Abstract
Uric acid (UA) is independently associated with the emergence of hypertension. Nocturnal nondipping pattern of hypertension is associated with a greater risk of cardiovascular, renal, and cerebrovascular complications than dippers. The aim of the present study was to evaluate the relationship between the circadian blood pressure rhythm and UA level in patients with newly diagnosed essential hypertension. The study included 112 essential hypertensive patients and 50 healthy controls. The hypertensive patients were divided into two groups according to the results of 24‐hour ambulatory blood pressure monitoring, including 60 dippers (35 men, 25 women; mean age, 52.6±15.8 years) and 52 nondippers (29 men, 23 women; mean age, 55.9±13.2 years). Nondippers had significantly higher serum UA levels than the dippers and controls (5.8±0.8, 5.1±0.9 and 4.2±0.9 mg/dL, respectively; P<.001). Serum high‐sensitivity C‐reactive protein levels were also significantly higher in the nondipper group than the other groups (P<.001) and significantly correlated with serum UA (r=0.358, P<.001). Multivariate logistic regression analysis revealed an independent positive association between serum UA levels and nondipper pattern (odds ratio, 2.28; 95% confidence interval, 1.33–3.94; P=.003). Serum UA is strongly and independently associated with the nondipper circadian pattern in essential hypertension.
Arterial hypertension is the most important risk factor for the development of cardiovascular morbidity and mortality. 1 While the diagnosis of hypertension is based on office blood pressure (BP), 2 24‐hour ambulatory BP monitoring (ABPM) provides additional information, such as 24‐hour systolic and diastolic BP load, BP short‐term variability, and the diurnal variations of BP. 3 Normal nighttime variation of BP is characterized by a ≥10% reduction in BP levels from daytime. 3 In hypertensive patients, as well as normotensive individuals, a <10% fall in nocturnal BP is defined as a “nondipping BP profile” and such patients are called nondippers. The presence of nondipping hypertension carries greater cardiovascular risk and worse prognosis compared with patients with a normal circadian rhythm. 4 , 5
Recently, elevated serum uric acid (UA) has emerged as an important independent risk factor for hypertension. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 UA is commonly elevated in patients with hypertension, especially in those with severe hypertension or kidney disease. 18 Serum UA is also commonly associated with different hypertensive conditions such as prehypertension, 19 gestational hypertension, 20 preeclampsia, 21 and hypertension in adolescence. 22 A study in adolescents presenting with hypertension found elevated serum UA in nearly 90% of patients, whereas it was infrequent in normal healthy patients. 23 Studies in laboratory animals have also reported that raising serum UA can induce hypertension by stimulating oxidative stress, impairing endothelial function, and stimulating the renin angiotensin system. 24 , 25 , 26 Experimentally, an elevated UA has been found to induce microvascular disease in the kidney (arteriolosclerosis), and once this develops, the animal acquires salt‐sensitive hypertension that will persist even if the UA is lowered. 27 , 28 Vascular disease is mediated in part by a direct effect of UA to stimulate vascular smooth muscle cell proliferation. 27 , 29 , 30 Pilot studies in humans also suggest a benefit of lowering UA on endothelial function with an improvement in BP. 31 , 32 , 33
Despite the importance of nondipper hypertension on cardiovascular outcomes, no studies have examined whether hyperuricemia is associated with nondipper hypertension. We therefore investigated the association between circadian BP pattern and serum UA together with high‐sensitivity C‐reactive protein (hs‐CRP) in patients with newly diagnosed essential hypertension.
Methods
Study Population
The study consisted of 112 consecutive newly diagnosed essential hypertensive patients. Fifty healthy patients (26 men, 24 women; mean age, 53.9±14.4 years) were enrolled in the control group. Hypertension was defined as a systolic BP (SBP) >140 mm Hg and/or a diastolic BP (DBP) >90 mm Hg (mean of 3 measurements on at least 2 visits). 34 After diagnosis of hypertension, 24‐hour ABPM was performed and patients were further divided into dipper and nondipper groups based on the results obtained from ABPM. Exclusion criteria included secondary hypertension, evidence of any concomitant inflammatory disease, neoplastic diseases, heart failure, gout, renal dysfunction (estimated glomerular filtration rate <90 mL/min/1.73 m2) or hepatic dysfunction and known coronary arterial or cerebrovascular disease. Patients using allopurinol or any other agents that may affect serum UA level were also excluded.
