Abstract
Objective
The incidence of IBS increases following enteric infections, suggesting a causative role for microbial imbalance. However, analyses of faecal microbiota have not demonstrated consistent alterations. Here, we used metaproteomics to investigate potential associations between mucus-resident microbiota and IBS symptoms.
Design
Mucus samples were prospectively collected from sigmoid colon biopsies from patients with IBS and healthy volunteers, and their microbial protein composition analysed by mass spectrometry. Observations were verified by immunofluorescence, electron microscopy and real-time PCR, further confirmed in a second cohort, and correlated with comprehensive profiling of clinical characteristics and mucosal immune responses.
Results
Metaproteomic analysis of colon mucus samples identified peptides from potentially pathogenic Brachyspira species in a subset of patients with IBS. Using multiple diagnostic methods, mucosal Brachyspira colonisation was detected in a total of 19/62 (31%) patients with IBS from two prospective cohorts, versus 0/31 healthy volunteers (p<0.001). The prevalence of Brachyspira colonisation in IBS with diarrhoea (IBS-D) was 40% in both cohorts (p=0.02 and p=0.006 vs controls). Brachyspira attachment to the colonocyte apical membrane was observed in 20% of patients with IBS and associated with accelerated oro-anal transit, mild mucosal inflammation, mast cell activation and alterations of molecular pathways linked to bacterial uptake and ion–fluid homeostasis. Metronidazole treatment paradoxically promoted Brachyspira relocation into goblet cell secretory granules—possibly representing a novel bacterial strategy to evade antibiotics.
Conclusion
Mucosal Brachyspira colonisation was significantly more common in IBS and associated with distinctive clinical, histological and molecular characteristics. Our observations suggest a role for Brachyspira in the pathogenesis of IBS, particularly IBS-D.
Keywords: irritable bowel syndrome, bacterial adherence, bacterial pathogenesis, mucus
Significance of this study.
What is already known on this subject?
IBS incidence increases after enteric infections, suggesting a possible causative role for microbial perturbation.
Studies of the faecal microbiota have failed to demonstrate consistent alterations associated with IBS symptoms.
What are the new findings?
This study represents the first comparison of the microbial community of the colonic inner mucus layer of patients with IBS and healthy volunteers.
Colonisation of the colonic epithelial surface or mucus layers by pathogenic Brachyspira species was detected in 40% of patients with IBS with diarrhoea but not in any healthy individual.
Brachyspira-associated IBS was linked to distinctive clinical, histological and molecular characteristics, suggesting that it should be considered a separate diagnostic entity.
Treatment paradoxically resulted in relocation of the Brachyspira into goblet cell secretory granules.
How might it impact on clinical practice in the foreseeable future?
The presence of Brachyspira may be used to identify a distinct subset of patients with IBS, who could potentially be responsive to eradication therapy.
The relocation of the Brachyspira into goblet cell mucus granules likely represents a novel bacterial strategy to evade antibiotics, which could inform our understanding of other persistent or recurrent mucosal infections.
Introduction
The incidence of IBS steeply increases following a gastroenteritis episode, suggesting a possible causative role for microbial perturbation.1 Still, previous investigations have not demonstrated consistent associations between intestinal microbiota and IBS symptoms.2 3
Gut microbiota composition studies overwhelmingly rely on faecal material. However, these samples largely reflect the luminal microbial community, which is spatially separated from the colonic epithelium and underlying immune cells through a two-tiered mucus barrier.4 By contrast, certain species have found a niche in the outer mucus layer, feeding on the abundant mucin O-glycans.5 Thus, faecal and mucus-associated bacteria represent distinctive populations, with the latter more likely to influence the epithelium. In particular, bacterial presence in the inner mucus layer might result in epithelial stress and immune activation. Here, we performed a metaproteomic analysis of inner mucus layer samples from patients with IBS and healthy individuals, to investigate potential associations between mucus-resident microbiota and IBS symptoms.
Materials and methods
Study design
Patients aged 18–65 years fulfilling the Rome III criteria for IBS were prospectively included at a secondary/tertiary care unit (Sahlgrenska Hospital, Gothenburg, Sweden) June 2012 through April 2018. Healthy volunteers were recruited through advertisements and screened by questionnaires and interviews to rule out gastrointestinal complaints and comorbidities. Exclusion criteria are listed in the online supplemental file. Patients with IBS were subtyped based on the Rome III criteria and the Bristol Stool Form scale, as follows: IBS with diarrhoea (IBS-D), IBS with constipation (IBS-C), IBS with mixed bowel habits (IBS-M) and unsubtyped IBS without diarrhoea or constipation (IBS-U). All participants provided written informed consent.
gutjnl-2020-321466supp001.pdf (8.4MB, pdf)
All study participants (IBS n=62, healthy n=31) underwent sigmoidoscopy with sampling of biopsies in methanol-Carnoy for future histology/immunohistochemistry and real-time PCR analysis (figure 1). Carnoy’s fixative enables optimal preservation of both the mucus layers and the tissue nucleic acids.6 Comprehensive clinical characterisation, including assessment of oro-anal transit time (OATT) and rectal sensitivity, was undertaken in patients with IBS.
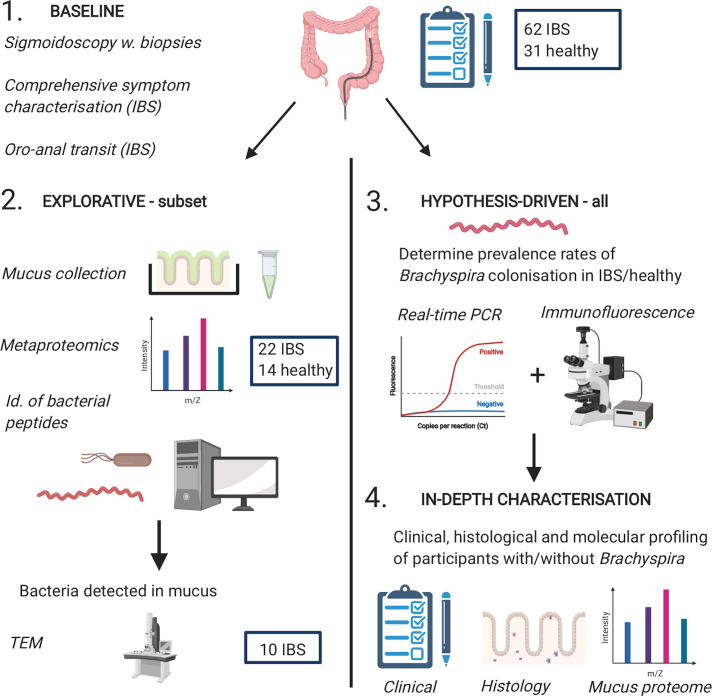
Figure 1.
Graphical summary of the study design. Schematic representation of the different phases of the study. In-depth profiling of clinical, histological and molecular characteristics (phase 4) was performed in participants where Brachyspira colonisation could be confirmed/rejected with high confidence, typically based on consistent results from two different methods. Quantification of mucosal immune cells by histology was performed in a representative subset of participants with good-quality biopsy sections, whereas analysis of the human mucus proteome was largely restricted to participants from the explorative cohort. The figure was created with BioRender.com.
In a randomly selected subset of participants (the first/explorative cohort, IBS n=22, healthy n=14), mucus was collected from ex vivo sigmoid colon biopsies and analysed by mass spectrometry (MS). The objectives of this explorative phase were twofold: to characterise the metaproteomic composition of the inner mucus layer of patients with IBS and healthy individuals, and to generate hypotheses to pursue in the entire study population. Patients in whose mucus/tissue samples bacteria were detected by MS (n=9), or, in certain cases, other methods (n=3), underwent repeat sigmoidoscopy, with biopsy sampling for electron microscopy, mucus collection and/or routine clinical histopathology.
The metaproteomic analysis identified a putative link between the Brachyspira genus and IBS. This association was validated in the explorative cohort, as well in the remainder of the study population—the second cohort, comprising 40 patients with IBS and 17 healthy individuals—using real-time PCR and immunofluorescence. Sampling and analysis were, whenever possible, performed with blinding to participant identity. Missing data due to technical failures or suboptimal biopsy specimens are tabulated in online supplemental table S1.
Patient and public involvement statement
Patients were not involved in the planning, design or evaluation of this study.
Patient-reported symptoms
Participants recorded the frequency and Bristol scale consistency of their bowel movements in a 2-week structured diary. Patients with IBS completed the IBS Severity Scoring System (IBS-SSS) questionnaire as well as an Extracolonic Symptom Severity Score (online supplemental file).7 8
Oro-anal transit time
OATTs were assessed for 60/62 (97%) patients with IBS (online supplemental file).
