Abstract
The N6-methyladenosine (m6A) modification is the most common mRNA modification in eukaryotes and exerts biological functions by affecting RNA metabolism. The m6A modification is installed by m6A methyltransferases, removed by demethylases and recognized by m6A-binding proteins. The interaction between these three elements maintains the dynamic equilibrium of m6A in cells. Accumulating evidence indicates that m6A RNA methylation has a significant impact on RNA metabolism and is involved in the pathogenesis of cancer. Lung cancer is the leading cause of cancer-related deaths worldwide. The treatment options for lung cancer have developed considerably over the past few years; however, the survival rate of patients with lung cancer still remains very low. Although diagnostic methods and targeted therapies have been rapidly developed in recent years, the underlying mechanism and importance of m6A RNA methylation in the pathogenesis of lung cancer remains ambiguous. The current review summarized the biological functions of m6A modification and considers the potential roles of m6A regulators in the occurrence and development of lung cancer.
Keywords: RNA methylation, N6-methyladenosine, lung cancer, translation, methyltransferase-like 3
1. Introduction
RNA modifications are used to fine-tune the structural features of infrastructural RNAs. In recent years, RNA modifications have been found to be reversible and involved in important biological processes, through continued efforts to map and quantify various RNA modifications in a transcriptome-wide manner (1). The N6-methyladenosine (m6A) methylation modification is the most prevalent internal modification of eukaryotic mRNA. The latest discoveries of the locations, functions, and mechanisms of m6A provide new insights into the regulation mechanism of RNA expression (2). Evidence supports the involvement of m6A modifications in precursor mRNA (pre-mRNA) splicing, mRNA stability, RNA structure, translation, and processing of primary transcripts of microRNAs (miRNAs) (3). The m6A sites are enriched near the stop codons and 3'-untranslated regions (UTRs), and an association exists between the m6A residues and the mRNA-binding site in the 3'-UTR (4,5).
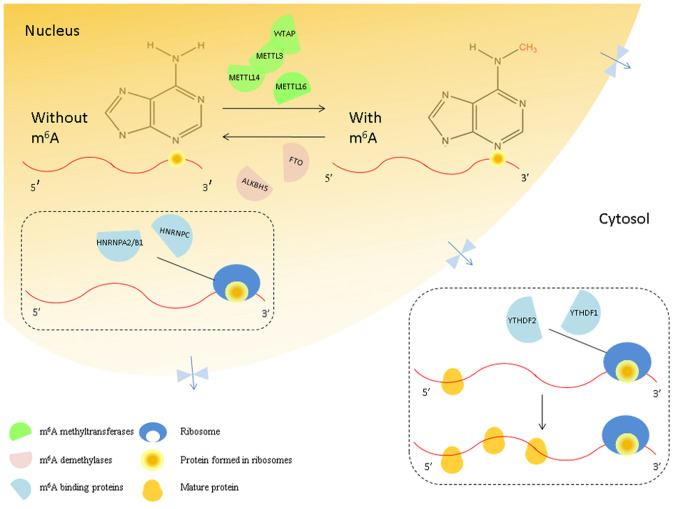
The m6A modification appears to be reversible under the combined action of the enzymes involved (6,7). The m6A modification is mediated, removed, and recognized by methyltransferases, demethylases, and m6A-binding proteins, respectively (8). Methyltransferase-like 3 (METTL3) (9), methyltransferase-like 14 (METTL14) (10), methyltransferase-like 16 (METTL16) (11), Wilms tumor 1-associating protein (WTAP) (12), RNA-binding motif protein 15/15B (RBM15/15B) (13), and vir-like m6A methyltransferase-associated protein (VIRMA/KIAA1429) (14) are considered to be the components of ‘writers’ that catalyze the formation of m6A; ‘erasers’ such as the obesity-associated protein (FTO) and alkB homolog 5 (ALKBH5) (15,16) remove the methyl code from target mRNAs; ‘readers’ such as the YTH domain protein families (YTHDF) and heterogeneous nuclear ribonucleoprotein (HNRNP) families (17,18) are capable of recognizing m6A methylation and generating a functional signal (19). YTH domain proteins read m6A through a conserved aromatic cage (20) and two other proteins, FMRP translational regulator 1 (FMR1) and leucine-rich pentatricopeptide repeat-containing (LRPPRC), can also recognize this modification (21,22). Therefore, the m6A modification is a highly dynamic and reversible process (Fig. 1) (23).
Figure 1.
Interaction between the mRNA methylation ‘writers,’ ‘erasers’ and ‘readers.’ In the nucleus, m6A methyltransferases (green semicircles) are named ‘writers’. Their binding proteins (blue semicircles) are named ‘readers,’ and the demethylases (pink semicircles) are named ‘erasers.’ The m6A ‘writers’ include METTL3/14/16 and WTAP, and the ‘erasers’ that can eliminate the m6A from mRNAs include FTO and ALKBH5. Furthermore, m6A is recognized by the ‘readers,’ such as YTHDF1/2/3, HNRNPA2/B1, and HNRNPC. YTHDF1/2/3 (yellow circles) promotes the translation of m6A-modified mRNAs in the cytosol. m6A, N6-methyladenosine; METTL, methyltransferase-like; WTAP, Wilms tumor 1-associating protein; FTO, Fat mass and obesity-associated protein; ALKBH5, alkB homolog 5; YTHDF, YTH domain protein families; HNRNP A2/B1, heterogeneous nuclear ribonucleoprotein A2/B1; HNRNPC, heterogeneous nuclear ribonucleoprotein C.
Lung cancer is the most common malignant tumor with high morbidity and mortality rates worldwide. Non-small cell lung cancer (NSCLC) accounts for 85-90% of all cases of lung cancer, including lung squamous cell carcinoma (LUSC), lung adenocarcinoma (LUAD), and large cell anaplastic carcinoma (LCAC) (24). According to the latest data on cancer incidence, the 5-year survival rate of patients with NSCLC is as low as approximately 15%, which can be attributed to atypical symptoms in the early phase of the disease and lack of effective treatment (25,26). The epigenetic m6A modifications are involved in the progression, auxiliary diagnosis, and prognosis of lung cancer (27). Moreover, m6A modification is an important factor affecting the growth, survival, and invasion of cancer cells (28,29). Here, we review and summarize the molecular mechanisms and functions of m6A RNA modification in lung cancer. Further, we discuss the role of m6A modification in lung cancer to provide a new theoretical basis for m6A research.
2. m6A writers in lung cancer
The m6A methyltransferase writer complex, which catalyzes the m6A mRNA methylation in lung cancer, primarily consists of METTL3, METTL14, METTL16, and WTAP. METTL3 (also known as MTA70) is the methyltransferase primarily responsible for the m6A modification. METTL3 and METTL14 form a stable heterodimer core complex of METTL3-METTL14, which affects the cellular m6A deposition on mammalian nuclear RNAs. WTAP does not exhibit methylation activity; however, it interacts with the METTL3-METTL14 complex to significantly impact the cellular m6A deposition (10).