Clinical and demographic characteristics of the study population including age, sex, and tobacco use were noted. In addition, serum levels of hs‐CRP, fasting blood glucose levels, creatinine levels, and fasting serum lipid status including total cholesterol, low‐density lipoprotein, high‐density lipoprotein, and triglyceride levels were also recorded. Body mass index was calculated as weight (kg) divided by height squared (m2). The local ethics committee approved the study protocol and informed consent was obtained from each of the individuals.
Laboratory Tests
The laboratory data were obtained from venous blood samples drawn after at least 12 hours of fasting. Lipid profile, glucose, and creatinine levels were measured according to standard methods. Serum UA levels were measured by an enzymatic colorimetric method (Cobas Integra Uric Acid Cassette; Roche Diagnostics, Indianapolis, IN) on an autoanalyzer (Cobas Integra 400; Roche Diagnostics). hs‐CRP levels were assessed using the BN2 model nephelometer (Cardio Phase hsCRP Assay; Dade Behring, Marburg, Germany).
Ambulatory BP Monitoring
Twenty‐four–hour noninvasive ABPM (Mobilograph, Stolberg, Germany) was performed as described elsewhere. BP readings were recorded at 20‐minute intervals during the daytime and at 30‐minute intervals during nighttime. All patients were asked to undertake their usual daily activities. Daytime and nighttime were defined as 6 am to 10 pm and from 10 pm to 6 am, respectively. Sleep and wake periods were assessed based on the information obtained from the patients. The recordings were analyzed using interactive software and patients were excluded from the study if ≥20% of the measurements were not recorded successfully. From the hourly averages of ambulatory BP recordings, daytime, nighttime, and 24‐hour averages of systolic, diastolic, and mean BPs were calculated for each patient. Patients with BP decline of ≥10% during nighttime were defined as dippers, whereas those with a recorded decline <10% were considered nondippers. 4 In addition to dipping status, we also defined the percentage decline in nocturnal SBP and DBP from day to night by implementing the formula: [(daytime BP mean) − (nighttime mean)]/daytime BP mean) × 100.
Statistical Analysis
Statistical analysis was performed by using SPSS 15.0 statistical software (SPSS Inc, Chicago, IL). The Kolmogorov‐Smirnov test was used to determine whether the continuous variables were normally distributed. Parametric tests were applied with normal distribution, whereas nonparametric tests were used without normal distribution. Normally distributed variables were given as mean±standard deviation, while those variables featured by non‐normal distribution were given as medians with interquartile ranges. One‐way analysis of variance or Kruskal‐Wallis tests were used for continuous variables, whereas chi‐square test was used for categorical variables to test the differences among the control, dipper, and nondipper groups. Differences between subgroups were revealed using Tukey’s multiple comparison post hoc test. Data were analyzed to identify whether serum UA was independently associated with nondipping pattern by using univariate logistic and stepwise multivariate logistic regression models. Odds ratios and 95% confidence intervals were estimated for the effect of independent variables on nondipper pattern. A P value <.05 was accepted to be statistically significant.
Results
The study consisted of 112 newly diagnosed essential hypertensive patients and 50 control patients. On the basis of the results of 24‐hour ABPM, the patients were divided into two subgroups: 60 dippers (35 men, 25 women; mean age, 52.6±15.8 years) and 52 nondippers (29 men, 23 women; mean age, 55.9±13.2 years). Office BP values were similar between nondipper hypertensive and dipper hypertensive patients (SBP, 154.9±9.9 mm Hg vs 151.3±10.8 mm Hg, P=.073; DBP, 93.8±9.0 mm Hg vs 92.2±8.7 mm Hg, P=.334). The nocturnal systolic, diastolic, and mean BP levels, together with nighttime mean heart rates, were found to be significantly greater in nondippers than dippers (Table I).
