Abstract
Peripheral inflammatory and neuropathic pain are closely related to the activation of purinergic receptor P2X ligand-gated ion channel 3 (P2X3) and transient receptor potential vanilloid 1 (TRPV1), but the interaction between P2X3 and TRPV1 in different types of pathological pain has rarely been reported. In this study, complete Freund’s adjuvant (CFA)-induced inflammatory pain and spared nerve injury (SNI)-induced neuropathic pain models were established in adult rats. The interactions between P2X3 and TRPV1 in the dorsal root ganglion were observed by pharmacological, co-immunoprecipitation, immunofluorescence and whole-cell patch-clamp recording assays. TRPV1 was shown to promote the induction of spontaneous pain caused by P2X3 in the SNI model, but the induction of spontaneous pain behaviour by TRPV1 was not completely dependent on P2X3 in vivo. In both the CFA and SNI models, the activation of peripheral P2X3 enhanced the effect of TRPV1 on spontaneous pain, while the inhibition of peripheral TRPV1 reduced the induction of spontaneous pain by P2X3 in the CFA model. TRPV1 and P2X3 had inhibitory effects on each other in the inflammatory pain model. During neuropathic pain, P2X3 facilitated the function of TRPV1, while TRPV1 had an inhibitory effect on P2X3. These results suggest that the mutual effects of P2X3 and TRPV1 differ in cases of inflammatory and neuropathic pain in rats.
Keywords: Transient receptor potential vanilloid 1, purinergic receptor P2X ligand-gated ion channel 3, complete Freund's adjuvant, spared nerve injury, correlation effect
Introduction
Pain is a subjective and complex psychological process that involves sensation, emotion and cognitive impairment and has complex underlying mechanisms.1 Conventional drugs can alleviate pain well, but due to many inevitable side effects, their curative effect remains unsatisfactory. Many receptors and ion channels, such as transient receptor potential receptors (TRPs) and purinergic receptors (P2Xs), play important roles in the generation and processes of pain.2,3 Among these receptors, P2X3 and TRPV1 have been confirmed to be closely related to pain.4–7
TRPV1, which is well known to be a capsaicin receptor, is easily activated by capsaicin, protons, heat, etc.8,9 Previous studies have suggested that TRPV1 can be activated in various ways, such as by increased phosphorylation and protein overexpression, to participate in the generation of pathological pain.10–13 P2X3, which has been reported to be a subtype of the P2X family, plays an important role in the process of pathological pain and has been confirmed to be co-expressed with TRPV1 in small- to medium-diameter primary sensory neurons.14–16 A preclinical study found one-way cross-desensitization between P2X purinoceptors and vanilloid receptors in adult rat dorsal root ganglion (DRG) neurons, which indicates that a physiological relationship may exist between P2X3 and TRPV1.17 Another study revealed a physiological inhibitory effect between TRPV1 and P2X3. The mechanism of the interaction between P2X3 and TRPV1 may be related to the promoter of the P2X3 carboxyl terminus.18 However, there have been few reports concerning the interaction between TRPV1 and P2X3 in the pathological state, particularly during the induction and development of pain. The purpose of this study was to investigate the possible correlation effect between P2X3 and TRPV1 in different types of pathological pain and to identify the critical ion channel and its relationship with pain formation.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats (160-180 g) (Shanghai Laboratory Animal Centre, Chinese Academy of Sciences (animal certificate no. SCXK (沪) 2013-0016)) raised by the Laboratory Animal Centre of Zhejiang (SYXK (浙)2013–0184) were used. The rats were freely fed standard pellets and water (provided by the experimental animal centre) and housed on a 12-h light/dark cycle at 6 animals per cage in a temperature- and humidity-controlled environment (25 ± 2°C, 55% ± 5%). All experimental procedures were approved by the Animal Ethics Committee of Zhejiang Chinese Medical University (ZSLL-2015-022).
Pain models
Complete Freund's adjuvant (CFA) model establishment
Inflammatory pain was produced by the injection of 0.1 ml of CFA (Sigma, USA) into the right plantar surface of the hind paw of each rat.
Spared nerve injury (SNI) model establishment19
SD rats were fasted for one day before the operation. Under 2% isoflurane anaesthesia, the right hind limb of each rat was shaved and disinfected. The skin was incised 1 cm above the middle point between the greater trochanter of the femur and the tibia head, and the three terminal branches of the sciatic nerve were exposed. The common peroneal and tibial nerves were ligated and cut, and the sural nerve was sutured. Penicillin (160,000 units) was administered for three days after the operation.
Experimental design
The animals were divided into two groups, the inflammatory pain model (CFA) and the neuropathic pain model (SNI), and each group was subjected to two experiments (in vivo and in vitro). Each experiment investigated two issues: (1) the influence of P2X3 on the function of TRPV1 and (2) the influence of TRPV1 on the function of P2X3. The in vivo study explored the correlation between P2X3 and TRPV1 in three parts. In part I, the rats were randomly divided into the SNI/CFA+ vehicle group, low-dose agonist group, medium-dose agonist group and high-dose agonist group to identify the appropriate agonist concentration for subsequent experiments. In part II, the rats were randomly divided into the agonist control group, low-dose antagonist group and high-dose antagonist group to identify the appropriate antagonist concentration for subsequent experiments. In part III, the rats were randomly divided into the basic agonist group, agonist + basic agonist group, and antagonist + basic agonist group. In vitro, we used whole-cell patch-clamp recordings to further determine the correlation between P2X3 and TRPV1 in different models.
Drug administration
The chemicals used in this study were capsaicin (TRPV1 agonist, St. Louis, USA), capsazepine (TRPV1 antagonist, Tocris, UK), α,β-methylene adenosine 5′-triphosphate lithium salt (α,β-meATP, P2X3 agonist, St. Louis, USA) and 2′,3′-O-(2,4,6-trinitrophenyl)adenosine-5′-triphosphate tetra(triethylammonium) salt (TNP-ATP, P2X3 antagonist, Tocris, UK). Capsaicin was dissolved in 100% dimethylsulfoxide (DMSO) and further diluted in saline containing 2% Tween 80 at low, medium and high concentrations of 3, 7.5 and 30 μg, respectively. Capsazepine was dissolved in 100% DMSO and further diluted in saline containing 5% Tween 80 at concentrations of 113 μg and 400 μg. α,β-meATP was dissolved in saline and further diluted in saline to concentrations of 10 nmol, 50 nmol, and 250 nmol. TNP-ATP (TNP) was dissolved and diluted in saline to final concentrations of 50 nmol and 250 nmol. All these drugs were subcutaneously injected at a volume of 25 μl into the dorsum of the right foot of CFA or SNI rats once 3 days after modelling.
Spontaneous pain behaviours
The rats were placed in a transparent observation box (30 cm x 40 cm x 40 cm) for 30 min for acclimatization and then placed into boxes and administered different concentrations of drugs once 3 days after modelling. The number of flinches and the licking times in 2-min intervals at 2, 4, 6, 8, 10, 12, 14, 16, 18, and 20 min were recorded by a digital video camera for statistical analysis.
Co-immunoprecipitation
Co-immunoprecipitation was performed using a previously described method.20 L4-6 DRGs from SD rats were harvested immediately after the behavioural test and lysed in RIPA buffer (Beyotime) for phacofragmentation and centrifugation to obtain the supernatant. Rabbit anti-TRPV1 (1:1000 in 5% normal donkey serum, Abcam, USA) or mouse anti-P2X3 (1:1000 in 5% normal donkey serum, Santa Cruz, USA) was added to the tissue lysates and incubated at 4°C overnight, and partial total protein lysates were used as the input. The other compound was incubated with resuspended protein A/G plus-agarose (Santa Cruz, USA) at 4°C for 4 hours and washed with phosphate-buffered saline (PBS). The agarose bead antigen-antibody complex was resuspended in loading buffer, and the boiled protein samples were then separated by 8% SDS-PAGE and transferred to polyvinyl difluoride (PVDF) membranes (Bio-Rad, USA). After incubation with an anti-TRPV1 (1:1000 in 5% normal donkey serum, Abcam, USA) or anti-P2X3 (1:1000 in 5% normal donkey serum, Abcam, USA) primary antibody, the membrane was washed and incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:10000, Santa Cruz, USA) or HRP-conjugated goat anti-mouse IgG H&L (1:1000, Abcam, USA) as the secondary antibody. An ECL kit (Pierce, USA) was used to visualize the band density, and the signals were captured with an Image Quant LAS 4000 system (EG, USA). The density of each band was measured using ImageQuant TL 7.0 analysis software (GE, USA).
