IN ADDITION TO THE SLEEP–WAKE CYCLE AND COGNITIVE FUNCTIONS SUCH as learning and memory, intrinsic clocks determine nearly all circadian cycles in physiology, such as daily variation in blood pressure, heart rate, hormone levels, respiratory and exercise capacity, and coagulation. Many pathologic events occur at specific times of day, indicating that circadian processes contribute to disease (Fig. 1). A central function of the clock system is to drive periods of energy acquisition and use in anticipation of the cycling of day and night. A molecular understanding of circadian time opens therapeutic insights that can help prevent and treat disease.
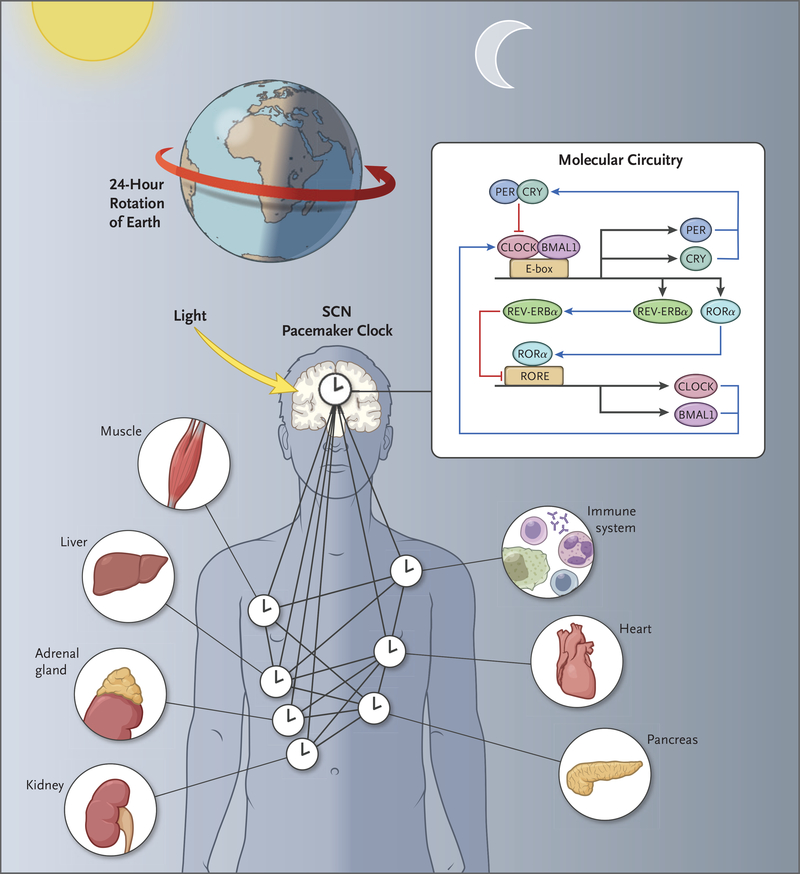
Figure 1. Circadian Networks and Geophysical Time.
The molecular circuitry of circadian clocks is encoded by an autoregulatory 24-hour transcription loop in the brain, where the clocks align sleep–wake and feeding cycles with the rotation of Earth on its axis. Clocks are also present in nearly all tissues of the body, composing a network of timekeepers that anticipate varying environmental conditions each day. Having evolved across all kingdoms of life, the molecular circuitry provides photosensitive species with a mechanism to enhance bioenergetic cycles and ensure escape from DNA-damaging effects of sunlight. BMAL1 denotes brain and muscle Arnt-like protein 1, CLOCK circadian locomotor output cycles kaput, CRY cryptochrome, PER period, RORE retinoic acid–related orphan receptor (ROR) response elements, and SCN suprachiasmatic nucle
CIRCADIAN ORGANIZATION OF PHYSIOLOGY
Circadian rhythms persist even in constant conditions with a period that is almost 24 hours (circa diem, about a day). (See the box for a brief summary.) Light entrains the clock to the 24-hour rotation of Earth (Fig. 1). The first forms of life to tell time according to the light–dark cycle were photosynthetic eubacteria. The evolution of these clocks coincided with the great oxygen expansion 3 billion years ago, fundamentally tying circadian processes with oxygenic respiration.1 Circadian clocks have probably evolved independently in each of the four kingdoms of life, suggesting that they are crucial for the fitness and survival of species.1
CIRCADIAN PACEMAKER NEURONS
Pacemaker neurons housing circadian clocks are the master node in a hierarchical network of internal clocks, driving sleep–wake rhythms and orchestrating clocks in peripheral tissues (Fig. 1). In mammals, classic experiments involving lesions of the hypothalamic suprachiasmatic nucleus (SCN) followed by transplantation have shown that the SCN, comprising approximately 20,000 neurons and glia, contains the pacemaker neurons that are both necessary and sufficient to drive these rhythms.2 Circadian pacemaker neurons display high-amplitude day–night variation in the spontaneous firing rate and resting membrane potential.3 The appropriately timed activity of resting sodium and potassium currents within pacemaker neurons, as well as spike-associated conductance, confer the requisite excitatory and inhibitory drives, respectively, for robust activity rhythms. The oscillations evident at the cellular level are coupled with the core transcriptional oscillator through daily transcript expression of ion channels4 or their regulators,5 including metabolic signals within the cell (e.g., nicotinamide adenine dinucleotide [NAD+]).6 Neuronal activity also serves to reset cellular autonomous molecular clocks in brain regions outside the pacemaker cells, maintaining synchronous 24-hour oscillations across this master neural network. The finding that the molecular and cellular basis of daily sleep–wake rhythms is shared among vertebrates and invertebrates suggests that, like the core clock itself, neural control of daily sleep–wake behavior has evolutionary roots dating back more than 500 million years.