Table I.
Comparison of Ambulatory Blood Pressure Monitoring Results Between Dipper and Nondipper Hypertensive
| Dippers (n=60) | Nondippers (n=52) | P Value | |
|---|---|---|---|
| 24‐h ambulatory SBP, mm Hg | 137.9±10.0 | 144.3±11.6 | .002 |
| 24‐h ambulatory DBP, mm Hg | 87.6±10.4 | 91.3±10.9 | .066 |
| 24‐h mean BP, mm Hg | 104.4±9.4 | 109±10.0 | .013 |
| 24‐h mean heart rate, beats per min | 73.6±9.7 | 76.7±10.3 | .081 |
| Daytime SBP, mm Hg | 142.2±10.1 | 144.8±11.6 | .201 |
| Daytime DBP, mm Hg | 90.9±10.8 | 91.8±11.6 | .644 |
| Daytime mean BP, mm Hg | 108±9.6 | 109.5±10.4 | .419 |
| Daytime mean heart rate, beats per min | 75.9±9.0 | 75.9±9.2 | .970 |
| Nighttime SBP, mm Hg | 123.8±12.4 | 143.6±14.4 | <.001 |
| Nighttime DBP, mm Hg | 75.7±9.9 | 89.7±11.1 | <.001 |
| Nighttime mean BP, mm Hg | 91.7±9.8 | 107.6±11.0 | <.001 |
| Nighttime mean heart rate, beats per min | 65.3±10.4 | 69.8±11.1 | .017 |
| The rate of systolic fall in nighttime | 13.0±5.6 | 0.8±6.8 | <.001 |
| The rate of diastolic fall in nighttime | 16.8±5.7 | 1.9±6.8 | <.001 |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Table II summarizes the demographic and clinical data of the study groups. The nondipper patients were older and their body mass indexes were greater than those in the dipper and control groups, but the difference did not reach statistical significance. There were also no significant differences between the groups in terms of sex distribution and presence of diabetes mellitus, yet smoking rates were higher in the nondipper group. A small but statistically significant difference was observed in the high‐density lipoprotein levels in the nondipper group compared with the dipper group (41.7±10.7 vs 46.6±11.4, P=.032). Nondippers also had higher hs‐CRP levels compared with the dipper and control groups (5.5±3.0 mg/dL, 3.0±0.9 mg/dL, and 1.7±0.9 mg/dL; P<.001, nondipper, dipper, and control groups, respectively).
Table II.
Demographic and Biochemical Data of the Patient and Control Groups
| Control (n=50) | Dippers (n=60) | Nondippers (n=52) | P Value | |
|---|---|---|---|---|
| Age, y | 53.9±14.4 | 52.6±15.8 | 55.9±13.2 | .497 |
| Men, No. (%) | 26 (52) | 35 (58.3) | 29 (55.8) | .467 |
| Body mass index, kg/m2 | 26.4±3.1 | 27.1±3.6 | 27.6±4.0 | .255 |
| Current smoker, No. (%) | 10 (20) | 13 (21.6) | 21 (40.4) | .033a,b |
| Diabetes mellitus, No. (%) | 7 (14) | 11 (18.3) | 8 (15.3) | .817 |