Immunofluorescence imaging
Immunofluorescence imaging was performed as previously described.21 After the behaviour test on day 3, the rats were anaesthetized by an intraperitoneal injection of 10% chloral hydrate (0.35 ml/100 g) and sacrificed. The animals were perfused with 4% paraformaldehyde in 0.1 M PBS. The L4-6 DRGs were quickly removed, placed in 4% paraformaldehyde and fixed for 6 h. Dehydration was performed in 15% and 30% sucrose solutions for 24 hours. The collected L4-6 DRGs were then embedded in OCT (SAKURA, USA). After complete immobilization, the tissue was sliced at a thickness of 12 μm. The samples were attached to positively charged slides, blocked with 5% donkey serum in TBST (0.1% Tween-20) and incubated at 37°C for 1 h. A rabbit anti-TRPV1-antibody (1:1000 in 5% normal donkey serum, Abcam, USA) and a guinea pig anti-P2X3-antibody (1:400, in 5% normal donkey serum, Abcam, USA) were used as the primary antibodies, and FITC-conjugated donkey anti-rabbit (1:400, Jackson) and Alexa Fluor 647-conjugated donkey anti-guinea pig (1:800, Jackson) were used as the secondary antibodies. The number of cells co-labelled with P2X3 and TRPV1 in the L4-6 DRGs was observed by a Nikon A1R laser confocal microscope under a 10× objective lens. Five sections were selected from each rat. The positive cells were counted by NIS Elements AR software, and the mean values were obtained.
Whole-cell patch-clamp recording
Whole-cell patch-clamp recording was performed as previously described.22 After intraperitoneal anaesthesia, the L4-6 DRGs were quickly removed and immediately placed in ice-cold oxygenated fresh dissecting solution (130 NaCl, 5 KCl, 2 KH2PO4, 1.5 CaCl2, 6 MgSO4, 10 glucose, and 10 HEPES; pH=7.2, osmolarity =305 mOsm) before being immediately removed and placed in pre-cooled DMEM. Then, the DRGs were transferred to digestive solution, which contained collagenase and trypsin (collagenase: 1 mg/mL, trypsin: 0.5 mg/mL), digested for 25 min at 37°C and washed for single cell suspension. The cells were plated on acid-cleaned coverslips. For patch-clamp recording, the neurons were perfused at room temperature with a normal external solution (130 NaCl, 5 KCl, 2 KH2PO4, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose; pH=7.2, adjusted with NaOH, osmolarity =295–300 mOsm). Recording pipettes were pulled with a P1000 control instrument and typically had a resistance of 3∼5 MΩ when filled with a solution containing 140 potassium gluconate, 10 NaCl, 10 HEPES, 10 glucose, 5 BAPTA, and 1 CaCl2 (pH=7.25 adjusted with KOH, osmolarity=295 mOsm). Currents induced by TRPV1 and P2X3 were recorded under current-clamp conditions. The cells were clamped at -60 mV, and 10 μM α,β-meATP and 0.3 μl of capsaicin were administered. The obtained data were stored and analysed by Clampfit 9.2 software. The entire experiment was carried out in a shielding cage at a room temperature of 22–24°C.
Statistical analysis
SPSS 19.0 software was used for statistical analysis. All experimental data are presented as the mean±s.e.m. Student’s t-test was used to compare two independent samples, whereas analysis of variance (ANOVA) followed by Bonferroni or Dunnett’s T3 multiple comparison tests was used to compare three or more samples. Differences were deemed statistically significant at P <0.05.
Results
Correlative effect of P2X3 and TRPV1 in SNI rats in vivo
Different concentrations of agonists and antagonists of TRPV1 and P2X3 were used in SNI rats in this study. Based on flinching and licking time comparisons, 3 μg was selected as the final concentration of the TRPV1 agonist capsaicin, and 400 μg was selected as the final concentration of the TRPV1 antagonist capsazepine for subsequent experiments on SNI rats. In addition, 50 nmol was chosen as the final concentration of the P2X3 agonist α,β-meATP, and 250 nmol was chosen as the final concentration of the P2X3 antagonist TNP-ATP for subsequent experiments on SNI rats (SFig1,2).
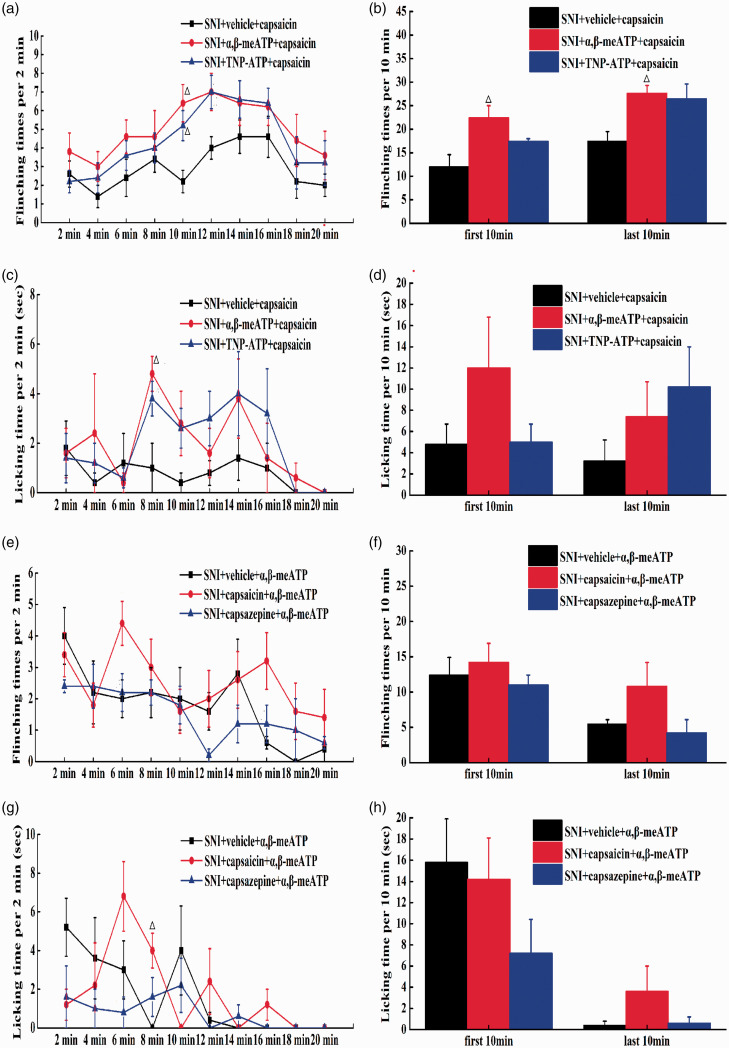
To observe whether P2X3 in the DRG can regulate the function of TRPV1 in vivo, α,β-meATP and TNP-ATP were intraplantarly injected into rats to regulate the spontaneous pain behaviours induced by capsaicin injection. Compared with vehicle, both α,β-meATP and TNP-ATP increased the flinching times and the licking time induced by capsaicin in SNI rats at 10 min after the injection (P<0.05) and the licking time at 8 min after the injection (P<0.05) (Figure 1(a) and (c)). Compared with vehicle, only α,β-meATP increased the flinching times induced by capsaicin in SNI rats during the first 10 min and the last 10 min. However, TNP-ATP had no effect on the spontaneous pain behaviours produced by TRPV1 in licking time (Figure 1(b) and (d)). These results indicated that activation or inhibition of peripheral P2X3 receptors can enhance the algogenic effect of TRPV1 in SNI rats.