Retinal rod and cone photoreceptors and specialized retinal ganglion cells (RGCs) that express the photopigment melanopsin convey light information to entrain SCN clocks.7 These intrinsically photosensitive RGCs project to the SCN and other brain regions, including those regulating mood, and can even entrain SCN clocks in perceptually blind persons.8 Melanopsin absorbs blue light, which is emitted by electronic devices more readily than broad-spectrum light. Artificial lighting in the evening can delay circadian clocks, resulting in misalignment with environmental cycles and increasing the risk of sleep disorders.9 The coincidence of light with the endogenous clock program in the SCN shifts as day length varies from summer to winter months, leading to seasonal changes in intrinsic cycles.
SCN pacemaker neurons regulate a myriad of physiological processes, including sleep, arousal, temperature regulation, autonomic nervous system tone, feeding cycles, reward pathways, mood, and movement. Neuroendocrine systems operate as homeostatic sensors that respond to environmental changes (e.g., the release of insulin in response to glucose, as well as the release of glucocorticoid in response to stress). In contrast, the circadian clock endows physiological systems with the ability to anticipate daily changes. (See additional references in the Supplementary Appendix, available with the full text of this article at NEJM.org.)
MOLECULAR CIRCUITRY OF THE CIRCADIAN CLOCK
A breakthrough in understanding how circadian clocks “tick” followed the discovery of the Period gene (Per) in the fruit fly Drosophila melanogaster and the Clock gene in the mouse. Surprisingly, Per encodes a protein that represses its own transcription, resulting in daily Per rhythms.10,11 Subsequently, the Per activator was discovered in mammals and named Clock, revealing that the gears of the clock are composed of activators that induce the expression of their own repressors, forming a negative feedback loop12 that is highly conserved from flies to humans. The core loop consists of basic helix–loop–helix (bHLH) and Per-Arnt-Sim (PAS) heterodimeric transcriptional activators (CLOCK [circadian locomotor output cycles kaput] or its paralog, NPAS2, with BMAL1 [brain and muscle Arnt-like protein 1]) (Fig. 1).12 The activators bind to E-box elements in the core clock repressors Period (Per1, Per2, or Per3) and Cryptochrome (Cry1 or Cry2) in mammals, which dimerize and then provide negative feedback to control their own transcription. The timing of feedback is tuned by means of post-transcriptional modifications (e.g., splicing and translation) and especially post-translational modifications. A common regulatory motif is rhythmic phosphorylation of clock components coupled with their rhythmic degradation, often through the ubiquitin–proteasome system. This core loop is embedded in other transcription feedback loops through CLOCK–BMAL1 activation of Rev-erbα and Rorα, which reinforce the core loop. Additional transcription factors provide feedback and regulate CLOCK activity, including USF1 and Dec1–Dec2. Studies in mice with core Clock disruption have shown that the rhythmicity of physiological processes arises from oscillating gene expression downstream of this core transcriptional oscillator.13
PERIPHERAL-TISSUE CLOCKS
Central pacemaker clocks drive the rhythmic activity of molecular clocks that are expressed in most cells throughout the body, termed peripheral clocks (Fig. 2). These peripheral clocks govern a vast array of molecular and cellular processes at virtually every level of regulation (Fig. 3). Transcriptional analyses in animals, including humans, have revealed that large fractions of the genome are clock-controlled; more than half of protein-encoding genes show circadian oscillation in distinct patterns across tissues.14–16 In addition to transcription, circadian regulation of cell physiology arises from the rhythmic control of post-transcriptional processes, including RNA splicing, protein translation, and post-translational processing.
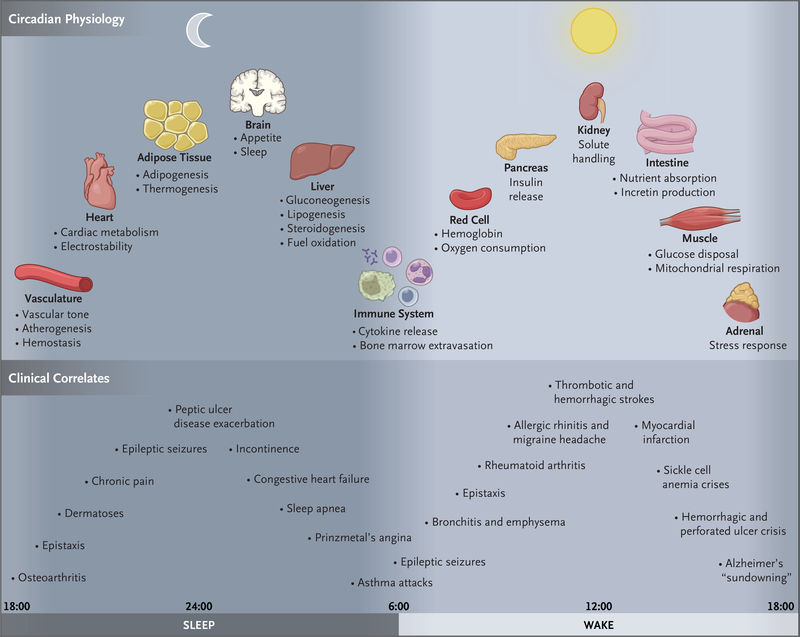
Figure 2. Circadian Timing of Physiological Processes and Disease.
