Abstract
Nondipping blood pressure (BP) pattern is a potential independent risk factor for chronic kidney disease (CKD). Bedtime administration of valsartan is considered to normalize circadian rhythm and protect the kidneys and heart in CKD patients. However, more clinical trials are needed to confirm this benefit. Sixty patients with nondipping BP pattern and thirty patients with dipping BP pattern were enrolled in this study, and the patients with nondipping BP pattern were randomly divided into two groups and treated with bedtime or awakening doses of valsartan (80–320 mg). Nondipping BP patients treated with bedtime doses of valsartan showed a greater reduction in 24‐hour proteinuria and bedtime proteinuria, a greater delayed decline in estimated glomerular filtration rate, and more protection against myocardial hypertrophy (P<.05) compared with patients with the nondipping BP pattern treated with the awakening dose (P<.05). This was similar to patients with dipping BP. No severe clinical complications were recorded in these patients. Valsartan with bedtime dosing in CKD patients with the nondipping BP pattern have better renal and cardiovascular protection. Antihypertensive “chronotherapy” may be useful in clinical practice for CKD patients.
The normal circadian rhythm of blood pressure (BP) is as follows: BP is lowest early in the morning, rises as the day progresses, and then dips down during the night. In healthy patients, there is a diurnal variation in BP that decreases by 10% to 20% during the nighttime compared with the daytime, which is known as the dipping pattern. While other patients’ BP levels do not dip or dip very little, which is known as the nondipping pattern. This reflects abnormal or inadequate mechanisms that regulate BP and can be the result of baroreflex or autonomic dysfunction, relative nocturnal volume overload, and abnormal sodium handling. Chronic kidney disease (CKD) has been associated with a higher prevalence of the nondipping pattern. Many studies have indicated that the nondipping BP pattern in CKD patients results in significant target organ damage such as subsequent deterioration in renal function 1 and more renal morphological changes and is an early predictor of later nephropathy in type 1 diabetes, 2 high incidence of cardiovascular (CV) diseases, and poor long‐term survival in hemodialysis patients. 3
The time of ingestion of antihypertensive medications can affect the circadian pattern of BP, independent of the terminal half‐life of each individual medication. Treatment at bedtime is the most cost‐effective and simplest strategy for successfully achieving therapeutic goals of adequate sleeping BP reduction and preserving or re‐establishing normal 24‐hour BP dipping pattern. 4 Studies on ingestion of valsartan, valsartan/hydrochlorothiazide, or olmesartan at bedtime were shown to be significantly more efficient than morning ingestion in reducing asleep mean systolic BP (SBP), thus significantly increasing the sleep time–relative SBP decline toward a more normal dipping pattern. 5 , 6 , 7 However, these studies focused on essential hypertension patients and not patients with CKD. Given that nocturnal nondipping BP is a potential independent risk factor for CKD progression and the development of cardiorenal syndrome, bedtime administration of antihypertensive drugs should be considered in patients with CKD. A significant reduction in asleep mean BP and decreased urinary albumin excretion were reported after changing one BP‐lowering medication from morning to evening in 32 uncontrolled nondipping patients with CKD. In one trial, urinary albumin excretion was greatly reduced in patients ingesting one antihypertensive medication at bedtime compared with those ingesting all medications upon awakening. 8 No control group was included and the duration of follow‐up was limited, although the results were encouraging and interesting. More studies are needed to confirm the effect of such a chronotherapeutic approach on glomerular filtration rate (GFR), proteinuria, and CV damage in patients with CKD. In this pilot study, we determined whether ingestion of valsartan at bedtime resulted in better kidney and heart protection than ingestion of valsartan at awakening in nondipping patients with CKD.
Materials and Methods
Patients
Inclusion Criteria. The following study inclusion criteria were required: (1) presence of CKD; 9 (2) age between 18 and 65 years; (3) estimated GFR (eGFR) <90 mL/min/1.73 m2 (using the Modification of Diet in Renal Disease Study equation) but >30 mL/min/1.73 m2; (4) 24‐hour proteinuria >0.5 g but <2.0 g; and (5) signed informed consent from participating patients. The study was approved by the ethical committee of the Third Hospital of Sun Yat‐Sen University.
