Abstract
The meta-analysis was performed to access efficacy of L-carnitine/L-acetyl-carnitine (LC/LAC) and N-acetyl-cysteine (NAC) in men with idiopathic asthenozoospermia. We researched PubMed, EMBASE, and Cochrane Library databases and references to related articles. Finally, seven articles including 621 patients were analyzed. The results indicated that LC/LAC and NAC had a considerable improvement in sperm motility (p = .03 and p < .0001, respectively) and normal morphology (p = .006, p = .0002, respectively) compared with the placebo group. Besides, NAC had a significantly greater increase in sperm concentration (p < .00001) and ejaculate volume (p = .002) compared with the placebo group, and there was no significant difference in LC/LAC. For the analysis of serum hormones, NAC had no obvious differences in improving the serum testosterone, luteinizing hormone, follicle-stimulating hormone, and prolactin compared with non-treatment group. Conclusively, LC/LAC and NAC showed a greater improvement in sperm motility and normal morphology. Moreover, NAC has a positive effect on sperm concentration and ejaculate volume, whereas no obvious effect was observed in serum hormones.
Keywords: L-carnitine, L-acetyl-carnitine, N-acetyl-cysteine, meta-analysis, idiopathic asthenozoospermia
It is reported that about half of childless couples with infertility are not pregnant due to male factors (Gnoth et al., 2005). Among them, 44% of infertile men had no known disease that could explain infertility and these patients were assigned as idiopathic male infertility (Pierik et al., 2000). As the pathogenesis of this disease is not completely clear, there is no evidence-based treatment for idiopathic male infertility, and most treatments depend on clinical experience (Dohle et al., 2012).
L-carnitine (LC) plays an important role in cellular energetic metabolism, and it acts as a shuttle of the acyl-CoA into the mitochondria (Bremer, 1983; Jeulin et al., 1994). High levels of LC in epididymal fluid plays an important role in sperm cell metabolism (Enomoto et al., 2002), and some studies report that the onset of sperm motility is related to the increase of LC in epididymal cavity and the concentration of L-acetyl-carnitine (LAC) in sperm cells (Radigue et al., 1996). N-acetyl-cysteine (NAC) as a derivative of L-cysteine is frequently regarded as antioxidant (Zafarullah et al., 2003). It was reported that NAC has the function of scavenging free radicals in vivo and in vitro (Ciftci et al., 2009; Erkkilä et al., 1998; Oeda et al., 1997). Compared with placebo, NAC orally daily can significantly improve sperm motility (Safarinejad & Safarinejad, 2009). Moreover, NAC can not only increase sperm concentration and acrosome reaction but also reduce reactive oxygen species (ROS) and sperm DNA oxidation (Comhaire et al., 2000).
The efficacy of LC / LAC and NAC in the treatment of idiopathic asthenospermia has not been evaluated by evidence-based medicine (EBM). Therefore, a meta-analysis was carried out.
Materials and Methods
Protocol
This meta-analysis strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) list (Moher et al., 2010).
Literature Sources and Search Strategy
Two reviewers searched dependently for all randomized controlled trials (RCTs) published until August 31, 2020, involving the efficacy of LC/LAC and NAC in men with idiopathic asthenozoospermia. They researched PubMed, EMBASE, and Cochrane Library databases and references of the included studies; Medical Subject Headings (MeSH) terms were “L-carnitine, L-acetyl-carnitine, N-acetyl-cysteine, NAC, LC, LAC, infertile, idiopathic, idiopathic infertile, men, male, idiopathic asthenozoospermia.”
Inclusion and Exclusion Criteria
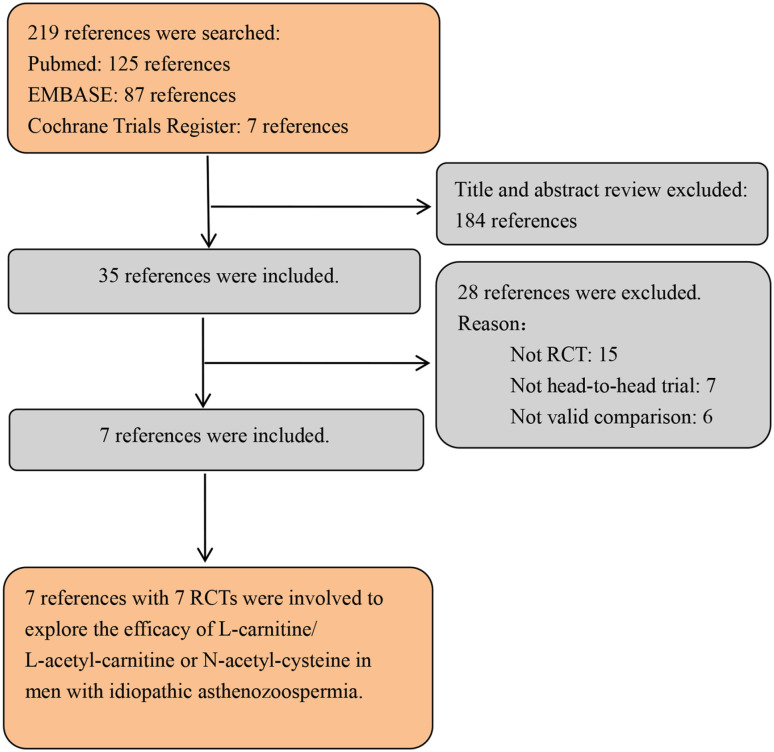
Inclusion criteria: there are no identifiable medical conditions that could explain infertility and these cases are classified as idiopathic male infertility—this study only included patients with idiopathic male infertility; LC/LAC and NAC in treating men with idiopathic asthenozoospermia were evaluated; full text available; data including the number of participants and the vary of each indicator; RCT. Exclusion criteria: not RCT, such as abstract, review, or comment; patients with urogenital bacterial infection, smoke, hypogonadism, varicocele and other comorbidities; other treatment. The PRISMA flowchart is illustrated in Figure 1.
