: Peter J. O'Dwyer
- Principal Investigator: Peter J. O'Dwyer
- IRB Approved: Yes
References
- 1. Dick FA, Goodrich DW, Sage J et al. Non‐canonical functions of the RB protein in cancer. Nat Rev Cancer 2018;18:442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clark AS, Karasic TB, DeMichele A et al. Palbociclib (PD0332991)‐a selective and potent cyclin‐dependent kinase inhibitor: A review of pharmacodynamics and clinical development. JAMA Oncol 2016;2:253–260. [DOI] [PubMed] [Google Scholar]
- 3. Turner NC, Slamon DJ, Ro J et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 2018;379:1926–1936. [DOI] [PubMed] [Google Scholar]
- 4. Schettini F, De Santo I, Rea CG et al. CDK 4/6 inhibitors as single agent in advanced solid tumors. Front Oncol 2018;8:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cancer Genome Atlas Research Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayakawa Y, Hirata Y, Nakagawa H et al. Apoptosis signal‐regulating kinase 1 and cyclin D1 compose a positive feedback loop contributing to tumor growth in gastric cancer. Proc Natl Acad Sci USA 2011;108:780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gopalan PK, Pinder MC, Chiappori A et al. A phase II clinical trial of the CDK 4/6 inhibitor palbociclib (PD0332991) in previously treated, advanced non‐small cell lung cancer (NSCLC) patients with inactivated CDKN2A. J Clin Oncol 2014;32(suppl 15):8077a. [Google Scholar]
- 8. Dickson MA, Schwartz GK, Keohan ML et al. Progression‐free survival among patients with well‐differentiated or dedifferentiated liposarcoma treated with CDK4 inhibitor palbociclib: A phase 2 clinical trial. JAMA Oncol 2016;2:937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Konecny GE, Hendrickson AEW, Jatoi A et al. A multicenter open‐label phase II study of the efficacy and safety of palbociclib a cyclin‐dependent kinases 4 and 6 inhibitor in patients with recurrent ovarian cancer. J Clin Oncol 2016;34(suppl 15):5557a. [Google Scholar]
- 10. Grande E, Teulé A, Alonso‐Gordoa T et al. The PALBONET trial: A phase II study of palbociclib in metastatic grade 1 and 2 pancreatic neuroendocrine tumors (GETNE‐1407). The Oncologist 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeMichele A, Clark AS, Tan KS et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: Phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res 2015;21:995–1001. [DOI] [PubMed] [Google Scholar]
- 12. Frankell AM, Jammula S, Li X et al. The landscape of selection in 551 esophageal adenocarcinomas defines genomic biomarkers for the clinic. Nat Genet 2019;51:506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klein ME, Kovatcheva M, Davis LE et al. CDK4/6 inhibitors: The mechanism of action may not be as simple as once thought. Cancer Cell 2018;34:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goel S, DeCristo MJ, Watt AC et al. CDK4/6 inhibition triggers anti‐tumour immunity. Nature 2017;548:471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deng J, Wang ES, Jenkins RW et al. CDK4/6 inhibition augments antitumor immunity by enhancing T‐cell activation. Cancer Discov 2018;8:216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schaer DA, Beckmann RP, Dempsey JA et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD‐L1 checkpoint blockade. Cell Rep 2018;22:2978–2994. [DOI] [PubMed] [Google Scholar]


