ABSTRACT
Although COVID-19 vaccines have recently been approved for emergency use, search for new vaccines are still urgent, since the access of the countries, especially the poorest, to the vaccines, has shown to be slower than the necessary to rapidly control the pandemic. We proposed a novel platform for vaccine using recombinant receptor binding domain (rRBD) from Sars-Cov-2 spike protein and Neisseria meningitidis outer membrane vesicles (OMVs). The antigen preparation produced a humoral and cellular immune response. Taken together our findings suggest a good immunostimulatory patter in response to immunization with rRBD plus N. meningitidis OMV.
KEYWORDS: SARS-Cov-2, OMV, Neisseria meningitidis, immune response
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a recently identified coronavirus that causes coronavirus disease 2019 (COVID-19)1 characterized by its severe ‘flu’-like symptoms that can progress to acute respiratory distress (ARDS), pneumonia, renal failure, and death.2 COVID-19 was declared by WHO a public health emergency of international concern on 30 January 2020. Due to the great pathogenicity and rapid spread of SARS-Cov-2, COVID-19 became the deadliest, fastest-moving pandemic since 1918, leading to hundreds of thousands of deaths and huge economic impact.3 Although the first vaccines have recently been approved for emergency use, the access of the countries, especially the poorest, to the vaccines, has shown to be slower than the necessary to rapidly reach the herd immunity and slowdown the pandemic. Thus, researches for new vaccines and adjuvants are urgent.
The spike (S) protein is a structural glycoprotein which plays a key role in the receptor recognition and cell membrane fusion process.4 This protein is composed of two subunits, S1 and S2. The S1 subunit possess the receptor-binding domain (RBD) that binds to the host cell receptor angiotensin-converting enzyme 2 (ACE2) mediating viral cell entry.5 Due to this characteristic, RBD is a critical target for neutralizing antibodies.4
The Outer membrane vesicles (OMVs) are nano-sized spherical blebs shed by Gram-negative bacteria that reflects outer membrane composition. It is composed of bacteria surface-exposed antigens in native conformation and orientation, and immunostimulatory molecules, such as lipopolysaccharide (LPS), lipoproteins or peptidoglycans. These vesicles mimic the outside of bacteria, resembling a pathogen but, as they are non-living, lack the ability to cause associated disease and have been proposed as vaccines since their discovery.6 Vaccines against Neisseria meningitidis serogroup B (MenB) based on OMVs have proved to be very useful against epidemic strains in Cuba,7 Norway8 and New Zealand.9 On the other hand, the 4CMenB (Bexsero) vaccine that also contains outer membrane vesicles (NZ OMV) and additionally contains three surface-exposed recombinant proteins (fHbp, NadA, and NHBA), shows important cross-reactivity with other MenB strains and it is estimated to provide 66–91% coverage against MenB strains worldwide.10 Besides being approved for use against MenB around the world, the OMV-based vaccines have also been tested against other diseases, such as N. gonorrhea, Mycobacterium tuberculosis, Klebsiella pneumoniae, Salmonella and has proved to be more immunogenic than classical vaccines.6
Here, we propose a preliminary study of a Covid-19 vaccine with rRBD as antigen and N. meningitidis OMV and aluminum hydroxide as adjuvants, with two intramuscular and two intranasal doses.
The critical role of S protein to Sars-Cov-2 cell invasion makes this protein an obvious vaccine candidate.11 Thus, the S protein, including RBD, is actually, the main target to anti-COVID-19 vaccines development,12 and its effectivity as immunogen has proved to be satisfactory even in phase III studies, with different effectiveness, depending on the vaccine platform used.13–15 Besides the choice of the antigenic target, the choice for a good adjuvant is crucial to vaccine development. OMVs from Gram-negative bacteria have shown to have adjuvant properties, since due to its membrane composition, it retains several pathogen-associated molecular pattern (PAMPs), recognized by pattern recognition receptors (PPRs) in antigen presenting cells (APC), what is crucial for a strong adaptive immune response.16 Its application to deliver heterologous vaccine antigens has been demonstrated to bacterial, viral, parasitic, and even cancer antigens.17–20
Aluminum hydroxide was chosen as a compound of our vaccine design, since it has been used as an adjuvant for the licensed vaccines containing OMV, such as Bexsero®21 and its use is allowed in humans. The intramuscular route of administration is the choice for inactivated vaccines that contain aluminum hydroxide in the preparation22 Boost with intranasal antigen23 free of aluminum hydroxide aimed to verify whether the animals would intensify or modulate the response after this administration.