| In‐office SBP, mm Hg | 126.3±5.8 | 151.3±10.8 | 154.9±9.9 | <.001c |
| In‐office DBP, mm Hg | 76.2±5.3 | 92.2±8.7 | 93.8±9.0 | <.001c |
| Fasting glucose, mg/dL | 93 (86–103) | 96 (89–102) | 99 (88–108) | .496 |
| Serum creatinine, mg/dL | 0.70 (0.60–1.00) | 0.80 (0.70–1.00) | 1.0 (0.70–1.3) | .013a,b |
| Serum uric acid, mg/dL | 4.2±0.9 | 5.1±0.9 | 5.8±0.8 | <.001a,b,d |
| Fasting lipid status, mg/dL | ||||
| Total cholesterol | 203.1±35.5 | 209.8±37.6 | 198.5±35.5 | .257 |
| HDL cholesterol | 46.1±9.3 | 46.6±11.4 | 41.7±10.7 | .032a |
| LDL cholesterol | 129.7±35.6 | 128.5±36.5 | 119.7±28.8 | .264 |
| Triglycerides | 151 (114–190) | 149 (109–188) | 132 (101–165) | .475 |
| Hemoglobin, g/L | 14.3±1.2 | 14.5±1.4 | 13.8±1.6 | .022a |
| Platelet count, ×109/L | 254.8±72.5 | 278.2±61.0 | 261.4±80.9 | .202 |
| White blood cell count, ×109/L | 7.4±1.7 | 7.5±1.7 | 8.1±1.7 | .060 |
| hs‐CRP, mg/L | 1.7±0.9 | 3.0±0.9 | 5.5±3.0 | <.001a,b,d |
Abbreviations: DBP, diastolic blood pressure; HDL, high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; SBP, systolic blood pressure. Data are expressed as mean±standard deviation or median (interquartile range). aBetween nondipper and dipper group. bBetween nondipper and conrol group. cBetween patient groups and control group. dBetween dipper and control group.
As shown in Figure 1, serum UA levels were higher in nondipper patients compared with dippers and controls (5.8±0.8 mg/dL, 5.1±0.9 mg/dL, and 4.2±0.9 mg/dL; P<.001, respectively). Serum UA negatively correlated with the fall in systolic and diastolic BP at night both in the dippers and the nondippers (Figure 2). Serum UA correlated with hs‐CRP levels in hypertensive patients (r=0.358, P<.001) (Figure 3). In univariate and stepwise multivariate analysis, serum UA (P=.003), hs‐CRP levels (P<.001), and smoking (P<.013) independently predicted nondipper status in hypertensive patients (Table III).
Figure 1.
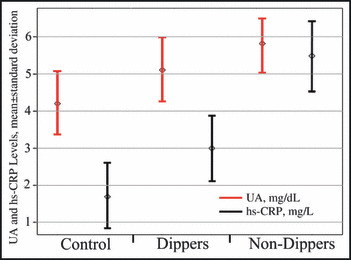
Mean serum uric acid (UA) and C‐reactive protein levels in patients with controls, dippers, and nondippers. hs‐CRP indicates high‐sensitivity C‐reactive protein.
Figure 2.
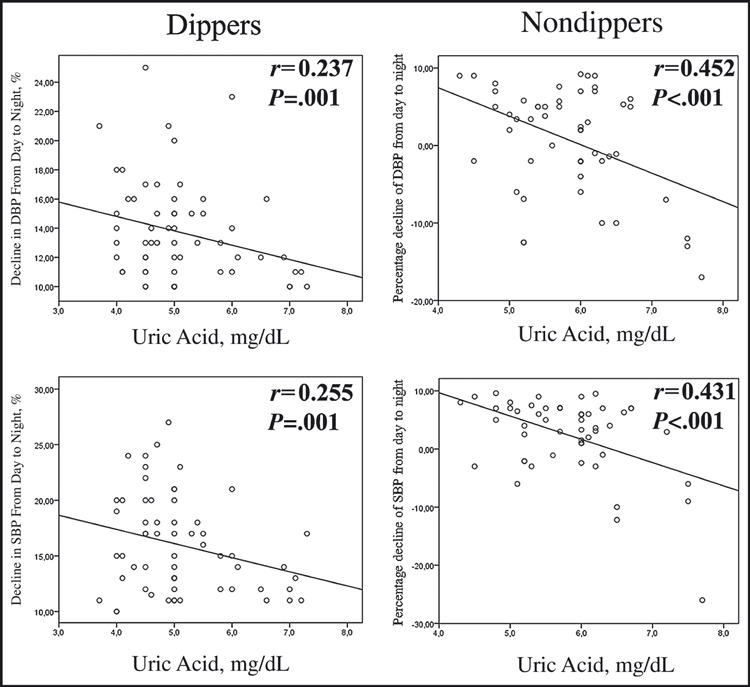
Percentage decline in blood pressure from day to night in relation to uric acid level in dippers and nondippers. SBP indicates systolic blood pressure; DBP, diastolic blood pressure.