Figure 1.
Flowchart of selection PRISMA.
Note. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta- Analyses; RCTs = randomized controlled trials.
Quality of Assessment
The quality of RCTs was accessed using the Cochrane Handbook for Systematic Reviews of Interventions v.5.3.0 (Cumpston et al., 2019). The RCTs were classified using the same Handbook (Cumpston et al., 2019). Three quality classification criteria: (+) RCT would be considered a low possibility of bias for conforming to almost all quality criteria; (?) RCT would be counted a secondary probability for fulfilling partial quality criteria or indistinct; (-) RCT would be considered a high possibility of bias for conforming to bare quality criteria.
Data Extraction
The relevant data are extracted as follows: characteristics of study; name of authors; study design and sample size; interventions among the groups; and evaluation index, such as sperm concentration, ejaculate volume, sperm motility, normal morphology, testosterone, luteinizing hormone, follicle-stimulating hormone, and prolactin. All authors checked the accuracy of the data. In addition, this research did not require ethical consent.
Statistical Analysis
Rev man 5.3.0 was used to analyze the data. Mean difference (MD) with 95% confidence interval (CI) was used to evaluate continuous index, and odds ratio (OR) with 95% CI was used to evaluate dichotomous index. The fixed effect model was used and considered to be homogeneous, if the result was p > .05 (DerSimonian & Laird, 1986). I2 statistic was applied to analyze inconsistent results. Random effect model was adopted when the index showed p < .05 or I2 > 50%. If the result showed p < .05, the results were considered statistically significant.
Results
Basic Characteristics and Search Process
The reviewers searched 219 studies in three databases. According to the inclusion criteria, 184 studies were excluded. Due to the lack of valid data, 28 studies were excluded. At last, seven RCTs (Ciftci et al., 2009; Jannatifar et al., 2019; Kopets et al., 2020; Lenzi et al., 2004; Micic et al., 2019; Safarinejad & Safarinejad, 2009; Sigman et al., 2006) were used to show the efficacy of LC/LAC and NAC in men with idiopathic asthenozoospermia. Table 1 presents the basic characteristics of each study.
Table 1.
The Details of Individual Studies.
| Study | Country | Study Design | Treatment |
Sample Size |
Follow-Up | Dose | Mode of Administration | Inclusion Criteria | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | Experimental | Control | |||||||
| Kopets et al. (2020) | Ukraine | Randomized controlled trial | LC/LAC | Placebo | 42 | 41 | 4 months | 1990 mg daily | Oral | Age 21–50 years, idiopathic male infertility defined as absence of conception in a couple having a regular unprotected intercourse for 12 months with a woman without evident pathology that could cause infertility, sperm concentration <15 million/mL and/or <32% forms with progressive motility and/or <4% of sperm cells with normal morphology. |
| Balercia et al. (2005) | Italy | Randomized controlled trial | LC/LAC | Placebo | 15 | 15 | 6 months | 2 g/1 g daily | Oral | Age 20–40 years, infertility ≥2 years after regular sexual intercourses with a fertile woman; normal rheologic characteristics (appearance, consistency, and liquefaction) of semen, and volume and pH in the normal range; sperm count 20 *10^6/mL, sperm motility <50%, and normal sperm morphologic features ≥30%. |
| Sigman et al. (2006) | USA | Randomized controlled trial | LC/LAC | Placebo | 12 | 9 | 6 months | 2 g/1 g daily | Oral | Males aged 18–65 years with infertility of at least 6 months duration, sperm concentration of at least five million sperm/mL, motility of 10%–50%. |
| Lenzi et al. (2004) | Italy | Randomized controlled trial | LC/LAC | Placebo | 30 | 26 | 6 months | 2 g/1 g daily | Oral | Normal rheological characteristics (appearance, consistency, and liquefaction), volume and pH in the normal range, sperm concentration of 10–40 *10^6/mL; forward motility, <15%; total motility, 10%–40%; atypical forms, <80%; semen leukocytes, <1*10^6/mL. |
| Safarinejad & Safarinejad (2009) | Iran | Randomized controlled trial | NAC | Placebo | 105 | 106 | 26 weeks | 600 mg daily | Oral | Sperm count ≥5*10^6/mL, no factors in their history with a possible influence for male infertility, ≥2 years of failed attempts at conception, no female factors. |
| Ciftci et al. (2009) | Turkey | Randomized controlled trial | NAC | Placebo | 60 | 60 | 3 months | 600 mg daily | Oral | Idiopathic infertility with normal sperm parameters, determined by medical history and physical and seminal examination findings. |
| Jannatifar et al. (2019) | Iran | Randomized controlled trial | NAC | Placebo | 50 | 50 | 3 months | 600 mg daily | Oral | The infertile couples with no previous report of pregnancy, normal female partner, and male partner defined as having asthenoteratozoospermia. |
Note. LC/LAC = L-carnitine/L-acetyl-carnitine; NAC = N-acetyl-cysteine.