METTL3
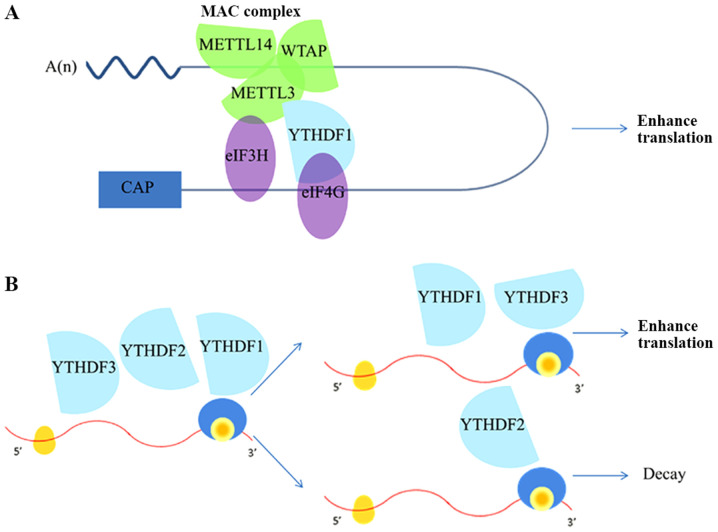
METTL3 levels are upregulated in lung cancer tissues, which are higher in advanced stage lung cancer patients (30). METTL3 increases the translation of target mRNAs by recruiting the eukaryotic translation initiation factor (eIF)3 to the translation initiation complex in H1299 cells (31). It directly interacts with certain components of the multi-subunit eIF3 complex. Meanwhile, the METTL3-eIF3H interaction is essential for promoting translation, formation of densely packed polyribosomes, and oncogenic transformation of A549 cells (Fig. 2A). Disruption of the METTL3-eIF3H interaction eliminates the ability of METTL3 to promote translation, influence polysome conformation, and enhance oncogenic transformation (32). Furthermore, Lin et al found that METTL3 enhanced RNA translation without the aid of methyltransferase and reader protein activity. METTL3 increases RNA translation by directly recruiting translation initiation factors. METTL3 knockdown inhibits the recruitment of eIF3 to both the cap-binding protein 80 (CBP80)- and eIF4E-cap binding proteins (33). Inhibition of m6A with METTL3 short hairpin RNA (shMETTL3) significantly decreases the expression of eIFs in lung cancer cells (34).
Figure 2.
Functional roles of m6A modification on mRNA expression. (A) RNA circularization. METTL3, an RNA methyltransferase located at the 3'-UTR, combines with METTL14 and WTAP to form the MAC complex, which increases the translation of specific mRNAs independently through its catalytic activity and recruitment of eIF3H. YTHDF1 binds to eIF4G at 5'-UTR, which promotes the process of RNA circularization. (B) YTHDF1 and YTHDF3 synergistically promote protein synthesis, while YTHDF2 affects the attenuation of methylated mRNA. YTHDF1/2/3 serves a key role in the metabolism of N6-methyladenosine-modified mRNAs in the cytoplasm. METTL, methyltransferase-like; UTR, untranslated region; WTAP, Wilms tumor 1-associating protein; MAC, m6A-METTL complex; eIF3H, eukaryotic translation initiation factor 3H; YTHDF, YTH domain protein families.
METTL3 regulates the translation of genes related to tumor progression and apoptosis. METTL3 knockdown in A549 cells decreases the expression of bromodomain containing 4 (BRD4) and other targets, and the cells expressing METTL3 are more sensitive to pharmacological BRD4 inhibition. METTL3 promotes translation only when it is tethered to the reporter mRNA at sites close to the stop codon and assists the mRNA looping mechanism for ribosome recycling and translational control (32). The mRNAs of several oncogenes, such as the epidermal growth factor receptor (EGFR), tafazzin (TAZ), MAPKAPK2 (MK2), and DNA methyltransferase 3 alpha (DNMT3A), have one or more m6A peaks near the stop codon. The analysis of m6A levels in EGFR revealed that METTL3 binds to the EGFR mRNA in A549 cells (33). METTL3 knockdown significantly increases E-cadherin expression and decreases the expression of Fibronectin and Vimentin in A549 and LC-2/ad cells. Moreover, it inhibits the expression changes of these epithelial-mesenchymal transition (EMT)-relate marker genes stimulated by transforming growth factor β (TGF-β) treatment. These results suggest the involvement of endogenous METTL3 in the transcriptional regulation of TGF-β-induced EMT program (35). Furthermore, the expression of metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), a transcript related to lung cancer metastasis and prognosis, is increased due to a higher level of m6A modification mediated by METTL3. Meanwhile, the METTL3/YTHDF3 complex increases the stability of MALAT1. METTL3 catalyzes the m6A methylation modification of the nuclear effector yes-associated protein (YAP) of the Hippo signaling pathway, promotes its translation, and mediates the proliferation and metastasis of NSCLC (31).
The characteristics of METTL3 in activation and posttranslational modification (such as SUMOylation) of METTL3 may directly affect the proliferation and xenograft tumor growth of lung cancer cells. SUMOylation of METTL3 mediates the m6A mRNA modification and subsequent differences in gene expression profiles (36).
miRNAs can be differentially expressed and act as oncogenic or tumor suppressor miRNAs, which are based on the roles of miRNA-regulated genes (37). The m6A modification may control arsenite-induced proliferation and apoptosis of cells by affecting miRNAs (38). Studies have provided new insights into the mechanism of METTL3 regulation by miRNAs, thus signifying the potential application of METTL3 as a therapeutic target in NSCLC. For example, miR-33a inhibits NSCLC cell proliferation by targeting the 3'-UTR of METTL3 mRNA (39). Moreover, miR-600 attenuates the METTL3 expression and regulates cell proliferation, metastasis, and apoptosis by regulating the PKB Protein Kinase (AKT) and β-catenin signaling pathways (40). METTL3 has been reported to increase the splicing of precursor miR-143-3p, accelerate the processing and maturation of miR-143-3p. Moreover, miR-143-3p/VASH1 axis acts as adverse prognosis factors for in vivo progression and overall survival rate of lung cancer (41). In summary, miRNA is an important bridge for m6A to influence the proliferation, metastasis, invasion, and apoptosis of lung cancer cells.
Other m6A methyltransferases
WTAP is the target of miRNAs and accelerates the progression of NSCLC (42). METTL16 is a recently confirmed m6A RNA methyltransferase that interacts with the 3'-terminal RNA triple helix of MALAT1 in lung cancer (43). The three-m6A-regulator signature (KIAA1429, METTL3 and IGF2BP1) is recognized as an independent prognostic model to categorize lung cancers into high- and low-risk groups for patient stratification, prognostic assessment, and personalized treatment in lung cancer. KIAA1429 and insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) are significantly associated with multiple biological processes, including proliferation, apoptosis, metastasis, energy metabolism, drug resistance, and recurrence; additionally, they target potential genes related to lung cancer (44). Proteinase activated-receptor 2 (PAR2) participates in cancer metastasis promoted by serine proteinases. Knock down of NOP2/sun domain family, member 2 (NSun2), a new RNA methyltransferase, blocks the reduction in miR-125b induced by PAR2. NSun2 is shown to interfere in the mature processing of miR-125b from pri- and pre-miR-125b2 in A549 cells. Furthermore, PAR2 activation increased the level of m6A-containing pre-miR-125b in NSun2-dependent manner (45). Overall, these data reveal the existence of a complex network of interactions between the m6A methylases and oncogenes that can regulate the proliferation, metastasis, invasion, and apoptosis of lung cancer cells.