Rectal sensitivity
Rectal sensitivity was evaluated in 45/62 (73%) patients with IBS, using an electronic barostat (online supplemental file).
Mucus collection
Sigmoidoscopy was performed without prior bowel preparation. Two biopsies per participant (explorative cohort n=36, second cohort n=4) were transported in oxygenated, ice-cold Krebs buffer, mounted in our in-house developed ex vivo mucus measurement chambers and allowed to secrete mucus for 1 hour.9 Mucus was then collected by gentle scraping (online supplemental figure S1). Since the outer, easily removable, mucus layer is lost during sampling and transport, sample microbial protein content was considered to reflect that of the inner mucus layer.
MS and data processing
Mucus samples were prepared for MS according to a modified version of the Filter-Aided Sample Preparation (FASP) protocol.10 Nano-liquid chromatography-tandem MS was performed on a Q-Exactive instrument (Thermo). Peptides were identified using the Andromeda search engine integrated into MaxQuant (V.1.3.0.5) and the MASCOT software (V.2.2).11 Searches were performed against all reviewed human and eubacteria sequences of the SwissProt-UniProt database (February 2016). Minimum one unique peptide at a threshold of 1% was required for protein identification. The identification threshold for a bacterial family/genus was generally defined as ≥3 proteins, each identified by at least one unique, family/genus-specific peptide. Detailed information is provided in the online supplemental file.
Histology and immunohistochemistry
One biopsy per participant (both cohorts) was fixed in methanol-Carnoy, paraffin-embedded and sectioned. Tissue sections were stained with either H&E, Alcian blue/periodic acid-Schiff (AB-PAS), toluidine blue, Gram stain or with antibodies for fluorescent microscopy (online supplemental file). Lamina propria and subepithelial/intraepithelial immune cell populations were counted in five high-power fields in H&E or toluidine blue-stained sections by two to three independent observers.
Transmission electron microscopy
Transmission electron microscopy analysis of biopsies from 10 participants was performed as detailed in the online supplemental file.
DNA isolation and real-time PCR analysis
DNA was isolated from methanol-Carnoy-fixed, paraffin-embedded tissue as outlined in the online supplemental file. In certain participants, DNA was also isolated from fresh frozen biopsies and/or faecal material (online supplemental file). Brachyspira species were identified using a melting curve-based method.12 To further validate our observations, a multiplex hydrolysis probe assay specific for Brachyspira aalborgi/hominis and Brachyspira pilosicoli was designed (online supplemental file).
16S rDNA sequencing
16S rDNA sequencing of faecal samples was performed as described in the online supplemental file.
Mucus penetrability analysis
Mucus penetrability was assessed by confocal microscopy, using fluorescent beads as surrogate markers for bacteria (online supplemental file).9
Antibiotic treatment—pilot study
The first four patients with IBS in the study to be diagnosed with epithelial Brachyspira colonisation/infection were treated with 500 mg metronidazole three times a day for 14 days.13 As the rationale for the treatment was clinical, the intervention was open-label and uncontrolled. Patients completed IBS-SSS questionnaires before treatment, and 2, 4, 6 and 8 weeks, as well as 6, 12 and 15 months after commencing antibiotics. They kept a diary of their bowel habits from 2 weeks before, until 8 weeks after, treatment, and also for 2 weeks at 6 and 12 months after metronidazole therapy, respectively. Patients underwent sigmoidoscopy with biopsy sampling for histology, immunohistochemistry and PCR 6 weeks post-treatment and collected faecal samples for probe-based PCR analysis 6 weeks, 6 months and 1 year after antibiotic therapy.
Statistics
Fisher’s exact test was used for categorical data. For continuous data, Welch’s t-test or the Mann-Whitney U test was used for two-group comparisons, depending on data distribution as verified by the Kolmogorov-Smirnov test. Kruskal-Wallis test was used for multigroup comparisons. P values are two-sided. The significance threshold (0.05) was adjusted according to the Bonferroni/Holm-Bonferroni methods in the event of multiple (>2) comparisons. Data was analysed with GraphPad Prism (V. 8).
Results
Study population
A graphical summary of the study design is provided in figure 1. In an explorative cohort of 22 patients with IBS and 14 healthy controls, inner mucus layer samples were collected from sigmoid colon biopsies and analysed by metaproteomics, revealing a tentative link between Brachyspira and IBS. This association was confirmed through histology, electron microscopy, immunofluorescence and targeted real-time PCR analysis, and further investigated in a second cohort of 40 patients with IBS and 17 healthy individuals, using immunofluorescence and PCR. Clinical and demographic characteristics of both cohorts are compiled in table 1. The number/proportion of individuals analysed by each method are summarised in table 1; participant-level data are provided in online supplemental data file S1. Missing data are tabulated in online supplemental table S1, stratified by IBS diagnosis and Brachyspira colonisation status. Demographic factors did not differ between patients with IBS and healthy individuals (table 1).
Table 1.
The study cohorts
| First (explorative) cohort | Second cohort | Study population* | ||||
| IBS patients | Controls | IBS patients | Controls | IBS patients | Controls | |
| Participants | 22 | 14 | 40 | 17 | 62 | 31 |
| Females | 15 (68%) | 8 (57%) | 33 (83%) | 10 (59%) | 48 (77%) | 18 (58%) |
| Median age (range) | 29 (22–55) | 29 (23–49) | 28 (20–62) | 27 (19–55) | 28 (20–62) | 29 (19–55) |
| IBS-D | 10 (45%) | NA | 15 (38%) | NA | 25 (40%) | NA |
| IBS-C | 5 (23%) | NA | 8 (20%) | NA | 13 (21%) | NA |
| IBS-M | 5 (23%) | NA | 14 (35%) | NA | 19 (31%) | NA |
| IBS-U | 2 (9%) | NA | 3 (8%) | NA | 5 (8%) | NA |
| Mucus samples analysed by proteomics | 22 (100%) | 14 (100%) | 3 (8%) | 1 (6%) | 25 (40%) | 15 (48%) |
| Tissue sections analysed by immunofluorescence | 19 (86%) | 12 (86%) | 37 (93%) | 16 (94%) | 56 (90%) | 28 (90%) |
| Biopsies analysed by real-time PCR | 18 (82%) | 9 (64%) | 36 (90%) | 16 (94%) | 54 (87%) | 25 (81%) |
| Patients treated with metronidazole | 3 (14%) | 0 | 1 (3%) | 0 | 4 (6%) | 0 |
*Entire study population (both cohorts).
IBS-C, IBS with constipation; IBS-D, IBS with diarrhoea; IBS-M, IBS with mixed bowel habits; IBS-U, unsubtyped IBS without diarrhoea or constipation; NA, not applicable.
gutjnl-2020-321466supp002.xlsx (19.7KB, xlsx)
Bacterial proteins were more frequently detected in mucus from patients with IBS
Using stringent criteria, bacteria were identified in mucus from 9/22 (41%) patients with IBS and 1/14 (7%) healthy controls (p=0.05; online supplemental figure S2A). The most frequently identified bacterial family was Pseudomonadaceae (online supplemental figure S2B‒D). Proteins from the Brachyspiraceae family, genus Brachyspira were detected in 3/22 (14%) patients with IBS but not in any controls (online supplemental figure S2C, D).
The Brachyspira genus includes putative human pathogens associated with intestinal spirochetosis. In this condition, tentatively linked to diarrhoea and abdominal pain, Brachyspira penetrates the mucus layers, frequently colonising the colonocyte apical membrane.12–15 Therefore, we focused our investigation on the potential link between Brachyspira and IBS. Numbers of identified peptides and proteins per family are compiled in online supplemental table S2 and Brachyspira peptide/protein identifications in online supplemental table S3.