Figure 1.
The correlation effect between P2X3 and TRPV1 of SNI rats in vivo. (a) After TRPV1 is activated, the flinching times (2 min intervals) of SNI rats with P2X3 agoinst/P2X3 antagonist. (b) After TRPV1 is activated, the flinching times (10 min intervals) of SNI rats with P2X3 agoinst/P2X3 antagonist. (c) After TRPV1 is activated, the licking time (2 min intervals) of SNI rats with P2X3 agoinst/P2X3 antagonist. (d) After TRPV1 is activated, the licking time (10 min intervals) of SNI rats with P2X3 agoinst/P2X3 antagonist. Compared with SNI+vehicle+capsaicin group, △P<0.05, △△P<0.01, compared with SNI+α,β-meATP+capsaicin group, #P<0.05, ##P<0.01, (SNI+vehicle+capsaicin group: n=5, SNI+α,β-meATP+capsaicin group: n=5, SNI+TNP-ATP+capsaicin group: n=5). (e) After P2X3 was activated, the flinching times (2 min intervals) of SNI rats with TRPV1 agoinst/TRPV1 antagonist. (f) After P2X3 was activated, the flinching times (10 min intervals) of SNI rats with TRPV1 agoinst/TRPV1 antagonist. (g) After P2X3 was activated, the licking time (2 min intervals) of SNI rats with of SNI rats with TRPV1 agoinst/TRPV1 antagonist. (h) After P2X3 was activated, the licking time (10 min intervals) of SNI rats with of SNI rats with TRPV1 agoinst/TRPV1 antagonist. Compared with SNI+vehicle+α,β-meATP group, △P<0.05, △△P<0.01, compared with SNI+ capsaicin +α,β-meATP group, #P<0.05, ##P<0.01, (SNI+vehicle+α,β-meATP group: n=5, SNI+capsaicin+α,β-meATP group: n=5, SNI+capsazepine +α,β-meATP group: n=5)
To determine whether TRPV1 could regulate the function of P2X3 in vivo, capsaicin or capsazepine was intraplantarly injected to impact the spontaneous pain behaviour induced by α,β-meATP injection. Compared with vehicle, capsaicin increased the licking time exhibited by SNI rats at 8 min after the injection (P<0.05). However, capsazepine had no effect on the spontaneous pain behaviours produced by P2X3 (P>0.05) (Figure 1(e) and (g)). During the first and the last 10 min, both capsaicin and capsazepine had no effect on the spontaneous pain behaviours produced by P2X3 (Figure 1(f) and (h)). All the above results indicated that the activation of TRPV1 can promote the algogenic effect of P2X3 in SNI rats and that the inhibition of TRPV1 may have no effect on the algogenic effect of P2X3.
Correlative effect of P2X3 and TRPV1 in the DRGs of CFA rats in vivo
Different concentrations of agonists and antagonists of TRPV1 and P2X3 were used in CFA rats in this study. Based on the flinching and licking time comparisons, 30 μg was selected as the final concentration of the TRPV1 agonist capsaicin, and 113 μg was selected as the final concentration of the TRPV1 antagonist capsazepine for subsequent experiments on CFA rats. In addition, 250 nmol was chosen as the final concentration of the P2X3 agonist α,β-meATP, and 250 nmol was chosen as the final concentration of the P2X3 antagonist TNP-ATP for subsequent experiments on CFA rats (SFigs 3, 4).
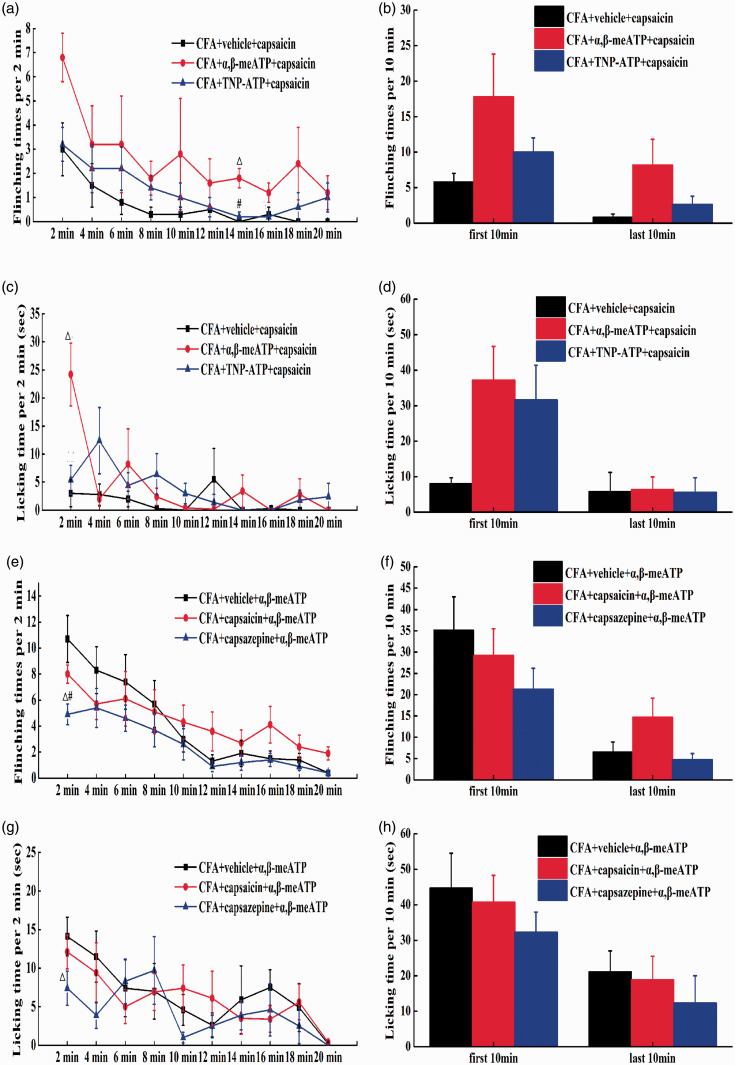
To further determine whether P2X3 could regulate the function of TRPV1 in vivo, α,β-meATP and TNP-ATP were intraplantarly injected to regulate the spontaneous pain behaviours induced by capsaicin injection. Compared with the vehicle, α,β-meATP increased the flinching times induced by capsaicin in CFA rats at 14 min after injection (P<0.05) and the licking time at 2 min after the injection (P<0.01); TNP-ATP had no effect on the spontaneous pain behaviours produced by TRPV1 (P>0.05) (Figure 2(a) and (c)). TNP-ATP and α,β-meATP had no effect on the spontaneous pain behaviours produced by TRPV1 in flinching times and licking time (Figure 2(b) and (d)).These results indicated that the activation of peripheral P2X3 receptors can enhance the algogenic effect of TRPV1 in CFA rats, while the inhibition of peripheral P2X3 receptors has no effect on the algogenic effect of TRPV1.
Figure 2.
The correlation effect between P2X3 and TRPV1 of CFA rats in vivo. (a) After TRPV1 was activated, the flinching times (2 min intervals) of CFA rats with P2X3 agoinst/P2X3 antagonist. (b) After TRPV1 was activated, the flinching times (10 min intervals) of CFA rats with P2X3 agoinst/P2X3 antagonist. (c) After TRPV1 was activated, the licking time (2 min intervals) of CFA rats with P2X3 agoinst/P2X3 antagonist. (d) After TRPV1 was activated, the licking time (10 min intervals) of CFA rats with P2X3 agoinst/P2X3 antagonist. Compared with CFA+vehicle+capsaicin group, △P<0.05, △△P<0.01, compared with CFA+α,β-meATP+capsaicin group, #P<0.05, ##P<0.01, (CFA+vehicle+capsaicin group: n=4, CFA+α,β-meATP+capsaicin group: n=5, CFA+TNP-ATP+capsaicin group: n=5). (e) After P2X3 was activated, the flinching times (2 min intervals) of CFA rats with TRPV1 agoinst/TRPV1 antagonist. (f) After P2X3 was activated, the flinching times (10 min intervals) of CFA rats with TRPV1 agoinst/TRPV1 antagonist. (g) After P2X3 was activated, the licking time (2 min intervals) of CFA rats with TRPV1 agoinst/TRPV1 antagonist. (h) After P2X3 was activated, the licking time (10 min intervals) of CFA rats with TRPV1 agoinst/TRPV1 antagonist. Compared with CFA+vehicle+α,β-meATP group,△P<0.05, △△P<0.01, compared with CFA+capsazepine+α,β-meATP group, #P<0.05, ##P<0.01, (CFA+vehicle+α,β-meATP group: n=5, CFA+capsaicin+α,β-meATP group: n=5, CFA+ capsazepine +α,β-meATP group: n=6).