Molecular clocks are present in most cells in the brain and throughout peripheral tissues of the body. Clocks are entrained by the brain pacemaker neuron to the environmental light–dark cycle and help maintain the constancy of the internal milieu through anticipation of alterations that occur as mammals undergo daily sleep–wake and fasting–feeding cycles. The top panel highlights a subset of 24-hour oscillating physiological processes across diverse tissues, all coordinated with the day–night cycle. The bottom panel shows the clinical correlates in humans that are associated with circadian disruption across the day and night. “Sundowning” refers to confusion or delirium during the evening or night that disappears or abates during daytime.
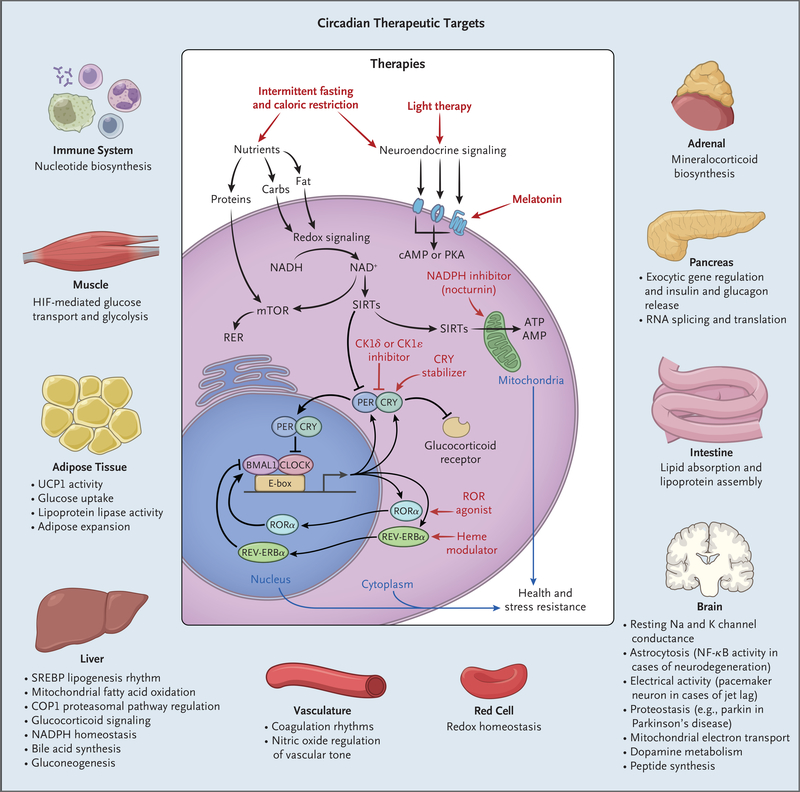
Figure 3. Potential Exploitation of Clock Pathways for Therapeutics.
Molecular clocks in nearly all cell types drive gene transcription in collaboration with tissue-specific factors. Local clocks generate robust oscillation in the expression of distinct rate-limiting factors according to the time of day. Therapies that affect either central clock activity (e.g., light or melatonin, each of which modulates the sleep–wake cycle) or peripheral-acting targets (e.g., modulators of nucleotide levels, cryptochrome [CRY] stability, and nuclear receptor activity) represent potential targets for manipulating circadian pathways in specific tissues. Shown are the pathways regulated in diverse tissues and, in the center, the localization of input signals and downstream therapeutic targets that are circadian outputs. Carbs denotes carbohydrates, cAMP cyclic AMP, CK1 casein kinase 1, ER endoplasmic reticulum, HIF hypoxia-inducible factor, mTOR mammalian target of rapamycin, NAD+ nicotinamide adenine dinucleotide, NF-κB nuclear f actor κB, PKA protein kinase A, SIRT sirtuin, and UCP1 uncoupling protein 1.
METABOLIC CUES FOR ENTRAINMENT
Exposing cultured cells to a pulse of serum (presumably the associated humoral factors) synchronizes robust 24-hour transcription cycles.17 Although peripheral clocks are normally entrained by the master SCN pacemaker, feeding can independently synchronize peripheral clocks in the liver and kidneys, leading to misalignment in clock cycles when food is consumed at the wrong time of day.18 Peripheral clocks reinforce rhythmic regulation at the local tissue level and can be entrained by timed meals, even in animals lacking the master brain clock.
Clock transcription factors are bifunctional proteins with a bHLH DNA-binding domain connected to a PAS domain (sometimes a ligand-binding domain). The bHLH–PAS transcription factors sense changes in the environment, and cross-talk between clock factors and other bHLH–PAS superfamily members, such as hypoxia-inducible factor 1α (HIF-1α), leads to metabolic signaling that is specific to the time of day. For example, cross-talk between CLOCK and HIF-1α coordinates oxygen sensing and circadian transcription cycles, contributing to day–night differences in exercise capacity. Clock factors also respond to changes in the partial pressure of oxygen through heme, which binds to the REV-ERBs,19 contributing to rhythmic skeletal-muscle metabolism.
Central circadian entrainment of peripheral clocks leads to robust daily rhythms across many physiological systems and complements homeostatic responses. One example is the rhythm in blood pressure regulated by interactions among the endogenous circadian system, sleep, and changes in posture that alter sympathovagal balance.20 In addition, circadian oscillation in renal solute handling adjusts intravascular volume to anticipate postural changes across sleep–wake states. A wide range of immunologic responses similarly have day–night variation, as discussed below.
