Abstract
Objective
To detect the expression of FK506-binding protein 5 (FKBP5) in human papillary thyroid carcinoma (PTC) tissues, and explore its possible role in the progression of PTC.
Methods
FKBP5 expression levels were assessed in 115 PTC tissues and corresponding normal tissues by immunohistochemistry. We also examined the correlations between FKBP5 expression and clinicopathological factors and survival in 75 patients with PTC. The effects of FKBP5 on the proliferation and apoptosis of PTC cells were detected by colony-formation, MTT, and flow cytometry assays, respectively. We further investigated the effects of FKBP5 on tumor growth in mice.
Results
We revealed high expression levels of FKBP5 in human PTC tissues compared with normal tissues. Furthermore, high FKBP5 expression was associated with an increased incidence of intraglandular dissemination, and lower overall and progression-free survival. FKBP5 depletion remarkably suppressed the proliferation and induced apoptosis of PTC cells in vitro. FKBP5 further contributed to the growth of PTC tumors in mice.
Conclusions
The results of this study demonstrated the potential involvement of FKBP5 in the progression of PTC, and confirmed FKBP5 as a novel therapeutic target for PTC treatment.
Keywords: FK506-binding protein 5, papillary thyroid carcinoma, proliferation, apoptosis, prognosis, survival
Introduction
Papillary thyroid carcinoma (PTC) is the most common type of primary endocrine malignancy of the thyroid in adults, with an estimated worldwide incidence rate of around 1.7% of total cancer diagnoses, and an increasing incidence rate in China.1,2 Surgery, chemotherapy, and radiotherapy are the main treatments for cancers, but most patients have poor outcomes with side effects.3–5 An understanding of the molecular mechanisms responsible for PTC progression is therefore crucial for its treatment.
FK506-binding protein 5 (FKBP5) is a 51-kDa protein with a C-terminal including a three-unit domain of tetratricopeptide repeat motifs that interact with several proteins, such as heat shock protein 90,6,7 and an N-terminal region including two FKBP-like domains: FK1, encompassing the peptidyl-proline isomerase (PPIase) function, and FK2, with no measurable PPIase activity.8,9 FKBP5 has been confirmed to act as a scaffolding protein to enhance PH domain leucine-rich repeat protein phosphatase (PHLPP)-Akt interaction and promote PHLPP-mediated dephosphorylation of Akt-Ser473.10 FKBP5 has multiple other cellular functions, and its involvement in the progression of multiple cancers has been widely revealed.11–12 However, the possible role of FKBP5 in the development of PTC is still unclear.
Here, we investigated the expression of FKBP5 in human PTC tissues by immunohistochemistry (IHC) and its association with prognosis and clinical features in patients with PTC. We also examined the effects of FKBP5 knockdown on the proliferation and apoptosis of PTC cells, and the effects of FKBP5 on tumor growth in mice. The results suggest that FKBP5 might provide a promising therapeutic target for the treatment of PTC.
Materials and methods
Patients and tissue specimens
PTC specimens were obtained from patients who underwent surgical resection, without chemotherapy or radiotherapy, at the School of Medicine, Xuchang University from September to December 2019. Samples and survival were analyzed for all patients with clinicopathological data relating to age, sex, TNM stage, tumor size, and lymph node metastasis. All studies were approved by the ethics committee of the School of Medicine, Xuchang University, and verbal informed consent was obtained from all patients.
Antibodies
The following antibodies were used for western blotting and IHC: FKBP5 (Abcam, Cambridge, MA, USA; ab2901, 1:1000 dilution for western blot, 1:200 dilution for IHC), β-actin (Abcam; ab5694, 1:1000 dilution), Ki67 (Abcam; ab16667, 1:1000 dilution), proliferating cell nuclear antigen (PCNA) (Abcam; ab18191, 1:500 dilution), caspase-3 (Abcam; ab13847, 1:500 dilution), caspase-7 (Abcam; ab32522, 1:1000 dilution), and caspase-9 (Abcam; ab32539, 1:500 dilution).
IHC
Formalin-fixed, paraffin-embedded tissue samples were cut at 4-µm thickness and prepared on charged glass slides. After deparaffinization and rehydration, the slides were immersed in 10 mM citrate buffer (pH 7.5) and microwaved at 750 W for 30 minutes for antigen retrieval. Endogenous peroxidase activity was blocked by adding 3% hydrogen peroxide. The sections were then incubated with diluted antibodies followed by polymer-conjugated horseradish peroxidase in a humidified chamber. Standard DAB staining was performed for chromogenic detection of the IHC targets, using a DAB staining kit (Abcam; ab64238), according to the manufacturer’s instructions.
The sections were scored as follows. The proportion of positively stained cells was assessed as 0, negative tumor cells; 1, 10% to 60% positive tumor cells; and 2, >60% positive staining cells. Staining intensity was assessed as 0 (negative staining), 1 (modest staining), or 2 (strong staining). FKBP5 expression levels were then calculated according to the staining score as staining intensity score + positive tumor cell staining score, with scores of 0, 1, and 2 considered low expression, and scores of 3 and 4 high expression.