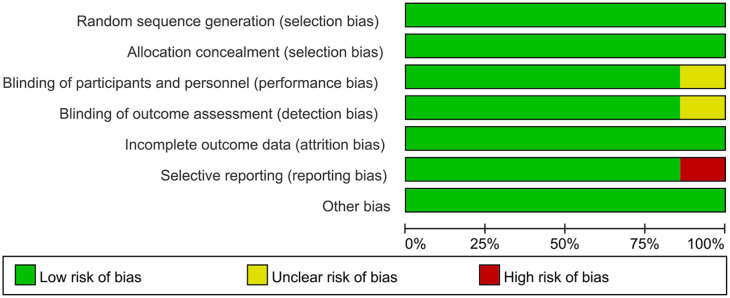
The Risk of Bias in Included Literature
All included studies were randomized controlled design with specific randomized protocols, and three study described the calculation of sample size. The outcomes of quality assessment are presented in Table 2. The risk of bias graph is presented in Figure 2.
Table 2.
The Quality Assessment of Each Study.
| Study | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias | Calculation of Sample Size | Statistical Analysis |
|---|---|---|---|---|---|---|---|---|---|
| Kopets et al. (2020) | + | + | + | + | + | + | + | Yes | ANCOVA |
| Balercia et al. (2005) | + | + | + | + | + | + | + | No | ANCOVA |
| Sigman et al. (2006) | + | + | + | + | + | + | + | No | Friedman test |
| Lenzi et al. (2004) | + | + | + | + | + | + | + | Yes | Independent-samples test; Student’s t-test; ANOVA; Mann–Whitney U test |
| Safarinejad & Safarinejad (2009) | + | + | + | + | + | + | + | Yes | ANOVA; Mann–Whitney U test |
| Ciftci et al. (2009) | + | + | ? | ? | + | - | + | No | Student’s t-test |
| Jannatifar et al. (2019) | + | + | + | + | + | + | + | No | t-test |
Note. ANCOVA = analysis of covariance.
Figure 2.
The risk of bias graph.
Sperm Concentration
LC/LAC Versus Placebo
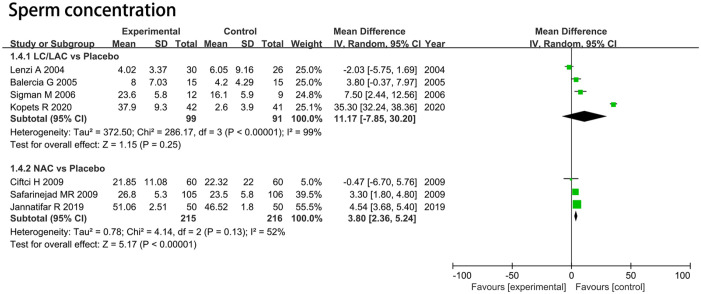
Four RCTs including 190 patients were included in the analysis. Forest plots drew an MD of 11.17 *10^6/mL and 95% CI [–7.85, 30.20] (p = .25), which indicated that LC/LAC did not have a better effect in raising sperm concentration compared with the placebo (Figure 3).
Figure 3.
Forest plots showing results in sperm parameters on the sperm concentration.
Note. SD = standard deviation; IV = inverse variance; CI = confidence interval; df = degrees of freedom.
NAC Versus Placebo
Three RCTs including 431 patients were included in the analysis. Forest plots drew an MD of 3.80 *10^6/mL and 95% CI [2.36, 5.24] (p < .00001), which indicated that NAC had a significantly greater increase in sperm concentration compared with the placebo (Figure 3).