Histology and immunohistochemistry confirmed proteomic Brachyspira identifications
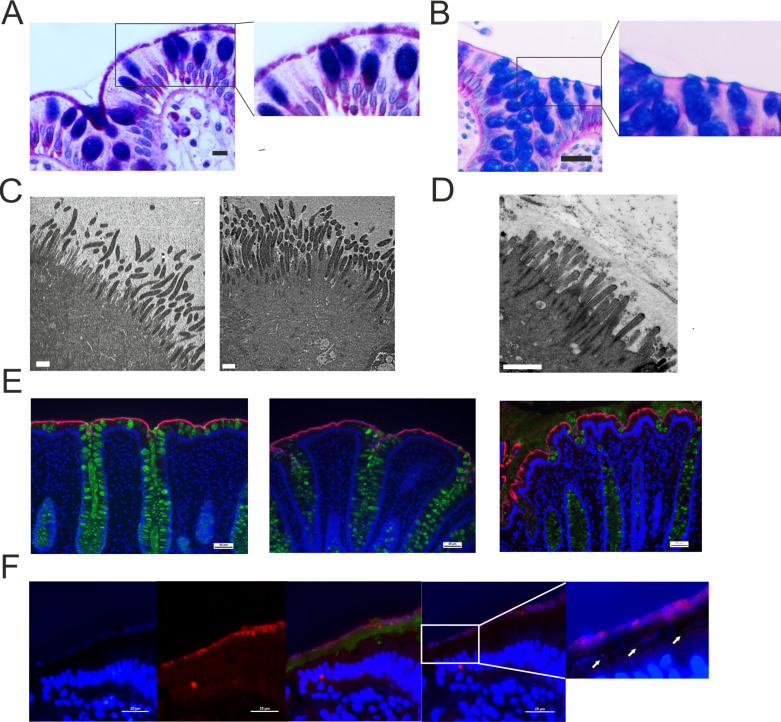
In the three patients with proteomic Brachyspira identifications, spirochetes could be visualised at the apical membrane by AB-PAS staining (figure 2A, B). Transmission electron microscopy showed Brachyspira densely colonising the epithelial surface, attaching between the microvilli (figure 2C, D). These observations were further confirmed by immunofluorescent staining with Brachyspira antiserum (figure 2E).14 When the immunofluorescence analysis was extended to the entire explorative cohort, Brachyspira colonisation of the colonocyte membrane (membrane-associated spirochetosis) was detected in three additional patients with IBS. In other cases, Brachyspira was observed in the mucus, occasionally invading the inner layer (figure 2F). By contrast, all healthy volunteer samples were negative for Brachyspira.
Figure 2.
Histology, immunohistochemistry and transmission electron microscopy demonstrated the presence of Brachyspira at the colonic epithelial surface. (A) Alcian blue-periodic acid-Schiff (PAS) stain showing Brachyspira bacteria at the apical membrane. Scale bar: 10 µm. (B) Representative Alcian blue-PAS stain from a patient with IBS without Brachyspira. Scale bar: 25 µm. The right-hand images represent zoomed sections from the left panel. (C) Transmission electron micrographs showing Brachyspira attaching to the epithelial surface between the (shorter and less electron-dense) microvilli. Scale bar: 1 µm. (D) Transmission electron micrograph from a patient without Brachyspira. Scale bar: 1 µm. (E) Sections from three patients with IBS where Brachyspira species were detected by proteomics, stained with Brachyspira antiserum (red) and co-stained for mucus with anti-CLCA1 (green). DNA was counterstained with Hoechst (blue). Scale bars: 50 µm. (F) In certain patients, Brachyspira colonisation was restricted to mucus. From left to right: (1) bacteria at a distance from the epithelium, visualised by DNA stain (Hoechst); (2) immunostaining for Brachyspira; (3) merge of images showing Brachyspira (red), mucus (anti-CLCA1, green) and DNA (Hoechst, blue) staining of nuclei and bacteria, demonstrating co-localisation of Brachyspira and DNA staining for bacteria in the outer mucus layer. (4) Brachyspira (red) and other bacteria (blue) could also be observed in the inner mucus layer, as indicated by arrows. Scale bars: 25 µm.
Remarkably, routine histological assessment of six individuals with membrane-associated spirochetosis failed to identify this condition in any patient. To further investigate why an association between Brachyspira and IBS has not previously been observed, 16S rDNA sequencing of faecal samples was undertaken in four patients with spirochetosis diagnosed by multiple methods (online supplemental data file S2); this approach also failed to detect Brachyspira in all cases.
gutjnl-2020-321466supp003.xlsx (15.5KB, xlsx)
Targeted analyses of colonic biopsies verified high prevalence of spirochetosis in IBS
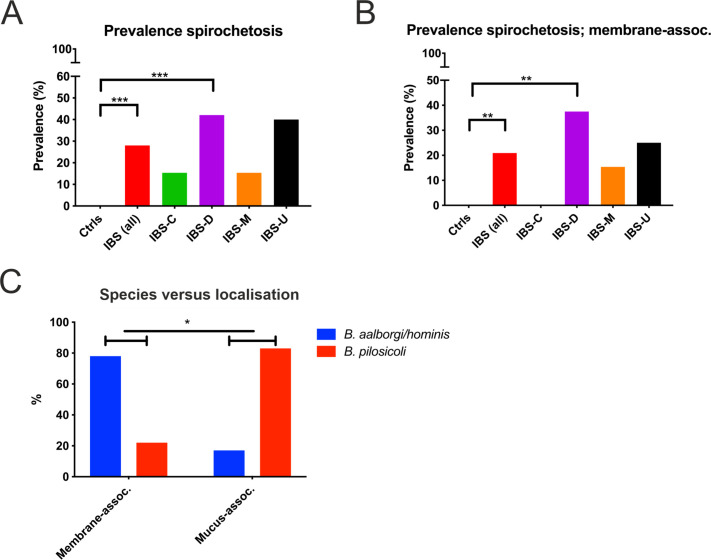
Two different methods for targeted real-time PCR analysis of biopsy material were used in conjunction with immunofluorescence to establish spirochetosis prevalence rates in both cohorts.12 14 In the entire study population, the prevalence of Brachyspira colonisation in IBS was 31% (19/62 patients), with no cases identified among the 31 healthy controls (p<0.001). To minimise the risk of false positive results, subsequent analyses were focused on cases that were either positive, or consistently negative for Brachyspira according to ≥2 methods. These criteria were fulfilled for 80/93 (86%) participants; 50 patients with IBS and 30 healthy individuals. With this restriction, the overall prevalence of Brachyspira colonisation in IBS was 28% (14/50; p<0.001; figure 3A). Brachyspira prevalence in IBS-D was 42%, with highly congruent results in the two cohorts (44% and 40%; p=0.01 for both comparisons with healthy volunteers; online supplemental table S4).
Figure 3.
Prevalence of intestinal spirochetosis in IBS, and association between species and colonisation pattern. (A) Overall prevalence rates of Brachyspira colonisation in healthy individuals and in different IBS subtypes. (B) Prevalence of membrane-associated spirochetosis. In (A) and (B) data from the entire study population (both cohorts) are shown; these are based on agreement between at least two independent diagnostic methods. Membrane-associated spirochetosis was diagnosed by immunohistochemistry, with Brachyspira colonisation confirmed by at least one additional method. Patients with mucus-associated spirochetosis are not included in the analysis in (B). (C) Comparison of the distribution of Brachyspira species among patients with membrane-associated and mucus-associated spirochetosis. Groups were compared by Fisher’s exact test: *p≤0.05 **p≤0.01 ***p≤0.001. IBS-C, IBS with constipation; IBS-D, IBS with diarrhoea; IBS-M, IBS with mixed bowel habits; IBS-U, unsubtyped IBS without diarrhoea or constipation.
A total of 43 patients with IBS and 27 controls had conclusive results from immunofluorescence analysis, which could be verified by at least one additional method. Membrane-associated spirochetosis was detected in 21% (9/43) of patients with IBS but not in any control (p=0.01; figure 3B). The prevalence of membrane-associated spirochetosis was 38% for IBS-D versus 0% for IBS-C (p=0.05). Prevalence rates stratified by cohort and IBS subtype are compiled in online supplemental table S4A‒D. Individual and combined results for the different methods for Brachyspira identification in patients with confirmed spirochetosis are provided in online supplemental table S5.
Different niche preferences for B. aalborgi/hominis and B. pilosicoli
According to species discrimination by real-time PCR, 50% of patients with spirochetosis were colonised by B. pilosicoli; others had either B. aalborgi or the closely related, unconfirmed, species B. hominis.12 Membrane-associated spirochetosis was linked to B. aalborgi/hominis in 78% of cases, whereas B. pilosicoli accounted for 83% of identifications restricted to mucus (p=0.04; figure 3C), suggesting that these species typically occupy different niches.
