Abstract
Objective
The majority of patients with cervical cancer in Ghana present with locally advanced disease. In October 2014, high-dose rate (HDR) brachytherapy was introduced at the National Center for Radiotherapy, Accra after years of using low-dose rate (LDR) brachytherapy. The aim of this study was to compare the treatment outcomes of patients treated with LDR versus HDR brachytherapy.
Methods
Patients with cervical cancer treated from January 2008 to December 2017 were reviewed. Those with stage IB–IIIB who received chemoradiation plus brachytherapy were included in the study. Post-operative patients and those with stage IV were excluded. The study end points were local control, disease-free survival, and overall survival at 2 years. Endpoints were estimated using the Kaplan–Meier method. Comparisons between treatment groups were performed using the log-rank test and Cox proportional hazards model.
Results
We included 284 LDR and 136 HDR brachytherapy patients. For stages IB, IIA, IIB, IIIA and IIIB disease, the 2-year local control for LDR versus HDR brachytherapy was 63% and 61% (p=0.35), 86% and 90% (p=0.68), 86% and 88% (p=0.83), 66% and 60% (p=0.56), and 77% and 40% (p=0.005), respectively. The 2-year disease-free survival for LDR versus HDR brachytherapy was 64% and 61% (p=0.50), 81% and 69% (p=0.18), 81% and 80% (p=0.54), 62% and 33% (p=0.82), and 71% and 30% (p=0.001) for stages IB, IIA, IIB, IIIA, and IIIB, respectively. The 2-year overall survival for LDR versus HDR brachytherapy was 94% and 93% (p=0.92), 98% and 68% (p=0.21), 89% and 88% (p=0.60), and 88% and 82% (p=0.34) for stages IB, IIA, IIB, and IIIB disease, respectively.
Conclusion
There was no difference between LDR and HDR brachytherapy in local control and disease-free survival for all stages of disease, except in stage IIIB. These findings highlight the need to refine this brachytherapy technique for this group of patients.
Keywords: cervical cancer, brachytherapy
HIGHLIGHTS.
There is no significant difference in outcomes of patients with cervical cancer treated with low-dose versus high-dose rate brachytherapy, except in stage IIIB disease.
Patients with stage IIIB disease had statistically inferior local control when treated with high-dose rate brachytherapy.
Patients with stage IIIB disease had statistically inferior disease-free survival when treated with high-dose rate brachytherapy.
Introduction
Cervical cancer is the the most common gynecological cancer in Ghana, accounting for approximately 70% of all gynecological cancer managed at the National Center for Radiotherapy, Accra. The majority of patients present with locally advanced disease attributable to poor screening practices.1 The standard curative treatment for locally advanced disease consists of concurrent chemoradiation plus intracavitary brachytherapy.2 Brachytherapy allows for dose escalation to the cervix and paracervical tissues while differentially sparing normal tissues from high radiation doses, significantly improving pelvic disease control and survival.3
Historically, the brachytherapy component has been delivered using low-dose rate (LDR) brachytherapy, delivering radiation at doses of 0.4–2 Gy/hour.4 Patients are typically hospitalized for up to 72 hours to allow for radiation delivery. Over the past few decades, high-dose rate (HDR) brachytherapy, which delivers a dose of 12 Gy/hour, has been explored for the treatment of cervical cancer and is currently the dominant brachytherapy delivery technique used in many developed nations.5 HDR brachytherapy addresses some of the drawbacks of LDR brachytherapy such as prolonged immobilization with its high cost, logistics, and risk of deep vein thrombosis and pulmonary embolism.
The outcome of LDR versus HDR brachytherapy after chemoradiation for cervical cancer has been compared in several studies including randomized trials and a meta-analysis, which found comparable outcomes for the two brachytherapy techniques.6 However, all these studies were conducted in developed regions and parts of Asia which may have superior healthcare systems compared with Africa. In October 2014, HDR brachytherapy with a cobalt-60 source was introduced for the management of cervical cancer at the Center after several years of using LDR with caesium-137. The change from LDR to HDR was necessitated by the need to treat more patients per week as LDR allowed for treatment of only two patients per week, each hospitalized for 3 days per insertion. Many resource-constrained countries including Ghana prefer cobalt-60 over iridium-192 sources because of its extended half-life of 5.26 years compared with 74 days for iridium-192.7
The objectives of this study were to compare treatment outcomes—namely, local control, disease-free survival, and overall survival at 2 years—in patients with cervical cancer who received LDR versus HDR brachytherapy.
Methods
Study Design
This is a retrospective study of patients with confirmed histological diagnoses of cervical cancer managed at the National Center for Radiotherapy from January 2008 to December 2017. Ethical approval for this research was obtained from the Institutional Review Board of the hospital.
