TO THE EDITOR
Hidradenitis suppurativa has been previously defined as a “chronic, inflammatory, recurrent, debilitating skin disease” of the hair follicle that typically presents postpuberty with “painful, deep-seated, inflamed lesions in apocrine gland-bearing areas of the body,” particularly the axillary, inguinal, and anogenital regions (Paus et al., 2008). Although the precise molecular pathogenesis remains unknown, some patients with familial hidradenitis suppurativa (acne inversa, OMIM. Johns Hopkins University, Baltimore, MD. MIM Number: 142690. http://www.ncbi.nlm.nih.gov/omim/) bear mutations in the protease complex, gamma-secretase (GS) (Griffith et al., 2015; Pink et al., 2012). Interestingly, previous studies of conditional knockout mice have shown GS and its downstream signaling target, Notch, to be important for maintenance of the pilosebaceous unit (Pan et al., 2004). Nonetheless, it remains to be seen whether GS-dependent follicular pathology, as observed in familial hidradenitis suppurativa in humans, occurs early in hair follicle development, arises in a puberty-dependent process, or can be conferred in adult skin simply by knocking out GS.
Adverse dermatological events in trials of targeted therapy provide opportunities to improve our understanding about the biology of targets in skin. Inhibitors of GS, including semagacestat and avagacestat, have previously been investigated as potential treatments for Alzheimer’s disease, owing to the role GS plays in cleaving amyloid precursor protein and generating pathognomonic amyloid plaques. Notably, the largest investigation of more than 1,500 patients randomized to treatment with GS inhibitor semagacestat (Doody et al., 2013) was terminated early because of an unfavorable ratio of minimal benefit to adverse events, among which unspecified “rash” constituted the most frequent adverse event cluster (Henley et al., 2014).
To pursue the relationship between pharmacologic inhibition of GS and skin pathology, we investigated a cohort of 17 adults who were enrolled in an institutional review board-approved phase II trial for targeted treatment of desmoid tumor/aggressive fibromatosis with the GS inhibitor PF-03084014 (niragacestat) (Kummar et al., 2017) and who provided written informed consent as well as permission to publish their images. Desmoid tumors are rare and rapidly growing mesenchymal neoplasms that do not metastasize but exert local mass effects that can restrict mobility, cause pain, and impair function of nearby tissues. They frequently recur after resection, prompting investigation into medical therapy that targets Notch via GS inhibition (Messersmith et al., 2015). From our cohort of patients with desmoid tumor, demographic information, the incidence of skin lesions, and treatment with antibiotics or local surgical intervention were recorded in a retrospective chart review (Supplementary Table S1 online). For the seven patients evaluated by the Dermatology Consult Service, information about the location of follicular and cystic lesions and more specific specialist descriptions of skin findings were noted and correlated with histopathology when available.
From the cohort of 17 patients, 12 patients (71%) reported CTCAE (v4.0) grade 1 and 2 skin adverse events during treatment, including follicular and cystic lesions (9/12 [75%]) and pruritic eruption (6/12 [50%]). Most patients with skin symptoms were successfully managed with topical and/or systemic antibiotics for skin findings (9/12 [75%]). In addition, five (42%) received local surgical intervention for follicular and cystic lesions. The frequency of skin toxicity noted in our GS inhibitor (niragacestat) trial is consistent with the reported incidence of skin and subcutaneous disorders noted in patients taking the GS inhibitor semagacestat for Alzheimer’s disease in the phase III IDENTITY trials (225/506 [44.4%] on 100 mg daily and 275/527 [52.1%] on 140 mg daily vs. 105/501 [20.9%] for placebo), including rash (146/506 [28.9%] on 100 mg daily and 187/140 [35.3%] on 140 mg daily vs. 69/501 [13.8%] for placebo) (Henley et al., 2014). For the patients who held or discontinued treatment with the GS inhibitor on our trial, cutaneous side effects resolved; however, it is not possible to separate the effects of drug hold or additional therapy (e.g., antibiotics, antihistamine) in our retrospective review.