Membrane-associated spirochetosis defined a clinically distinctive subset of patients with IBS
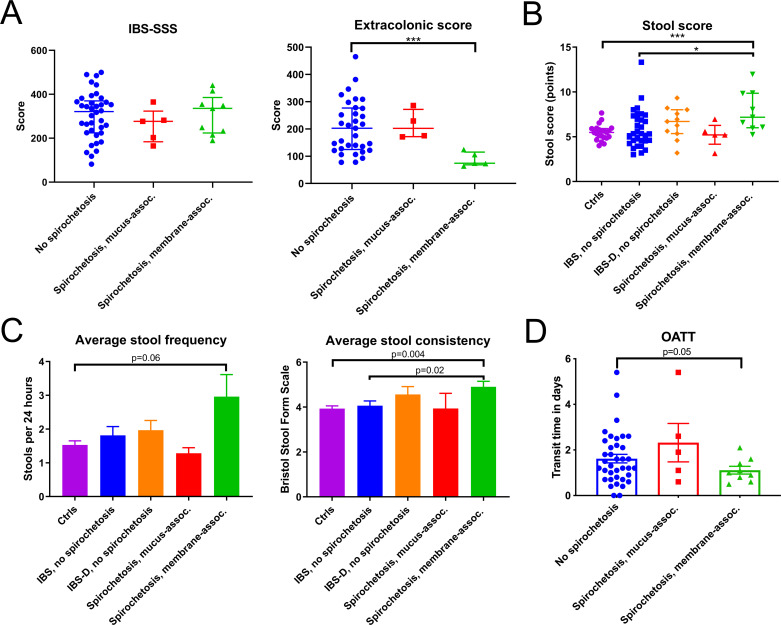
For the analysis of clinical characteristics and immune responses, the two cohorts were merged. The analysis was restricted to participants with consistent results from ≥2 methods for Brachyspira identification (n=80) or with negative results from real-time PCR with no other tests performed (n=3). The demographic distribution of patients with IBS with versus without spirochetosis did not differ significantly, nor did total and component IBS-SSS scores or rectal sensitivity (table 2, figure 4A, online supplemental figure S3A, B).7 By contrast, Extracolonic Symptom Severity Scores were significantly lower in membrane-associated spirochetosis (figure 4A).8 To quantify diarrhoeal symptoms, a ‘stool score’, representing the sum of average stool frequency and Bristol scale consistency, was constructed for each participant. This score was higher in membrane-associated spirochetosis than in patients with IBS without Brachyspira and controls (p=0.02 and <0.001, respectively; figure 4B). Separate comparisons of stool frequency and consistency are shown in figure 4C. Oro-anal transit was also accelerated in membrane-associated spirochetosis as compared with other patients with IBS (p=0.03; figure 4D).
Table 2.
Demographic and clinical characteristics of patients with IBS with and without intestinal spirochetosis
| No spirochetosis* | Spirochetosis; all† | Spirochetosis; mucus-associated‡ | Spirochetosis; membrane-associated‡ | |
| No of participants | 36 | 14 | 5 | 9 |
| No of females (%) | 27 (75) | 10 (71) | 4 (80) | 6 (67) |
| Median age (p25–p75)§ | 31 (24–42) | 28 (24–31) | 29 (28–45) | 26 (23–30) |
| Median duration, years (p25–p75)§ | 15 (7–24) | 10 (4–13) | 10 (7–18) | 10 (1–10) |
| No of IBS-D (%) | 11/36 (31) | 8/14 (57) | 2/5 (40) | 6/9 (67) |
| No of IBS-C (%) | 11/36 (31) | 2/14 (14) | 2/5 (40) | 0/9 (0) |
| No of IBS-M (%) | 11/36 (31) | 2/14 (14) | 0/5 (0) | 2/9 (22) |
| No of IBS-U (%) | 3/36 (8) | 2/14 (14) | 1/5 (20) | 1/9 (11) |
| Median IBS-SSS (p25–p75)§ | 318 (231–365) | 280 (220–354) | 277 (203–282) | 336 (233–355) |
| Median extracolonic SSS (p25–p75)§ | 189 (123–271) | 125 (75–175) | 202 (174–244) | 75 (73–106) |
| Median OATT5 (p25–p75)§ | 1.3 (0.8–2.4) | 1.1 (0.8–1.8) | 1.9 (1.1–2.6) | 1.0 (0.8–1.4) |
| Median rectal pain threshold, mm Hg (p25–p75)§ | 28 (20–28) | 28 (20–32) | 32 (27–37) | 20 (20–32) |
*Patients with IBS negative for Brachyspira according to at least two different methods, with no positive results.
†Patients with IBS positive for Brachyspira according to at least two methods.
‡The stratification of mucus-associated and membrane-associated spirochetosis was based on immunofluorescence analysis of biopsy sections.
§p25, 25th percentile; p75, 75th percentile.
IBS-C, IBS with constipation; IBS-D, IBS with diarrhoea; IBS-M, IBS with mixed bowel habits; IBS-U, unsubtyped IBS without diarrhoea or constipation; OATT, oro-anal transit time (days); SSS, Severity Scoring System.
Figure 4.
Individuals with membrane-associated spirochetosis constitute a clinically distinctive subgroup of patients with IBS. (A) Patient-reported IBS symptom severity according to the IBS Severity Scoring System (IBS-SSS) did not differ between patients with and without spirochetosis. However, Extra-colonic Symptom Scores were lower in membrane-associated spirochetosis (Mann-Whitney U=11.5; p<0.001). (B) Stool scores (summed average stool frequency and average Bristol scale consistency) were higher in membrane-associated spirochetosis than in patients with IBS without Brachyspira, indicating more diarrhoeal symptoms (U=68.0; p=0.02). In (A) and (B) individual observations are overlaid by the median and interquartile range. The significance threshold was adjusted by the Bonferroni method. *p≤0.025, ***p≤0.001. (C) Average stool frequency and consistency for controls and the different patient groups. Results for patients with IBS with diarrhoea (IBS-D) without Brachyspira (n=11) are separately displayed, as well as included in the category of all patients with IBS without spirochetosis. (D) Oro-anal transit times (OATT) for patients with IBS with versus without Brachyspira. OATT were shorter in membrane-associated spirochetosis as compared with IBS without spirochetosis (p=0.05) and patients with IBS without Brachyspira colonisation of the epithelial membrane (p=0.03; Welch-corrected t=2.3). For (C) and (D) bars represent the mean and error bars the SE of the mean (SEM); groups were compared by unpaired Welch’s t-tests. For all analyses of clinical characteristics, the two study cohorts were merged. The analysis was restricted to participants where Brachyspira colonisation could be confirmed or rejected by at least two independent methods or cases where real-time PCR was negative for Brachyspira with no conclusive results from other methods.
Brachyspira colonisation was linked to alterations of molecular pathways associated with membrane remodelling and ion–fluid homeostasis
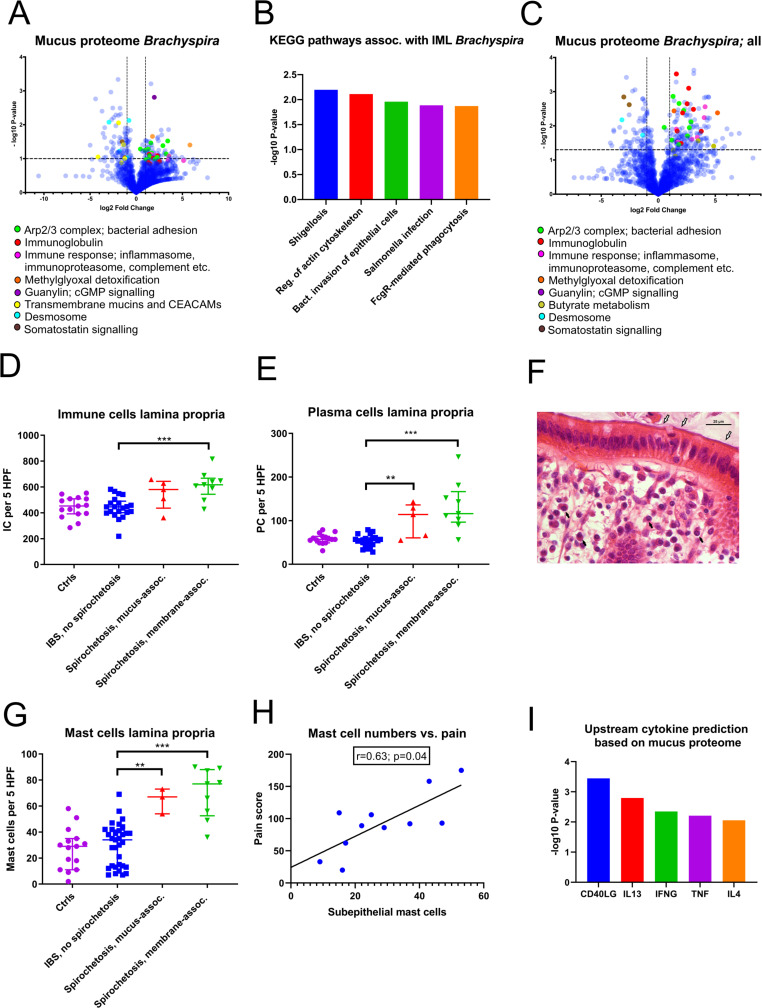
To delineate the underlying mechanisms behind the symptoms observed in spirochetosis, the host mucus proteome was analysed (online supplemental data file S3). A global increase in proteins associated with bacterial adhesion and invasion, notably the Arp2/3 complex, was observed in patients with proteomic Brachyspira identifications (figure 5A). Furthermore, inflammatory mediators—complement factors, inflammasome and immunoproteasome components and particularly immunoglobulins—were strongly induced. Consequently, the most enriched gene ontology biological pathways were associated with antibody-mediated phagocytosis (online supplemental figure S4A). An overlay with the Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway library revealed distinct resemblances with enteric infections, such as shigellosis and salmonellosis (figure 5B).