In our study, although immunization with rRBD alone induced a small production of sera with IgG directed toward SARS-CoV-2, we observed an increase in IgG production when rRBD was mixed to OMV plus aluminum hydroxide, in both, prep1 and prep2, 15 and 45 days after the first immunization dose (Figure 1(a)). As a versatile delivery system, OMVs can be used in a simple mixture with the antigen,24 chemically conjugated to them25 or can be genetically engineered to express the antigen proteins.26 Although there are some evidence that chemical conjugation improves the OMV adjuvant property,25 the simple mixture of the OMV with antigens, as we have done in this work, is capable of inducing serum production of IgG heterologous to OMV, that is, specific to the vaccine antigen.17,18,24,27 We have recently demonstrated that this vaccine schedule induces anti-RBD IgG with intermediary avidity,28 what is not completely surprising, since, even natural infection has shown to fail in inducing high avidity antibody directed toward SARS‐CoV‐2 antigens, probably due to incomplete avidity maturation.29 Since the interaction between SARS-CoV-2 and human ACE2 possesses strong affinity, the induction of high avidity anti- SARS-CoV-2 antibodies are desirable and can be necessary to truly block SARS-CoV-2 adherence and infection30 and can be a challenge. We also observed IgA production, but only 37 days after the first immunization dose, i.e., only after the first boost with intranasal antigen for both prep1 and prep2 immunization (Figure 1(b)), what is interesting since IgA seems to dominate the early neutralizing antibody response to SARS-CoV-231 We also verified the anti-rRBD IgG production in the supernatant of the splenocytes stimulated in vitro with rRBD. In this experiment, IgG production was higher in the groups immunized with prep1 and prep2, compared to the animals immunized with rRBD alone. Although the total amount of antibody in animals immunized with prep1 was slightly higher than in animals immunized with prep2, no statistical significance was observed (Figure 1(c)).
Figure 1.
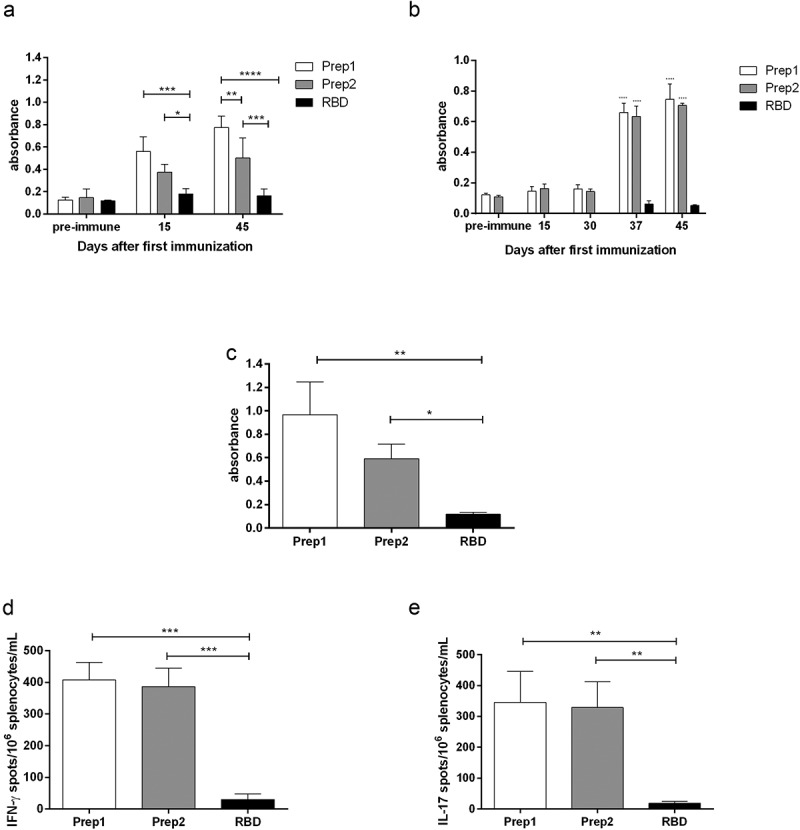
Immune response after immunization with rRBD from Sars-Cov-2 with aluminum hydroxide plus OMV from Neisseria meningitidis strains B:8:P1.6 (prep1) or C:2a.P1.5 (prep2). The IgG production in sera collected 15 and 45 days after the first immunization dose was measured by ELISA and can be observed in (a). The IgA production was accessed 15, 30, 37 and 45 days after the first immunization dose (b). Additionally, 45 days after the first immunization dose, the animals were euthanized and the splenocytes were cultured under rRBD stimuli. The IgG production in culture supernatant was evaluated by ELISA (c) and the number of cells/106 splenocytes producing IFN-γ (d) or IL-17 (e) was evaluated through ELISpot