To determine whether TRPV1 could regulate the function of P2X3 in vivo, capsaicin or capsazepine was intraplantarly injected to observe the regulation of spontaneous pain behaviour induced by α,β-meATP injection. Compared with the vehicle, capsazepine decreased both the flinching and licking times exhibited by CFA rats at 2 min after the injection (P<0.05). However, capsaicin failed to have an effect on the spontaneous pain behaviours produced by P2X3 (P>0.05) (Figure 2(e) and (g)). During the first and the last 10 min, both capsaicin and capsazepine had no effect on the spontaneous pain behaviours produced by P2X3 (Figure 2(f) and (h)). All the above results indicated that the inhibition of peripheral TRPV1 can relieve the algogenic effect of P2X3 on spontaneous pain in CFA rats and that the activation of TRPV1 may not impact the algogenic effect of P2X3.
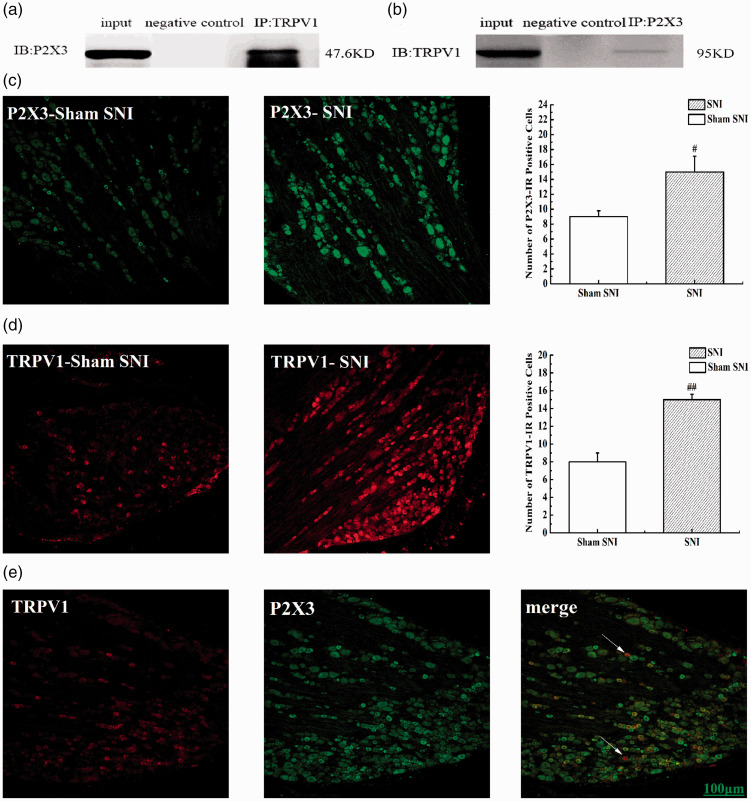
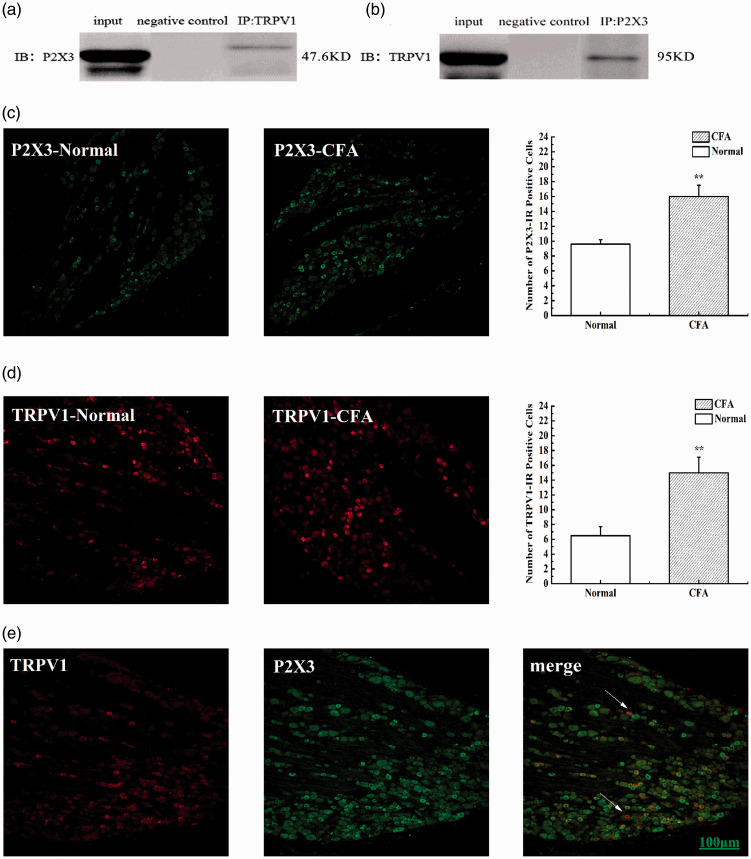
Co-expression and co-immunoprecipitation of TRPV1 and P2X3 in SNI rats
Co-immunoprecipitation was used to observe any interaction between P2X3 and TRPV1 in the DRGs of SNI rats. Immunoblotting with an anti-TRPV1 antibody revealed a strong P2X3-immunoreactive band (Figure 3(a)), and immunoblotting with an anti-P2X3 antibody revealed a strong TRPV1-immunoreactive band (Figure 3(b)), which suggested a correlation between P2X3 and TRPV1 in SNI rats. We also used immunofluorescence imaging to detect neurons that were positively stained for P2X3 and TRPV1, and compared with that in the sham SNI group, the number of P2X3-IR-positive and TRPV1-IR-positive neurons was increased in the SNI group (P<0.05) (Figure 3(c) and (d)), and P2X3 and TRPV1 were widely co-expressed in small- and medium-diameter DRG neurons (Figure 3(e)).
Figure 3.
TRPV1 is physically associated with P2X3 in SNI rats. (a and b) Representative co-immunoprecipitation bands between P2X3 and TRPV1 in L4-6 DRG of SNI rats. Negative control: IgG control. (c) The number of P2X3-IR positive cells in L4-6 DRG of SNI rats. (d) The number of TRPV1-IR positive cells in L4-6 DRG of SNI rats. (e) Immunofluorescence confocal micrographs of P2X3, TRPV1 and merge in L4–6 DRG of SNI rats. Sections show immunohistochemical red labeling for TRPV1 positive neurons, green labeling for P2X3 positive neurons. Scale bars=100μm.