Exclusion Criteria. Exclusion criteria were: (1) treatment with steroids or hormonal therapy; (2) acute changes in eGFR >30% in the past 3 months; (3) orthostatic hypotension or severe hypertension (SBP >160 mm Hg diastolic BP [DBP] >100 mm Hg); (4) pregnancy; (5) history of drug or alcohol abuse; (6) night‐ or shift‐work employment; (7) presence of acquired immunodeficiency syndrome; (8) CV disorders (unstable angina pectoris, heart failure, life‐threatening arrhythmia, atrial fibrillation, kidney failure, and grade III or IV retinopathy); (9) intolerance to ambulatory BP monitoring (ABPM); and (10) inability to communicate and comply with all of the study requirements.
Withdrawal Criteria. Withdrawal criteria included side effects such as CV disorders (unstable angina pectoris, heart failure, life‐threatening arrhythmia, atrial fibrillation, kidney failure, and grade III or IV retinopathy), acute changes in eGFR >30% after treatment, severe orthostatic hypotension, or repeat hyperkalemia.
Diagnostic Criteria. Diagnosis of hypertension was based on accepted ABPM criteria: when nocturnal SBP fell by ≥10% of the diurnal BP, the patient was defined as a “dipper.” Similarly, a patient whose nocturnal SBP fell by approximately 10% or increased was defined as a “nondipper.” 10
Study Design and Protocols. The study was performed from May 2010 to May 2012 in the Division of Nephrology, Third Hospital of Sun Yat‐Sen University. Patients who met the inclusion criteria were divided into two groups according to BP pattern. Any patients who had any antihypertension medication at bedtime would withdrawal the drugs for 4 weeks before ABPM. Patients with nondipping BP were then randomly divided into two groups and received a bedtime or awakening dose of valsartan. Patients with dipping BP were called the dipper group, while the patients with nondipping BP were called the awakening dose group or bedtime dose group, according to the time of drug administration.
In this chronotherapy trial, after a 2‐ to 4‐week washout period when required, patients were randomly assigned to single daily valsartan monotherapy (80–320 mg/d) either in the morning (on awakening) or at bedtime. Patients were also given nifedipine controlled‐release tablets (30 to 60 mm Hg) when their BP was higher than the recommended target (BP target values <130/80 mm Hg for patients with CKD and <120/75 mm Hg in patients with proteinuria >1 g/d) 14 after receiving the highest dose of valsartan for 4 weeks. Patients in the dipper group and in the awakening group received valsartan in the morning, while patients in the bedtime group received valsartan at bedtime.
Ambulatory BP Monitoring
Patients underwent 24‐hour ABPM using a TM‐2430 monitor (A&D Company, Tokyo, Japan). Cuff size was chosen on the basis of the patient’s arm circumference, and the cuff was fixed to the nondominant arm. Three BP readings were obtained in the morning (7 am–10 am) concomitant with sphygmomanometric measurements to ensure that the average of the two sets of values did not differ by >5 mm Hg. BP was recorded every 15 minutes from 7 am to 10 pm and every 30 minutes from 10 pm to 7 am. The daytime and nighttime periods were derived from diaries recorded by the patients during ABPM. Monitoring was always performed on a working day. Patients had no access to ABPM values. Strenuous physical activity was discouraged in all patients during the monitoring period and their daily activities were comparable. BP series were eliminated from analysis when >30% of the measurements were lacking, when they had missing data for >3‐hour spans, or when they were collected from patients while they were experiencing an irregular rest‐activity schedule or a nighttime sleep span <6 hours or >12 hours during monitoring.
Ultrasonography Assessment. Left ventricular volumes, mass, systolic function, and diastolic function were assessed using 2‐dimensional echocardiography. Left ventricular mass was calculated using the Duvereux method. 11 Left ventricular mass index (LVMI) was obtained by calculating left ventricular mass to height2.7. 12 Left ventricular systolic function was assessed by left ventricular ejection fraction, and diastolic function was assessed by early mitral inflow filling velocity (E), peak mitral filling velocity at atrial contraction (A), E/A deceleration time of the mitral E wave, and tissue Doppler velocity of the mitral annulus. 13
Carotid intima‐media thickness was determined by averaging 3 measurements taken on each carotid artery (anteriorly, laterally, and posteriorly), measuring the distance between the leading edge of the lumen‐intima interface and the leading edge of the collagenous upper layer of the adventitia using high‐resolution B‐mode ultrasonography. Measurements were taken in areas free of obvious atherosclerotic plaque around the level of the carotid bifurcation.