3. m6A erasers in lung cancer
m6A is deposited by the methyltransferase complex and cleared by the demethylases FTO and ALKBH5. These demethylases participate in the biological processes of lung cancer (46,47). m6A in the nuclear RNA is a substrate of FTO, and FTO causes an enzymatic alteration that may be related to mRNA transcription (48). ALKBH5, a primary m6A demethylase, plays important roles in lung cancer by regulating proliferation, migration, invasion, metastasis, and tumor growth (49).
FTO
Increased METTL3 and decreased FTO levels demonstrate that the dysregulated writer and/or eraser may affect the m6A content in both the cells and tissues of LUAD patients (34). RNA sequencing analysis has revealed that some genes are influenced by m6A demethylation, most of which are associated with lung cancer, such as laminin γ2, nerve growth factor inducible, integrin alpha 11, thrombospondin 1, and proprotein convertase subtilisin/kexin type 9. FTO enhances LUAC cell progression by activating cell migration (15).
In LUSC, FTO acts as a prognostic factor responsible for aberrant m6A modifications (50). The proliferation and invasion of cells in LUSD was found to be effectively decreased by FTO knockdown. Furthermore, overexpression of FTO rather than its mutant form promotes the malignant phenotype of cells. Mechanism analysis demonstrated that FTO decreases the m6A modification of the myeloid zinc finger 1 (MZF1) transcript and strengthens its stability, resulting in increased MZF1 expression, as well as promotion of the occurrence and development of lung cancer (47).
In addition, FTO represses the m6A levels and strengthens the mRNA stability of ubiquitin-specific protease 7 (USP7), which relies on the demethylase activity of FTO. FTO downregulation inhibits proliferation and growth of NSCLC cells by facilitating the expression of USP7(46). Therefore, overexpression of FTO promotes the proliferation, migration, and invasion abilities of lung cancer cells.
ALKBH5
ALKBH5 is upregulated in NSCLC and is closely associated with a poor prognosis. Functionally, ALKBH5 facilitates proliferation and inhibits apoptosis of the NSCLC cells in vitro, whereas ALKBH5 knockdown reduces tumor growth in vivo (16). The overexpression of ALKBH5 results in the increase translation efficiency of factor forkhead box M1 (FOXM1) mRNA by decreasing the level of m6A in FOXM1, which promotes the growth of LUAD cells (51). Mechanistically, ALKBH5 knockout inhibits the growth and invasion of A549 and NCI-H566 cells under intermittent hypoxia by downregulating the m6A modification of FOXM1 and increasing the FOXM1 levels (51). Mechanistically, methylated RNA immunoprecipitation sequencing revealed that ALKBH5 targets the 3'-UTR of tissue inhibitor of metalloproteinase 3 (TIMP3). ALKBH5 inhibits the TIMP3 transcript stability, thereby reducing its translation (16). Due to the upregulation of ALKBH5 in NSCLC, the oncogene ubiquitin conjugating enzyme E2C (UBE2C) is stabilized epitranscriptionally with the remaining lower m6A levels within its mature RNAs. Activation of UBE2C is associated with adverse prognosis and enhances proliferation, clonogenicity, and invasive growth of NSCLC cells (52). Furthermore, ALKBH5 restrains tumor growth and metastasis by decreasing the expression and activity of YAP in a YTHDF- and miR-107/large tumor suppressor kinase 2 (LATS2)-mediated manner. YAP expression is negatively correlated with ALKBH5 expression and plays an opposite role in the regulation of cellular proliferation, invasion, migration, and EMT of NSCLC cells (53). Collectively, m6A demethylases affect the proliferation, invasion, and apoptosis of lung cancer cells by downregulating the m6A modification of mRNA.
4. m6A readers in lung cancer
m6A also affects biological processes by recruiting reader proteins that specifically recognize m6A RNA methylation and affect the downstream functions (54,55). YTH N6-methyladenosine RNA-binding protein (YTHDF)1, YTHDF2, YTHDF3, YTH domain containing 1 (YTHDC)1, and YTHDC2 read mRNA with the m6A modification specifically in the cytoplasm (56). In the cytoplasm, the m6A-binding protein YTHDF1 promotes the translation of m6A-modified mRNAs, and YTHDF2 facilitates the decay of m6A-modified transcripts. YTHDF3 accelerates protein synthesis in synergy with YTHDF1 and impacts the methylated mRNA decay mediated by YTHDF2. Cells deficient in all three YTHDFs show the most dramatic accumulation of m6A-modified transcripts (8). In addition to the YTH domain m6A readers, other readers such as the heterogeneous nuclear ribonucleoprotein A2/B1 (HNRNP A2/B1) and heterogeneous nuclear ribonucleoprotein C (HNRNPC) do not directly interact with m6A but bind to the transcripts containing m6A (57).
YTH domain family
The expression of YTHDF1 and YTHDF3 is higher, but that of YTHDF2 is lower, in human lung cancer tissues compared to adjacent normal lung tissues. These alterations are related to the functions of mRNA translation and decay of the pre-mRNA target genes (Fig. 2B). Similarly, YTHDF1 knockdown partly blocks the pre-mRNA processing factor 6 (PRPF6) expression and cell growth (34). YTHDF1 positively facilitates protein synthesis by interacting with the translation machinery (58). YTHDF1 knockdown restrains NSCLC cell proliferation and xenograft tumor formation by regulating the translational efficiency of cyclin-dependent kinase (CDK)2, CDK4, and cyclin D1, whereas YTHDF1 depletion inhibits lung cancer progression. High expression of YTHDF1 is related to better clinical outcomes, with its depletion rendering cancer cells resistant to cisplatin treatment. Mechanistic studies identified the Keap1-Nrf2 axis as the downstream mediator of YTHDF1(59). YTHDF2 is upregulated in lung cancer tissues, promotes lung cancer cell growth and enhances the pentose phosphate pathway (PPP) flux, which is important for tumor growth. Mechanistically, YTHDF2 directly binds to the m6A modification site of 6-phosphogluconate dehydrogenase(6-PGD) 3'-UTR to promote 6-PGD mRNA translation in lung cancer cells (60). YTHDF3 with METTL3, YTHDF1, and eIF3b directly promotes YAP translation via interaction with the translation initiation machinery in NSCLC. Meanwhile, MALAT1 stability is increased by the METTL3/YTHDF3 complex. Both YAP and MALAT1 promote carcinogenic activity and are associated with the occurrence of lung cancer (31). YTHDF1 and YTHDF2 competitively interacted with YTHDF3 in an m6A-independent manner to regulate YAP expression. YTHDF2 facilitated YAP mRNA decay via the AGO2 system, whereas YTHDF1 promoted YAP mRNA translation by interacting with eIF3a; both these activities are regulated by m6A modification (53).
In addition, the m6A-binding protein YTHDC2 is associated with tumor progression in lung cancer (27). YTHDC2 is frequently suppressed in LUAD. Downregulation of YTHDC2 is associated with poor clinical outcome of LUAD. Moreover, YTHDC2 exhibits antitumor activity in human LUAD cells. Mechanistically, YTHDC2, through its m6A-recognizing YTH domain, inhibits cystine uptake and blocked the downstream antioxidant program. Furthermore, solute carrier 7A11 (SLC7A11), the catalytic subunit of cystine transporter system Xc-, is identified to be the direct target of YTHDC2. YTHDC2 destabilizes SLC7A11 mRNA in an m6A-dependent manner because YTHDC2 preferentially bound to m6A-modified SLC7A11 mRNA and thereafter promotes its decay (61). In summary, these YTH domain family proteins may influence the fundamental biological processes in an integrated and cooperative manner in lung cancer.