Patient Characteristics and Staging
We reviewed records of all patients with cervical cancer 2009 International Federation of Gynecology and Obstetrics (FIGO) stages IB2–IIIB who received definitive chemoradiation plus brachytherapy within the 10-year period. The FIGO 2009 staging was not adjusted to the 2018 edition as the majority of patients were staged with physical examinations, chest X-rays, and abdominopelvic ultrasounds and any adjustments could lead to discrepancies. Patients who received post-operative and palliative radiotherapy were excluded from the study.
Treatment
All patients received concurrent chemotherapy with weekly cisplatin at doses of 40 mg/m2, maximum of five doses, with external beam radiation (EBRT).
External Beam Field Design
Patients were simulated in the supine position with their legs on a leg rest and arms above their heads. Anterior–posterior (AP) and posterior–anterior (PA) isocentric fields were used for the majority, except for patients with AP separation exceeding 22 cm where four-field box techniques were used which included the AP/PA fields and two lateral-opposing fields. The 50% depth dose for cobalt-60 teletherapy units of 12 cm informed our choice of AP/PA or four fields guided by the patient’s separation.8 Plans were manually generated.
The superior and inferior borders of the fields were placed at the L4/L5 intervertebral disc space and the ischial tuberosity, respectively. The inferior border was extended 3 cm below any palpable tumor marked by a radio-opaque marker with lower third vagina involvement. The lateral borders of the AP/PA fields were extended 2 cm lateral to the pelvic brim. The entire sacrum was included in the posterior border, and the anterior border was placed in front of the pubic symphysis for the lateral fields.
The EBRT dose was 46 Gy in 23 fractions, prescribed to the midpoint on cobalt-60 teletherapy unit from Best Theratronic (Equinox 100). An EBRT sidewall boost of 6–8 Gy in 3–4 fractions was delivered following 46 Gy to the pelvis using a reduced AP/PA field with a 4 cm midline block, with the superior border dropped to the mid-sacroiliac joint for patients with stage IIB–IIIB disease. Customized Cerrobend blocks were used to block the femoral heads, part of the iliac wings, and bowel to minimize the dose to those areas. Typical field sizes were 18 cm × 18 cm for AP/PA fields, 15 cm × 18 cm for lateral fields, and 7 cm × 12 cm for sidewall boost fields.
Intracavitary Brachytherapy
Applicator placement and treatment planning were similar in both cohorts, and performed in a mini theater with patients under conscious sedation using midazolam and morphine injections. Fletcher–Suit applicators were loaded using the Manchester-based loading system. A two-dimensional planning technique was used and radiation doses were prescribed to point A delineated through the use of two orthogonal films with contrast in the bladder and rectum. International Commission on Radiation Units and Measurements 38 recommended doses to point A, point B, bladder, and rectal points were recorded.9
In the LDR arm, a tandem and ovoids were used for all cases, and where the lower vagina was involved with disease, a midline block was not placed with the EBRT sidewall boost. The dose prescription was 40 Gy in two insertions, with the first insertion performed after completion of the EBRT and the second 1–2 weeks after the first. In the HDR brachytherapy cohort, a tandem and vaginal cylinder applicators were used when the lower third of the vagina was involved, otherwise all patients had a tandem and ovoids inserted. The dose prescription was 7 Gy per insertion, four insertions at weekly intervals with the first insertion after at least 20 Gy of EBRT. Patients with bulky disease completed at least 40 Gy of EBRT before their first HDR insertion. No chemotherapy or EBRT was administered on HDR brachytherapy treatment days. We aimed to complete the overall treatment within 8 weeks, considering the association between extended overall treatment time and poor outcome.10
Follow-up
Following treatment completion, patients were assessed for response, toxicity, and disease recurrence by history and physical examinations every 3 months for 2 years, then every 4–6 months until year 5, and then annually until year 10. Imaging was requested only on suspicion of disease recurrence.
For this study, local control was defined as objective local tumor response in addition to absence of local disease progression in the treated field, as demonstrated by clinical examination. Disease-free survival was defined as the length of time after completion of treatment that the patient lived without any signs or symptoms of the disease. It was measured from the time of completion of treatment to the date any sign of disease recurrence was documented. Overall survival was defined as the length of time from the date of histological diagnosis to survival status of the patient at the date the patient was documented to be alive or dead.
Statistical Analysis
Statistical Package for Social Sciences version 22 and Excel 2013 were used for data cleaning and analysis. The primary endpoints of the study were assessed using the Kaplan–Meier method. Adjusted Cox proportional hazard and log-rank tests were used where applicable. Confidence levels were set at 95% and significance at 0.05.