ROLE OF CIRCADIAN DISRUPTION IN DISEASE
There is evidence that circadian misalignment due to artificial light, shift work, and jet travel is common in modern life and contributes to a wide range of human diseases (Fig. 2). Light exposure at the incorrect time of day shifts the phase of pacemaker neuronal clocks and peripheral-tissue clocks and can impair cognitive performance.21 Irregular sleep and eating schedules can misalign clocks in metabolic organs, leading to obesity and diabetes. In addition, the incidence of disease-related events, such as myocardial infarction, and responses to drugs are often influenced by the time of day. Treatments may be most effective if dose administration is synchronized with these daily changes.
SLEEP DISORDERS
General circadian rhythm sleep disorders are characterized by misalignment between intrinsic circadian cycles and the environmental light–dark cycle (Fig. 2). These disorders can be due to conditions, such as travel across time zones or exposure to artificial light, or to intrinsic disorders of clock function, such as those due to mutations in core clock genes. One severe subtype of circadian rhythm sleep disorder occurs in persons who are blind as a result of bilateral enucleation, a condition in which loss of the intrinsically photosensitive RGCs impedes the reception of light signals by the SCN. These persons have free-running circadian sleep–wake rhythms that are desynchronized from 24-hour schedules. Behavioral interventions and treatment with pharmacologic agents (melatonin agonists) can facilitate realignment.22
Because the human central circadian pacemaker can shift by only about 1 hour per day, rapid air travel across multiple time zones results in misalignment between the destination environment and the internal clock. Since the intrinsic period of the circadian clock is slower than 24 hours,23 this problem is more evident with travel in an eastward direction, which would require a faster clock to readjust to the new time zone. Jet lag is associated with impaired motor performance and symptoms of malaise such as gastrointestinal disorders. In fact, shifts requiring even a 1-hour advance of the clock, such as “spring forward” for daylight saving time, have been associated with a wide range of adverse clinical events,24,25 including an increased incidence of myocardial infarction,26 and impaired performance, resulting in car accidents.27
Social jet lag refers to a pattern of inconsistent sleep time between work days and days off from work. This problem can be exacerbated by exposure to phase-delaying blue light in the evening from electronic devices or other artificial lighting. Shift-work sleep disorder is defined by insomnia or excessive sleepiness occurring in relation to work scheduled during normal sleep time. Recovery sleep occupies normal free time and interferes with social relationships. Severe disorders lead to impaired work performance. Persons with extreme chronotypes, who may be at increased risk for these disorders, can be identified with the use of questionnaires that evaluate “morningness–eveningness” type on the basis of bedtime and wake-up preferences. Such subjective characterization can be validated with the use of melatonin measurements to track the endogenous circadian cycle, with the timing of peak levels differing by up to 4 hours between the extremes of early and late chronotypes.
In addition to circadian rhythm disorders associated with modern living, some families with heritable chronotypes characterized by an extremely advanced (i.e., early) onset of sleep have been shown to transmit variants in the core clock genes that encode casein kinase 1 delta and its target, PER2.28 Gain-of-function mutations in Cry that reduce activity of core clock activators (CLOCK and BMAL1) have been identified as a cause of delayed sleep onset and a prolonged period of wakefulness, a disorder also associated with polymorphisms in Clock and Per3.29 Sleep–wake cycle disturbances in humans involve mechanisms similar to those identified through genetic analyses in D. melanogaster.
PSYCHIATRIC AND NEURODEGENERATIVE DISEASES
Perhaps one of the greatest therapeutic opportunities for the application of circadian knowledge is in neurodegenerative diseases, for which we largely lack effective disease-modifying treatments. Disrupted daily rhythms at the level of sleep–wake behavior, hormones (e.g., melatonin), and gene expression (e.g., Per) are evident in patients with a wide range of neurodegenerative diseases, including Huntington’s disease, Parkinson’s disease, and Alzheimer’s disease.30 Neuropathological damage is also evident in the SCN and had been assumed to be a consequence of rather than a contributor to these disorders. However, in many cases, circadian disruption is evident even before the onset of pathognomonic symptoms.30 Indeed, preclinical and clinical studies have correlated circadian disruption with the accumulation of neurotoxic proteins and neurodegeneration itself.31 Clock control of astrocyte and microglia function may also contribute to neurodegenerative disease, the latter through REV-ERBα.32 The interplay between circadian and neurodegenerative processes may also occur through clock control of sleep and rhythmic clearance of neurotoxic proteins through the glymphatic system33 or sleep-driven changes in cerebrospinal fluid flow.34 An emerging theme is that circadian control of oxidative or proteotoxic stress may play a part in neurodegeneration31 (Fig. 3). Such findings point to potential new treatments for neurodegenerative diseases.
Circadian clock disruption has been observed in schizophrenia35 and many other psychiatric disorders.36 Probably the most prominent and most well understood mechanistically is the circadian link to mood disorders, such as seasonal affective disorder. These disorders are accompanied by a reduced amplitude or altered phase in a wide range of rhythms, including sleep–wake rhythms, blood pressure, hormonal rhythms (cortisol and melatonin), and the 24-hour rhythmicity of circadian clock gene expression.37 The social zeitgeber (environmental cue) theory, originally described in relation to bipolar disorder, hypothesizes that disruption in the timing of daily social routines (as a result of jet lag or shift work, for example) can exacerbate bipolar depression and other mood disorders. Remarkably, the efficacy of therapeutics for mood disorders is correlated with their ability to alter circadian rhythms. In fact, the antidepressant agomelatine directly targets the circadian system, acting as a mixed agonist–antagonist: a melatonin-receptor agonist and a 5-hydroxytryptamine 2C (5-HT2C) receptor antagonist.38 Human genetic studies, including genomewide association studies, have identified circadian clock gene variants that substantially contribute to the risk of mood disorders.