Cell culture, cell lines, and transfection
The human PTC cell lines TPC-1 and KTC-1 were purchased from the American Type Culture Collection (Manassas, VA, USA) and maintained in RPMI-1640 medium (Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco), 100 ng/mL streptomycin, and 100 U/mL penicillin (Gibco) at 37°C with 5% CO2. When the cells reached 50% to 70% confluency, they were transfected with FKBP5 or control short hairpin RNA (shRNA) using lentivirus purchased from ViGene Bioscience (#SH802465, Rockville, MD, USA), according to the manufacturers’ instructions, to produce FKBP5-knockdown and control cells, respectively. The target sequence for FKBP5 shRNA used in this study was AAAGTTTATGTCCATTACAAAGG.
RNA isolation and quantitative reverse transcription polymerase chain reaction (RT-qPCR)
The expression of FKBP5 mRNA was detected by RT-qPCR assays. Total RNA was isolated from cells using TRIzol reagent (Invitrogen, Waltham, MA, USA) and used for first-strand cDNA synthesis, using a Reverse Transcription System (Roche, Indianapolis, IN, USA) following the manufacturer’s protocol. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. The primer sequences were: GAPDH, forward 5′-AACGGATTTGGTCGTATTGGG-3′ and reverse 5′-TCGCTCCTGGAAGATGGTGAT-3′; FKBP5, forward 5′-ATGAAGAAAGCCCCACAGC-3′ and reverse 5′-CCTCACCATTCCCCACTCT-3′.
Western blot analysis
TPC-1 and KTC-1 cells were washed with phosphate-buffered saline (PBS) and lysed in RIPA buffer (50 mM Tris-HCl/pH 7.4; 1% NP-40; 150 mM NaCl; 1 mM EDTA; 1 mM proteinase inhibitor; 1 mM Na3VO4; 1 mM NaF; 1 mM okadaic acid; and 1 mg/mL aprotinin, leupeptin, and pepstatin). Protein samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes at 4°C (250 mA, 2 hours). The membranes were then blocked with 5% dry milk for 2 hours, incubated with antibodies as above, at room temperature for 2 hours, and washed with TBST. The membranes were finally developed in enhanced chemiluminescence mixture using an ECL kit (Abcam; ab65623) and visualized using ImageJ software (version 1.8.0; National Institute of Health, MD, USA). The gray values of the blots were counted using ImageJ, normalized to the gray values of the control group as 1.
Colony assays
Approximately 1,000 TPC-1 or KTC-1 stable FKBP5-knockdown cells and 1,000 control cells transfected with control shRNA plasmids were seeded in six-well plates, with three replicates. After 4 weeks, colonies were fixed with paraformaldehyde for 30 minutes at room temperature, stained with 0.5% crystal violet for 20 minutes, and photographed under a microscope, and the colony number was counted manually.
MTT and Cell Counting Kit-8 (CCK-8) assays for cell proliferation
Cell proliferation was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma, St. Louis, MO, USA) proliferation or CCK-8 assays (Abcam; ab228554), according to the manufacturers’ instructions. Each experiment was performed in triplicate and repeated three times.
Flow cytometry detection of apoptosis
FKBP5-knockdown and negative control cells (about 3 × 106/mL) were collected and centrifuged at 500 × g for 5 minutes and the supernatant was discarded. The cells were washed in 3 mL PBS, centrifuged, and fixed with ice-cold 70% ethanol at 4°C for 1 to 2 hours. The cells were centrifuged again and the fixative was discarded, and the cells were resuspended in 3 mL PBS for 5 minutes, followed by further centrifugation with a 400-mesh sieve at 1000 × g for 5 minutes. The PBS was discarded and the cells were stained with 1 mL propidium iodide (PI) in the dark for 30 minutes at 4°C. The PI was excited by argon ion fluorescence, using a laser at a 488-nm wavelength, with an emission wavelength >630 nm.
In vivo xenograft assays
Sterilized female Balb/c nude mice (5 weeks old, 18–22 g) were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and housed in pathogen-free animal facilities (four per group). The mice were randomized into two groups and injected subcutaneously with 5 × 106 KTC-1 control or KTC-1 FKBP5-depleted cells. After injection, the animals were housed for 7 weeks and tumor volume was measured every 7 days from the third week using Vernier calipers. Tumor volume was calculated as follows: tumor volume (mm3) = maximum tumor length (mm) × maximum tumor width (mm)2/2.
Statistical analysis
Data were analyzed using GraphPad Prism 6.0 software (GraphPad Software Inc., La Jolla, CA, USA). All results were presented as mean ± standard deviation. Survival analysis was carried out using the Kaplan–Meier method. Correlations between clinical results and protein expression were calculated using Fisher’s exact test and χ2 analysis. Student’s t-test was used for statistical comparisons. P < 0.05 was considered statistically significant.