Among the seven patients evaluated by Dermatology, six had follicular and cystic lesions in intertriginous locations, including axilla (four patients), inguinal crease (three patients), labia (three patients), buttocks (three patients), and medial thigh (two patients). Patients did not present with nor report personal or family history of hidradenitis suppurativa or other comorbidities with reported associations with hidradenitis suppurativa (Fimmel and Zouboulis, 2010). The corresponding histology for these lesions was available for two patients. From the first patient who was a 30-year-old white woman with intermittent symptomatic cysts on the buttocks noted at Dermatology consult 8 months into the trial (Figure 1a), a biopsy taken in month 21 from the buttocks showed an inflamed follicular cyst (Figure 1b). The second patient, a 24-year-old white woman with discrete purple-brown papules and pustules on the shins (Figure 1c), was found to have a follicular cyst, infundibular type (Figure 1d), and a follicular pustule with lichenoid inflammation (Figure 1e). These results support clinical suspicions that lesions were localized to the hair follicle. Additional skin findings are presented in Supplementary Table S1.
Figure 1. Clinical and histopathologic findings in patients treated with PF-03084014.
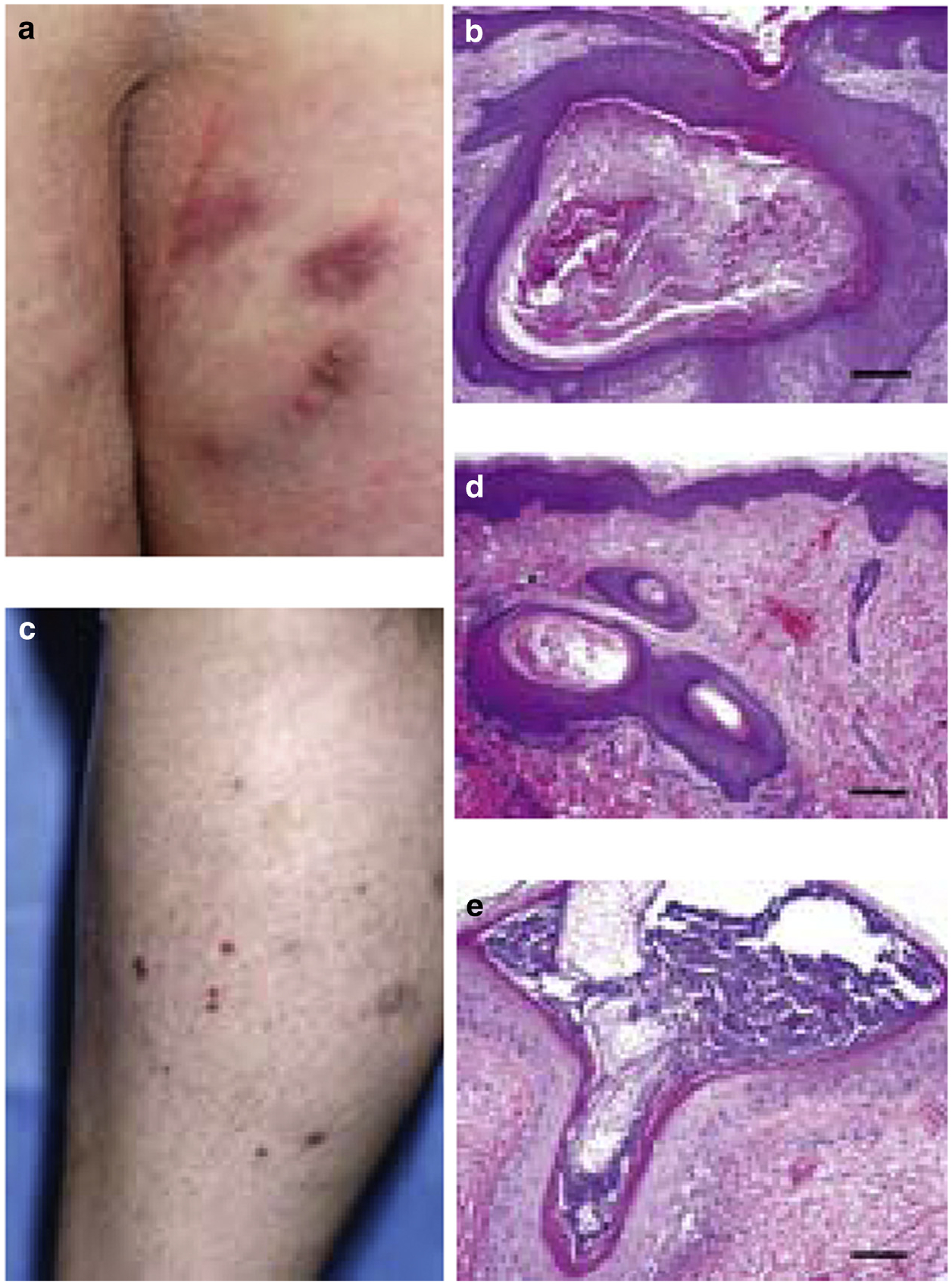
(a) Scarred erythematous plaques with occasional pustules on buttocks and (b) hematoxylin and eosin biopsy specimen from buttocks revealed a follicular cyst, infundibular type (original magnification, ×10, scale bar = 200 μm). (c) Keratotic follicular papules on anterior tibia at trial month 10. Hematoxylin and eosin-stained biopsy specimens from anterior tibia biopsies showed (d) follicular cyst, infundibular type (original magnification, ×10, scale bar = 200 μm) and (e) follicular pustule with lichenoid chronic inflammation (original magnification, ×20, scale bar = 100 μm). Photographs published with patient permission.