Other Data Collected. We collected 24‐hour urine samples within a 7‐day period of ABPM measurement and measured urinary protein, creatinine, and sodium excretion. We also separately collected urine samples at 7 am to 10 pm and 10 pm to 7 am to detect proteinuria and sodium excretion, which were termed awakening and bedtime urine proteinuria and sodium excretion. Patients were asked to empty their bladder at 7 am and 10 pm to ensure valid results. In addition, medical history, including history of CV events, demographic and laboratory data (serum creatinine, cholesterol, triglycerides, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, fasting glucose), and current therapy were obtained at the initial study visit.
Follow‐Up. The same evaluation procedure as described above, including conventional clinic BP measurement, was performed every 1 month; 24‐hour ABPM were scheduled every 3 months; and ultrasonography assessment was carried out every 6 months. Registered events included the following: death from all causes, myocardial infarction, angina pectoris, heart failure, acute arterial occlusion of lower extremities, thrombotic occlusion of the retinal artery, hemorrhagic stroke, ischemic stroke, and transient ischemic attack.
Statistical Methods
Data were analyzed using SPSS version 15.0 (SPSS Inc, Chicago, IL). Continuous variables are expressed as mean±standard deviation. Paired Student t test, analysis of variance, and χ2 tests were used to compare variable changes before and after treatment. Categorical variables are expressed as percentage and compared using the χ2 test. All values quoted are two‐tailed, and significance was defined as P<.05.
Results
Baseline Parameters
There were no significant differences between patients with regard to age, sex, duration of disease, number of patients with diabetic mellitus, body mass index, mean 24‐hour SBP and DBP, 24‐hour proteinuria and urinary sodium excretion, renal function, serum lipid levels, and fasting glucose (P>.05), while the nighttime BP, the ratio of bedtime/awakening proteinuria, LVMI, carotid intima‐media thickness, and the frequency of plaque in the nondipper group were higher than the dipper group (P<.05), these data were not different between the awakening and bedtime groups (P>.05). These results are shown in I, II.
Table I.
Patient Basal Clinical Characteristics
| Dipper | Nondipper Group | |||
|---|---|---|---|---|
| Group | Total | Awakening Dose | Bedtime Dose | |
| Age, y | 34±7 | 36±9 | 35±9 | 37±9 |
| Male:female ratio | 2:1 | 3:2 | 8:7 | 2:1 |
| Duration, mo | 17±12 | 15±14 | 15±14 | 15±14 |
| Diabetic/nondiabetic | 0:30 | 1:14 | 1:14 | 1:14 |
| BMI, kg/m2 | 22±4 | 24±3 | 24±3 | 23±2 |
| Valsartan dose, mg | 200±68 | 194±55 | 180±36 | 209±69 |
| Number of use nifedipine controlled‐release tablets | 3 | 8 | 3 | 5 |
Abbreviation: BMI, body mass index. Total=awakening dose group + bedtime dose group.
Table II.