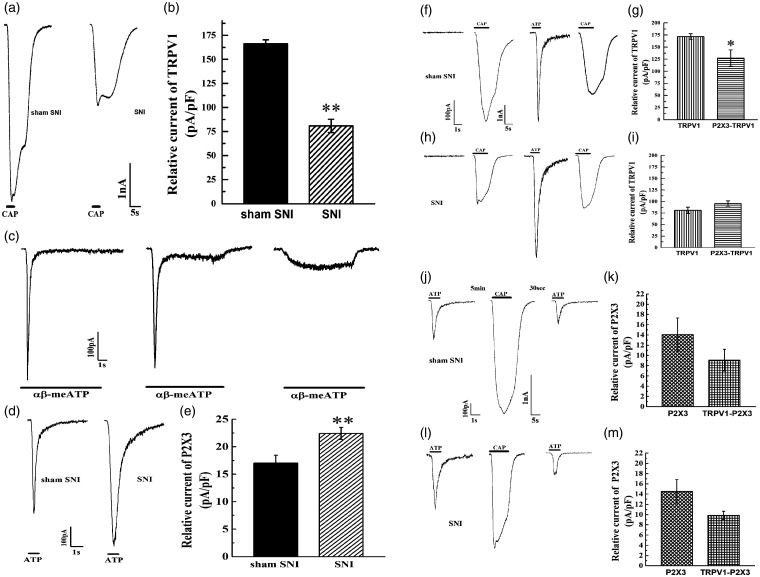
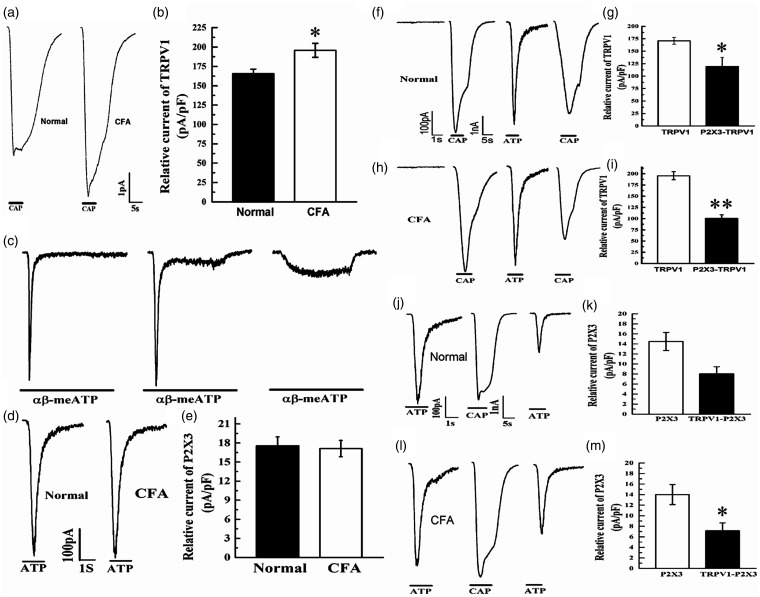
Correlative effect of TRPV1 and P2X3 in neurons from SNI rats in vitro
Whole-cell patch-clamp recordings were used to observe the interaction between P2X3 and TRPV1 in the DRGs of SNI rats. Compared with that of sham SNI rats, the inward TRPV1 current of SNI rats was decreased (Figure 4(a) and (b)) (P<0.01). Because P2X3 and P2X2 are usually expressed in the same neurons and because both are likely to be activated by α,β-meATP, DRG neurons with specific P2X3 receptor expression were selected based on the waveform of inward current induced by the agonist. In the initial study, we found that P2X3 activated by α,β-meATP produced a fast inward current, P2X2 produced a slow inward current, and heterogeneous P2X3/P2X2 receptors produced a combined inward current (Figure 4(c)), which was consistent with a previous study.23 In this study, we examined fast inward currents. Compared with that in the sham SNI group, the inward current of P2X3 in the SNI group was significantly increased (P<0.01) (Figure 4(d) and (e)). Then, the effect of P2X3 on TRPV1 was investigated, revealing that the inward TRPV1 current was decreased by pre-treatment with α,β-meATP in the sham SNI group (P<0.05) (Figure 4(f) and (g)), which indicated that P2X3 inhibited the effect of TRPV1 in DRGs in the physiological state. However, in the SNI group, there was no difference in the inward TRPV1 current after modelling or in the absence of α,β-meATP (P>0.05) (Figure 4(h) and (i)). Finally, the effect of TRPV1 on P2X3 was detected. The administration of capsaicin did not affect the inward P2X3 current induced by α,β-meATP in either the sham SNI group or the SNI group (P>0.05) (Figure 4(j) to (m)). These results indicated that in the physiological state, peripheral TRPV1 may have no direct effect on P2X3 but it may inhibit pain induced by P2X3 under pathological conditions.
Figure 4.
The correlation effect of TRPV1 and P2X3 of SNI rats in vitro. (a and b) Relative current of TRPV1 in Sham SNI group and SNI group. (c) Screening of P2X3 specific DRGs. (d and e) Relative current of P2X3 in Sham SNI group and SNI group. (f to i) The effects of P2X3 on TRPV1 in Sham SNI group and SNI group. (j to m) The effects of TRPV1 on P2X3 in Sham SNI group and SNI group. Compared with Sham SNI group, *P<0.05, **P<0.01.
Co-expression and co-immunoprecipitation of TRPV1 and P2X3 in CFA rats
Co-immunoprecipitation was used to observe interactions between P2X3 and TRPV1 in CFA rats. Immunoblotting with an anti-TRPV1 antibody revealed a P2X3-immunoreactive band, and immunoblotting with an anti-P2X3 antibody revealed a TRPV1-immunoreactive band, suggesting a correlation between TRPV1 and P2X3 in the DRGs of CFA rats (Figure 5(a) and (b)). We also used immunofluorescence imaging to detect P2X3- and TRPV1-positive neurons; compared with that in the normal group, the number of P2X3-IR-positive and TRPV1-IR-positive neurons was increased in the CFA group (P<0.01) (Figure 5(c) and (d)), and P2X3 and TRPV1 were widely co-expressed in small- and medium-diameter DRG neurons (Figure 5(e)).
Figure 5.
TRPV1 is physically associated with P2X3 in CFA rats. (a and b) Representative co-immunoprecipitation bands between P2X3 and TRPV1 in L4-6 DRG of CFA rats. Negative control: IgG control. (c) The number of P2X3-IR positive cells in L4-6 DRG of CFA rats. (d) The number of TRPV1-IR positive cells in L4-6 DRG of CFA rats. (e) Immunofluorescence confocal micrographs of P2X3, TRPV1 and merge in L4–6 DRG of CFA rats. Sections show immunohistochemical red labeling for TRPV1 positive neurons, green labeling for P2X3 positive neurons. Scale bars=100μm.
Correlative effect of TRPV1 and P2X3 in CFA rats in vitro
Whole-cell patch-clamp recordings were used to detect the interaction between P2X3 and TRPV1 in CFA rats. Compared with that in the normal group, the inward TRPV1 current was increased in the CFA group (P>0.05) (Figure 6(a) and (b)). DRG neurons with specific P2X3 receptor expression were selected as described above (Figure 6(c)). The inward P2X3 current in the CFA group did not differ from that in the normal group (P>0.05) (Figure 6(d) and (e)). After TRPV1 was activated by capsaicin, the α,β-meATP-induced inward current was decreased in both the normal group and the CFA group (P<0.05) (Figure 6(f) to (i)), which indicated that P2X3 inhibited the effect of TRPV1 under both physiological and pathological conditions. Next, the effect of TRPV1 on P2X3 was investigated. After capsaicin stimulation, the inward P2X3 current was not altered in the normal group (P>0.05) but was decreased in the CFA group (P<0.05) (Figure 6(j) to (m)). These results indicated that at the physiological level, peripheral TRPV1 had no direct effect on P2X3, but TRPV1 inhibited the effect of P2X3 in the CFA model.
Figure 6.
The correlation effect of TRPV1 and P2X3 of CFA rats in vitro. (a and b) Relative current of TRPV1 in normal group and CFA group. (c) Screening of P2X3 specific DRGs. (d and e) Relative current of P2X3 in normal group and CFA group. (f to i) The effects of P2X3 on TRPV1 in normal group and CFA group. (j to m) The effects of TRPV1 on P2X3 in normal group and CFA group. Compared with Normal group, *P<0.05, **P<0.01.