Figure 5.
Spirochetosis was linked to distinct mucosal responses at the molecular and cellular level. (A) Volcano plot showing host mucus proteome alterations in samples where Brachyspira was detected by metaproteomics (n=6, vs n=18 samples from individuals without Brachyspira). The transformed p values were obtained by Welch’s t-tests. (B) Top five predicted Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathways based on mucus protein enrichment in individuals with metaproteomic Brachyspira identifications. Proteins at least twofold upregulated with a p value <0.10 were included in the analysis. Pathway p values were obtained through a modified version of Fisher’s exact test (EASE score), using DAVID bioinformatics tool. IML, inner mucus layer. (C) Host mucus proteome alterations in samples (n=17) from patients where Brachyspira could be detected by two independent methods (some of whom were negative for Brachyspira in the mass spectrometry analysis). (D) Increased immune cell infiltration of the lamina propria of patients with membrane-associated spirochetosis compared with patients with IBS without Brachyspira (Mann-Whitney U=17.0; p<0.001). The graph shows results from patients with membrane-associated spirochetosis (n=9), mucus-associated spirochetosis (n=5), patients with IBS without Brachyspira (n=21) and healthy volunteers (n=15). (E) Plasma cell counts were increased in spirochetosis (membrane-associated spirochetosis vs IBS without Brachyspira: U=9.0; p<0.001). (F) Plasma cells (black arrows) in an H&E-stained sigmoid colon section from a patient with membrane-associated Brachyspira (white arrows). Scale bar: 25 µm. (G) Mast cell expansion in the lamina propria of patients with spirochetosis (membrane-associated spirochetosis vs IBS without Brachyspira: U=19.5; p<0.001). The graph shows results from 9 patients with IBS with membrane-associated spirochetosis, 3 patients with mucus-associated spirochetosis, 33 patients with IBS without Brachyspira and 15 healthy volunteers. (H) Correlation between subepithelial mast cell numbers and pain (summed pain frequency and intensity scores from the IBS Severity Scoring System questionnaire). The line was fitted using linear regression analysis. Spearman’s rho: 0.63 (95% CI: 0.02 to 0.90). Immune cells were counted in five high-power fields (MAG ×600) in H&E or toluidine blue (mast cells) stained sigmoid colon sections; individual observations are overlaid by the median and IQR. Groups were compared by the Mann-Whitney U test: **p≤0.01, ***p≤0.001. (I) Upstream activation of cytokines in patients with proteomic Brachyspira identifications, predicted from Ingenuity Pathway Analysis of the host mucus proteome. Data were analysed through the use of IPA (Qiagen, https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis). Minimum twofold upregulated proteins with a p value <0.15 were included in the analysis. P values for cytokine prediction were obtained by Fisher’s exact test. CEACAMs, carcinoembryonic antigen-related cell adhesion molecule; IC, immune cell; PC, plasma cell.
gutjnl-2020-321466supp004.xlsx (772.7KB, xlsx)
Other significantly dysregulated proteins were linked to guanylate cyclase/cGMP (guanylin, online supplemental figure S4B) and somatostatin (SST, PCSK1N; online supplemental figure S4C) signalling, as well as to methylglyoxal detoxification (GLO1, PARK7; online supplemental figure S4D). Guanylin is an endogenous ligand of guanylate cyclase-C (GC-C), also known as the heat-stable enterotoxin receptor.16 GC-C signalling attenuates, whereas somatostatin promotes, fluid reabsorption.17 Methylglyoxal is a minor by-product of human glycolysis, as well as a toxic metabolite of several intestinal bacterial species.18 While guanylin and proteins related to methylglyoxal toxicity were increased in spirochetosis, somatostatin levels were decreased (online supplemental figure S4B‒D).
In patients with proteomic Brachyspira identifications, indicating dense membrane colonisation, glycocalyx and desmosomal proteins were reduced, suggesting multilevel mucosal barrier weakening (figure 5A). Indications of membrane remodelling were less conspicuous in individuals with no, or patchy, epithelial colonisation (figure 5C, online supplemental figure S4E). By contrast, inflammatory mediators did not substantially differ depending on colonisation pattern.
Spirochetosis was associated with mild mucosal inflammation and mast cell activation
Histology revealed a modest increase in lamina propria immune cells (p<0.001), particularly plasma cells (p<0.001), in membrane-associated spirochetosis compared with IBS without Brachyspira (figure 5D–F). Intraepithelial/subepithelial eosinophils were also augmented (p=0.002; online supplemental figure S5A, B). Full results of the differential counting of mucosal immune cells are provided in online supplemental tables S6A, B.
Strikingly, total and activated mast cells were significantly more abundant in patients with IBS with Brachyspira, based on toluidine blue staining (figure 5G, online supplemental figure S5C, D). Subepithelial mast cell counts correlated closely with abdominal pain in spirochetosis (figure 5H, online supplemental figure S5E) but not in patients with IBS without Brachyspira. In the proteomic analysis, mast cell proteins tryptase, chymase and/or beta-hexosaminidase were more prevalent in mucus from patients with spirochetosis (p=0.09), whereas enzymes involved in histamine metabolism were more abundant (p=0.04 for the comparison with patients with IBS without Brachyspira; online supplemental figure S5F, online supplemental data file S3). Based on the overall mucus proteome alterations, cytokines CD40LG, IL13 and IL4 were predicted as major upstream regulators (figure 5I). Taken together, our observations suggest the immune response in Brachyspira infection to be predominantly Th2-driven.
Brachyspira colonisation was linked to mucus barrier failure and crypt invasion by other bacteria
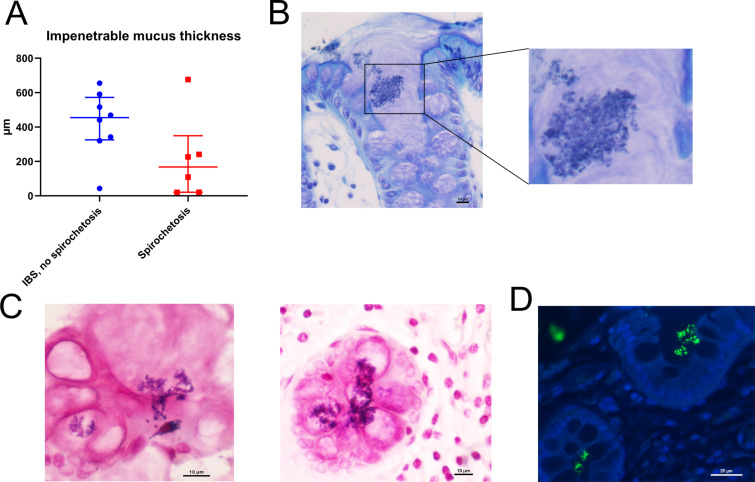
While the main component of the mucus, the MUC2 mucin, tended to be more abundant in spirochetosis, the thickness of the impenetrable mucus was reduced (figure 6A, online supplemental figure S6A). Moreover, bacterial invasion into crypts and goblet cells was occasionally detected (figure 6B, C, online supplemental figure S6B‒D). These observations did not match Brachyspira staining (online supplemental figure S6E). Instead, at least some of the invading bacteria appeared Gram positive, which was verified through staining with anti-lipoteichoic acid (figure 6C, D).
Figure 6.
Brachyspira colonisation was associated with mucus barrier failure. (A) Impenetrable mucus thickness tended to be reduced in patients with spirochetosis (p=0.11; Mann-Whitney U test). Toluidine blue (B) and Gram (C) staining of sigmoid colon sections from patients with spirochetosis showed bacterial invasion of crypt lumina and goblet cells. Some of the invading bacteria appeared Gram positive. Scale bars: 10 µm. (D) Section from a patient with spirochetosis stained with anti-lipoteichoic acid (green) and counterstained for DNA with Hoechst (blue), showing Gram-positive bacteria inside crypts. Scale bar: 25 µm.