Clinical Parameters of Different Group After Therapy
| Dipper Group | Total | Nondipper Group | |||||
|---|---|---|---|---|---|---|---|
| Baseline | End | Awakening Dose | Bedtime Dose | ||||
| Baseline | End | Baseline | End | ||||
| 24 h‐SBP, mm Hg | 123±16 | 112±67 | 127±17 | 126±18 | 113±12 | 129±16 | 112±9 |
| 24 h‐DBP, mm Hg | 77±11 | 67±6a | 78±10 | 77±8 | 68±7a | 79±11 | 70±6a |
| Awakening SBP, mm Hg | 127±16 | 116±9a | 129±17 | 128±19 | 116±13a | 130±16 | 118±11a |
| Awakening DBP, mm Hg | 81±11 | 70±7a | 79±10 | 79±9 | 71±6a | 80±11 | 73±7a |
| Bedtime SBP, mm Hg | 109±15 | 102±7a | 123±16b | 121±17 | 107±11a | 124±16 | 102±7a |
| Bedtime DBP, mm Hg | 68±11 | 62±6a | 75±13b | 76±13 | 63±5a | 75±13 | 63±6a |
| Proteinuria, g/24 h | 1.1±0.3 | 0.4±0.3a | 1.0±0.2 | 1.1±0.2 | 0.7±0.3a | 1.0±0.2 | 0.4±0.3a |
| Ratio of bedtime/awakening time proteinuria | 0.4±0.1 | 0.4±0.2 | 0.6±0.2b | 0.5±0.2a | 0.5±0.1 | 0.6±0.2 | 0.4±0.2a |
| Urinary sodium excretion, mmol | 134±48 | 138±51 | 135±40 | 124±35 | 120±22 | 146±44 | 136±45 |
| Ratio of bedtime/awakening time urinary sodium excretion | 0.4±0.1 | 0.4±0.2 | 0.7±0.3b | 0.6±0.2 | 0.6±0.1 | 0.9±0.5 | 0.6±0.4a |
| Serum creatinine, μmol/L | 94±40 | 99±42 | 107±43 | 96±45 | 107±50 | 119±38 | 119±51 |
| GFR, mL/min/1.73 m2 | 82±23 | 77±26 | 75±29 | 80±31 | 70±28a | 70±26 | 67±26 |
| Cholesterol, mmol/L | 4.8±1.1 | 4.7±1.0 | 4.9±0.9 | 5.3±0.3 | 5.2±0.4 | 4.4±0.8 | 4.3±0.6 |
| Triglyceride, mmol/L | 1.8±0.9 | 1.7±1.0 | 1.9±0.9 | 1.9±0.8 | 2.0±0.7 | 2.0±1.0 | 1.9±0.8 |
| LDL‐C, mmol/L | 3.5±1.9 | 3.4±1.5 | 3.1±0.8 | 3.4±0.7 | 3.4±0.4 | 2.7±0.8 | 2.9±0.6 |
| HDL‐C, mmol/L | 1.3±0.5 | 1.3±0.6 | 1.2±0.3 | 1.3±0.3 | 1.3±0.4 | 1.0±0.2 | 0.9±0.3 |
| Glucose, mmol/L | 4.6±0.6 | 4.4±0.6 | 4.6±0.7 | 4.7±0.6 | 4.6±0.6 | 4.4±0.9 | 4.4±0.5 |
| LVEF, % | 70±4 | 70±5 | 69±7 | 70±6 | 69±8 | 69±8 | 68±5 |
| LVMI, g/m2.7 | 35±11 | 36±10 | 44±11# | 41±9 | 49±10a | 47±13 | 45±12 |
| E/A ratio | 1.3±0.2 | 1.3±0.3 | 1.1±0.3 | 1.1±0.3 | 1.0±0.4 | 1.0±0.3 | 1.0±0.5 |
| CIMI, mm | 0.5±0.1 | 0.6±0.1 | 0.6±0.2b | 0.6±0.2 | 0.7±0.2 | 0.7±0.2 | 0.8±0.4 |
| Plaque | 2/30 | 2/30 | 18/60b | 8/30 | 18/30a | 10/30 | 10/30 |
Abbreviations: A, peak mitral filling velocity at atrial contraction; CIMI, carotid intima‐media thickness; DBP, diastolic blood pressure; E, early mitral inflow filling velocity; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; SBP, systolic blood pressure. Total=awakening dose group + bedtime dose group. aControl with baseline parameters within different group P<.005. bControl with baseline parameters in dipper group P<.005.
Effect of Time of Valsartan Administration on BP Pattern and Ratio of Bedtime/Awakening BP
Valsartan administration at both bed and awakening time significantly decreased SBP and DBP in these three groups (P<.05); however, valsartan with a bedtime dose decreased nighttime SBP compared with patients with awakening dose in the nondipper group (P<.05). The ratio of bedtime/awakening SBP and DBP in the nondipper group treated with the bedtime dose of valsartan for 1 year was similar to that of the dipper group but significantly different from the awakening group (P<.05). Most of the patients in bedtime group shifted to dipper status in the bedtime group, which was higher than that in patients in the awakening group (80% vs 30%, P<.05). These results are shown in Figure 1 and Table II.