Discussion
Studies have shown that the activation of the TRPV1 receptor leads to a large influx of Ca2+, which further promotes the release of inflammatory mediators in peripheral sensory terminals and, in turn, promotes pain sensitivity.24–26 In our study, we found that the number of TRPV1-positive neurons increased and the response of TRPV1 channels to capsaicin was significantly reduced after SNI modelling (Figures 3 and 4), which indicated that neuropathic pain did not increase the sensitivity of TRPV1 to capsaicin in the short term or reduce the sensitivity of TRPV1. These results confirmed that TRPV1 might mediate pain sensitization in rats through other mechanisms of activation, such as increased protein, phosphorylation and mRNA levels, in cases of neuropathic pain.11,27–29 However, in the CFA model, the number of TRPV1-positive neurons increased, and the response of TRPV1 channels to capsaicin increased (Figures 5 and 6), which indicated that TRPV1 might mediate pain sensation during inflammatory pain.
Many studies have confirmed that P2X3 is involved in inflammatory and neuropathological pain. Our previous study30 showed that in CFA-induced inflammatory pain, both P2X3 protein expression and the number of P2X3-positive neurons were increased. Studies have also confirmed that P2X3 mRNA and protein expression levels are increased in the DRG neurons of rats of different neuropathological pain models.31–33 In our present study, the number of P2X3-positive neurons was increased in the CFA model, which was consistent with our previous study, and the response of P2X3 channels to ATP was not increased in the CFA-induced inflammatory pain model but was increased in the SNI-induced neuropathological pain model. These results indicated that P2X3 might mediate pain sensitization in rats through other mechanisms of activation, such as increased positive cell numbers, in cases of inflammatory pain.
In addition, we found that P2X3 and TRPV1 were widely co-expressed in small- and medium-diameter DRG neurons (Figures 3 and 5), which was consistent with our previous study,34 and determined that a correlation existed between TRPV1 and P2X3 via co-immunoprecipitation. Our laboratory already reported an interaction between TRPV1 and P2X3 at the peripheral neuron level in naïve rats. The P2X3 agonist did not alleviate pain behaviour induced by the TRPV1 agonist, but the P2X3 inhibitor did. The TRPV1 agonist increased pain behaviour induced by the P2X3 agonist, and the TRPV1 inhibitor did not reduce pain behaviour induced by the P2X3 agonist.34 Stanchev et al.18 found that TRPV1 and P2X3 interacted with each other in an inhibitory manner in vitro via whole-cell patch-clamp recording. However, there have been few studies on the relationship between TRPV1 and P2X3 under pathological conditions. Previous studies have found that P2X3 interacts differently with TRPV1 under different pathological conditions. Fang et al.35 found that the P2X3 protein expression in the DRGs of CFA rats was significantly increased in TRPV1 knockout mice compared with wild-type mice, which indicates that TRPV1 might mediate peripheral pain sensitivity by antagonizing P2X3 receptor expression during inflammatory pain. Kiyatkin et al.36 found that the combination of TRPV1 and P2X3 antagonists reduced peripheral mechanical pain sensitivity in the colon and rectum to a greater extent than one antagonist alone, which suggests a synergistic effect of TRPV1 and P2X3. Saloman et al.37 found that in P2X3/TRPV1-positive cells, the transient Ca2+ current induced by capsaicin increased significantly after P2X3 receptors were activated and that the activation of P2X3 receptors could induce the serine phosphorylation of TRPV1 in trigeminal ganglia cultured in vitro, which confirms that P2X3 and TRPV1 interact in a facilitated manner in the peripheral sensitization mechanism in masseter pain and that multiple protein kinases may be involved.
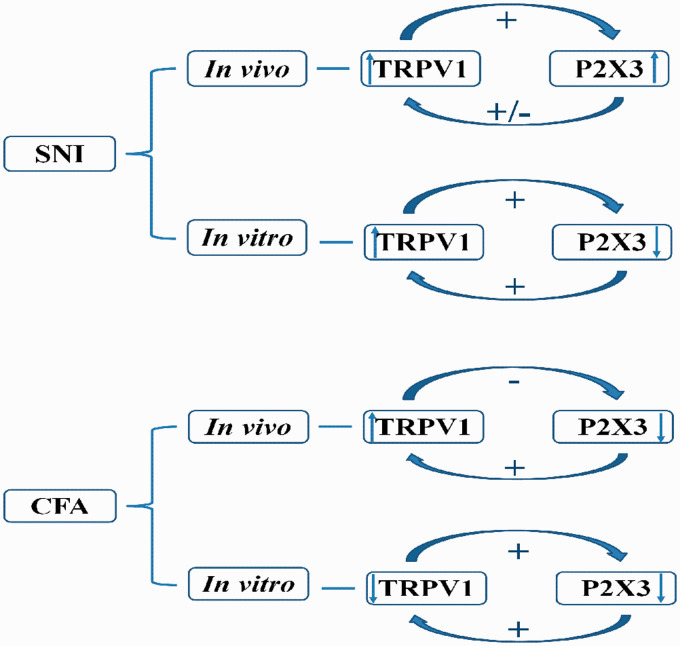
In the present study, we studied the correlative effect of P2X3 and TRPV1 in the DRGs of SNI and CFA models in vivo and in vitro (Figure 7). In vivo, the activation of TRPV1 promoted the algogenic effect of P2X3, but the effect of TRPV1 was not completely dependent on P2X3 in the SNI model. In the CFA model, the activation of peripheral P2X3 receptors enhanced the algogenic effect of TRPV1, and the inhibition of peripheral TRPV1 alleviated the algogenic effect of P2X3 during spontaneous pain. All the above results indicated that the interaction between TRPV1 and P2X3 has different effects in vivo under different pathological conditions.
Figure 7.
Schematic diagram of the interaction between P2X3 and TRPV1 in the dorsal root ganglia of adult rats with different pathological pains in vivo and in vitro.
Whole-cell patch-clamp recordings were used to reveal the correlation between P2X3 and TRPV1 in vitro (Figures 4 and 6). Our results showed that P2X3 may promote the effect of TRPV1 in SNI rats, which is consistent with Saloman et al.’s finding,37 and TRPV1 may antagonize the effect of P2X3. In CFA rats, we found a mutual inhibitory effect of P2X3 and TRPV1 on inflammatory pain. These results differ from our behavioural findings in vivo. This discrepancy may be attributed to a number of factors. α,β-meATP had been confirmed to be an agonist of P2X2/3 and P2X3.38 In vivo, α,β-meATP may also increase the sensitivity of P2X2/3, a pain receptor, which may also play a role in the sensitization of TRPV1. Studies have confirmed that the activation of TRPV1 and P2X3 receptors during inflammatory and neuropathic pain is related to the release of CGRP, PKC and other substances.39–42 While these neurotransmitters increase the sensitivity of TRPV1, additional transmitters can also simultaneously enhance the role of P2X3 receptors. However, although these transmitter signal sources are retained in vivo, they are reduced when cultured in vitro, which potentially attributes to the correlative effect of P2X3 and TRPV1 in vivo and in vitro. Moreover, TRPV1 and P2X3 are known to exhibit different functional expression patterns, such as those of protein expression, phosphorylation, membrane translocation and channel opening, during the occurrence and maintenance of pain.13,42,43 The level of TRPV1 serine phosphorylation in trigeminal ganglia increases significantly after P2X3 activation, suggesting that the interaction between TRPV1 and P2X3 receptors may be related to protein phosphorylation.37
However, our research had limitation: in vivo administration, the correlation of P2X3, TRPV1 and other substances could not be excluded. So, the results were not completely consistent with those in vitro. Therefore, the difference in the relationship in vivo and in vitro may be related to the pathological bases of different types of pain, different factors that affect transmitters, different pathological locations, different active expression forms and the pathological mechanisms of TRPV1 and P2X3 in rats under different pathological conditions. The experiments in vitro may be more straightforward to illustrate the interaction between P2X3 and TRPV1 in different pathological pains, but the specific mechanism needs further exploration.