Results
The total number of patients treated during the 10-year study period was 1882; however, only 284 LDR and 136 HDR brachytherapy patients were analyzed for this study. The remaining patients were excluded because they presented with stage IV disease, were not eligible to receive chemotherapy, were treated with post-operative or palliative radiotherapy, abandoned treatment, or were lost to follow-up after registration.
The median age in the LDR and HDR brachytherapy groups was 55 years (range 22–93) and 56 years (range 26–86), respectively. The most common histology was squamous cell carcinoma in both groups: 93% in the LDR group and 89.7% in the HDR group. The median tumor size in the LDR and HDR groups was 5 cm (range 2–8) and 5 cm (range 1–10), respectively. Stage IIB was the the most common stage in the LDR group (46.1%) compared with the HDR group (27.2%), whereas stage IIIB was the most common stage in the HDR group (45.6%) compared with the LDR group (27.5%).
The mean±SD point A dose (EQD2) was 80.4±8.4 and 81.4±8.4 in the LDR and HDR groups, respectively.
Patient and treatment characteristics are shown in Table 1.
Table 1.
Clinical and treatment characteristics
| Low-dose rate | High-dose rate | P value | 95% CI | |
| Total | 284 (68%) | 136 (32%) | ||
| Median follow-up, months | 22 | 11 | ||
| Range | 1–132 | 1–41 | ||
| Age, years | 0.22 | −2.817 to 2.262 | ||
| Mean±SD | 56.1±12 | 56.4±13.2 | ||
| Median (range) | 55 (22–93) | 56(26–86) | ||
| FIGO stage | 0.001 | 0.000 to 0.007 | ||
| IB1 | 8 (2.8%) | 6 (4.4%) | ||
| IB2 | 15 (5.3%) | 15 (11%) | ||
| IIA | 33 (11.6%) | 9 (6.6%) | ||
| IIA1 | 1 (0.4%) | 0 (0%) | ||
| IIA2 | 1 (0.4%) | 1 (0.7%) | ||
| IIB | 131 (46.1%) | 37 (27.2%) | ||
| IIIA | 17 (6%) | 6 (4.4%) | ||
| IIIB | 78 (27.5%) | 62 (45.6%) | ||
| Tumor size (cm) | 0.42 | −1.76 to 1.88 | ||
| <4 | 30 (10.6%) | 18 (13.2%) | ||
| >4 | 254 (89.4%) | 118 (86.8%) | ||
| Median | 5 (2–8) | 5 (1–10) | ||
| Chemotherapy cycles | 0.19 | −1.051 to 1.1713 | ||
| <5 weeks | 102 (34.9%) | 40 (29.4%) | ||
| At least 5 weeks | 182 (64.1%) | 96 (70.6%) | ||
| Histology | 0.16 | 0.149 to 0.223 | ||
| Squamous cell | 264 (93%) | 122 (89.7%) | ||
| Adenocarcinoma | 19 (6.7%) | 11 (8.1%) | ||
| Others | 1 (0.4%) | 3 (2.2%) | ||
| Radiation dose (EQD2), mean±SD | ||||
| Total point A dose | 80.4±8.4 | 81.4±8.4 | ||
| Point B dose contribution from brachytherapy only | 8.5±2 | 8.9±1.9 | ||
| Total point B dose | 57.8±4.7 | 58.2±5.7 |
EQD2, equivalent dose at 2 Gy.
Discussion
Summary of Main Results
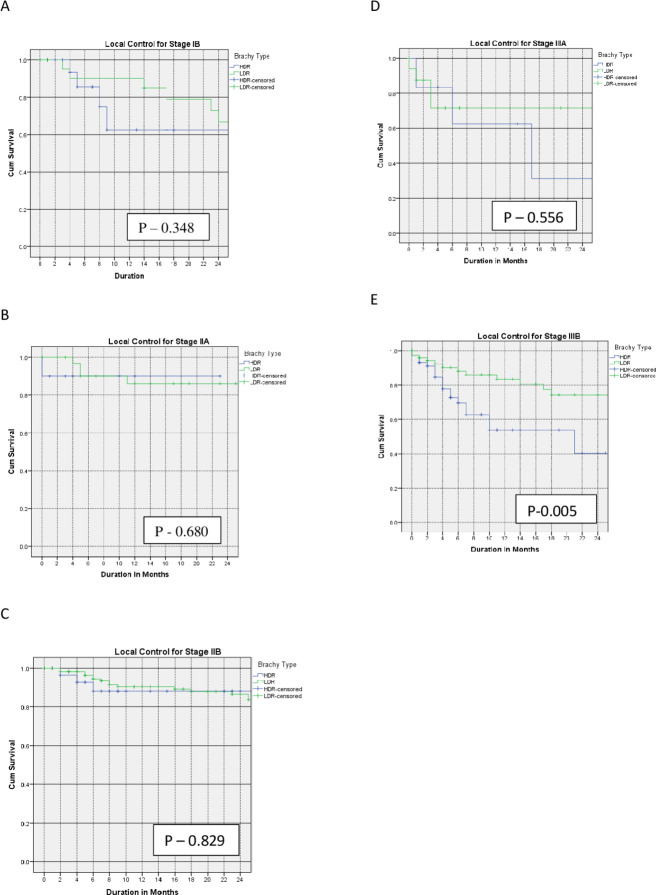
The 2-year local control for stages IB, IIA, IIB, IIIA, and IIIB were 63% and 61% (p=0.35), 86% and 90% (p=0.68), 86% and 88% (p=0.83), 66% and 60% (p=0.56), and 77% and 40% (p=0.005) for LDR and HDR, respectively.