We found an increase in IFN-γ producing cells in splenocytes culture from animals immunized with prep1 and prep2. The animals immunized with preparations containing OMV produced around 13 times more IFN-γ producing cells in splenocytes culture, than animals immunized with rRBD alone (Figure 1(d)). This finding is in accordance to others, that also find higher number of IFN-γ-producing cells32 or higher levels of IFN-γ in the sera33 or culture supernatant34 of the animals receiving OMV in their vaccine preparations. We also found an increase in IL-17 producing cells in splenocytes derived from animals immunized with prep1 and prep2 after stimuli with rRBD (Figure 1(e)). Kim et al.,34 although used OMV as a homologous vaccine, also found an increase in IL-17 production by culture splenocytes. These authors suggest the protection against bacteria-induced lethality occurs via Th1 and Th17 cell responses, since they also found increase in IFN-γ production. Il-17 production was elicited after immunization with SARS-CoV-2 pre-fusion stabilized (S-2P) spike protein adjuvanted with CAF®01 but not with aluminum hydroxide, nor a squalene-based oil-in-water emulsion system.35 Although IL-17 has been strongly associated with immunopathology, it also has an important role in host defense.36 This cytokine plays a key role in defense from both extracellular bacteria and viruses that infect airway mucous membranes, regulates homeostasis, and contributes to the repair of epithelial cells, damaged previously by an extracellular inflammatory stimulus.37 The exact role of IL-17 against Sars-CoV-2 is not completely elucidated. Its production seems to be enhanced in Sars-CoV-2 infected people with heterogeneous commitment degrees, but its association with disease severity is not clear.38,39
Although our data are preliminary, and more studies are necessary specially to improve avidity of anti-SARS-CoV-2 antibodies, taken together our findings suggest a good immunostimulatory pattern in response to immunization with rRBD plus N. meningitidis OMV, showing a promising platform to anti-COVID-19 vaccine development. Additional studies with animal models as OMV without adjuvants or others are required to assess the potential safety and effectiveness of this vaccine approach in humans.