In conclusion, we found that in vivo, TRPV1 could promote the induction of spontaneous pain caused by P2X3, but the induction of spontaneous pain behaviour by TRPV1 was not completely dependent on P2X3 in the SNI model. In the CFA model, the activation of peripheral P2X3 enhanced the effect of TRPV1 on spontaneous pain, while the inhibition of peripheral TRPV1 reduced the induction of spontaneous pain by P2X3. However, in vitro, P2X3 facilitated the function of TRPV1, while TRPV1 had an inhibitory effect on P2X3 in the SNI model, and TRPV1 and P2X3 had inhibitory effects on each other in the CFA model.
Supplemental Material
Supplemental material, sj-pdf-1-mpx-10.1177_17448069211011315 for The interaction between P2X3 and TRPV1 in the dorsal root ganglia of adult rats with different pathological pains by Jie Yu Junying Du, Junfan Fang, Yingjun Liu, Xuaner Xiang, Yi Liang, Xiaomei Shao and Jianqiao Fang in Molecular Pain
Supplemental material, sj-pdf-2-mpx-10.1177_17448069211011315 for The interaction between P2X3 and TRPV1 in the dorsal root ganglia of adult rats with different pathological pains by Jie Yu Junying Du, Junfan Fang, Yingjun Liu, Xuaner Xiang, Yi Liang, Xiaomei Shao and Jianqiao Fang in Molecular Pain
Supplemental material, sj-pdf-3-mpx-10.1177_17448069211011315 for The interaction between P2X3 and TRPV1 in the dorsal root ganglia of adult rats with different pathological pains by Jie Yu Junying Du, Junfan Fang, Yingjun Liu, Xuaner Xiang, Yi Liang, Xiaomei Shao and Jianqiao Fang in Molecular Pain
Supplemental material, sj-pdf-4-mpx-10.1177_17448069211011315 for The interaction between P2X3 and TRPV1 in the dorsal root ganglia of adult rats with different pathological pains by Jie Yu Junying Du, Junfan Fang, Yingjun Liu, Xuaner Xiang, Yi Liang, Xiaomei Shao and Jianqiao Fang in Molecular Pain
Footnotes
Author Contributions: Jie Yu: performed the animal experiment, analysed the data and wrote the original draft; Junying Du: performed the co-immunoprecipitation and immunofluorescence imaging experiments and wrote the original draft; Junfan Fang: performed the whole-cell patch-clamp recordings, analysed the data and reviewed and edited the manuscript; Yingjun Liu: performed the animal experiment; Xuaner Xiang: performed the animal experiment; Yi Liang: reviewed and edited the manuscript; Xiaomei Shao: reviewed and edited the manuscript; and Jianqiao Fang: reviewed and edited the manuscript and provided supervision.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81473772, 81603690, 81603692), the Zhejiang Provincial Natural Science Fund of China (LY19H270003), and the Talent Project of Zhejiang Association for Science and Technology (2017YCGC004).
ORCID iD: Jie Yu https://orcid.org/0000-0002-9225-1901
Supplemental material: Supplementary material for this article is available online.
References
- 1.Tiemann L, Heitmann H, Schulz E, Baumkotter J, Ploner M. Dopamine precursor depletion influences pain affect rather than pain sensation. PLoS One 2014; 9: e96167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill RZ, Morita T, Brem RB, Bautista DM. S1PR3 mediates itch and pain via distinct TRP channel-dependent pathways. J Neurosci 2018; 38: 7833–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lashermes A, Boudieu L, Barbier J, Sion B, Gelot A, Barnich N, Ardid D, Carvalho FA. Adherent-invasive E. coli enhances colonic hypersensitivity and P2X receptors expression during post-infectious period. Gut Microbes 2018; 9: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duo L, Hu L, Tian N, Cheng G, Wang H, Lin Z, Wang Y, Yang Y. TRPV1 gain-of-function mutation impairs pain and itch sensations in mice. Mol Pain 2018; 14: 1744806918762031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honda K, Shinoda M, Kondo M, Shimizu K, Yonemoto H, Otsuki K, Akasaka R, Furukawa A, Iwata K. Sensitization of TRPV1 and TRPA1 via peripheral mGluR5 signaling contributes to thermal and mechanical hypersensitivity. Pain 2017; 158: 1754–1764. [DOI] [PubMed] [Google Scholar]
- 6.Lu YF, Yang Y, Li CL, Wang Y, Li Z, Chen J. The locus coeruleus-norepinephrine system mediates empathy for pain through selective up-regulation of P2X3 receptor in dorsal root ganglia in rats. Front Neural Circuits 2017; 11: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teixeira JM, Bobinski F, Parada CA, Sluka KA, Tambeli CH. P2X3 and P2X2/3 receptors play a crucial role in articular hyperalgesia development through inflammatory mechanisms in the knee joint experimental synovitis. Mol Neurobiol 2017; 54: 6174–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000; 288: 306–313. [DOI] [PubMed] [Google Scholar]
- 9.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997; 389: 816–824. [DOI] [PubMed] [Google Scholar]
- 10.Jiang YL, Yin XH, Shen YF, He XF, Fang JQ. Low frequency electroacupuncture alleviated spinal nerve ligation induced mechanical allodynia by inhibiting TRPV1 upregulation in ipsilateral undamaged dorsal root ganglia in rats. Evid Based Complement Alternat Med 2013; 2013: 170910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Du J, Yang Y, Wang Y. Phosphorylation of TRPV1 by cyclin-dependent kinase 5 promotes TRPV1 surface localization, leading to inflammatory thermal hyperalgesia. Exp Neurol 2015; 273: 253–262. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Wang Y, Wang J, Fan Q, Zhu J, Yang L, Rong W. TLR4 mediates upregulation and sensitization of TRPV1 in primary afferent neurons in 2,4,6-trinitrobenzene sulfate-induced colitis. Mol Pain 2019; 15: 1744806919830018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao X, Zhao XT, Xu LC, Yue LP, Liu FY, Cai J, Liao FF, Kong JG, Xing GG, Yi M, Wan Y. Shp-1 dephosphorylates TRPV1 in dorsal root ganglion neurons and alleviates CFA-induced inflammatory pain in rats. Pain 2015; 156: 597–608. [DOI] [PubMed] [Google Scholar]
- 14.Kage K, Niforatos W, Zhu CZ, Lynch KJ, Honore P, Jarvis MF. Alteration of dorsal root ganglion P2X3 receptor expression and function following spinal nerve ligation in the rat. Exp Brain Res 2002; 147: 511–519. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa H, Hiura A. Capsaicin, transient receptor potential (TRP) protein subfamilies and the particular relationship between capsaicin receptors and small primary sensory neurons. Anat Sci Int 2006; 81: 135–155. [DOI] [PubMed] [Google Scholar]
- 16.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 1999; 11: 946–958. [DOI] [PubMed] [Google Scholar]
- 17.Piper AS, Docherty RJ. One-way cross-desensitization between P2X purinoceptors and vanilloid receptors in adult rat dorsal root ganglion neurones. J Physiol 2000; 3: 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanchev D, Blosa M, Milius D, Gerevich Z, Rubini P, Schmalzing G, Eschrich K, Schaefer M, Wirkner K, Illes P. Cross-inhibition between native and recombinant TRPV1 and P2X(3) receptors. Pain 2009; 143: 26–36. [DOI] [PubMed] [Google Scholar]
- 19.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000; 87: 149–158. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Bao W, Qiu M, Liao Y, Che Q, Yang T, He X, Qiu H, Wan X. Forkhead-box A1 suppresses the progression of endometrial cancer via crosstalk with estrogen receptor alpha. Oncol Rep 2014; 31: 1225–1234. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Li GW, Wang C, Gu Y, Huang LY. Mechanisms underlying enhanced P2X receptor-mediated responses in the neuropathic pain state. Pain 2005; 119: 38–48. [DOI] [PubMed] [Google Scholar]