Figure 3.
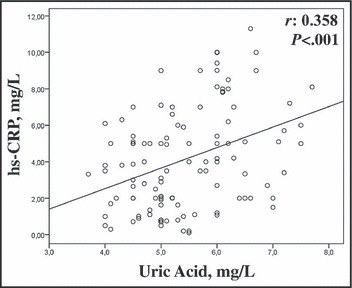
Correlation between serum uric acid levels and inflammatory activity. hs‐CRP indicates high‐sensitivity C‐reactive protein.
Table III.
Significant Predictors of Nondipping Status in Univariable and Stepwise Multivariable Logistic Regression Analysis
| Variable | Univariable | Stepwise Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| HDL | 0.96 (0.93–1.00) | .03 | – | – |
| Hemoglobin | 0.71 (0.55–0.93) | .01 | – | – |
| Uric acid | 2.63 (1.61–4.31) | <.001 | 2.28 (1.33–3.94) | .003 |
| Creatinine | 3.88 (1.09–13.83) | .036 | – | – |
| hs‐CRP | 1.46 (1.23–1.73) | <.001 | 1.37 (1.13–1.64) | .001 |
| Smoking | 2.45 (1.07–5.60) | .034 | 3.57 (1.30–9.79) | .013 |
Abbreviations: CI, confidence interval; HDL, high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; OR, odds ratio.
Discussion
In this study we demonstrate that serum UA level is significantly increased in patients with nondipper hypertension compared with hypertensive dippers and normotensive controls. Serum UA levels negatively correlated with the fall of systolic and diastolic BP at night.
Hypertension is the most common chronic disease and has a causative role in the death of almost 7.5 million people per year. 35 The etiology of essential hypertension is multifactorial and the number of factors involved in the pathogenesis of hypertension are constantly increasing. Epidemiological and experimental studies have shown that there is a strong relationship between UA and hypertension. 36 Furthermore, elevated serum UA levels are associated with a risk for developing hypertension, independent of other risk factors. 36 The relationship between hypertension and UA was first described in 1874, 37 and studies linking this association have been noted primarily in the past 5 decades. In 1966, Cannon and colleagues 18 demonstrated hyperuricemia in 25% to 50% of untreated essential hypertensive patients, and the relationship between UA level and hypertension was significant. Compared with normotensive patients, serum UA levels were significantly elevated in hypertensive patients. 38 Conversely, in asymptomatic patients with hyperuricemia, investigators have found that incidence of hypertension is around 50%. 39 Studies also suggested that serum UA level was an independent predictor for the development of new‐onset hypertension. Three decades ago, Kahn and colleagues 6 reported that increased serum UA was an independent risk factor for hypertension and serum UA was positively correlated with SBP. In a study conducted in 3329 Framingham study participants who had been free of hypertension, Sundstrom and colleagues 16 demonstrated that serum UA level was an independent predictor for hypertension incidence and longitudinal BP progression during a 4‐year follow‐up. In another large, community‐based cohort study, Nagahama and colleagues 13 found that serum UA was an independent predictor of developing hypertension in both men and women. In the Bogalusa Heart Study, Alper and colleagues 7 followed up 577 children for a 12‐year period. Childhood serum UA levels and their rates of change from childhood into adulthood were strongly and independently correlated with BP levels in adulthood. In addition, UA is a powerful risk marker for future cardiovascular disease and all‐cause mortality in patients with essential hypertension. 40
Twenty‐four–hour ABPM helps establish an accurate diagnosis of hypertension in patients with suspected hypertension and also provides clinicians with more valuable information in patients with established hypertension compared with either repeated clinic or home BP measurements. 3 Based on the ABPM results, hypertension can be classified as dipper or nondipper pattern. The nondipper pattern is associated with an increased risk for cardiovascular, cerebrovascular, and renal complications. 4 , 5 , 41 Although the exact mechanisms of nondipping pattern have yet to be wholly elucidated, studies suggest that nondippers display impaired autonomic dysfunction, higher sympathetic activity, and higher inflammatory activity. 42 , 43 , 44 , 45 While the relationship between UA and hypertension is not debated, 36 currently there is no information on whether UA is related to nondipper status in essential hypertension. In our study, increased serum UA was an independent and strong predictor of nondipper hypertension. Additionally, we found that inflammatory activity seemed to be increased in nondippers and hs‐CRP level was significantly correlated with serum UA level (r=0.357, P<.001).