Results
FKBP5 was highly expressed in advanced thyroid cancer tissues and was associated with a poor prognosis
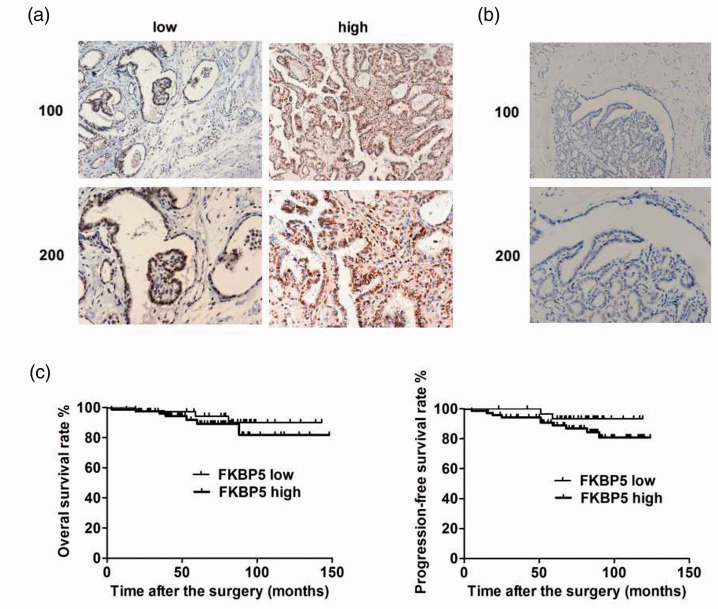
We determined the expression levels of FKBP5 in 115 tumor and normal tissue samples from patients with PTC using IHC (Figure 1a and 1b). Expression levels of FKBP5 were significantly lower in normal thyroid tissues (Figure 1b) compared with PTC tissues (Figure 1a) (P < 0.05). Using the median expression of FKBP5 as the cut-off value, high expression of FKBP5 (n = 59) was more common in patients with advanced-stage compared with those with early-stage thyroid cancer (Table 1, P = 0.015). High expression of FKBP5 was also associated with more intraglandular dissemination (Table 1, P = 0.016). We also demonstrated that patients with high expression of FKBP5 tended to have a poorer prognosis, with significantly lower 5-year overall survival and lower 5-year progression-free survival rates compared with patients with low FKBP5 expression levels (Figure 1c) (P < 0.05), using the median level of FKBP5 expression as the cut-off. These results suggested that FKBP5 had clinicopathological significance in terms of thyroid cancer progression.
Figure 1.
Expression of FKBP5 was increased in advanced thyroid cancer and was associated with a poor prognosis. (a) Immunohistochemistry (IHC) detection of FKBP5 expression in thyroid cancer specimens. High and low expression were defined according to the median cut-off value. Representative images shown at ×100 and ×200 magnification. (b) IHC detection of FKBP5 expression in normal thyroid tissues. Representative images shown at ×100 and ×200 magnification. (c) Kaplan–Meier curves for overall and progression-free survival rates in patients with thyroid cancer in relation to FKBP5 expression level, with median expression level as the cut-off. *P < 0.05.
Table 1.
Relationships between of FKBP5 expression and clinicopathological characteristics in 75 patients with papillary thyroid carcinoma.
| Feature | All (n = 75) | FKBP5 expression |
|||
|---|---|---|---|---|---|
| Low n = 16 | High n = 59 | χ2 | P-value | ||
| Age (years) | 2.111 | 0.146 | |||
| <45 | 26 | 8 | 18 | ||
| ≥45 | 49 | 8 | 41 | ||
| Sex | 2.061 | 0.151 | |||
| Male | 16 | 6 | 10 | ||
| Female | 59 | 10 | 49 | ||
| TNM stage | 6.305 | 0.012 | |||
| T1–T2 | 31 | 11 | 20 | ||
| T3–T4 | 44 | 5 | 39 | ||
| Tumor size | 0.949 | 0.330 | |||
| <2 cm | 23 | 7 | 16 | ||
| ≥2 cm | 52 | 9 | 43 | ||
| Lymph node metastasis | 0.033 | 0.857 | |||
| Yes | 36 | 8 | 28 | ||
| No | 39 | 8 | 31 | ||
| Intraglandular dissemination | 5.800 | 0.016 | |||
| Yes | 34 | 3 | 31 | ||
| No | 41 | 13 | 28 | ||