- 22.Zhou YL, Jiang GQ, Wei J, Zhang HH, Chen W, Zhu H, Hu S, Jiang X, Xu GY. Enhanced binding capability of nuclear factor-kappaB with demethylated P2X3 receptor gene contributes to cancer pain in rats. Pain 2015; 156: 1892–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitahara S, Yamashita M, Ikemoto Y. Effects of pentobarbital on purinergic P2X receptors of rat dorsal root ganglion neurons. Can J Physiol Pharmacol 2003; 81: 1085–1091. [DOI] [PubMed] [Google Scholar]
- 24.Ambrosino P, Soldovieri MV, Di Zazzo E, Paventi G, Iannotti FA, Mosca I, Miceli F, Franco C, Canzoniero LMT, Taglialatela M. Activation of Kv7 potassium channels inhibits intracellular Ca(2+) increases triggered by TRPV1-mediated pain-inducing stimuli in f11 immortalized sensory neurons. Int J Mol Sci 2019; 20. DOI: 10.3390/ijms20184322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma W, Quirion R. Inflammatory mediators modulating the transient receptor potential vanilloid 1 receptor: therapeutic targets to treat inflammatory and neuropathic pain. Expert Opin Ther Targets 2007; 11: 307–320. [DOI] [PubMed] [Google Scholar]
- 26.Yuksel E, Naziroglu M, Sahin M, Cig B. Involvement of TRPM2 and TRPV1 channels on hyperalgesia, apoptosis and oxidative stress in rat fibromyalgia model: protective role of selenium. Sci Rep 2017; 7: 17543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui YY, Xu H, Wu HH, Qi J, Shi J, Li YQ. Spatio-temporal expression and functional involvement of transient receptor potential vanilloid 1 in diabetic mechanical allodynia in rats. PLoS One 2014; 9: e102052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirai T, Enomoto M, Kaburagi H, Sotome S, Yoshida-Tanaka K, Ukegawa M, Kuwahara H, Yamamoto M, Tajiri M, Miyata H, Hirai Y, Tominaga M, Shinomiya K, Mizusawa H, Okawa A, Yokota T. Intrathecal AAV serotype 9-mediated delivery of shRNA against TRPV1 attenuates thermal hyperalgesia in a mouse model of peripheral nerve injury. Mol Ther 2014; 22: 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinoda M, Asano M, Omagari D, Honda K, Hitomi S, Katagiri A, Iwata K. Nerve growth factor contribution via transient receptor potential vanilloid 1 to ectopic orofacial pain. J Neurosci 2011; 31: 7145–7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang JQ, Du JY, Fang JF, Xiao T, Le XQ, Pan NF, Yu J, Liu BY. Parameter-specific analgesic effects of electroacupuncture mediated by degree of regulation TRPV1 and P2X3 in inflammatory pain in rats. Life Sci 2018; 200: 69–80. [DOI] [PubMed] [Google Scholar]
- 31.Guo J, Wang C, Niu X, Zhou F, Li H, Gao W. Effects of resveratrol in the signaling of neuropathic pain involving P2X3 in the dorsal root ganglion of rats. Acta Neurol Belg 2019. DOI: 10.1007/s13760-019-01126-2 [DOI] [PubMed]
- 32.Li L, Sheng X, Zhao S, Zou L, Han X, Gong Y, Yuan H, Shi L, Guo L, Jia T, Liu S, Wu B, Yi Z, Liu H, Gao Y, Li G, Li G, Zhang C, Xu H, Liang S. Nanoparticle-encapsulated emodin decreases diabetic neuropathic pain probably via a mechanism involving P2X3 receptor in the dorsal root ganglia. Purinergic Signal 2017; 13: 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao J, Liu L, Fan Y, Wang M, Li L, Zou L, Yuan H, Shi L, Yang R, Liang S, Liu S. Role of hesperidin in P2X3 receptor-mediated neuropathic pain in the dorsal root ganglia. Int J Neurosci 2019; 129: 784–793. [DOI] [PubMed] [Google Scholar]
- 34.Du J, Fang J, Xiang X, Xu Z, Jianqiao F. Regulation mechanism of peripheral pain sensation in rats based on the interaction between TRPV1 and P2X3. Acta Laboratorium Animalis Scientia Sinica 2019; 27: 485–492. [Google Scholar]
- 35.Fang X, Shi X-H, Huang L-B, Rong W-F, Bei M. Upregulation of P2X3 receptors in dorsal root ganglion of TRPV1 knockout female mice. Acta Physiologica Sinica 2014; 4: 431–437. [PubMed] [Google Scholar]
- 36.Kiyatkin ME, Feng B, Schwartz ES, Gebhart GF. Combined genetic and pharmacological inhibition of TRPV1 and P2X3 attenuates colorectal hypersensitivity and afferent sensitization. Am J Physiol Gastrointest Liver Physiol 2013; 305: G638–G648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saloman JL, Chung MK, Ro JY. P2X(3) and TRPV1 functionally interact and mediate sensitization of trigeminal sensory neurons. Neuroscience 2013; 232: 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adachi K, Shimizu K, Hu JW, Suzuki I, Sakagami H, Koshikawa N, Sessle BJ, Shinoda M, Miyamoto M, Honda K, Iwata K. Purinergic receptors are involved in tooth-pulp evoked nocifensive behavior and brainstem neuronal activity. Mol Pain 2010; 6: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabbretti E, D'Arco M, Fabbro A, Simonetti M, Nistri A, Giniatullin R. Delayed upregulation of ATP P2X3 receptors of trigeminal sensory neurons by calcitonin gene-related peptide. J Neurosci 2006; 26: 6163–6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song C, Liu P, Zhao Q, Guo S, Wang G. TRPV1 channel contributes to remifentanil-induced postoperative hyperalgesia via regulation of NMDA receptor trafficking in dorsal root ganglion. J Pain Res 2019; 12: 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Joseph J, Ro JY, Chung MK. Modality-specific mechanisms of protein kinase C-induced hypersensitivity of TRPV1: S800 is a polymodal sensitization site. Pain 2015; 156: 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou YF, Ying XM, He XF, Shou SY, Wei JJ, Tai ZX, Shao XM, Liang Y, Fang F, Fang JQ, Jiang YL. Suppressing PKC-dependent membrane P2X3 receptor upregulation in dorsal root ganglia mediated electroacupuncture analgesia in rat painful diabetic neuropathy. Purinergic Signal 2018; 14: 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arribas-Blazquez M, Olivos-Ore LA, Barahona MV, Sanchez de la Muela M, Solar V, Jimenez E, Gualix J, McIntosh JM, Ferrer-Montiel A, Miras-Portugal MT, Artalejo AR. Overexpression of P2X3 and P2X7 receptors and TRPV1 channels in adrenomedullary chromaffin cells in a rat model of neuropathic pain. Int J Mol Sci 2019; 20. DOI: 10.3390/ijms20010155 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-mpx-10.1177_17448069211011315 for The interaction between P2X3 and TRPV1 in the dorsal root ganglia of adult rats with different pathological pains by Jie Yu Junying Du, Junfan Fang, Yingjun Liu, Xuaner Xiang, Yi Liang, Xiaomei Shao and Jianqiao Fang in Molecular Pain
Supplemental material, sj-pdf-2-mpx-10.1177_17448069211011315 for The interaction between P2X3 and TRPV1 in the dorsal root ganglia of adult rats with different pathological pains by Jie Yu Junying Du, Junfan Fang, Yingjun Liu, Xuaner Xiang, Yi Liang, Xiaomei Shao and Jianqiao Fang in Molecular Pain
Supplemental material, sj-pdf-3-mpx-10.1177_17448069211011315 for The interaction between P2X3 and TRPV1 in the dorsal root ganglia of adult rats with different pathological pains by Jie Yu Junying Du, Junfan Fang, Yingjun Liu, Xuaner Xiang, Yi Liang, Xiaomei Shao and Jianqiao Fang in Molecular Pain
Supplemental material, sj-pdf-4-mpx-10.1177_17448069211011315 for The interaction between P2X3 and TRPV1 in the dorsal root ganglia of adult rats with different pathological pains by Jie Yu Junying Du, Junfan Fang, Yingjun Liu, Xuaner Xiang, Yi Liang, Xiaomei Shao and Jianqiao Fang in Molecular Pain