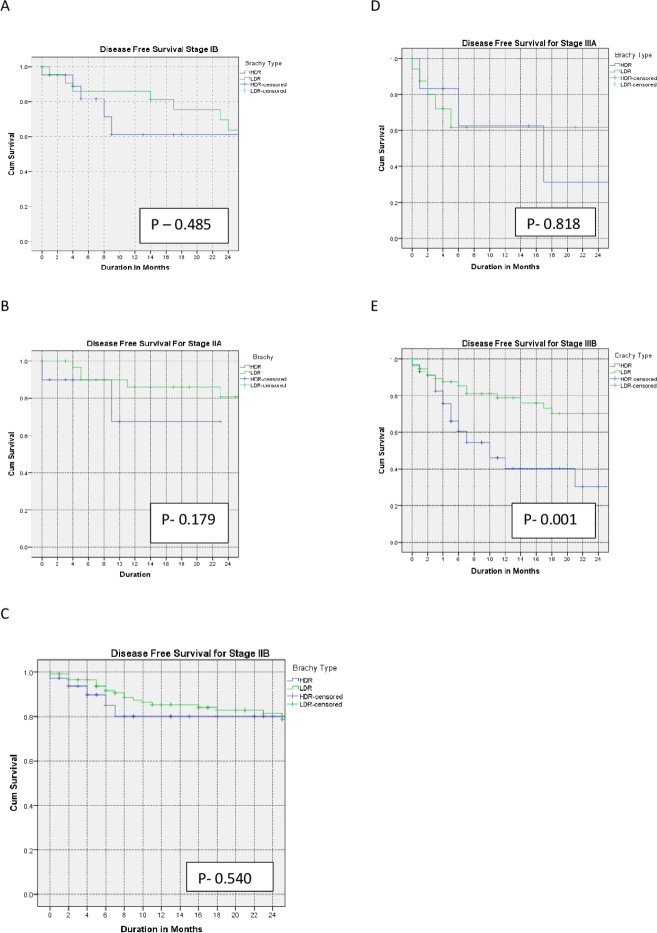
The 2-year disease-free survival for LDR and HDR were 64% and 61% (p=0.50), 81% and 69% (p=0.18), 81% and 80% (p=0.54), 62% and 33% (p=0.82), and 71% and 30% (p=0.001) for stages IB, IIA, IIB, IIIA, and IIIB, respectively.
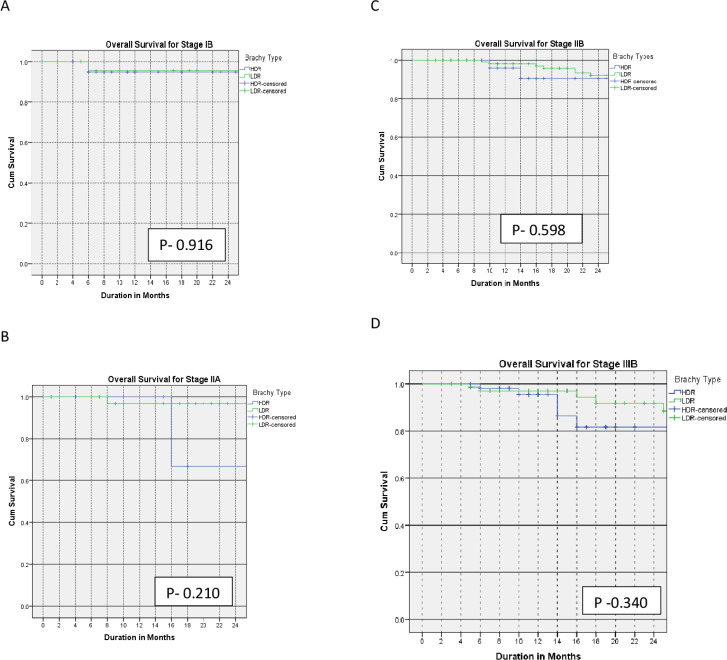
The 2-year overall survival for LDR and HDR were 94% and 93% (p=0.92), 98% and 68% (p=0.21), 89% and 88% (p=0.60), and 88% and 82% (p=0.34) for stages IB, IIA, IIB, and IIIB disease, respectively.