Light exposure has long been recognized as a factor in affective disorders, in addition to its association with mania. Persons who have depression during the short days of winter and manic symptoms during long summer days have a disorder at one end of the spectrum of light-sensitive mood disturbances. At the epidemiologic level, the incidence of depression and mania also corresponds with regional extremes in latitude.39 Seasonal behavioral disorders may be amenable to blue-light therapy, which activates retinal melanopsin cells, thereby directly stimulating regions that regulate mood independent of central pacemaker neuron activity.
The mechanisms by which light exposure and endogenous clocks modulate mood are multifactorial and incompletely understood. Studies in animal models have revealed specific molecular, cellular, and physiological pathways (e.g., monoaminergic neurotransmission and the hypothalamic–pituitary–adrenal axis) that may link the circadian clock to mood regulation, in addition to metabolic and immune pathways (Figs. 2 and 3). Circadian clock genes directly regulate tyrosine hydroxylase and monoamine oxidase A, rate-limiting enzymes that produce and degrade dopamine, respectively. Given the prominent role of dopamine in schizophrenia, as well as in a host of other psychiatric disorders, mechanisms of clock regulation may be susceptible to new treatments.
CANCER
Epidemiologic and experimental studies provide evidence that cancer is associated with shift work and circadian disruption. Landmark findings from the Nurses’ Health Study indicated an increased risk of breast cancer in association with night-shift work, in addition to evidence of an increased risk of colorectal disease.40 More recent studies have implicated exposure to light at night in the risk of melanoma. At the cellular level, desynchrony in the circadian control of DNA replication, transcription, and cell metabolism may contribute to cancer. For example, in mice, the damaging effect of sunlight on epidermis is greatest in the morning, when DNA excision repair is at its nadir.41 The circadian period proteins form complexes with the cryptochromes and have been implicated as regulators of the cell cycle and of the tumor suppressor p53, which is important in lung cancer.42 In addition, interplay between the oncogenic bHLH transcription factor MYC and CLOCK has been shown to coregulate glycolytic genes, possibly facilitating cancer progression in MYC-driven cancers such as neuroblastoma.43 Cross-talk between CLOCK–BMAL1 and HIF-1α represents an additional node for coregulation of circadian and metabolic pathways that may be involved in HIF-dependent cancers.44 In addition to the connection between circadian disruption and cancer initiation, interference with rhythms may contribute to the DNA damage response and other aspects of cancer progression. For example, a major output of the clock involves the rhythmic control of enzymes involved in the biosynthesis of NAD+, a cofactor for the DNA repair pathway involving poly(ADP-ribose) polymerase (PARP) enzymes and sirtuin deacetylases. Furthermore, CRY1 and CRY2 inhibit nuclear receptors involved in endocrine cancer, such as the androgen receptor, pointing to potential therapeutic targets in prostate cancer.45
INFECTION, INFLAMMATION, AND CARDIOVASCULAR DISEASE
Understanding the molecular actions of the circadian clock provides insight into rhythmic patterns of inflammatory disease. Responses to pathogens show circadian variation in circulating cells of the innate immune system. Sympathetic nervous system rhythms also generate rhythmic variation in the endotoxin response. Within the epidermis, mast cells show circadian variation in IgE-mediated cutaneous anaphylactic reactions. Clock expression in pulmonary epithelial cells generates rhythmic variation in Streptococcus pneumoniae infection.46 Inflammatory bowel disease has rhythmic variation that may be related to circadian control of the canonical repressor NFIL3 involved in type 17 helper T-cell regulation.47 In patients with rheumatoid arthritis, joint symptoms in the morning have been attributed to the accumulation of inflammatory cytokines during the previous night. Experimental studies in mice indicate that alterations in clock function may elicit inflammation within mesenchymal cells lining the joint.48
At the molecular level, nuclear factor κB, a central mediator of immune-cell activity, inhibits the clock repressor PER2. The interdependence of circadian and inflammatory pathways indicates that the two processes may be homeostatically connected.49,50 For instance, REV-ERBα, a core component of the molecular clock, represses the production of proinflammatory cytokines in macrophages. In addition, direct circadian control of glucocorticoid signaling51 may point to new therapeutics for inflammatory disease (Fig. 3).
Cardiovascular and thrombotic processes have an inflammatory component and are influenced by additional circadian factors at both the tissue and systemic levels. Epidemiologic evidence shows a temporal spike in myocardial infarction and aortic rupture in the morning and when clocks change with daylight saving time.52–54 Physiological rhythms underlying morning cardiovascular events include platelet activation, endothelial-cell nitric oxide and thromboxane production,55 prothrombotic plasminogen activator inhibitor 1 production,56 and a rise in catecholamine levels.57 Intrinsic electrical conduction and arrhythmogenic abnormalities also peak in the early daytime.58,59 Recent evidence suggests that the risk of ischemia–reperfusion injury with cardiopulmonary bypass may be greatest in the early-morning hours.60 Abnormal blood-pressure dipping at nighttime is a prognostic sign of cardiovascular risk that is independent of daytime blood pressure. Enhanced prevention and treatment of hypertension may be achieved through more widespread application of circadian monitoring.