Sperm Volume
LC/LAC Versus Placebo
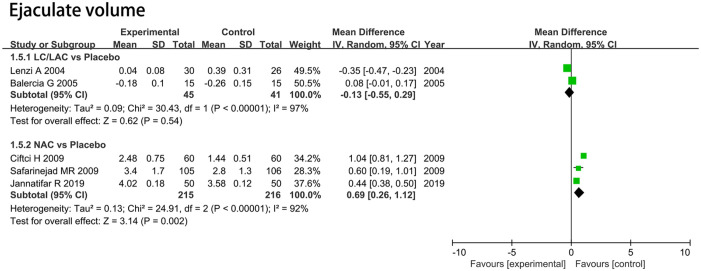
Two RCTs including 86 patients were included in the analysis. Forest plots drew an MD of 11.17 mL and 95% CI [–7.85, 30.20] (p = .25), which indicated that LC/LAC did not have a better effect in raising sperm volume compared with the placebo (Figure 4).
Figure 4.
Forest plots showing results in sperm parameters on the sperm volume.
Note. SD = standard deviation; IV = inverse variance; CI = confidence interval; df = degrees of freedom.
NAC Versus Placebo
Three RCTs including 431 patients were included in the analysis. Forest plots drew an MD of 0.69 ml and 95% CI [0.26, 1.12] (p = .002), which indicated that NAC had a significantly greater increase in sperm volume compared with the placebo (Figure 4).
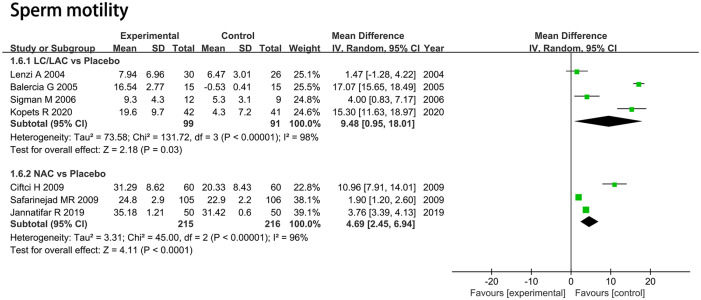
Sperm Motility (%)
LC/LAC Versus Placebo
Four RCTs including 190 patients were included in the analysis. Forest plots drew an MD of 9.48 and 95% CI [0.95, 18.01] (p = 0.03), which indicated that LC/LAC had a better effect in raising sperm motility compared with the placebo (Figure 5).
Figure 5.
Forest plots showing results in sperm parameters on the sperm motility.
Note. SD = standard deviation; IV = inverse variance; CI = confidence interval; df = degrees of freedom.
NAC Versus Placebo
Three RCTs including 431 patients were included in the analysis. Forest plots drew an MD of 4.69 and 95% CI [2.45, 6.94] (p < .0001), which indicated that NAC had a significantly greater increase in sperm motility compared with the placebo (Figure 5).
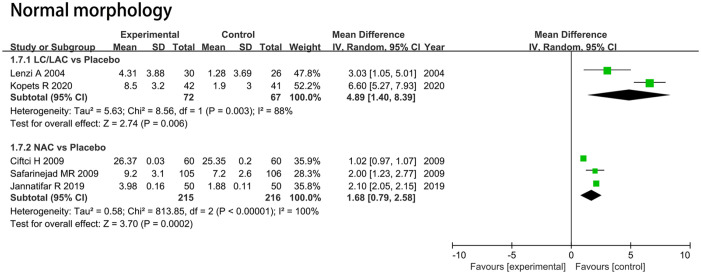
Normal Morphology (%)
LC/LAC Versus Placebo
Two RCTs including 139 patients were included in the analysis. Forest plots drew an MD of 4.89 and 95% CI [1.40, 8.39] (p = .006), which indicated that LC/LAC had a better effect in raising normal morphology compared with the placebo (Figure 6).
Figure 6.
Forest plots showing results in sperm parameters on the normal morphology.
Note. SD = standard deviation; IV = inverse variance; CI = confidence interval; df = degrees of freedom.
NAC Versus Placebo
Three RCTs including 431 patients were included in the analysis. Forest plots drew an MD of 1.68 and 95% CI [0.79, 2.58] (p = .0002), which indicated that NAC had a significantly greater increase in normal morphology for infertile men compared with the placebo (Figure 6).