The Kaplan–Meier curves for the primary outcomes are shown in Figures 1–3 and summarized in Table 2.
Figure 1.
Kaplan–Meier curves of 2-year local control for patients with stage IB (A), IIA (B), IIB (C), IIIA (D), and IIIB (E) treated with low-dose versus high-dose rate brachytherapy.
Figure 2.
Kaplan–Meier curves of 2-year disease-free survival for patients with stage IB (A), IIA (B), IIB (C), IIIA (D), and IIIB (E) treated with low-dose versus high-dose rate brachytherapy.
Figure 3.
Kaplan–Meier curves of 2-year overall survival for patients with stage IB (A), IIA (B), IIB (C), and IIIB (D) treated with low-dose versus high-dose rate brachytherapy.
Table 2.
Primary outcomes
| Outcome | LDR | HDR | P value |
| Local control at 2 years | |||
| IB | 63% | 61% | 0.35 |
| IIA | 86% | 90% | 0.68 |
| IIB | 86% | 88% | 0.83 |
| IIIA | 66% | 60% | 0.56 |
| IIIB | 77% | 40% | 0.005 |
| Disease-free survival at 2 years | |||
| IB | 64% | 61% | 0.50 |
| IIA | 81% | 69% | 0.18 |
| IIB | 81% | 80% | 0.54 |
| IIIA | 62% | 33% | 0.82 |
| IIIB | 71% | 30% | 0.001 |
| Overall survival at 2 years | |||
| IB | 94% | 93% | 0.92 |
| IIA | 98% | 68% | 0.21 |
| IIB | 89% | 88% | 0.60 |
| IIIB | 88% | 82% | 0.34 |
HDR, high-dose rate brachytherapy; LDR, low-dose rate brachytherapy.
Results in Context of Published Literature
The 2-year local control rates in our study were comparable in the LDR and HDR brachytherapy groups for patients with stage I and II disease. This is consistent with findings from several studies. Petereit et al reported a comparable local control rate for stage IB (91% vs 85%) and stage II (78% vs 80%) for LDR versus HDR brachytherapy, respectively.11 In a study by Kuipers et al the local control rate for stage I and II disease was 88% versus 90% and 89% versus 85% for LDR versus HDR brachytherapy, respectively.12 Patel et al also reported comparable local control for LDR versus HDR brachytherapy for stage I and II disease.13
The better local control rates for patients with stage IIIB treated with LDR brachytherapy in this study are consistent with reports by Petereit et al where the local control rates for patients with stage IIIB disease treated with LDR brachytherapy were better than those treated with HDR brachytherapy (75% and 44%, respectively).11 Similarly, Ferrigno et al reported better local control rates for patients with stage III disease treated with LDR than HDR brachytherapy (58% and 50%, respectively).14 However, a randomized trial by Hareyama et al reported comparable local control rates for patients with stage III disease receiving LDR versus HDR brachytherapy.15 Also, Lertsanguansinchai et al and Akine et al reported equivalent local control rates of 92.8% versus 93.7% and 61% versus 64% for LDR versus HDR brachytherapy, respectively, for patients with stage IIIB disease.16 17
Our study shows equivalent disease-free survival rates for stage I and II disease treated with LDR versus HDR brachytherapy, which is consistent with other reports. Petereit et al reported comparable disease-free survival of 81% versus 85% and 61% versus 69% for stage IB and II disease with LDR versus HDR brachytherapy, respectively.11 Similarly, a meta-analysis reported by Viani showed comparable disease-free survival for patients with stage I and II disease, as reported in our study.6 On the other hand, patients with stage IIIB disease had statistically significant improved disease-free survival when treated with LDR compared with HDR brachytherapy, consistent with the report by Petereit et al in which the 3-year disease-free survival for stage III disease was 63% and 30% for LDR and HDR brachytherapy, respectively.11 Ferrigno et al also reported that the 5-year disease-free survival for patients with stage IIIB disease was better when treated with LDR than with HDR brachytherapy.14 Our finding is, however, in contrast to several studies including those reported by Romano et al and Patel et al who reported equivalence for the two techniques.13 18 Also, Teshima et al and Hareyama et al reported equivalent disease-free survival for patients with stage III disease (47% vs 53% and 60% vs 51% for LDR vs HDR brachytherapy, respectively).15 19 Thus, the outcome of stage III cervical cancer in terms of the local control and disease-free survival varies across studies, and this may be attributed to differences in techniques used or timing of brachytherapy.