The proinflammatory properties of UA have been shown in experimental and clinical studies. UA stimulates the synthesis of monocyte chemoattractant protein‐1 in rat vascular smooth muscle cells. 46 In humans, soluble UA associated positively with interleukin 6 and tumor necrosis factor‐α and negatively with interleukin 1β 47 and stimulates secretion of CRP in human vascular cells. 30 A relationship between serum UA level and inflammation has also been shown in clinical studies using various circulating inflammatory markers, such as fibrinogen, 48 monocyte chemoattractant protein 1, 49 interleukin 6, 47 tumor necrosis factor‐α, 47 and CRP. 47 , 48 , 49 , 50 , 51
In hypertensive individuals, early identification of the nondipping pattern can aid the physician in identifying individuals who are at increased risk for cardiovascular disease. To our knowledge, this is the first study to evaluate UA in nondipper hypertension. The aforementioned correlation between UA and inflammation may elucidate the relationship between UA and nondipping status. These studies suggest that serum UA may provide a useful biomarker for identifying who is prone to nighttime nondipping hypertensive pattern and, hence, poorer cardiovascular outcomes.
Study Limitations
Limitations included the fact that the study was cross‐sectional and represented only a single‐center experience. Nondipper hypertensive patients were not followed for the development of cardiovascular events. Serum UA and hs‐CRP levels were measured only at the beginning of the study. The levels of these markers have a possibility to change over time in a given person. Although a significant association between elevated serum UA and nondipping pattern was identified, we could not establish the precise mechanism of this association.
Conclusions
Increased serum UA and hs‐CRP levels are independent markers for nondipper status in patients with essential hypertension. A large prospective study aiming at lowering serum UA levels should be performed to determine whether such treatments would improve adequate reduction of BP levels at nighttime.
Disclosures: Dr RJ Johnson is an inventor on a patent for allopurinol in the treatment of hypertension (University of Washington and Merck, Inc) and also has patent applications related to the lowering of uric acid in the metabolic syndrome. The other authors declared no conflicts of interest.