HNRNP family
HNRNP A2/B1 is overexpressed in lung cancer and in other cancers, such as liver cancer, breast cancer, and pancreatic cancer (62,63). This overexpression is not associated with the histological classification of lung cancer but with the clonal expansion of the tumor in NSCLC patients (64,65). He et al demonstrated that hnRNP A2/B1 formed complexes with the transcripts of many of the verified downstream genes, suggesting that HNRNP A2/B1 contributes to the regulation of these genes (66). The expression of HNRNP A2/B1 protein is correlated with the expression of anexeleto (AXL). The expression of HNRNP A2/B1 and AXL affects the prognosis of patients with NSCLC (67). CACNA1G-AS1 (CAS1) increases the level of HNRNP A2/B1, which enhances cancer cell invasion and migration in NSCLC (68). In addition, expression of HNRNP A2/B1 may affect the function of EMT by regulating the E-cadherin expression in non-epithelial lung cancer cell lines (62). These results reinforce the conclusion that HNRNP A2/B1 is associated with cellular processes that affect the cell cycle and proliferation.
HNRNPC is another RNA-binding protein ‘reader’ of m6A methylation, and is related to the progression of various cancers. HNRNPC is upregulated in progressed lung cancer (27). HNRNPC expression is significantly related to poor overall survival in patients with LUAD, indicating that HNRNPC may be a cancer-promoting factor and a potential prognostic biomarker in LUAD (69). Overexpression of HNRNPC significantly enhances lung cancer cell proliferation, migration, and invasion both in vitro and in vivo. In NSCLC cell lines, HNRNPC interacts with KH-type splicing regulatory protein (KHSRP), which is considered to be a metastasis-associated candidate molecule. KHSRP and HNRNPC are significantly associated with advanced tumor progression and metastasis (both lymph node and distant) and may induce invasion and metastasis in human lung cancer (70).
The other m6A binding proteins
IGF2BPs, a class of RNA-binding proteins, including IGF2BP1, IGF2BP2, and IGF2BP3, are considered to be the ‘reader’ of m6A methylation and remarkably affects cancer occurrence and development. Studies have proved that IGF2BP3 has prognostic potential in multiple public databases compared with other members of the IGF2BPs family. IGF2BP3 is abnormally highly expressed in LUAD tissue, and can lead to worse overall survival. IGF2BP3 expression could serve for independently predicting the prognosis of LUAD patients. In summary, IGF2BP3 may be an oncogene and potential prognostic biomarker of LUAD (71).
m6A regulators regulate the expression of the downstream target genes by mediating the mRNA stability, translation efficiency, and mRNA decay to affect the proliferation, migration, and invasion of lung cancer cells (Table I). Knowledge about the mechanism of m6A methylation is limited, and the supplementary discoveries of regulatory patterns mediated by m6A in lung cancer are worth verifying in future studies.
Table I.
Potential mechanisms and target genes of m6A regulators in lung cancer.
| A, Writer m6A components | |||
|---|---|---|---|
| Proteins | Related targets | Roles in lung cancer | (Refs.) |
| METTL3 | EGFR, TAZ, eIF, CBP80, BRD4, DNMT3A, JUNB, EZH2, ATG7, LC3B, SQSTM1 | Promotes growth, translation, survival and invasion of lung cancer cells | (28,30,25,49) |
| METTL14 | Unknown | Forms complex with METTL3 | (10) |
| METTL16 | MALAT | Combines with metastasis-related lung adenocarcinoma transcripts to promote lung cancer activity | (85) |
| WTAP | Unknown | Forms complex with METTL3 | (86) |
| B, Eraser m6A components | |||
| Proteins | Related targets | Roles in lung cancer | (Refs.) |
| FTO | USP7, MZF1 | Promotes growth of lung cancer cells | (46,47) |
| ALKBH5 | FOXM1, YAP UBE2C, TIMP3 | Promotes growth and invasion of lung adenocarcinoma cells and stabilize mRNA transcripts | (16,53) |
| C, Reader m6A components | |||
| Proteins | Related targets | Roles in lung cancer | (Refs.) |
| YTHDF1 | eIF, G3BP1 | Promotes translational efficiency | (58) |
| YTHDF2 | 6-PGD | Reduces the stability of the target transcript | (60) |
| YTHDF3 | YAP | Regulates the stability of mRNA and cooperates with YTHDF1 to promote protein synthesis | (53) |
| HNRNP A2/B1 | AXL, COX-2, PGE2 | Regulates expression of transcription-related factors and determines cell fate transition | (64,87,88) |
| HNRNPC | KHSRP, uPAR | Promotes proliferation, migration, and invasion of lung cancer cells | (70,89) |
6-PGD, 6-phosphogluconate dehydrogenase; ATG, autophagy protein; AXL, anexeleto; BRD4, bromodomain-containing protein 4; CBP80, cap-binding protein 80; COX-2, cytochrome c oxidase subunit II; DNMT3A, DNA methyltransferase 3 alpha; EGFR, epidermal growth factor receptor; eIF, eukaryotic translation initiation factors; EZH2, enhancer of zest homolog 2; FOXM1, factor forkhead box M1; G3BP1, GTPase-activating protein-(SH3domain)-binding protein 1; JUNB, recombinant Jun B proto oncogene; KHSRP, KH-type splicing regulatory protein; LC3B, light chain 3B; MZF1, myeloid zinc finger 1; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; PGE2, prostaglandin E2; SQSTM1, Sequestosome 1; TAZ, tafazzin; TIMP3, tissue inhibitors of metalloproteinase 3; UBE2C, ubiquitin conjugated enzyme E2C; uPAR, urokinase plasminogen activator receptor; USP7, ubiquitin-specific protease 7, YAP, yes-associated protein.
5. m6A as a potential therapeutic target in lung cancer
The treatment options for lung cancer have developed considerably over the past years; however, most patients are diagnosed at an advanced stage of the disease due to the insidious symptoms of early-stage lung cancer. Thus, the survival rate of lung cancer patients remains low (72). Most RNA methylation regulators had distinct expressions in tumor tissues and adjacent tissues. The patients in the high-risk group were more likely to have a higher stage, more lymph node metastases, and distant metastases, showing a poor clinical outcome. Different molecular phenotypes constructed by RNA methylation regulators can be independent ri7sk factors for the prognosis of LUAD (73). The expression of m6A methylation regulators between high- and low-risk LUSC patients is significantly different, and the high-risk LUSC patients have significantly low levels of ALKBH5, METTL3, HNRNPC, and KIAA1429. Thus, m6A methylation regulators may result in a poor prognosis in patients with low-risk LUSC (74).