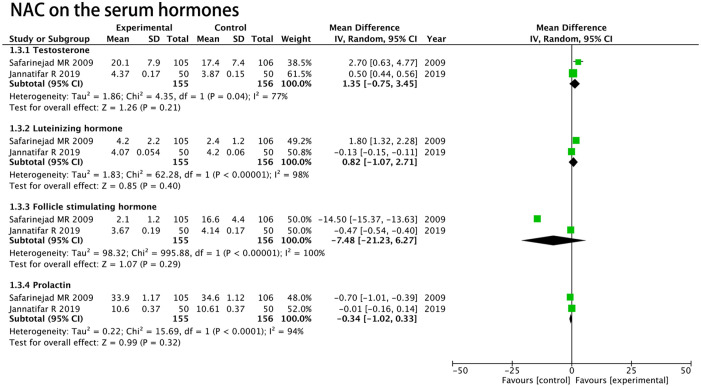
Hormonal Analysis
Two RCTs including 311 patients (155 patients in the NAC group and 156 patients in the placebo group) were used to estimate the level of testosterone, luteinizing hormone, follicle-stimulating hormone, and prolactin. The random model showed that there were no significant differences between NAC and placebo in raising the level of testosterone (MD, 1.35 ng/mL; 95% CI [–0.75, 3.45]; p = .21; Figure 7), luteinizing hormone (MD, 0.82 mIU/mL; 95% CI [–1.07, 2.71]; p = .40; Figure 7), follicle-stimulating hormone (MD, –7.48 mIU/mL; 95% CI [–21.23, 6.27]; p = .29; Figure 7), and prolactin (MD, –0.34 ng/mL; 95% CI [–1.02, 0.33]; p = .32; Figure 7) for infertile men compared with the placebo.
Figure 7.
Forest plots showing results in hormonal analysis.
Note. SD = standard deviation; IV = inverse variance; CI = confidence interval; df = degrees of freedom.
Discussion
This meta-analysis aimed to confirm the efficacy of LC/LAC and NAC in men with idiopathic asthenozoospermia from the perspective of EBM. The results indicated that LC/LAC and NAC had a considerable improvement in sperm motility and normal morphology compared with the placebo group. Besides, NAC had a significantly greater increase in sperm concentration and ejaculate volume compared with the placebo group, and there was no significant difference in LC/LAC. For the analysis of serum hormones, NAC had no obvious differences in improving the serum levels of testosterone, luteinizing hormone, follicle-stimulating hormone, and prolactin compared with the placebo group.
Infertility is an important medical and social problem, which has a huge impact on health (Agarwal et al., 2020). Among them, the treatment of idiopathic male infertility is still controversial, and most of the clinical treatment still depends on empirical methods (Agarwal et al., 2020). In recent years, the application of LC/LAC in the treatment of oligoasthenoteratozoospermia of unknown origin has been proposed. Many studies have reported that their effects on sperm epididymal maturation and energy metabolism are an intracellular mechanism, and their effects on testicular epididymal microenvironment are an indirect antioxidant effect (Lenzi et al., 1992).
Free LC, named 3-hydroxy-4-N-trymethylaminobutyric acid, plays a key role in cell energy production. Free LC is a necessary molecule in the process of mitochondrial oxidation of long chain fatty acids. Fatty acids must combine with coenzyme A (CoA) to form acyl-CoA to enter the mitochondria. Long chain acyl-CoA can’t pass through the internal mitochondrial membrane, and hence this progress needs a specific enzymatic mechanism, which uses LC as a shuttle (Bremer, 1983; Jeulin et al., 1994; Jeulin & Lewin, 1996). After transport into the mitochondria, acyl is transferred to the mitochondrial CoA by acyl-carnitine. This initiates oxidation with the product adenosine triphosphate and finally exits as LAC to start a new transport cycle. Carnitine can not only protect cell membrane and DNA from oxygen free radical damage but can also prevent protein oxidation and pyruvate and lactate oxidative damage (Arduini, 1992). The activation of sperm motility occurred simultaneously with the increase of carnitine concentration in the epididymal lumen and LAC in the spermatozoa (Radigue et al., 1996). Epididymal epithelium secretes LC into the lumen through a specific active transport system (Enomoto et al., 2002). Considering their mechanisms and functions, LC and LAC have been proposed as a possible treatment in selected forms of oligoasthenoteratozoospermia (Lenzi et al., 2003; Vicari et al., 2002).
NAC is a N-acetyl derivative of natural amino acid L-cysteine. Because it can significantly reduce disulfide bond, NAC is used to reduce viscosity and elasticity of mucus (Aitken et al., 1993). Like many thiols, NAC is a good scavenger for hydroxyl radicals (Orzechowski et al., 2002). In the included literature, all patients were treated with 600 mg/d NAC for at least 3 months, which was considered as a safe drug dose with a higher median lethal dose (Ciftci et al., 2009; Jannatifar et al., 2019; Safarinejad & Safarinejad, 2009). The study reported that although sperm density and sperm activity did not improve, sperm function tended to improve and ROS level decreased significantly after NAC administration (Akiyama, 1999). Different from the results of this study, an RCT found that sperm motility was improved after NAC treatment. In this meta-analysis, compared with the control group, the viscosity, liquefaction time, and sperm volume of NAC group were significantly increased.