Figure 1.
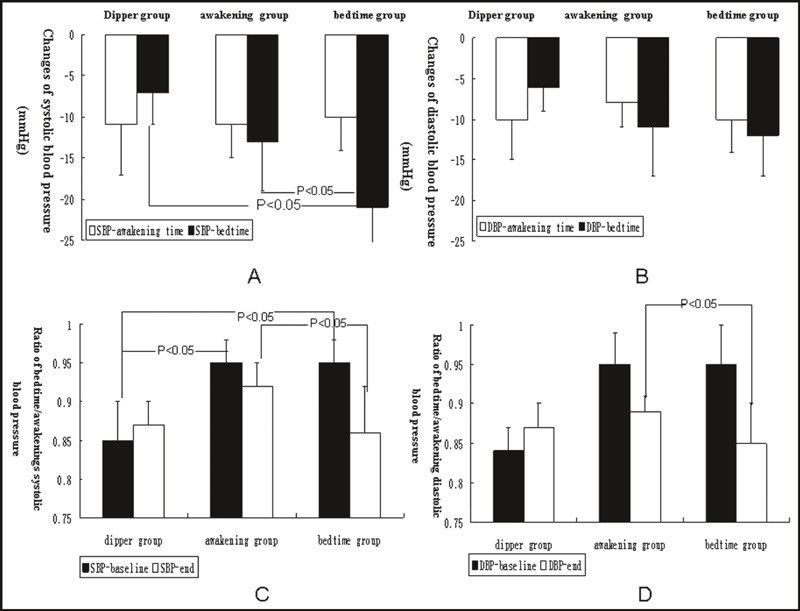
Comparison of blood pressure after valsartan therapy in the three groups. (A) Changes in systolic blood pressure after valsartan therapy in the three groups. (B) Changes in diastolic blood pressure after valsartan therapy in the three groups. (C) Comparison of the ratio of bedtime/awakening systolic blood pressure after valsartan therapy in the three groups. (D) Comparison of the ratio of bedtime/awakening diastolic blood pressure after valsartan therapy in the three groups.
Effect of Time of Valsartan Administration on eGFR and Proteinuria
Valsartan administration in nondipper patients with awakening dose did not delay deterioration of eGFR, and changes of eGFR in this group were significantly higher than those in patients in the dipper and bedtime groups (P<.05). In addition, valsartan administration at both awakening and bedtime significantly lowered proteinuria in these patients (P<.05), but the changes of proteinuria in nondipping patients receiving the bedtime dose were higher than in patients receiving the awakening dose (P<.05). Results are shown in Figure 2 and Table II.
Figure 2.
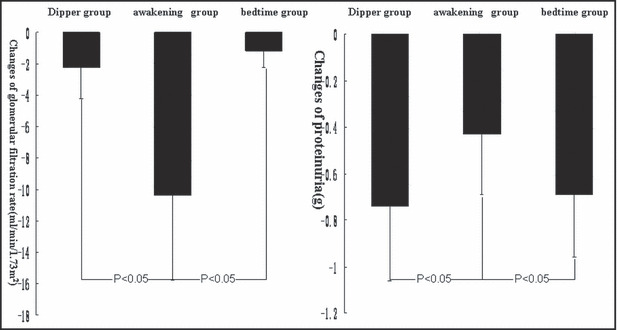
Comparison of the changes in glomerular filtration rate and proteinuria after valsartan therapy in the three groups. (A) Changes in glomerular filtration rate after valsartan therapy in the three groups. (B) Changes in proteinuria after valsartan therapy in the three groups.
Effect of Time of Valsartan Administration on Urine Sodium Excretion
The 24‐hour urine sodium excretion in the nondipper group was not different from patients in the dipper group, while the ratio of bedtime/awakening urine sodium excretion in the nondipper group was higher than that in the dipper group (P<.05). Valsartan administered at bedtime significantly decreased the ratio of bedtime/awakening urine sodium excretion compared with patients in the nondipper with awakening dose (P<.05). These results are shown in Table II.