Recently, cancer immunotherapy has become involved in treating all forms of cancer and has changed the landscape of cancer care. LUAD is the most common histological subtype in lung cancer. LUAD subtypes are identified on the basis of the immunogenomic profiling of 29 immune signatures. There are three LUAD subtypes: Immunity High, Immunity Medium, and Immunity Low. The Immunity High subtype exhibits more sensitivity to immunotherapy and chemotherapy. Immunity High is significantly associated with decreased gene expression, such as METTL3, RBM15, YTHDC1, YTHDF1, and YTHDF2, which are involved in m6A mRNA methylation. And the level of m6A RNA methylation, associates with cancer initiation and progression, is reduced in the Immunity High subtype (75). Interleukin-37 (IL-37) plays a crucial protective role in lung cancer. Treatment of IL-37 in lung cancer cells induced widespread and dynamic RNA m6A methylation. It could lead to changes in m6A methylation level and relates molecule expression level in A546 cells, such as METTL3, METTL14, WTAP, ALKBH5 and FTO, and may downregulate the proliferation by inhibiting Wnt5a/5b pathway in A549 cells. It concludes that IL-37 suppresses tumor growth through regulation of RNA m6A methylation in lung cancer cells (76). These findings suggest that m6A RNA methylation is important determinants of initiation, progression and prognosis in lung cancer and may provide potential prognostic biomarker or therapeutic target for immunotherapeutic and chemotherapeutic development.
m6A performs multi-functional roles in EMT modulation, suggesting the critical roles of m6A in cancer progression regulation. EMT plays a critical role in lung cancer progression; thus, it is important to identify the factors that inhibit EMT in lung cancer treatment (77). METTL3 downregulation in lung cancer tissues influences EMT via m6A modification of the enhancer of zeste homolog 2 (EZH2), contributes to the macrophage recruitment, and reduces the malignant progression of lung cancer (30). Knockdown of METTL3 inhibits the TGF-β-induced morphological conversion of the cell and increases the cell migration potential as well as changes in the expression of EMT-related marker genes (35). TGF-β1-induced EMT is inhibited in METTL3 knockdown cells. The expression of TGF-β1 is up-regulated, while self-stimulated expression of TGF-β1 is suppressed in METTL3 cells. m6A promotes TGF-β1 mRNA decay, but impairs TGF-β1 translation progress. Besides this, the autocrine of TGF-β1 is disrupted in METTL3 cells through interrupting TGF-β1 dimer formation. Snail, which is down-regulated in METTL3 cells, is a key factor responding to TGF-β1-induced EMT (78). In addition, YTHDF1 is positively correlated with the growth, invasion, and EMT of NSCLC cells, while YTHDF2 plays an opposite role in these cell processes (53). Recent studies have demonstrated the inhibitory effect of simvastatin on tumor cell proliferation. Simvastatin causes METTL3 downregulation in lung cancer tissues, resulting in EMT via m6A modification of mRNA, thus restraining the malignant progression of lung cancer (30). These results indicate that m6A regulators can be potential therapeutic targets for EMT in lung cancer cells.
In recent years, epigenetics, especially m6A RNA modification, has been further understood and explored with the rapid advances in detection methods and high-throughput sequencing techniques (79). It has been widely illustrated that the dysregulation of m6A RNA modification is related to various types of cancers, as well as the drug resistance to anti-tumor therapy (80). In three LUAD cell lines, treatment with ammonium tetra thiomolybdate (ATTM) at high concentrations inhibited the cell growth, while at low ATTM concentrations the cell growth was promoted. Treatment with ATTM significantly increased the level of METTL3 but reduced the FTO levels. Additionally, ATTM upregulates METTL14 expression, which is not consistent with The Cancer Genome Atlas (TCGA) analysis. This difference may be due to the uncertainty in gene expression between the mRNA and protein levels. This unique expression contributes to ATTM-induced increase in m6A in A549 cells (34). METTL3 promotes the translation of important oncogenes like EGFR in lung cancer. EGFR inhibitors, such as gefitinib and erlotinib, have gained approval for the treatment of patients with NSCLC (81). FTO inhibitor rhein enhances the antitumor activity of pemetrexed through influencing autophagy and apoptosis by modulating the p PI3K-AKT-mTOR pathway and B-cell lymphoma-2 (Bcl-2) family of proteins in A549 cells. It demonstrates that the potential application of rhein as a candidate drug in combination with pemetrexed is promising for treatment of the human lung cancer (82). m6A methyltransferase METTL3-mediated autophagy plays an important role in reversing gefitinib resistance by β-elemene in NSCLC cells. Mechanistically, β-elemene can reverse gefitinib resistance by inhibiting the late stage of autophagy in a manner of chloroquine, which inhibits the maturation of autophagosomes into autolysosomes through attenuating the lysosomal acidification. In this reversing process, METTL3 can positively regulate this autophagy process by targeting autophagy protein (ATG5), ATG7, light chain 3B (LC3B) and Sequestosome 1 (SQSTM1) (83). Functional enrichment analysis of the m6A-modified genes revealed that m6A methylation might modify the cell cycle to influence the response to afatinib. Furthermore, these m6A-modified genes are over-represented in the putative drug resistance-associated genes and FDA-approved drug targets and have a higher average degree and clustering coefficient than other genes in the protein–protein interaction network (84). Overall, the action of m6A regulators may contribute to drug resistance in tumor therapy and prognosis.
6. Conclusion
m6A methylation has a huge impact on RNA production/metabolism and is involved in the pathogenesis of many diseases, including cancer. In the occurrence and development of lung cancer, m6A-modified mRNA regulates RNA transcription, splicing, processing, translation, and decay. Accumulating evidence reveals that m6A regulators and their mechanisms of action play vital roles in lung cancer. The m6A modification directly or indirectly affects cell proliferation, metastasis, invasion, and apoptosis. Systematic study of the functions and potential molecular mechanisms of m6A regulators will further improve our understanding of the complex networks associated with lung cancer. Inhibitors targeting m6A regulators may have great therapeutic potential in the treatment of lung cancer. In addition to the known modulating effects of m6A methylation, the underlying mechanism of m6A modification in lung cancer needs to be further investigated.
Acknowledgements
Not applicable.
Funding Statement
Funding: This work was supported by grants from the project of innovation plan for graduate students of Beihua University (grant no. 2019021, 2019027), the National Student's Training Program for Innovation and Entrepreneurship (grant no. 201913706015), the Scientific Technology Research Project of the Education Department of Jilin Province (grant nos. JJKH20191068KJ and JJKH20200076KJ), and the Jilin Science and Technology Innovation Development Program (grant no. 20190601177).
Availability of data and materials
Not applicable.