Antibiotic treatment in Brachyspira-associated IBS might be linked to partial clinical improvement over time
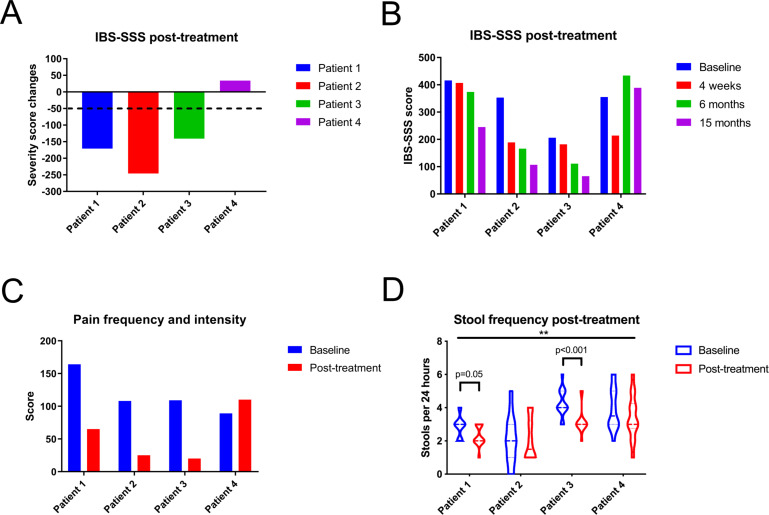
Based on current recommendations, four patients with membrane-associated spirochetosis received metronidazole treatment (online supplemental table S7).13 Although initial responses were variable, three patients could potentially be classified as long-term responders, with a reduction in overall IBS-SSS scores >50 points 1 year post-treatment (figure 7A, B).7 In particular, pain, bloating and IBS interference with quality of life were reduced (figure 7C, online supplemental figure S7A, B). Bowel habit dissatisfaction and stool consistency did not conclusively improve (online supplemental figure S7C, D). However, in two responders, stool frequency was significantly reduced post-treatment, while the third responder had a normal frequency at baseline (figure 7D, online supplemental figure S7E).
Figure 7.
Three in four patients with spirochetosis experienced partial clinical improvement following antibiotic treatment. (A) Three patients had a sustained clinical response, defined as a reduction of their overall scores from the IBS Severity Scoring System (IBS-SSS) of at least 50 points, after 15 months. (B) Overall IBS-SSS scores at baseline and at 1, 6 and 15 months post-treatment. (C) Summed IBS-SSS pain frequency and intensity values at baseline and at 15 months post-treatment. (D) Violin plot of stool frequency at baseline and 1 year post-treatment. The midline represents the median and the upper and lower lines the interquartile range (25th and 75th percentile). Time points were compared through a two-way analysis of variance, with the horizontal bar on top referring to the overall comparison of stool frequency before and after treatment (F=7.0; p=0.01). Subsequent intraindividual comparisons were performed by Mann-Whitney U tests, with an adjusted significance threshold of 0.0125.
Spirochaete relocation into crypts and goblet cells post-antibiotics
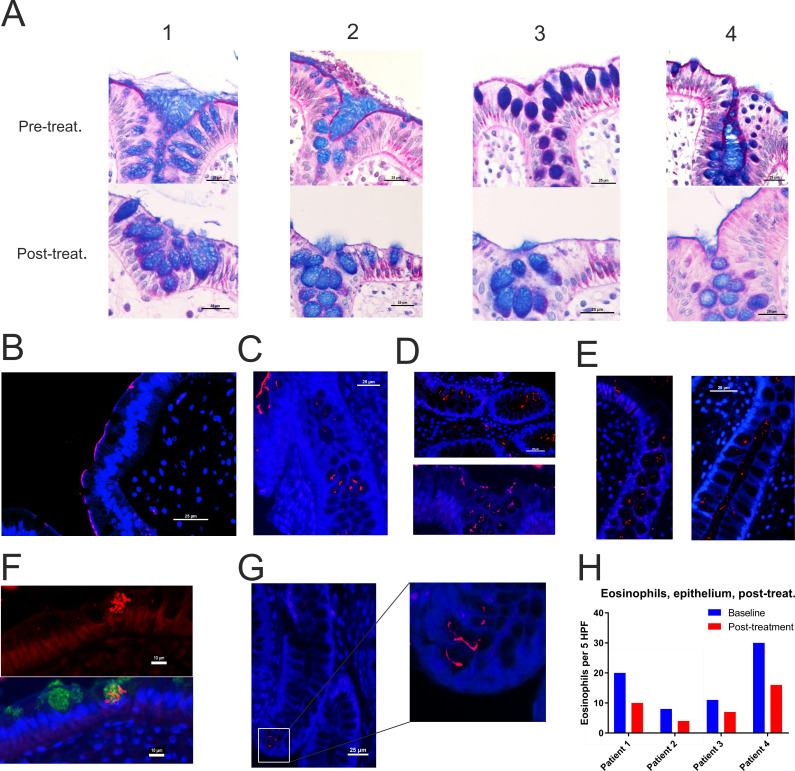
In all four cases, AB-PAS staining of tissue sections demonstrated clearance of the Brachyspira from the epithelium 6 weeks post-treatment (figure 8A). Real-time PCR of colonic biopsies and faecal samples corroborated a drastic reduction in Brachyspira (online supplemental table S8A, B).
Figure 8.
Invasion of spirochetes into crypts and goblet cells post-treatment. (A) Alcian blue-periodic acid-Schiff stain of (from left to right) patients 1–4 before and 6 weeks after the completion of treatment. Staining with Brachyspira antiserum (red) revealed a persistent, faint/patchy staining of the apical membrane after treatment in the non-responder ((B), patient 4) and in patient 1 (C). Furthermore, Brachyspira (red) was observed in crypt lumina and goblet cells in all cases, as shown for patients 1 (C), 2 (D), 3 (E) and 4 (see online supplemental figure S8). (F) Brachyspira (red) co-localised with anti-MUC2 staining (green) inside goblet cells. The figure shows images of the same area that were obtained from adjacent sections, manually fitted and superimposed. (G) Brachyspira (red) penetrating into the crypt base. In all immunofluorescent images, nucleic acids were counterstained with Hoechst (blue). Scale bars in (F) are 10 µm, all other scale bars 25 µm. (H) There was a reduction of subepithelial/intraepithelial eosinophils post-treatment in all cases.
However, results from immunostaining for Brachyspira in biopsies obtained 6 weeks post-metronidazole were less encouraging. Although the spirochetes had largely disappeared from the epithelial surface, Brachyspira invasion into crypts and goblet cell mucus granules was observed in all four patients (figure 8B–G, online supplemental figure S8A). Gram staining did not indicate a similar relocation of Gram-positive bacteria post-treatment (online supplemental figure S8B), suggesting a specific rather than general response.
While overall mucosal immune cell numbers remained unchanged after treatment, there was a reduction in plasma cell, mast cell and eosinophil counts (online supplemental figure S8C‒F, figure 8H). Taken together, the cellular composition appeared to deviate from a Th2-like to a Th1-like pattern—possibly reflecting the Brachyspira shift to an intracellular lifestyle.
Discussion
The increased incidence of IBS after a gastroenteritis episode suggests microbial perturbation as an underlying factor.1 However, studies of faecal microbiota in IBS have not demonstrated reproducible alterations.2 In this investigation, potentially pathogenic Brachyspira species were identified in the colonic mucosa of 31% of patients with IBS but not in any healthy individual. Brachyspira attachment to the epithelial brush border was observed in every fifth patient with IBS and associated with diarrhoea, accelerated oro-anal transit, mild mucosal inflammation and mast cell activation. Hence, Brachyspira colonisation defines a sizeable and distinctive subgroup of patients with IBS that might be responsive to antibiotic therapy. However, metronidazole treatment paradoxically resulted in spirochaete invasion into crypts and goblet cells. Thus, our observations suggest a role for Brachyspira in IBS-D pathogenesis but also urge caution with regard to antibiotic therapy in IBS.
While Brachyspira species are well-established pathogens in veterinary medicine, reports on the relationship between spirochetosis and symptoms in humans are contradictory.15 19–23 Variable, frequently partial, responses to treatment have added to the controversy.13 15 20 Potentially human-pathogenic Brachyspira species are notoriously difficult to culture. Therefore, the diagnosis typically relies on histology, where perpendicular attachment of the Brachyspira to the apical membrane may appear as a distinctive ‘fringe’.14 15 19–21 24 Reported prevalence rates of intestinal spirochetosis range from 0.5% to 3% in industrialised countries.13 15 21–23 Here, based on results from two independent prospective cohorts, Brachyspira were observed in contact with the epithelium in 20%–40% of patients with IBS-D but not in patients with predominant constipation or healthy individuals.