FKBP5 promoted the proliferation of TPC-1 and KTC-1 thyroid cancer cells in vitro
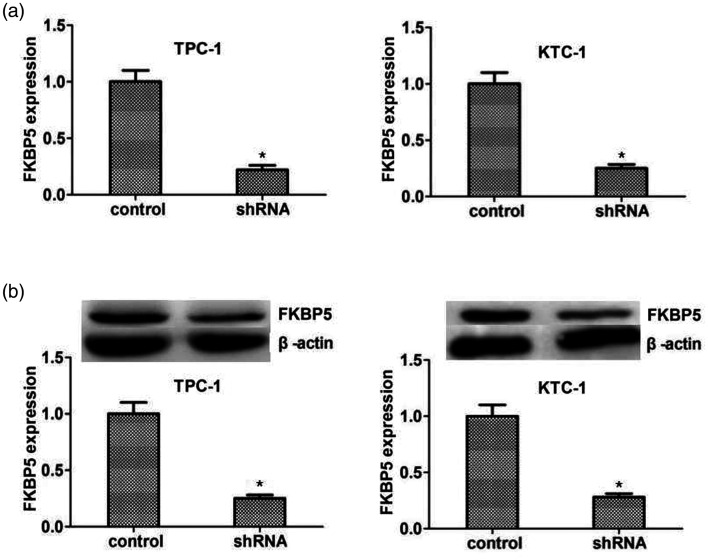
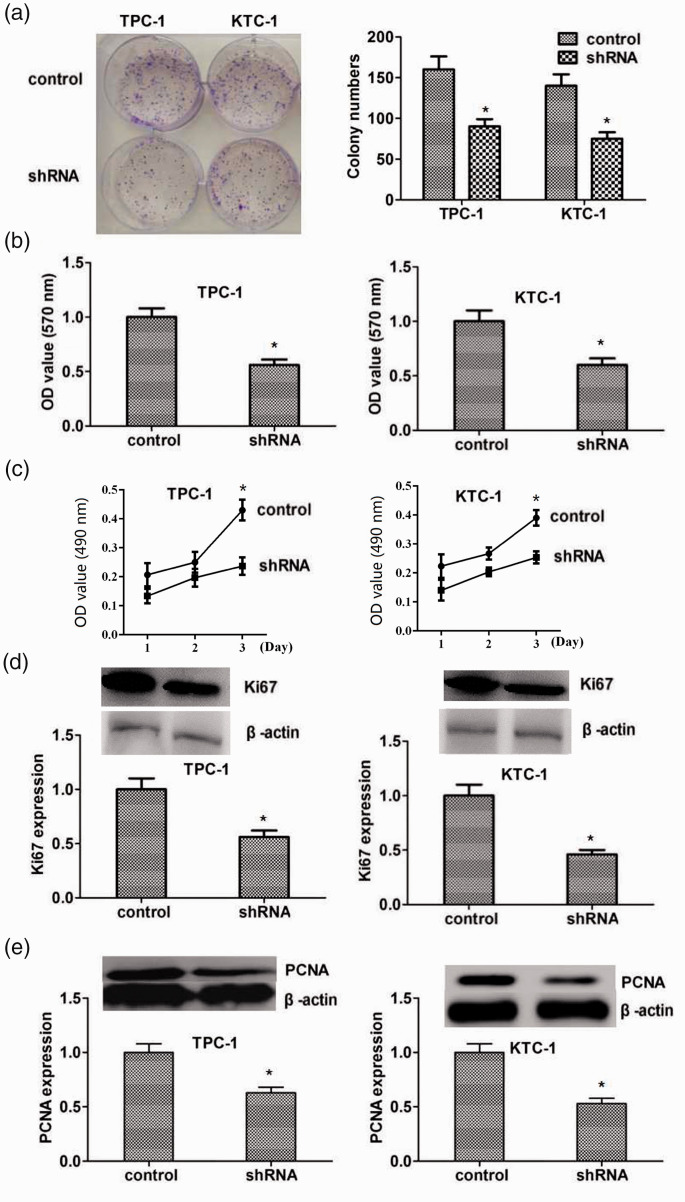
We further investigated the cellular functions of FKBP5 in thyroid cancer in FKBP5-knockdown TPC-1 and KTC-1 cell lines. FKBP5 expression was significantly decreased after transfection of FKBP5 shRNA, as shown by qPCR assays (Figure 2a). Immunoblotting showed similar declines in FKBP5 expression in the two cell lines (Figure 2b). To determine the impact of FKBP5 on the growth of thyroid cancer cells, we performed colony-formation assays in FKBP5-knockdown TPC-1 and KTC-1 cell lines with FKBP5 depletion (Figure 3a). Loss of FKBP5 suppressed the growth of both cell lines compared with the negative control cohort, as shown by MTT and CCK-8 assays (Figure 3b, c). Expression levels of the proliferation markers PCNA and Ki67 decreased significantly after knockdown of FKBP5 in both TPC-1 and KTC-1 cells, as shown by immunoblotting (Figure 3d, e), indicating the facilitating role of FKBP5 in PTC cell proliferation.
Figure 2.
FKBP5 was effectively depleted in TPC-1 and KTC-1 thyroid cancer cell lines. (a) Quantitative polymerase chain reaction detection of FKBP5 expression in TPC-1 and KTC-1 cells transfected with control or FKBP5 shRNA plasmids. (b) Western blot detection of FKBP5 expression in TPC-1 and KTC-1 cells transfected with control or FKBP5 shRNA plasmids (upper). Expression levels of β-actin and FKBP5 were evaluated using ImageJ with integrated optical density based on the results of immunoblotting. Relative FKBP5 expression was normalized to β-actin level. *P<0.05
shRNA, short hairpin RNA.
Figure 3.
FKBP5 promoted the proliferation of TPC-1 and KTC-1 thyroid cancer cell lines. (a) TPC-1 and KTC-1 cell growth after FKBP5 depletion was determined by colony-formation assays. (b, c) MTT (b) and CCK-8 (c) assays of TPC-1 and KTC-1 cells after FKBP5 depletion. The optical density was determined after 48 hours. (d) Immunoblot detection of Ki67 protein expression after FKBP5 depletion (upper). Expression levels of β-actin and Ki67 were evaluated using ImageJ with integrated optical density (IOD) based on the results of western blotting. Relative Ki67 expression was normalized to β-actin level. (e) Western blot detection of proliferating cell nuclear antigen (PCNA) protein expression after FKBP5 ablation (upper). Expression levels of β-actin and PCNA were evaluated using ImageJ with IOD based on the results of western blotting. Relative PCNA expression was normalized to β-actin level. *P < 0.05
OD, optical density; shRNA, short hairpin RNA; PCNA, proliferating cell nuclear antigen.