Authors' contributions
YW and XS designed the review. MZ, MX, YC, and ZL were involved in the collection and collation of references. YW wrote, reviewed, and edited the manuscript. WZ critically revised the manuscript for intellectual content. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Liu N, Pan T. N6-methyladenosine-encoded epitranscriptomics. Nat Struct Mol Biol. 2016;23:98–102. doi: 10.1038/nsmb.3162. [DOI] [PubMed] [Google Scholar]
- 2.Jonkhout N, Tran J, Smith M, Schonrock N, Mattick J, Novoa E. The RNA modification landscape in human disease. RNA. 2017;23:1754–1769. doi: 10.1261/rna.063503.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Fan Y, Leng R, Pan H, Ye D. Potential link between m6A modification and systemic lupus erythematosus. Mol Immunol. 2018;93:55–63. doi: 10.1016/j.molimm.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Meyer K, Saletore Y, Zumbo P, Elemento O, Mason C, Jaffrey S. Comprehensive analysis of mRNA methylation reveals enrichment in 3'UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Lu Z, Gomez A, Hon G, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boccaletto P, Machnicka M, Purta E, Piatkowski P, Baginski B, Wirecki T, de Crécy-Lagard V, Ross R, Limbach P, Kotter A, et al. MODOMICS: A database of RNA modification pathways 2017 update. Nucleic Acids Res. 2018;46:D303–D307. doi: 10.1093/nar/gkx1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han Z, Niu T, Chang J, Lei X, Zhao M, Wang Q, Cheng W, Wang J, Feng Y, Chai J. Crystal structure of the FTO protein reveals basis for its substrate specificity. Nature. 2010;464:1205–1209. doi: 10.1038/nature08921. [DOI] [PubMed] [Google Scholar]
- 8.Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumann U, Shafik A, Preiss T. METTL3 Gains R/W access to the Epitranscriptome. Mol Cell. 2016;62:323–324. doi: 10.1016/j.molcel.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2013;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu F, Cheng W, Zhao F, Tang M, Diao Y, Xu R. Association of N6-methyladenosine with viruses and related diseases. Virol J. 2019;16(133) doi: 10.1186/s12985-019-1236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang W, Adhikari S, Shi Y, Lv Y, Chen YS, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer K, Jaffrey S. Rethinking m6A Readers, Writers, and Erasers. Annu Rev Cell Dev Biol. 2017;33:319–342. doi: 10.1146/annurev-cellbio-100616-060758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz S, Mumbach M, Jovanovic M, Wang T, Maciag K, Bushkin G, Mertins P, Ter-Ovanesyan D, Habib N, Cacchiarelli D, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5'sites. Cell Rep. 2014;8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Y, Qi N, Wang K, Huang Y, Liao J, Wang H, Tan A, Liu L, Zhang Z, Li J, et al. FTO facilitates lung adenocarcinoma cell progression by activating cell migration through mRNA Demethylation. Onco Targets Ther. 2020;13:1461–1470. doi: 10.2147/OTT.S231914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Z, Qian Q, Zhao X, Ma L, Chen P. N 6-methyladenosine ALKBH5 promotes non-small cell lung cancer progress by regulating TIMP3 stability. Gene. 2020;731(144348) doi: 10.1016/j.gene.2020.144348. [DOI] [PubMed] [Google Scholar]
- 17.Han S, Tang Y, Smith R. Functional diversity of the hnRNPs: Past, present and perspectives. Biochem J. 2010;430:379–392. doi: 10.1042/BJ20100396. [DOI] [PubMed] [Google Scholar]
- 18.Patil D, Pickering B, Jaffrey S. Reading m 6 A in the Transcriptome: M 6 A-binding proteins. Trends Cell Biol. 2018;28:113–127. doi: 10.1016/j.tcb.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller S, Glaß M, Singh A, Haase J, Bley N, Fuchs T, Lederer M, Dahl A, Huang H, Chen J, et al. IGF2BP1 promotes SRF-dependent transcription in cancer in a m6A- and miRNA-dependent manner. Nucleic Acids Res. 2019;47:375–390. doi: 10.1093/nar/gky1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu C, Liu K, Ahmed H, Loppnau P, Schapira M, Min J. Structural basis for the discriminative recognition of N6-methyladenosine RNA by the Human YT521-B homology domain family of proteins. J Biol Chem. 2015;290:24902–24913. doi: 10.1074/jbc.M115.680389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arguello A, DeLiberto A, Kleiner R. RNA chemical proteomics reveals the N 6-methyladenosine (m6A)-regulated protein-RNA Interactome. J Am Chem Soc. 2017;139:17249–17252. doi: 10.1021/jacs.7b09213. [DOI] [PubMed] [Google Scholar]
- 22.Edupuganti RR, Geiger S, Lindeboom RGH, Shi H, Hsu PJ, Lu ZY, Wang SY, Baltissen MPA, Jansen PWTC, Rossa M, et al. N 6-methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nat Struct Mol Biol. 2017;24:870–878. doi: 10.1038/nsmb.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu D, Li M, Tian W, Wang S, Cui L, Li H, Wang H, Ji A, Li Y. Hydrogen sulfide acts as a double-edged sword in human hepatocellular carcinoma cells through EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Sci Rep. 2017;7(5134) doi: 10.1038/s41598-017-05457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakelee H, Kelly K, Edelman M. doi: 10.14694/EdBook_AM.2014.34.177. 50 Years of progress in the systemic therapy of non-small cell lung cancer. Am Soc Clin Oncol Educ Book: 2014: 177-189 doi: 10.14694/EdBook_AM.2014.34.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel R, Miller K, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 26.Lin JJ, Cardarella S, Lydon CA, Dahlberg SE, Jackman DM, Jänne PA, Johnson BE. Five-year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J Thorac Oncol. 2016;11:556–565. doi: 10.1016/j.jtho.2015.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhuang Z, Chen L, Mao Y, Zheng Q, Li H, Huang Y, Hu Z, Jin Y. Diagnostic, progressive and prognostic performance of m6A methylation RNA regulators in lung adenocarcinoma. Int J Biol Sci. 2020;16:1785–1797. doi: 10.7150/ijbs.39046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen XY, Zhang J, Zhu JS. The role of m6A RNA methylation in human cancer. Mol Cancer. 2019;18(103) doi: 10.1186/s12943-019-1033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z, Li L, Sun H, Liu S. Link between m6A modification and cancers. Front Bioeng Biotechnol. 2018;6(89) doi: 10.3389/fbioe.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen WW, Qi JW, Hang J, Wu JX, Zhou XX, Chen JZ, Wang J, Wang HH. Simvastatin is beneficial to lung cancer progression by inducing METTL3-induced m6A modification on EZH2 mRNA. Eur Rev Med Pharmacol Sci. 2020;24:4263–4270. doi: 10.26355/eurrev_202004_21006. [DOI] [PubMed] [Google Scholar]