In summary, our results show that the majority of patients taking this GS inhibitor developed skin toxicity, most frequently follicular and cystic lesions consistent with a role for GS in hair follicle maintenance demonstrated in mice. Although other studies of early hidradenitis suppurativa have identified histologic features including the absence of sebaceous glands (Kamp et al., 2011), the biopsies in this cohort were from developed lesions and demonstrated scarring that could independently cause loss of adnexa. Of clinical note, the chronicity of GS treatment for patients with desmoid tumor or other oncology patients will make anticipating skin toxicities an important priority in care. The salient involvement of the axilla, inguinal crease, buttocks, and other sites commonly afflicted by hidradenitis suppurativa supports the importance of GS loss of function, either through germline mutation or pharmacologic inhibition, in the pathogenesis of this disease. Our results suggest that the relationship between GS loss of function and hidradenitis suppurativa-like lesions does not depend on GS insufficiency in development or puberty, but rather can be recapitulated in developmentally normal, postpubertal skin with targeted inhibition of GS.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by the National Cancer Institute’s Intramural Research Program and the Division of Cancer Treatment and Diagnosis. This research was also made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, and other private donors. For a complete list, visit the foundation website at http://www.fnih.org.
Abbreviation:
- GS
gamma-secretase
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2017.09.051.
REFERENCES
- Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med 2013;369:341–50. [DOI] [PubMed] [Google Scholar]
- Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa). Derma-toendocrinol 2010;2:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith R, Simmons B, Abyaneh M-A, Bray F, Falto-Aizpurua L, Nouri K. y-Sec and AD HS in AA family. JAMA Dermatol 2015;151:668–70.25693063 [Google Scholar]
- Henley DB, Sundell KL, Sethuraman G, Dowsett SA, May PC. Safety profile of semagacestat, a gamma-secretase inhibitor: IDENTITY trial findings. Curr Med Res Opin 2014;30:2021–32. [DOI] [PubMed] [Google Scholar]
- Kamp S, Fiehn AM, Stenderup K, Rosada C, Pakkenberg B, Kemp K, et al. Hidradenitis suppurativa: a disease of the absent sebaceous gland? Sebaceous gland number and volume are significantly reduced in uninvolved hair follicles from patients with hidradenitis suppurativa. Br J Dermatol 2011;164:1017–22. [DOI] [PubMed] [Google Scholar]
- Kummar S, Coyne G, Do KT, Turkbey B, Meltzer PS, Polley E, et al. Clinical activity of the γ-secretase inhibitor PF-03084014 in adults with desmoid tumors (aggressive fibromatosis). J Clin Oncol 2017;35:1561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messersmith WA, Shapiro GI, Cleary JM, Jimeno A, Dasari A, Huang B, et al. A phase I, dose-finding study in patients with advanced solid malignancies of the oral γ-secretase inhibitor PF-03084014. Clin Cancer Res 2015;21:60–7. [DOI] [PubMed] [Google Scholar]
- Pan Y, Lin M-H, Tian X, Cheng H-T, Gridley T, Shen J, et al. γ-Secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev Cell 2004;7: 731–43. [DOI] [PubMed] [Google Scholar]
- Paus L, Kurzen H, Kurokawa I, Jemec GBE, Emtestam L, Sellheyer K, et al. What causes hidradenitis suppurativa? Exp Dermatol 2008;17:455–6. [DOI] [PubMed] [Google Scholar]
- Pink AE, Simpson MA, Desai N, Trembath RC, Barker JNW. γ-Secretase mutations in hidradenitis suppurativa: new insights into disease pathogenesis. J Investig Dermatol 2012;133: 601–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