Methodology
Outer membrane vesicles (OMV) from N. meningitidis strains, serogroup B: (B:8:P1.6) and serogroup C (C:2a.P1.5) were obtained as previously described.40 These two strains have been used for our group to produce OMVs with protein compounds slightly different (data not shown), so they deserved to be tested in separated vaccine preparations. Moreover, they belonged to two different serogroups, B and C. Five Swiss female mice per group were immunized with 3 µg/animal of recombinant receptor-binding domain (rRBD) from SARS-Cov-2 protein S complexed to aluminum hydroxide at 0.1 mM plus 10 μg/ml of OMV from N. meningitidis strains B:8:P1.6 (prep1) or C:2a.P1.5 (prep2) or rRBD alone, as control. The OMVs were detoxified as previously described.41 Animals were immunized with two doses of antigenic preparations intramuscullarlly (IM), 15 days apart and two doses intranasally (IN), 7 days apart.28 The intranasal inoculation was performed with RBD plus OMV, without aluminum hydroxide. Blood samples were obtained by retro-orbital plexus puncture in anesthetized animals, 15 and 45 days after the first immunization dose. Forty-five days after immunization the animals were anesthetized with Dopalen and Anasedan (CEVA, Brazil), sacrificed by cervical dislocation and the spleens were collected for the ELISpot assay. The supernatant from splenocyte culture was collected to IgG measurement. All procedures involving animals were performed following the guidelines of the Brazilian Code for the Use of Laboratory Animals and approved by the ethics committee of CEUA-IAL/Pasteur (protocol number 03/2012 extended to 2022).
Enzyme-Linked Immunosorbent assay (ELISA) from mice sera or from splenocyte supernatant was performed as previously described,40 except for the adsorption step, in which polystyrene plates (Maxisorp Nunc – Thermo Fisher Scientific, Rochester, NY, USA) were adsorbed with 100 μL/well of a solution containing 1 μg/mL of rRBD diluted in 0.05 M carbonate-bicarbonate buffer pH 9.6, and incubated overnight at 4°C, in a humid chamber. The secondary antibodies (dilution) used were anti-mouse IgG (γ) (1:5.000) or IgA(α)(1:2000) (Kirkegaard & Per Laboratories, KPL, Gaithersburg, MD, EUA).
The Enzyme-Linked Immunosorbent Spot (ELISpot) assay was also performed as described by Trzewikoswki de Lima et al.,40 with minor modifications. The splenocytes derived from immunized animals were added to the wells at 1 × 106 cells/mL and the cells were stimulated with 2 µg/mL of rRBD, 10 µg/mL concanavalin A (ConA) (Sigma Chemical Company, St. Louis, MO, USA) or added without stimulus.
The significance of the results was evaluated by ANOVA with Tukey post test. P values were considered significant when p ≤ 0.05.
Acknowledgments
The research was supported with grants by FAPESP (18/04202-0 e 12/15568-0). The receptor-binding domain (RBD) antigen was kindly donated by Dr. Florian Krammer, from Icahn School of Medicine, Mount Sinai, Nova York, NY, EUA.
Funding Statement
The research was supported with grants by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (18/04202-0 e 12/15568-0).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Sun P, Lu X, Xu C, Sun W, Pan B.. Understanding of COVID-19 based on current evidence. J Med Virol. 2020;92(6):548–51. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020;41(12):1100–15. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, Agha M, Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–93. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y, Yang C, Xu X-F, Xu W, Liu S-W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141–49. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5(4):562–69. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micoli F, MacLennan CA. Outer membrane vesicle vaccines. Semin Immunol. 2020;50:101433. doi: 10.1016/j.smim.2020.101433. [DOI] [PubMed] [Google Scholar]
- 7.Sierra GV, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, Sotolongo PF, Casanueva GV, Rico CO, Rodriguez CR, Terry MH, et al. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14(2):195–207. discussion 8–10. [PubMed] [Google Scholar]