- 31.Jin D, Guo J, Wu Y, Du J, Yang L, Wang X, Di W, Hu B, An J, Kong L, et al. m(6)A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug resistance and metastasis. J Hematol Oncol. 2019;12(135) doi: 10.1186/s13045-019-0830-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Choe J, Lin S, Zhang W, Liu Q, Wang L, Ramirez-Moya J, Du P, Kim W, Tang S, Sliz P, et al. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature. 2018;561:556–560. doi: 10.1038/s41586-018-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell. 2016;62:335–345. doi: 10.1016/j.molcel.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Li N, Huang L, Xu S, Zheng X, Hamsath A, Zhang M, Dai L, Zhang H, Wong JJ, et al. Is hydrogen sulfide a concern during treatment of lung adenocarcinoma with ammonium tetrathiomolybdate? Front Oncol. 2020;10(234) doi: 10.3389/fonc.2020.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wanna-Udom S, Terashima M, Lyu H, Ishimura A, Takino T, Sakari M, Tsukahara T, Suzuki T. The m6A methyltransferase METTL3 contributes to transforming growth Factor-beta-induced epithelial-mesenchymal transition of lung cancer cells through the regulation of JUNB. Biochem Biophys Res Commun. 2020;524:150–155. doi: 10.1016/j.bbrc.2020.01.042. [DOI] [PubMed] [Google Scholar]
- 36.Du Y, Hou G, Zhang H, Dou J, He J, Guo Y, Li L, Chen R, Wang Y, Deng R, et al. SUMOylation of the m6A-RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res. 2018;46:5195–5208. doi: 10.1093/nar/gky156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Chang W, Hsiao M. Aberrant expression of microRNA clusters in head and neck cancer development and progression: Current and future translational impacts. Pharmaceuticals (Basel) 2021;14(194) doi: 10.3390/ph14030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu S, Sun D, Dai H, Zhang Z. N 6-methyladenosine mediates the cellular proliferation and apoptosis via microRNAs in Arsenite-transformed cells. Toxicol Lett. 2018;292:1–11. doi: 10.1016/j.toxlet.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 39.Du M, Zhang Y, Mao Y, Mou J, Zhao J, Xue Q, Wang D, Huang J, Gao S, Gao Y. MiR-33a suppresses proliferation of NSCLC cells via targeting METTL3 mRNA. Biochem Biophys Res Communs. 2017;482:582–589. doi: 10.1016/j.bbrc.2016.11.077. [DOI] [PubMed] [Google Scholar]
- 40.Wei W, Huo B, Shi X. miR-600 inhibits lung cancer via downregulating the expression of METTL3. Cancer Manag Res. 2019;11:1177–1187. doi: 10.2147/CMAR.S181058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Deng Q, Lv Z, Ling Y, Hou X, Chen Z, Dinglin X, Ma S, Li D, Wu Y, et al. N6-methyladenosine induced miR-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol Cancer. 2019;18(181) doi: 10.1186/s12943-019-1108-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Wu LS, Qian JY, Wang M, Yang H. Identifying the role of Wilms tumor 1 associated protein in cancer prediction using integrative genomic analyses. Mol Med Rep. 2016;14:2823–2831. doi: 10.3892/mmr.2016.5528. [DOI] [PubMed] [Google Scholar]
- 43.Ruszkowska A, Ruszkowski M, Dauter Z, Brown J. Structural insights into the RNA methyltransferase domain of METTL16. Sci Rep. 2018;8(5311) doi: 10.1038/s41598-018-23608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li N, Zhan X. Identification of pathology-specific regulators of m6A RNA modification to optimize lung cancer management in the context of predictive, preventive, and personalized medicine. EPMA J. 2020;11:485–504. doi: 10.1007/s13167-020-00220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang L, Ma Y, Han W, Li W, Cui L, Zhao X, Tian Y, Zhou Z, Wang W, Wang H. Proteinase-activated receptor 2 promotes cancer cell migration through RNA methylation-mediated repression of miR-125b. J Biol Chem. 2015;290:26627–26637. doi: 10.1074/jbc.M115.667717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Han Y, Zhang H, Qian Z, Jia W, Gao Y, Zheng H, Li B. The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem Biophys Res Commun. 2019;512:479–485. doi: 10.1016/j.bbrc.2019.03.093. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Ren D, Du Z, Wang H, Zhang H, Jin Y. m6A demethylase FTO facilitates tumor progression in lung squamous cell carcinoma by regulating MZF1 expression. Biochem Biophys Res Commun. 2018;502:456–464. doi: 10.1016/j.bbrc.2018.05.175. [DOI] [PubMed] [Google Scholar]
- 48.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li F, Wang H, Huang H, Zhang L, Wang D, Wan Y. m6A RNA methylation regulators participate in the malignant progression and have clinical prognostic value in lung adenocarcinoma. Front Genet. 2020;11(994) doi: 10.3389/fgene.2020.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao X, Yang Y, Sun BF, Shi Y, Yang X, Xiao W, Hao YJ, Ping XL, Chen YS, Wang WJ, et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403–1419. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chao Y, Shang J, Ji W. ALKBH5-m6A-FOXM1 signaling axis promotes proliferation and invasion of lung adenocarcinoma cells under intermittent hypoxia. Biochem Biophys Res Commun. 2020;521:499–506. doi: 10.1016/j.bbrc.2019.10.145. [DOI] [PubMed] [Google Scholar]
- 52.Guo J, Wu Y, Du J, Yang L, Chen W, Gong K, Dai J, Miao S, Jin D, Xi S. Deregulation of UBE2C-mediated autophagy repression aggravates NSCLC progression. Oncogenesis. 2018;7(49) doi: 10.1038/s41389-018-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin D, Guo J, Wu Y, Yang L, Wang X, Du J, Dai J, Chen W, Gong K, Miao S, et al. m6A demethylase ALKBH5 inhibits tumor growth and metastasis by reducing YTHDFs-mediated YAP expression and inhibiting miR-107/LATS2-mediated YAP activity in NSCLC. Mol Cancer. 2020;19(40) doi: 10.1186/s12943-020-01161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng X, Su R, Weng H, Huang H, Li Z, Chen J. RNA N(6)-methyladenosine modification in cancers: Current status and perspectives. Cell Res. 2018;28:507–517. doi: 10.1038/s41422-018-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yue Y, Liu J, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29:1343–1355. doi: 10.1101/gad.262766.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Y, Hsu PJ, Chen YS, Yang YG. Dynamic transcriptomic m(6)A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28:616–624. doi: 10.1038/s41422-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi Y, Fan S, Wu M, Zuo Z, Li X, Jiang L, Shen Q, Xu P, Zeng L, Zhou Y, et al. YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat Commun. 2019;10(4892) doi: 10.1038/s41467-019-12801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheng H, Li Z, Su S, Sun W, Zhang X, Li L, Li J, Liu S, Lu B, Zhang S, Shan C. YTH domain family 2 promotes lung cancer cell growth by facilitating 6-phosphogluconate dehydrogenase mRNA translation. Carcinogenesis. 2019;41:541–550. doi: 10.1093/carcin/bgz152. [DOI] [PubMed] [Google Scholar]
- 61.Ma L, Chen T, Zhang X, Miao Y, Tian X, Yu K, Xu X, Niu Y, Guo S, Zhang C, et al. The m(6)A reader YTHDC2 inhibits lung adenocarcinoma tumorigenesis by suppressing SLC7A11-dependent antioxidant function. Redox Biol. 2021;38(101801) doi: 10.1016/j.redox.2020.101801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tauler J, Zudaire E, Liu H, Shih J, Mulshine J. hnRNP A2/B1 modulates epithelial-mesenchymal transition in lung cancer cell lines. Cancer Res. 2010;70:7137–7147. doi: 10.1158/0008-5472.CAN-10-0860. [DOI] [PubMed] [Google Scholar]