FKBP5 suppressed apoptosis of TPC-1 and KTC-1 thyroid cancer cells
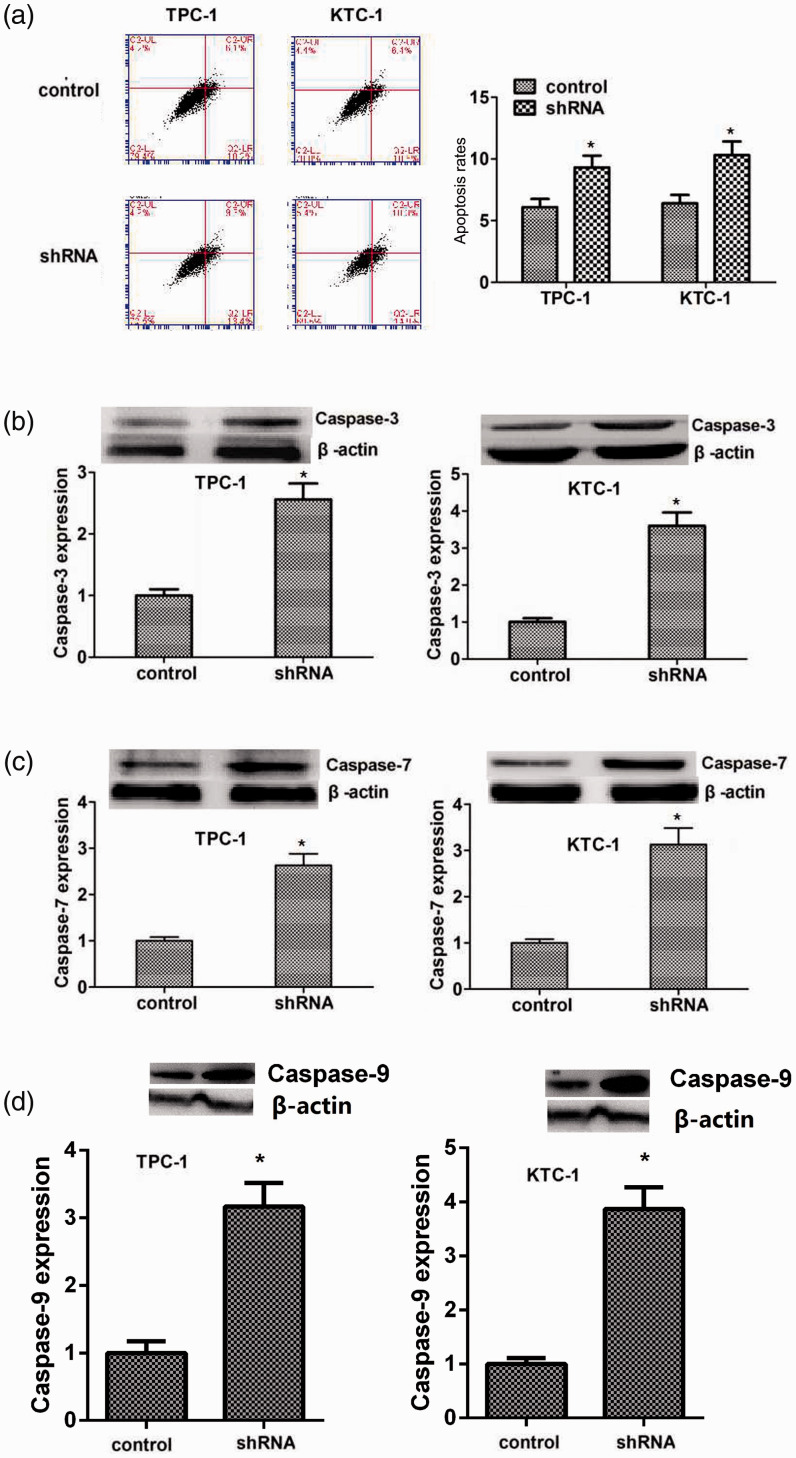
We confirmed the effects of FKBP5 on apoptosis of TPC-1 and KTC-1 cells by flow cytometry analysis of the two cell lines after transfection with control and FKBP5 shRNA plasmids. Apoptosis was increased in FKBP5-knockdown TPC-1 and KTC-1 cells compared with negative control cells (Figure 4a). Caspase-3, 7, and 9 are activated during the early stage of apoptosis, and these were all accordingly significantly increased in line with apoptosis induced by FKBP5 depletion (Figure 4b–d).
Figure 4.
FKBP5 suppressed the apoptosis of TPC-1 and KTC-1 thyroid cancer cells. (a) Apoptosis of TPC-1 and KTC-1 cells transfected with FKBP5 shRNA plasmids was detected by colony-formation assays. (b) Immunoblot detection of caspase-3 protein expression after FKBP5 ablation (upper). Expression levels of β-actin and caspase-3 were evaluated using ImageJ with integrated optical density (IOD) based on the results of immunoblotting. Relative caspase-3 expression was normalized to β-actin level. (c) Immunoblotting of caspase-7 protein expression after FKBP5 knockdown (upper). Expression levels of β-actin and caspase-7 were evaluated using ImageJ with IOD based on the results of western blotting. Relative caspase-7 expression was normalized to β-actin level. (d) Immunoblotting of caspase-9 protein expression after FKBP5 knockdown (upper). *P < 0.05
shRNA, short hairpin RNA.