The 2-year overall survival rate in this study was similar for all stages in the two cohorts. Shrivastava et al, Pantankar et al and others support this finding.20 21 However, Ferrigno et al and Petereit et al reported a statistically significant improved overall survival outcome for patients with stage III disease treated with LDR compared with HDR brachytherapy.11 14 The finding that the overall survival was equivalent in both arms despite the inferior local control and disease-free survival rates for the stage IIIB group treated with HDR brachytherapy is not surprising. Possible reasons include the short duration of follow-up in the HDR brachytherapy arm. Furthermore, patients in whom recurrence occurs receive additional treatment, usually chemotherapy, to control the disease.
Strengths and Weaknesses of the Study
To the best of our knowledge, this is the largest study comparing the outcome of patients with cervical cancer treated with LDR versus HDR brachytherapy in Africa, where the incidence is very high but resources are limited. The main limitation of the study is its retrospective nature and the absence of a toxicity assessment in this report.
Implications for Practice and Future Research
Intracavitary brachytherapy generally has a higher dose inhomogeneity compared with EBRT because of the rapid dose fall-off due to the inverse square law as one moves away from the radiation sources. This inhomogeneity is compounded by the radial dose function of the radioactive source used for the brachytherapy.22 The radial dose function of caesium-137 has been shown to be comparable to that of iridium-192, and does not fall off sharply within the first 10 cm radial distance, making its dose distribution fairly homogenous, especially in bulky tumors.22–24 However, the fall-off of cobalt-60 is steep within the first 10 cm radial distance, making its dose distribution especially in bulky tumors inhomogeneous.22–24 Although the results of this study showed that point B, which represents the lateral parametrium, received an adequate dose as recommended by the American Brachytherapy Society, the dose distribution in bulky disease may not have been homogeneous in the cobalt-60 HDR brachytherapy cohort.25 This may have contributed to the poor local control observed in the stage IIIB HDR brachytherapy cohort. It may be necessary to allow patients with this stage of disease to complete all the EBRT dose including the side wall boost to maximize tumor response before the first insertion of HDR brachytherapy to optimize the dose at brachytherapy. These patients should then have two fractions of HDR brachytherapy per week rather than one fraction in order to keep up with the overall treatment duration of 56 days.
The use of interstitial needles combined with HDR intracavitary brachytherapy has been shown to provide a prescription dose of up to 15 mm lateral to point A, increasing the target coverage and improving the 5-year local control rates for stage III disease up to about 79%.26 27 Thus, the use of interstitial needles with HDR intracavitary brachytherapy can obviate the differences observed in the outcomes of stage IIIB patients treated with LDR versus HDR brachytherapy.
Recent studies have shown that the outer margins of tumors may be missed with HDR brachytherapy if the dose prescription is optimized to point A without three-dimensional (3D) image guidance, especially if the tumor has not regressed significantly at the time of brachytherapy.28–30 Image-guided planning allows for better tumor localization and delineation with more accurate optimized planning to improve dose inhomogeneity, especially in patients with bulky disease.
In 2020 the Center adopted 3D image guided planning after acquiring a linear accelerator and accompanying planning systems with Intensity Modulated Radiation Therapy (IMRT) capability. Furthermore, a radiation oncologist from the Center is currently receiving training in Canada to attain competencies in advanced radiotherapy techniques including the use of interstitial needles for the management of gynecologic malignancies.
Conclusions
This study shows that patients with cervical cancer treated in our center with LDR versus HDR brachytherapy have comparable 2-year local control, disease-free survival, and overall survival for all disease stages, except for those with stage IIIB disease who had statistically significant inferior local control and disease-free survival when treated with HDR brachytherapy. Refinement of the HDR brachytherapy technique is necessary to achieve a better outcome for patients with bulky disease treated with HDR brachytherapy.
Acknowledgments
We acknowledge the support from the staff of the National Center for Radiotherapy and Nuclear Medicine, Accra, Ghana. Special thanks to Dr Hannah Ayettey-Anie for her guidance. Special recognition to the Radiation Medicine Program of the Princess Margaret Cancer Center, Toronto, Canada, especially the Gynecology group for supporting our member of staff with fellowship. We are in their debt.