Effect of Time of Valsartan Administration on Cardiac Structure and Function
The awakening dose of valsartan for 1 year significantly lowered the changes in LVMI (P<.05) in the dipper group; however, the awakening dose in patients with a nondipping pattern did not prevent deterioration of LVMI. In addition, bedtime administration of valsartan prevented the deterioration of LVMI (P<.05), while the changes in left ventricular ejection fraction and the E/A ratio were not significantly different among the three groups. No severe CV diseases such as myocardial infarction, angina pectoris, or heart failure were recorded in the three groups of patients. These results are shown in Figure 3.
Figure 3.
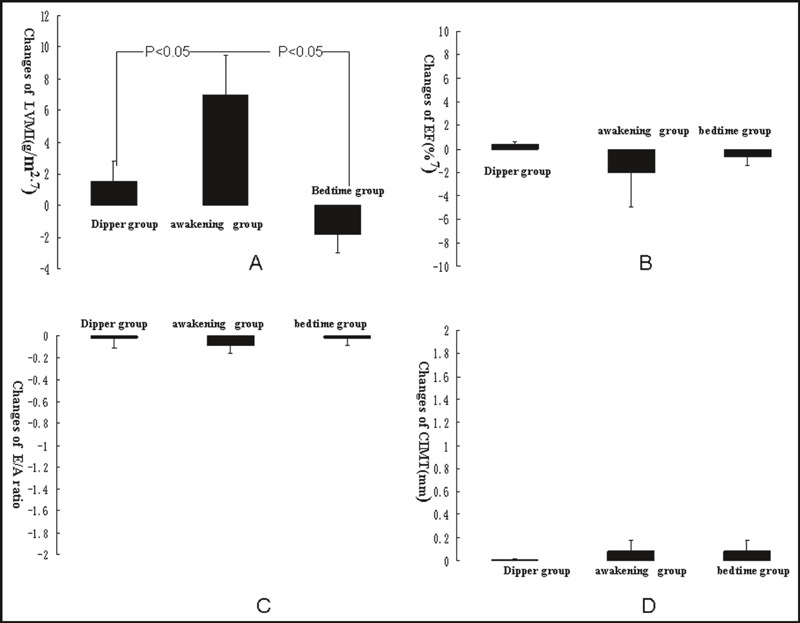
Comparison of ultrasonography parameters after valsartan therapy in the three groups. (A) Changes in left ventricular mass index after valsartan therapy in the three groups. (B) Changes in left ventricular ejection fraction after valsartan therapy in the three groups. (C) Changes in E/A ratio after valsartan therapy in the three groups. (D) Changes in carotid intima‐media thickness after valsartan therapy in the three groups.
Effect of Time of Valsartan Administration on Carotid Structure
Valsartan administration at both awakening and bedtime can significantly prevent deterioration of carotid intima‐media thickness, and changes of the carotid intima‐media thickness were not different in these three groups. Administration of the bedtime dose in nondipping patients reduced the appearance of plaque in the carotid artery compared with patients with the nondipping pattern receiving the awakening dose. These results are shown in Figure 3.
Discussion
In this pilot clinical study, we found that patients with the nondipping BP pattern had a higher ratio of bedtime/awakening proteinuria and urinary sodium excretion, more severe target organ damage such as LVMI, carotid intima‐media thickness, and plaque ratio. When these patients were given valsartan at bedtime, the elevation in nighttime BP was significantly decreased and more patients changed to the dipping BP pattern compared with patients receiving the awakening dose of valsartan. At the same time, valsartan administration at bedtime had better renal protection, including a slower decline in eGFR, decreased proteinuria, and better target organ protection in nondipping BP patients compared with awakening administration of valsartan.