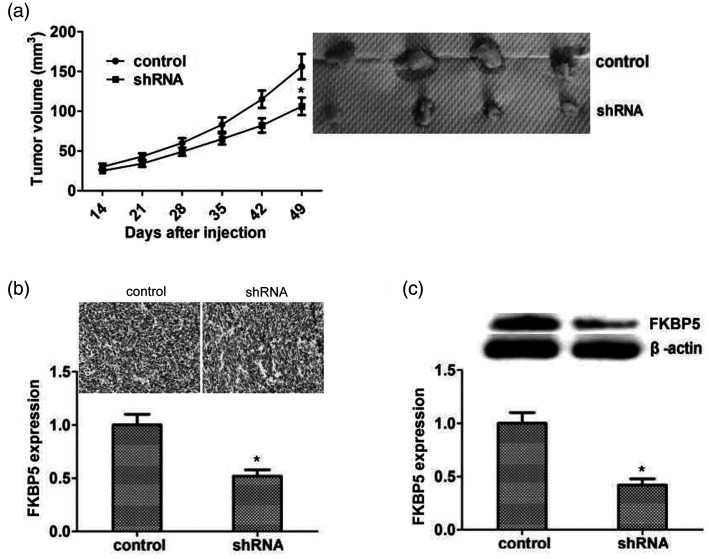
Knockdown of FKBP5 inhibited tumor growth of KTC-1 cells in mice
To explore the potential roles of FKBP5 in thyroid cancer, we injected KTC-1 cells into 6-week-old female Balb/c mice (n = 8). Control mice (n = 4) were injected with cells transfected with lentivirus carrying control shRNA and treated mice (n = 4) were injected with cells transfected with lentivirus carrying FKBP5 shRNA plasmid. Injections were carried out weekly for 5 weeks. KTC-1 tumor growth was significantly reduced in mice injected with FKBP5-knockdown cells compared with control cells (P < 0.05; Figure 5a). IHC and immunoblot assays demonstrated a significant decrease in FKBP5 expression in the FKBP5-depletion cohort (P < 0.05; Figure 5b and c). These results indicated that FKBP5 promoted PTC cell survival and growth in vivo, by stimulating proliferation and inhibiting apoptosis.
Figure 5.
Knockdown of FKBP5 inhibited growth of KTC-1 tumor cells in vivo. (a) Suppression of papillary thyroid carcinoma tumor growth in mice injected with cells transfected with FKBP5 shRNA (n=4) or scrambled shRNA (n=4) for 49 days. Tumor size was measured (left) and tumors were imaged at the time of tumor removal (right). (b) FKBP5 expression in the two groups determined by immunohistochemistry. Six fields per group were chosen at random and counted under a microscope at ×400 magnification. Representative images and statistical analysis are shown. Evaluation was not blinded. (c) Western blot detection of FKBP5 expression in the indicated tumor specimens. Expression levels of β-actin and FKBP5 were evaluated using ImageJ with integrated optical density based on the results of western blotting. Relative FKBP5 expression was normalized to β-actin level. *P<0.05
shRNA, short hairpin RNA.
Discussion
Thyroid cancer is one of the hardest human malignancies to treat, with high metastasis and mortality rates. PTC is a particularly malignant sub-type of thyroid cancer,3 and traditional treatments, such as chemotherapy, often fail to achieve clinical effects in patients with PTC.1 Although targeted therapy and immunotherapy have recently shown promising results in the treatment of advanced PTC, novel therapeutic molecular targets are still needed.2 In this study, we demonstrated high expression levels of FKBP5 in human PTC tissues, and showed that FKBP5 depletion led to the inhibition of cell proliferation and induction of cell apoptosis in vitro, and inhibited PTC tumor growth in mice. These results suggest that FKBP5 may be a novel and promising therapeutic target for the treatment of PTC; however, the regulatory mechanisms need further study.
IHC detection and analysis of 115 human PTC tissue samples and their corresponding adjacent normal samples demonstrated significantly higher expression levels of FKBP5 in tumor tissues, and FKBP5 expression levels were also significantly associated with clinical features, including intraglandular dissemination (P = 0.016). FKBP5 was also associated with a poor prognosis in patients with PTC in terms of survival. Similarly, previous studies reported that FKBP5 was an independent prognostic indicator in patients with esophageal adenocarcinoma,13 and a promising biomarker for Akt inhibitors and gemcitabine in pancreatic cancer.14 Overall, these results evaluated the potential role of FKBP5 in the histopathology and progression of tumorigenesis. We further confirmed that FKBP5 served as a trigger of PTC progression via the regulation of cell proliferation and apoptosis. Consistent with our results, FKBP5 also blocked the proliferation and promoted apoptosis of glioma cells15 and promoted the proliferation of LNCaP prostate cancer cells.16 In the current study, we also demonstrated decreased Ki67 and PCNA expression in cancer cells, further confirming the key role of FKBP5 in the regulation of PTC cell proliferation.