References
- 1. Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996;275:1571–1576. [PubMed] [Google Scholar]
- 2. Ogedegbe G, Pickering T. Principles and techniques of blood pressure measurement. Cardiol Clin. 2010;28:571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanbay M, Turkmen K, Ecder T, Covic A. Ambulatory blood pressure monitoring: from old concepts to novel insights. Int Urol Nephrol. 2012;44:173–182. [DOI] [PubMed] [Google Scholar]
- 4. Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. [DOI] [PubMed] [Google Scholar]
- 5. de la Sierra A, Redon J, Banegas JR, et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466–472. [DOI] [PubMed] [Google Scholar]
- 6. Kahn HA, Medalie JH, Neufeld HN, et al. The incidence of hypertension and associated factors: the Israel ischemic heart disease study. Am Heart J. 1972;84:171–182. [DOI] [PubMed] [Google Scholar]
- 7. Alper AB Jr, Chen W, Yau L, et al. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension. 2005;45:34–38. [DOI] [PubMed] [Google Scholar]
- 8. Dyer AR, Liu K, Walsh M, et al. Ten‐year incidence of elevated blood pressure and its predictors: the CARDIA study. Coronary Artery Risk Development in (Young) Adults. J Hum Hypertens. 1999;13:13–21. [DOI] [PubMed] [Google Scholar]
- 9. Grayson PC, Kim SY, Lavalley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta‐analysis. Arthritis Care Res. 2011;63:102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hunt SC, Stephenson SH, Hopkins PN, Williams RR. Predictors of an increased risk of future hypertension in Utah. A screening analysis. Hypertension. 1991;17(6 Pt 2):969–976. [DOI] [PubMed] [Google Scholar]
- 11. Jossa F, Farinaro E, Panico S, et al. Serum uric acid and hypertension: the Olivetti heart study. J Hum Hypertens. 1994;8:677–681. [PubMed] [Google Scholar]
- 12. Masuo K, Kawaguchi H, Mikami H, et al. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42:474–480. [DOI] [PubMed] [Google Scholar]
- 13. Nagahama K, Inoue T, Iseki K, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res. 2004;27:835–841. [DOI] [PubMed] [Google Scholar]
- 14. Nakanishi N, Okamoto M, Yoshida H, et al. Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003;18:523–530. [DOI] [PubMed] [Google Scholar]
- 15. Shankar A, Klein R, Klein BE, Nieto FJ. The association between serum uric acid level and long‐term incidence of hypertension: population‐based cohort study. J Hum Hypertens. 2006;20:937–945. [DOI] [PubMed] [Google Scholar]
- 16. Sundstrom J, Sullivan L, D’Agostino RB, et al. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28–33. [DOI] [PubMed] [Google Scholar]
- 17. Taniguchi Y, Hayashi T, Tsumura K, et al. Serum uric acid and the risk for hypertension and Type 2 diabetes in Japanese men: the Osaka Health Survey. J Hypertens. 2001;19:1209–1215. [DOI] [PubMed] [Google Scholar]
- 18. Cannon PJ, Stason WB, Demartini FE, et al. Hyperuricemia in primary and renal hypertension. N Engl J Med. 1966;275:457–464. [DOI] [PubMed] [Google Scholar]
- 19. Syamala S, Li J, Shankar A. Association between serum uric acid and prehypertension among US adults. J Hypertens. 2007;25:1583–1589. [DOI] [PubMed] [Google Scholar]
- 20. Bellomo G, Venanzi S, Saronio P, et al. Prognostic significance of serum uric acid in women with gestational hypertension. Hypertension. 2011;58:704–708. [DOI] [PubMed] [Google Scholar]
- 21. Redman CW, Beilin LJ, Bonnar J, Wilkinson RH. Plasma‐urate measurements in predicting fetal death in hypertensive pregnancy. Lancet. 1976;1:1370–1373. [DOI] [PubMed] [Google Scholar]
- 22. Loeffler LF, Navas‐Acien A, Brady TM, et al. Uric acid level and elevated blood pressure in US Adolescents: National Health and Nutrition Examination Survey, 1999–2006. Hypertension. 2012;59:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sanchez‐Lozada LG, Soto V, Tapia E, et al. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol. 2008;295:F1134–F1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanchez‐Lozada LG, Tapia E, Lopez‐Molina R, et al. Effects of acute and chronic L‐arginine treatment in experimental hyperuricemia. Am J Physiol. 2007;292:F1238–F1244. [DOI] [PubMed] [Google Scholar]
- 26. Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal‐independent mechanism. Hypertension. 2001;38:1101–1106. [DOI] [PubMed] [Google Scholar]