- 63.Wu C, Li W, Chen W, Xu D, Wei D, Zhou Q. Expression of hnRNP A2/B1 in human lung cancer cell lines. Chin J Lung Cancer. 2004;7:121–124. doi: 10.3779/j.issn.1009-3419.2004.02.10. [DOI] [PubMed] [Google Scholar]
- 64.Dai X, Yang H. Cloning of hnRNP A2/B1 gene and detection of its expression in lung cancer tissues. Chin J Lung Cancer. 2005;8:266–269. doi: 10.3779/j.issn.1009-3419.2005.04.03. [DOI] [PubMed] [Google Scholar]
- 65.Zhou J, Nong L, Wloch M, Cantor A, Mulshine J, Tockman M. Expression of early lung cancer detection marker: hnRNP-A2/B1 and its relation to microsatellite alteration in non-small cell lung cancer. Lung Cancer. 2001;34:341–350. doi: 10.1016/s0169-5002(01)00254-9. [DOI] [PubMed] [Google Scholar]
- 66.He Y, Rothnagel J, Epis M, Leedman P, Smith R. Downstream targets of heterogeneous nuclear ribonucleoprotein A2 mediate cell proliferation. Mol Carcinog. 2009;48:167–179. doi: 10.1002/mc.20467. [DOI] [PubMed] [Google Scholar]
- 67.Qu X, Liu J, Zhong X, Li X, Zhang Q. Insights into the roles of hnRNP A2/B1 and AXL in non-small cell lung cancer. Oncol Lett. 2015;10:1677–1685. doi: 10.3892/ol.2015.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu P, Kang A, Jing L, Wang Y. Long non-coding RNA CACNA1G-AS1 promotes cell migration, invasion and epithelial-mesenchymal transition by HNRNPA2B1 in non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2018;22:993–1002. doi: 10.26355/eurrev_201802_14381. [DOI] [PubMed] [Google Scholar]
- 69.Guo W, Huai Q, Zhang G, Guo L, Song P, Xue X, Tan F, Xue Q, Gao S, He J. Elevated heterogeneous nuclear ribonucleoprotein C expression correlates with poor prognosis in patients with surgically resected lung adenocarcinoma. Front Oncol. 2020;10(598437) doi: 10.3389/fonc.2020.598437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan M, Sun L, Li J, Yu H, Lin H, Yu T, Zhao F, Zhu M, Liu L, Geng Q, et al. RNA-binding protein KHSRP promotes tumor growth and metastasis in non-small cell lung cancer. J Exp Clin Cancer Res. 2019;38(478) doi: 10.1186/s13046-019-1479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo W, Huai Q, Wan H, Guo L, Song P, Gao S, He J. Prognostic impact of IGF2BP3 expression in patients with surgically resected lung adenocarcinoma. DNA Cell Biol. 2021;40:316–331. doi: 10.1089/dna.2020.6136. [DOI] [PubMed] [Google Scholar]
- 72.Miller K, Nogueira L, Mariotto A, Rowland J, Yabroff K, Alfano C, Jemal A, Kramer J, Siegel R. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 73.Sun L, Liu W, Du X, Liu X, Li G, Yao Y, Han T, Li W, Gu J. Large-scale transcriptome analysis identified RNA methylation regulators as novel prognostic signatures for lung adenocarcinoma. Ann Transl Med. 2020;8(751) doi: 10.21037/atm-20-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu F, Zhang H, Chen J, Lin L, Chen Y. Immune signature of T follicular helper cells predicts clinical prognostic and therapeutic impact in lung squamous cell carcinoma. Int Immunopharmacol. 2020;81(105932) doi: 10.1016/j.intimp.2019.105932. [DOI] [PubMed] [Google Scholar]
- 75.Xu F, Chen J, Yang X, Hong X, Li Z, Lin L, Chen Y. Analysis of lung adenocarcinoma subtypes based on immune signatures identifies clinical implications for cancer therapy. Mol Ther Oncolytics. 2020;17:241–249. doi: 10.1016/j.omto.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mu X, Zhao Q, Chen W, Zhao Y, Yan Q, Peng R, Zhu J, Yang C, Lan K, Gu X, Wang Y. IL-37 confers anti-tumor activity by regulation of m6A methylation. Front Oncol. 2020;10(526866) doi: 10.3389/fonc.2020.526866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu L, Kim HJ, Park MK, Byun HJ, Kim EJ, Kim B, Nguyen MT, Kim JH, Kang GJ, Lee H, et al. Ethacrynic acid, a loop diuretic, suppresses epithelial-mesenchymal transition of A549 lung cancer cells via blocking of NDP-induced WNT signaling. Biochem Pharmacol. 2020;183(114339) doi: 10.1016/j.bcp.2020.114339. [DOI] [PubMed] [Google Scholar]
- 78.Li J, Chen F, Peng Y, Lv Z, Lin X, Chen Z, Wang H. N6-methyladenosine regulates the expression and secretion of TGFβ1 to affect the epithelial-mesenchymal transition of cancer cells. Cells. 2020;9(296) doi: 10.3390/cells9020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gu C, Shi X, Dai C, Shen F, Rocco G, Chen J, Huang Z, Chen C, He C, Huang T, et al. RNA m6A modification in cancers: Molecular mechanisms and potential clinical applications. Innovation. 2020;1(100066) doi: 10.1016/j.xinn.2020.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frye M, Harada B, Behm M, He C. RNA modifications modulate gene expression during development. Science. 2018;361:1346–1349. doi: 10.1126/science.aau1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Modjtahedi H, Essapen S. Epidermal growth factor receptor inhibitors in cancer treatment: Advances, challenges and opportunities. Anticancer Drugs. 2009;20:851–855. doi: 10.1097/CAD.0b013e3283330590. [DOI] [PubMed] [Google Scholar]
- 82.Bu T, Wang C, Jin H, Meng Q, Huo X, Sun H, Sun P, Wu J, Ma X, Liu Z, Liu K. Organic anion transporters and PI3K-AKT-mTOR pathway mediate the synergistic anticancer effect of pemetrexed and rhein. J Cell Physiol. 2020;235:3309–3319. doi: 10.1002/jcp.29218. [DOI] [PubMed] [Google Scholar]
- 83.Liu S, Li Q, Li G, Zhang Q, Zhuo L, Han X, Zhang M, Chen X, Pan T, Yan L, et al. The mechanism of m6A methyltransferase METTL3-mediated autophagy in reversing gefitinib resistance in NSCLC cells by β-elemene. Cell Death Dis. 2020;11(969) doi: 10.1038/s41419-020-03148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meng Q, Wang S, Zhou S, Liu H, Ma X, Zhou X, Liu H, Xu C, Jiang W. Dissecting the m6A methylation affection on afatinib resistance in non-small cell lung cancer. Pharmacogenomics J. 2019;20:227–234. doi: 10.1038/s41397-019-0110-4. [DOI] [PubMed] [Google Scholar]
- 85.Brown JA, Kinzig CG, DeGregorio SJ, Steitz JA. Methyltransferase-like protein 16 binds the 3'-terminal triple helix of MALAT1 long noncoding RNA. Proc Natl Acad Sci USA. 2016;113:14013–14018. doi: 10.1073/pnas.1614759113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fish L, Navickas A, Culbertson B, Xu Y, Nguyen HCB, Zhang S, Hochman M, Okimoto R, Dill BD, Molina H, et al. Nuclear TARBP2 drives oncogenic dysregulation of RNA splicing and decay. Mol Cell. 2019;75:967–981.e9. doi: 10.1016/j.molcel.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kwon J, Jo Y, Namgoong S, Kim N. Functional roles of hnRNPA2/B1 regulated by METTL3 in mammalian embryonic development. Sci Rep. 2019;9(8640) doi: 10.1038/s41598-019-44714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xuan Y, Wang J, Ban L, Lu JJ, Yi C, Li Z, Yu W, Li M, Xu T, Yang W, et al. hnRNPA2/B1 activates cyclooxygenase-2 and promotes tumor growth in human lung cancers. Mol Oncol. 2016;10:610–624. doi: 10.1016/j.molonc.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shetty S. Regulation of urokinase receptor mRNA stability by hnRNP C in lung epithelial cells. Mol Cell Biochem. 2005;272:107–118. doi: 10.1007/s11010-005-7644-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.