Footnotes
Contributors: Conception and design: AAS and JY. Administrative support: AAS, JY, VV, KA, CAA, TO-M. Provision of study materials: AAS, JY, MA, SNB. Collection and assembly of data: AAS, KA, TO-M, MA, SNB. Data analysis and interpretation: AAS, JY, VV, CAA. Manuscript writing: all authors. Final approval of manuscript: all authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data for this study will be made available upon reasonable request after data sharing agreement is made. All patients are anonymised.
References
- 1. Vulpe H, Asamoah FA, Maganti M, et al. External beam radiation therapy and brachytherapy for cervical cancer: the experience of the National Centre for Radiotherapy in Accra, Ghana. Int J Radiat Oncol Biol Phys 2018;100:1246–53. 10.1016/j.ijrobp.2017.12.270 [DOI] [PubMed] [Google Scholar]
- 2. Todo Y, Watari H. Concurrent chemoradiotherapy for cervical cancer: background including evidence-based data, pitfalls of the data, limitation of treatment in certain groups. Chin J Cancer Res 2016;28:221–7. 10.21147/j.issn.1000-9604.2016.02.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banerjee R, Kamrava M. Brachytherapy in the treatment of cervical cancer: a review. Int J Womens Health 2014;6:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gaur R, Singh OP, Kumar M, et al. Comparison of low and high dose brachytherapy in carcinoma cervix: results from a randomized study. Indian J Clin Pract 2012;23:203–11. [Google Scholar]
- 5. Viswanathan AN, Beriwal S, De Los Santos JF, et al. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: High-dose-rate brachytherapy. Brachytherapy 2012;11:47–52. 10.1016/j.brachy.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Viani GA, Manta GB, Stefano EJ, et al. Brachytherapy for cervix cancer: low-dose rate or high-dose rate brachytherapy - a meta-analysis of clinical trials. J Exp Clin Viswanathan A, Thomadsen B Cancer Res 2009;28:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strohmaier S, Zwierzchowski G. Comparison of 60 CO and 192 IR sources in HDR brachytherapy. J Contemp Brachytherapy 2011;4:199–208. 10.5114/jcb.2011.26471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pötter R, Van Limbergen E, Gerstner N, et al. Survey of the use of the ICRU 38 in recording and reporting cervical cancer brachytherapy. Radiother Oncol 2001;58:11–18. 10.1016/S0167-8140(00)00266-8 [DOI] [PubMed] [Google Scholar]
- 9. Viswanathan A, Thomadsen B. American Brachytherapy Society Cervical Cancer Brachytherapy Task Group. May, 2014. Available: http://www.americanbrachytherapy.org/guidelines/cervical_cancer_taskgroup.pdf
- 10. Podgorsak EB. External photon beams: physical aspects. radiation oncology physics: a Handbook for teachers and students. Vienna: International Atomic Energy Agency, 2005: 161–217. [Google Scholar]
- 11. Petereit DG, Sarkaria JN, Potter DM, et al. High-dose-rate versus low-dose-rate brachytherapy in the treatment of cervical cancer: analysis of tumor recurrence—the University of Wisconsin experience. Int J Radiat Oncol Biol Phys 1999;45:1267–74. 10.1016/S0360-3016(99)00262-X [DOI] [PubMed] [Google Scholar]
- 12. KK F, Phillips TL. High-dose-rate versus low-dose-rate intracavitary brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys 1990;19:791–6. [DOI] [PubMed] [Google Scholar]
- 13. Patel FD, Sharma SC, Negi PS, et al. Low dose rate vs. high dose rate brachytherapy in the treatment of carcinoma of the uterine cervix: a clinical trial. Int J Radiat Oncol Biol Phys 1994;28:335–41. 10.1016/0360-3016(94)90055-8 [DOI] [PubMed] [Google Scholar]
- 14. Ferrigno R, Nishimoto IN, Ribeiro dos Santos Novaes PE, et al. Comparison of low and high dose rate brachytherapy in the treatment of uterine cervix cancer. retrospective analysis of two sequential series. Int J Radiat Oncol Biol Phys 2005;62:1108–16. 10.1016/j.ijrobp.2004.12.016 [DOI] [PubMed] [Google Scholar]
- 15. Hareyama M, Sakata K-ichi, Oouchi A, et al. High-dose-rate versus low-dose-rate intracavitary therapy for carcinoma of the uterine cervix. Cancer 2002;94:117–24. 10.1002/cncr.10207 [DOI] [PubMed] [Google Scholar]