Valsartan is an orally active, specific, and selective angiotensin receptor blocker. After a single oral morning dose, the onset of its BP‐lowering action is within 2 hours, with a peak effect occurring within 4 to 6 hours. Different administration times have different peak effect times, and the drug shows a better effect when the peak effect time is the same as the peak in BP. Valsartan may reduce nocturnal BP level more efficiently when administered in the evening, thereby achieving partial restoration of the physiological nocturnal BP dipping pattern even more than compounds with recommended once‐daily administration based on their pharmacokinetics. Hermida and colleagues 15 first reported that valsartan administered at bedtime, as opposed to at awakening, resulted in a highly significant average increase of 6% in the diurnal‐nocturnal ratio of BP. This corresponded to a 73% relative reduction in the number of nondipping patients. These findings confirm that the time of treatment can be chosen according to the dipper status of a patient to optimize the effect of antihypertensive therapy. The administration of a valsartan fixed combination such as valsartan/amlodipine or valsartan/hydrochlorothiazide can also improve the sleep time–relative BP decline toward a more normal dipping pattern without loss of 24‐hour efficacy. 5 , 6 This phenomenon is not specific to valsartan, as it is also found in olmesartan. 7 Nifedipine (30 mg/d) efficiently reduced BP for 24 hours, and this reduction was significantly greater after bedtime administration. A significant reduction in nighttime BP, a decrease in the prevalence of the nondipping BP pattern, and a significant decrease in morning BP surge were found with bedtime as compared with morning administration. 16 Therefore, the time of ingestion of antihypertensive medications needs to be considered to efficiently reduce BP and improve the circadian pattern of BP without additional cost. Our results also indicate that drug administration according to BP pattern may successfully achieve the therapeutic goals of adequate asleep BP reduction and preserve or re‐establish the normal 24‐hour BP dipping pattern, which is more significant in clinical practice considering that most CKD patients show a nondipping BP pattern. 17 CKD patients benefitted from valsartan therapy without additional costs when we used drugs according to ABPM, considering its better renal and CV protection without additional drugs.
The mechanism of CKD in patients with the nondipping BP pattern remains unclear. Recent reports indicate that several factors such as volume‐dependent hypertension exacerbated by recumbent posture and abnormal sodium handling might be attributed to an abnormal pattern. One study has shown that hypertension, especially elevated nighttime BP, is associated with increased nighttime sodium excretion. The correlation between nighttime sodium excretion and nighttime BP is stronger than the correlation between daytime sodium excretion and daytime BP. 18 Our results also indicated that nondipping BP patients have a higher ratio of bedtime/awakening urine sodium excretion compared with patients with the dipping BP pattern, and the ratio can be lowered following bedtime administration of valsartan for 1 year. These results indicated that valsartan administration at bedtime changes the BP pattern by altering sodium excretion.
To date, antihypertensive “chronotherapy has not been formally demonstrated to affect CKD progression. However, there are some reports that the antiproteinuric efficacy of the angiotensin receptor blocker valsartan or candesartan was associated with an increased day to night BP level ratio on ambulatory or home BP monitoring induced by bedtime dosing. Other important trials have focused on the effect of bedtime dosing of antihypertensive medication on CV risk in CKD. They found that patients receiving bedtime treatment demonstrated a similar significant reduction in risk for a composite outcome of CV death, myocardial infarction, and stroke. 19 We believe that bedtime dosing of antihypertensive medication might offer better renal and CV protection based on theory analysis and small size of trials. Our results suggest that valsartan ingestion at bedtime in patients with a nondipping BP pattern showed better renal protection (slower deterioration of eGFR and greater reduction in proteinuria) and CV protection (greater reduction in LVMI) compared with patients with the awakening dose and these parameters were similar to those in dipping patients. Due to limited sample size and time, we did not find any difference in left ventricular ejection fraction, E/A ratio, and onset of CV attack between different groups; however, these results indicate that antihypertensive chronotherapy may demonstrate better clinical results according to the findings of ABPM. We recommend ABPM in CKD patients because it allows the acquisition of valuable information on not only the average 24‐hour BP, but also the variations in BP values that occur during the course of daily life.
Conclusions
Our results indicate that bedtime dosing of valsartan in CKD patients with a nondipping BP pattern showed better renal and CV protection. Further studies on this topic are required.