It is found that semen mucinosis is closely related to male infertility (Elzanaty et al., 2004). Semen viscosity plays an important role in the process of pregnancy because the sperm may adhere to semen fiber or mucus, which prevents the sperm from migrating from seminal plasma to the cervix and fertilization site. NAC can reduce the effect of ROS activity in the sperm, which was a factor for reducing sperm viscosity (Orzechowski et al., 2002).
This study reported the positive effects of the use of the LC/LAC and NAC for treating male infertility caused by oxidative stress on the semen parameters. The latest report stated that administrating the exogenous antioxidants can cause oxidative stress induced by the antioxidant paradox or reductive stress accompanied by diminishments in the endogenous oxidants, which are required to induce the physiological pathways that suppress functions like sperm capacitation (Beygi et al., 2020). Thus, these antioxidants should be received cautiously and patients should take necessary tests for their redox status prior to the treatment with a supplement of specific formulation. Notably, the treatment should be specifically personalized to achieve normal redox status (Henkel et al., 2019).
Spermatogenesis is highly controlled by hormonal environment, in addition to affecting the speed and quality of spermatogenesis; any change of hormone distribution may profoundly affect the chromosome ploidy and the integrity of sperm chromatin (Safarinejad & Safarinejad, 2009). The increase of testosterone has a negative feedback effect on the secretion of hypothalamus and pituitary, which leads to the decrease of GnRH pulse frequency and pituitary response to GnRH, and finally the decrease of gonadotropin release (Hayes et al., 2000). Khan et al. (2013) reported that the level of total antioxidant capacity (TAC) in seminal plasma of patients with asthenospermia was significantly lower than that of healthy men. Jannatifar et al. (2019) reported that the concentration of seminal malondialdehyde (MDA) decreased significantly, and MDA was a specific marker of lipid peroxidation, while TAC increased significantly after NAC treatment. Therefore, NAC reduces the severity of oxidative stress, thus reducing lipid peroxidation and DNA fragmentation.
We need to acknowledge the limitations of this analysis. There are deficiencies in the quality of the trials used for analysis, mainly including fewer trials included and inconsistent basic indicators of various studies. However, the studies included in this study are all RCTs, which increases the strength of the research results. In addition, selection bias and subjective bias may affect the final results of this study.
Conclusions
LC/LAC and NAC showed a greater improvement in sperm motility and normal morphology. Moreover, NAC has a positive effect on sperm concentration and ejaculate volume, whereas no obvious effect in serum hormones.
Footnotes
Author Contribution: Conceptualization: Yong Zhang. Data curation: Guangzhu Wei, Zhongbao Zhou, Yuanshan Cui. Formal analysis: Guangzhu Wei, Zhongbao Zhou. Funding acquisition: Yong Zhang, Yuanshan Cui. Investigation: Yongjin Huang, Zijin Wan, Xuanyan Che, Yumeng Cai. Methodology: Guangzhu Wei, Zhongbao Zhou, Yongjin Huang, Zijin Wan, Xuanyan Che. Project administration: Yong Zhang. Resources: Zijin Wan, Xuanyan Che, Yumeng Cai. Software: Guangzhu Wei, Zhongbao Zhou. Supervision: Yong Zhang. Writing – original draft: Guangzhu Wei, Yuanshan Cui, Yongjin Huang. Writing – review & editing: Yong Zhang, Yuanshan Cui.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Beijing Municipal Administration of Hospitals’ Ascent Plan, Code: DFL20190502; Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support, Code: ZYLX201820; National Nature Science Foundation of China, Code: 81801429.
Ethical statement: No ethics to disclose.