- 16. Lertsanguansinchai P, Lertbutsayanukul C, Shotelersuk K, et al. Phase III randomized trial comparing LDR and HDR brachytherapy in treatment of cervical carcinoma. Int J Radiat Oncol Biol Phys 2004;59:1424–31. 10.1016/j.ijrobp.2004.01.034 [DOI] [PubMed] [Google Scholar]
- 17. Akine Y, Arimoto H, Ogino T, et al. High-dose-rate intracavitary irradiation in the treatment of carcinoma of the uterine cervix: early experience with 84 patients. Int J Radiat Oncol Biol Phys 1988;14:893–8. 10.1016/0360-3016(88)90011-9 [DOI] [PubMed] [Google Scholar]
- 18. Romano KD, Pugh KJ, Trifiletti DM, et al. Transition from LDR to HDR brachytherapy for cervical cancer: evaluation of tumor control, survival, and toxicity. Brachytherapy 2017;16:378–86. 10.1016/j.brachy.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 19. Teshitma T, Inoue T, Ikeda H, et al. High‐dose rate and low-dose rate intracavitary therapy for carcinoma of the uterine cervix. Final results of Osaka University Hospital. Cancer 1993;72:2409–14. [DOI] [PubMed] [Google Scholar]
- 20. Shrivastava S, Dinshaw K, Mahantshetty U, et al. Comparing low dose rate and high dose rate intracavitary brachytherapy in carcinoma cervix: results from a randomized controlled study. Int J Radiat Oncol Biol Phys 2006;66:S 42. 10.1016/j.ijrobp.2006.07.102 [DOI] [Google Scholar]
- 21. Patankar SS, Tergas AI, Deutsch I, et al. High versus low-dose rate brachytherapy for cervical cancer. Gynecol Oncol 2015;136:534–41. 10.1016/j.ygyno.2014.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Venselaar JLM, van der Giessen PH, Dries WJF. Measurement and calculation of the dose at large distances from brachytherapy sources: Cs-137, Ir-192, and Co-60. Med Phys 1996;23:537–43. 10.1118/1.597811 [DOI] [PubMed] [Google Scholar]
- 23. Strohmaier S, Zwierzchowski G. Comparison of 60Co and 192Ir sources in HDR brachytherapy. J Contemp Brachytherapy 2011;3:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meigooni AS, Nath R. A comparison of radial dose functions for 103Pd, 125I, 145Sm, 241Am, 169Yb, 192Ir, and 137Cs brachytherapy sources. Int J Radiat Oncol Biol Phys 1992;22:1125–30 http://www.sciencedirect.com/science/article/pii/0360301692908194 10.1016/0360-3016(92)90819-4 [DOI] [PubMed] [Google Scholar]
- 25. Nag S, Chao C, Erickson B, et al. The American Brachytherapy Society recommendations for low-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys 2002;52:33–48. 10.1016/S0360-3016(01)01755-2 [DOI] [PubMed] [Google Scholar]
- 26. Demanes DJ, Rodriguez RR, Bendre DD, et al. High dose rate transperineal interstitial brachytherapy for cervical cancer: high pelvic control and low complication rates. Int J Radiat Oncol Biol Phys 1999;45:105–12. 10.1016/S0360-3016(99)00124-8 [DOI] [PubMed] [Google Scholar]
- 27. Kirisits C, Pötter R, Lang S, et al. Dose and volume parameters for MRI-based treatment planning in intracavitary brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2005;62:901–11. 10.1016/j.ijrobp.2005.02.040 [DOI] [PubMed] [Google Scholar]
- 28. Chakrabarti B, Basu-Roy S, Kar SK, et al. Comparison of dose volume parameters evaluated using three forward planning-optimization techniques in cervical cancer brachytherapy involving two applicators. J Contemp Brachytherapy 2017;5:431–45 https://www.ncbi.nlm.nih.gov/pubmed/29204164 10.5114/jcb.2017.70677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim RY, Pareek P. Radiography-based treatment planning compared with computed tomography (CT)-based treatment planning for intracavitary brachytherapy in cancer of the cervix: analysis of dose-volume histograms. Brachytherapy 2003;2:200–6. 10.1016/j.brachy.2003.06.001 [DOI] [PubMed] [Google Scholar]
- 30. Kim H, Beriwal S, Houser C, et al. Dosimetric analysis of 3D image-guided HDR brachytherapy planning for the treatment of cervical cancer: is point A-based dose prescription still valid in image-guided brachytherapy? Medical Dosimetry 2011;36:166–70. 10.1016/j.meddos.2010.02.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this study will be made available upon reasonable request after data sharing agreement is made. All patients are anonymised.