The management of PTC is a popular topic, with individualized targeted therapy being the main issue. There is thus an urgent need to identify clinical markers and therapeutic targets to facilitate the early diagnosis and effective treatment of PTC. The present study provides the first report of the clinicopathological role of FKBP5 in the progression of PTC. Human FKBP5 is highly expressed in multiple tissues, such as skeletal muscle, placenta, and peripheral blood, and its expression can be induced by activation of the progesterone receptor and androgen receptor.17 Notably, over-expression and down-regulation of FKBP5 have been identified in several cancers13,14,18–23: data in the Oncomine database indicates that FKBP5 is overexpressed in many cancers, including brain and prostate cancer, while previous studies revealed that FKBP5 was downregulated in pancreatic cancer.10 In this study, we investigated the expression of FKBP5 in PTC by IHC, and confirmed that FKBP5 expression was upregulated in human PTC compared with normal tissues. Ni et al.24 reported that FKBP5 stimulated androgen-dependent transcription and cell proliferation in prostate cancer. Similarly, we found that knockdown of FKBP5 suppressed the proliferation and promoted the apoptosis of KTC-1 and TPC-1 PTC cells. Through in vivo assays, we also confirmed that targeting FKBP5 inhibited the growth of PTC, suggesting the therapeutic significance of FKBP5. These previous studies, together with our current findings, confirm the important role of FKBP5 in cancer progression. However, further studies are needed to clarify the precise molecular mechanism underlying FKBP5-induced PTC growth.
Excessive cell proliferation and impaired apoptosis can lead to the development of tumors,25 and these processes can have synergistic effects in exacerbating tumor progression and metastasis.26 In this study, we showed that FKBP5 promoted apoptosis in the development of PTC. We also demonstrated that caspase-3, -7, and -9 were up-regulated in human PTC tissues, consistent with previous results,11,12 suggesting multiple roles of FKBP5 in cancer progression. Caspase-3, -7, and -9 are indicators of apoptosis and have been reported to be changed in multiple cancers caused by the induction of cell apoptosis.27 Notably, Croton gratissimus leaf extracts prevented cancer cell growth by promoting the activation of caspases 3/7, with additional anti-inflammatory and antioxidant activities.28 The tumor-promotion roles of FKBP5 have thus been widely revealed; however, the effects of FKBP5 on cancer cell migration and invasion are still unclear and need further study.
Previous studies also indicated that FKBP5 acted as an androgen-dependent gene in prostate cancer and a modulator of androgen receptor activity,29 promoting cell growth and acting as a target of FK506 in prostate cancer cells.28,30 We therefore hypothesize that FKBP5 might be activated and upregulated by androgens and involved in the progression of PTC. However, FKBP5 inhibited cell proliferation in colorectal adenocarcinoma, attributed to its action on the glucocorticoid receptor.31 In contrast, the current results showed that depletion of FKBP2 inhibited cell proliferation and promoted apoptosis of PTC cells. These studies thus indicate that FKBP5 has complex roles in cancer progression.
This study was limited by the lack of overexpression assays, which meant that we were unable to determine the effects of FKBP5 overexpression on PTC cell proliferation. Additionally, the precise mechanisms underlying FKBP5 promotion of PTC progression still need further study.
Conclusions
In this study, using TPC-1 and KPC-1 cell models, we confirmed the involvement of FKBP5 in the progression of PTC, via suppressing apoptosis and supporting the proliferation of PTC cells. Collectively, the results suggest that FKBP5 might be a promising therapeutic target for the treatment of PTC.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Zhenya Gao https://orcid.org/0000-0003-0134-9944
References
- 1.Sherman SI, Wirth LJ, Droz JP, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med 2008; 359: 31–42. [DOI] [PubMed] [Google Scholar]
- 2.Dong WW, Zhang H, Zhang P, et al. The changing incidence of papillary thyroid carcinoma in Shenyang, China before and after universal salt iodization. Med Sci Monit 2013; 19: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddad R, Sherman SI, Shah JP, et al. New frontiers and treatment paradigms for papillary thyroid carcinoma. Clin Adv Hematol Oncol 2014; 12: 3–21. [PubMed] [Google Scholar]
- 4.Mladěnka A, Mladěnka P, Simetka O, et al. Intra-operative and postoperative complications depend on operating approach and body mass index in patients with malignant uterus body neoplasm. Eur J Gynaecol Oncol 2019; 40: 593–598. [Google Scholar]
- 5.Yang Y, Zhao Z, Xie CW, et al. Dual-targeting liposome modified by glutamic hexapeptide and folic acid for bone metastatic breast cancer. Chem Phys Lipids 2020; 228: 104882. [DOI] [PubMed] [Google Scholar]