Although one previous publication has suggested a link between IBS and intestinal spirochetosis, this is the first report of increased prevalence of Brachyspira colonisation in IBS.21 Patients with IBS-D routinely undergo recto-/colonoscopy with histology. Moreover, 16S rDNA sequencing of faecal samples has been performed in several large-scale studies.2 3 Thus, it may be surprising that this association should have gone undetected. Here instead, the initial observations were made through unbiased metaproteomic analysis of mucus samples and corroborated by immunostaining and targeted PCR analysis. By contrast, routine histology and faecal 16S rDNA sequencing failed to detect Brachyspira in these patients. The ability of standard 16S amplicon sequencing to identify Brachyspira species was recently shown to be hampered by primer incompatibility.25 This underlines the importance of innovative and complementary methods to identify novel associations between gut microbiota and human disease.
The effects of Brachyspira attachment on the epithelium and underlying tissue have traditionally been regarded as minimal.15 24 Here, in-depth analysis of host mucosal responses revealed a striking increase in inflammatory mediators and proteins associated with membrane remodelling. Unbiased functional annotation analysis mapped these alterations to pathways associated with bacterial adhesion and phagocytosis, detecting mechanistic resemblances to enteric infections such as salmonellosis and shigellosis. Histology confirmed a modest but distinctive inflammatory response, with expansion of plasma cells, eosinophils and mast cells. The cellular composition, in conjunction with upstream cytokine prediction based on the proteomic analysis, indicated that the inflammation may be predominantly Th2-driven. Further studies are required to establish a causal relationship between spirochetosis and concomitant mucosal inflammation. Still, our observations suggest Brachyspira as a potential human pathogen rather than merely a harmless commensal.
While diarrhoea has previously been related to spirochetosis, the mechanistic link has not been explored.15 19 Here, based on analysis of host responses, several molecular shifts that may synergistically induce diarrhoea were identified. First, remodelling of the apical membrane might reduce the surface available for fluid re-uptake. Second, a pronounced increase in the mucus abundance of guanylin was observed in membrane-associated spirochetosis. Guanylin is a ligand of the apical GC-C receptor, which is also the target of heat-stable Escherichia coli enterotoxin.16 GC-C signalling attenuates sodium uptake by NHE3 (SLC9A3) and promotes chloride secretion by cystic fibrosis transmembrane conductance regulator (CFTR), resulting in impaired fluid absorption.16 Accordingly, activating mutations of GC-C is a rare cause of IBS-D.16 Moreover, endogenous GC-C signalling is induced in certain enteric infections and may contribute to pathogen clearance.26 Another potential cause of diarrhoea could be decreased somatostatin signalling, as suggested by the proteomic analysis. Somatostatin promotes intestinal fluid absorption, possibly via the NHE8 (SLC9A8) transporter, and is used to treat refractory diarrhoea.17 Somatostatin-producing cells are highly susceptible to Helicobacter pylori-related inflammation and might conceivably also be particularly affected by spirochetosis. Finally, histamine secretion from activated mast cells may promote secretomotor diarrhoea.27 In conclusion, this study identified several mechanistic clues to the association between spirochetosis and IBS-D, supporting the biological relevance of this unexpected observation.
Antibiotic treatment of patients with spirochetosis was associated with alleviated abdominal pain and bloating, suggesting that Brachyspira may contribute to IBS symptoms other than diarrhoea. Mast cell activation has previously been linked to IBS-related abdominal pain, although reports are conflicting.28 29 In this study, mucosal mast cell counts were selectively increased in Brachyspira-associated IBS and correlated closely with abdominal pain scores in patients with spirochetosis but not in IBS without Brachyspira. Thus, the controversial link between mast cells and IBS symptomatology might in part depend on Brachyspira colonisation status.
Spirochetosis was associated with mucus barrier failure, decreased glycocalyx components and sporadic crypt invasion by other genera. Furthermore, there were indications of enhanced mucosal responses to bacterial metabolites—including methylglyoxal that is likely not produced by Brachyspira. Methylglyoxal has been linked to visceral hypersensitivity, for example, through the induction of nociceptor ion channel TRPA1.18 30 Hence, inappropriate host contact with commensal microbiota could aggravate symptoms in patients with spirochetosis and may partly explain variations of the clinical picture.
Several studies have reported positive effects of non-absorbable antibiotics in a subset of patients with IBS.2 31 In our investigation, three in four patients with spirochetosis experienced partial, but sustained, symptom relief following metronidazole treatment. The response correlated with improvement of objective parameters, such as stool frequency and mast cell counts. Results from this small-scale, open-label intervention must obviously be interpreted with caution. The effects of Brachyspira eradication in IBS will hopefully be the topic of future, appropriately powered, placebo-controlled trials.
Our observations tentatively suggest that stratification of patients with IBS with spirochetosis for antibiotic treatment might result in improved response rates. Nonetheless, there are obvious caveats, including growing resistance problems reported from veterinary medicine.32 In this study, the therapeutic intervention had to be discontinued due to spirochaete relocation into goblet cells. Concerningly, Brachyspira were also detected in the crypt base post-antibiotics, with the potential to directly influence the stem cell reservoir. Thus, therapeutic strategies other than antibiotics may have to be considered to treat IBS-associated spirochetosis. These might include short-term laxative treatment, bismuth subsalicylate or probiotics/prebiotics, used alone or as adjuvants to antibiotics.
While bacterial invasion of goblet cell mucus granules post-antibiotics is a novel phenomenon, it is partly reminiscent of goblet cell-associated antigen passages (GAPs).33 In mice, GAPs mediate translocation of live bacteria through the colonic epithelium in response to dysbiosis, for instance during metronidazole treatment.33 Nevertheless, the time lapse since antibiotic therapy and the massive scale of Brachyspira relocation rather support an adaptation of the spirochetes. Thus, certain Brachyspira presumably have a tropism for colonising mucus granules—a trait that may have been favoured by antibiotic treatment. Although metronidazole easily diffuses into cells, the densely packed granule contents might restrict its access to the Brachyspira. Future studies should investigate whether antibiotics promote similar adaptations in other pathogenic or commensal bacterial species.
The study has some limitations. Suboptimal biopsy specimens and technical failures resulted in a small proportion of missing data for certain methods, although with no systematic bias between participant categories. Most importantly, the observational nature of this study did not allow us to establish a causal relationship between Brachyspira and IBS symptoms. Other unanswered questions concern the potential contribution of epidemiological factors to Brachyspira colonisation—including antibiotic treatment, which may be more frequent in patients with IBS and has been linked to increased risk of post-infectious IBS.1 The exact molecular mechanisms of the Brachyspira-host interaction also require further investigation. However, as the main species associated with intestinal spirochetosis, B. aalborgi, is exceptionally difficult to culture and infects only primates, experimental models to address this question are lacking.14 24 Hence, there are major challenges to overcome in order to fully elucidate the role of Brachyspira in IBS-D pathogenesis.
In conclusion, we report a novel, strong association between the Brachyspira genus and IBS. Based on results from two independent prospective cohorts, mucosal Brachyspira colonisation was observed in 40% of patients with IBS-D but not in any healthy individual. Brachyspira-associated IBS was linked to distinctive clinical, histological and molecular characteristics, suggesting that it should be considered a separate diagnostic entity. Thus, Brachyspira eradication therapy could conceivably have substantial effects on the overall IBS morbidity burden. However, the increased invasiveness of the spirochetes post-treatment urges caution with regard to antibiotic therapy in IBS. Strikingly, the invasion of goblet cell mucus granules could represent a novel bacterial strategy to survive antibiotics, which may have bearings on other recurrent and chronic infections originating at mucosal surfaces.
Acknowledgments
The authors wish to thank Ms Gunilla Naslin (Department of Gastroenterology and Hepatology, Sahlgrenska University Hospital, Gothenburg) for excellent assistance with sample collection and the administration of patient follow-up. Dr Liisa Arike (Department of Medical Biochemistry, University of Gothenburg) is gratefully acknowledged for her assistance with the mass spectrometry analysis. We would also like to thank Dr Valentina Tremaroli (Department of Molecular and Clinical Medicine, University of Gothenburg) for kindly providing her time and expertise to assist us with the 16S rDNA sequencing analysis. Professor Lars Engstrand (Department of Microbiology, Karolinska Institute, Stockholm) is acknowledged for the generous provision of the Brachyspira antiserum. The authors thank the Center for Cellular Imaging (CCI) at the University of Gothenburg for help with sample preparation for electron microscopy and Histocenter, Gothenburg, for assistance with preparation of sections for histology.