ORCID iD: Zhongbao Zhou  https://orcid.org/0000-0002-9810-8145
https://orcid.org/0000-0002-9810-8145
References
- Agarwal A., Baskaran S., Parekh N., Cho C. L., Henkel R., Vij S., Arafa M., Selvam M. K. P., Shah R. (2020). Male infertility. Lancet. 10.1016/s0140-6736(20)32667-2 [DOI] [PubMed]
- Aitken R. J., Buckingham D., Harkiss D. (1993). Use of a xanthine oxidase free radical generating system to investigate the cytotoxic effects of reactive oxygen species on human spermatozoa. Journal of Reproduction and Infertility, 97(2), 441–450. 10.1530/jrf.0.0970441 [DOI] [PubMed] [Google Scholar]
- Akiyama M. (1999). In vivo scavenging effect of ethylcysteine on reactive oxygen species in human semen. Nihon Hinyokika Gakkai Zasshi, 90(3), 421–428. 10.5980/jpnjurol1989.90.421 [DOI] [PubMed] [Google Scholar]
- Arduini A. (1992). Carnitine and its acyl esters as secondary antioxidants? American Heart Journal, 123(6), 1726–1727. 10.1016/0002-8703(92)90850-u [DOI] [PubMed] [Google Scholar]
- Balercia G., Regoli F., Armeni T., Koverech A., Mantero F., Boscaro M. (2005). Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertility and Sterility, 84(3), 662–671. 10.1016/j.fertnstert.2005.03.064 [DOI] [PubMed] [Google Scholar]
- Beygi Z., Forouhari S., Mahmoudi E., Hayat S. M. G., Nourimand F. (2020). Role of oxidative stress and antioxidant supplementation in male fertility. Current Molecular Medicine. 10.2174/1566524020999200831123553 [DOI] [PubMed]
- Bremer J. (1983). Carnitine–metabolism and functions. Physiological Reviews, 63(4), 1420–1480. 10.1152/physrev.1983.63.4.1420 [DOI] [PubMed] [Google Scholar]
- Ciftci H., Verit A., Savas M., Yeni E., Erel O. (2009). Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology, 74(1), 73–76. 10.1016/j.urology.2009.02.034 [DOI] [PubMed] [Google Scholar]
- Comhaire F. H., Christophe A. B., Zalata A. A., Dhooge W. S., Mahmoud A. M., Depuydt C. E. (2000). The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 63(3), 159–165. 10.1054/plef.2000.0174 [DOI] [PubMed] [Google Scholar]
- Cumpston M., Li T., Page M. J., Chandler J., Welch V. A., Higgins J. P., Thomas J. (2019). Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Database of Systematic Reviews, 10, Ed000142. 10.1002/14651858.Ed000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. (1986). Meta-analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Dohle G. R., Diemer T., Kopa Z., Krausz C., Giwercman A., Jungwirth A. (2012). European Association of Urology guidelines on vasectomy. European Urology, 61(1), 159–163. 10.1016/j.eururo.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Elzanaty S., Malm J., Giwercman A. (2004). Visco-elasticity of seminal fluid in relation to the epididymal and accessory sex gland function and its impact on sperm motility. International Journal of Andrology, 27(2), 94–100. 10.1046/j.1365-2605.2003.00455.x [DOI] [PubMed] [Google Scholar]
- Enomoto A., Wempe M. F., Tsuchida H., Shin H. J., Cha S. H., Anzai N., Goto A., Sakamoto A., Niwa T., Kanai Y., Anders M. W., Endou H. (2002). Molecular identification of a novel carnitine transporter specific to human testis. Insights into the mechanism of carnitine recognition. The Journal of Biological Chemistry, 277(39), 36262–36271. 10.1074/jbc.M203883200 [DOI] [PubMed] [Google Scholar]
- Erkkilä K., Hirvonen V., Wuokko E., Parvinen M., Dunkel L. (1998). N-acetyl-L-cysteine inhibits apoptosis in human male germ cells in vitro. The Journal of Clinical Endocrinology and Metabolism, 83(7), 2523–2531. 10.1210/jcem.83.7.4949 [DOI] [PubMed] [Google Scholar]
- Gnoth C., Godehardt E., Frank-Herrmann P., Friol K., Tigges J., Freundl G. (2005). Definition and prevalence of subfertility and infertility. Human Reproduction, 20(5), 1144–1147. 10.1093/humrep/deh870 [DOI] [PubMed] [Google Scholar]
- Hayes F. J., Seminara S. B., Decruz S., Boepple P. A., Crowley W. F., Jr. (2000). Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. The Journal of Clinical Endocrinology and Metabolism, 85(9), 3027–3035. 10.1210/jcem.85.9.6795 [DOI] [PubMed] [Google Scholar]
- Henkel R., Sandhu I. S., Agarwal A. (2019). The excessive use of antioxidant therapy: A possible cause of male infertility? Andrologia, 51(1), e13162. 10.1111/and.13162 [DOI] [PubMed] [Google Scholar]
- Jannatifar R., Parivar K., Roodbari N. H., Nasr-Esfahani M. H. (2019). Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reproductive Biology and Endocrinology, 17(1), 24. 10.1186/s12958-019-0468-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeulin C., Dacheux J. L., Soufir J. C. (1994). Uptake and release of free L-carnitine by boar epididymal spermatozoa in vitro and subsequent acetylation rate. Journal of Reproduction and Fertility, 100(1), 263–271. 10.1530/jrf.0.1000263 [DOI] [PubMed] [Google Scholar]