- 27. Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure‐independent mechanism. Am J Physiol. 2002;282:F991–F997. [DOI] [PubMed] [Google Scholar]
- 28. Watanabe S, Kang DH, Feng L, et al. Uric acid, hominoid evolution, and the pathogenesis of salt‐sensitivity. Hypertension. 2002;40:355–360. [DOI] [PubMed] [Google Scholar]
- 29. Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet‐derived growth factor A‐chain expression. J Biol Chem. 1991;266:8604–8608. [PubMed] [Google Scholar]
- 30. Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid‐induced C‐reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. [DOI] [PubMed] [Google Scholar]
- 31. Kanbay M, Ozkara A, Selcoki Y, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227–1233. [DOI] [PubMed] [Google Scholar]
- 32. Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6:1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on the blood pressure of adolescents with newly diagnosed essential hypertension. JAMA. 2008;300:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 35. World Health Organization . Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: World Health Organization; 2009. [Google Scholar]
- 36. Mazzali M, Kanbay M, Segal MS, et al. Uric acid and hypertension: cause or effect? Curr Rheumatol Rep. 2010;12:108–117. [DOI] [PubMed] [Google Scholar]
- 37. Mohamed FA. The etiology of Bright’s disease and the prealbuminuric state. Med Chir Trans. 1874;39:197–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmidt MI, Watson RL, Duncan BB, et al. Clustering of dyslipidemia, hyperuricemia, diabetes, and hypertension and its association with fasting insulin and central and overall obesity in a general population. Atherosclerosis Risk in Communities Study Investigators. Metabolism. 1996;45:699–706. [DOI] [PubMed] [Google Scholar]
- 39. Johnson RJ, Kang DH, Feig D, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. [DOI] [PubMed] [Google Scholar]
- 40. Verdecchia P, Schillaci G, Reboldi G, et al. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension. 2000;36:1072–1078. [DOI] [PubMed] [Google Scholar]
- 41. Kario K, Pickering TG, Matsuo T, et al. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. [DOI] [PubMed] [Google Scholar]
- 42. Mann S, Altman DG, Raftery EB, Bannister R. Circadian variation of blood pressure in autonomic failure. Circulation. 1983;68:477–483. [DOI] [PubMed] [Google Scholar]
- 43. Sherwood A, Routledge FS, Wohlgemuth WK, et al. Blood pressure dipping: ethnicity, sleep quality, and sympathetic nervous system activity. Am J Hypertens. 2011;24:982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaya MG, Yarlioglues M, Gunebakmaz O, et al. Platelet activation and inflammatory response in patients with non‐dipper hypertension. Atherosclerosis. 2010;209:278–282. [DOI] [PubMed] [Google Scholar]
- 45. Ermis N, Yagmur J, Acikgoz N, et al. Serum gamma‐glutamyl transferase (GGT) levels and inflammatory activity in patients with non‐dipper hypertension. Clin Exp Hypertens. 2012;34:311–315. [DOI] [PubMed] [Google Scholar]
- 46. Kanellis J, Watanabe S, Li JH, et al. Uric acid stimulates monocyte chemoattractant protein‐1 production in vascular smooth muscle cells via mitogen‐activated protein kinase and cyclooxygenase‐2. Hypertension. 2003;41:1287–1293. [DOI] [PubMed] [Google Scholar]
- 47. Lyngdoh T, Marques‐Vidal P, Paccaud F, et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population‐based Colaus study. PLoS ONE. 2011;6:e19901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Coutinho Tde A, Turner ST, Peyser PA, et al. Associations of serum uric acid with markers of inflammation, metabolic syndrome, and subclinical coronary atherosclerosis. Am J Hypertens. 2007;20:83–89. [DOI] [PubMed] [Google Scholar]
- 49. Wasilewska A. Is duration of breastfeeding in infancy associated with serum uric acid levels and markers of low‐grade inflammation in children. Acta Paediatr. 2012;March 27. [Google Scholar]
- 50. Vekic J, Jelic‐Ivanovic Z, Spasojevic‐Kalimanovska V, et al. High serum uric acid and low‐grade inflammation are associated with smaller LDL and HDL particles. Atherosclerosis. 2009;203:236–242. [DOI] [PubMed] [Google Scholar]
- 51. Meisinger C, Koenig W, Baumert J, Doring A. Uric acid levels are associated with all‐cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population: the MONICA/KORA cohort study. Arterioscler Thromb Vasc Biol. 2008;28:1186–1192. [DOI] [PubMed] [Google Scholar]