In addition to the vascular effects of impaired rhythmic control, deregulation of clock function within adipose tissue, liver, and muscle may secondarily promote cardiometabolic disorders.6 In muscle, clock function regulates glucose uptake and exercise capacity, factors that may influence cardiovascular risk in the long term. Many rate-limiting enzymes in cholesterol and bile acid metabolism in the liver have diurnal patterns, and misalignment of these endogenous cycles with food consumption may contribute to dyslipidemia. In addition, misalignment of feeding with circadian cycles modulating adipose insulin sensitivity, nutrient storage, inflammation, and thermogenesis may contribute to metabolic complications of obesity, as discussed below.
DISRUPTION OF GLUCOSE HOMEOSTASIS AND TIME-RESTRICTED FEEDING
Impaired glucose tolerance represents a major systemic effect of circadian disruption in both epidemiologic and clinical studies. Glucose tolerance is lower at night in healthy persons,61 and in patients with diabetes, the “dawn phenomenon” (i.e., high morning glucose levels) reflects sustained glucose production in the liver and reduced glucose uptake related to growth hormone, cortisol, and adrenergic stimulation during sleep.
Insulin release by pancreatic beta cells requires the expression of clock genes within glucose-sensing islet cells. Clock transcription factors regulate genes involved in insulin secretion and generate maximal secretory capacity to coincide with wakefulness.62 In contrast, circadian control of glucagon production by alpha cells may be important in the maintenance of constant glucose levels during sleep.63 The autonomic nervous system may entrain the local alpha- and beta-cell clocks to the light–dark cycle, in addition to signaling by the sleep-associated hormone melatonin.62 In humans, melatonin 1B receptor variants are associated with glucose levels, although whether melatonin acts in the brain or in the islet to control glucose remains unclear.6 Melatonin levels rise at night and may increase incretin-mediated insulin release the next day.64 The clock system is central to hepatic glucose production, skeletal-muscle glucose disposal, and thermogenesis — integrated processes that direct cycles of storage and use of glucose for energy.6
Disruption of meal timing in animals and humans,65 either genetically or simply by providing a high-fat diet,66,67 leads to impaired glucose tolerance and weight gain,68 whereas time-restricted feeding ameliorates metabolic disorders related to diet-induced obesity.69 Restricting feeding to limited periods not only provides protection against obesity but also reproduces the metabolic profile found with caloric restriction, raising the possibility that feeding time, like a hypocaloric diet, may promote healthy aging.70 In the hospital setting, the normal circadian routine is frequently disrupted. For example, the light–dark cycle may be disrupted and nutritional supplementation may be provided without alignment of nutrient delivery with endogenous circadian cycles that are normally entrained to light; these factors potentially contribute to inflammation and insulin resistance.
DIAGNOSING CLOCK DISRUPTION
Despite our knowledge of the far-reaching effect of the circadian clock on human disease, a major barrier to leveraging this understanding in clinical medicine is the difficulty of detecting and diagnosing circadian disruption. The standard method is to assay the timed onset of plasma melatonin levels. However, this requires serial sampling and typically must occur in the evening and in dim light, precluding sample collection during a typical office visit. Peripheralblood “omics” assays have been combined with computational tools to identify signatures of circadian time, which can provide an accurate assessment of the circadian phase, even from a single sample.71,72 These studies could open the door to routine clinical assessments of circadian phenotypes that may be useful for prediction of drug responses, as well as disease diagnosis and prognosis (Fig. 3). An example is the range of circadian variables involved in blood-pressure regulation, which may reflect chronotype differences between persons.
Detecting defined alterations in physiological rhythms holds promise for identifying persons at increased risk for disease, as well as those most likely to benefit from circadian-based therapeutics. Many pharmacologic targets are the products of intrinsically time-dependent RNAs, including those involved in treatments to induce sleep or wakefulness and those related to rhythmic production of hormones such as glucocorticoids.73 For instance, the clock repressor Cryptochrome rhythmically modulates the activity of nuclear receptors involved in neuroendocrine disease, including prostate cancer.
ADAPTING TREATMENT TO CIRCADIAN RHYTHMS
Beyond the potential pathophysiological role of circadian pathways, emerging evidence indicates that synchronizing drug delivery with endogenous physiological rhythms may be used to optimize treatment efficacy. For example, adjusting the administration of oxaliplatin, a cis-platinum derivative, to the time of day reduces off-target side effects in patients with colorectal cancer.74 More recent evidence has revealed significant diurnal variation in the metabolism of a small-molecule receptor tyrosine kinase inhibitor, suggesting that consideration of endogenous clock time may enhance the efficacy of this and perhaps other chemotherapeutic agents.75 Rhythmic expression of central mitochondrial enzymes in the liver that are important in the activation or catabolism of lipid-soluble drugs may influence pharmacokinetics at different times of the day. Alternatively, drug targets themselves may peak at different times. An example is the rate-limiting enzyme of cholesterol biosynthesis, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which peaks at night in humans. This observation led to the recommendation that short-acting statins be administered in the evening. Many agents with a half-life shorter than 12 hours are most effective when delivery is adjusted for intrinsic circadian time.
FUTURE CHALLENGES
Discovery of the circadian clock first established a genetic basis for behavior, and our understanding of circadian rhythms has since expanded to provide molecular insights into physiology and disease. Yet the challenge remains to translate these insights regarding the role of circadian clocks in cells and tissues into the practice of clinical medicine. Exploiting knowledge of the molecular clock in a disease-specific context may one day lead to more precise “temporal” diagnostics and aid in multiple levels of management. Investigation into core mechanisms may provide therapies to reset or amplify circadian signals. A mechanistic understanding of the link between the molecular clock and disease can be leveraged to identify the appropriate timing of therapies, as well as new treatment targets (Fig. 3). We anticipate that as these molecular links are revealed, new interventions will be developed and applied across the wide swath of systems affected by the circadian clock. Only time will tell.