- Jeulin C., Lewin L. M. (1996). Role of free L-carnitine and acetyl-L-carnitine in post-gonadal maturation of mammalian spermatozoa. Human Reproduction Update, 2(2), 87–102. 10.1093/humupd/2.2.87 [DOI] [PubMed] [Google Scholar]
- Khan R. U., Rahman Z. U., Javed I., Muhammad F. (2013). Effect of vitamins, probiotics and protein level on semen traits and seminal plasma biochemical parameters of post-moult male broiler breeders. British Poultry Science, 54(1), 120–129. 10.1080/00071668.2012.753511 [DOI] [PubMed] [Google Scholar]
- Kopets R., Kuibida I., Chernyavska I., Cherepanyn V., Mazo R., Fedevych V., Gerasymov S. (2020). Dietary supplementation with a novel l-carnitine multi-micronutrient in idiopathic male subfertility involving oligo-, astheno-, teratozoospermia: A randomized clinical study. Andrology, 8(5), 1184–1193. 10.1111/andr.12805 [DOI] [PubMed] [Google Scholar]
- Lenzi A., Lombardo F., Gandini L., Dondero F. (1992). Metabolism and action of L-carnitine: Its possible role in sperm tail function. Archivio Italiano di Urologia, Nefrologia, Andrologia, 64(2), 187–196. [PubMed] [Google Scholar]
- Lenzi A., Lombardo F., Sgrò P., Salacone P., Caponecchia L., Dondero F., Gandini L. (2003). Use of carnitine therapy in selected cases of male factor infertility: A double-blind crossover trial. Fertility and Sterility, 79(2), 292–300. 10.1016/s0015-0282(02)04679-4 [DOI] [PubMed] [Google Scholar]
- Lenzi A., Sgrò P., Salacone P., Paoli D., Gilio B., Lombardo F., Santulli M., Agarwal A., Gandini L. (2004). A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia. Fertility and Sterility, 81(6), 1578–1584. 10.1016/j.fertnstert.2003.10.034 [DOI] [PubMed] [Google Scholar]
- Micic S., Lalic N., Djordjevic D., Bojanic N., Bogavac-Stanojevic N., Busetto G. M., Virmani A., Agarwal A. (2019). Double-blind, randomised, placebo-controlled trial on the effect of L-carnitine and L-acetylcarnitine on sperm parameters in men with idiopathic oligoasthenozoospermia. Andrologia, 51(6), e13267. 10.1111/and.13267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. (2010). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. International Journal of Surgery, 8(5), 336–341. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Oeda T., Henkel R., Ohmori H., Schill W. B. (1997). Scavenging effect of N-acetyl-L-cysteine against reactive oxygen species in human semen: A possible therapeutic modality for male factor infertility? Andrologia, 29(3), 125–131. 10.1111/j.1439-0272.1997.tb00305.x [DOI] [PubMed] [Google Scholar]
- Orzechowski A., Łokociejewska M., Muras P., Hocquette J. F. (2002). Preconditioning with millimolar concentrations of vitamin C or N-acetylcysteine protects L6 muscle cells insulin-stimulated viability and DNA synthesis under oxidative stress. Life Sciences, 71(15), 1793–1808. 10.1016/s0024-3205(02)01942-2 [DOI] [PubMed] [Google Scholar]
- Pierik F. H., Van Ginneken A. M., Dohle G. R., Vreeburg J. T., Weber R. F. (2000). The advantages of standardized evaluation of male infertility. International Journal of Andrology, 23(6), 340–346. 10.1046/j.1365-2605.2000.00250.x [DOI] [PubMed] [Google Scholar]
- Radigue C., Es-Slami S., Soufir J. C. (1996). Relationship of carnitine transport across the epididymis to blood carnitine and androgens in rats. Archives of Andrology, 37(1), 27–31. 10.3109/01485019608988499 [DOI] [PubMed] [Google Scholar]
- Safarinejad M. R., Safarinejad S. (2009). Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: A double-blind, placebo controlled, randomized study. The Journal of Urology, 181(2), 741–751. 10.1016/j.juro.2008.10.015 [DOI] [PubMed] [Google Scholar]
- Sigman M., Glass S., Campagnone J., Pryor J. L. (2006). Carnitine for the treatment of idiopathic asthenospermia: A randomized, double-blind, placebo-controlled trial. Fertility and Sterility, 85(5), 1409–1414. 10.1016/j.fertnstert.2005.10.055 [DOI] [PubMed] [Google Scholar]
- Vicari E., La Vignera S., Calogero A. E. (2002). Antioxidant treatment with carnitines is effective in infertile patients with prostatovesiculoepididymitis and elevated seminal leukocyte concentrations after treatment with nonsteroidal anti-inflammatory compounds. Fertility and Sterility, 78(6), 1203–1208. 10.1016/s0015-0282(02)04350-9 [DOI] [PubMed] [Google Scholar]
- Zafarullah M., Li W. Q., Sylvester J., Ahmad M. (2003). Molecular mechanisms of N-acetylcysteine actions. Cellular and Molecular Life Sciences, 60(1), 6–20. 10.1007/s000180300001 [DOI] [PMC free article] [PubMed] [Google Scholar]