Footnotes
Contributors: KSJ, MS and GCH conceived the original idea; KSJ, BD, LE, CW, AE and ÅJ performed experimental procedures and data analysis; HT and MS supervised subject enrolment; KSJ, LE, HT and MS analysed clinical data, KSJ drafted the manuscript with input from all authors.
Funding: This work was supported by the National Institute of Allergy and Infectious Diseases (U01AI095473), Knut and Alice Wallenberg Foundation, European Research Council (ERC), Swedish Research Council, AFA insurance, IngaBritt and Arne Lundberg Foundation, Sahlgrenska University Hospital (ALF), Wilhelm and Martina Lundgren Foundation, Adlerbert Research Foundation. The funders were not involved in the decision to publish; and had no role in the design and execution of the study, or in the preparation of the manuscript.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Competing interests: MS has received unrestricted research grants from Danone Nutricia Research and Ferring Pharmaceuticals, has served as a Consultant/Advisory Board member for AstraZeneca, Danone, Nestlé, Almirall, Allergan, Albireo, Glycom, and Shire, and as a speaker for Tillotts, Menarini, Takeda, Shire, Allergan, and Almirall.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the Gothenburg Ethical Review Board.
References
- 1. Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology 2009;136:1979–88. 10.1053/j.gastro.2009.02.074 [DOI] [PubMed] [Google Scholar]
- 2. Simrén M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome Foundation report. Gut 2013;62:159–76. 10.1136/gutjnl-2012-302167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tap J, Derrien M, Törnblom H, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology 2017;152:111–23. 10.1053/j.gastro.2016.09.049 [DOI] [PubMed] [Google Scholar]
- 4. Johansson MEV, Phillipson M, Petersson J, et al. The inner of the two MUC2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 2008;105:15064–9. 10.1073/pnas.0803124105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li H, Limenitakis JP, Fuhrer T, et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun 2015;6:8292. 10.1038/ncomms9292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol 2002;161:1961–71. 10.1016/S0002-9440(10)64472-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395–402. 10.1046/j.1365-2036.1997.142318000.x [DOI] [PubMed] [Google Scholar]
- 8. Gonsalkorale WM, Houghton LA, Whorwell PJ. Hypnotherapy in irritable bowel syndrome: a large-scale audit of a clinical service with examination of factors influencing responsiveness. Am J Gastroenterol 2002;97:954–61. 10.1111/j.1572-0241.2002.05615.x [DOI] [PubMed] [Google Scholar]
- 9. Gustafsson JK, Ermund A, Johansson MEV, et al. An ex vivo method for studying mucus formation, properties, and thickness in human colonic biopsies and mouse small and large intestinal explants. Am J Physiol Gastrointest Liver Physiol 2012;302:G430–8. 10.1152/ajpgi.00405.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiśniewski JR, Zougman A, Nagaraj N, et al. Universal sample preparation method for proteome analysis. Nat Methods 2009;6:359–62. 10.1038/nmeth.1322 [DOI] [PubMed] [Google Scholar]
- 11. Cox J, Neuhauser N, Michalski A, et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res 2011;10:1794–805. 10.1021/pr101065j [DOI] [PubMed] [Google Scholar]
- 12. Westerman LJ, Stel HV, Schipper MEI, et al. Development of a real-time PCR for identification of Brachyspira species in human colonic biopsies. PLoS One 2012;7:e52281. 10.1371/journal.pone.0052281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Esteve M, Salas A, Fernández-Bañares F, et al. Intestinal spirochetosis and chronic watery diarrhea: clinical and histological response to treatment and long-term follow up. J Gastroenterol Hepatol 2006;21:1326–33. 10.1111/j.1440-1746.2006.04150.x [DOI] [PubMed] [Google Scholar]
- 14. Kraaz W, Pettersson B, Thunberg U, et al. Brachyspira aalborgi infection diagnosed by culture and 16S ribosomal DNA sequencing using human colonic biopsy specimens. J Clin Microbiol 2000;38:3555–60. 10.1128/JCM.38.10.3555-3560.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsinganou E, Gebbers J-O. Human intestinal spirochetosis--a review. Ger Med Sci 2010;8:Doc01. 10.3205/000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waldman SA, Camilleri M. Guanylate cyclase-C as a therapeutic target in gastrointestinal disorders. Gut 2018;67:1543–52. 10.1136/gutjnl-2018-316029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang C, Xu H, Chen H, et al. Somatostatin stimulates intestinal NHE8 expression via p38 MAPK pathway. Am J Physiol Cell Physiol 2011;300:C375–82. 10.1152/ajpcell.00421.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang S, Jiao T, Chen Y, et al. Methylglyoxal induces systemic symptoms of irritable bowel syndrome. PLoS One 2014;9:e105307. 10.1371/journal.pone.0105307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hampson DJ. The spirochete Brachyspira pilosicoli, enteric pathogen of animals and humans. Clin Microbiol Rev 2018;31:e00087–17. 10.1128/CMR.00087-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weisheit B, Bethke B, Stolte M. Human intestinal spirochetosis: analysis of the symptoms of 209 patients. Scand J Gastroenterol 2007;42:1422–7. 10.1080/00365520701245629 [DOI] [PubMed] [Google Scholar]
- 21. Walker MM, Talley NJ, Inganäs L, et al. Colonic spirochetosis is associated with colonic eosinophilia and irritable bowel syndrome in a general population in Sweden. Hum Pathol 2015;46:277–83. 10.1016/j.humpath.2014.10.026 [DOI] [PubMed] [Google Scholar]
- 22. Tanahashi J, Daa T, Gamachi A, et al. Human intestinal spirochetosis in Japan; its incidence, clinicopathologic features, and genotypic identification. Mod Pathol 2008;21:76–84. 10.1038/modpathol.3800987 [DOI] [PubMed] [Google Scholar]
- 23. Westerman LJ, de Boer RF, Roelfsema JH, et al. Brachyspira species and gastroenteritis in humans. J Clin Microbiol 2013;51:2411–3. 10.1128/JCM.01069-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hovind-Hougen K, Birch-Andersen A, Henrik-Nielsen R, et al. Intestinal spirochetosis: morphological characterization and cultivation of the spirochete Brachyspira aalborgi gen. nov., sp. nov. J Clin Microbiol 1982;16:1127–36. 10.1128/JCM.16.6.1127-1136.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thorell K, Inganäs L, Backhans A, et al. Isolates from colonic spirochetosis in humans show high genomic divergence and potential pathogenic features but are not detected using standard primers for the human microbiota. J Bacteriol 2019;201:e00272–19. 10.1128/JB.00272-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mann EA, Harmel-Laws E, Cohen MB, et al. Guanylate cyclase C limits systemic dissemination of a murine enteric pathogen. BMC Gastroenterol 2013;13:135. 10.1186/1471-230X-13-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang YZ, Cooke HJ, Su HC, et al. Histamine augments colonic secretion in guinea pig distal colon. Am J Physiol 1990;258:G432–9. 10.1152/ajpgi.1990.258.3.G432 [DOI] [PubMed] [Google Scholar]
- 28. Braak B, Klooker TK, Wouters MM, et al. Mucosal immune cell numbers and visceral sensitivity in patients with irritable bowel syndrome: is there any relationship? Am J Gastroenterol 2012;107:715–26. 10.1038/ajg.2012.54 [DOI] [PubMed] [Google Scholar]
- 29. Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 2007;132:26–37. 10.1053/j.gastro.2006.11.039 [DOI] [PubMed] [Google Scholar]
- 30. Huang Q, Chen Y, Gong N, et al. Methylglyoxal mediates streptozotocin-induced diabetic neuropathic pain via activation of the peripheral TRPA1 and Nav1.8 channels. Metabolism 2016;65:463–74. 10.1016/j.metabol.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 31. Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011;364:22–32. 10.1056/NEJMoa1004409 [DOI] [PubMed] [Google Scholar]
- 32. Hampson DJ, Lugsomya K, La T, et al. Antimicrobial resistance in Brachyspira - An increasing problem for disease control. Vet Microbiol 2019;229:59–71. 10.1016/j.vetmic.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 33. Knoop KA, McDonald KG, Kulkarni DH, et al. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 2016;65:1100–9. 10.1136/gutjnl-2014-309059 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2020-321466supp001.pdf (8.4MB, pdf)
gutjnl-2020-321466supp002.xlsx (19.7KB, xlsx)
gutjnl-2020-321466supp003.xlsx (15.5KB, xlsx)
gutjnl-2020-321466supp004.xlsx (772.7KB, xlsx)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.