Supplementary Material
Circadian Rhythms.
The term circadian originates from the Latin circa diem, about a day. Animals that are active during daylight are referred to as diurnal, and species that are active at night are classified as nocturnal. When placed in constant darkness, both nocturnal and diurnal species show 24-hour periodicity in behavior and physiology, a hallmark of the endogenous circadian rhythms. Unlike most biochemical processes, these rhythms do not vary according to temperature. In contrast, many daily rhythms do not have 24-hour periodicity under constant conditions, including metabolic rhythms that shift with changes in the fasting–feeding cycle or temperature.
Acknowledgments
We thank Grant Barish, Lisa Beutler, Jonathan Cedernaes, and John Hogenesch for critical discussion and Billie Marcheva for assistance with earlier versions of the figures.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Ravi Allada, Department of Neurobiology, Northwestern University, Evanston, Illinois
Joseph Bass, Department of Medicine, Division of Endocrinology, Metabolism, and Molecular Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois
REFERENCES
- 1.Gehring W, Rosbash M. The coevolution of blue-light photoreception and circadian rhythms. J Mol Evol 2003; 57: Suppl 1: S286–S289. [DOI] [PubMed] [Google Scholar]
- 2.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science 1990;247:975–8. [DOI] [PubMed] [Google Scholar]
- 3.Paul JR, Davis JA, Goode LK, et al. Circadian regulation of membrane physiology in neural oscillators throughout the brain. Eur J Neurosci 2020;51:109–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meredith AL, Wiler SW, Miller BH, et al. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci 2006;9:1041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flourakis M, Kula-Eversole E, Hutchison AL, et al. A conserved bicycle model for circadian clock control of membrane excitability. Cell 2015;162:836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science 2016;354:994–9. [DOI] [PubMed] [Google Scholar]
- 7.LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci 2014;15:443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czeisler CA, Shanahan TL, Klerman EB, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med 1995;332:6–11. [DOI] [PubMed] [Google Scholar]
- 9.Chang A-M, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A 2015; 112: 1232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A 1971;68:2112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardin PE, Hall JC, Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 1990;343:536–40. [DOI] [PubMed] [Google Scholar]
- 12.King DP, Zhao Y, Sangoram AM, et al. Positional cloning of the mouse circadian clock gene. Cell 1997;89:641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 2020;21:67–84. [DOI] [PubMed] [Google Scholar]
- 14.Panda S, Antoch MP, Miller BH, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 2002;109:307–20. [DOI] [PubMed] [Google Scholar]
- 15.Fang B, Everett LJ, Jager J, et al. Circadian enhancers coordinate multiple phases of rhythmic gene transcription in vivo. Cell 2014;159:1140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koike N, Yoo S-H, Huang H-C, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 2012;338:349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 1998;93:929–37. [DOI] [PubMed] [Google Scholar]
- 18.Izumo M, Pejchal M, Schook AC, et al. Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. Elife 2014;3:e04617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin L, Wu N, Curtin JC, et al. Reverbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 2007;318:1786–9. [DOI] [PubMed] [Google Scholar]
- 20.Thosar SS, Butler MP, Shea SA. Role of the circadian system in cardiovascular disease. J Clin Invest 2018;128:2157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeGates TA, Altimus CM, Wang H, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 2012;491:594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skene DJ, Arendt J. Circadian rhythm sleep disorders in the blind and their treatment with melatonin. Sleep Med 2007;8:651–5. [DOI] [PubMed] [Google Scholar]
- 23.Duffy JF, Cain SW, Chang A-M, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A 2011; 108: Suppl 3:15602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Dahlén T, Khan A, Edgren G, Rzhetsky A. Measurable health effects associated with the daylight saving time shift. PLoS Comput Biol 2020;16(6):e1007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poteser M, Moshammer H. Daylight saving time transitions: impact on total mortality. Int J Environ Res Public Health 2020;17:1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manfredini R, Fabbian F, Cappadona R, et al. Daylight saving time and acute myocardial infarction: a meta-analysis. J Clin Med 2019;8:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martín-Olalla JM. Traffic accident increase attributed to daylight saving time doubled after Energy Policy Act. Curr Biol 2020;30:R298–R300. [DOI] [PubMed] [Google Scholar]
- 28.Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 2001;291:1040–3. [DOI] [PubMed] [Google Scholar]
- 29.Patke A, Murphy PJ, Onat OE, et al. Mutation of the human circadian clock gene CRY1 in familial delayed sleep phase disorder. Cell 2017;169(2):203–215.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol 2019;18:307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu F, Kula-Eversole E, Iwanaszko M, Hutchison AL, Dinner A, Allada R. Circadian clocks function in concert with heat shock organizing protein to modulate mutant Huntingtin aggregation and toxicity. Cell Rep 2019;27(1):59–70.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKee CA, Lananna BV, Musiek ES. Circadian regulation of astrocyte function: implications for Alzheimer’s disease. Cell Mol Life Sci 2020;77:1049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hablitz LM, Plá V, Giannetto M, et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun 2020;11:4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fultz NE, Bonmassar G, Setsompop K, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019;366:628–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yates NJ. Schizophrenia: the role of sleep and circadian rhythms in regulating dopamine and psychosis. Rev Neurosci 2016;27:669–87. [DOI] [PubMed] [Google Scholar]