- 8.Bjune G, Høiby EA, Grønnesby JK, Arnesen Ø, Fredriksen JH, Lindbak A-K, Nøkleby H, Rosenqvist E, Solberg LK, Closs O, et al. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338(8775):1093–96. doi: 10.1016/0140-6736(91)91961-S. [DOI] [PubMed] [Google Scholar]
- 9.Arnold R, Galloway Y, McNicholas A, O’Hallahan J. Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine. 2011;29(40):7100–06. doi: 10.1016/j.vaccine.2011.06.120. [DOI] [PubMed] [Google Scholar]
- 10.O’Ryan M, Stoddard J, Toneatto D, Wassil J, Dull PM. A multi-component meningococcal serogroup B vaccine (4CMenB): the clinical development program. Drugs. 2014;74(1):15–30. doi: 10.1007/s40265-013-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Kutateladze TG. Molecular structure analyses suggest strategies to therapeutically target SARS-CoV-2. Nat commun. 2020;11(1):2920.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO . Draft landscape and tracker of COVID-19 candidate vaccines: WHO; 2021. [accessed 2021 Aug 2]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 13.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–81. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mancini F, Rossi O, Necchi F, Micoli F. OMV vaccines and the role of TLR agonists in immune response. Int J Mol Sci. 2020;21(12):4416. doi: 10.3390/ijms21124416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koeberling O, Giuntini S, Seubert A, Granoff DM. Meningococcal outer membrane vesicle vaccines derived from mutant strains engineered to express factor H binding proteins from antigenic variant groups 1 and 2. Clin Vaccine Immunol: CVI. 2009;16(2):156–62. doi: 10.1128/CVI.00403-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins HC, Rappazzo CG, Higgins JS, Sun X, Brock N, Chau A, Misra A, Cannizzo JPB, King MR, Maines TR, et al. Safe recombinant outer membrane vesicles that display M2e elicit heterologous influenza protection. Mol Therapy: J Am Soc Gene Therapy. 2017;25(4):989–1002. doi: 10.1016/j.ymthe.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scaria PV, Rowe CG, Chen BB, Muratova OV, Fischer ER, Barnafo EK, Anderson CF, Zaidi IU, Lambert LE, Lucas BJ, et al. Outer membrane protein complex as a carrier for malaria transmission blocking antigen Pfs230. NPJ Vaccines. 2019;4(1):24. doi: 10.1038/s41541-019-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grandi A, Tomasi M, Zanella I, Ganfini L, Caproni E, Fantappiè L, Irene C, Frattini L, Isaac SJ, König E, et al. Synergistic protective activity of tumor-specific epitopes engineered in bacterial outer membrane vesicles. Front Oncol. 2017;7:253. doi: 10.3389/fonc.2017.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.FDA. BEXSERO® US prescribing information [internet]. FDA; 2015. [uploaded 2015; cited 2021 Mar 23]. Available from https://www.fda.gov/media/90996/download.
- 22.Cook IF. Evidence based route of administration of vaccines. Hum Vaccin. 2008;4(1):67–73. doi: 10.4161/hv.4.1.4747. [DOI] [PubMed] [Google Scholar]
- 23.Guy B, Fourage S, Hessler C, Sanchez V, Millet MJ. Effects of the nature of adjuvant and site of parenteral immunization on the serum and mucosal immune responses induced by a nasal boost with a vaccine alone. Clin Diagn Lab Immunol. 1998;5(5):732–36. doi: 10.1128/CDLI.5.5.732-736.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sardiñas G, Reddin K, Pajon R, Gorringe A. Outer membrane vesicles of Neisseria lactamica as a potential mucosal adjuvant. Vaccine. 2006;24(2):206–14. doi: 10.1016/j.vaccine.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 25.Micoli F, Alfini R, Di Benedetto R, Necchi F, Schiavo F, Mancini F, Carducci M, Palmieri E, Balocchi C, Gasperini G, et al. GMMA is a versatile platform to design effective multivalent combination vaccines. Vaccine. 2020;8(3):540. doi: 10.3390/vaccines8030540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen DJ, Osterrieder N, Metzger SM, Buckles E, Doody AM, DeLisa MP, Putnam D. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc Natl Acad Sci. 2010;107(7):3099–104. doi: 10.1073/pnas.0805532107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DH, Kim S-H, Kang W, Choi YS, Lee S-H, Lee S-R, You S, Lee HK, Chang K-T, Shin E-C, et al. Adjuvant effect of bacterial outer membrane vesicles with penta-acylated lipopolysaccharide on antigen-specific T cell priming. Vaccine. 2011;29(46):8293–301. doi: 10.1016/j.vaccine.2011.08.102. [DOI] [PubMed] [Google Scholar]