Disclosures: No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1. Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent declinein glomerular filtration rate. Arch Intern Med. 2006;166:846–852. [DOI] [PubMed] [Google Scholar]
- 2. Liu M, Takahashi H, Morita M, et al. Non‐dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in haemodialysis patients. Nephrol Dial Transplant. 2003;18:563–569. [DOI] [PubMed] [Google Scholar]
- 3. Torbjornsdotter TB, Jaremkl GA, Berg UB. Nondipping and its relation to glomerulopathy and hyperfiltration in adolescents with type 1 diabetes. Diabetes Care. 2004;27:510–516. [DOI] [PubMed] [Google Scholar]
- 4. Tamura K, Kanaoka T, Ohsawa M, et al. Emerging concept of anti‐hypertensive therapy based on ambulatory blood pressure profile in chronic kidney disease. Am J Cardiovasc Dis. 2011;1:236–243. [PMC free article] [PubMed] [Google Scholar]
- 5. Hermida RC, Ayala DE, Fontao MJ, Mojón A, Fernández JR. Chronotherapy with valsartan/amlodipine fixed combination: improved blood ressure control of essention hypertension with bedtime dosing. Chronobiol Int. 2010;27:1287–1303. [DOI] [PubMed] [Google Scholar]
- 6. Hermida RC, Ayala DE, Mojón A, Fontao MJ, Fernández JR. Chronotherapy with valsartan/hydrochlorothiazide combination in essential hypertension: improved sleep‐time blood pressure control with bedtime dosing. Chronobiol Int. 2011;28:601–610. [DOI] [PubMed] [Google Scholar]
- 7. Hermida RC, Ayala DE, Chayan L, Mojón A, Fernández JR. Administration‐time‐dependent effects of olmesartan of the ambulatory blood pressure of essential hypertension patients. Chronobiol Int. 2009;26:61–79. [DOI] [PubMed] [Google Scholar]
- 8. Minutolo R, Gabbai FB, Borrelli S, et al. Changing the timing of antihypertensive therapy to reduce nocturnal blood pressure in CKD: an 8‐week uncontrolled trial. Am J Kidney Dis. 2007;50:908–917. [DOI] [PubMed] [Google Scholar]
- 9. National Kidney Foundation . K/DOQI clinical practice guidelines on chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;3:S1–S266. [PubMed] [Google Scholar]
- 10. Mancia G, De Baker G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension. The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 11. Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantification of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 12. Zoccali C, Benedetto FA, Mallamaci F, et al. Prognostic impact of the indexation of left ventricular mass in patients undergoing dialysis. J Am Soc Nephrol. 2001;12:2768–2774. [DOI] [PubMed] [Google Scholar]
- 13. Ommen SR, Nishimura RA. A clinical approach to the assessment of left ventricular diastolic function by Doppler echocardiography: update 2003. Heart. 2003;89:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ravera M, Re M, Deferrari L, Vettoretti S, Deferrari G. Importance of blood pressure cntrol in chronic kidney disease. J Am Soc Nephrol. 2006;17:S98–S103. [DOI] [PubMed] [Google Scholar]
- 15. Hermida RC, Carlos C, Ayala DE, et al. Administration time–dependent effects of valsartan on ambulatory blood pressure in hypertensive subjects. Hypertension. 2003;42:283–290. [DOI] [PubMed] [Google Scholar]
- 16. Hermida RC, Ayala DE, Mojón A, Fernández JR. Reduction of morning blood pressure surge after treatment with nifedipine GITS at bedtime, but not upon awakening, in essential hypertension. Blood Press Monit. 2009;14:152–159. [DOI] [PubMed] [Google Scholar]
- 17. Pogue V, Rahman M, Lipkowitz M, et al. Disparate estimates of hypertension control from ambulatory and clinicical blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53:20–27. [DOI] [PubMed] [Google Scholar]
- 18. Hermida RC, Calvo C, Ayala DE, López JE. Decrease in urinary albumin excretion associated to the normalization of nocturnal blood pressure in hypertensive subjects. Hypertension. 2005;46:960–968. [DOI] [PubMed] [Google Scholar]
- 19. Hermida RC, Ayala DE, Mojón A, Fernández JR. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol. 2011;22:2313–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]