- 6.Baughman G, Wiederrecht GJ, Campbell NF, et al. FKBP51, a novel T-cell-specific immunophilin capable of calcineurin inhibition. Mol Cell Biol 1995; 15: 4395–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallo LI, Lagadari M, Piwien-Pilipuk G, et al. The 90-kDa heat-shock protein (Hsp90)-binding immunophilin FKBP51 is a mitochondrial protein that translocates to the nucleus to protect cells against oxidative stress. J Biol Chem 2011; 286: 30152–30160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinars CR, Cheung-Flynn J, Rimerman RA, et al. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc Natl Acad Sci USA 2003; 100: 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nair SC, Rimerman RA, Toran EJ, et al. Molecular cloning of human FKBP51 and comparisons of immunophilin interactions with Hsp90 and progesterone receptor. Mol Cell Biol 1997; 17: 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pei H, Li L, Fridley BL, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell 2009; 16: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romano S, D'Angelillo A, Staibano S, et al. Immunomodulatory pathways regulate expression of a spliced FKBP51 isoform in lymphocytes of melanoma patients. Pigment Cell Melanoma Res 2015; 28: 442–452. [DOI] [PubMed] [Google Scholar]
- 12.Gaali S, Gopalakrishnan R, Wang Y, et al. The chemical biology of immunophilin ligands. Curr Med Chem 2011; 18: 5355–5379. [DOI] [PubMed] [Google Scholar]
- 13.Smith E, Palethorpe HM, Ruszkiewicz AR, et al. Androgen receptor and androgen-responsive gene FKBP5 are independent prognostic indicators for esophageal adenocarcinoma. Dig Dis Sci 2016; 61: 433–443. [DOI] [PubMed] [Google Scholar]
- 14.Ellsworth KA, Eckloff BW, Li L, et al. Contribution of FKBP5 genetic variation to gemcitabine treatment and survival in pancreatic adenocarcinoma. PLoS One 2013; 8: e70216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Zhang QX, Pei DS, et al. FK506-binding protein 5 inhibits proliferation and stimulates apoptosis of glioma cells. Arch Med Sci 2015; 11: 1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin ML, Kim YW, Jeong KW. BAF53A regulates androgen receptor-mediated gene expression and proliferation in LNCaP cells. Biochem Biophys Res Commun 2018; 505: 618–623. [DOI] [PubMed] [Google Scholar]
- 17.Ratajczak T, Ward BK, Minchin RF. Immunophilin chaperones in steroid receptor signalling. Curr Top Med Chem 2003; 3: 1348–1357. [DOI] [PubMed] [Google Scholar]
- 18.Linnstaedt SD, Riker KD, Rueckeis CA, et al. A functional riboSNitch in the 3' untranslated region of FKBP5 alters microRNA-320a binding efficiency and mediates vulnerability to chronic post-traumatic pain. J Neurosci 2018; 38: 8407–8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suppli NP, Bukh JD, Moffitt TE, et al. Genetic variants in 5-HTTLPR, BDNF, HTR1A, COMT, and FKBP5 and risk for treated depression after cancer diagnosis. Depress Anxiety 2017; 34: 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun NK, Huang SL, Chang PY, et al. Transcriptomic profiling of taxol-resistant ovarian cancer cells identifies FKBP5 and the androgen receptor as critical markers of chemotherapeutic response. Oncotarget 2014; 5: 11939–11956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang JI, Chung HC, Jeung HC, et al. FKBP5 polymorphisms as vulnerability to anxiety and depression in patients with advanced gastric cancer: a controlled and prospective study. Psychoneuroendocrinology 2012; 37: 1569–1576. [DOI] [PubMed] [Google Scholar]
- 22.Hou J, Wang L. FKBP5 as a selection biomarker for gemcitabine and Akt inhibitors in treatment of pancreatic cancer. PLoS One 2012; 7: e36252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Lou Z, Wang L. The role of FKBP5 in cancer aetiology and chemoresistance. Br J Cancer 2011; 104: 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni L, Yang CS, Gioeli D, et al. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol Cell Biol 2010; 30: 1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lima JSB, Miola AC, Marques MEA, et al. Patterns of proliferation and apoptosis in different subtypes of basal cell carcinoma, adjacent epidermis, and recurrent forms. An Bras Dermatol 2019; 94: 108–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barman SA, Li X, Haigh S, et al. Galectin-3 is expressed in vascular smooth muscle cells and promotes pulmonary hypertension through changes in proliferation, apoptosis and fibrosis. Am J Physiol Lung Cell Mol Physiol 2019; 316: L784–L797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fassan M, Cui R, Gasparini P, et al. miR-224 is significantly upregulated and targets caspase-3 and caspase-7 during colorectal carcinogenesis. Transl Oncol 2019; 12: 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Njoya EM, Eloff JN, McGaw LJ. Croton gratissimus leaf extracts inhibit cancer cell growth by inducing caspase 3/7 activation with additional anti-inflammatory and antioxidant activities. BMC Complement Altern Med 2018; 18: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stechschulte LA, Sanchez ER. FKBP51-a selective modulator of glucocorticoid and androgen sensitivity. Curr Opin Pharmacol 2011; 11: 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Periyasamy S, Hinds T, Jr, Shemshedini L, et al. FKBP51 and Cyp40 are positive regulators of androgen-dependent prostate cancer cell growth and the targets of FK506 and cyclosporin A. Oncogene 2010; 29: 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukaide H, Adachi Y, Taketani S, et al. FKBP51 expressed by both normal epithelial cells and adenocarcinoma of colon suppresses proliferation of colorectal adenocarcinoma. Cancer Invest 2008; 26: 385–390. [DOI] [PubMed] [Google Scholar]