- 28.Gaspar EB, De Gaspari E. Avidity assay to test functionality of anti-SARS-Cov-2 antibodies. Vaccine. 2021;39(10):1473–75. doi: 10.1016/j.vaccine.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauer G, Struck F, Schreiner P, Staschik E, Soutschek E, Motz M. The challenge of avidity determination in SARS-CoV-2 serology. J Med Virol. 2021;93(5):3092–3104. doi: 10.1002/jmv.26863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khatri I, Staal FJT, Van Dongen JJM. Blocking of the high-affinity interaction-synapse between SARS-CoV-2 spike and human ACE2 proteins likely requires multiple high-affinity antibodies: an immune perspective. Front Immunol. 2020;11(2258). doi: 10.3389/fimmu.2020.570018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, Quentric P, Fadlallah J, Devilliers H, Ghillani P. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Trans Med. 2021;13(577). doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watkins HC, Pagan CL, Childs HR, Posada S, Chau A, Rios J, Guarino C, DeLisa MP, Whittaker GR, Putnam D, et al. A single dose and long lasting vaccine against pandemic influenza through the controlled release of a heterospecies tandem M2 sequence embedded within detoxified bacterial outer membrane vesicles. Vaccine. 2017;35(40):5373–80. doi: 10.1016/j.vaccine.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Xu K, Zhao Q, Wen X, Wu R, Wen Y, Huang X, Huang Y, Yan Q, Han X, Ma X, et al. A trivalent Apx-fusion protein delivered by E. coli outer membrane vesicles induce protection against Actinobacillus pleuropneumoniae of serotype 1 and 7 challenge in a murine model. PLoS One. 2018;13(1):e0191286. doi: 10.1371/journal.pone.0191286. PMID: 29373591; PMCID: PMC5786296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim OY, Hong BS, Park K-S, Yoon YJ, Choi SJ, Lee WH, Roh T-Y, Lötvall J, Kim Y-K, Gho YS, et al. Immunization with escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J Immunol. 2013;190(8):4092–102. doi: 10.4049/jimmunol.1200742. [DOI] [PubMed] [Google Scholar]
- 35.Wørzner K, Sheward DJ, Schmidt ST, Hanke L, Zimmermann J, McInerney G, Karlsson Hedestam GB, Murrell B, Christensen D, Pedersen GK, et al. Adjuvanted SARS-CoV-2 spike protein elicits neutralizing antibodies and CD4 T cell responses after a single immunization in mice. EBioMed. 2021;63:103197. doi: 10.1016/j.ebiom.2020.103197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veldhoen M. Interleukin 17 is a chief orchestrator of immunity. Nat Immunol. 2017;18(6):612–21. doi: 10.1038/ni.3742. [DOI] [PubMed] [Google Scholar]
- 37.Casillo GM, Mansour AA, Raucci F, Saviano A, Mascolo N, Iqbal AJ, Maione F. Could IL-17 represent a new therapeutic target for the treatment and/or management of COVID-19-related respiratory syndrome? Pharmacol Res. 2020;156:104791. doi: 10.1016/j.phrs.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghazavi A, Ganji A, Keshavarzian N, Rabiemajd S, Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine. 2021;137:155323. doi: 10.1016/j.cyto.2020.155323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Zhang C, Huang F, Yang Y, Wang F, Yuan J, Zhang Z, Qin Y, Li X, Zhao D, et al. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. Natl Sci Rev. 2020;7(6):1003–11. doi: 10.1093/nsr/nwaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trzewikoswki De Lima G, Rodrigues TS, Portilho AI, Correa VA, Gaspar EB, De Gaspari E. Immune responses of meningococcal B outer membrane vesicles in middle-aged mice. Pathog Dis. 2020;78(5). doi: 10.1093/femspd/ftaa028. [DOI] [PubMed] [Google Scholar]
- 41.De Oliveira Santos FA, Lincopan N, De Gaspari E. Evaluation of intranasal and subcutaneous route of immunization in neonatal mice using DODAB-BF as adjuvant with outer membrane vesicles of Neisseria meningitis B. Immunobiol. 2018;223(12):750–60. doi: 10.1016/j.imbio.2018.07.021. [DOI] [PubMed] [Google Scholar]