- 36.Carr O, Saunders KEA, Tsanas A, et al. Variability in phase and amplitude of diurnal rhythms is related to variation of mood in bipolar and borderline personality disorder. Sci Rep 2018;8:1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li JZ, Bunney BG, Meng F, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci U S A 2013;110:9950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor D, Sparshatt A, Varma S, Olofinjana O. Antidepressant efficacy of agomelatine: meta-analysis of published and unpublished studies. BMJ 2014;348:g1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mersch PP, Middendorp HM, Bouhuys AL, Beersma DG, van den Hoofdakker RH. Seasonal affective disorder and latitude: a review of the literature. J Affect Disord 1999;53:35–48. [DOI] [PubMed] [Google Scholar]
- 40.Wegrzyn LR, Tamimi RM, Rosner BA, et al. Rotating night-shift work and the risk of breast cancer in the Nurses’ Health Studies. Am J Epidemiol 2017;186:532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaddameedhi S, Selby CP, Kaufmann WK, Smart RC, Sancar A. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci U S A 2011;108:18790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papagiannakopoulos T, Bauer MR, Davidson SM, et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab 2016;24:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altman BJ, Hsieh AL, Sengupta A, et al. MYC disrupts the circadian clock and metabolism in cancer cells. Cell Metab 2015;22:1009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peek CB, Levine DC, Cedernaes J, et al. Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab 2017;25:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kriebs A, Jordan SD, Soto E, et al. Circadian repressors CRY1 and CRY2 broadly interact with nuclear receptors and modulate transcriptional activity. Proc Natl Acad Sci U S A 2017;114:8776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibbs J, Ince L, Matthews L, et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med 2014;20:919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu X, Rollins D, Ruhn KA, et al. TH17 cell differentiation is regulated by the circadian clock. Science 2013;342:727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hand LE, Dickson SH, Freemont AJ, Ray DW, Gibbs JE. The circadian regulator Bmal1 in joint mesenchymal cells regulates both joint development and inflammatory arthritis. Arthritis Res Ther 2019;21:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho H, Zhao X, Hatori M, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 2012;485:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lam MTY, Cho H, Lesch HP, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature 2013;498:511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamia KA, Papp SJ, Yu RT, et al. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 2011;480:552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985;313:1315–22. [DOI] [PubMed] [Google Scholar]
- 53.Vitale J, Manfredini R, Gallerani M, et al. Chronobiology of acute aortic rupture or dissection: a systematic review and a meta-analysis of the literature. Chronobiol Int 2015;32:385–94. [DOI] [PubMed] [Google Scholar]
- 54.Jiddou MR, Pica M, Boura J, Qu L, Franklin BA. Incidence of myocardial infarction with shifts to and from daylight savings time. Am J Cardiol 2013;111:631–5. [DOI] [PubMed] [Google Scholar]
- 55.Bridges AB, McLaren M, Saniabadi A, Fisher TC, Belch JJ. Circadian variation of endothelial cell function, red blood cell deformability and dehydro-thromboxane B2 in healthy volunteers. Blood Coagul Fibrinolysis 1991;2:447–52. [DOI] [PubMed] [Google Scholar]
- 56.Scheer FAJL, Shea SA. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood 2014;123:590–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller JE, Tofler GH, Willich SN, Stone PH. Circadian variation of cardiovascular disease and sympathetic activity. J Cardiovasc Pharmacol 1987; 10: Suppl 2:S104–S109. [PubMed] [Google Scholar]
- 58.Twidale N, Taylor S, Heddle WF, Ayres BF, Tonkin AM. Morning increase in the time of onset of sustained ventricular tachycardia. Am J Cardiol 1989;64:1204–6. [DOI] [PubMed] [Google Scholar]
- 59.Jeyaraj D, Haldar SM, Wan X, et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 2012;483:96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montaigne D, Marechal X, Modine T, et al. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet 2018;391:59–69. [DOI] [PubMed] [Google Scholar]
- 61.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest 1991;88:934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perelis M, Marcheva B, Moynihan Ramsey K, et al. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 2015;350(6261):aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petrenko V, Saini C, Giovannoni L, et al. Pancreatic α- and β-cellular clocks have distinct molecular properties and impact on islet hormone secretion and gene expression. Genes Dev 2017;31:383–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kemp DM, Ubeda M, Habener JF. Identification and functional characterization of melatonin Mel 1a receptors in pancreatic beta cells: potential role in incretin-mediated cell function by sensitization of cAMP signaling. Mol Cell Endocrinol 2002;191:157–66. [DOI] [PubMed] [Google Scholar]
- 65.Wehrens SMT, Christou S, Isherwood C, et al. Meal timing regulates the human circadian system. Curr Biol 2017;27(12):1768–1775.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science 2005;308:1043–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kohsaka A, Laposky AD, Ramsey KM, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 2007;6:414–21. [DOI] [PubMed] [Google Scholar]
- 68.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 2012;15:848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilkinson MJ, Manoogian ENC, Zadourian A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab 2020;31(1):92–104.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laing EE, Möller-Levet CS, Poh N, Santhi N, Archer SN, Dijk D-J. Blood transcriptome based biomarkers for human circadian phase. Elife 2017;6:e20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braun R, Kath WL, Iwanaszko M, et al. Universal method for robust detection of circadian state from gene expression. Proc Natl Acad Sci U S A 2018;115:E9247–E9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 2014;111:16219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lévi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. Lancet 1997;350:681–6. [DOI] [PubMed] [Google Scholar]
- 75.Salem AH, Koenig D, Carlson D. Pooled population pharmacokinetic analysis of phase I, II and III studies of linifanib in cancer patients. Clin Pharmacokinet 2014;53:347–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.